Abstract
Recent electrophysiological and neuroimaging studies showed the possibility to detect command-specific changes in electroencephalography (EEG) or functional magnetic resonance imaging (fMRI) signals independent of any motor pathway. These techniques could help in the improvement of the diagnosis in patients with disorders of consciousness (DOC; often suffering severe motor disabilities), providing motor-independent evidence of command following and even, in some cases, permitting communication. We here review the first results obtained by BCI-like applications in patients with DOC and discuss the challenges facing BCI research. One application which has been rarely thought for BCI is the use of these systems as diagnosis tools. Indeed, a BCI may help to detect signs of consciousness and communication in patients lacking the ability to move or speak. In this chapter, we will present the first applications of BCI approaches to detect signs of consciousness in patients with DOC. We will then highlight the main challenges that will need to be overcome in future research and some clues from studies in healthy controls and patients with locked-in syndrome (LIS).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Challenge of Diagnosis in Patients with Disorders of Consciousness
Following severe brain damage, patients may fall into a coma (i.e. absence of eye opening, reflex responses). After some days or weeks, they may awaken (i.e., open their eyes) but still fail to show voluntary behaviors. This syndrome is known as “unresponsive wakefulness syndrome” (UWS; Laureys et al. 2010), formerly coined “vegetative state” (VS). Some patients will remain unresponsive for decades; other patients may evolve to a minimally conscious state, i.e., showing more than reflex behaviors such as visual pursuit (MCS minus; Bruno et al. 2012) or command following (MCS plus) but lacking functional communication (Giacino et al. 2002). Others may awaken and be fully aware but paralyzed and mute, i.e., locked-in syndrome (LIS; Plum and Posner 1966). Nowadays, the clinical diagnosis of patients with disorders of consciousness (DOC) such as unresponsive wakefulness syndrome (UWS) and minimally conscious state (MCS), and therefore their access to rehabilitation, is mainly based on behavioral observations. Keystones in diagnosis are the acquisition of voluntary responses such as command following and functional communication, which indicate emergence from the UWS and the MCS, respectively. Command following and functional communication also distinguish LIS from UWS patients. However, the difficulty of distinguishing reflex from voluntary responses makes the assessment very challenging for clinicians, particularly in a population often suffering from motor disabilities associated with brain damage.
Some electrophysiological and neuroimaging studies have been proposed to probe residual brain function in DOC. They aimed at providing diagnosis and prognosis markers. Some, in line with BCI studies, aim at detecting command-specific changes in electroencephalography (EEG) or functional magnetic resonance imaging (fMRI) signals providing motor-independent evidence of conscious thoughts.
In the context of patients with DOC, the first goal of a BCI is to establish, beyond reasonable doubt, that a patient is able to follow a command. To do so, the patient would need to be able to understand the task requirements, which ideally should be as simple as “squeeze my hand”, and execute the task multiple times. Then, the software and hardware could be extendable to test communication with “responders”. The patient would therefore need to be able to attend to stimuli/questions while retaining task information in working memory. Current BCIs require much greater capacities from the patient than behavioral testing but they are a unique opportunity to establish an early diagnosis of LIS in patients who cannot behaviorally express their consciousness.
When looking at the results obtained in studies in patients with DOC, we need to take into account the number of patients showing command following with the system, but also how many of them were able to follow a command at the bedside and could not be detected by the system. Indeed, this ratio would give us important information about the false negatives in the studied population with a given paradigm. We will therefore always give this information for every study reviewed below.
2 BCIs as a Diagnostic Tool in Patients with DOC
The first case study using BCI as a diagnostic tool of consciousness was a patient diagnosed as being in an UWS, who was instructed to “imagine playing tennis” and “walking through her house” during an fMRI session (Owen et al. 2006). The paradigm consisted of a 30-s period of mental imagery followed by a resting period of 30 s. Each imagery task was repeated ten times. This patient displayed similar brain activations compared to control subjects for both tasks. A few months after the study, the patient behaviorally evolved into MCS. In a follow-up study (Monti et al. 2010) including 54 patients (23 UWS and 31 MCS), five (four UWS) were able to willfully modulate their brain activity. One of them was even able to answer simple questions, e.g. “Is your father’s name Alexander?”, using one task for “yes” and the other for “no”. However, out of 18 patients showing command following at the bedside, only one could be identified with the system (false negative: 94 %). Bardin et al. (2011) investigated the use of a different imagery task instructing patients to imagine themselves swimming or playing tennis with their right hand, using a protocol very close to the one used by Owen et al. (2006) and Monti et al. (2010). Out of six patients, three of them were able to follow commands with the system. However, if five patients were able to follow commands at the bedside, two of them could not be identified with the system (40 %). In the same idea, Monti et al. reported preserved working memory abilities in an MCS patient exceeding expectations based on the standard behavioral assessment, using an active task in fMRI (counting target-neutral words in an auditory sequence of non-target words) (Monti et al. 2009). This patient was able to follow a command and communicate intentionally at the bedside. Nevertheless, this study only included one patient and the results have not been replicated yet, preventing any interpretation in terms of the false negative rate.
Despite the many advantages of fMRI, this technique is limited in terms of availability, affordability, and ease of use in this population. On the other hand, EEG can potentially lead to the development of relatively cheap and compact systems that can be readily deployed at the bedside. Schnakers et al. proposed using an auditory P300 for EEG-driven commands following detection (Schnakers et al. 2008). The advantage of the P300 is that it can be elicited by meaningful stimuli requiring only a limited workload from the patient. However, some of the most successful P300-based BCI systems are based on visual P300 whereas patients with DOC often suffer from gaze fixation impairments, and hence cannot react to visual stimulations. Consequently, auditory P300 is more likely to be usable by a greater number of patients (Chatelle et al. 2012). Schnakers et al. instructed patients to count the number of times a name (subject’s own name or unfamiliar name) was presented within an auditory sequence of random names (Schnakers et al. 2008). Results showed that, out of 14, five MCS patients showed significantly better P300 responses when actively counting the occurrence of their own name as compared to when only passively listening. Interestingly, four other patients showed a response only when they were asked to count an unfamiliar name as compared to passive listening. Since both sessions were recorded at the same time, these results could highlight an important fluctuation of vigilance in this population. On the other hand, the eight UWS patients did not show any response to the active task. Moreover, when administered in a complete LIS patient behaviorally diagnosed as being comatose, they also observed a significant difference between the passive and the active task (Schnakers et al. 2009). Using this paradigm, two (22 %) out of nine patients showing command following at the bedside that could not be detected with the system.
Building on the auditory P300, Lulé et al. tested a four-choice auditory P300-based BCI on 13 MCS, three UWS, and two LIS patients (Lule et al. 2013). After a training phase, each patient had to answer ten questions by concentrating on repetitions of “yes” or “no” presented in a stream of words. One LIS patient had a significant correct response rate of 60 % while the other LIS patient had a response rate of 20 % and could not use the BCI for communication. No MCS patient could communicate through the BCI. Out of six patients showing command following at the bedside, five (83 %) could not be detected with the BCI. It is important to highlight that this paradigm was designed as a communication protocol, and it would therefore be interesting to adapt it to first study command following in a population with DOC.
Another well studied BCI is based on motor imagery. Imagination of movement is well known to be associated with a power decrease in the sensorimotor or mu-rhythm (8–15 Hz; SMR; Pfurtscheller et al. 1997; Neuper et al. 2005), called event-related desynchronization, focused in the motor region implicated in the target movement (Pfurtscheller and Lopes da Silva 1999). For these BCIs, the stimulation can be effectively delivered auditorily (Chatelle et al. 2012). Goldfine and colleagues (Goldfine et al. 2011) recorded EEG from three patients showing command following at the bedside (MCS, MCS/exit-MCS and LIS), while they were involved in motor imagery and spatial navigation tasks. A session alternated eight 15-s periods of mental imagery with 15-s periods of rest. All patients demonstrated the capacity to generate mental imagery on the same tasks, via independent fMRI studies. With univariate comparisons (individual frequencies), Goldfine and colleagues were able to show evidence of significant differences between the frequency spectra accompanying the two imagery tasks in one MCS patient (however inconsistently) and one LIS patient (33 % not detected with the system). Multivariate comparisons (patterns across the frequency range) using linear discriminant analysis did not lead to any evidence of brain-related activation in any patient (100 % undetected).
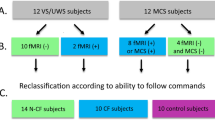
In another study from Cruse et al., motor imagery task was investigated in 16 UWS and 23 MCS patients (Cruse et al. 2012), showing that eight of them (three UWS, five MCS) were able to voluntarily control their brain activity in response to a command (“imagine squeezing your right hand” vs “imagine moving all your toes”). Out of 15 patients showing command following, 13 could not be identified by the system (87 %). However, these results have to be taken with caution as the methodology emphasizes the need for powerful statistical tests for that kind of BCI application (Cruse et al. 2013; Goldfine et al. 2013).
3 Future Perspectives
The high rate of false negatives achieved using current BCIs highlight the need to develop more sensitive tools for diagnosing DOC patients (Table 3.1). Indeed, a system which is not sensitive enough to detect patients diagnosed as conscious at the bedside could not be reliably used in a population with unclear diagnosis. Currently, research on BCI in patients with DOC will have to overcome a number of challenges. First, there is the barrier associated with the lesions leading to awareness fluctuation, fatigue, and limited attention span commonly observed in these patients, especially in MCS patients (Giacino et al. 2002). Hence, task/stimulus complexity and duration is an important factor to consider when evaluating BCI applications. Moreover, multiple repetition of the BCI session must be considered to ensure a reliable diagnosis. In terms of communication, evaluation should be assessed with simple questions as severely brain-damaged patients may have difficulty giving accurate answers even to trivial yes/no questions (Nakase-Richardson et al. 2009). Furthermore, brain injury is often associated with sensory deficits (such as cortical blindness or deafness). When research on BCI in healthy participants seems to highlight better performance with a visual BCI as compared to auditory or tactile BCIs (Pham et al. 2005; Kübler et al. 2009; Halder et al. 2010), the key challenge here will be to develop sensitive systems offering stimuli, instructions, and/or questions through multiple channels.
Third, feedback and motivation – known factors influencing BCI results – must be considered with care as we cannot distinguish a patient not paying attention to the task (lacking motivation), an unsuccessful patient, and an unconscious patient. Fourth, suboptimal data quality due to movement and ocular and respiration artifacts in these challenging populations may also be confounding factors that need to be overcome using appropriate statistical analyses. In addition, the suitability of different BCI designs for individual patients is significantly variable and will need to be comparatively assessed in each case. While some patients have been shown to be able to generate reliable P300s in response to task-relevant stimuli, others have demonstrated the ability to consistently perform mental imagery in response to commands.
One last main challenge that also needs to be pointed out is that in EEG, the classification accuracy achieved with a BCI naturally depends on the quality and inter-trial consistency of the data used to train the classifier. This is problematic for most patients with DOC, particularly those in MCS, who are prone to frequent and prolonged bouts of fatigue and attention fluctuation. This would effectively render them unable to pay attention for sufficiently long periods. For many patients, this limitation will adversely affect the statistical power of the classifiable patterns latent in their EEG data (e.g. dependency). It is therefore important to design protocols accordingly (avoid using blocks of one stimulation and long-lasting sessions, assess the patient at different time periods (Cruse et al. 2013; Goldfine et al. 2013)).
Amongst the different designs developed in healthy controls and tested in DOC, motor imagery BCIs are less hindered by problems of stimulation modality. There is relatively little stimulation that needs to be presented and this can be effectively delivered auditorily. Results on their use in some DOC patients have produced promising results (Monti et al. 2010; Goldfine et al. 2011). This knowledge, along with the fact that motor imagery (e.g. playing tennis vs. spatial navigation imagery) in fMRI has already allowed some patients to communicate when unable to do so at the bedside (Monti et al. 2010), bodes well for similar BCI variants. However, as motor imagery relies on the user’s ability to learn mappings between intention and movement imagery, they require adequate training before reliable performance can be achieved, which poses a significant challenge in a population of DOC patients, as illustrated by the high rate of false negatives achieved in previous studies on imagery tasks. In this context, P300-based BCI designs could be of interest since they rely on “automatic” responses of the brain to salient stimuli and hence require relatively little explicit user training. As highlighted earlier, previous findings by Schnakers et al. (2008) and Monti et al. (2009) have shown that some patients with DOC can generate consistent changes in EEG and fMRI when asked to selectively respond to task-relevant stimuli. Moreover, the P300 paradigm has shown to be the least sensitive to false negatives as compared to the other designs studied recently. Potentially, if successful with a patient, a P300-based BCI for spelling words and sentences using a predictive language support program could provide a true, multi-class system with relatively high efficiency. Moreover, a study on the P300 in healthy subjects has reported 89 % of healthy controls being able to use it with an accuracy of 80–100 % (Guger et al. 2009), compared to only 20 % of users with a motor-imagery-based BCI (Guger et al. 2003). Since we know the most successful P300-based BCI is visually based, it would also be possible to adapt a visually-based BCI for patients with eye control disabilities using one by one stimulus presentation, as successfully developed, although not tested on DOC patients, by Hoffmann et al. (2008). On the other hand, future studies should take into account the topographic and latency variabilities observed in the P300-based BCI in healthy subjects to interpret patients’ data (Bianchi et al. 2010; Kaufmann et al. 2011).
Other kinds of BCIs were only studied in healthy controls and LIS patients, but can be of interest for patients with DOC. Steady-state visually evoked potentials (SSVEPs; Regan 1989; Vialatte et al. 2010) are the oscillatory electrical responses of neurons in the visual cortex to stimuli that are repeatedly presented (or flashed) at frequencies above 6 Hz. SSVEPs are easy to detect, as their frequency content is completely determined by the visual stimuli used to elicit them. The advantage of this response is that it has high signal-to-noise ratios and is nearly completely free of eye movement (Perlstein et al. 2003) and electromyographic artifacts (Regan 1966; Gray et al. 2003). If this response has been shown to allow healthy subjects and motor-disabled patients to successfully use the system for communication (Parini et al. 2009), it will have to be tested in DOC using alternative approaches based on covert attention to solve the problem of eye motor control disabilities (Lesenfants et al. 2011).
Birbaumer and colleagues (Elbert et al. 1980; Birbaumer et al. 1999, 2000) have worked on the development of slow cortical potentials (SCPs)-based BCIs, slow voltage changes generated in the cortex, occurring over periods of 0.5–10.0 s. Usually, negative SCPs are associated with motor movement and other functions involving increased cortical activation, while positive SCPs are more associated with reduced cortical activation (Birbaumer 1997). Further, this system has been tested in people with late-stage amyotrophic lateral sclerosis (ALS) and has proved capable of providing basic communication capacities (Kübler et al. 1999). However, the main problem is again that the most successful system uses visually-based feedback (Birbaumer et al. 2000; Pham et al. 2005) and a relatively long period of training is needed (Birbaumer 2006). On the other hand, SCPs have the advantage of being the most stable over long periods (Chatelle et al. 2012).
4 Conclusion
In this chapter, we highlighted several studies performed on patients with DOC that consider the use of diagnostic tools. These results could have a significant impact on rehabilitation strategies, quality of life, and prognosis. However, for all of the BCI designs discussed here, results from patients with DOC will need to be interpreted with great caution. Indeed, results from these studies showed that the likelihood that a covertly aware patient might go undetected (i.e. the false negative rate) is likely to vary significantly across different paradigms. Hence, none of these tests applied individually to look for command following can currently be used to interpret negative results without combining findings from multiple testing methods to mitigate the level of uncertainty. Future research will need to overcome several challenges limiting current BCI applications in DOC patients, in order to create a more sensitive tool for diagnosis. Studies on BCIs in healthy participants could be used as a basis for the development of new paradigms, but there is a need to conduct extensive testing with patients likely to benefit from various BCI systems in their daily lives (Kübler et al. 2006), since we know it is often the case that results from controls do not generalize well to patient groups (Hill et al. 2006).
References
Bardin, J.C., J.J. Fins, et al. 2011. Dissociations between behavioural and functional magnetic resonance imaging-based evaluations of cognitive function after brain injury. Brain 134(Pt 3): 769–782.
Bianchi, L., S. Sami, et al. 2010. Which physiological components are more suitable for visual ERP based brain-computer interface? A preliminary MEG/EEG study. Brain Topography 23(2): 180–185.
Birbaumer, N. 1997. Slow cortical potentials: Their origin, meaning, and clinical use. In Brain and behavior past, present, and future, ed. G.J.M. van Boxtel and K. Böcker, 25–39. Tilburg: Tilburg University Press.
Birbaumer, N. 2006. Breaking the silence: Brain-computer interfaces (BCI) for communication and motor control. Psychophysiology 43(6): 517–532.
Birbaumer, N., N. Ghanayim, et al. 1999. A spelling device for the paralysed. Nature 398(6725): 297–298.
Birbaumer, N., A. Kübler, et al. 2000. The thought translation device (TTD) for completely paralyzed patients. IEEE Transactions on Rehabilitation Engineering 8(2): 190–193.
Bruno, M.A., S. Majerus, et al. 2012. Functional neuroanatomy underlying the clinical subcategorization of minimally conscious state patients. Journal of Neurology 259(6):1087–98.
Chatelle, C., S. Chennu, et al. 2012. Brain-computer interfacing in disorders of consciousness. Brain Injury 26(12):1510–22.
Cruse, D., S. Chennu, et al. 2011. Bedside detection of awareness in the vegetative state. The Lancet 378(9809): 2088–2094.
Cruse, D., S. Chennu, et al. 2012. The relationship between aetiology and covert cognition in the minimally-conscious state. Neurology 78(11): 816–822.
Cruse, D., S. Chennu, et al. 2013. Response to: Reanalysis of ‘Bedside detection of awareness in the vegetative state: A cohort study’. The Lancet 381(9863): 291–292.
Elbert, T., B. Rockstroh, et al. 1980. Biofeedback of slow cortical potentials. I. Electroencephalography and Clinical Neurophysiology 48(3): 293–301.
Giacino, J., S. Ashwal, et al. 2002. The minimally conscious state: Definition and diagnostic criteria. Neurology 58(3): 349–353.
Goldfine, A.M., J.D. Victor, et al. 2011. Determination of awareness in patients with severe brain injury using EEG power spectral analysis. Clinical Neurophysiology 122(11):2157–68.
Goldfine, A.M., J.C. Bardin, et al. 2013. Reanalysis of Bedside detection of awareness in the vegetative state: A cohort study. The Lancet 381(9863): 289–291.
Gray, M., A.H. Kemp, et al. 2003. Cortical neurophysiology of anticipatory anxiety: An investigation utilizing steady state probe topography (SSPT). NeuroImage 20(2): 975–986.
Guger, C., G. Edlinger, et al. 2003. How many people are able to operate an EEG-based brain-computer interface (BCI)? IEEE Transactions on Neural Systems and Rehabilitation Engineering 11(2): 145–147.
Guger, C., S. Daban, et al. 2009. How many people are able to control a P300-based brain-computer interface (BCI)? Neuroscience Letters 462(1): 94–98.
Halder, S., M. Rea, et al. 2010. An auditory oddball brain-computer interface for binary choices. Clinical Neurophysiology 121(4): 516–523.
Hill, N.J., T.N. Lal, et al. 2006. Classifying EEG and ECoG signals without subject training for fast BCI implementation: Comparison of nonparalyzed and completely paralyzed subjects. IEEE Transactions on Neural Systems and Rehabilitation Engineering 14(2): 183–186.
Hoffmann, U., J.M. Vesin, et al. 2008. An efficient P300-based brain-computer interface for disabled subjects. Journal of Neuroscience Methods 167(1): 115–125.
Kaufmann, T., E. Hammer, et al. 2011. ERPs contributing to classification in the P300 BCI. In Proceedings of the 5th international brain-computer interface conference. Graz: Graz University of Technology.
Kübler, A., B. Kotchoubey, et al. 1999. The thought translation device: A neurophysiological approach to communication in total motor paralysis. Experimental Brain Research 124(2): 223–232.
Kübler, A., V.K. Mushahwar, et al. 2006. BCI meeting 2005–workshop on clinical issues and applications. IEEE Transactions on Neural Systems and Rehabilitation Engineering 14(2): 131–134.
Kübler, A., A. Furdea, et al. 2009. A brain-computer interface controlled auditory event-related potential (p300) spelling system for locked-in patients. Annals of the New York Academy of Sciences 1157: 90–100.
Laureys, S., G.G. Celesia, et al. 2010. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Medicine 8: 68.
Lesenfants, D., N. Partoune, et al. 2011. Design of a novel covert SSVEP-based BCI. In Proceedings of the 5th international brain-computer interface conference. Graz: University of Technology Publishing House.
Lule, D., Q. Noirhomme, et al. 2013. Probing command following in patients with disorders of consciousness using a brain-computer interface. Clinical Neurophysiology 124: 101–106.
Monti, M.M., M.R. Coleman, et al. 2009. Executive functions in the absence of behavior: Functional imaging of the minimally conscious state. Progress in Brain Research 177: 249–260.
Monti, M.M., A. Vanhaudenhuyse, et al. 2010. Willful modulation of brain activity in disorders of consciousness. New England Journal of Medicine 362(7): 579–589.
Nakase-Richardson, R., S.A. Yablon, et al. 2009. Emergence from minimally conscious state: Insights from evaluation of posttraumatic confusion. Neurology 73(14): 1120–1126.
Neuper, C., R. Scherer, et al. 2005. Imagery of motor actions: Differential effects of kinesthetic and visual-motor mode of imagery in single-trial EEG. Brain Research. Cognitive Brain Research 25(3): 668–677.
Owen, A.M., M.R. Coleman, et al. 2006. Detecting awareness in the vegetative state. Science 313(5792): 1402.
Parini, S., L. Maggi, et al. 2009. A robust and self-paced BCI system based on a four class SSVEP paradigm: Algorithms and protocols for a high-transfer-rate direct brain communication. Computational Intelligence and Neuroscience 864564 doi:10.1155/2009/864564.
Perlstein, W.M., M.A. Cole, et al. 2003. Steady-state visual evoked potentials reveal frontally-mediated working memory activity in humans. Neuroscience Letters 342(3): 191–195.
Pfurtscheller, G., and F.H. Lopes da Silva. 1999. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clinical Neurophysiology 110(11): 1842–1857.
Pfurtscheller, G., C. Neuper, et al. 1997. EEG-based discrimination between imagination of right and left hand movement. Electroencephalography and Clinical Neurophysiology 103(6): 642–651.
Pham, M., T. Hinterberger, et al. 2005. An auditory brain-computer interface based on the self-regulation of slow cortical potentials. Neurorehabilitation and Neural Repair 19(3): 206–218.
Plum, F., and J. Posner. 1966. The diagnosis of stupor and coma. Philadelphia: F.A. Davis & Co.
Regan, D. 1966. Some characteristics of average steady-state and transient responses evoked by modulated light. Electroencephalography and Clinical Neurophysiology 20(3): 238–248.
Regan, D. 1989. Human brain electrophysiology: Evoked potentials and evoked magnetic fields in science and medicine. New York: Elsevier.
Schnakers, C., F. Perrin, et al. 2008. Voluntary brain processing in disorders of consciousness. Neurology 71: 1614–1620.
Schnakers, C., F. Perrin, et al. 2009. Detecting consciousness in a total locked-in syndrome: An active event-related paradigm. Neurocase 4: 1–7.
Vialatte, F.B., M. Maurice, et al. 2010. Steady-state visually evoked potentials: Focus on essential paradigms and future perspectives. Progress in Neurobiology 90(4): 418–438.
Acknowledgments
We gratefully acknowledge Audrey Vanhaudenhuyse and Martin Monti for their collaboration on the patients’ data information. This work was supported by the Belgian Fonds National de la Recherche Scientifique (FNRS), European Commission, Mind Science Foundation, James McDonnell Foundation, French Speaking Community Concerted Research Action, Fondation Léon Fredericq, Public Utility Foundation “Université Européenne du Travail” and “Fondazione Europea di Ricerca Biomedica”. This work is supported by the European ICT Programme Projects FP7-247919 DECODER. The text reflects solely the views of its authors. The European Commission is not liable for any use that may be made of the information contained therein.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Chatelle, C., Laureys, S., Noirhomme, Q. (2014). Brain-Computer Interfaces and Diagnosis. In: Grübler, G., Hildt, E. (eds) Brain-Computer-Interfaces in their ethical, social and cultural contexts. The International Library of Ethics, Law and Technology, vol 12. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8996-7_3
Download citation
DOI: https://doi.org/10.1007/978-94-017-8996-7_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8995-0
Online ISBN: 978-94-017-8996-7
eBook Packages: Humanities, Social Sciences and LawPhilosophy and Religion (R0)