Abstract
Purpose
The purpose of the study was to assess the differences in the concentration and function of urinary proteins between patients with cystine stones (CYS) and healthy controls (HC). We postulated that CYS and HC groups would demonstrate different proteomic profiles.
Methods
A pilot study was performed comparing urinary proteomes of 10 patients with CYS and 10 age- and gender-matched HC, using liquid chromatography-mass spectrometry. Proteins which met the selection criteria (i) ≥ 2 unique peptide identifications; (ii) ≥ twofold difference in protein abundance; and (iii) ≤ 0.05 p value for the Fisher’s Exact Test were analyzed using Gene Ontology classifications.
Results
Of the 2097 proteins identified by proteomic analysis, 398 proteins were significantly different between CYS and HC. Of those, 191 were involved in transport processes and 61 in inflammatory responses. The majority were vesicle-mediated transport proteins (78.5%), and 1/3 of them were down-regulated; of those, 12 proteins were involved in endosomal transport (including 6 charged multivesicular body proteins (CHMP) and 3 vacuolar sorting-associated proteins) and 9 in transmembrane transport. Myosin-2 and two actin-related proteins were significantly up-regulated in the vesicle-mediated transport group.
Conclusion
We provide proteomic evidence of impaired endocytosis, dysregulation of actin and myosin cytoskeleton, and inflammation in CYS. Endosomal transport proteins were down-regulated mainly through defective CHMP. These findings may contribute to further understanding of the pathogenesis of CYS, potentially affecting its management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cystinuria is a rare genetic disease that is characterized by impaired transport of cystine and dibasic amino acids in the proximal renal tubule [1]. About half of all cases are transmitted in an autosomal recessive fashion, caused by mutations in the SLC3A1 gene. This gene encodes rBAT, one of the two protein components of the neutral and basic amino acid transporter [2]. The other half are autosomal dominant with incomplete penetrance, caused by mutations in SLC7A9 gene, which encodes the light component, the catalytic protein, of the transporter, b0,+ AT.
Patients with cystinuria often present with kidney stones owing to the low solubility of cystine in urine. Their likelihood of forming stones is about 50% [3]. Cystine stones are large and bilateral in the majority of cases. Even though cystine stones represent only 1–2% of all cases with stones, the morbidity is high due to increased recurrence rate, need for multiple surgeries and the risk of developing chronic kidney disease [4]. Renal function in patients with cystine kidney stones is reduced compared to those with non-cystine stones [5], but the cause of this decline is not entirely clear.
The gene mutations identified in cystinuria cannot fully explain kidney stone activity. It has been shown that not all patients with two mutated SLC3A1 genes will form a stone. Additionally, patients with identical SLC3A1 or SLC7A9 genotypes may have onset of stone disease at different ages and with different severity. Therefore, other unidentified factors seem to contribute to kidney stone formation. These factors may include endogenous proteins that could serve as promoters or inhibitors of cystine precipitation, aggregation or epithelial adherence. Proteomics allows simultaneous examination of the patterns of multiple urinary proteins and has become one of the most promising tools in nephrology and urology [6, 7]. Using a proteomic approach, we aimed to assess the differences in the concentration and function of urinary proteins between cystinuria and renal stones (CYS) and healthy controls (HC). We postulated that CYS and HC groups would demonstrate different proteomic profiles. We also presumed that CYS patients would have a significant number of proteins involved in abnormal cellular processes including stress, inflammation and immune response, as well as others yet to be identified.
Methods
We compared urinary proteomes of 10 patients with CYS and 10 age- and gender-matched HC, using liquid chromatography-mass spectrometry (LC–MS/MS).
Patient selection
We prospectively enrolled 10 consecutive patients with CYS (35.4 ± 11.2 years, 5 males and 5 females) who presented for routine follow-up visit at New York University Langone Medical Center 9 patients), and at Children’s Hospital of Michigan (1 patient). All were established patients with cystinuria who had a history of nephrolithiasis. The diagnosis of cystinuria was made when a stone composed of cystine was obtained, and confirmed when urine cystine levels were found to be higher than 75 mg in a 24-h urine collection analyzed by Litholink Corporation (LabCorp, Chicago, IL).
Random mid-stream fresh urine samples were collected from all participants. At the time of urine collection, patients were asymptomatic (i.e., were not experiencing episodes of urinary tract obstruction and/or urinary tract infections), and were not taking antibiotics. The patients were taking citrate (9/10), tiopronin (5/10), or d-penicillamine (2/10).
HC group consisted of 10 age- and gender-matched volunteer individuals. Random mid-stream fresh urine samples were collected after a detailed history and physical examination was performed. The HC participants denied history of kidney stones, had normal urinalysis by dipstick and were not taking any medications. The urine was obtained for routine urinalysis for clinical care, and would have then been discarded; it was sent to the proteomics laboratory without any personal health identification information. Under these circumstances, analysis of the urine is not considered human subjects research.
Sample collection and preparation
Random mid-stream fresh urine samples were obtained in sterile cups, prepared within 3 h of collection (centrifuged at 2500 rpm for 15 min) at both institutions and stored as recommended by standardized protocols (developed by the Human Urine and Kidney Proteome Project, HUKPP, and the European Urine and Kidney Proteomics, EuroKUP Initiatives) until use [8]. Following preparation at NYU, de-identified urine specimens and data were sent to our institution. Aliquots were stored at − 80 °C until all urine specimens were received and processed for proteomic analysis. Each aliquot was used just once. Proteins in each sample were concentrated in a Centricon-type filter. Albumin and IgG were removed by anti-HAS/IgG resin (Sartorius). Protein concentration in each sample was measured by the BCA protein assay (Pierce).
Protein digestion and tandem mass tag (TMT) labeling
For HC and CYS groups, 10 µg of depleted urine protein from 10 individual samples were pooled together for 100 µg total, and then dried. Samples were resuspended in 10 µl of 1% SDS, and denatured for 5 min at 95 °C. Samples were reduced with 2 mM DTT in 50 mM TEAB for 30 min at 65 °C, followed by alkylation with 6 mM iodoacetamide for 30 min at RT. Proteins were digested with 2 µg trypsin overnight at 37 °C. Each sample was uniquely labeled using an isobaric TMT tag (Pierce, 1.2 mg) in 50% acetonitrile for 1 h at RT, followed by quenching with 0.5% hydroxylamine. Samples were purified over detergent-removal columns (Pierce) and dried.
2D LC–MS/MS
Peptides were resuspended in 5% acetonitrile/0.1% formic acid/0.005% trifluoroacetic acid and desalted/separated over a 5-µm reverse phase PLRP-S column (Agilent) using mobile phases A (2% acetonitrile) and B (98% acetonitrile), with both buffers adjusted to pH 10.0 using ammonium hydroxide. Thirty first dimension fractions were collected using a TriVersa NanoMate (Advion) system paired to a LTQ-XL mass spectrometer (Thermo). Second dimension peptide separation was performed using reverse phase chromatography (Acclaim PepMap RSLC column, Thermo) under acidic conditions (0.1% formic acid) with an EASY nLC-1000 uHPLC system (Thermo). Peptides were separated over a 90-min gradient and analyzed with an OrbiTrap Fusion mass spectrometer (Thermo). MS1 scans were performed in profile mode in the orbitrap at 120,000 resolution. Data-dependent MS2 acquisitions were triggered on the top 10 most abundant ions (charge states 2–7) and fragmented using CID at 35% collision energy in the linear ion trap. Dynamic exclusion was turned on (40 s). TMT tag quantitative was obtained in MS3 using Synchronous Precursor Selection. For this scan, the top 10 fragment ions were fragmented using HCD at 65% collision energy in the orbitrap at a resolution of 60,000 and m/z range of 100–500.
Protein identification and quantitation
LC–MS/MS data were analyzed using Proteome Discoverer (Thermo, version 1.4). Peptide identifications were scored using Sequest HT against a reviewed human protein database (Uniprot; downloaded on 2018-04-06; 20,260 entries) and simultaneously against a matched decoy database to determine the false discovery rate (FDR). Searches included up to 2 missed tryptic cleavages and 10 PPM/0.6 Da mass tolerances for parent and fragment ions, respectively. TMT label on peptide N-termini and internal lysine residues, and iodoacetamide derivative of cysteine were specified as fixed modifications. Oxidation of methionine was specified as a variable modification. Peptides were considered a positive identification if they achieved a ≤ 0.1 FDR using the Percolator algorithm. Ratios of reporter intensities were calculated as CYS/HC, and p values were calculated using a modified Fisher’s exact test. Proteins were considered significant if they displayed a ≥ twofold increase or decrease in abundance compared with controls, contained at least 2 unique peptide identifications, and had a p value of ≤ 0.05.
Pathway analysis
Proteins which met these criteria were analyzed for inclusion into Gene Ontology (GO) classifications. GO is a bioinformatics platform which provides a statistical likelihood that a group of proteins are enriched within a specific biological classification. Protein interaction mapping was used to create a network that indicates the relationship between proteins with increased or decreased abundance using the Reactome FI plugin for Cytoscape (version 3.5).
Results
Patient’s characteristics
Serum creatinine values were available in 6 out of the 10 CYS patients, with a mean of 1.09 ± 0.31 mg/dl (range 0.71–1.59 mg/dl). Mean eGFR for these patients was 92 ± 38.1 ml/min/1.73 m2 (range 46–146 ml/min/1.73 m2). The data regarding comorbidities were available only in 1 patient and included obesity. However, CYS was represented by a relatively young group of people who, besides having cystinuria, are relatively healthy. None of the HC had any comorbidities and their serum creatinine concentrations were not checked.
Proteomic analysis
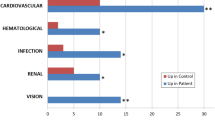
A total of 2097 proteins were identified by proteomic analysis. Of those, 398 proteins (216 up-regulated and 182 down-regulated) were found to be significantly different between CYS and HC. These significantly and differentially abundant proteins were used to analyze the group differences for biological processes. The top 7 most relevant biological processes over-represented in CYS patients compared to controls by GO analysis are shown in Fig. 1. Nearly half of proteins identified in CYS group (191 out of 398) were found to participate in transport processes. The majority of transport proteins were involved in vesicle-mediated transport (150 out of 191) (Fig. 2), and 1/3 of them were down-regulated (49/150) (Table 1, Supplementary Material). Of those 49, 12 proteins were involved in endosomal transport (Table 1), 9 in transmembrane transport (Table 2), and two were Ras-related proteins, RALA and Rab 5C. Among endosomal transport-related proteins, there were 6 charged multivesicular body protein (CHMP 1A, 1B, 2A, 2B, 4B, and 12A) and 3 vacuolar sorting-associated proteins (4B, 37D, VTA). Myosin-2 (ratio 12.86) and two actin-related proteins (subunit 2, ratio 7.62 and subunit 1B, ratio 4.9) were highly represented among up-regulated proteins in the vesicle-mediated transport group.
Gene ontology (GO) analysis of urinary proteins with relative increased (green) or decreased (red) abundance in adults with cystinuria (Cys) and kidney stones compared to healthy adults. Proteins which had a twofold difference in abundance, at least 2 unique spectral identifications and p ≤ 0.05 were mapped against GO term lists to determine which biological processes were enriched. *p value ≤ 0.05
A large number of proteins were found to be involved in inflammation (56 up-regulated and 5 down-regulated proteins) and immune response (98 up-regulated and 41 down-regulated) (Table 2, Supplementary Material), and 52 of those were related to complement activation. We identified 10 complement proteins (C1-C9 and complement factor I). Of those, C1 was the most abundant with all 3 subunits C1Q, R, and S being over-represented in CYS group.
Protein–protein interaction network modeling of proteins with overall increased and decreased abundance in CYS patients compared to HC is shown in Figure of supplementary material. In this representation, proteins are shown as key nodes or clusters, and known interactions are shown as edges. Consensus GO term for each cluster revealed six biological processes that play a major role in CYS patients: (1) complement activation, (2) regulation of complement activation, (3) endosomal transport, (4) receptor-mediated endocytosis, (5) extracellular matrix organization, (6) G-protein coupled receptor signaling. Of those, there were two major down-regulated processes, endosomal transport and G protein coupled receptor signaling pathways. Complement activation and receptor mediated endocytosis were the major up-regulated processes, while extracellular matrix organization included both up- and down-regulated proteins.
Discussion
In this study, we demonstrate that patients with CYS have a different urine proteomic profile than HC, as hypothesized. Our finding that the transport of proteins is one of the most significant biological processes affected in patients with cystinuria is not surprising, since cystinuria is a disease caused by a defective transport protein. Among various forms of transport, we detected a significant number of urinary proteins involved in vesicle-mediated transport. As part of these transport processes, almost all proteins that participate in endosomal transport were down-regulated. Among those, there were six down-regulated multivesicular bodies proteins (CHMP), and three vacuolar proteins. All these proteins were part of the endosomal transport cluster in the protein mapping, suggesting their interaction. Rab-related protein, another protein involved in vesicle-mediated transport [9], was also found to be down-regulated. All these proteins are part of the endocytic pathway and mediate autophagy [9]. CHMP play an essential role in destroying damaged proteins and other particles by incorporating them into intraluminal vesicles and by fusing with lysosomes [9]. CHMP contain endocytic markers such as Rabs, small GTP-binding proteins that participate in the proximal tubule apical endocytic cascade. Additionally, endocytosis is dependent on the integrity of the actin and myosin cytoskeleton, both of which were up-regulated in our study. Myosin and actin play a role in receptor-mediated endocytosis in the proximal renal tubule [10].
The importance of CHMP dysfunction in cystinuric patients is unclear. However, it may indicate an impairment of uptake of cystine crystals into vesicles preventing their degradation in lysosomes and facilitating their accumulation in the tubular cell with subsequent transfer to the interstitial space where they may initiate plaque formation. Indeed, Randall plaque was initially described in patients with CaOx stones [11] and later also demonstrated in cystinuric stone patients [12]. In a similar fashion, CaOx and CaP crystals have been shown to dissolve within lysosomes following binding and internalization of cultured renal cells [13], but the involvement of CHMP was not described. Indeed, none of the endosomal proteins we found in CYS were part of the urinary proteomes of our patients with nephrolithiasis and hypercalciuria [14], nor were described by others in patients with hyperoxaluria [15], indicating their unique significance in CYS.
Within transmembrane transport processes, we found sodium-amino acid and ammonium transporters to be down-regulated, while chloride channels were upregulated. We know that rBAT is a high-affinity protein that, as a component of the transport process at the apical membrane, facilitates sodium-independent uptake of cystine and dibasic amino acids [16]. Our study indicates that other sodium-dependent amino acid transporters may have an important role in cystinuria. The significance of chloride channel up-regulation is unclear but is likely related to defective endocytosis, since the endosomal chloride concentration regulates endocytic efficiency in proximal tubular cells.
We found that a significant number of urinary proteins are involved in inflammatory responses, complement activation and leukocyte mediated immunity in our cystinuric patients. These proteins were part of two clusters on the protein mapping and showed interaction with myosin-2, indicating dysregulation of the cell’s cytoskeleton. An increased number of up-regulated inflammatory proteins were also reported in a proteomic analysis of urinary exosomes obtained from 8 cystinuria patients compared to 10 controls [17]. Of those, 18/38 were circulating inflammatory proteins (e.g., complement C3 and C8; fibrinogen alpha, beta, and gamma chain; and serum paraoxonase 1), and 14/38 were derived from neutrophils. Additionally, significant inflammation was found in the urine proteome of patients with nephrolithiasis and hypercalciuria [14], and type 1 primary hyperoxaluria [15]. Altogether, these findings indicate that inflammation plays an important role in the pathogenesis of kidney stone disease irrespective of its metabolic cause.
The major limitation of this study is the small number of samples that were analyzed. This is mainly due to the low incidence of cystinuria in the general population. However, this was intended as a pilot study to generate hypotheses and identify novel biomarkers and intracellular pathways that will require further investigation. Validation using highly specific methods such as ELISA and Western blotting is needed. Another study limitation is the lack of serum creatinine results in about 1/3 of the CYS patients and all HC. However, this should have not affected the results because our study was designed to include pooled urine samples and not individual ones, and to involve healthy individuals as controls. Lastly, most of the CYS patients were on medication that potentially could have affected the urine proteome profile. However, based on their mechanisms of action, it is unlikely that these drugs could change the protein composition of the urine. Thiol drugs bind only to cystine disulfide bridges to form more soluble cysteine-drug complexes without affecting other proteins in the urine. Moreover, there is no reason that citrate, which would change the urine pH in a relatively narrow range, would have affected the proteomic results.
In conclusion, we provide proteomic evidence of impaired endocytosis, dysregulation of actin and myosin cytoskeleton, and inflammation in patients with cystinuria and kidney stone. Endosomal transport proteins were down-regulated mainly through defective CHMP. We can speculate that dysfunction of actin and myosin cytoskeleton facilitates crystal adhesion and engulfment/endocytosis into renal tubular cell, while defective CHMP prevents crystal degradation in lysosomes. It is unclear how the impaired endocytic pathway and CHMP involvement contributes to the development of cystinuria stone disease. Answering these questions may facilitate further understanding of the pathogenesis of the disease which may affect its management.
References
Chillarón J, Font-Llitjós M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacín M (2010) Pathophysiology and treatment of cystinuria. Nat Rev Nephrol 6(7):424–424
Pras E, Arber N, Aksentijevich I, Katz G, Schapiro JM, Prosen L, Gruberg L, Harel D, Liberman U, Weissenbach J (1994) Localization of a gene causing cystinuria to chromosome 2p. Nat Genet 6(4):415–419
Knoll T, Zollner A, Wendt-Nordahl G, Michel MS, Alken P (2005) Cystinuria in childhood and adolescence: recommendations for diagnosis, treatment, and follow-up. Pediatr Nephrol 20:19–24
Dahlberg PJ, Van den Berg CJ, Kurtz SB, Wilson DM, Smith LH (1977) Clinical features and management of cystinuria. Mayo Clin Proc 52:533–542
Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ (2002) The impact of cystinuria on renal function. J Urol 168:27–20
Thongboonkerd V (2008) Proteomics and kidney stone disease. Contrib Nephrol 160:142–148
Cadieux PA, Beiko DT, Watterson JD, Burton JP, Howard JC, Knudsen BE, Gan BS, McCormick JK, Chambers AF, Denstedt JD, Reid G (2004) Surface- enhanced laser desorption/ionization-time of flight-mass spectrometry (SELDI-TOF-MS): a new proteomic urinary test for patients with urolithiasis. J Clin Lab Anal 18(3):170–175
Thongboonkerd V (2007) Practical points in urinary proteomics. J Proteome Res 6(10):3881–3880
Piper RC, Katzmann DJ (2007) Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol 23:519–547
Eshbach ML, Weisz OA (2017) Receptor-mediated endocytosis in the proximal tubule. Annu Rev Physiol 79:425–448
Bagga HS, Chi T, Miller J, Stoller ML (2013) New insights into the pathogenesis of renal calculi. Urol Clin N Am 40(1):1–12
Evan AP, Coe FL, Lingeman JE, Shao Y, Matlaga BR, Kim SC, Bledsoe SB, Sommer AJ, Grynpas M, Phillips CL, Worcester EM (2006) Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int 69(12):2227–2235
Sabharanjak S, Sharma P, Parton RG (2002) GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independeent pinocytic pathway. Dev Cell 2:411–423
Kovacevic L, Lu H, Caruso JA, Lakshmanan Y (2016) Renal tubular dysfunction in pediatric urolithiasis: proteomic evidence. Urology 92:100–105
Brooks ER, Hoppe B, Milliner DS, Salido E, Rim J, Krevitt LM, Olson JB, Price HE, Vural G, Langman CB (2016) Assessment of urine proteomics in type 1 primary hyperoxaluria. Am J Nephrol 43(4):293–303
Nagamori S, Wiriyasermkul P, Guarch ME, Okuyama H, Nakagomi S, Tadagaki K, Nishinaka Y, Bodoy S, Takafuji K, Okuda S, Kurokawa J, Ohgaki R, Nunes V, Palacín M, Kanai Y (2016) Novel cystine transporter in renal proximal tubule identified as a missing partner of cystinuria-related plasma membrane protein rBAT/SLC3A1. Proc Natl Acad Sci USA 113(3):775–780
Bourderioux M, Nguyen-Khoa T, Chhuon C, Jeanson L, Tondelier D, Walczak M, Ollero M, Bekri S, Knebelmann B, Escudier E, Escudier B, Edelman A, Guerrera IC (2014) A new workflow for proteomic analysis of urinary exosomes and assessment in cystinuria patients. J Proteome Res 14(1):567–777
Funding
This study was funded by the Rare Kidney Stone Consortium (5U54DK083908-05), a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), National Center for Advancing Translational Sciences’ (NCATS). This consortium is funded through a collaboration between NCATS, and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The Wayne State University Proteomics Core is supported through the NIH Center Grant P30 ES 020957, the NIH Cancer Center Support Grant P30 CA 022453 and the NIH Shared Instrumentation Grant S10 OD 010700. We are thankful to Frank Modersitzki for his help with urine specimen collection and processing and with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
D.S. Goldfarb is a consultant for Retrophin and is owner of Ravine Group. The rest of the authors have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
The urine was obtained for routine urinalysis for clinical care, and would have then been discarded; it was sent to the proteomics laboratory without any personal health identification information. Under these circumstances, analysis of the urine is not considered human subjects research. and therefore informed consent was not required from the participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kovacevic, L., Caruso, J.A., Lu, H. et al. Urine proteomic profiling in patients with nephrolithiasis and cystinuria. Int Urol Nephrol 51, 593–599 (2019). https://doi.org/10.1007/s11255-018-2044-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11255-018-2044-1