Abstract
This article reviews research activities at the system level on (i) energy harvesting with wearable devices from the human body and (ii) powering up implant devices. The first part reviews wearable devices to harvest energy from the human body for biomedical and portable devices. Harvestable human body energy sources can be classified into two categories, voluntary and involuntary. Voluntary sources are capable of providing high power levels, up to several watts, but are only available when the wearer is active. Involuntary sources are constantly available, but provide much smaller amounts of energy, of the order of milliwatts. The latter part of the article reviews research activities to power up devices implanted in the human body. There are two approaches to power up implant devices. The first approach is to harvest energy from the body or ambient sources. The energy sources are essentially limited to kinetic energy of the body, body heat, and solar. The second approach is to transmit power to implant devices wirelessly. The power level available to implanted devices through harvesting or power transmission is small, often of the order of microwatts.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Technological advances in low-power electronics have increased the feasibility of powering them from ambient energy sources including solar, thermal, kinetic, and RF. Energy harvested from these sources can be used to charge or replace batteries in portable devices. A human body is a bountiful source of energy, which can be harvested to power biomedical devices such as body sensor networks, implants, and even long-range wireless sensor nodes. Harvesting body energy increases the operational life of medical devices or enables perpetual machines without the need for battery replacement or recharging.
A human body releases harvestable energy through two different mechanisms: heat and motion. Some body energy, such as motion from breathing and body heat, is released continuously, regardless of the person’s intention. This kind of energy source, called involuntary, does not require a conscious action. In contrast, voluntary sources, such as walking, jumping, or other spontaneous activities, can generate a much larger amount of energy than involuntary sources, but depend on the human to intentionally provide the energy.
The first part of this article reviews recent research activities in the area of wearable human energy harvesters, which can be applicable to biomedical circuits. All of these devices harness the energy from body heat, voluntary, or involuntary motion. A wide variety of transducers including thermoelectric generators, electromagnetic generators, electrostatic generators, and piezoelectric transducers are considered to harness the energy.
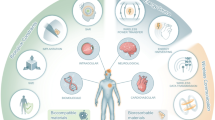
Implantable electronic devices enable us to monitor the human body in real time, and their applications on the human body are expected to expand rapidly as illustrated in Fig. 1 [1]. Several key challenges must be addressed to realize these scenarios: implantable devices should be minimally invasive, completely biocompatible, and with low thermal dissipation and power autonomy. In particular, the power autonomy is critical, since replacement of batteries for implant devices is costly and may involve surgery. The capacity of batteries has increased continuously, but fails to meet the power requirement of most implant devices. Also, size constraints of implant devices may limit the use of batteries.
Diversity of implantable device applications [1]. (Reproduced with permission from Springer)
There are two promising approaches for the power autonomy of implant devices. The first approach is to harvest power from the human body or ambient sources, and the other is to transmit power wirelessly from external sources to implant devices. Harvestable energy sources for the first approach include knee, body, and head movement, heart, artery, muscle, body heat, glucose fuel cell, and solar. Wireless power transmission to implant devices can be accomplished by various means including inductive link, RF transmission, infrared, and ultrasonic.
Both fields, energy harvesting from human body and powering up implant devices, are rapidly growing owing to advancements in transducers, integrated circuit technology, and energy storage devices. Various energy sources and power transmission methods pose distinct technical challenges at various levels such as transducer, circuit, and system. Intensive research will address these problems, but it is still in an early stage. It is expected that the research in this area will be intensified in the next decade.
This chapter reviews the major recent research activities on the two topics: energy harvesting with wearable devices from the human body and powering up implant devices at the system level. The chapter is organized as follows: Sect. 2 describes energy harvesting from voluntary sources with wearable devices followed by involuntary ones. It is organized by the type of energy sources. Section 3 covers energy harvesting from the human body with implant devices, and it is also organized by the type of energy sources. Section 4 describes wireless power transmission to implant devices. Section 5 summarizes the chapter.
2 Wearable Devices
Voluntary human motion includes any intentional movement such as walking or moving an arm. This energy is available typically in large quantities, but not continuously. A major goal of energy harvesting from voluntary motion is to incur minimal impact on the wearer, which leads to harvesting only a small portion of the available energy. In many instances, research focuses on harvesting energy without incurring any additional metabolic cost. This section covers energy harvesting from the foot, knee, torso, and arm.
2.1 Foot
Foot-mounted kinetic energy harvesters can harvest from heel strikes, sole compression and, foot forward motion. Of these three, heel strikes generate the largest amount of energy. For a typical person walking two steps per second and a heel displacement of 5 cm, 70 W is expended [2]. The rubber soles of shoes absorb and dissipate mechanical energy as heat, while elastically returning some portion of the energy to walking or running.
2.1.1 Heel-Strike Generators
An electromagnetic heel-strike harvester uses a rack and pinion to convert the almost-linear motion of a heel into high-speed rotational motion [2]. The rotational motion spins an electromagnetic generator, which converts mechanical energy into electrical energy. The electromagnetic heel-strike harvester generates 1.8 W from a person walking two steps per second. The power management circuit consists of a DC converter supplying power through a full-wave rectifier to charge a 0.2 F capacitor. A 13 Ω load resistor and a cell phone are in parallel with the capacitor for their experiments. The cell phone extracts usable energy from the capacitor. The resistor dissipates energy in an attempt to reduce voltage ripple.
The PZT (lead zirconate titanate) dimorph device in Fig. 2 shows a heel-strike harvester installed inside the sole of a shoe [3]. The device is made of a PZT dimorph and a metal mid-plate. Though it is feasible to harvest several watts from walking, harvesting the level of energy would severely hamper the feel of a normal shoe. Shenck et al. harvested 8.4 mW from a heel-strike piezoelectric generator in a Navy work boot [3–5]. The generator was imperceptible to the wearer, but sufficient to power a radio identification transmitter.
Piezoelectric material placement inside a shoe [3]. (Reproduced with permission from IEEE)
2.1.2 Sole Compression/Decompression Generators
Piezoelectric materials generate electricity under mechanical strain. There are two different approaches to use piezoelectric materials in shoes for energy harvesting. The first approach replaces most or all of the heel rubber with a piezoelectric structure that has similar elasticity to the original rubber [3–5]. This causes a slight change in the “feel” of the shoe when walking. The second approach uses a flat sheet of piezoelectric material inside the shoes under the foot that compresses and decompresses every step [3, 5]. One example of this approach is use of a flexible polyvinylidene fluoride (PVDF) stave as shown in Fig. 2. The mechanical design is simple, and it offers potential for large energy harvesting. The shoe-sole piezoelectric harvester demonstrated produces an average of 1.3 mW [3, 5].
2.1.3 Foot Motion Generators
Research groups investigated the use of linear electromagnetic generators in shoes to harvest energy from the forward acceleration of a foot. The harvester proposed by Zeng et al. is shown in Fig. 3. It consists of stationary coils and a movable permanent magnet plate to induce current [6]. They used a boost converter with maximum power point tracking (MPPT) to charge a supercapacitor, while a cascaded buck converter charges a lithium ion battery. Their devices achieve an energy density of 8.5 mW/cm3 [6]. In contrast, Stamenkovic et al. use a movable plate of coils and stationary permanent magnets and, simulation results show that it achieves the energy density of 5.379 mW/cm3 [7].
Cross-sectional view of a shoe-mounted linear electromagnetic generator [6]. (Reproduced with permission from IEEE)
2.2 Knee
A human knee is capable of generating a large amount of energy. However, energy harvesting from a knee should not increase the person’s metabolic cost, which incurs strain on the body and requires additional food intake. Both of them would be detrimental to the wearer’s comfort and health.
Donelan et al. focused on helping the knee decelerate without inhibiting acceleration [8]. Gears embedded in the electromagnetic generator increase rotational speed of the generator by several times, so that it produces a higher voltage to increase the system efficiency. In addition, it helps to produce a more stable voltage. A one-way clutch or ratchet allows energy harvesting from the knee only when the leg is extending. A controller measures the acceleration of the knee with the aid of a potentiometer, so that it harvests only when the knee is decelerating, which happens when the leg is almost fully extended. This regenerative braking ideally helps the knee and should not increase the wearer’s energy consumption. In reality, a mechanical generator still costs metabolic energy during the period when the energy is not harvested. The energy harvester generates 4.8 W with only 5.0 W of additional metabolic energy cost. If the harvester harvests energy during the acceleration period from forward motion, the metabolic cost would be several times that of the harvested power [8].
Luciano et al. worked on a brushless electromagnetic generator to be embedded inside a prosthetic knee [9, 10]. This research focused on the optimal combination of the magnets and the coil. The generator has six magnets and one coil. As the knee bends, the six magnets pass over the coil in sequence. Preliminary test results at 1 Hz show 2 V peak voltage over a load of 1 MΩ, but average power is not provided [9, 10].
A knee-generator shown in Fig. 4 is based on piezoelectric bimorph cantilevers [11]. A bimorph generates maximum power when it oscillates at its resonance frequency, typically ranging from several tens to hundreds of Hz, which is far higher than the frequency of the knee motion. Plectra around the circumference of the device actuate bimorph cantilevers at a higher frequency than that of the knee motion. Simulations predict generation of up to 7 mW under steady walking. Theoretical and experimental results in [11] are based on a fixed resistor load. Usable electrical energy is lower when the loss of the power management circuit (for rectification and voltage regulation) is considered [11].
Knee-mounted piezoelectric generator plucks multiple bimorphs to operate at resonance [11]. (Reproduced with permission from M. Zhu)
A dielectric elastomer is essentially a plate capacitor whose capacitance changes with the distance between the two plates and distortion of the dielectric material. Stretching the material converts mechanical energy into electric field energy. A dielectric elastomer generator could be attached to clothing on the front or back of the knee and cause very little discomfort to the wearer. Regular walking or running flexes the elastomer to generate electricity. The authors reported 10–50 µJ of energy harvested per knee extension [12].
2.3 Torso
Intensive research has been conducted on harvesting energy from human torso motion during walking or running [13–15]. An electromagnetic generator was developed to produce electricity from low-frequency human motion [13]. The device, shown in Fig. 5, consists of a magnetic ball contained in a nonconductive cage. A wire is wrapped around the cage, so that the moving ball induces AC voltage in the coils. The device can be placed anywhere on the human body such as a backpack or a pocket. It was shown that two sets of coils close to the ends of the cage (which is the right one in the figure) are more effective than one coil at the equator (the left one in the figure). The optimal number of the turns, ball diameter, and ball-to-cavity diameter ratio were determined for the particular test scenarios [13]. The electromagnetic generator achieves a power density of 0.5 mW/cm3 with optimal parameters, but efforts to make the generator smaller dramatically reduced its power density [13].
Two spherical generator prototypes. The equator-wrapped coil is on the left and the offset-wrapped coil is on the right [13]. (Reproduced with permission from D.P. Arnold)
Unlike the cage type, a linear electromagnetic generator shown in Fig. 6 has a tube with wire coils and a free-sliding magnetic mass inside the tube. Either fixed magnets or springs are attached at the ends of the tube, which make the sliding mass to oscillate [14, 15]. Two prototypes were tested on a shaker and a torso. The first prototype with fixed magnets at both ends generates 1.86 mW, while running with an oscillation frequency of 2.75 Hz. The second with only one fixed magnet generates 2.46 mW. One of the prototypes, the paper does not specify which, attached to a torso generates average 0.9 mW for 1 h of walking, which is sufficient to power a wearable wireless sensor node [15].
Linear electromagnetic generator to convert kinetic energy into electricity [14]. (Reproduced with permission from Trans Tech Publications)
Fujita et al. investigated vibration energy harvesting from forward and backward motion of the waist, wrist, and ankle using an electrostatic generator [16]. An electrostatic generator is similar to a dielectric elastomer generator, where moving electrodes convert the motion into electric field energy between the electrodes. Researchers mounted a prototype electrostatic energy harvester on the waist of a user and logged the amount of energy harvested. Over the course of an 8-minute walk across a college campus, the peak power was 0.5 nW, average power 61 pW, and the total energy extracted 32 nJ [16].
2.4 Arm
The energy level from an arm varies greatly from finger movement to forearm flexing. Typical arm movements are intermittent and low frequency, which necessitate unique transducers [17–20].
The piezoelectric shell transducer in Fig. 7 was investigated to increase the slope and magnitude of the generated impulse voltage. The piezoelectric shell structure was formed by attaching PVDF film to a curved thin polyester film, which provides a stiff structure for the piezoelectric shell generator [17]. As force is applied to a shell structure, it resists bending until it reaches a point and then all of a sudden bends quickly to generate a sharp impulse. A sharp impulse increases the peak voltage. A higher voltage, even if it may carry the same amount of energy per pulse, reduces the power loss across diodes and resistance in the current path [17]. Test results show that the shell structure generates a peak impulse of 30 V, while a flat PVDF of only 5 V. When eight shell structures were inserted into an arm-cuff worn around a person’s elbow, it exhibited peaks of 20 V, while flat PVDF films caused only 10 V peaks.
Plastic shell with a piezoelectric transducer [17]. (Reproduced with permission from IEEE)
Impact-based generators were also considered to sharpen the impulse and increase the peak voltage. An impact-based generator moves a mass at a slow speed, which strikes against piezoelectric transducers. The energy of the moving mass is transferred to the transducer in a short period and then the transducer resonates after the impact. A mathematical model predicts that a 1 cm3 device is capable of generating 40 µW on the wrist of a walking person [18]. Renaud et al. later presented a prototype generator consisting of two piezoelectric cantilevers, a free-moving mass, and a case. Such a generator could be worn on a wrist to power electronic devices. Electricity is generated by the impact of the mass on the piezoelectric cantilevers at each end of the frame. The device generates 47 µW in a resistive load at rotation of 30 rpm and 600 µW under 10 cm linear displacement at 10 Hz [19].
A piezoelectric wire, often bonded to a flexible substrate, can generate electricity by stretching and contracting. A single wire generator (SWG) is a piezoelectric wire manufactured on a polymer film. An SWG can harvest energy from low-frequency deformation such as air flow and human motion [20]. Yang et al. demonstrated SWGs harvesting energy from a finger and a live hamster. The finger-mounted SWG produces peaks of 20 mV and 150 pA from minute finger movements [20] (Fig. 8).
Using an SWG to harvest energy from a human index finger. a Mechanical design and flexing. b Open circuit voltage [20]. (Reproduced with permission from American Chemical Society)
2.5 Breathing
Chest expansion due to breathing provides a high amount of energy. An added benefit of harvesting from respiration is that the frequency of input power is the rate of respiration, which eliminates the need for a separate respiratory rate monitor. Padasdao et al. demonstrated a prototype energy harvester strapped around a chest. It converts chest expansion into linear motion initially and then into high-speed rotational motion using a gearbox to drive an electromagnetic generator [21]. The generator is a modified servomotor, which includes a gearbox and sturdy case. The concept of the approach is shown in Fig. 9. Chest expansion from breathing pulls on the chest strap, which causes an electromagnetic generator to rotate. The performance of the prototype was measured for normal, fast, and very slow breathing. The electromagnetic generator produces 1.4 V peak to peak and 84.8 mA peak to peak or average power of 15 mW under the normal breathing condition. The high rotational speed obtained with gears attributes high efficiency to the prototype [21].
Top view of chest and electromagnetic generator harvesting from respiration [21]. (Reproduced with permission from IEEE)
Shahhaidar et al. also developed a prototype energy harvester, where the approach is essentially the same as in the above [22]. The prototype is composed of two DC motors, a gearbox, springs, and a chest belt. The springs store energy in each expansion and keep the belt tight when the chest contracts. Despite the gearbox, the generator suffers from low rotational speed and produces 100 mV at the load. According to estimations, 0.2 Hz breathing can generate up to 30 mW. The prototype presented was able to harvest 2 mW [22].
2.6 Body Heat
Human bodies lose a tremendous amount of energy daily as heat, but harvesting a good amount of energy from body heat is challenging. The challenge stems from the fact that temperature gradient between the body and the surrounding air is small, typically 1°–2°. It is due to lack of air flow around the thermoelectric generator (TEG) worn on a body as well as a relatively small temperature difference between the body and the atmosphere. A TEG converts thermal energy into electrical energy because a temperature gradient applied to a p–n junction causes current to flow due to the Seebeck effect. Energy harvesting research from the human body focuses on three major areas: TEG design, low-voltage power electronics, and system design [23–30]. This section covers only the system design.
Several research groups focused on TEG systems to test their effectiveness and comfort for wearable electronics [25, 26]. Leonov et al. tested different textile configurations and situations to measure the system performance [30]. Figure 10 depicts the four different textile configurations, in which the power generated by a TEG (denoted as “1” in Fig. 10a) was measured. The configuration in Fig. 10a has an inner TEG metal plate touching the wearer’s skin, and an outside metal plate exposed to the outside air. It would be the most effective in terms of the temperature gradient, but least comfortable for the wearer. The configuration in Fig. 10b covers the outside metal plate with cotton. This causes only 5 % reduction in power, as long as there is no air gap between the cotton and the TEG. The configuration in Fig. 10c adds a carbon fiber heat-spreading layer between the cotton and the TEG. The carbon layer increases performance by approximately 30 %. The configuration in Fig. 10d adds another cotton layer between the skin and the TEG. It greatly increases comfort and completely hides the TEG, but slightly reduces performance. Fourteen units adopting the second configuration generated an average power of 4 mW while walking in a 17 °C environment [30].
Four different configurations to integrate TEGs in garments [30]. (Reproduced with permission from IEEE)
3 Implant Devices
This section reviews recent research activities on energy harvesting with implant devices. Potential energy sources for harvesting include kinetic energy from the human body, body heat, fuel cells, and ambient sources specifically solar. It describes research activities for each energy source type in the following.
3.1 Knee
A knee can be exposed to force up to three times higher than the body weight [31, 32]. A typical transducer used for knee bending is a piezoelectric element or electromagnetic generator. Platt et al. investigated the use of three piezoelectric elements inside the orthopedic implants [31, 32]. A knee implant is shown in Fig. 11. The femoral component is a highly polished hard surface attached to the femur. Relative motion between the femoral and bearing surfaces allows the knee to function. The tibial tray supports a low-friction polyethylene bearing surface. Force applied by the femoral component is applied to the bearing surface and then to the three piezoelectric stacks on the distribution plate. When 900 N of force is applied over one piezoelectric stack (whose dimension is 10 × 10 × 20 mm) placed inside the prototype in a laboratory setup, it generates up to 1.6 mW of raw power, a total of 4.8 mW for the three stacks. The height of the piezoelectric stacks is 20 mm, which significantly increases the thickness of the tibial tray. Consequently, the quantity of tibial and femoral bone loss also increases during a knee arthroplasty.
Almouahed et al. investigated a new generation of a knee implant shown in Fig. 12 through modeling and experimental trials [33, 34]. The knee implant is more sophisticated than the earlier one in the above, and it uses four smaller piezoelectric elements of dimension 10 × 10 × 4 mm. The power generated is estimated to be about 1 mW for a single piezoelectric element with 50 kΩ resistive load, and the total power generated would be about 4 mW. Finally, Chen et al. reported PZT ceramics (of dimension 5 × 5 × 18 mm) and an associated circuit applicable to orthopedic implants [35]. Four piezoelectric elements can generate about 1 mW of power, which is sufficient to power a wireless monitoring system.
The above works adopt piezoelectric elements to generate electric energy from knee bending [31–35]. Luciano et al. investigated use of a miniaturized electromagnetic generator shown in Fig. 13, which can be implantable in a human knee prosthesis [9, 36]. Figure 13a depicts the upper portion of the knee (femur), in which permanent magnets are installed. Figure 13b shows a coil at the top of the tibia or the lower part of the knee. When the knee flexes, the magnets move relative to and induce current in the coil. The system with a power conditioning circuit produces output energy of about 22.1 μJ in 7 s for a gait frequency of 1.02 Hz emulated with an electric motor.
3.2 Body Movement
Nasiri et al. investigated a linear permanent magnet generator implanted in the abdominal muscles as shown in Fig. 14 [37]. The linear generator consists of two layers of permanent magnets and one layer of coils. It generates power from multidirectional body movements. The generator was tested under different conditions including movements at different frequencies and amplitudes, resonance frequency test, and a walking test. The magnetic generator generates 1.1 mW of power at the resonant frequency of near 0.3 Hz. The peak output voltage drops from 1.5 V at the resonant frequency to 0.7 V at 0.1 Hz and 0.9 V at 6 Hz. It harvested 9 mJ from 22.5 s of walking motion.
Pocket for the linear permanent magnetic generator [37]. (Reproduced with permission from IEEE)
Morais et al. reported a nonlinear electromagnetic generator implantable in a hip prosthesis [38]. As shown in Fig. 15, the generator consists of a Teflon tube with one or two external coils and a neodymium magnet attached to a spring. A magnetic brake is attached at the bottom of the tube to avoid collisions of the magnet against the bottom of the Teflon tube itself. This device intends to harvest energy from the human gait to power a telemetric system inserted in a smart hip prosthesis implant for early detection of loosening and implant failure. The device with a power management circuit is able to power the telemetric system for 9.2 s after charging for 34.8 s from a walking speed of 1.3 Hz.
Electromagnetic generator implantable in a hip prosthesis [38]. (Reproduced with permission from Elsevier)
3.3 Heart
A system based on a piezoelectric generator is reported in [39], which harvests energy from heartbeats to power cardiac implant devices. The output power follows the acceleration spectrum of heartbeats as shown in Fig. 16. The achievable power level and design parameters are determined from a spectral analysis to about 100 μW before electronics. The system is about 15 × 7 × 5 mm3, which fits all the components as well as accommodates the proof mass travel range.
Simulated output power per gram of proof mass for a piezoelectric generator as the transducer [39]. (Reproduced with permission from IEEE)
Martin et al. present a more comprehensive design, fabrication, and tests of a microspiral-shaped piezoelectric energy harvester and its associated microfabricated packaging that collects energy from ordinary blood pressure variations in the cardiac environment [40], as shown in Fig. 17. A prototype of 10-µm-thin ultra-flexible electrodeposited micro-bellows (6 mm diameter, 21 mm3 volume) is a new type of implant packaging. It enables direct blood pressure harvesting and permits high-efficiency energy transfer to a transducer operating in a quasi-static mode and hence adaptable and unaffected by frequent heartbeat frequency changes. Spiral-shaped piezoelectric transducers are introduced for their flexibility and large incoming mechanical energy. Nontrivial optimal electrodes placement and best spiral design parameters are studied and discussed. Three types of spiral prototypes (11 mm3 volume each) with double-sided microstructured electrode patterns are presented and characterized. A power of 3 µJ/cm3/heartbeat and a transduction efficiency of 5.7 × 10−3 have been obtained for the best design at 1.5 Hz.
Schematic of the implantable packaged energy harvester scavenging energy from blood pressure variation [40]. (Reproduced with permission from IEEE)
An electrostatic generator harvesting energy from ventricular wall motion was developed to power a cardiac pacemaker perpetually [41], and it employs a honeycomb structure. A test setup was constructed with an accelerometer placed on a dog’s heart. The same amount of acceleration sensed from the heart drives the generator. The dog’s heart beats at 180 per minute, and the average power recorded for more than 2 h is 36 μW.
3.4 Artery
Potkat et al. investigated an implantable energy harvester from the expansion and contraction of an artery [42]. A PVDF thin film embedded within a flexible, self-curling medical-grade silicone cuff converts the expansion and contraction of the artery into electrical energy. The cuff is rolled around a latex tube mimicking an artery as shown in Fig. 18. A peak voltage of 1.2 V, a maximum instantaneous power of 16 nW, and an average power of 6 nW were measured. The microfabricated version of this implantable device would generate more than 1.0 μW for in vivo tests.
PVDF thin film energy harvester for the artery [42]. (Reproduced with permission from IEEE)
3.5 Muscle
Lewandowski et al. reported an implantable piezoelectric generator to harvest energy from muscles [43]. As shown in the conceptual block diagram in Fig. 19, the device is attached in series with a muscle tendon. The system targets individuals with extensive paralysis, where the electrically stimulated muscle would not interfere with natural muscle contractions or activities. Electrically stimulated muscle contractions exert force on the piezoelectric generator producing a charge, which is collected in energy storage circuitry and used to power the stimulator and other loads. It is argued that the required power to artificially excite a muscle is minimal in comparison with the power generated by the muscle and hence such a device is justified. Experimental results indicate that a small PZT stack prototype (5 × 5 × 18 mm3) generates 80 μW of power under application of force of 250 N.
Conceputal block diagram for an implanted piezoelectric generator for muscles [43]. (Reproduced with permission from Springer)
3.6 Body Heat
Yang et al. exploited the thermal gradient between a skin surface and the inner body and harvested thermal energy with an implanted TEG as shown in Fig. 20 [44]. They performed numerical simulations on three-dimensional bioheat transfer of a human body with implanted TEGs in different depths and configurations. To increase generation of energy, they also proposed and evaluated several approaches such that there is intentional cooling and heating of the skin surface. In their in vivo experiment, a TEG was implanted in the abdomen of a rabbit. The temperature difference stabilized at around 1.3° after 260 s with the TEG voltage of about 5 mV. When the rabbit skin surface was covered with an ice water bag, the temperature difference increased up to 5.5 °C and the TEG voltage increased to 25 mV.
Thermal energy harvesting with an implanted TEG and the model of a tissue [44]. (Reproduced with permission from J. Liu)
Nagel et al. investigated an implanted TEG to power an artificial accommodation system shown in Fig. 21 [45]. They considered characteristics of high-performance thermoelectric materials and the temperature distribution within the human eye to estimate power generated by a TEG as a part of the artificial accommodation system. It is shown that power ranges from 4.96 μW in the worst case to 24.4 μW in the best case.
Implanted artificial accommodation system within an eye [45]. (Reproduced with permission from IEEE)
3.7 Glucose Fuel Cell
A fuel cell is an electrochemical device that generates current through the reaction of two chemicals flowing into it—the fuel on the anode site and the oxidant on the cathode site. Implantable fuel cells that use glucose as a reactant are probably the most studied biofuel cells, due to the high availability of glucose in body fluids. Glucose fuel cells can be divided into two groups: (1) abiotically catalyzed and (2) enzymatically catalyzed. The former group utilizes nonbiological catalysts such as noble metals or activated carbon. The latter group, instead, uses enzymes, such as glucose oxidase or laccase, as catalysts to enable the electrode reactions. These devices are summarized in [46]. Figure 22 shows one example of flow-through type fuel cells intended for blood stream implantation. During in vitro experiments, glucose fuel cells abiotically catalyzed can generate up to 50 μW.
Simplified schematics of flow-through type fuel cells intended for bloodstream implantation. a Fuel cell with hydrophobic cathode membrane. b Concentrically arranged fuel cell with oxygen-selective cathode catalyst [46]. (Reproduced with permission from Elsevier)
3.8 Inner Ear
Bandyopadhyay et al. investigated endocochlear potential (EP), the 70–100 mV electrochemical biopotential inside the mammalian ear shown in Fig. 22 [47]. Due to the anatomical constraints inside the inner ear, the total extractable power from the EP is limited close to 1.1–6.25 nW. An nW boost converter is used to increase the input voltage (30–55 mV) to a higher voltage (0.8–1.1 V) usable by CMOS circuits in the sensor. The power management unit can sustain itself, and a duty-cycled ultralow-power load while extracting power from the EP of a live guinea pig. The power management unit circuits have been implemented on a 0.18 μm CMOS process (Fig. 23).
Endocochlear potential [47]. (Reproduced with permission from IEEE)
3.9 Solar Energy
All the implant devices described above harvest human body energy, which comes from the food intake. In contrast, solar energy is external and hence harvesting solar energy does not affect the body. Outdoor solar energy provides the highest power density among ambient energy sources, but the light penetration is greatly reduced by the human body tissue. A solar energy harvester for implant devices gathers the energy within the therapeutic window wavelengths, where the optical absorption is small [48]. Implanted subcutaneous photovoltaic cells shown in Fig. 24 can harvest microwatts of power in bright ambient light condition [48].
Solar energy harvester [48]. (Reproduced with permission from IEEE)
Ayzian et al. present an energy-autonomous, photovoltaic-driven, and MRI-compatible CMOS implantable sensor, as shown in Fig. 25 [49]. On-chip P +/N- well diode arrays are used as CMOS-compatible photovoltaic cells to harvest power from the light that penetrates into the tissue. In this 2.5 mm by 2.5 mm microwatt integrated system, the in vivo physiological signals are first measured by using a subthreshold ring oscillator-based sensor. The acquired data is then modulated into a frequency-shift keying signal, and finally transmitted neuromorphically to the skin surface by using a pair of polarized electrodes.
A photovoltaic-driven energy-autonomous CMOS implantable sensor [49]. (Reproduced with permission from IEEE)
4 Wireless Power Transmission to Implant Devices
Wireless power transmission to implant devices can rely on various means including inductive link, RF transmission, infrared, and ultrasonic and is described in this section.
4.1 Inductive Link
The use of inductive links to power implant devices has been actively investigated in the past decade. An inductive link consists of two coils: a primary coil and a secondary coil. The primary coil placed outside the body generates a variable magnetic field, which causes the change of the magnetic flux for the secondary coil planted underneath the skin. Power is transferred wirelessly through the body tissue. The ratio of the energy captured by the secondary coil to the energy transmitted by the primary ranges between 0.01 and 0.1. The simplified model of the inductively coupled system is shown in Fig. 26. The left side of this model represents the outside components of the system and the primary coil, while the right side includes a basic model of the implanted system. Here, R1 represents the parasitic resistance in the coil, C1 is the tuning capacitance used to raise the coil voltage, and RL is the load on the system. The primary coil L1 is driven by an RF amplifier supplying current i1 at frequency ω.
Most inductive link works reported utilize frequencies of the order of a few megahertz or lower. This frequency range minimizes the power absorption by the tissues, yielding higher transmission efficiency [50–52]. Sauer et al. in [50] present a telemetry chip shown in Fig. 27, which is powered by inductive coupling. The two coils are used to transmit both power and data. The chip fabricated in 0.5 μm CMOS technology supplies 1.7 mA at 3.3 V, over a distance up to 25 mm between coils.
An implant sensor system powered through inductive link [50]. (Reproduced with permission from IEEE)
Lenaerts et al. investigated an inductive link to power a wireless camera capsule for noninvasive visual inspection of the small bowel (small intestine) [51]. Up to 150 mW of usable dc power can be delivered to the capsule for the entire duration of its travel along the gastric track. Figure 28 shows parts of the power receiver. The outer dimensions of the power receiver compartment inside the capsule are 10 × 13 mm. The power efficiency of the link is measured to be 1 % under worst-case geometrical conditions.
The disassembled power receiver: three orthogonal coils and receiver electronics [51]. (Reproduced with permission from Elsevier)
Niu et al. in [52] investigated a microelectromechanical system (MEMS)-based spiral piezoelectric energy harvester, which harvests kinetic energy from patient’s shoes during walking. The harvested energy supplies power to an implant device, specifically an artificial heart, through inductive link as shown in Fig. 29. The regulated output power can reach up to 1 μW.
Basic structure of the transcutaneous energy transmission system [52]. (Reproduced with permission from IEEE)
4.2 RF Transmission
Cao et al. investigated a device for gastroesophageal reflux disease (GERD) monitoring [53]. The conceptual design and sensor system are shown in Fig. 30. The system consists of an implantable, batteryless, and wireless transponder with integrated impedance and pH sensors. The transponder implant with the size of 0.4 × 0.8 × 3.8 cm harvests RF energy to operate dual-sensor and load-modulation circuitry. The external reader can store the data in a memory card and/or send it to a base station wirelessly. The device and system were tested at the bench, in a mannequin and in live pigs, which demonstrates the functionality, feasibility, accuracy, and reliability in detecting various stimulated reflux episodes.
Passive wireless system using dual sensors in a single capsule to monitor GERD symptoms [53]. (Reproduced with permission from IEEE)
Faul et al. developed a wireless implant lens system powered by RF and is shown in Fig. 31 [54]. The power for the implant system is derived from the 915 MHz RF signal transmitted by the reader, and the RF signal is converted into a regulated DC voltage with two-stage half-wave rectifier. A small antenna fits into an area less than 10 × 10 mm, and the system has an operating range of nearly 100 cm.
External reader interfacing an intraocular (implanted) intraocular pressure sensor [54]. (Reproduced with permission from IEEE)
4.3 Infrared
Goto et al. exploited the infrared spectrum to transmit power to implanted devices [55]. The device shown in Fig. 32 can supply power ranging from hundreds of microwatts to a few milliwatts to implanted devices. When illuminated with the power density of 22 mW/cm2 for 17 min, the photodiode array generates sufficient power for a 20 μA cardiac pacemaker to operate for 24 h. The photothermal effect at the photodiode array raises the temperature at the irradiated skin by 1.4 °C for the power density, which does not pose any safety issues.
Schematic diagram of the near-infrared power supply system [55]. (Reproduced with permission from IEEE)
4.4 Ultrasonic
Ozeri et al. considered ultrasonic traveling waves to transfer power toward the receiver, whose energy is reconverted by the implanted receiver into electrical energy [56]. The recommended operating frequency is 100–1000 kHz. Piezoelectric generators convert the acoustic vibration energy into electrical power for implanted electronics devices as reported by [57–61]. Kim et al. investigated a piezoelectric energy harvester for low-frequency components of musical vibrations [57], which powers a wireless interrogation device. The system operates in two phases. A piezoelectric cantilever converts the sound vibration into electrical power and charges a capacitor. The stored charge is dumped into an LC tank, forcing it to oscillate at the resonance frequency and emit the energy to an outside receiver. A prototype transponder shown in Fig. 33 was tested using a PZT cantilever with a mechanical resonant frequency of 435 Hz encapsulated in a glass capsule (length = 40 mm, diameter = 8 mm) along with a rectifier circuitry and a storage capacitor. RF pulses can be picked up at distances of up to 7 cm without the tight requirement on alignment between the receiver and the transponder coils.
3-D drawing of implanted inductive pressure sensor with acoustic energy harvesting [57]. (Reproduced with permission from IEEE)
Shaul et al. proposed an ultrasonic transcutaneous energy transfer, which uses an external transmitting transducer attached to the skin surface facing an implanted receiving transducer [58]. The harvested power charges a capacitor during the charging period until it reaches a DC threshold voltage. Upon reaching the threshold level, the device starts to operate, while the capacitor being discharged. The implant sensing system shown in Fig. 34 consists of a small (2.2 × 2.3 mm) varactor, together with 10 turns of 30 gage wire wound over the implanted piezoelectric transducer.
Ultrasonic transcutaneous energy transfer system [58]. (Reproduced with permission from IEEE)
Anthony et al. present design and characterization of a MEMS-based energy harvester with target applications including implanted biomedical sensors and actuators [59]. The harvester converts ultrasonic waves from an external transmitter to mechanical excitation and then the vibration of a central mass structure into electrical energy with an electrostatic transducer. The device features a novel 3 degrees of freedom design, which enables the harvester in any orientation to produce energy. The harvester is fabricated using a conventional silicon-on-insulator MEMS process. An example of the experimental setup is shown in Fig. 35. The experimental results show that the system is able to generate 24.7, 19.8, and 14.5 nW via the device’s x-, y-, and z-axis, respectively, over 15 s.
Ultrasonic transcutaneous energy transfer system [59]. (Reproduced with permission from IEEE)
Francesco et al. present the design of an ultrasound link for deep implanted medical devices [60]. To avoid biological side effects such as cavitations, the operating frequency of 1 MHz is set for the system, and a class-E power amplifier as the front-end circuit drives external transducers. A novel synchronous rectifier is proposed to maximize energy extraction from the implanted transducer. The link efficiency is characterized in water for a transmitter–receiver distance of 105 mm, and a system efficiency of 2.3 % is achieved without using any phantom material. The link efficiency drops to 1.6 % when a phantom material is used.
5 Summary
The first part of this article reviews recent research activities on wearable energy harvesters, in which the target energy sources are limited to human bodies. The latter part of the article reviews the means to power implant devices. An implant device can be powered by harvesting energy from the human body, ambient sources, or through power transmission from external sources. Harvestable human body energy includes voluntary motion, involuntary motion, and heat. Each type of energy harvesting faces different technical challenges.
5.1 Energy Harvesting with Wearable Devices
Harvestable energy sources of a human body with wearable devices include foot, knee, torso, chest, and body heat. A comparison of power harvested from different body parts or types of energy (kinetic and thermal) is shown in Table 1. The power figure in the table is the maximum power obtained for each harvesting method, i.e., the highest power reported in a paper for the transducer type. The table indicates that electromagnetic generators are most effective for kinetic energy harvesting, followed by piezoelectric transducers of the order of milliwatts and dielectric elastomers of the order of tens of microwatts down to picowatts. Thermoelectric generator harvesting varies greatly depending on the environment and application ranging from hundreds of microwatts to a few milliwatts.
5.2 Powering Implant Devices
Implant devices can be powered through energy harvesting or transmission of power from external sources. Harvestable energy sources to power up implant devices include knee, heart, artery, muscle, body heat, and solar. Table 2 compares the maximum power reported for each harvesting method. The piezoelectric harvesters seem most promising in terms of the power produced. In particular, the harvesters in [31–35] generate power greater than 1 mW. However, these devices intended for the knees require significant force to generate power. Electromagnetic harvesters produce lower power than the above-mentioned piezoelectric devices, but they do not require large forces, and hence are applicable for any body part. The electrostatic harvesters produce much lower power than electromagnetic harvesters and are limited to low-power devices. Thermoelectric harvesters should be implanted close to the skin surface, where the temperature gradient is large. Large difference in the reported power is due to different sizes and thermal gradients used for the testing.
Implant devices can be powered up through various power transmission means including inductive link, RF transmission, infrared, and ultrasonic. The delivered power level of wireless power transmission methods is sensitive to the distance between the transducer and the implant. Table 3 compares various methods covered in this article. The inductive link power transmission reported in [51] delivers largest power, but its size is large. The infrared method may cause the skin temperature to increase, which should be carefully examined for safety and long-term effects. Overall, each method has unique characteristics and technical challenges to address.
5.3 Final Remarks
Modern research in energy harvesting from the human body is a broad field and is making steady progress in several areas. Most energy harvesting research focus on increasing the power level to harvest, and it will continue and be accomplished by improving transducers and power management circuits. Wearer’s comfort during energy harvesting is another important topic for wearable energy harvesters. Transducer design for comfort relies on flexible materials and unobtrusive hardware. Increased comfort can also be attained by using wireless sensor networks instead of wired sensors.
Implant devices will be pervasive as the wellbeing of humans becomes ever important. Powering implant devices is a major issue for current and future implant devices. Energy harvesting and wireless power transmission will be pursued to address the problem. Another important issue for energy harvesters and power transmission devices for implant devices is health hazard, and it should be addressed before deployment of any such devices.
References
Wei X, Liu J (2008) Power sources and electrical recharging strategies for implantable medical devices. Front Energy Power Eng Chin 2:1–13
Verma NK, Singla P, Roy A (2012) Energy harvesting by foot-propelled battery charger using shoe-model. Adv Mater Res 488–489:1268–1273
Shenck NS, Paradiso JA (2001) Energy scavenging with shoe-mounted piezoelectrics. IEEE Micro 21(3):30–42
Orecchini G, Yang L, Tentzeris MM, Roselli L (2011) Wearable battery-free active paper printed RFID tag with human-energy scavenger. In: Microwave Symposium Digest (MTT), 2011 IEEE MTT-S International, pp 1–4
Kymissis J, Kendall C, Paradiso J, Gershenfeld N (1998) Parasitic power harvesting in shoes. In: Second International Symposium on Wearable Computers, Digest of Papers, pp 132–139
Zeng P, Chen H, Yang Z, Khaligh A (2011) Unconventional wearable energy harvesting from human horizontal foot motion. In: Applied Power Electronics Conference and Exposition (APEC), 2011 Twenty-Sixth Annual IEEE, pp 258–264
Stamenkovic I, Milivojevic N, Zheng C, Khaligh A (2010) Three phase linear permanent magnet energy scavenger based on foot horizontal motion. In: Applied Power Electronics Conference and Exposition (APEC), 2010 Twenty-Fifth Annual IEEE, pp 2245–2250
Donelan JM, Li Q, Naing V, Hoffer JA, Weber DJ, Kuo AD (2008) Biomechanical energy harvesting: generating electricity during walking with minimal user effort. Science 319(5864):807–810
Luciano V, Sardini E, Serpelloni M, Baronio G (2012) Analysis of an electromechanical generator implanted in a human total knee prosthesis. In: Sensors Applications Symposium (SAS), 2012 IEEE, pp 1–5
Luciano V, Sardini E, Serpelloni M (2014) An Electromechanical generator implanted in human total knee prosthesis. In: Baldini F, D’Amico A, Natale CD, Siciliano P, Seeber R, Stefano LD, Bizzarri R, Andò B (eds) Sensors. Springer, New York, pp 25–30
Pozzi M, Zhu M (2011) Plucked piezoelectric bimorphs for knee-joint energy harvesting: modelling and experimental validation. Smart Mater Struct 20(5):055007
Lai H, Tan CA, Xu Y (2011) Dielectric elastomer energy harvesting and its application to human walking. ASME Conference Proceedings 2011(54884):601–607
Bowers BJ, Arnold DP (2009) Spherical, rolling magnet generators for passive energy harvesting from human motion. J Micromech Microeng 19(9):094008
Sun K, Liu GQ, Xu XY (2011) No-Load Analysis of Permanent Magnet Spring Nonlinear Resonant Generator for Human Motion Energy Harvesting. Appl Mech Mater 130–134:2778–2782
Saha CR, O’Donnell T, Wang N, McCloskey P (2008) Electromagnetic generator for harvesting energy from human motion. Sens Actuators, A 147(1):248–253
Fujita T, Onishi T, Fujii K, Kanda K, Maenaka K, Higuchi K (2012) Evaluation of the human vibration for autonomous power source. In: World Automation Congress (WAC), pp 1–4
Yang B, Yun K-S (2011) Efficient energy harvesting from human motion using wearable piezoelectric shell structures. In: Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), 2011 16th International, pp 2646–2649
Renaud M, Sterken T, Fiorini P, Puers R, Baert K, van Hoof C (2005) Scavenging energy from human body: design of a piezoelectric transducer. In: The 13th International Conference on Solid-State Sensors, Actuators and Microsystems, Digest of Technical Papers, TRANSDUCERS ’05, vol 1, pp 784–787
Renaud M, Fiorini P, van Schaijk R, van Hoof C (2009) Harvesting energy from the motion of human limbs: the design and analysis of an impact-based piezoelectric generator. Smart Mater Struct 18(3):035001
Yang R, Qin Y, Li C, Zhu G, Wang ZL (2009) Converting biomechanical energy into electricity by a muscle-movement-driven nanogenerator. Nano Lett 9(3):1201–1205
Padasdao B, Boric-Lubecke O (2011) Respiratory rate detection using a wearable electromagnetic generator. In: 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, pp 3217–3220
Shahhaidar E, Boric-Lubecke O, Ghorbani R, Wolfe M (2011) Electromagnetic generator: as respiratory effort energy harvester. In: IEEE Power and Energy Conference at Illinois (PECI), pp 1–4
Wang Z, Leonov V, Fiorini P, Van Hoof C (2007) Micromachined thermopiles for energy scavenging on human body. In: Solid-State Sensors, Actuators and Microsystems Conference, 2007, TRANSDUCERS 2007, International, pp 911–914
Wang Z, Leonov V, Fiorini P, Van Hoof C (2009) Realization of a wearable miniaturized thermoelectric generator for human body applications. Sens Actuators A 156(1):95–102
Jung S, Lauterbach C, Strasser M, Weber W (2003) Enabling technologies for disappearing electronics in smart textiles. In: IEEE International Solid-State Circuits Conference (ISSCC), Digest of Technical Papers, vol 1, pp 386–387
Jo SE, Kim MK, Kim MS, Kim YJ (2012) Flexible thermoelectric generator for human body heat energy harvesting. Electron Lett 48(16):1013–1015
Koplow M, Chen A, Steingart D, Wright PK, Evans JW (2008) Thick film thermoelectric energy harvesting systems for biomedical applications. In: 5th International Summer School and Symposium on Medical Devices and Biosensors (ISSS-MDBS), pp 322–325
Zhang F, Zhang Y, Silver J, Shakhsheer Y, Nagaraju M, Klinefelter A, Pandey J, Boley J, Carlson E, Shrivastava A, Otis B, Calhoun B (2012) A batteryless 19 µW MICS/ISM-band energy harvesting body area sensor node SoC. In: IEEE International Solid-State Circuits Conference (ISSCC), Digest of Technical Papers, pp 298–300
Ramadass YK, Chandrakasan AP (2011) A Battery-Less thermoelectric energy harvesting interface circuit with 35 mV startup voltage. IEEE J Solid-State Circuits 46(1):333–341
Leonov V (2013) Thermoelectric energy harvesting of human body heat for wearable sensors. IEEE Sens J 13(6):2284–2291
Platt SR, Farritor S, Haider H (2005) On low-frequency electric power generation with PZT ceramics. IEEE/ASME Trans Mechatronics 10(2):240–252
Platt SR, Farritor S, Garvin K, Haider H (2005) The use of piezoelectric ceramics for electric power generation within orthopedic implants. IEEE/ASME Trans Mechatronics 10(4):455–461
Almouahed S, Gouriou M, Hamitiouche C, Stindel E, Roux C (2011) The use of piezoceramics as electrical energy harvesters within instrumented knee implant during walking. IEEE Trans Mechatron 16(5):799–807
Almouahed S, Gouriou M, Hamitiouche C, Stindel E, Roux C (2011) Design and evaluation of instrumented smart knee implant. IEEE Trans Biomed Eng 58(4):971–982
Chen H, Liu M, Jia C, Wang Z (2009) Power harvesting using PZT ceramics embedded in orthopedic implants. IEEE Trans Ultrason Ferroelectr Freq Control 56(9):2010–2014
Luciano V, Sardini E, Serpelloni M, Baronio G (2014) An energy harvesting converter to power sensorized total human knee prosthsis. J IOPscience Meas Sci Technol 25(2)
Nasiri A, Zabalawi SA, Jeutter DC (2011) A linear permanent magnet generator for powering implanted electronic devices. IEEE Trans Power Electon 26(1):192–199
Morais R, Silva NM, Santos PM, Frias CM, Ferreira JAF, Ramos AM, Simoes JAO, Baptista JMR, Reis MC (2011) Double permanent magnet vibration power generator for smart hip prosthesis. J Sens Actuators Phys 172(1):259–268
Deterre M, Boutaud R, Dalmolin R, Bosseau S, Chaillout JJ, Lefeuvre E, Gergram ED (2011) Energy harvesting system for cardiac implant applications. In: Proceeding of Design, Test, Integration and Packaging of MEMS (DTIP), pp 387–391
Deterre M, Lefeuvre E, Zhu Y, Woytasik M, Boutaud B, Molin RD (2014) Micro blood pressure energy harvester for intracardiac pacemaker. J Microelectromech Syst 23(3):651–660
Tashiro R, Kabei N, Katayama K, Tsuboi F, Tsuchiya K (2002) Development of an electrostatic generator for a cardiac pacemaker that harnesses the ventricular wall motion. Artif Organs 5:239–245
Potkat JA, Brooks K (2008) An arterial cuff energy scavenger for implanted microsystems. In: Proceedings of Bioinformatics and Biomedical Engineering (ICBBE), pp 1580–1583
Lewandowski BE, Kilgore KL, Gustafson KJ (2007) Design considerations for an implantable, muscle powered piezoelectric system for generating electrical power. Ann Biomed Eng 35(4):631–641
Yang Y, Wei X, Liu J (2007) Suitability of a thermoelectric power generator for implantable medical electronic devices. J Phys D Appl Phys 40:5790–5800
Nagel JA, Sieber I, Gengenbach U, Guth H, Bretthauer G, Guthoff RF (2010) Investigation of a thermoelectric power supply for the artificial accommodation system. In: Proceeding of Applied Sciences in Biomedical and Communication Technologies (ISABEL), pp 1–5
Kerzenmacher S, Ducre J, Zengerle R, Stetten FV (2008) Energy harvesting by implantable abiotically catalyzed glucose fuel cells. J Power Sources 182(1):1–17
Bandyopadhyay S, Mercier PP, Lysaght AC, Stankovic KM, Chandrakasan AP (2014) A 1.1 nW Energy-Harvesting System with 544 pW Quiescent Power for Next-Generation Implants. IEEE J Solid-State Circuits 49(12):2812–2824
Ayazian S, Hassibi A (2011) Delivering optical power to subcutaneous implanted devices. In: Proceeding of IEEE Engineering in Medicine and Biology Society (EMBC), pp 2874–2877
Ayazian S, Akhavan VA, Soenen E, Hassibi A (2012) A photovoltaic-driven and energy-autonomous CMOS implantable sensor. IEEE Trans Biomed Circuits Syst 6(4):336–343
Sauer C, Stanacevic M, Cauwenberghs G, Thakor N (2005) Power harvesting and telemetry in CMOS for implanted devices. IEEE Trans Biomed Circuits Syst 52(12):2605–2613
Lenaerts B, Puers R (2007) An inductive power link for a wireless endoscope. Biosens Bioelectron 22(7):1390–1395
Niu Q, Wang L, Dong T, Yang H (2009) Application of MEMS-based energy harvester for artificial heart wireless energy transmission. In: Proceeding of Computing, Communication, Control, and Management (CCCM), pp 38–41
Cao H, Landge V, Tata U, Seo Y, Rao S, Tand S et al (2012) An implantable, batteryless, and wireless capsule with integrated impedance and pH sensors for gastroesophageal reflux monitoring. IEEE Trans Biomed Eng 59(11):3131–3139
Faul A, Turner M, Naber J (2011) Implantable wireless microsystems for the measurement of intraocular pressure. In: Proceeding of IEEE International Midwest Symposium on Circuits and Systems (MWSCAS), Aug 2011, pp 1–4
Goto K, Nakagawa T, Nakamura O, Kawata S (2001) An implantable power supply with an optical rechargeable lithium battery. IEEE Trans Biomed Eng 48(7):830–833
Ozeri S, Shmilovitz D (2010) Ultrasonic transcutaneous energy transfer for powering implanted devices. Ultrasonics 50(6):556–566
Kim A, Maleki T, Ziaie B (2012) A novel electromechanical interrogation scheme for implantable passive transponders. In: Proceedings of IEEE MEMS, pp 31–34
Shaul O, Boaz S, Doron S (2012) Non-invasive sensing of the electrical energy harvested by medical implants powered by an ultrasonic transcutaneous energy transfer link. In: Proceeding of IEEE Industrial Electronics (ISIE), pp 1153–1157
Fowler AG, Moheimani SOR, Behrens S (2014) An omnidirectional MEMS ultrasonic energy harvester for implanted devices. J Microelectromech Syst 23(6):1454–1462
Mazzilli F, Lafon C, Dehollain C (2014) A 10.5 cm ultrasound link for deep implanted medical devices. IEEE Trans Biomed Circuits Syst 8(5):738–750
Mazzilli F, Thoppay PE, Praplan V, Dehollain C (2012) Ultrasound energy harvesting system for deep implanted-medical-devices (IMDs). In: Proceeding of IEEE International Symposium on Circuits and Systems (ISCAS), pp 2865–2868
Acknowledgments
This work is supported by the Center for Integrated Smart Sensors funded by the Ministry of Science, ICT and Future Planning as the Global Frontier Project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Kerley, R., Huang, X., Ha, D.S. (2016). Energy Harvesting from the Human Body and Powering up Implant Devices. In: Kyung, CM. (eds) Nano Devices and Circuit Techniques for Low-Energy Applications and Energy Harvesting. KAIST Research Series. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9990-4_5
Download citation
DOI: https://doi.org/10.1007/978-94-017-9990-4_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9989-8
Online ISBN: 978-94-017-9990-4
eBook Packages: EngineeringEngineering (R0)