Abstract
Renal development process in human is divided into 3 successive stages: pronephros, mesonephros, and metanephros. The tubules continue to mature for 1–2 year after birth. Research of urinary proteome during human renal development is still lacking. Most urine proteome studies focus on postnatal renal maturation period. A comparison between full-term infant and adult urinary protein pattern identified 648 infant-enriched protein spots, of which most were involved in cellular turnover and metabolism. The study of preterm infant urinary proteome compared with term infants suggests elevated IGFBP-1, IGFBP-2, and IGFBP-6, monocyte chemotactic protein-1, CD14, and sialic acid-binding Ig-like lectin 5 during nephrogenesis. Research in several congenital kidney and urinary tract anomalies, ureteropelvic junction obstruction and autosomal dominant polycystic kidney disease, has discovered novel biomarkers, which may help to imply the mechanisms underlying inherited disorders. Future exploration of urinary proteome evolution during renal maturation is needed and will help to find novel biomarkers specially suiting pediatric renal diseases.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Renal development
- Urinary proteome
- Biomarkers
- Ureteropelvic junction obstruction
- Autosomal dominant polycystic kidney disease
1 Introduction
Discovery of novel therapeutic targets and strategies to slow or reverse kidney injury process require an understanding of the molecular mechanisms that underlie kidney development [12]. In the past few decades, researchers mainly rely on molecular and biochemical techniques to explore individual genes and molecular pathways involved in normal renal development. The information has reinforced our understanding of different developmental disorders such as renal agenesis and renal dysplasia.
As a newly emerged discipline, urinary proteome analysis has been extensively applied to the discovery of diagnostics and prognostic disease biomarkers. In humans, while all segments of the nephron are present at birth, maturation of the tubule continues in the postnatal period [3]. Although there is a lack of enough literature toward the research of the urinary proteome during renal development, previous work on normal rat urine has demonstrated that the urinary proteome undergoes significant changes from birth (0 days) to adulthood (>30 days) [10].Thus, the human urinary protein patterns may change over time during late nephrogenesis and early postnatal renal maturation. Analysis of urinary proteins will offer a unique window into dynamically changes in renal developmental processes.
2 Human Renal Development and Maturation
Like other vertebrates, human kidney derives from the intermediate mesoderm of the urogenital ridge, a structure along the posterior wall of the abdomen in fetus [18]. Three successive stages, the pronephros, the mesonephros, and the metanephros, are gone through and mature kidney eventually emerges. From the beginning of pronephros development at 22-day gestation to the completion of nephrogenesis and tubulogenesis at 32- to 36-week gestation, full-term infants will get a complete endowment of nephrons, varying widely from 250,000 to 1.8 million per kidney, and do not form new nephrons after birth [8, 16].
Up to this point, the whole process is defined as renal development, of which metanephros transformation attracts the most research interest. From 5 week, a series of reciprocal inductive interactions occur between metanephric mesenchyme (MM) and epithelial ureteric bud (UB). Upon invasion of UB into the MM, signals from MM cause UB to undergo dichotomous branching, giving rise to the urinary collecting system. MM is also induced by the signals back from UB to condense along the surface of UB, fulfilling the mesenchymal–epithelial transition [12]. Simultaneously, interactions between epithelial and mesenchymal cells lead to a coordinated development of multiple highly specialized stromal, vascular, and epithelial cell types [15]. Previous molecular studies have implied many growth factors and signal pathways are involved in this process.
Tubules of neonatal kidney continue to maturate for an additional 1–2 year in the postnatal period to achieve the adult glomerular filtration rate, which is approximately 50 times that of neonatal kidney [1]. Some nephron segments change in abundance of transporters, while others change in transporter isoforms, paracellular permeability, or intracellular signaling that regulate the transporter [3]. All these mechanisms work together to achieve the expansion of glomerular filtration function.
3 Proteomic Analysis of Human Renal Development and Maturation
Urinary proteome can change under pathological conditions or alter because of normal physiologic variations. However, to date, few studies have examined the effect of normal renal development and maturation process on complete urine protein expression. Obviously, the bottleneck for conducting developmental urine proteome research is the basic fact that researchers cannot even obtain qualified urine samples before the full completion of glomerulogenesis and tubulogenesis. Currently, most published urine proteome studies focus on postnatal renal maturation period.
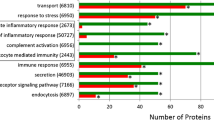
Froehlich et al. [7] published their work in 2013. Their group compared the urinary proteome of 6 healthy adult males (mean age: 31.7 years) with 6 healthy full-term infant males (mean age: 1.0 year) and identified three datasets. Seven hundred and eight proteins were commonly identified in both cohorts; 228 proteins were detected only in the adult samples, and 648 proteins were identified exclusively in infants. Of the 708 commonly presented proteins, 136 were significantly enriched in urine from adults and 94 were significantly enriched in urine from infants. Using gene ontology, it turned out that infant-enriched or infant-specific subproteome (743 proteins) had an over representation of proteins that are involved in translation and transcription, cellular growth and metabolic processes. In contrast, the adult-enriched or adult-specific subproteome (364 proteins) showed an overexpression of proteins involved in immune response and cell adhesion.
This study suggests cellular turnover and metabolism are increased in infants, in accordance with the postnatal tubular maturation theory. Further explorations are needed to identify biomarkers associated with renal maturation. Moreover, these data highlight the importance of age matching in urinary proteomics.
Due to the technical bottleneck mentioned above, there is only one study focusing on the renal development in fetus up to now, which was conducted by Charlton et al. [4]. They collected urine samples at birth and over 12 month from preterm (33–35 week) and term (38–40 week) infants. Utilizing a combination of G2000 antibody array and enzyme-linked immunosorbent assay, preterm infants at birth were found to have relatively elevated levels of insulin-like growth factor binding protein (IGFBP)-1, IGFBP-2 and IGFBP-6, monocyte chemotactic protein-1, CD14, and sialic acid-binding Ig-like lectin 5 (Siglec-5). Elevated levels of multiple members of the IGF family support the critical role for this family of growth factors during nephrogenesis, which is consistent with the results of previous animal studies [5]. MCP-1, CD14, and Siglec-5 are considered markers of inflammation and were unanticipated findings.
As nephron formation continues until 36 week gestation, these 33- to 35-week preterm infants could possibly offer biomarker clues for renal development. Yet, it should be noted that variations seen in the markers may also represent changes in adaptations to extra-uterine environmental stresses that are related to preterm delivery, which is actually abnormal situations.
4 Biomarker Discovery from Urine Proteome in Congenital Anomalies of Kidneys and Urinary Tract
Studies of urine proteome profiles under the pathological renal developmental processes may help to imply the mechanisms underlying inherited anomalies of kidneys and urinary tract and discover novel biomarkers for clinical diagnoses and prognosis evaluation. Of this recently emerged research area, the most prominent researches are as follows:
-
1.
Ureteropelvic junction obstruction (UPJO):
UPJO occurs during early kidney development and affects renal morphogenesis, maturation, and growth [19], which is the most common prenatally detected disease leading to hydronephrosis [13]. Previous studies showed obstruction may affect the formation of nephrons. Although the decreased obstructed kidney function later normalizes, preexisting structural changes may in later years reduce the functional capacity of the kidney [6]. Researchers are challenging to distinguish those patients with severe diseases needing urgent surgical intervention out from others under rather stable conditions by utilizing urinary biomarkers. Previously, investigators have examined the cytokines already known to be up- or downregulated in UPJO or other nephropathy to find potential urinary biomarkers. Several widely known biomarkers screened out in this way are summarized in Mia Gebauer Madsen et al’s review published in 2010 [11]. They are transforming growth factor β1 (TGF-β1), N-Acetyl-β-D-glucosaminidase (NAG), monocyte chemotactic peptide-1 (MCP-1), epidermal growth factor (EGF), and endothelin-1 (ET-1) (Table 10.1). Some of these biomarkers have also been tested in clinical studies.
Table 10.1 Potential urinary biomarkers in prenatally diagnosed unilateral hydronephrosis [11] Recently, Hrair-George O. Mesrobian et al. obtained urine specimens from 21 healthy infants with normal maternal/fetal ultrasound and 25 infants with grade IV unilateral ureteropelvic junction obstruction. Samples from the 2 groups were subjected to liquid chromatography/tandem mass spectrometry analysis. They found 31 proteins significantly different in abundance at 1 to 6 months and 18 at 7 to 12 months compared to age-matched controls. All of the 5 biomarkers in Table 10.1 were observed with the notable exception of TGF-β1 [14]. This study utilizes the most advanced urinary proteome analysis technology to find more information about specific proteins and peptides in UPJO, which may allow for more accurate diagnosis and disease stratification. Moreover, these dynamically changing protein profiles between UPJO and control groups also provide clues into the pathological mechanism underlying clinical manifestation.
-
2.
Autosomal dominant polycystic kidney disease (ADPKD):
ADPKD is an inherited disorder affecting 1 in 1,000 people and responsible for 10 % of cases of the end-stage renal disease (ESRD) [2]. The disease is caused by mutations in the PKD1 (85 % of cases) or PKD2 gene (15 % of cases). The precise processes leading to cyst formation and loss of renal function remain incompletely understood. Early diagnosis would be of benefit for efficient planning of therapy. Kistler [9] and other colleagues published their results in 2009. Using capillary electrophoresis and mass spectrometry, they analyzed urinary samples from 17 ADPKD patients and compared with 86 samples from age- and sex-matched apparently healthy controls. After a series of selecting and eliminating procedures, 38 proteins were eventually identified as biomarkers, most of which were collagen fragments. This suggests that there is high turnover of extracellular matrix proteins. Uromodulin peptides, previously implicated in tubular injury, were also found in the urine specimens. A support vector machine (SVM)-based model was then created by combining these 38 biomarkers and including additional controls to enable high specificity. This model applied to an independent masked dataset of 24 cases and 35 healthy controls discriminated ADPKD from controls with 87.5 % sensitivity and 97.5 % specificity (AUC: 0.95). Moreover, the model remained with a high sensitivity and specificity when additionally tested in normal controls, patients with different chronic renal diseases, with bladder cancer, with renal cell cancer and elderly group (aged >60).
Although the diagnosis of ADPKD is in most cases easily established based on an age-dependent cystic renal phenotype and a positive family history [17], there is considerable inter- and intrafamilial variability in the rate of progression to kidney failure. Physicians could utilize this technique of urinary proteome to select patients with rapidly progressive disease for more radical treatments, while avoiding exposing patients with mild disease to expensive and unnecessary therapies with potential side effects.
5 Conclusions and Future Place
Urinary proteome analysis has been increasingly investigated to provide promising results concerning molecules participating human renal development, adding to our understanding of the mechanisms and pathophysiology underlying diseases. We expect more determination of important protein elements in signaling pathways. Developmental urine proteome is especially widely applied in investigating biomarkers of congenital renal anomalies. Since differences of urinary proteome patterns exist among different age groups, a deeper exploration of urinary proteome evolution during renal maturation will help to find novel biomarkers specially suiting pediatric renal diseases. Future contributions are still needed to better understand the series of events culminating in the formation of mature metanephros in humans. Emerging knowledge in this area will link top basic research to clinical setting in the coming years.
References
Arant BS Jr (1978) Developmental patterns of renal functional maturation compared in the human neonate. J Pediatrics 92:705–712
Bakun M, Niemczyk M, Domanski D, Jazwiec R, Perzanowska A, Niemczyk S, Kistowski M, Fabijanska A, Borowiec A, Paczek L, Dadlez M (2012) Urine proteome of autosomal dominant polycystic kidney disease patients. Clin Proteomics 9:13
Baum M, Quigley R, Satlin L (2003) Maturational changes in renal tubular transport. Curr Opin Nephrol Hypertens 12:521–526
Charlton JR, Norwood VF, Kiley SC, Gurka MJ, Chevalier RL (2012) Evolution of the urinary proteome during human renal development and maturation: variations with gestational and postnatal age. Pediatr Res 72:179–185
Doublier S, Amri K, Seurin D, Moreau E, Merlet-Benichou C, Striker GE, Gilbert T (2001) Overexpression of human insulin-like growth factor binding protein-1 in the mouse leads to nephron deficit. Pediatr Res 49:660–666
Eskild-Jensen A, Frokiaer J, Djurhuus JC, Jorgensen TM, Nyengaard JR (2002) Reduced number of glomeruli in kidneys with neonatally induced partial ureteropelvic obstruction in pigs. J Urol 167:1435–1439
Froehlich JW, Vaezzadeh AR, Kirchner M, Briscoe AC, Hofmann O, Hide W, Steen H, Lee RS (2013). An in-depth comparison of the male pediatric and adult urinary proteomes. Biochimica et biophysica acta 1844:1044–1050
Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF (2003) A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 63:S31–37
Kistler AD, Mischak H, Poster D, Dakna M, Wuthrich RP, Serra AL (2009) Identification of a unique urinary biomarker profile in patients with autosomal dominant polycystic kidney disease. Kidney Int 76:89–96
Lee RS, Monigatti F, Lutchman M, Patterson T, Budnik B, Steen JA, Freeman MR, Steen H (2008) Temporal variations of the postnatal rat urinary proteome as a reflection of systemic maturation. Proteomics 8:1097–1112
Madsen MG, Norregaard R, Frokiaer J, Jorgensen TM (2011) Urinary biomarkers in prenatally diagnosed unilateral hydronephrosis. J Pediatr Urol 7:105–112
Maezawa Y, Kreidberg J, Quaggin SE (2012) Embryology of the Kidney. In Brenner and Rector’s the Kidney (9th Edition). Elseiver, New York, pp 1–23
Mesrobian HG, Mirza SP (2012) Hydronephrosis: a view from the inside. Pediatr Clin North Am 59:839–851
Mesrobian HG, Mitchell ME, See WA, Halligan BD, Carlson BE, Greene AS, Wakim BT (2010) Candidate urinary biomarker discovery in ureteropelvic junction obstruction: a proteomic approach. J Urol 184:709–714
Michos O (2009). Kidney development: from ureteric bud formation to branching morphogenesis. Curr Opin Genet Dev 19:484–490
Osathanondh V, Potter EL (1963) Development of Human Kidney as Shown by Microdissection. III. Formation and Interrelationship of Collecting Tubules and Nephrons. Archives Pathol 76:290–302
Ravine D, Gibson RN, Walker RG, Sheffield LJ, Kincaid-Smith P, Danks DM (1994) Evaluation of ultrasonographic diagnostic criteria for autosomal dominant polycystic kidney disease 1. Lancet 343:824–827
Saxen L (1987) Organogenesis of the kidney. Cambridge University Press, Cambridge
Wen JG, Ringgaard S, Jorgensen TM, Stodkilde-Jorgensen H, Djurhuus JC, Frokiaer J (2002) Long-term effects of partial unilateral ureteral obstruction on renal hemodynamics and morphology in newborn rats: a magnetic resonance imaging study. Urol Res 30:205–212
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Wang, Z., Li, M. (2015). Evolution of the Urinary Proteome During Human Renal Development and Maturation. In: Gao, Y. (eds) Urine Proteomics in Kidney Disease Biomarker Discovery. Advances in Experimental Medicine and Biology, vol 845. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9523-4_10
Download citation
DOI: https://doi.org/10.1007/978-94-017-9523-4_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9522-7
Online ISBN: 978-94-017-9523-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)