Abstract
The concept of tumor dormancy has been developed to explain a prolonged interval between primary therapy and the recurrence of metastatic disease. The process of dormancy may be driven or supported by several different mechanisms including angiogenesis and the tumor microenvironment, cell cycle arrest, immune surveillance, and autophagy. One xenograft model for dormancy in ovarian cancer emphasizes the importance of an effective and stable tumor microvasculature. In another xenograft model of dormancy in ovarian cancer, autophagy appears to be important for sustaining dormant cancer cells under nutrient poor conditions. Autophagy plays an ambiguous role in cancer pathophysiology. As a cellular defense mechanism, loss of autophagy has been implicated in tumor progression in several models, but autophagy can also provide a survival mechanism for dormant cancer cells. ARHI (DIRAS3) is a maternally imprinted tumor suppressor gene that is downregulated in 60% of human ovarian cancers associated with decreased progression free survival. Re-expression of ARHI blocks proliferation, inhibits migration, prevents angiogenesis and induces autophagy and tumor dormancy. Treatment of mice bearing ARHI-induced dormant xenografts with chloroquine, a functional inhibitor of autophagy, delays the outgrowth of tumor transplants, consistent with a role for autophagy in sustaining nutrient deprived cancer cells. This mechanism may extend beyond ovarian cancer, as downregulation of ARHI has been found in breast, lung, prostate, pancreatic, and thyroid cancers. This review considers the role of multiple mechanisms for dormancy in epithelial ovarian cancers that may provide new targets for eliminating dormant cancer cells.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
The concept of tumor dormancy has been developed to explain the prolonged interval – sometimes many years – between primary therapy and the recurrence of metastatic disease. This phenomenon was noted as early as 1934 by Willis and has been observed in several different malignancies including carcinomas of the breast, ovary and kidney, as well as lymphomas and melanomas, where recurrence can be observed 5–20 years following initial removal of the primary tumor (Willis 1934). Possible mechanisms that could contribute to tumor dormancy include: (1) an exit from the cell cycle or cell cycle arrest; (2) a balance between cell proliferation and cell death; (3) a prolonged block in effective angiogenesis, (4) a requirement for additional mutations or epigenetic events to permit the progression of metastatic clones, and (5) immune suppression of cancer cell growth. Dormancy can occur following primary surgery or radiation therapy, but is now observed most frequently after systemic chemotherapy or hormonal therapy. By definition, dormant cancer cells have survived primary therapy and generally contain a subpopulation of drug resistant tumor initiating cells.
For epithelial cancers that arise from several different sites, attempts to eliminate dormant cancer cells with additional maintenance chemotherapy have not been effective. This is particularly true for ovarian cancer where maintenance therapy with paclitaxel (Markman et al. 2009) or bevacizumab (Perren et al. 2011) can prolong progression free survival, but not overall survival or the rate of cure.
Clinical Biology of Ovarian Cancer
Ovarian cancer is neither a common nor a rare disease. The lifetime risk for a woman in the United States is 1 in 70 compared to 1 in 8 for breast cancer. Among women, approximately 3% of cancers arise from the ovary and these malignancies account for 6% of cancer deaths (Jemal et al. 2002). Ovarian cancers have been thought to develop from epithelial cells that cover the ovary or that line cysts immediately below the ovarian surface. Recent studies suggest that a fraction of high grade serous “ovarian” cancers actually arise from the fimbriae of the fallopian tube (Crum et al. 2007). Like breast or lung cancer, ovarian cancer can metastasize through blood or lymph. Most frequently, however, ovarian cancer cells metastasize from the ovary or fallopian tube across the abdominal cavity, producing multiple tumor nodules on the parietal and visceral peritoneum. When ovarian cancers are detected prior to metastasis, more than 90% can be cured with currently available therapy. When disease has spread throughout the abdominal cavity or above the diaphragm, long term survival falls to less than 30%. Despite promising research, there is at present no proven strategy for early detection and less than a quarter are diagnosed while still limited to the ovaries (Stage I) (Lu et al. 2013; Menon et al. 2009).
Primary treatment of newly diagnosed ovarian cancer involves “cytoreductive surgery” followed by combination chemotherapy. Gynecologic surgeons with special training remove not only the ovaries, fallopian tubes, uterus and omentum, but as much metastatic cancer as possible, even when complete resection is not feasible. Small metastatic deposits less than 1 cm in diameter are often left after surgery and chemotherapy is required for their elimination. Ovarian cancer is chemo-responsive, but much less chemo-curable. Treatment with six cycles of carboplatin and paclitaxel will produce a response in 70% of patients, but less than 30% remain free from recurrent disease. In the majority, disease recurs within 1–3 years, but in some cases recurrence has been documented 5–10 years following primary therapy. Median survival now extends to 4–5 years with optimal and aggressive care, but the fraction of women cured remains less than 30% and has not changed over the last two decades.
Recurrence of metastatic disease occurs most often within the peritoneal cavity. In the past, “second look” operations have been performed after primary surgery and chemotherapy to detect residual disease. Ovarian cancer cells have been found in small, poorly vascularized fibrotic nodules on the peritoneal surface. In this setting, dormant ovarian cancer cells are likely to be hypoxic and nutrient deprived. Survival of dormant cancer cells in this setting is likely to depend upon multiple mechanisms, including autophagy. Compartmentalization and self-digestion of long-lived cellular proteins and organelles yield amino acids and fatty acids that can provide needed energy under nutrient poor conditions.
Models for Human Ovarian Cancer Dormancy
Studies with two human ovarian cancer xenograft models in immunosuppressed mice suggest that the induction or maintenance of dormancy relates to a block in the development of stable vasculature. Gilead and colleagues grew spheroids from human MLS ovarian cancer cells in culture. Ovarian cancer spheroids were injected subcutaneously into genetically immunosuppressed female CD-1 nu/nu mice. Tumor growth and angiogenesis were monitored sequentially with magnetic resonance imaging (MRI). Maturity of vessels was judged by their response to oxygen and carbon dioxide which depends upon the growth of pericytes and stromal cells around the endothelial cells that line nascent tumor vessels. In three of ten xenografts, tumors grew promptly and progressively. In seven of ten xenografts, small subcutaneous tumor nodules remained stable from 13 to 54 days before growing progressively, providing a model for tumor dormancy (Gilead et al. 2004; Gilead and Neeman 1999). Vessels appeared at the periphery of nodules within 2 days after injection of spheroids. Both mature and immature vessels formed, but underwent up to six cycles of growth and regression. Emergence from dormancy and progressive tumor growth correlated with penetration of new vessels and stroma into the cancer spheroids. These observations are compatible with a model where VEGF is produced by hypoxic ovarian cancer cells, attracting endothelial cells and pericytes. Development of tumor vessels provides sufficient oxygen to suppress VEGF expression. In the absence of tumor-derived VEGF, tumor vessels regress, deceasing oxygen delivery and inducing VEGF once again.
Our own group had developed the first inducible model for tumor dormancy in ovarian cancer. ARHI (DIRAS3) is a maternally imprinted tumor suppressor gene that encodes a 26 kD GTPase with 50–60% homology to Ras and RAP. ARHI is downregulated in ovarian cancers as well as many other tumor types including breast, lung, prostate, pancreatic and thyroid carcinomas (Dalai et al. 2007; Huang et al. 2010; Lin et al. 2011; Lu et al. 2001; Wang et al. 2003; Wu et al. 2013; Yu et al. 1999). ARHI expression was down-regulated in 63% of 407 invasive cancers and could not be detected in 47%, associated with decreased progression free survival but not overall survival (Rosen et al. 2004).
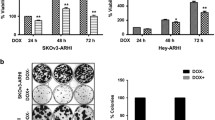
Sublines of SKOv3 and HEY ovarian cancer cells – designated SKOv3-ARHI and HEY-ARHI – have been developed that re-express ARHI at physiologic levels in the presence of doxycycline. Re-expression of ARHI blocks proliferation, upregulates p21, arrests the cell cycle in G1, and inhibits motility (Badgwell et al. 2012). Doxycycline-driven, re-expression of ARHI at physiologic levels eliminates clonogenic growth of SKOv3-ARHI through autophagic type II cell death (Lu et al. 2008). Subcutaneous xenografts of the SKOv3-ARHI ovarian cancer cell line were established in Balb/c nu/nu mice. Oral administration of doxycycline in drinking water to tumor bearing mice increased ARHI in xenografts, arrested tumor growth, blocked angiogenesis and induced autophagy, but did not kill ovarian cancer cells. When doxycycline was withdrawn after 6–8 weeks, ARHI levels declined, tumor vasculature was established and SKOv3-ARHI xenografts grew rapidly and progressively (Fig. 8.1). Once angiogenesis occurred, re-institution of doxycycline treatment failed to inhibit xenograft growth (Lu et al. 2008; In preparation: Established angiogenesis will inhibit ARHI (DIRAS3) induced tumor dormancy), consistent with the importance of a block in angiogenesis to maintain tumor dormancy.
ARHI-induced autophagy results in tumor dormancy in xenografts. (a–d) BalB/c nu/nu mice were injected with SKOv3 (a), SKOv3-ARHI (b) or SKOv3-NTD (c) cells and provided with or without DOX in their drinking water. (d) DOX withdrawn after 32 (green triangles) or 42 (blue diamonds) days of treatment shows re-growth of tumor. (e–i) TEM Images of tumor xenografts indicating autophagosomes. (j) Inhibition of autophagy blocks tumor dormancy (Lu et al. 2008)
Angiogenesis and the Tumor Microenvironment in Ovarian Cancer Dormancy
The process of angiogenesis has been well studied and has been found to be a vital component of tumor progression. Without the recruitment and formation of functional blood vessels to support tumor growth by providing oxygen and nutrients, tumors remain in a dormant state. If successful angiogenesis is inhibited, the tumors remain avascular and microscopic in size (Gimbrone et al. 1972; Hart 1999; Holmgren et al. 1995; O’Reilly et al. 1996). Small tumor size is not, however, necessarily due to lack of proliferation. Dormant tumor cells typically exhibit increased apoptosis to counteract the elevated proliferation, producing angiogenic dormancy (Holmgren et al. 1995; Naumov et al. 2006). Thus, the tumor microenvironment is a key component in maintaining this dormant state. The balance between proangiogenic factors (e.g., PDGF, VEGF and FGF) and antiangiogenic factors (e.g., angiostatin, endostatin, and vasculostatin) regulate the switch which controls angiogenic dormancy. Re-expression of ARHI downregulates, but does not eliminate VEGF expression.
The tumor microenvironment can also contribute to regulating autophagic cell death. In the SKOv3-ARHI dormancy model, VEGF, IL-8 and IGF have all been detected in tumor xenografts. VEGF and IL-8 are expressed by the human ovarian cancer cells and released into the peritumoral space, whereas IGF is produced by the murine stroma. Addition of these peptide growth factors and cytokines to SKOv3-ARHI cells cultured in the presence of doxycycline, recues ARHI-induced ovarian cancer cells from autophagic death (Lu et al. 2008). Thus, VEGF, IL-8 and IGF may act as survival factors, regulating and limiting autophagic damage to cancer cells. Survival of dormant cells may require a balance where sufficient autophagy is maintained to meet minimal energy requirements, but where autophagy stops short of inducing cell death (Fig. 8.2).
Cell Cycle Arrest in Ovarian Cancer Dormancy
Cancer dormancy has been attributed to a balance between cell proliferation and cell death that limits expansion of the tumor cell population. Alternatively, dormancy could result from growth arrest where cancer cells enter and remain in G0-G1 and do not proliferate (Aguirre-Ghiso 2007). Non-proliferating cancer cells can exhibit a low metabolic rate. Computer simulations have been used to explore whether balanced proliferation or growth arrest was more likely to underlie dormancy (Wells et al. 2013). Growth arrest and quiescence were found to be more likely to occur in dormant cancer cells. If growth arrest occurs more frequently, cytotoxic chemotherapy that targets cycling cells is unlikely to eliminate dormant cancer cells and different strategies will be required to achieve cure. In the two ovarian cancer xenograft models for tumor dormancy, cell cycle arrest is likely to play a role. Although cell cycle events were not studied after injection of MEL spheroids into nu/nu mice (Gilead et al. 2004), other reports with large pancreatic cancer cell spheroids (500–600 uM) indicate that cells proliferate only in outer cell layers and not at the center of spheroids (Laurent et al. 2013). Induction of ARHI in the SKOv3-ARHI model produced growth arrest in G1 (Lu et al. AACR 2013 Abstract #1680, ARHI induces autophagy and enhances chemosensitivity to cisplatin in ovarian cancer cell lines and xenografts).
Immunity in Ovarian Cancer Dormancy
Nu/nu mice are deficient in T and B cell function. Consequently, studies in xenograft models cannot assess the influence of antigen specific immunity on dormancy. Primary autochthonous ovarian cancers have been induced in immuno-competent genetically engineered mice (Mullany and Richards 2012) and these models may permit evaluation of the immune response in regulating dormancy. Although studies of ARHI as a modulator of immune surveillance have not yet been performed, recent reports suggest that autophagy can affect both innate and adaptive immunity, as well as, inflammation though several different mechanisms. Autophagy has been shown to increase resistance to CD8+ T lymphocyte-mediated lysis of human breast cancer cells (Akalay et al. 2013). Autophagy is important for cell-autonomous elimination of intracellular microbes by modulating activation of inflammatory cytokines, Toll-like and Nod-like receptors, antigen presentation, and mature T cell development and homeostasis (Deretic 2012). When tumor bearing mice were treated with chemotherapy, autophagy-competent, but not autophagy-deficient, cancers attracted dendritic cells and T lymphocytes into the tumor bed to establish tumor specific immunity. Autophagy was found to be essential for the release of ATP from dying cancer cells to attract dendritic cells and T lymphocytes and to establish immunity that could enhance the effects of chemotherapy (Michaud et al. 2011).
Autophagy in Ovarian Cancer Dormancy
Autophagy is a physiological process that digests warn organelles and long lived proteins, releasing amino acids and fatty acids which are catabolized yielding ATP. During nutrient deprivation, autophagy is induced, providing energy and promoting cell survival. Excessive autophagy can, however, result in cell death. The process of autophagy has been conserved in eukaryotes from yeast to mammals (Fig. 8.3) (Reggiori and Klionsky 2002). Autophagy is induced by downregulation of mammalian target of rapamycin (mTOR) which results in decreased phosphorylation of ATG13. Inhibition of mTOR is regulated in mammalian cells by the tuberous sclerosis complex (TSC1/2) which is upregulated by inhibition of Class I PI3K or by activation of AMP kinase. Once autophagy is induced, an autophagy inhibition complex (AIC) is assembled to nucleate autophagic vesicles. The AIC contains Beclin1, UVRAG and Vps34 Class III PI3K. Prior to induction, Beclin1 exists as a homo-dimer bound to Bcl2. An important step in regulating autophagy is the dissociation of Beclin1 from Bcl2 and the monomerization of Beclin1. Prompted by the AIC, double membrane structures elongate, enclosing warn mitochondria, endoplasmic reticulum and long-lived proteins. Autophagic vesicles are decorated with Atg5 and Atg12. Atg4, a cysteine protease, is activated and upregulated, cleaving microtubule associated protein (MAP) LC3-1. Cleaved LC3-1 is then lipidated, forming LC3-2 (LC3-II) that attaches to the developing autophagosome, displacing Atg5. Mature autophagosomes then dock and fuse with lysosomes forming autophagolysosomes, a step requiring lamp 1, lamp 2 and Rab7. Autophagolysosomes are acidified, activating proteases and lipases which digest proteins and lipids. Chloroquine passes readily into these structures and raises the pH, functionally inhibiting autophagy.
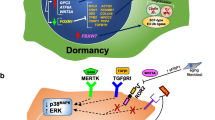
ARHI (DIRAS3) enhances autophagy at several critical steps. Through inhibition of the PI3K/Akt signaling pathway, ARHI upregulates TSC1/2 and inhibits mTOR inducing autophagy. ARHI acts on several additional targets in the autophagic process, as knockdown of ARHI blocks mTOR induced autophagy. Recently, our laboratory has found that ARHI acts as a critical switch in the displacement of Bcl-2 from Beclin1 and in dissociating the Beclin1 homodimer (Lu et al. 2014b). ARHI further contributes to forming the AIC by increasing the association of Vps34 and ATG14 with Beclin1. Moreover, ARHI induces the Atg4 cysteine protease that cleaves LC3-1 (LC3-I), while co-localizing directly with LC3-II in the membrane of the developing autophagosome during elongation. ARHI-mediated downregulation of PI3K/Akt and Ras/ERK signaling decreases phosphorylation of FOXO3a and sequesters the transcription factor in the nucleus, upregulating Atg4 and MAP-LC3-I required for autophagosome maturation and upregulating Rab7 required for fusion of the autophagosome with the lysosome (Lu et al. 2014a). Thus, re-expression of ARHI in ovarian cancer can contribute to the induction of autophagy though several different mechanisms and clinically is expressed in small deposits of ovarian cancer that persist following primary surgery and chemotherapy, associated with punctate LC3. This phenotype is consistent with autophagy in resistant, dormant ovarian cancer cells and is the main mechanism by which ARHI induces tumor dormancy (Figs. 8.4 and 8.5).
ARHI (DIRAS3) plays a critical role in many steps of autophagosome formation. ARHI inhibits mTOR through suppression of the PI3K pathway initiating the induction of autophagosome formation. ARHI affects the autophagosome initiation complex (AIC) by disrupting the Beclin-1 homodimer and displacing Bcl-2. ARHI directly affects the elongation stage of autophagosome formation by up-regulating ATG4
The clinical relevance of these observations with cell lines and xenografts is supported by observations with primary and “second look” surgical specimens (Lu et al. 2014b). All patients were in a complete clinical remission at the completion of adjuvant chemotherapy defined by normal CA-125 level, normal CT scans of the abdomen and pelvis, and a normal physical exam. When immunohistochemical analysis was performed, ARHI staining (>2–3) was observed in 41% of primary cancers and 97% of second-look cases. LC3 staining (>2–3) was observed in 35% of primary cancers and 85% of second-look cases. Punctate ARHI staining was observed in 23% of primary cancers and 84% of second-look cases, whereas punctate LC3 staining was observed in 21% of primary cancers and 81% of second-look cases. Positive staining of both total and punctate ARHI and LC3 was significantly higher in second-look than in primary cases (P < 0.0001).
Despite substantial homology to Ras, ARHI inhibits Ras transformation (Sutton et al. In preparation; The role of DIRAS family tumor suppressors in Ras transformation), blocks growth and motility of transformed cells while inducing autophagy and tumor dormancy. At a structural level, ARHI is distinguished from Ras by a 34 amino acid N-terminal extension that is required for most functions, including autophagy (Lu et al. 2008; Luo et al. 2003). Re-expression of ARHI at physiologic levels leads to programmed cell death of ovarian cancer cells in culture with, at most, low levels of apoptosis. This cell death was preceded by the development of autophagy as judged by increased catabolism of long-lived vesicles that are decorated with LC3-II, and visualization of autophagosome formation by electron microscopy. Re-expression of an N-terminal deleted construct with a Dox-inducible promoter did not induce autophagy or suppress tumor growth. This structure-function correlation also observed in vivo. Induction of the full length ARHI at physiological levels in ovarian cancer xenografts suppressed remained the growth of dormant ovarian cancer cells for weeks and xenografts grew promptly when ARHI was downreguated. R-expression of the N-terminal deleted ARHI mutant protein had no effect on xenograft growth compared when compared to controls that were not fed doxycycline (Lu et al. 2008).
Autophagy plays an ambiguous role in cancer pathophysiology. As a cellular defense mechanism, dysregulated autophagy has been implicated in tumor progression for many disease sites. Enhanced development of breast cancers occur in genetically engineered hemizygous Beclin +/− mice with impaired autophagy, suggesting that the process of autophagy can suppress carcinogenesis (Qu et al. 2003; Yue et al. 2003). In pancreatic cancer, unresolved inflammation and disrupted regulation of autophagy are common features in the pathogenesis of pancreatitis and subsequently pancreatic cancer (Gukovsky et al. 2013). Altered expression of other autophagy proteins have been observed in human cancer specimens. In prostate carcinomas, Atg5 expression was markedly up-regulated in prostate intraepithelial neoplasms and prostate cancer cells determined by immunohistochemical analysis, whereas, but no somatic mutations of ATG5 were found (Kim et al. 2011). Cytoplasmic LC3 punctate staining has been observed in a variety of human cancers, including lung adenocarcinomas, breast adenocarcinomas, hepatocellular carcinoma, testicular seminoma, and melanoma. These authors observed increased LC3 punctate staining in the majority of human cancers compared with normal tissues from corresponding organs. Cancers of the same histological type showed substantial heterogeneity in the number and intensity of LC3 puncta per cell (Ladoire et al. 2012).
Conclusion
Thus, autophagy can suppress the induction of cancer and can cause death of transformed cells, but can also serve as a survival mechanism contributing to the survival of dormant cancer cells in nutrient poor conditions. Context matters. Manipulation of autophagy in either direction, positive or negatively, could plausibly be utilized as a therapeutic approach to eradicate cancer (Ryter and Choi 2010). Clinically, these strategies have been employed by using chloroquine and its derivative, hydroxychloroquine, (inhibitors of lysosomal acidification) to enhance chemotherapeutic efficacy in many clinical trials across several disease sites including prostate, pancreatic, breast and non-small-cell lung cancers (Choi et al. 2013). For ovarian cancer, the modulation of autophagy for a therapeutic effect has recently been observed where VEGF therapies have been effective in delaying tumor re-growth, possibly by affecting dormant cells. The role of ARHI (DIRAS3) in promoting autophagy and tumor dormancy may very well extend beyond ovarian cancer as downregulation of this imprinted tumor suppressor gene has been found in breast, lung, prostate, pancreatic and thyroid cancers (Huang et al. 2010; Lin et al. 2011; Lu et al. 2001; Wang et al. 2003; Yu et al. 1999). Overall, a greater understanding of the mechanisms surrounding autophagy and tumor dormancy as related to cancer pathogenesis will provide insight to new targets for therapeutic and diagnostic approaches.
References
Aguirre-Ghiso JA (2007) Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7(11):834–846. doi:10.1038/nrc2256
Akalay I, Janji B, Hasmim M et al (2013) Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res 73(8):2418–2427. doi:10.1158/0008-5472.CAN-12-2432
Badgwell DB, Lu Z, Le K et al (2012) The tumor-suppressor gene ARHI (DIRAS3) suppresses ovarian cancer cell migration through inhibition of the Stat3 and FAK/Rho signaling pathways. Oncogene 31(1):68–79. doi:10.1038/onc.2011.213
Choi AM, Ryter SW, Levine B (2013) Autophagy in human health and disease. N Engl J Med 368(19): 1845–1846. doi:10.1056/NEJMc1303158
Crum CP, Drapkin R, Kindelberger D et al (2007) Lessons from BRCA: the tubal fimbria emerges as an origin for pelvic serous cancer. Clin Med Res 5(1):35–44. doi:10.3121/cmr.2007.702
Dalai I, Missiaglia E, Barbi S et al (2007) Low expression of ARHI is associated with shorter progression-free survival in pancreatic endocrine tumors. Neoplasia 9(3):181–183
Deretic V (2012) Autophagy: an emerging immunological paradigm. J Immunol 189(1):15–20. doi:10.4049/jimmunol.1102108
Gilead A, Neeman M (1999) Dynamic remodeling of the vascular bed precedes tumor growth: MLS ovarian carcinoma spheroids implanted in nude mice. Neoplasia 1(3):226–230
Gilead A, Meir G, Neeman M (2004) The role of angiogenesis, vascular maturation, regression and stroma infiltration in dormancy and growth of implanted MLS ovarian carcinoma spheroids. Int J Cancer 108(4):524–531. doi:10.1002/ijc.11583
Gimbrone MA Jr, Leapman SB, Cotran RS et al (1972) Tumor dormancy in vivo by prevention of neovascularization. J Exp Med 136(2):261–276
Gukovsky I, Li N, Todoric J et al (2013) Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 144(6):1199–1209. doi:10.1053/j.gastro.2013.02.007, e1194
Hart IR (1999) Perspective: tumour spread – the problems of latency. J Pathol 187(1):91–94. doi:10.1002/(SICI)1096-9896(199901)187:1<91::AID-PATH234>3.0.CO;2-J
Holmgren L, O’Reilly MS, Folkman J (1995) Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med 1(2):149–153
Huang S, Chang IS, Lin W et al (2010) ARHI (DIRAS3), an imprinted tumour suppressor gene, binds to importins and blocks nuclear import of cargo proteins. Biosci Rep 30(3):159–168. doi:10.1042/BSR20090008
Jemal A, Murray T, Thun M (2002) Cancer statistics. Cancer J Clin 52:23–47
Kim MS, Song SY, Lee JY et al (2011) Expressional and mutational analyses of ATG5 gene in prostate cancers. APMIS 119(11):802–807. doi:10.1111/j.1600-0463.2011.02812.x
Ladoire S, Chaba K, Martins I et al (2012) Immunohistochemical detection of cytoplasmic LC3 puncta in human cancer specimens. Autophagy 8(8):1175–1184. doi:10.4161/auto.20353
Laurent J, Frongia C, Cazales M et al (2013) Multicellular tumor spheroid models to explore cell cycle checkpoints in 3D. BMC Cancer 13:73. doi:10.1186/1471-2407-13-73
Lin D, Cui F, Bu Q et al (2011) The expression and clinical significance of GTP-binding RAS-like 3 (ARHI) and microRNA 221 and 222 in prostate cancer. J Int Med Res 39(5):1870–1875
Lu ZH, Chen J, Gu LJ et al (2001) ARHI mRNA and protein expression in pancreatic cancers. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 23(4):324–327
Lu Z, Luo RZ, Lu Y et al (2008) The tumor suppressor gene ARHI regulates autophagy and tumor dormancy in human ovarian cancer cells. J Clin Invest 118(12):3917–3929. doi:10.1172/JCI35512
Lu KH, Skates S, Hernandez MA et al (2013) A 2-stage ovarian cancer screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer. doi:10.1002/cncr.28183
Lu Z, Yang H, Sutton MN, Yang M, Clarke CH, Liao WA, Bast RC (2014a) ARHI (DIRAS3) induces autophagy in ovarian cancer cells by downregulating the epidermal growth factor receptor, inhibiting PI3K and Ras/MAP signaling and activating FOXo3a-mediated induction of Rab 7. Cell Death Differ 21(8):1275–1289
Lu Z, Baquero MT, Yang H, Yang M, Reger AS, Kim C, Levine DA, Clarke CH, Liao WS, Bast RC (2014b) DIRAS3 regulates the autophagosome initiation complex in dormant ovarian cancer cells. Autophagy 10(6):1071–1092
Luo RZ, Fang X, Marquez R et al (2003) ARHI is a Ras-related small G-protein with a novel N-terminal extension that inhibits growth of ovarian and breast cancers. Oncogene 22(19):2897–2909. doi:10.1038/sj.onc.1206380
Markman M, Liu PY, Moon J et al (2009) Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum-paclitaxel: follow-up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecol Oncol 114(2):195–198. doi:10.1016/j.ygyno.2009.04.012
Menon U, Gentry-Maharaj A, Hallett R et al (2009) Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol 10(4):327–340. doi:10.1016/S1470-2045(09)70026-9
Michaud M, Martins I, Sukkurwala AQ et al (2011) Autophagy-dependent anticancer immune responses induced by chemotherapeutic agents in mice. Science 334(6062):1573–1577. doi:10.1126/science.1208347
Mullany LK, Richards JS (2012) Minireview: animal models and mechanisms of ovarian cancer development. Endocrinology 153(4):1585–1592. doi:10.1210/en.2011-2121
Naumov GN, Akslen LA, Folkman J (2006) Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle 5(16):1779–1787
O’Reilly MS, Holmgren L, Chen C et al (1996) Angiostatin induces and sustains dormancy of human primary tumors in mice. Nat Med 2(6):689–692
Perren TJ, Swart AM, Pfisterer J et al (2011) A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 365(26):2484–2496. doi:10.1056/NEJMoa1103799
Qu X, Yu J, Bhagat G et al (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112(12):1809–1820. doi:10.1172/JCI20039
Reggiori F, Klionsky DJ (2002) Autophagy in the eukaryotic cell. Eukaryot Cell 1(1):11–21
Rosen DG, Wang L, Jain AN et al (2004) Expression of the tumor suppressor gene ARHI in epithelial ovarian cancer is associated with increased expression of p21WAF1/CIP1 and prolonged progression-free survival. Clin Cancer Res 10(19):6559–6566. doi:10.1158/1078-0432.CCR-04-0698
Ryter SW, Choi AM (2010) Autophagy in the lung. Proc Am Thorac Soc 7(1):13–21. doi:10.1513/pats.200909-101JS
Wang L, Hoque A, Luo RZ et al (2003) Loss of the expression of the tumor suppressor gene ARHI is associated with progression of breast cancer. Clin Cancer Res 9(10 Pt 1):3660–3666
Wells A, Griffith L, Wells JZ et al (2013) The dormancy dilemma: quiescence versus balanced proliferation. Cancer Res. doi:10.1158/0008-5472.CAN-13-0356
Willis RA (1934) The spread of tumours in the human body. J. & A. Chuchill, London
Wu X, Liang L, Dong L et al (2013) Effect of ARHI on lung cancer cell proliferation, apoptosis and invasion in vitro. Mol Biol Rep 40(3):2671–2678. doi:10.1007/s11033-012-2353-x
Yu Y, Xu F, Peng H et al (1999) NOEY2 (ARHI), an imprinted putative tumor suppressor gene in ovarian and breast carcinomas. Proc Natl Acad Sci U S A 96(1):214–219
Yue Z, Jin S, Yang C et al (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 100(25):15077–15082. doi:10.1073/pnas.2436255100
Acknowledgments
This chapter was supported in part by grants from the National Cancer Institute P01 CA064602 and R01 CA135354, by the M.D. Anderson SPORE in Ovarian Cancer NCI P50 CA83639, the Shared Resources of the M.D. Anderson CCSG NCI P30 CA16672, the Ovarian Cancer Research Fund, the National Foundation for Cancer research and philanthropic support from the Zarrow Foundation and Stuart and Gaye Lynn Zarrow.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Sutton, M.N., Lu, Z., Bast, R.C. (2014). The Role of Angiogenesis, Growth Arrest and Autophagy in Human Ovarian Cancer Xenograft Models for Tumor Dormancy. In: Hayat, M. (eds) Tumor Dormancy, Quiescence, and Senescence, Vol. 3. Tumor Dormancy and Cellular Quiescence and Senescence, vol 3. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-9325-4_8
Download citation
DOI: https://doi.org/10.1007/978-94-017-9325-4_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-9324-7
Online ISBN: 978-94-017-9325-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)