Abstract
There is a clinical need for the replacement or repair of damaged and diseased blood vessels; hence, artificial materials are engineered to form replacement tissue analogues. In order to achieve functionality of these grafts, the process of endothelialisation is required. This forms a natural confluent barrier between the artificial substrate and the contacting blood flow, preventing immune responses, plaque formation or stenosis. Endothelialisation is a difficult vascular biology technique to achieve; this chapter focuses on the use of structural cues from the underlying material to enhance the functionality of the engineered vessel. We introduce the use of artificial vessels, describe the importance of the endothelialisation process and explain the options to achieve this. Methodologies to examine the effects of these structures upon the endothelial cell responses are detailed, including microscopy, image analysis, and staining for cell marker expression of endothelial cell-material samples. A focus on the use of electrospinning combined with these techniques, and sample results, is also provided.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Endothelial cells
- Endothelialisation
- Artificial blood vessels
- Tissue engineering
- Electrospinning
- Structural properties
- Polymers
- Cell behaviour
1 Introduction: The Need for Artificial Blood Vessel Analogues
We will describe the importance of endothelialisation of artificial blood vessels, discuss the techniques that can be utilised to enhance this cell layer, with a focus on using the underlying structural cues from the engineered materials, and detail methodology used to examine the effects of these properties upon the endothelial cell behaviour.
The processes of angiogenesis and vasculogenesis are important for the formation and regeneration of tissue and organ structures. However, they are not always naturally possible due to these functions being impaired by damage or disease. The fabrication of engineered blood vessels to artificially create angio- or vasculo-genesis can therefore be employed [1, 2].
Problems requiring the use of an engineered/artificial blood vessel (graft) include occluded vessels due to stenosis, damaged vessels resulting from trauma or aneurysm, and the formation of a new tissue structure through regenerative therapies. Both the replacement and the repair of blood vessels are viable treatment options.
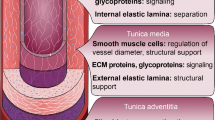
Currently, the “gold standard” option is to use naturally occurring vessels such as the saphenous vein; however, this brings inherent problems including additional surgery for the patient, and the frequent unsuitability or limited availability of their veins due to systemic disease. There is also a lack of viable treatment options when the blood vessel is less than 6 mm in diameter [2–4, 6]. Hence, artificial blood vessels, following either biomaterial or tissue engineered approaches, are utilised.
The artificial vessel, regardless of its construction method/material, should closely match the structure and properties of the natural tissue it is replacing. This is to maximise the functionality and viability of the substitute tissue and the contained blood flow. A significant contributing factor towards achieving this is the vascular biology technique of endothelialisation.
2 Endothelialisation of (Artificial) Blood Vessels
Endothelialisation is the formation of a layer of endothelial cells on the luminal surface of natural blood vessels; with regard to engineered grafts, these cells form a confluent barrier between the artificial material and the circulating blood to prevent the initiation of the clotting cascade, immune response, thrombus/plaque formation and stenosis. The ultimate aim is for cells to align preferentially in the direction of blood flow, with non-activated, non-inflamed, stable phenotype, thus allowing the blood vessels to function naturally demonstrating thromboresistivity and high patency [2–4, 7, 8].
3 Current Methodologies in Optimising Artificial Blood Vessels: Use of Endothelialisation
In order to achieve this, endothelialisation has to be addressed as a vascular biology strategy- although promising animal trial data has been produced, there is a lack of inherent mechanism in humans for re-endothelialisation to occur over sustained surface areas. There is also evidence that human endothelial cells show low adhesion, are slow to populate the disturbed vessel surfaces, and are easily removed and damaged by blood shear stresses [2, 4, 7, 9].
This endothelialisation of the artificial vessels can be approached using three potential strategies (or a combination of): preseeding; precoating; in situ endothelialisation. Each has advantages and disadvantages to their use:
-
Preseeding is an in vitro approach, which uses the structure and/or chemistry of the graft material to produce a layer of adhered, oriented and spread endothelial cells on the luminal surface. Significant bodies of research have demonstrated a direct link between the underlying material and the induced resulting cellular behaviour [1, 2, 4, 10]. The cells are seeded onto the materials, in optimised densities, in sterile laboratory conditions and cultured. This seeded material is then implanted as a whole construct. Some researchers are trying to move away from/modify this approach due to its associated cost, time and cell sourcing issues [6, 11].
-
Precoating is the use of a covering layer upon the luminal surface of the material comprising the blood vessel wall, usually a biological material such as collagen. This enhances the cellular adhesion, growth and proliferation upon the artificial structure; however, the underlying structure is still ideally detectable through this layer to aid in directing the cell growth. This coating itself introduces material effects, such as hydrolysis, and is potentially unstable in its attachment to the material; it is also difficult to achieve with small diameter grafts. This strategy can be used in combination with preseeding or implanted directly [2, 4, 12, 13].
-
The in situ method aims to coat the artificial materials with a natural endothelial layer after implantation; either using uncoated material or in combination with the precoating technique. However, this approach is subject to the previously mentioned issues for of slow cell adhesion, coverage of only small distances and shear stress effects from the flowing blood. Hence, enhancement of the inherent regrowth mechanisms of the endothelial cells is required. There is also a risk of, in the initial implantation stages, that the uncovered material will induce clotting and/or immune responses [2, 4, 14, 15].
It can be seen that all of these methods, from some aspect, require the contribution of the underlying material structure and chemistry, in order to produce the required cellular mechanisms and growth/adhesion. There is a large bulk of research looking at the effect of the material chemistry, and which, in combination with surface coatings, has shown the benefit of carefully selecting the material (and its associated surface charges and hydrophobicity) to produce the optimum cell adhesion and growth patterns [6, 10, 16–19]. However, there are also studies that indicate the critical effect of the structural cues upon the same cell behaviour [8, 20–27]. Structural cues can be used regardless of material choice, and are a significant research area within cell biology; the effect of a cell’s environment upon its behaviour has been widely reported, with the nano- and micro-environments of the natural extracellular matrices and basement membranes providing contact guidance for the cell responses [28, 29]. These properties are also an important factor if considering the performance of currently used clinical vascular grafts, with protein coatings such as collagen/albumin, used to reduce blood loss through the pores; although the structures are still detected and are an influencing factor upon the contacting endothelial cells [5–8, 12, 19, 23, 30]. The ultimate aim is to replicate the signalling and guidance provided in the natural environment through the manipulation of the underlying artificial material [9, 31–34].
Hence, here we describe basic protocols for developing improved endothelialisation of artificial/engineered blood vessels through the use of structural cues, and how to analyse the effects of these modifications. The chapter will end with a focussed example concerning the topical, and increasingly used, electrospinning technique [8, 20, 35–41].
4 Methodologies Using Structural Cues
The surface properties most identified in the literature as having significant effects upon the contacting cellular behaviour are: pore size, “fibre” diameter, surface roughness, porosity (including interconnectivity), fibre orientation and material stiffness (Young’s Modulus). The significance of the property varies with the fabrication method used. In general terms, studies have shown that endothelial cell proliferation is affected by the amount of material (including void space and fibre diameter; increased material raises the proliferation); material fibre orientation affects cellular alignment (increased orientation increases alignment) but not proliferation; cell retention under shear stress (blood flow) is also influenced by fibre orientation although there is an additional link to the amount of material present, with more complicated dual-effects in place (increased fibre orientation increases cell retention although this varies with combined increased surface area of material); lower surface roughness increased endothelial cell adhesion; increased topography increases the cytoskeletal involvement of the cells and so the ability to adapt to shear stress effects (again there are complex inter-linked effects from the different properties- the aim is to produce high amounts of both focal contacts and F-actin to enable adhesion but also movement in combination with low height profiles) [3, 7, 8, 20]. These overall trends are affected by the class of structure produced by the selected fabrication method, e.g. fibrous electrospun scaffolds, or porous extruded foams [19, 21, 23, 24, 35, 39].
In order to determine the specific material effects upon induced/controlled cell responses, a wide range of cell behaviours can be investigated: changes in cell morphology, adhesion, proliferation, viability, cell area and expressed signalling/inflammatory markers. These can be analysed within a simple panel of laboratory tests performed upon the cell-seeded material samples, as outlined below. The experiments performed are applicable for static and dynamic (peri- and/or post-culture) cell culture samples, as an initial stage of testing before animal models and tissue samples are used, and can be used as whole panel or as individual tests (depending on the focus of the experiments/research).
5 Basic Protocol
All materials used and their source companies are listed throughout the detailed protocol sections. For all sections, a minimum of four repeats is recommended to increase statistical accuracy and the assessment of relationships between the altered structural parameters and the induced cellular behaviour.
-
Step 1: Fabricate a range of material samples with systematically altered and controlled structures
Fabricate the selected polymer into an artificial blood vessel construct using the production method of choice (e.g. extrusion, electrospinning); systematically alter the fabrication parameters to produce a range of structures with variation across the property under focus (e.g. pore size) (for guidance regarding potentially significant structural properties to initially investigate please see Sect. 25.4 of this chapter).
-
Step 2: Analyse the pre-culture material properties
Cut 1 cm2 sized pieces of the material- these are then analysed to determine the pre-culture analysis of the material structural properties (see later sections for more detail).
-
Step 3: Perform cell culture tests on the material samples
Cut appropriate sized samples for cell culture experiments; these will typically consist of 1 cm2 flat pieces for static or flat dynamic tests, or 5–10 cm lengths of tubes for dynamic (e.g. bioreactor) tests.
Sterilise the samples using a non-reactive method (polymer-dependent), i.e. your samples should not shrink/swell during the process.
Secure the sample in place in a 24-well plate (these experiments should be run simultaneously across the range of selected structural property, and further repeats performed at future dates); cell suspension rings or sterile glue can be used.
Seed the samples with endothelial cells; a seeding density of 5 × 104 cells per sample allows a range of culture periods to be analysed without over-proliferation of the cells. Seeding with the cells suspended in a very small volume of media (approximately 100–200 μl), leaving to settle in the incubator for 20–30 min then adding the required 1–2 ml of media ensures the adhesion to the material rather than tissue culture polystyrene well surface. (Medium consists of 40 % DMEM (Dulbecco’s Modified Eagles Medium), 40 % 199 (containing modified Earle’s salts) (Gibco™, Invitrogen Corporation, Paisley, UK), 20 % bovine fetal calf serum (FCS) (Cambrex, Nottingham, UK), 1 % non-essential amino acids (NEAA), 1 % sodium pyruvate, 1 % streptomycin and penicillin.)
Samples are cultured for periods of 3, 7, 14 and 28 days, with media changes every 3 days.
-
Step 4: Remove the cell-seeded material samples from culture conditions and perform post-culture analysis to acquire data
After the designated time period, samples are removed from the culture conditions, the media removed and a gentle rinse with phosphate buffered saline (PBS) (Dulbecco’s, d.PBS) performed.
Samples are prepared according to their method of testing- see separate sections in Sect. 25.6 for a more detailed, focussed example of their use. Cell-material analysis would typically consist of assays for: morphology; viability; cell number; cell proliferation; cell coverage/area; cell spreading; cell orientation; cell signalling and marker expression (including examination of activation, inflammation and endothelial phenotype) (all or a selection of these studies depending on the focus of the investigation).
Statistical analysis is then performed, with averages and standard deviations calculated, and one-way ANOVA tests of variance executed to determine significant differences between sample groups and observed behaviour. Tukey post-hoc analysis with a 95 % confidence interval is used to rank these significances; Pearson correlation coefficient is utilised to determine correlative effects from the underlying structural properties upon the induced material-cell interactions.
6 Detailed Protocol with a Focus on the Investigation of the Effect of Structural Cues Provided from Electrospun Scaffolds on Endothelial Cells (Human Umbilical Vein Endothelial Cells (HUVECs)) (Some Details Adapted from [8, 20, 41])
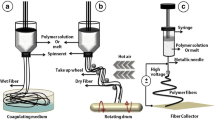
6.1 Production of a Range of Fibrous Scaffolds Using Electrospinning
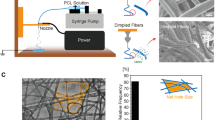
The polymer of choice was dissolved in an appropriate solvent (e.g. 12.5 w/v % Tecoflex® SG-80A polyurethane (Thermedics, Woburn, USA) with 1:1.68 dimethylacetamide (DMAC): 2-butanone (methyl ethyl ketone) (MEK) (Aldrich, Gillingham, UK)).
The spinning (process) parameters were systematically altered between spinning runs to produce a predictable range of scaffolds with varying structural components [41]. Parameters included: flow rate (flow of solution from nozzles); spray height (the relative vertical distance of the nozzles from the mandrel); spray distance (the horizontal distance of the nozzles from the mandrel); traverse speed (the constant linear speed of the traverse); mandrel speed (the constant rotational speed of the mandrel); applied voltage and temperature/relative humidity. 1 cm2 samples were cut for use in further analysis and tests.
6.2 Characterisation of Pre-culture Scaffold Structural Properties
The pre-culture scaffolds were characterized for inter-fibre separation (ifs), fibre diameter (f.dia), void fraction (VF), surface roughness (SR) and fibre orientation (f.orn).
Ifs, f.dia and f.orn measurements were taken using a field emission scanning electron microscope (SEM), with a working distance of 8 mm, an acceleration gun voltage of 5 kV and the secondary electron detector. Samples were first sputter-coated with chromium (approximately 50 nm coating) for 2 min at 125 mA in an argon atmosphere. Measurements were taken of the structures at approximately ×1,000 magnification, using SEM digital measuring tools, from the top layer of fibres as indicated by their overlapping on the images.
VF was also analysed using the SEM images, combined with the use of the imaging software, using macro programs to analyse the stored microscope images (software can include the proprietary microscope package such as KS400 or AxioVision (Zeiss, Welwyn Garden City, UK) or ImageJ (NIH, USA)).
Atomic force microscopy (AFM) was used to quantify the SR of the scaffolds. The probe was fitted with a silicone-nitride (Si2N4) tip, operated in contact mode. Measurements were taken on an individual fibre basis, with scan sizes set to 15 μm, line scanned at a rate of 1 Hz, and a z range of 4.69 μm.
Ifs measurements were repeated 20 times, f.dia 14 times, SR and VF 5 times and f.orn 20 times per sample.
6.3 Cell Culture of Electrospun Material Samples
As described in Steps 2 and 3 in Sect. 25.5 of this chapter.
6.4 Analysis of Post-Culture Cell-Material Samples with Induced Cell-Material Interactions
6.4.1 General Cell Morphology, Area and Spreading Imaging and Analysis
SEM Analysis
Cell-samples were immersed in 2.5 % glutaraldehyde (VWR, Poole, UK) solution for 1 h, washed in PBS and distilled water, and dehydrated in alcohol (70, 90 and 100 % ethanol for 15 min respectively, each stage performed twice). Samples were then dried, preferably critical point dried to enhance the surface features of the cells (although careful air-drying can be used), followed by sputter-coating with chromium (details previously described). The same SEM parameters as detailed in Sect. 25.6.2 of this chapter were used.
Confocal Staining and Analysis
Cell-seeded samples were fixed (after rinsing with d.PBS) using 4 % formaldehyde, 2 % sucrose fixative solution at 37 °C, 5 % CO2, humidified, for 10 min, then rinsed with PBS. Samples were permeabilized using 0.5 % Triton X100 solution at 4 °C for 5 min, then rinsed again with PBS. Primary antibodies were added and incubated for 1 h at 37 °C: 0.22 mg/ml monoclonal mouse anti-human vinculin (Serotec, Oxford, UK). This was followed by a trio of wash steps performed in the dark at room temperature for 3 min each, using 0.1 % Tween 20® solution (ICN Biomedicals Inc., Aurora, Ohio, USA). Secondary antibodies were added for 1 h at 37 °C: 0.1 mg/ml rhodamine (rhodamine-conjugated goat IgG fraction to mouse IgG whole molecule) (Sigma, Gillingham, UK). The Tween 20® wash step was then repeated. Oregon-green phalloidin (Molecular Probes, Leiden, The Netherlands) was added at a concentration of 5 μg/ml for 30 min at 4 °C. Samples were washed with PBS before mounting with fluorescence stabilising mountant containing DAPI stain (Vectashield® with DAPI H-1200) (Vector Laboratories Inc., Burlingame, CA, USA). Samples were kept in the dark, at 4 °C, until analysis.
Images were obtained from a confocal laser scanning microscope (LSM 510) (Zeiss, Welwyn Garden City, UK). The DAPI nuclear stain (both cell types) was visualised by UV excitation at λ = 364 nm, a HeNe, λ = 568 nm laser was used to visualise vinculin for focal contacts (L929 cells), λ = 543 nm for HUVECs, and oregon green phalloidin for F-actin was excited with an Argon laser λ = 488 nm. All samples were examined at x100 magnification. Z-stack (imaging through the vertical plane) measurements, through the use of the confocal computer software, were also taken of the cells to determine their height profiles on the three different materials.
Analysis of all microscope images was performed using an imaging software package (as Sect. 25.6.2). Programmed macros assessed and calculated cell area, coverage (defined as the percentage of scaffold surface covered with cells), spreading (the index of the degree of spreading of the cells, calculated by dividing the cell coverage by the cell number), and orientation (the angle of the cell measured along the long axis; degrees).
6.4.2 Cell Numbers and Proliferation Analysis
Samples were washed with d.PBS and stored (dry) at −80 °C. Upon analysis, samples were thawed at room temperature, and the CyQuant assay performed to determine cell number according to manufacturer’s instructions (Molecular Probes, Eugene, Oregon, USA); fluorescence was read using a plate reader. Standard curves (fluorescence versus cell number) were produced from cell pellet data, and used to convert fluorescence into cell number for the material-cell samples.
6.4.3 Cell Viability Analysis
Samples were washed with PBS and kept in a wet state under a layer of PBS whilst preparing reagents. Live:dead assay (Life Technologies, UK) is used to produce a ratio of percentage live and dead cells through two colour fluorescent staining and image analysis, or fluorescent plate reader data acquisition. Full details should be followed according to the manufacturer’s protocol.
6.4.4 Analysis of Cell Signalling and Marker Expression
Cell-seeded material scaffolds were rinsed in d.PBS, fixed in 4 % formaldehyde 2 % sucrose solution (VWR, Poole, UK) for 10 min at 37 °C, 5 % CO2, humidified, then rinsed again with d.PBS. Samples were then stained with sterile filtered 0.4 % methylene blue for 12 min (VWR, Poole, UK).
Each sample was cut into smaller areas and incubated with rabbit serum for 30 min at room temperature, then with a 1:200 dilution of mouse anti-human primary antibodies in PBS containing 1 % bovine serum albumin (BSA) at room temperature for 1 h. The primary antibodies used were: collagen I, elastin, fibronectin, CD54, CD106, CD51/61, CD49c; CD31, CD62E/P, vWF (see Table 25.1 for more details on this immunostaining panel). An IgG1 isotype control and PBS negative control were used throughout all staining procedures. Two PBS buffer rinses followed. Secondary antibody solution of rabbit anti-mouse immunoglobulin (biotinylated) (E0464) (Dako A/S, Denmark) (25 μl in 5 ml PBS) was added for 30 min at room temperature; again followed by PBS rinses. Samples were then incubated with Vectastain ABC-AP kit (AK-500) (Vector Laboratories Inc., Burlingame, CA, USA) for 30 min. This was followed by a PBS wash. Incubation in Alkaline Phosphatase substrate (Kit 1, SK-5100) (Vector Laboratories Inc., Burlingame, CA, USA) followed (Trizma® Base (Tris[hydroxymethyl]aminomethane) (Tris) (Sigma, Gillingham, UK) solution of 1.2 g Tris in 100 ml distilled water (pH range 8.2–8.5) combined with kit reagents). Samples were incubated with this solution for 30 min in the dark, followed by a final wash step in distilled water prior to mounting in a fluorescence stabilising mountant containing DAPI nuclear stain (Vectashield® with DAPI) (Vector Laboratories Inc., Burlingame, CA, USA). Samples were kept in the dark, at 4 °C, until analysis.
Positive/negative expression of extracellular matrix and adhesion molecules was determined by reflective light microscopy and laser scanning confocal microscopy (methodology already detailed) (methylene blue and DAPI nuclear stains confirmed the correct location of positive staining and provided the ability to co-image cell area and quantity/location of positive marker expression); quantified results were obtained through the image analysis of the images (described in Sects. 25.6.2 and 25.6.4.1).
6.5 Further Analysis to Determine Correlative Relationships and Develop the Data Obtained
Statistical analysis was then performed as detailed in Sect. 25.5 (step 4) to determine significant differences in the produced cell behaviour, and then specific correlative relationships, i.e. an induced effect upon the cell behaviour directly from the changes made to the underlying material structures.
Optimised structural properties to enhance endothelialisation can then be selected and the “designed”, fabricated grafts further investigated for specific aspects of functionality.
7 Sample Results
The examination of two commonly used clinical vascular graft materials (PET and ePTFE) showed significant changes to the cellular behaviour resulting from the underlying substrate structures. This altered the cellular ability to adapt to the shear stresses and so the retention of the endothelial layer, having a direct effect on the performance and functionality of the artificial vessel [8] (Figs. 25.1 and 25.2).
Investigation of two commonly used vascular graft materials and an electrospun scaffold. (a) Images (light microscope and SEM) showing surfaces of PET, ePTFE and electospun PU (left to right); (b) confocal microscope images showing cell morphology and cytoskeletal involvement across the three materials; (c) graph showing the cell height profiles; (d) graph showing the comparative levels of three cell markers (Adapted from [8])
Further investigation of the electrospun materials, using a range of scaffolds with varying structures. (a) Charts to show positive/negative expression of cell markers at 7 and 28 days (top to bottom); (b) confocal microscope images showing cell morphology, cell orientation and cytoskeletal involvement across two of the electrospun materials (left- 22D; right 48A); (c) graphs showing the cellular behavior (left- cell coverage; centre- cell number; right- cell spreading) (Adapted from [20])
As a result of these findings, electrospun materials (PU) were investigated in more detail, with a range of structures included in the tests. Fundamental mechanisms relating the scaffold features to the induced contacting cell behaviour were established (based on data and statistical analysis): initial cell adhesion relied on the physical amount of material present; increased cell proliferation was affected by scaffold material and orientation of physical features; features influencing cell spreading and coverage included void size, surface roughness and fibre orientation; cell-cell interactions were influenced to different degrees by all scaffold features [20].
8 Developing the Application of These Methodologies
The results of using these methodologies can be extended and applied to further research. For example, in order to determine the optimum structure for a specific vascular application/location, these tests can be used to find the best properties to fabricate and those parameters then used and researched in greater detail regarding the functionality, biocompatibility and immunological responses provoked (for example using ELISAs, Western Blotting, or Real-Time PCR). The ideal aim is to use these tests as a way of determining the optimum fabrication parameters to produce a material that has (structural) properties that enhance endothelialisation. Hence, the artificial blood vessel can be produced in such a way to mimic the natural tissue it is replacing, i.e. replicates the natural basement membrane that produces and sustains endothelial layers.
Another important area directly connected to this development of artificial blood vessels is that of haemodynamics. This field is worthy of further attention, with significant potential and need for advancement alongside the use of material structural cues to enhance endothelialisation. In order to achieve this, the haemodynamic parameters applied to the cells from the contacting blood flow should be known as fully as possible [5, 7, 8, 16, 17, 19, 42].
As mentioned throughout this chapter, endothelialization in engineered/artificial grafts is a crucial process to maximise the functionality and viability of the vessel and the contained blood flow and has significant effects on the success of implantation. Endothelial cells lining the artery wall have the ability to act as the fluid dynamic wall-shear biosensor and can minimize the development of plaque.
Currently, one of the most important haemodynamic parameters in cardiovascular problems is the ‘shear stress’. In fluid mechanics, shear stress is defined as the friction between two layers of the fluid moving at different velocities. In blood vessels, the shear stress also arises at the interplay between blood and endothelial layer where it induces a shearing deformation of the endothelial cells. Nowadays, thanks to the increasing power-to-cost ratio of computers and the advent of methods for subject-specific modelling of cardiovascular mechanics, it is possible to calculate shear stress-related forces using Computational Fluid Dynamics (CFD).
In general, CFD is a technique to analyse fluid flow, heat transfer and associated phenomena, using computer-based simulation and has recently shown great potential for the calculation of various haemodynamic parameters in patient-specific models including Time-Averaged Wall Shear Stress (TAWSS), Oscillatory Shear Index (OSI) and Relative Residence Time (RRT), amongst others. CFD also provides an alternative to invasive or non-invasive flow measurements of blood flow by and/or ex vivo experimental flow measurement techniques, which can be very expensive and time consuming. Further details on the use of CFD in cardiovascular problems and haemodynamic parameters can be found in Chap. 27.Footnote 1
In artificial grafts, the shear stress at the endothelial wall (also known as the Wall Shear Stress – WSS), has a fundamental role in the endothelialization process. For example, it is known that high shear stress could limit the endothelialization process, therefore, the calculation of shear stress within the whole artificial blood vessel is considered to play an important role in its design and optimization [42].
Apart from the importance of WSS in the endothelialization process, WSS can also help to better explain some of the common graft failures. For instance, in their computational simulations, Kouhi et al. [43] reported a dramatic drop in the magnitude of WSS in critical areas of graft anastomosis such as toe, heel, and suture lines which could initiate the promotion of intimal hyperplasia and cause early graft failure after coronary artery bypass graft.
Another graft failure is due to atherosclerosis, in and around the downstream anastomosis. Atherosclerotic lesions occur predominantly at sites of low shear, whereas regions of the vasculature exposed to a physiologic shear are protected [44]. A developing plaque can modify the local shear stress around the lesion. Lumen narrowing would result in an increase in the blood velocity and shear stress over the plaque, low shear stress in the upstream region, and disturbed flow in the form of directionally OSI in the downstream zone of the plaque [45, 46].
9 Conclusions
There is a clinical need to enhance endothelialisation of artificial blood vessels used to repair or replace damaged/diseased blood vessels. The increased understanding of successful techniques that can be used to achieve this, regardless of the material, fabrication method or design used, would be a significant achievement in the fields of vascular biology, biomaterials and tissue engineering. This chapter has detailed a variety of methodologies that can be used as individual or a panel of tests to determine the effect of altering the underlying material structures upon the endothelial cells. These results provide the means to determine correlative relationships between the structural properties and the cell behaviour; these can then be incorporated into the fabrication design and progressed to develop improved strategies for artificial blood vessels with enhanced functionality, creating engineered tissue analogues.
Notes
- 1.
Note for the editor: this is a reference to another Chapter submitted as part of this book entitled “Vascular Flow Modelling using Computational Fluid Dynamics”, authored by A. Keshmiri and K. Andrews.
References
Tian L, George SC (2011) Biomaterials to prevascularize engineered tissues. J Cardiovasc Transl Res 4:685–698
Vara DS, Salacinski KJ, Kannan RY et al (2005) Cardiovascular tissue engineering: state of the art. Pathol Biol 53:599–612
Andrews KD, Hunt JA (2009) Developing smaller-diameter biocompatible vascular grafts. In: Di Silvio L (ed) Cellular responses to biomaterials (section II: cell responses & regenerative medicine). Woodhead, Cambridge
Menu P, Stoltz JF, Kerdjoudf H (2013) Progress in vascular graft substitute. Clin Hemorheol Microcirc 53:117–129
Spadaccio C, Rainer A, Barbato R et al (2013) The fate of large-diameter Dacron® vascular grafts in surgical practice: are we really satisfied? Int J Cardiol 168:5028–5029
Sarkar S, Schmitz-Rixen T, Hamilton G et al (2007) Achieving the ideal properties for vascular bypass grafts using a tissue engineered approach: a review. Med Biol Eng Comput 45:327–336
Feugier P, Black RA, Hunt JA et al (2005) Attachment, morphology and adherence of human endothelial cells to vascular prosthesis materials under the action of shear stress. Biomaterials 26:1457–1466
Andrews KD, Feugier P, Black RA et al (2008) Vascular prostheses: performance related to cell-shear responses. J Surg Res 149:39–46
Cittadella G, de Mel A, Dee R et al (2013) Arterial tissue regeneration for pediatric applications: inspiration from up-to-date tissue-engineered vascular bypass grafts. Artif Organs 37:423–434
Kurobe H, Maxfield MW, Breuer CK et al (2012) Concise review: tissue-engineered vascular grafts for cardiac surgery: past, present, and future. Stem Cells Transl Med 1:566–571
Fernandez CE, Achneck HE, Reichert WM et al (2014) Biological and engineering design considerations for vascular tissue engineered blood vessels (TEBVs). Curr Opin Chem Eng 3:83–90
Fernandez P, Deguette A, Pothuaud L et al (2005) Quality control assessment of ePTFE precoating procedure for in vitro endothelial cell seeding. Biomaterials 26:5042–5047
Assmann A, Delfs C, Munakata H et al (2013) Acceleration of autologous in vivo recellularization of decellularized aortic conduits by fibronectin surface coating. Biomaterials 34:6015–6026
Avci-Adali M, Perle N, Ziemer G et al (2011) Current concepts and new developments for autologous in vivo endothelialisation of biomaterials for intravascular applications. Eur Cells Mater 21:157–176
Avci-Adali M, Paul A, Ziemer G et al (2008) New strategies for in vivo tissue engineering by mimicry of homing factors for self-endothelialisation of blood contacting materials. Biomaterials 29:3936–3945
Thomas LV, Lekshmi V, Nair PD (2013) Tissue engineered vascular grafts- preclinical aspects. Int J Cardiol 167:1091–1100
Sarkar S, Sales KM, Hamilton G et al (2007) Addressing thrombogenicity in vascular graft construction. J Biomed Mater Res B Appl Biomater 82B:100–108
de Valence S, Tille J-C, Chaabane C et al (2013) Plasma treatment for improving cell biocompatibility of a biodegradable polymer scaffold for vascular graft applications. Eur J Pharm Biopharm 85:78–86
Wong CS, Sgarioto M, Owida AA et al (2006) Polyethyleneterephthalate provides superior retention of endothelial cells during shear stress compared to polytetrafluoroethylene and pericardium. Heart Lung Circ 15:371–377
Andrews KD, Hunt JA (2008) Upregulation of matrix and adhesion molecules induced by controlled topography. J Mater Sci Mater Med 19:1601–1608
Isenberg BC, Backman DE, Kinahan ME et al (2012) Micropatterned cell sheets with defined cell and extracellular matrix orientation exhibit anisotropic mechanical properties. J Biomech 45:756–761
Chen J-H, Laiw R-F, Jiang S-F et al (1999) Microporous segmented polyetherurethane vascular graft: I. dependency of graft morphology and mechanical properties on compositions and fabrication conditions. J Biomed Mater Res 48:235–245
Blinder YJ, Mooney DJ, Levenberg S (2014) Engineering approaches for inducing blood vessel formation. Curr Opin Chem Eng 3:56–61
Ahmed M, Ghanbari H, Cousins BG et al (2011) Small caliber polyhedral oligomeric silsesquioxane nanocomposite cardiovascular grafts: influence of porosity on the structure, hemocompatibility and mechanical properties. Acta Biomater 7:3857–3867
Koens MJW, Faraj KA, Wismans RG et al (2010) Controlled fabrication of triple layered and molecularly defined collagen/elastin vascular grafts resembling the native blood vessel. Acta Biomater 6:4666–4674
Norotte C, Marga FS, Niklason LE et al (2009) Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 30:5910–5917
Kang T-Y, Hong JM, Kim BJ et al (2013) Enhanced endothelialization for developing artificial vascular networks with a natural vessel mimicking the luminal surface in scaffolds. Acta Biomater 9:4716–4725
McNamara LE, Burchmore R, Riehle MO et al (2012) The role of microtopography in cellular mechanotransduction. Biomaterials 33:2835–2847
Biggs MJP, Richards G, Dalby MJ (2010) Nanotopographical modification: a regulator of cellular function through focal adhesions. Nanomed: Nanotechnol Biol Med 6:619–633
Vitte J, Benoliel AM, Pierres A et al (2004) Is there a predictable relationship between surface physical-chemical properties and cell behavior at the interface? Eur Cell Mater 7:52–63
Boccafoschi F, Mosca C, Ramella M et al (2013) Biological evaluation of materials for cardiovascular application: the role of the short-term inflammatory response in endothelial regeneration. J Biomed Mater Res A 101A:3131–3140
Filová E, Brynda E, Riedel T et al (2014) Improved adhesion and differentiation of endothelial cells on surface-attached fibrin structures containing extracellular matrix proteins. J Biomed Mater Res A 102A:698–712
Kirton JP, Xu Q (2010) Endothelial precursors in vascular repair. Microvasc Res 79:193–199
Li W, Wang H, Kuang C-Y et al (2012) An essential role for the Id1/PI3K/Akt/NFkB/surviving signaling pathway in promoting the proliferation of endothelial progenitor cell in vitro. Mol Cell Biochem 363:135–145
Hasan A, Memic A, Annabi N et al (2014) Electrospun scaffolds for tissue engineering of vascular grafts. Acta Biomater 10:11–25
Fullana MJ, Wnek GE (2012) Electrospun collagen and its applications in regenerative medicine. Drug Deliv Transl Res 2:313–322
Dargaville BL, Vaquette C, Rasoul F et al (2013) Electrospinning and crosslinking of low-molecular-weight poly(trimethylene carbonate-co-L-lactide) as an elastomeric scaffold for vascular engineering. Acta Biomater 9:6885–6897
Wong CS, Liu X, Xu Z et al (2013) Elastin and collagen enhances electrospun aligned polyurethane as scaffolds for vascular graft. J Mater Sci Mater Med 24:1865–1874
Hu J-J, Chao W-C, Lee P-Y et al (2012) Construction and characterization of an electrospun tubular scaffold for small-diameter tissue-engineered vascular grafts: a scaffold membrane approach. J Mech Behav Biomed Mater 13:140–155
Kuwabara F, Narita Y, Yamawaki-Ogata A et al (2012) Long-term results of tissue-engineered small-caliber vascular grafts in a rat carotid arterial replacement model. J Artif Organs 15:399–405
Andrews KD, Hunt JA, Black RA (2008) Technology of electrostatic spinning for the production of polyurethane tissue engineering scaffolds. Polym Int 57:203–210
Gui L, Niklason LE (2014) Vascular tissue engineering: building perfusable vasculature for implantation. Curr Opin Chem Eng 3:68–74
Do H, Owida AA, Yang W, Morsi YS (2011) Numerical simulation of the haemodynamics in end-to-side anastomoses. Int J Nume Methods Fluids 67:638–650. doi:10.1002/fld
Kouhi E, Morsi YS, Masood SH (2008) Haemodynamic analysis of coronary artery bypass grafting in a non-linear deformable artery and Newtonian pulsatile blood flow. Proc Inst Mech Eng H 222:1273–1287. doi:10.1243/09544119JEIM459
Dai G, Kaazempur-Mofrad MR, Natarajan S et al (2004) Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and -resistant regions of human vasculature. Proc Natl Acad Sci U S A 101:14871–14876. doi:10.1073/pnas.0406073101
Cecchi E, Giglioli C, Valente S et al (2011) Role of hemodynamic shear stress in cardiovascular disease. Atherosclerosis 214:249–256. doi:10.1016/j.atherosclerosis.2010.09.008
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Andrews, K., Keshmiri, A. (2015). Enhancing Endothelialisation of Artificial/Engineered Blood Vessels Using Structural Cues. In: Slevin, M., McDowell, G. (eds) Handbook of Vascular Biology Techniques. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9716-0_25
Download citation
DOI: https://doi.org/10.1007/978-94-017-9716-0_25
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9715-3
Online ISBN: 978-94-017-9716-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)