Summary
The immobilization is a process of catalyst (cells) attachment to the matrix. It separates effectively the cells from the liquid and gas phases, allowing a significant increase in the culture density. This review describes various approaches used for immobilization of photosynthetic cells. The main attention is focused on advantages and limitations of immobilized systems for hydrogen photoproduction.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Molecular hydrogen is an ideal energy carrier for the future world. It can be produced from a wide range of sources and by a number of different technologies that includes: water electrolysis, reforming of natural gas, coal gasification, thermochemical production and biomass gasification. Hydrogen gas production by the cultures of phototrophic microorganisms is considered as one of the most promising and ecologically friendly approaches. The process occurs under ambient temperatures, requires sunlight, water and minimal amounts of macro and micronutrients. However, due to some limitations discussed in this and other chapters of the book it is still in the research and development stage. Recently obtained research data show promise in the practical application. However, there are a number of problems that should be solved before H2 gas production by phototrophic microorganisms becomes a commercially competitive technology. Among the most important limitations are the low rates of hydrogen gas production and difficulties in maintaining and processing suspension cultures. These challenges might be addressed by immobilizing microbial cells.
When compared to suspensions, immobilized cultures have higher volumetric rates of hydrogen production; the separation of gas, liquid, and solid phases is natural; the catalyst (microbial cells) has higher stability due to a diffusion barrier of the matrix. These and some other advantages make immobilization a very suitable approach for biohydrogen applications. This chapter reviews the modern methods available for immobilization of photosynthetic microorganisms, describes criteria for selection of these methods, gives some recommendations for designing photobioreactors (PhBRs) with immobilized cells and presents examples of already described systems for hydrogen production by immobilized photosynthetic microorganisms.
II. Methods of Immobilization
Immobilization in biotechnology is defined as “the confinement or localization of viable microbial cells to a certain defined region of space in such a way as to exhibit hydrodynamic characteristics, which differ from those of the surrounding environment” (Azbar and Kapdan 2011). Methods of immobilization can be divided into two large groups: artificial cell entrapments that assume application of matrices or substrates for attachment, entrapment within the matrix, or encapsulation of microorganisms, and natural cell entrapments, which allow microorganisms to form biofilm or granules. Independently on the nature, the ideal immobilization method should satisfy the following requirements:
-
Neutrality to cell metabolism. The immobilization matrix should be non-toxic to the cells and stable against cellular activities.
-
Stability in time and in space. Mechanical properties of the matrix should be strong enough to withstand against shear stress, but gentle to avoid mechanical inhibition of the cells.
-
Porousness of the matrix or/and supporting substrate. Associated diffusion barriers should not limit the target reaction, but should be high enough to realize a high resistance of immobilized cells against toxic compounds and non-optimal environmental parameters like sharp pH changes.
-
Absence of cell leakage outside of the immobilization space.
-
Simplicity in production and operation.
-
Low cost.
-
Renewability of materials, if possible.
In addition to general requirements listed above, the immobilization method for photosynthetic microorganisms should allow the light penetration to the cells. Therefore, the matrix and support for the matrix should be transparent (translucent for foam and porous structures).
A. Artificial Immobilization
Microorganisms can be fixed in space using a variety of methods, which include: ionic adsorption on water-insoluble matrices, gel entrapments and encapsulations with solid or liquid membranes.
Ionic Adsorption on Water-Insoluble Matrices
Ionic adsorption of microorganisms can be non-specific and specific. An example of non-specific interactions is the cyanobacterial cells readily attached to hydrophilic polyvinyl or polyurethane foam (Hall and Rao 1988). Specific adsorption is developed due to surface charges. The surface of many bacteria has negative charge. When a substrate is charged positively, for example by activating the glass with aminosilane (Tsygankov et al. 1993), bacteria occupy up to 40 % of the substrate surface. This method is quick and simple, but produces monolayer of cells. If not followed by colonization, the process is reversible. Nevertheless, this approach is good for accelerating the natural immobilization (see below).
Gel Entrapments
A variety of natural and synthetic, organic and inorganic substances can be used for the entrapment of microorganisms.
a. Polysaccharides
Polysaccharides are excellent materials for entrapping microorganisms. They satisfy almost all requirements. Matrices with immobilized cells can be formed into any shape, including beads (Hallenbeck 1983), cubes (Saetang and Babel 2009) and fibers (Park et al. 1991). The main disadvantage of polysaccharides is a low chemical and mechanical stability of their matrices, which requires application of additional supporting substrates: glass plates, synthetic fibers, steel or plastic screens. The following groups of natural and modified polysaccharides are frequently used in immobilization (Stolarzewicz et al. 2011):
-
polyuronides (polymers of uronic acids): alginate and pectins;
-
galactans (galactose polymers): agar, agarose, carrageenan;
-
glucans (polymers of D-glucose and its derivatives): chitosan, an amino polysaccharide derivative of chitin, starch, cellulose and its alkyl and carboxylic derivatives;
-
some polysaccharides containing natural products like cashew apple bagasse, corn starch gel or orange peel.
The immobilization process using the above mentioned matrices is usually carried out by encapsulating the cells or entrapping them within gels of different shapes. Solidification (polymerization) of the gel is achieved by several physical and chemical treatments, largely depending on properties of the used polymer. In some cases, polymerization occurs in the presence of bivalent ions (alginic acid) and cationic compounds: metal ions, amines, amino acid derivates and organic solvents (carrageenan) or by changing the pH (alginic acid, chitosan, pectins). In other cases, gel thickening is driven by cooling the liquid polymer to the temperature of solidification (agar, agarose). The most commonly used polysaccharides for entrapping within gels are alginate, a linear unbranched polymer containing 1-4-linked-β-D-mannuronic acid and α-L-guluronic acid residues in different proportions and sequences, and carrageenan, a linear sulfated polysaccharide consisting of alternating 3-linked-β-D-galactopyranose and 4-linked-α-D-galactopiranose units.
b. Proteins
Similar to polysaccharides, proteins form hydrocolloids and may also be very effective for immobilization of enzymes and whole cells (Kourkoutas et al. 2004). The most often employed proteins are: albumin, gelatin, gluten and silk fibroin (Krastanov 1997; Gugerli et al. 2004). Encapsulation and entrapment of the cells inside fibers are commonly used for protein immobilization approaches. Protein hydrocolloids are transparent in visible part of spectrum and, therefore, can be used for immobilization of photosynthetic cells in biohydrogen applications. The main drawback of these matrices is their high cost.
c. Polyvinyl Alcohol
Polyvinyl alcohol (PVA) is another excellent, but synthetic material, for entrapping microorganisms due to its neutrality, stability, transparency, simplicity of operation, cost, and commercial availability. Two methods of matrix polymerization are often used for microbial immobilization. The first is “freezing-thawing”, and the second is UV-light polymerization. PVA is not mechanically strong enough to create foam. For this purpose, the method of preparing macroporous PVA foam with improved stability was suggested, which involves adding calcium carbonate as a pore-forming agent and epichlorohydrin as a chemical crosslinking agent (Bai et al. 2010).
d. Polyacrylamide
Acrylic polymers obtained from acrylic or methacrylic acid and their derivatives, such as amides, esters and others, and cross-linked with N,Nʹ-methylenebisacrylamide form transparent, mechanically and chemically stable matrices, which have a high diffusion rate regulated by cross-linking. These matrices are often used for immobilization of microorganisms (Stolarzewicz et al. 2011). The polymerization procedure (by irradiation or by chemical activation) is not neutral for cells and, therefore, should be tested for the influence on the target activity before any application for the particular microorganisms.
Thin-Layer Immobilization
The main limiting factor affecting the productivity of photosynthetic microorganisms is the light distribution within the culture (Torzillo et al. 2003). The immobilized cells are subject to the same rule. With increasing depth of the matrix, the lower cell layers experience shading, which can be significant in the dense culture. On the contrary, upper cell layers are subject to photoinhibition, especially under high light. As a result, overall light utilization efficiency by the culture is low. The problem can be addressed in part by immobilizing photosynthetic microorganisms in thin-layer matrices. Thin-layer immobilization allows a precise control of the matrix thickness and makes possible a more uniform light distribution to the cells. Although different transparent polymers can be applied for entrapping photosynthetic microorganisms within thin-layer films, the most frequently used polymers in this approach are sol-gels, latexes and alginates. Except for sol-gels, these materials are cheap and available in industrial quantities. In addition, alginates, as natural polymers, are renewable materials.
a. Sol-Gel Encapsulation
Starting from the first description of sol-gel application for the immobilization of microorganisms (Carturan et al. 1989) this technology is developed for encapsulation of different enzymes and cells, including photosynthetic microorganisms and plant cells (Rooke et al. 2008). Silica sol–gels are chemically inert, mechanically stable, transparent and can be used not only for encapsulation but also for entrapment (Kandimalla et al. 2006). One drawback is the procedure of evaporation during xerogel preparation since drying reduces the viability of cells immobilized within sol–gel matrices (Baca et al. 2007). Another drawback of sol-gel application is the formation of alcohols from alkosilane matrices (Meunier et al. 2010). Sol-gel materials based on alumina, titania and other compounds have similar drawbacks. To avoid cells drying during xerogel formation, several methods were applied. Fukushima et al. (1988) entrapped whole cells in alginate-silica gels with very promising results. Other authors employed biocompatible short chain phospholipids to direct the formation of an ordered silica mesophase during evaporative processes (Baca et al. 2007). To increase bacterial viability during sol-gel preparation, different authors used encapsulation of microorganisms by alginate (Pannier et al. 2011), PVA (Liu et al. 2009) with following coating by sol-gel. The addition of cryoprotectors like glycerol, quorum-sensing molecules or combination of them increased viability of bacteria in sol-gel (Meunier et al. 2010).
Different kinds of precursors have been developed to obtain the sol-gel matrices with improved properties like reduced brittle nature, high transparency, improved hydrophilicity, flexible and tuned porosity, etc. Nevertheless, the ideal matrix has not been developed. For example, inorganic sol-gels are good in transparency, but have low porosity in xerogels restricts their application. Organically modified sol–gels have good tunable porosity, but have limited optical transparency (Kandimalla et al. 2006) that limits their application for photosynthetic cells.
b. Latex Coatings
Although photosynthetic microbes were first immobilized in latex coatings nearly 20 years ago (Martens and Hall 1994), the latex polymers have been applied mainly for entrapping heterotrophic bacteria (Lyngberg et al. 2001; Flickinger et al. 2007). The entrapment of photosynthetic microorganisms into latex matrices has attracted more attention only recently with increasing demands of immobilizing H2-photoproducing cultures. The main advantage of latex polymers compared to many other materials used for cell entrapments is their ability to form mechanically stable nanoporous coatings (Flickinger et al. 2007). The coating thickness can easily be controlled within 10–250 μm (Gosse et al. 2007). Using this advantage, Gosse et al. (2007) found the optimal thickness for H2-producing phototrophic bacterium, Rhodopseudomonas palustris entrapped in latex coatings of around 50 μm (at 34 μE m−2 s−1 PAR). As expected, an increase in the thickness led to the loss in photoreactivity due to the self-shading effect. Immobilization of bacteria into the latex polymer also improved greatly their catalytic stability. The latex coatings of Rp. palustris produced H2 gas for over 4,000 h (Gosse et al. 2010). They also remained active after hydrated storage for greater than 3 months in the dark and over 1 year when stored at −80 ° C (Gosse et al. 2007).
c. Alginate Films
Latex polymerization requires drying that leads to coalescence of latex particles. Commercial latex polymer formulations may also contain biocides and other toxic additives. As a result, not all microbial cultures can survive in latex coatings. In those cases, alginate and likely other hydrogels may replace latex polymers in the thin-layer immobilization approach. Recently, Kosourov and Seibert (2009) entrapped green alga, Chlamydomonas reinhardtii within thin Ca2+-alginate films (Fig. 14.1). Since alginate is not mechanically stable polymer, authors introduced an additional polymeric screen for the mechanical support that improved the film longevity for up to 1 month. The immobilization of cells within alginate films has several advantages. The pH of a 4–6 % alginate solution is close to 7, and the polymerization process does not shift the pH inside the matrix, which is important for optimal survival of microbial cells. Furthermore, the polymerization process occurs at room temperature in the presence of added non-toxic divalent ions like Ca2+ and Ba2+. The material is cheap and can be produced in industrial quantities from a renewable resource.
The mechanical stability of alginate films can further be improved by cross-linking alginate with other polymers, such as: chitosan, polyacrylic acid, polyurethane, polyvinyl alcohol and polyvinylamine or by coating the surface of alginate films with poly-L-lysine, polyethyleneimine or glutaraldehyde.
Covalent Attachment
Immobilization based on the formation of covalent bonds is widely used for enzymes. Covalent methods in general can be divided in two groups: activation of the matrix by addition of a reactive function to the polymer and modification of the polymer backbone to produce an activated group (Brena and Batista-Viera 2006). A wide variety of reactions have developed depending on the functional groups available on the matrix. Treatments with tresyl chloride, cyanogen bromide, epoxides, epichlorohydrin, glutaraldehyde, glycidol-glyoxyl are among them (Brena and Batista-Viera 2006). Covalent coupling of whole cells as immobilization method is often applied in scanning electron microscopy. This method and methods based on cross-linking and co-cross linking with neutral molecules are not applicable for long-term immobilization of whole cells producing valuable products due to negative influence on cell surface and leakage of cells into suspension due to growth and division. However, there is a possibility to apply mild covalent binding as a first step accelerating natural immobilization (see below).
All artificial immobilization methods have advantages and drawbacks. For example, adsorption is simple, cheap and effective but creates monolayers with limited applications and frequently reversible; covalent attachment and cross-linking are effective and durable, but expensive and often affect microorganisms viability; gel entrapment and encapsulation have inherent diffusion problems. Furthermore, the existence of many different immobilization methods indicates that no universal method exists for different microorganisms and various processes.
B. Natural Immobilization
Bacteria exhibit two different behavioral strategies: a planktonic state, in which individual cells move freely in liquid medium, or a benthic state, in which they are tightly clamp. If no support exists, benthic bacteria form mats, which can be seen in different ecological niches. If bacteria attach to the surface, they form biofilms. Most of physiological, biochemical, and genetic researches have been based on the assumption that bacterial populations consist of the individual cells with identical characteristics or of the colonies originating from a single cell. Development of microscopy and molecular genetic techniques promoted the understanding that natural bacterial populations exist mostly in the attached state, in which bacteria may exchange signals and exhibit coordinated activity with creation of subpopulations for specialized functions (Smirnova et al. 2010).
Cells in biofilms have enhanced resistance to solvents and toxins as compared to suspension cells (Dagher et al. 2010; Smirnova et al. 2010). Development of mat or biofilm communities is one of the main strategies for survival of bacteria in a certain ecological niche under different stresses. Due to a higher resistance, biofilms cause chronic infections and persistent diseases, food spoilage, metal corrosion that can wear out or block water pipes, and cause other common problems associated with surfaces exposed to water.
Apart from the nature and problems associated with formation of biofilms, they have found application in biotechnology as an immobilization method. Natural immobilization is widely used in industrial applications for the treatment of wastewater, gas desulfurization, and in food production (Dagher et al. 2010). Therefore, a number of publications dealing with different aspects of biofilm formation is growing.
Biofilm Formation
Different authors divide the process of biofilm formation into different stages, but all of them agree that three main stages exist: attachment, colonization with extracellular polymer production and growth with maturation of the biofilm (Dagher et al. 2010; Smirnova et al. 2010; Cheng et al. 2010).
a. Attachment
The first stage, named attachment, is greatly affected by the surface properties (i.e. roughness, porosity, hydrophobicity and charge) and the growth rate of microorganisms being transported to the surface. The hydrodynamic conditions of the medium can also affect adherence to the surface by increasing or decreasing cell shearing. Many microorganisms react to excessive turbulence and shearing forces by inducing a global genetic response that causes a complete modification of cell surface components including flagella, fimbriae, pili, capsule, and other cell-wall polysaccharides (Dagher et al. 2010). The attachment is the reversible process and cell surface structures like flagella, adhesins, fimbria, and pili participate in irreversible adsorption together with extracellular polymers synthesis (Smirnova et al. 2010).
b. Colonization
When the cell is irreversibly attached to the surface it continues growing and dividing. As a result, microcolonies of microorganisms appear on the support. Simultaneously, depending on the microorganism and environmental conditions cells continue synthesizing exopolymeric substances like polysaccharides, proteins, carbohydrates, DNA and lipids (Dagher et al. 2010). Alternatively, the monolayer of cells is formed by irreversible attachment of moving cells along the surface (Smirnova et al. 2010). Evidently, this event depends greatly on the environmental conditions, such as: availability of substrates and different gradients near the surface, turbulence of liquid, presence of toxins etc. When conditions favor biofilm formation, both events can take place simultaneously.
At the colonization stage, the polymer matrix between the surface and cells starts forming. The bulk of the matrix consists of extracellular polysaccharides (EPS). In some cases, the matured biofilm contains 85 % EPS (Romanova et al. 2011). Nevertheless, other constituents of the polymer matrix are also very important. For example, extracellular DNA participates in polymer matrix formation and addition of DNA-ase to the biofilm destroys the polymer matrix (Romanova et al. 2011).
EPS build bridges between negatively charged cells providing them with a natural matrix. The EPS matrix consists mainly of homo- and heteropolysaccharides. The EPS contain uronic acids, mostly glucuronic and aminosugars. For some bacteria the structure of EPS is already known. For example, Pseudomonas species produce alginate, Escherichia coli synthesizes colonic acid, and Bacillus cepacia – cepacian (Smirnova et al. 2010).
During the colonization stage of biofilm formation, concentration of the cells is enough for them to start interacting each other. The bacterial cell-to-cell communications occur on chemical and physical (via pili) levels. Cells can produce and sense molecules that allow the whole population initiating a concerted action of structural and metabolic changes once a critical concentration (which depends on the population density) of the signaling molecule is reached, a phenomenon known as quorum sensing. Autoinducer 2 (AI-2) is suggested to be a universal bacterial signaling molecule, which is synthesized by the luxS product (Dagher et al. 2010). The quorum sensing is a possibility for the cells to sense a combination of cell density, mass-transfer properties of the environment and spatial cell distribution, to estimate the efficiency of producing extracellular effectors and to respond only when the response is efficient (Carnes et al. 2010).
c. Biofilm Maturation
With increasing cell density in the matrix, the distribution of cells becomes non-homogeneous. In addition, the diffusion nature of interactions between the matrix and the environment creates local gradients of substrates, products, AI-2, pH, and other growth components along the biofilm. The last process results in changes of cell morphology (Smirnova et al. 2008) and leads to the differentiation of the microbial population inside the biofilm in subpopulations with specialized functions (Dagher et al. 2010). Continuous growth of the biofilm forms its 3-dimensional structure. The thickness of biofilm varies from a few microns to centimeters depending on microbial species, biofilm age, nutrient availability and environmental liquid hydrodynamic parameters (Cheng et al. 2010).
Simultaneously with biofilm formation, the dispersal of planktonic cells and the detachment of biofilm pieces happen. When the rate of biofilm formation is equal to the rate of the cell dispersal and the detachment of biofilm pieces, the biofilm reaches a steady state.
Acceleration of Natural Immobilization
Similar to artificial immobilization, natural immobilization or biofilm formation is the process of cell entrapment in polysaccharide matrix. At the start of the operation, artificial immobilization using appropriately adapted cells realizes higher rates of the target reactions. However, artificial immobilization has the long-term stability lower than natural biofilms. This conclusion comes from the consideration that cells in natural biofilms already have morphology and metabolism adapted to the immobilized state (Smirnova et al. 2010) and surrounded by the polymers of their own metabolism. The biofilms have optimal thickness adapted to the particular environment, substrates, toxins, and product concentrations. Therefore, natural immobilization is a preferable approach for long-term operation. Unfortunately, biofilm formation is a slow process. For example, the natural biofilm of cyanobacteria in polyurethane foam cubes was formed for 2 weeks (Park et al. 1991), the biofilm of microalgae on glass tissue was formed for 2–3 weeks (Laurinavichene et al. 2006), the biofilm of purple bacteria on glass beads – 60 days (Tian et al. 2010). Therefore, acceleration of biofilm formation is required for adopting this process in practical applications.
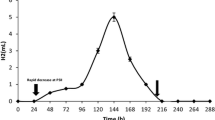
Looking at different stages of biofilm formation, it becomes evident that the attachment of the cells is the most difficult and time-consuming stage. If the attached cells are kept in the appropriate medium, the rate of biofilm formation is determined by the growth rate of microorganisms. Several ways for accelerating the attachment exist. The simplest is to use the support, which is favorable for the cell attachment. Here, different polymers with the positively charged surface are used. For example, cyanobacteria attach readily to hydrophilic polyvinyl or polyurethane foams due to non-specific interaction (Hall and Rao 1988). The next way is modification of the substrate surface. For example, glass has the same, negative, charge as microbial cells. There is a possibility to modify it by aminosilanes. For example, glass surface activated by 3-(2-aminoethylaminopropyl)-trimetoxysilane was occupied by cells of purple bacterium, Rhodobacter sphaeroides after 2 h (Tsygankov et al. 1993). In contrast, pure glass surface did not contain bacteria during the same incubation. After 3 days of continuous medium flow, the biofilm on the glass surface was formed with stable rate of hydrogen production at least for 40 days (Tsygankov et al. 1994). It was shown later that not only purple bacteria but also green algae and cyanobacteria attach readily to the activated glass surface (Tsygankov et al. 1998b). Unfortunately, this procedure is not cheap and requires incubation of bacteria in distilled water for avoiding positive charges shading by different ions. Also, other methods of glass activation were studied: treatment of the glass surface with sulfuric acid, with hydrophobic silane reagent, and with sodium hydroxide (Tekucheva et al. 2011). After 4 days of continuous medium flow, glass textile treated with sodium hydroxide contained highest quantity of bacteria as measured by the content of bacteriochlorophyll a (Fig. 14.2). Other authors also used sodium hydroxide glass treatment (Tian et al. 2010). Therefore, simple and cheap procedure for accelerating the first stage of biofilm formation on glass surfaces is available.
III. Mechanical Support and Photobioreactors for Immobilized Photosynthetic Microorganisms
Ionic adsorption, gel entrapment, and biofilms need a mechanical support. For immobilization of photosynthetic microorganisms this support should not absorb light energy. In the case of smooth surfaces, it means that the surface should be transparent. When the support has complicated surface (foam, pores, cavities for the surface enhancement) even glass is not transparent but translucent. This mechanical support for biofilm formation in some cases is called as a matrix for immobilization (Tsygankov 2004). However, it is not correct: biofilm creates its own matrix on the support. A variety of inert light penetrable supports are available for immobilization: glass, polyurethane, polyvinyl chloride, other transparent polymers in different forms and shapes as sheets (Fedorov et al. 1998), hollow fibers (Park et al. 1991; Markov et al. 1993), polymeric screens (Kosourov and Seibert 2009), textiles made of glass fibers (Laurinavichene et al. 2006). Alternatively, in the case of entrapment of bacteria in gels, there is a possibility to form beads (Sasikala et al. 1992) or cubes (Kannaiyan et al. 1994) that can be placed directly in PhBRs.
The support with immobilized cultures should be placed in the vessel, which is called a photobioreactor. For immobilized heterotrophic bacteria a variety of bioreactors with high performance exists (Cheng et al. 2010). They can be divided in two categories: fixed bed and expanded bed bioreactors. Fixed bed reactors, where immobilized bacteria are fixed on static media, can be divided into submerged beds in which the biofilm particles are completely immersed in the liquid; trickling filters in which the liquid flows downward through the biofilm bed; rotating biological contactor, in which the biofilm develops on the surface of a partially submerged surfaces; membrane biofilm reactors in which the microbial layer is formed on a porous gas-permeable membrane. Expanded bed reactors contain biofilm with continuously moving media. They are divided into fluidized beds in which particles (bacterial granules or pieces of the support with immobilized bacteria) move up and down within the expanded bed and moving beds in which the whole expanded bed circulates throughout the reactors, such as air-lift reactor and circulating bed reactors.
Unfortunately, in the present state these reactors are not applicable for photosynthetic microorganisms due to the absence of light delivery. The problem of light delivery comes from the fact of light absorption by photosynthetic microorganisms. As a result, deeper layers of photosynthetic microorganisms receive less light energy. In practice, suspensions with approximately 1 g dry biomass of photosynthetic bacteria per liter decrease light intensity by 90 % after 1 cm of optical path. Therefore, PhBRs with immobilized photosynthetic microorganisms should be constructed in such a way that incident light goes through them as short as possible. The following types of PhBRs are already described in the literature:
-
The column packed with beads or cubes of a support with immobilized microorganisms; light is delivered through transparent walls (Park et al. 1991).
-
The column packed with hollow fibers; light is delivered through transparent walls while hydrogen (the product of cyanobacterial photosynthesis) is extracted to the inner space of hollow fibers by decreased pressure (Markov et al. 1995).
-
The column packed with optical fibers; light is delivered through optical fibers and cells are immobilized around them (Matsunaga et al. 1991).
-
The column packed with optical fibers; light is delivered through optical fibers and cells are immobilized around them. Optical fibers are covered by stainless steel mesh to increase the surface for biofilm formation (Guo et al. 2011).
-
Plate type PhBRs with sheets of porous glass (Tsygankov et al. 1994) or glass textile (Laurinavichene et al. 2006) for biofilm and with partitions for directed medium flow.
Unfortunately, the geometry of the PhBR itself cannot define the efficiency of hydrogen production by immobilized microorganisms for several reasons:
-
The efficiency of light delivery to the whole set of microorganisms is defined by interplay between the thickness of illuminated layer and cell density. For PhBRs with suspension cultures, particular criteria for estimating this parameter were created (Tsygankov 2001a). The simplest one is the ratio of illuminated surface to the volume. However, it is not very accurate for scaling up (Tsygankov 2001a). In practice, nobody applied this criterion for comparison of PhBRs with immobilized cells. It remains unclear how to compare efficiencies of light delivery to PhBRs with different shapes and sizes.
-
The efficiency of substrate delivery, the product outflow, and protection of cultures against toxins and non-optimal environmental conditions depend on the PhBR geometry, mode of the process (batch or continuous), homogeneity of the liquid distribution, the rate of the process, as well as the concentration of immobilized cells. All these parameters depend on each other. Therefore, the interaction of liquid and solid phases in the PhBR should be analyzed separately for each PhBR.
-
The concentration of immobilized cells should be as high as possible, taking into account the efficient light delivery and mass exchange between the cells and the liquid phase. This parameter defines the geometry of the support that should have as high surface as possible and the volume very close to the PhBR volume, taking into account light delivery and solid-liquid mass exchange. Otherwise, the volume of the PhBR will be used inefficiently and the volumetric rate will be lower than possible.
-
As a matter of fact, different strains of microorganisms have different abilities for hydrogen production, distinct demands for the medium and the light supplement. If a wrong culture is used, the best PhBR satisfying all the other criteria above will not produce hydrogen with the highest rate.
Analyzing the above-listed considerations, one could suggest that the rate of hydrogen production by immobilized photosynthetic bacteria measured per unit of the volume is the result of very complicated interplay between the shape of the PhBR, the efficiency of its illumination, its mass transfer between solid, liquid, and gas phases, the shape of the support, concentration of cells in the support and in the PhBR, as well as the activity of cells.
IV. Hydrogen Production by Purple Bacteria
Purple non-sulfur bacteria are anoxygenic phototrophs. Due to the presence of only one photosystem they cannot use water as electron donor for photosynthesis and, therefore, do not produce oxygen. Instead of water, they utilize simple organics, reduced sulfur compounds, molecular hydrogen and some other reduced substrates.
Under combined nitrogen deficiency purple non-sulfur bacteria synthesize nitrogenase. This enzyme in reaction of nitrogen fixation produces 1 mol of H2 per 1 mol of fixed N2:
When N2 is absent, all electron flows to nitrogenase are directed to H2 synthesis:
Purple non-sulfur bacteria belong to the most active nitrogen fixers (Vignais et al. 1985). Under the light and diazotrophic conditions, they produce H2 at high rates. Coupling of nitrogen fixation to anoxygenic photosynthesis allows purple bacteria producing H2 gas at high rates that are accelerated in the absence (or deficiency) of nitrogen. Simultaneously, they consume simple organics. These physiologic peculiarities allow researchers considering purple bacteria as biocatalysts in systems of wastewater treatment with simultaneous H2 production. Extensive studies on mechanisms (Vignais et al. 1985) and major factors influencing the process (for review see Akkerman et al. 2002), as well as optimization of hydrogen photoproduction rates (Tsygankov et al. 1998a) were done using suspension cultures.
It was proved that immobilization of microorganisms is an efficient way of increasing the volumetric hydrogen production rates (Brodelius and Vandamme 1987). The first attempts of entrapping the purple bacteria were done more than 30 years ago (Vincenzini et al. 1981, 1982a, b, 1986; Zurrer and Bachofen 1985; Tsygankov et al. 1998a; Zhu et al. 1999a). These publications demonstrate that immobilized purple bacteria can be packed at significantly higher concentrations than suspension cultures. As a result, immobilized systems produce H2 gas with higher volumetric rates than suspensions (Tsygankov 2001b). They also showed more stable H2 production than suspensions and the time of operation close to 1,000 h (Tsygankov et al. 1994, 1998a; Tsygankov 2004).
At present, the list of available supports and matrices for immobilization of purple bacteria includes: gels like agar, agarose, carrageenan, alginate (Planchard et al. 1984; Fissler et al. 1995), PVA (Tian et al. 2009), agar with chitosan (Zhu et al. 1999b), PVA with carrageenan and alginate (Wang et al. 2010), clay (Chen and Chang 2006), latex (Gosse et al. 2007). The range of supports for biofilms of purple bacteria expands from porous glass and smooth glass surfaces (Tsygankov et al. 1993) to polyurethane foam (Fedorov et al. 1998), glass textile (Tekucheva et al. 2011), glass beads (Tian et al. 2010), plastic optical fibers with additional mesh support around them (Guo et al. 2011). As reported in the papers, all matrices and supports showed good characteristics. From a wide range of different methods used in immobilization of purple bacteria, it seems that the choice of the matrix or support is rather a preference of the authors than a necessity.
As a matter of fact, no any significant advances in the volumetric rate of hydrogen production by immobilized purple bacteria have been reported in the last two decades. In most cases, it is impossible to explain this phenomenon. In some cases, however, after a thorough analysis of published data one can conclude that authors did not take into account one or several interplaying parameters that influence the final process.
V. Hydrogen Production by Immobilized Microalgae
Despite a quite extensive list of different techniques and approaches described for immobilization of microalgae, they were mainly devised for water purification and production of some valuable metabolites, but not for H2 photoproduction (Mallick 2002, 2006). As an example, the different species of Chlorella, Scenedesmus, Chlamydomonas and Dunaliella immobilized in alginate or carrageenan beads and screens, in polyvinyl or polyurethane foams and on hollow cellulose fibers were successfully applied for reduction of nitrogen and phosphorus contents in farm and industrial wastewater effluents (Travieso et al. 1992, 1996; Kaya and Picard 1995; Robinson 1998; Jimenez-Perez et al. 2004; Shi et al. 2007). In some cases, the immobilized cells removed up to 95 % of inorganic nitrogen and up to 99 % of phosphates (Lau et al. 1998). The immobilized microalgae were also applied for biosorption of heavy metals (Garnham et al. 1992; Moreno-Garrido et al. 2005) and biodegradation of industrial pollutants, including biocides, hydrocarbons and surfactants (Zhang et al. 1998; Semple et al. 1999). As biocatalysts, they showed promise in de novo biosynthesis of glycerol (Leon and Galvan 1995), hydrogen peroxide (Scholz et al. 1995) and (R)-1,2-propanediol (Hatanaka et al. 1999). More details on the environmental and industrial applications of immobilized microalgae can be found in recently published reviews (Moreno-Garrido 2008; de-Bashan and Bashan 2010).
The use of immobilized microalgae for H2 photoproduction was limited until recently by an extremely low yield of H2 gas in algal cultures. As a result, the early research efforts were focused mainly on investigating the mechanisms of H2 evolution in suspensions. A few dozen algal species were tested for their ability to photoproduce H2 gas after the period of dark anaerobic adaptation (Boichenko and Hoffmann, 1994). It was discovered that some of them, but not all, are capable of direct water biophotolysis, a biochemical reaction resulting in simultaneous accumulation of molecular hydrogen and oxygen in the same volume:
The first reaction is typical to all oxygenic phototrophs, including plants and cyanobacteria. This process results in the release of oxygen in photosystem II (PSII) and simultaneous production of NADPH and ATP that are further utilized mainly in the CO2 fixation pathway. The reaction (14.4) is possible only under anaerobic conditions. The role of this process in the physiology of green algae is still a matter of debate. Most probably, it serves as a regulatory valve preventing overreduction of photosynthetic apparatus in algae during their transition from dark anaerobic to light aerobic conditions (Appel and Schulz 1998; Boichenko et al. 2004). The reaction is driven by a special enzyme, [FeFe]-hydrogenase, that is extremely sensitive to O2 (Ghirardi et al. 1997). The reaction proceeds at high initial rates and high light to hydrogen conversion efficiencies that raise a question about industrial applicability of the process in future (Boichenko et al. 2004). At the current point, however, water biophotolysis cannot be sustained due to a rapid (within seconds) inactivation of [FeFe]-hydrogenase by O2 co-evolved in photosynthesis (Ghirardi et al. 1997).
The immobilization of green algae for long-term H2 photoproduction has become possible after the discovery of partial inactivation of O2–evolving activity in algal cells in the absence of some essential nutrients, mainly sulfur and phosphorus (Wykoff et al. 1998). Taking into account the experimental data obtained by Wykoff and co-authors, the collaborative group of researchers from UC Berkeley and National Renewable Energy Laboratory successfully applied a sulfur deprivation procedure to sustain H2 production in C. reinhardtii cultures (Melis et al. 2000). In these experiments, the long-term H2 photoproduction was possible due to a metabolic switch occurring in sulfur-deprived algal cells that separated temporarily the O2-evolving (the reaction 14.3 above) and H2-producting (the reaction 14.4) stages in the same culture (Ghirardi et al. 2000). The later studies showed that the same principle works for phosphorus- (Batyrova et al. 2012) and nitrogen-depleted (Philipps et al. 2012) microalgae. Although the overall efficiency of H2 evolution in nutrient-deprived cultures was shown far below the capacity of direct biophotolysis (Melis 2007; Ghirardi et al. 2000), this approach allowed sustaining the process for several days (Melis et al. 2000; Kosourov et al. 2002). As a result, the nutrient-deprivation protocol has become a platform for testing the performance of a variety of algal mutants, growth conditions and other engineering factors, including different immobilization techniques.
The original sulfur deprivation procedure requires repetitive washing of cells in sulfur-free medium by centrifugation (Melis et al. 2000). Furthermore, H2 photoproduction in the batch cultures can be repeated several times by rejuvenating the cells in sulfur-containing medium (Ghirardi et al. 2000). The fact that algae are suspended in a liquid phase makes it difficult to cycle the batch system between rounds of sulfur deprivation and sulfur re-addition without large energy input for the required centrifugation steps. In attempting to overcome this barrier and increase the overall duration of the process, two independent research groups immobilized sulfur-deprived C. reinhardtii cultures using different solid supports (Laurinavichene et al. 2006, 2008; Hahn et al. 2007).
Laurinavichene et al. (2006) immobilized a wild-type C. reinhardtii strain, 137C mt + and a non-motile mutant, CC-1036 pf18 mt + on glass fiber matrices having a linen-like structure. The alga with paralyzed flagella was supposed to have a better attachment to the glass surface. Both strains, however, exhibited similar immobilization properties. Different glass fiber matrices with different water absorption properties were tested and the best (TR-03) was selected for further work. All of these matrices are available in the industrial scale at very low cost. The authors used two methods for attachment. In the quick immobilization procedure, glass was activated by 3-(2-aminoethyl-aminopropyl)-trimethoxysilane (Tsygankov et al. 1994) and matrices were placed in cell suspensions for 2.5 h. This approach resulted in the glass matrices having below 60 mg total Chl per m2. In the second procedure, algae were allowed to colonize glass surfaces in a natural way. The matrices were incubated with cells for about 2 weeks during the growth phase on a regular medium. This approach produced matrices with significantly higher cell densities (about 570 mg total Chl per m2). Independently of the technique used, immobilization of algal cells on glass fiber matrices significantly increases the duration of H2 photoproduction in sulfur-deprived algae (up to 4 weeks) with the specific rate similar to suspension cultures. Both approaches use the property of microalga to form biofilm. However, in the first one the biofilm formation was accelerated (see above). In the best case, the immobilized cells produced H2 gas with the rate of about 6.5 μmol H2 (mg Chl h)−1. In comparison, sulfur-deprived suspension cultures produce H2 with a maximum specific rate usually ranging from ~4 to ~6 μmol H2 (mg Chl h)−1 (Kosourov et al. 2002), although under the most favorable conditions, rates as high as 9.5 μmol H2 (mg Chl h)−1 have been observed (Kosourov et al. 2003). The average rate in algal cultures immobilized on glass fiber matrices on a per volume basis was around 4 ml H2 (LPhBR h)−1 and the maximum was 9.2 ml H2 (LPhBR h)−1 (Laurinavichene at al. 2006). The following studies with either a constant flow of the medium containing micromolar sulfate concentrations or cycling immobilized cells between minus and plus sulfate conditions improved the duration of H2 production up to at least 3 months (Laurinavichene at al. 2008). Nevertheless, due to irregular colonization of glass fibers by the algal cells, the system showed significant physical and physiological heterogeneities in different parts of the matrix, resulting in irregular light and nutrient distributions. The authors found that algae had a very high photochemical activity in some parts of the matrix and evolved oxygen instead of hydrogen. Produced O2 inhibited H2 photoproduction activities in other algae. As a result, the efficient H2 production in this system required continuous argon flow for mixing and gas removal.
Hahn et al. (2007) attached C. reinhardtii cells to the fumed silica particles. The study was based on the principle that the fixed cells can be cycled between sulfate reach and sulfate free environments via filtration, thus, eliminating the expensive centrifugation steps. Similar to Laurinavichene et al. approach, the immobilization was done through a natural colonization of algal cells on the silica particles during the growth phase. In the initial experiment, the authors also tried glass beads as a support without success. The study showed that the algae bound to the fumed silica particles produce H2 gas at a very similar rate to free-floating algae. It is important to note here that these experiments were also done with a suspension of algae/silica particles that should not give any significant advantage in the light utilization as compared to free-floating algae. Unfortunately, the authors did not provide any information about the rates of H2 production allowing the precise estimation of H2 photoproduction efficiency. However, based on the total H2 photoproduction yields anyone can conclude that the suspended silica particles carrying microalgae utilize light not better than the free-floating cells.
Although very cheap, natural immobilization techniques resulted only in a very slight improvement in the light to hydrogen conversion efficiency in the nutrient-deprived microalgae as compared to the suspension cultures (Ghirardi 2006), but led to a significant prolongation of H2 photoproduction period (Laurinavichene at al. 2008). Trying to improve the light absorption properties of immobilized microalgae, Kosourov and Seibert (2009) entrapped C. reinhardtii cells within thin alginate films. The technique was based on the idea of thin layer cell immobilization into the thin nanoporous latex coatings (Flickinger et al. 2007; Lyngberg et al. 2001). The entrapment of phototrophic cells into the polymer allows a very precise control of the cell density inside the coatings and their thicknesses that, in the ideal case, must provide the immobilized cells with the best environment for light distribution. Indeed, Gosse and co-authors (2007) observed an improvement in the rate of H2 photoproduction in phototrophic bacterium, Rp. palustris, entrapped within thin nanoporous latex coatings as compared to suspension cultures. Unfortunately, viability of green algae could not be maintained during the drying process inherent to regular latex film formation (JL Gosse, S Kosourov, M Seibert and MC Flickinger, unpublished, 2013). Therefore, Kosourov and Seibert (2009) used alginate for entrapment of H2-producing C. reinhardtii cells. The switch to alginate significantly improved the cell viability and reactivity of the coatings, but decreased their mechanical stability, as expected. The mechanical stability of the system was improved by introducing a special template consisting of a polymer insect screen placed over the sticky side of a wide adhesive tape. In this configuration, the alginate films with entrapped algae survived for up to 1 month. They also demonstrated high cell densities (up to 2,000 μg Chl per mL of the matrix) resulting in the specific rate of H2 evolution up to 12.5 μmol H2 (mg Chl h)−1, which is almost three-times higher than in suspensions (~4 to ~6 μmol H2 (mg Chl h)−1; Kosourov et al. 2002), but the rates in suspensions were obtained at significantly higher light intensities (200 μE m−2 s−1 PAR in suspensions vs. 62 μE m−2 s−1 PAR in alginate films). As a result, the conversion efficiency of incident light energy into energy of the H2 gas in alginate films at 62 μE m−2 s−1 PAR was above 1.5 % for the period of the maximum H2-production rate and was close to 1 % for the whole period of nutrient deprivation. These values are significantly higher than the values reported for immobilized on glass fibers (0.36 %) and suspension (0.24 %) C. reinhardtii cultures, but calculated at much higher light intensities: 120 and 200 μE m−2 s−1 PAR, respectively (Ghirardi 2006). The above efficiencies, however, are given only for comparison reasons and could not be used for the estimation of the real light to H2 conversion efficiency in microalgal cultures since in all cases they were calculated in the presence of acetate in the medium.
As discussed above, entrapment of microbial cells into the alginate polymer protects them against adverse environmental conditions. Following this common property of gel entrapments, microalgae immobilized within alginate films showed a high resistance of their H2-photoproducing system to inactivation by atmospheric oxygen. In well-mixed suspensions even trace amounts of O2 in the PhBR headspace inhibit H2 photoproduction (Ghirardi et al. 2000). In contrast, the algal cells entrapped in alginate films and placed in vials containing 21 % O2 in the headspace evolved up to 67 % of the H2 gas produced under anaerobic conditions (Kosourov and Seibert 2009). The lower susceptibility of the immobilized algal H2-producing system to inactivation by O2 was caused by the higher rate of respiration in the dense film and the capability of the alginate polymer itself to effectively separate the entrapped cells from O2 in the liquid and headspace and restrict O2 diffusion into the matrix. The alginate polymer, however, slows down diffusion of O2 not only from the atmosphere to the film, but also from the film to the atmosphere. Under high light conditions, this affects immobilized algae oppositely preventing the efficient release of O2 originated in PSII from the cells and decreasing the overall H2-photoproduction performance of alginate films (Kosourov et al. 2011). In the paper mentioned above, the authors partly solved the problem by immobilizing the C. reinhardtii mutants with truncated light-harvesting antennae. Although the CC-4169 strain affected in the TLA1 gene also experienced photoinhibition under high light, it produced significantly more H2 gas than the parental CC-425 strain. Unfortunately, CC-425 itself showed very low specific rates of H2 photoproduction under all light intensities tested, thus, making difficult any comparison of the daughter CC-4169 strain with better H2-producers. Nevertheless, this work was the first report on the increase performance of H2 photoproduction under high light in the alga with truncated light-harvesting antenna complexes.
The low mechanical stability of the alginate polymer, especially in the presence of such chelating agents as phosphates that are very important for the cell metabolism, continued the efforts of the researchers to find a more stable and cheap material for microalgae immobilization. The most interesting advance in this direction was done recently with immobilization of cells into the latex polymer (Gosse et al. 2012). The authors described a latex wet coalescence method for gas-phase immobilization of microorganisms on a filter paper, which does not require drying for adhesion. In this approach, the mixture of algal cells or other microorganisms in latex is dropped on the top of the filter paper strip. Then, the paper strip is partly submerged into the medium allowing the coated cells staying in the vial headspace. Since the paper is wet throughout the experiment, the full latex polymerization does not occur, but latex does allow binding of the cells to the paper surface. This method is applicable for microorganisms that do not tolerate desiccation stress during latex drying. Interestingly, in contrast to a regular latex immobilization technique, wet C. reinhardtii coatings retain cell reactivity throughout the experiment (~250 h) as measured by oxygen gas evolution or CO2 consumption (Gosse et al. 2012). More interestingly, C. reinhardtii cells showed even higher CO2 consumption and O2 evolution rates than the cyanobacterium, Synechococcus sp. PCC7002 placed under the same conditions. As an example, the Chlamydomonas and Synechococcus coatings consumed CO2 at the rate of about 3.9 and 3.6 mmol CO2 m−2 h−1 and produced O2 at the rate of about 10.2 and 5.0 mmol O2 m−2 h−1, respectively. The coatings also tolerated 20 % CO2 in the PhBR headspace. These observations indicate that the latex polymer itself is not toxic to the cells and that the viability of algal cells in the latex coatings depends drastically on the presence of water. The reactivity of wet coatings can further be improved by increasing the coating surface area and cell density in the latex emulsion. Another advantage of wet cell binding to the filter paper is that the technique significantly increases the transfer rates for the gases allowing faster consumption of CO2 by the cells and faster release of O2 and H2 gases from the cells to the PhBR headspace. Since H2 photoproduction in microalgae decreases significantly with increasing the H2 partial pressure (Kosourov et al. 2012), the faster release of H2 gas from the coatings may further improve the rates and yields of H2 photoproduction. Taking into account the above findings, the latex wet coalescence method should be considered as a suitable approach for generation of H2 gas by immobilized microalgae, especially under autotrophic conditions.
The vast majority of experiments on H2 production by nutrient-deprived microalgae have been done so far with C. reinhardtii cultures. However, other species of green algae also produce H2 gas under this condition (Winkler et al. 2002; Skjanes et al. 2008; Meuser et al. 2009). Some of these strains show very efficient H2 photoproduction in an immobilized state. For example, Song et al. (2011) immobilized Chlorella sp. into the square (0.5 × 0.5 × 0.5 cm) pieces of agar. Although the system was not perfect in terms of light and nutrients distribution as compared to the thin-layer immobilization approach, sulfur-deprived Chlorella cells produced H2 gas with the maximum rate above 23 ml H2 h−1 per LPhBR. This rate, however, was observed for a short period of time (less than 10 h) in the presence of 30 mM glucose. Nevertheless, under these conditions immobilized Chlorella cultures yielded around 500 mL H2 gas per LPhBR for less than 40 h. Under photoautotrophic conditions, when only CO2 was used as a carbon source, the alga yielded up to 160 ml H2 h−1 per LPhBR that corresponds to the average rate of ~3 ml H2 h−1 per LPhBR. The system also demonstrated the multiple cycles of H2 photoproduction after periodic restorations of algal cultures in the full (sulfur-containing) medium both under photoheterotrophic (+glucose) and photoautotrophic (+CO2) conditions. In the presence of glucose, authors observed up to ten cycles of H2 production in immobilized Chlorella cultures with the average yield of 460–480 mL H2 per LPhBR for the each cycle. Information on other species of microalgae capable of H2 photoproduction in immobilized state is very limited. There are data that the marine green alga, Platymonas subcordiformis produces H2 gas when entrapped in alginate beads (Guan et al. 2003). Similar to C. reinhardtii, algae released H2 gas in the two-stage process. However, the efficient H2 photoproduction in Platymonas was only observed in the presence of carbonyl cyanide m-chlorophenylhydrazone (CCCP), an uncoupler of photophosphorylation (Guan et al. 2004).
From all the above-mentioned examples it becomes obvious that the immobilization approach can bring significant advances to H2 gas photoproduction in microalgae. It not only improves the volumetric rate of hydrogen production, but also allows their easy cycling between nutrient-replete and nutrient deprived stages. In addition, immobilization simplifies a continuous flow of liquid media through the PhBR. As a result, microalgae produce H2 gas more efficiently and for longer periods of time as compared to the suspensions. Nevertheless, at the current state the rates of H2 photoproduction in immobilized algal cells are still not high enough for the industrial H2 production systems to be viable. Here, the most important barrier is a high sensitivity of the process to molecular oxygen, either atmospheric or co-evolved in photosynthesis. Although entrapments of algal cells into the alginate polymer showed us how to defend the H2-producing system from inactivation by atmospheric O2, immobilization itself cannot protect the hydrogenase enzymes from O2 originated in photosynthesis. The situation is more dramatic in autotrophic cells, where O2 respiration depends solely on the level of stored carbohydrates (Tsygankov et al. 2006). In this case, the PhBR with immobilized algae should be constructed in the way allowing a fast release of O2 from the cells to the atmosphere, but, if possible, preventing its back diffusion to the cells. The next issue, which is waiting for technological solution, is the direct dependence of H2 photoproduction in microalgae on the H2 partial pressure in the gas phase above the culture (Kosourov et al. 2012). Inhibition of H2 photoproduction in algal cells by increasing levels of H2 gas can be eliminated in the hybrid system (Dante 2005), where H2 production is tightly coupled to its consumption in a fuel cell for electricity generation.
VI. Hydrogen Production by Immobilized Cyanobacteria
Cyanobacteria, phototrophic O2-evolving prokaryotes, are other good candidates for generation of H2 gas in the artificial systems. These organisms perform oxygenic photosynthesis using the same pathway as green algae and high plants, which includes four major multiprotein complexes: PSII, cytochrome b6f, PSI and ATP synthase. Similar to green algae, they split water in the light and store energy in the form of NADPH and ATP that are consumed in CO2 fixation via the Calvin-Benson-Bassham cycle and used in other energy-dependent metabolic pathways. Cyanobacteria display a relatively wide range of morphological diversity, including unicellular, filamentous, and colonial forms (Tamagnini et al. 2007). Some filamentous strains form differentiated cells called heterocysts that are specialized in N2 fixation.
The vast majority of cyanobacterial strains are capable to indirect water biophotolysis resulting in the production of H2 gas either in the light or in the dark. The light-dependent process of H2 production is usually linked to N2 fixation and is catalyzed by the nitrogenase enzyme (see Eq. 14.1 above). The release of 1 mol of H2 per 1 mol of fixed N2 occurs only under optimal conditions (Tsygankov 2007). If the amount of nitrogen is insufficient, hydrogen is released at higher rates. Under the condition of complete absence of nitrogen, nitrogenases catalyze solely the reduction of protons to H2, thus decreasing the ATP requirement of the process from 16 to 4 mol per mole of H2 produced (see Eq. 14.2). Although H2 photoproduction via nitrogenases is rather efficient process, the wild-type strains do not accumulate H2 gas in the cultures under normal conditions due to the presence of uptake (hup-encoded) hydrogenases in the cells that recycle the produced H2 gas (Tamagnini et al. 2002). In some non-N2-fixing cyanobacteria, molecular hydrogen is produced in the light by the [NiFe]-bidirectional (hox-encoded) hydrogenase in a manner similar to H2 production in green algae (see the reactions 14.3 and 14.4 above). However, in contrast to the green algal [Fe-Fe]-hydrogenases, the hox-encoded hydrogenases are NADH/NADPH-dependent enzymes (Vignais and Billoud 2007; Carrieri et al. 2011). Hydrogen photoproduction via [NiFe]-bidirectional hydrogenases in cyanobacteria is a transient phenomenon usually lasting less than 30 s in the light and is followed by H2 uptake (Appel et al. 2000; Cournac et al. 2004). It serves as an electron valve for the disposal of low-potential electrons generated at the onset of illumination, thus preventing over-reduction of the photosynthetic electron-transport chains or for additional electron donation to the electron-transport chains in the presence of H2 (Appel and Schulz 1998; Appel et al. 2000). In addition to the light-dependent H2 evolution, some cyanobacteria are capable of releasing H2 gas in the dark via the hox-encoded hydrogenases (Tsygankov 2007). This process regenerates NADP+ from NADPH during fermentation allowing catabolism of endogenous carbohydrates to proceed (Carrieri et al. 2011). Some species, like Gloeocapsa alpicola and Arthrospira maxima, evolve significant amounts of H2 gas via the hox-encoded hydrogenases, especially under the stress conditions (Troshina et al. 2002; Ananyev et al. 2008).
Due to a significant diversity of H2 metabolism in cyanobacteria and extensive literature on the topic, in the current review we will consider only the most interesting and promising approaches used for immobilization of H2-producing cyanobacteria. Starting from the natural colonization techniques, we should first mention a series of works on immobilization of the N2-fixing heterocystous cyanobacterium Anabaena variabilis on hollow fibers (Markov et al. 1993, 1995). Entrapment of algal cells on hollow fibers has a numerous different advantages. The material is relatively cheap and non-toxic to the cells. It also has a very large surface-to-volume ratio, which allows the design of compact systems. Markov et al. (1995) tested the degree of cell immobilization on the different hollow fibers and found the better attachment of Anabaena cells to hydrophilic cellulosic over than hydrophobic polysulphone fibers. Without CO2 re-additions, the immobilized cells produced H2 gas at rates of 0.02–0.2 ml H2 h−1 per mg dry weight for up to 5 months (Markov et al. 1993). However, the maximum rate was observed only in the first 3 days. The following decrease in the rate of H2 photoproduction to a low steady-state level was caused by the lack of CO2 in the system. Re-additions of CO2 to the PhBR not only solved the problem, but also improved the rate up to 20 ml H2 h−1 per mg dry weight (Markov et al. 1995). In this system, however, Anabaena cells did not produce H2 gas during the photosynthetic, CO2-consuming phase that usually lasts for few days. Such a two-phase system was maintained continuously for over 1 year. The importance of CO2 for hydrogen photoproduction in N2-fixing cyanobacteria emphasizes the role of photosynthetic apparatus of vegetative cells in satisfying the high-energy demand of nitrogenase-driven processes in heterocysts. The long-term experiments with Anabaena cells entrapped onto hollow fibers also demonstrated the importance of periodic re-additions of nitrogen to the system since it is required to support cell metabolism.
A variety of cyanobacteria show a high natural adhesion to glass. This property is used for the attachment of cyanobacterial cells to different glass substrates. When attached, cultures start growing and making a very thin biofilm on the surface of the glass. Using the high adhesion property of the cells, Serebryakova and Tsygankov (2007) successfully entrapped unicellular Gloeocapsa alpicola CALU 743 on the glass fiber matrices. When grown on a regular BG11 medium, cells uniformly covered the surface of glass within 7–8 days. The total density of the culture achieved ~765 mg Chl a per m2 matrix that is significantly higher than in immobilized C. reinhardtii cultures (570 mg total Chl per m2 of the same matrix). Gloeocapsa alpicola cells produce H2 gas via the hox-encoded hydrogenase in the dark during fermentation of endogenous glycogen (Serebryakova et al. 1998). Nitrate limitation significantly improves H2 production rates since under this condition cells accumulate glycogen up to 50 % of dry weight (Troshina et al. 2002). For efficient H2 production, Serebryakova and Tsygankov (2007) applied a two-stage principle allowing effectively accumulate glycogen under nitrate starvation during the photosynthetic stage and utilize it for H2 during the dark period. Up to ten cycles of H2 production were demonstrated by the authors. The experiments were done under continuous flow of the medium (15 mL h−1) with periodic (during the dark period) purging of the bioreactor by argon (400–800 mL h−1). Increase in the argon flow rate improved the rate of H2 production since the hox-encoded hyrogenase catalyzes the reversible process, which depends significantly on the H2 partial pressure. The argon and medium flows also removed CO2 and acetate from the system, thus preventing their negative influence on the process. Under the best conditions, Gloeocapsa alpicola cells immobilized on glass fiber matrices produced H2 gas at the average rate of about 20 mL H2 h−1 L−1 matrix, which is significantly higher than the rates observed in suspensions (6 mL H2 h−1 L−1 with argon flow rate of 2 L h−1).
The entrapment of cyanobacterial cells into the polymeric materials in the form of beads or films also significantly improves the volumetric rates of H2 production. Here, the N2-fixing cyanobacteria are good candidates for immobilization since the entrapment of the cells into the polymer matrix provides additional protection of their nitrogenase enzyme from inactivation by atmospheric O2. A study of H2 photoproduction by the non-heterocystous filamentous marine cyanobacterium, Oscillatoria sp. immobilized in rectangular (1 × 1 × 0.2 cm) pieces of 1.5 % agar showed that the rate and longevity of H2 evolution increased significantly compared to the free cell suspensions (Phlips and Mitsui 1986). The authors observed the rates above 13 μL H2 g−1 dry weight h−1. Immobilization sustained the process for at least 3 weeks and allowed to drive the process under outdoor light conditions. Interestingly, in contrast to other N2-fixing cyanobacteria, Oscillatoria sp. Miami BG7 has a negligible H2 uptake activity (Kumazawa and Mitsui 1985) that prevents recycling of H2 gas accumulated in cells and in the agar matrix. As a result, cells photoproduce H2 more efficiently. The enhanced H2 photoproduction, as compared to the suspension cultures, was also observed in another non-heterocystous filamentous cyanobacterium, Plectonema boryanum immobilized in alginate beads (Sarkar et al. 1992). H2 production in this strain was accompanied by the release of ammonia in the medium. Another interesting observation was that the additions of 24 % N2 into the gas phase containing 72 % Ar and 4 % CO2 stimulated the H2 photoproduction rate twofold as compared to the PhBRs containing only argon and CO2 in the headspace. Usually, the presence of N2 inhibits H2 evolution driven by the nitrogenase system (see the reactions 14.1 and 14.2 above). Although the authors did not explain this effect, one could suggest that stimulation was caused by the decreased diffusion of N2 through the alginate matrix, limiting the steady-state level of N2 inside the cells. Nitrogen at low concentrations, however, supports the cell metabolism and may prolong H2 photoproduction period. Indeed, the authors observed such prolongation. In the presence of N2, immobilized cultures produced H2 gas for up to 12 days (7 days in Ar/CO2 atm).
The cyanobacteria capable of H2 release in the dark via a two-stage process were entrapped into the gels as well. Unicellular non-N2-fixing cyanobacterium, Microcystis aeruginosa is good example (Rashid et al. 2009, 2012). Similar to Gloeocapsa alpicola, this organism accumulates considerable amounts of glycogen during the photosynthetic stage. Subsequent fermentation in the dark produces H2 gas. In contrast to experiments with G. alpicola, where nitrate starvation was used, Rashid et al. (2009) applied sulfur deprivation to the cultures entrapped into 1.5 % agar pieces during the H2 production stage. Since the rates of H2 evolution were not very significant (1.5–2 mL H2 h−1 L−1), the authors introduced glucose to the medium. The externally added glucose improved the maximum H2 production rate in the immobilized cultures up to 17 mL H2 h−1 L−1 (34 mL H2 h−1 L−1 matrix). Under these conditions, the average rate was about 13 mL H2 h−1 L−1. The system also demonstrated the multiple cycles of H2 production (Rashid et al. 2009, 2012).
The entrapment of cells in thin films instead of beads or rectangular pieces leads to a more efficient light utilization. Following this idea, Leino et al. (2012) immobilized cells of two N2-fixing heterocystous cyanobacteria, Calothrix 336/3 and Anabaena PCC 7120 (wild-type and ΔhupL strains) within thin alginate films. In all cases, efficient H2 photoproduction was observed in vials containing 6 % CO2 in argon. Re-additions of CO2 gas into vials were important for restoration of the H2 photoproduction activity in cells and allowed several cycles of H2 photoproduction to occur. Without CO2 supplementations, the immobilized cultures of Calothrix and Anabaena stopped H2 photoproduction after the first cycle. Cyanobacteria entrapped in alginate films release H2 gas simultaneously with O2 evolution. Simultaneous production of molecular H2 and O2 is typical to all heterocystous cyanobacteria and observed in suspensions as well (Tsygankov et al. 2002). As expected, the wild-type Anabaena strain having the uptake hydrogenase produced significantly less H2 gas than the ΔhupL since alginate slows down the release of H2 from the film to atmosphere, thus allowing re-cyclization of produced H2 gas via the uptake hydrogenase, especially at high O2 concentrations. On the contrary, Calothrix 336/3, which also possesses hupSL genes, in some cases demonstrated even higher the H2 photoproduction activity than the ΔhupL mutant without uptake hydrogenase. Most probably, Calothrix cells do not have the real H2-uptaking activity despite the presence of hupSL genes. Immobilization in alginate films had a positive effect on cell viability. All three strains, the Calothrix 336/3, wild-type Anabaena and ΔhupL cells, were viable for over 10 months in the initial nutrient media without additions of CO2. Interestingly, the alginate films with entrapped cyanobacteria were more mechanically stable than the films with entrapped green algae. The maximum specific rates of H2 photoproduction were 35, 9 and 30 μmol H2 (mg Chl a h)−1 for Calothrix 336/3, wild-type Anabaena and ΔhupL cultures, respectively. These rates are significantly higher than the rates observed in C. reinhardtii cultures immobilized approximately under the same conditions.
Another interesting technique developed for immobilization of H2-producing cyanobacteria is the entrapment of cells within sol-gel silica matrices. This approach has never been reported successful for immobilization of H2-producing green algae. However, some non-H2-producing microalgae, like Haematococcus pluvialis survived through the immobilization procedure (Fiedler et al. 2007). Recently, Dickson et al. (2009) immobilized the cyanobacterium, Synechocystis sp. PCC6803 and its mutant, M55 deficient in the NDH-1 complex into silica matrices using tetraethoxysilane (TEOS), tetramethoxysilane (TMOS) and methyltriethoxysilane (MTES) as precursors in the sol-gel reaction. Glycerol and polyethylene glycol (PEG 400) were used as additives helping to increase porosity of the matrix and reduce osmotic stress on the encapsulated cells. H2 photoproduction driven by the hox-encoded hydrogenase was measured under the light/dark exposure (2 min on/18 min off) for up to 5 days. The results showed that H2 photoproduction rates from encapsulated cells in most cases were comparable to the rates in suspensions, confirming the activity of encapsulated cells. Although the process is currently far from optimized, it provides a proof of the concept demonstrating the ability to achieve measurable amounts of H2 gas from sol-gel encapsulated cells.
Despite extensive investigations over the last few decades, a real potential of cyanobacterial species to produce H2 gas has not been fully explored. If compared to other phototrophs, these organisms have several significant advantages. The first and the most important is that they are capable of producing H2 gas under truly autotrophic conditions. Although green algae can do the same, at the current state efficient H2 evolution in their cultures requires the presence of acetate or glucose in the medium. As a rule, H2 photoproduction in N2-fixing cyanobacteria is very stable to O2 inactivation due to a range of different protection mechanisms. Some cyanobacteria can produce H2 gas in the presence of atmospheric levels of O2 and above. In addition, cyanobacteria can easily adapt to periodic light environment and drive H2 production under solar light intensities. This makes the process possible under outdoor conditions. These advantages emphasize the importance of future research in this direction, including application of different immobilization techniques.
VII. Concluding Remarks
Although many photosynthetic microorganisms are capable of producing H2 gas utilizing either water or organic substrates, so far, there are no any commercial applications of the process. One of the major bottlenecks for a large-scale H2 generation using phototrophs is the low volumetric rate of H2 photoproduction for all known pathways. From a practical point of view, however, the application of photosynthetic microorganisms is a great advantage since their cultures require only solar energy and relatively small amounts of other inputs for growth and operation. Presumably everyone will agree that a significant improvement in the rate of H2 gas generation will be possible only after understanding all barriers limiting H2 photoproduction in the particular group of photosynthetic microorganisms and generating mutants capable of overcoming these barriers. Nevertheless, there is still a space for technological improvements.
For example, the problem of inhomogeneous light distribution in the suspension culture with constantly increasing biomass can be partly solved by immobilizing the cells. As discussed above, thin-layer cell immobilization has already improved the light to H2 conversion efficiency above 1 % in green algal cultures and slightly increased the specific rates of H2 photoproduction both in green algae and purple bacteria. A combination of thin-layer immobilization with appropriate photobioreactor geometry significantly increased volumetric rate of hydrogen production, as was shown for purple bacteria (Tsygankov et al. 1994). The light to H2 conversion efficiency can further be improved by co-immobilizing different mutants (for example, the wild-type strain and mutants with truncated light-harvesting antennae) or different organisms (for example, purple bacteria and green algae). In addition, cell immobilization significantly simplifies the culture maintenance in the PhBR and, thus, decreases the overall cost of the system operation. Nevertheless, the material for entrapping phototrophic cultures should be non-toxic to the cells, transparent, stable, available in industrial quantities, made from a renewable source and cheap. At this point, there are no such materials that satisfy all requirements. Therefore, the future efforts should also be concentrated on the screening of new materials and substrates for immobilization of photosynthetic microorganisms.
Abbreviations
- Chl –:
-
Chlorophyll;
- EPS –:
-
Extracellular polysaccharides;
- Fd –:
-
Ferredoxin;
- PAR –:
-
Photosynthetic active radiation;
- PhBR(s) –:
-
Photobioreactor(s);
- PSI –:
-
Photosystem I;
- PSII –:
-
Photosystem II;
- PVA –:
-
Polyvinyl alcohol
References
Akkerman I, Janssen M, Rocha J, Wijffels RH (2002) Photobiological hydrogen production: photochemical efficiency and bioreactor design. Int J Hydrog Energy 27:1195–1208
Ananyev G, Carrieri D, Dismukes GC (2008) Optimization of metabolic capacity and flux through environmental cues to maximize hydrogen production by the cyanobacterium Arthrospira (Spirulina) maxima. Appl Environ Microbiol 74:6102–6113
Appel J, Schulz R (1998) Hydrogen metabolism in organisms with oxygenic photosynthesis: hydrogenases as important regulatory devices for a proper redox poising? J Photochem Photobiol B 47:1–11
Appel J, Phunpruch S, Steinmuller K, Schulz R (2000) The bidirectional hydrogenase of Synechocystis sp. PCC 6803 works as an electron valve during photosynthesis. Arch Microbiol 173:333–338
Azbar N, Kapdan IK (2011) Use of immobilized cell systems in biohydrogen production. In: Levin D, Azbar N (eds) State of the art and progress in production of biohydrogen. Bentham Science Publishers, Bussum, pp 228–250
Baca HK, Carnes E, Singh S, Ashley C, Lopez D, Brinker CJ (2007) Cell-directed assembly of bio/nano interfaces – a new scheme for cell immobilization. Acc Chem Res 40:836–845
Bai X, Ye ZF, Li YF, Ma YX (2010) Macroporous poly(vinyl alcohol) foam crosslinked with epichlorohydrin for microorganism immobilization. J Appl Polym Sci 117:2732–2739
Batyrova K, Tsygankov A, Kosourov SN (2012) Sustained hydrogen photoproduction by phosphorus-deprived Chlamydomonas reinhardtii cultures. Int J Hydrog Energy 37:8834–8839
Boichenko VA, Hoffmann P (1994) Photosynthetic hydrogen production in prokaryotes and eukaryotes: occurrence, mechanism, and functions. Photosynthetica 30:527–552
Boichenko VA, Greenbaum E, Seibert M (2004) Hydrogen production by photosynthetic microorganisms. In: Archer MD, Barber J (eds) Molecular to global photosynthesis: photoconversion of solar energy. Imperial College Press, London, pp 397–452
Brena BM, Batista-Viera F (2006) Immobilization of enzymes. In: Guisan JM (ed) Methods in biotechnology. Immobilization of enzymes and cells. Humana Press, Totowa, pp 15–30
Brodelius P, Vandamme EJ (1987) Immobilized cell systems. In: Kennedy JF (ed) Biotechnology, vol 7a: Enzyme technology. VCH Publication, New York, pp 405–464
Carnes EC, Lopez DM, Donegan NP, Cheung A, Gresham H, Timmins GS, Brinker CJ (2010) Confinement-induced quorum sensing of individual Staphylococcus aureus bacteria. Nat Chem Biol 6:41–45
Carrieri D, Wawrousek K, Eckert C, Yu J, Maness P-C (2011) The role of the bidirectional hydrogenase in cyanobacteria. Bioresour Technol 102:8368–8377
Carturan G, Campostrini R, Dire S, Scardi V, DeAlteriis E (1989) Inorganic gels for immobilization of biocatalysts. Inclusion of invertase-active whole cells of yeast (Saccharomyces cerevisiae) into thin layers of SiO2 gel deposited on glass sheets. J Mol Catal 57:L13–L16
Chen CY, Chang JS (2006) Enhancing phototropic hydrogen production by solid-carrier assisted fermentation and internal optical-fiber illumination. Process Biochem 41:2041–2049
Cheng K-C, Demirci A, Catchmark JM (2010) Advances in biofilm reactors for production of value-added products. Appl Microbiol Biotechnol 87:445–456
Cournac L, Guedeney G, Peltier G, Vignais PM (2004) Sustained photoevolution of molecular hydrogen in a mutant of Synechocystis sp. strain PCC 6803 deficient in the type I NADPH-dehydrogenase complex. J Bacteriol 186:1737–1746
Dagher SF, Ragout AL, Sineriz F, Bruno-Barcena JM (2010) Cell immobilization for production of lactic acid: biofilms do it naturally. In: Laskin AI, Sariaslani S, Gadd GM (eds) Advances in applied microbiology, vol 71. Elsevier Academic Press, San Diego, pp 113–148
Dante R (2005) Hypotheses for direct PEM fuel cells applications of photobioproduced hydrogen by Chlamydomonas reinhardtii. Int J Hydrog Energy 30:421–424
de-Bashan LE, Bashan Y (2010) Immobilized microalgae for removing pollutants: review of practical aspects. Bioresour Technol 101:1611–1627
Dickson D, Page C, Ely R (2009) Photobiological hydrogen production from Synechocystis sp. PCC 6803 encapsulated in silica sol–gel. Int J Hydrog Energy 34:204–215
Fedorov A, Tsygankov A, Rao KK, Hall DO (1998) Hydrogen photoproduction by Rhodobacter capsulatus immobilized on polyurethane foam. Biotechnol Lett 20:1007–1009
Fiedler D, Hager U, Franke H, Soltmann U, Bottcher H (2007) Algae biocers: astaxanthin formation in sol-gel immobilised living microalgae. J Mater Chem 17:261–266
Fissler J, Kohring GW, Giffhorn F (1995) Enhanced hydrogen production from aromatic acids by immobilized cells of Rhodopseudomonas palustris. Appl Biochem Biotechnol 44:43–46
Flickinger MC, Schottel JL, Bond DR, Aksan A, Scriven LE (2007) Painting and printing living bacteria: engineering nanoporous biocatalytic coatings to preserve microbial viability and intensify reactivity. Biotechnol Progr 23:2–17
Fukushima Y, Okamura K, Imai K, Motai H (1988) A new immobilization technique of whole cells and enzymes with colloidal silica and alginate. Biotechnol Bioeng 32:584–594
Garnham GW, Codd GA, Gadd GM (1992) Accumulation of cobalt, zinc and manganese by the estuarine green microalga Chlorella salina immobilized in alginate microbeads. Environ Sci Technol 26:1764–1770
Ghirardi ML (2006) Hydrogen production by photosynthetic green algae. Indian J Biochem Biophys 43:201–210
Ghirardi ML, Togasaki RK, Seibert M (1997) Oxygen sensitivity of algal H2-production. Appl Biochem Biotechnol 63:141–151
Ghirardi ML, Zhang L, Lee JW, Flynn T, Seibert M, Greenbaum E (2000) Microalgae: a green source of renewable H2. Trends Biotechnol 18:506–511
Gosse JL, Engel BJ, Rey FE, Harwood CS, Scriven LE, Flickinger MC (2007) Hydrogen production by photoreactive nanoporous latex coatings of nongrowing Rhodopseudomonas palustris CGA009. Biotechnol Progr 23:124–130
Gosse JL, Engel BJ, Hui JC-H, Harwood CS, Flickinger MC (2010) Progress toward a biomimetic leaf: 4,000 h of hydrogen production by coating-stabilized nongrowing photosynthetic Rhodopseudomonas palustris. Biotechnol Progr 26:907–918
Gosse JL, Chinn MS, Grunden AM, Bernal OI, Jenkins JS, Yeager C, Kosourov S, Seibert M, Flickinger MC (2012) A versatile method for preparation of hydrated microbial-latex biocatalytic coatings for gas absorption and gas evolution. J Ind Microbiol Biotechnol 39:1269–1278
Guan YF, Zhang W, Yu XJ, Deng MC (2003) Two-stage photo hydrogen production using immobilized marine green alga Platymonas subcordiformis. Abstracts of marine biotechnology: basics and applications. Biomol Eng 20:37–82
Guan YF, Zhang W, Deng MC, Jin MF, Yu XJ (2004) Significant enhancement of photobiological H2 evolution by carbonylcyanide m-chlorophenylhydrazone in the marine green alga Platymonas subcordiformis. Biotechnol Lett 26:1031–1035
Gugerli R, Breguet V, von Stockar U, Marison IW (2004) Immobilization as a tool to control fermentation in yeast-leavened refrigerated dough. Food Hydrocoll 18:703–715
Guo CL, Zhu X, Liao Q, Wang Y-Z, Chen R, Lee D-J (2011) Enhancement of photo-hydrogen production in a biofilm photobioreactor using optical fiber with additional rough surface. Bioresour Technol 102:8507–8513
Hahn JJ, Ghirardi ML, Jacoby WA (2007) Immobilized algal cells used for hydrogen production. Biochem Eng J 37:75–79
Hall DO, Rao KK (1988) Immobilized photosynthetic membranes and cells for the production of fuels and chemicals. In: Gaber BP, Schnur JM, Chapman D (eds) Biotechnological applications of lipid structures. Plenum Press, New York/London, pp 225–245
Hallenbeck PC (1983) Immobilized microorganisms for hydrogen and ammonia production. Enzyme Microb Technol 5:171–180
Hatanaka Y, Kudo T, Miyataka M, Kobayashi O, Higashihara M, Hiyama K (1999) Asymmetric reduction of hydroxyacetone to propanediol in immobilized halotolerant microalga Dunaliella parva. J Biosci Bioeng 88:281–286
Jimenez-Perez MV, Sanchez-Castillo P, Romera O, Fernadnez-Moreno D, Perez-Martinez C (2004) Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzyme Microb Technol 34:392–398
Kandimalla VB, Tripathi VS, Ju HX (2006) Immobilization of biomolecules in sol-gels: biological and analytical applications. Crit Rev Anal Chem 36:73–106
Kannaiyan S, Rao KK, Hall DO (1994) Immobilization of Anabaena azollae from Azolla filiculoides in polyvinyl foam for ammonia production in a photobioreactor system. World J Microbiol Biotechnol 10:55–58
Kaya VM, Picard G (1995) The viability of Scenedesmus bicellularis cells immobilized on alginate screens following nutrient starvation in air at 100 percent relative humidity. Biotechnol Bioeng 46:459–464
Kosourov SN, Seibert M (2009) Hydrogen photoproduction by nutrient-deprived Chlamydomonas reinhardtii cells immobilized within thin alginate films under aerobic and anaerobic conditions. Biotechnol Bioeng 102:50–58
Kosourov S, Tsygankov A, Seibert M, Ghirardi ML (2002) Sustained hydrogen photoproduction by Chlamydomonas reinhardtii: effects of culture parameters. Biotechnol Bioeng 78:731–740
Kosourov S, Seibert M, Ghirardi ML (2003) Effects of extracellular pH on the metabolic pathways of sulfur-deprived, H2-producing Chlamydomonas reinhardtii cultures. Plant Cell Physiol 44:146–155
Kosourov SN, Ghirardi ML, Seibert M (2011) A truncated antenna mutant of Chlamydomonas reinhardtii can produce more hydrogen than the parental strain. Int J Hydrog Energy 36:2044–2048
Kosourov SN, Batyrova K, Petushkova EP, Tsygankov A, Ghirardi ML, Seibert M (2012) Maximizing the hydrogen photoproduction yields in Chlamydomonas reinhardtii cultures: the effect of the H2 partial pressure. Int J Hydrog Energy 37:8850–8858
Kourkoutas Y, Bekatorou A, Banat IM, Marchant R, Koutinas AA (2004) Immobilization technologies and support materials suitable in alcohol beverages production: a review. Food Microbiol 21:377–397
Krastanov A (1997) Continuous sucrose hydrolysis by yeast cells immobilized to wool. Appl Microbiol Biotechnol 47:476–481
Kumazawa S, Mitsui A (1985) Comparative amperometric study of uptake hydrogenase and hydrogen photoproduction activities between heterocystous cyanobacterium Anabaena cylindrica B629 and nonheterocystous cyanobacterium Oscillatoria sp. strain Miami BG7. Appl Environ Microbiol 50:287–291
Lau PS, Tam NFY, Wong YS (1998) Effect of carrageenan immobilization on the physiological activities of Chlorella vulgaris. Bioresour Technol 63:115–121
Laurinavichene TV, Fedorov AS, Ghirardi ML, Seibert M, Tsygankov AA (2006) Demonstration of sustained hydrogen photoproduction by immobilized, sulfur-deprived Chlamydomonas reinhardtii cells. Int J Hydrog Energy 31:659–667
Laurinavichene TV, Kosourov SN, Ghirardi ML, Seibert M, Tsygankov AA (2008) Prolongation of H2 photoproduction by immobilized, sulfur-limited Chlamydomonas reinhardtii cultures. J Biotechnol 134:275–277
Leino H, Kosourov SN, Saari L, Sivonen K, Tsygankov A, Aro E-M, Allahverdiyeva Y (2012) Extended H2 photoproduction by N2-fixing cyanobacteria immobilized in thin alginate films. Int J Hydrog Energy 37:151–161
Leon R, Galvan F (1995) Glycerol photoproduction by free and Ca-alginate entrapped cells of Chlamydomonas reinhardtii. J Biotechnol 42:61–67
Liu L, Shang L, Guo S, Li D, Liu C, Qi L, Dong S (2009) Organic-inorganic hybrid material for the cells immobilization: long-term viability mechanism and application in BOD sensors. Biosens Bioelectron 25:523–526
Lyngberg OK, Ng CP, Thiagarajan V, Scriven LE, Flickinger MC (2001) Engineering the microstructure and permeability of thin multilayer latex biocatalytic coatings containing E. coli. Biotechnol Progr 17:1169–1179
Mallick N (2002) Biotechnological potential of immobilized algae for wastewater N, P and metal removal: a review. Biometals 15:377–390
Mallick N (2006) Immobilization of microalgae. In: Guisan JM (ed) Immobilization of enzymes and cells. Humana Press, Totowa, pp 373–391
Markov SA, Lichtl R, Rao KK, Hall DO (1993) A hollow-fiber photobioreactor for continuous production of hydrogen by immobilized cyanobacteria under partial vacuum. Int J Hydrog Energy 18:901–906
Markov SA, Bazin MJ, Hall DO (1995) Hydrogen photoproduction and carbon dioxide uptake by immobilized Anabaena variabilis in a hollow-fiber photobioreactor. Enzyme Microb Technol 17:306–310
Martens N, Hall EAH (1994) Immobilization of photosynthetic cells based on film-forming emulsion polymers. Anal Chim Acta 292:49–63
Matsunaga T, Takeyama H, Sudo H, Oyama N, Ariura S, Takano H, Hirano M, Burgess JG, Sode K, Nakamura N (1991) Glutamate production from CO2 by marine cyanobacterium Synechococcus sp. using a novel biosolar reactor employing light-diffusing optical fibers. Appl Biochem Biotechnol 28:157–167
Melis A (2007) Photosynthetic H2 metabolism in Chlamydomonas reinhardtii (unicellular green algae). Planta 226:1075–1086
Melis A, Zhang L, Forestier M, Ghirardi ML, Seibert M (2000) Sustained photobiological hydrogen gas production upon reversible inactivation of oxygen evolution in the green alga Chlamydomonas reinhardtii. Plant Physiol 122:127–136
Meunier CF, Dandoy P, Su BL (2010) Encapsulation of cells within silica matrixes: towards a new advance in the conception of living hybrid materials. J Colloid Interface Sci 342:211–224
Meuser JE, Ananyev G, Wittig LE, Kosourov S, Ghirardi ML, Seibert M, Dismukes GC, Posewitz MC (2009) Phenotypic diversity of hydrogen production in chlorophycean algae reflects distinct anaerobic metabolisms. J Biotechnol 142:21–30
Moreno-Garrido I (2008) Microalgae immobilization: current techniques and uses. Bioresour Technol 99:3949–3964
Moreno-Garrido I, Campana O, Lubian LM, Blasco J (2005) Calcium alginate immobilized marine microalgae: experiments on growth and short-term heavy metal accumulation. Mar Pollut Bull 51:823–929
Pannier A, Oehm C, Fischer AR, Werner P, Soltmann U, Bottcher H (2011) Biodegradation of fuel oxygenates by sol-gel immobilized bacteria Aquincola tertiaricarbonis L108. Enzyme Microb Technol 47:291–296
Park IH, Rao KK, Hall DO (1991) Photoproduction of hydrogen, hydrogen-peroxide and ammonia using immobilized cyanobacteria. Int J Hydrog Energy 16:313–318
Philipps G, Happe T, Hemschemeier A (2012) Nitrogen deprivation results in photosynthetic hydrogen production in Chlamydomonas reinhardtii. Planta 235:729–745
Phlips J, Mitsui A (1986) Characterization and optimization of hydrogen production by a salt water blue-green alga Oscillatoria sp. Miami BG 7. II. Use of immobilization for enhancement of hydrogen production. Int J Hydrog Energy 11:83–89
Planchard A, Mignot L, Jouenne T, Junter G-A (1984) Photoproduction of molecular hydrogen by Rhodospirillum rubrum immobilized in composite agar layer/microporous membrane structures. Appl Microbiol Biotechnol 31:49–54
Rashid N, Song W, Park J, Jin H-F, Lee K (2009) Characteristics of hydrogen production by immobilized cyanobacterium Microcystis aeruginosa through cycles of photosynthesis and anaerobic incubation. J Ind Eng Chem 15:498–503
Rashid N, Choi W, Lee K (2012) Optimization of two-staged bio-hydrogen production by immobilized Microcystis aeruginosa. Biomass Bioenergy 36:241–249
Robinson PK (1998) Immobilized algal technology for wastewater treatment purposes. In: Wong Y-S, Tam NFY (eds) Wastewater treatment with algae. Springer and Landes Bioscience, New York, pp 1–16
Romanova YM, Didenko LV, Tolordava ER, Ginzburg AL (2011) Biofilms of pathogenic bacteria: a role in chronic of infectious process and search of agents of struggle. Vestnik RAMN (Russ) 10:31–39
Rooke JC, Meunier C, Leonard A, Su BL (2008) Energy from photobioreactors: bioencapsulation of photosynthetically active molecules, organelles, and whole cells within biologically inert matrices. Pure Appl Chem 80:2345–2376
Saetang J, Babel S (2009) Effect of leachate loading rate and incubation period on the treatment efficiency by T. versicolor immobilized on foam cubes. Int J Environ Sci Technol 6:457–466
Sarkar S, Pandey KD, Kashyap AK (1992) Hydrogen photoproduction by filamentous nonheterocystous cyanobacterium Plectonema boryanum and simultaneous release of ammonia. Int J Hydrog Energy 17:689–694
Sasikala K, Ramana CV, Rao PR (1992) Photoproduction of hydrogen from the waste-water of a distillery by Rhodobacter sphaeroides OU 001. Int J Hydrog Energy 17:23–27
Scholz W, Galvan F, de la Rosa FF (1995) The microalga Chlamydomonas reinhardtii CW-15 as a solar cell for hydrogen peroxide photoproduction: comparison between free and immobilized cells and thylakoids for energy conversion efficiency. Sol Energy Mater Sol Cell 39:61–69
Semple KT, Cain RB, Schmidt S (1999) Biodegradation of aromatic compounds by microalgae. FEMS Microbiol Lett 170:291–300
Serebryakova LT, Tsygankov AA (2007) Two-stage system for hydrogen production by immobilized cyanobacterium Gloeocapsa alpicola CALU 743. Biotechnol Progr 23:1106–1110
Serebryakova L, Sheremetieva M, Tsygankov A (1998) Reversible hydrogenase activity of Gloeocapsa alpicola in continuous culture. FEMS Microbiol Lett 166:89–94
Shi J, Podola B, Melkonian M (2007) Removal of nitrogen and phosphorus from wastewater using microalgae immobilized on twin layers: an experimental study. J Appl Phycol 19:417–423
Skjanes K, Knutsen G, Kallqvist T, Lindblad P (2008) H2 production from marine and freshwater species of green algae during sulfur deprivation and considerations for bioreactor design. Int J Hydrog Energy 33:511–521
Smirnova TA, Didenko LV, Andreev AL, Alekseeva NV, Stepanova TV, Romanova YM (2008) Electron microscopic study of Burkholderia cepacia biofilms. Microbiology 77:55–61
Smirnova TA, Didenko LV, Azizbekyan RR, Romanova YM (2010) Structural and functional characteristics of bacterial biofilms. Microbiology 79:413–423
Song W, Rashid N, Choi W, Lee K (2011) Biohydrogen production by immobilized Chlorella sp. using cycles of oxygenic photosynthesis and anaerobiosis. Bioresour Technol 102:8676–8681
Stolarzewicz I, Bialecka-Florjanczyk E, Majewska E, Krzyczkowska J (2011) Immobilization of yeast on polymeric supports. Chem Biochem Eng Q 25:135–144
Tamagnini P, Axelsson R, Lindberg P, Oxelfelt F, Wunschiers R, Lindblad P (2002) Hydrogenases and hydrogen metabolism of cyanobacteria. Microbiol Mol Biol Rev 66:1–20
Tamagnini P, Leitao E, Oliveira P, Ferreira D, Pinto F, Harris D, Heidorn T, Lindblad P (2007) Cyanobacterial hydrogenases: diversity, regulation and applications. FEMS Microbiol Rev 31:692–720
Tekucheva DN, Laurinavichene T, Seibert M, Tsygankov A (2011) Immobilized purple bacteria for light-driven H2 production from starch and potato fermentation effluents. Biotechnol Progr 27:1248–1256
Tian X, Liao Q, Liu W, Wang YZ, Zhu X, Li J, Wang H (2009) Photo-hydrogen production rate of a PVA-boric acid gel granule containing immobilized photosynthetic bacteria cells. Int J Hydrog Energy 34:4708–4717
Tian X, Liao Q, Zhu X, Wang Y, Zhang P, Li J, Wang H (2010) Characteristics of a biofilm photobioreactor as applied to photo-hydrogen production. Bioresour Technol 101:977–983
Torzillo G, Pushparaj B, Masojidek J, Vonshak A (2003) Biological constraints in algal biotechnology. Biotechnol Bioprocess Eng 8:338–348
Travieso L, Benitez F, Dupeiron R (1992) Sewage treatment using immobilized microalgae. Bioresour Technol 40:183–187
Travieso L, Benitez F, Weiland P, Sanchez E, Dupeyron R, Dominguez AR (1996) Experiments on immobilization of microalgae for nutrient removal in wastewater treatments. Bioresour Technol 55:181–186
Troshina O, Serebryakova L, Sheremetieva M, Lindblad P (2002) Production of H2 by the unicellular cyanobacterium Gloeocapsa alpicola CALU 743 during fermentation. J Gen Microbiol 27:1283–1289
Tsygankov AA (2001a) Laboratory scale photobioreactors. Appl Biochem Microbiol 37:333–341
Tsygankov AA (2001b) Hydrogen production by purple bacteria: immobilized vs. suspension cultures. In: Miyake J, Matsunaga T, San Pietro A (eds) Biohydrogen 2. An approach to environmentally acceptable technology. Pergamon Press, Amsterdam, pp 229–244
Tsygankov A (2004) Hydrogen production by suspension and immobilized cultures of phototrophic microorganisms. Technological aspects. In: Miyake J, Igarashi H, Rogner M (eds) Biohydrogen III. Renewable energy system by biological solar energy conversion. Elsevier, Amsterdam, pp 57–74
Tsygankov AA (2007) Nitrogen-fixing cyanobacteria: a review. Appl Biochem Microbiol 43:279–288
Tsygankov AA, Hirata Y, Miyake M, Asada Y, Miyake J (1993) Immobilization of the purple nonsulfur bacterium Rhodobacter sphaeroides on glass surfaces. Biotechnol Tech 77:575–578
Tsygankov AA, Hirata Y, Miyake M, Asada Y, Miyake J (1994) Photobioreactor with photosynthetic bacteria immobilized on porous glass for hydrogen photoproduction. J Ferment Bioeng 77:575–578
Tsygankov AA, Fedorov AS, Laurinavichene TV, Gogotov IN, Rao KK, Hall DO (1998a) Actual and potential rates of hydrogen photoproduction by continuous culture of the purple non-sulphur bacterium Rhodobacter capsulatus. Appl Microbiol Biotechnol 49:102–107
Tsygankov AA, Fedorov AS, Talipova IV, Laurinavichene TV, Miyake J, Gogotov IN (1998b) Use of immobilized phototrophic microorganisms for wastewater treatment and simultaneous production of hydrogen. Appl Biochem Microbiol 34:362–366
Tsygankov AA, Fedorov AS, Kosourov SN, Rao KK (2002) Hydrogen production by cyanobacteria in an automated outdoor photobioreactor under aerobic conditions. Biotechnol Bioeng 80:777–783
Tsygankov AA, Kosourov SN, Tolstygina IV, Ghirardi ML, Seibert M (2006) Hydrogen production by sulfur-deprived Chlamydomonas reinhardtii under photoautotrophic conditions. Int J Hydrog Energy 31:1574–1584
Vignais PM, Billoud B (2007) Occurrence, classification and biological function of hydrogenases: an overview. Chem Rev 107:4206–4272
Vignais PM, Colbeau A, Willison JC, Jouanneau Y (1985) Hydrogenase, nitrogenase, and hydrogen metabolism in the photosynthetic bacteria. Adv Microb Physiol 26:155–234
Vincenzini M, Balloni W, Mannelli D, Florenzano G (1981) A bioreactor for continuous treatment of waste waters with immobilized cells of photosynthetic bacteria. Experientia 37:710–711
Vincenzini M, Materassi R, Tredici MR, Florenzano G (1982a) Hydrogen production by immobilized cells. I. Light-dependent dissimilation of organic substances by Rhodopseudomonas palustris. Int J Hydrog Energy 7:231–236
Vincenzini M, Materassi R, Tredici MR, Florenzano G (1982b) Hydrogen production by immobilized cells. II. H2-photoevolution and wastewater treatment by agar-entrapped cells of Rhodopseudomonas palustris and Rhodospirillum molischianum. Int J Hydrog Energy 7:725–728
Vincenzini M, Materassi R, Sili C, Florenzano G (1986) Hydrogen production by immobilized cells. III. Prolonged and stable H2 photoevolution by Rhodopseudomonas palustris in light-dark cycles. Int J Hydrog Energy 11:623–626
Wang YZ, Liao Q, Zhu X, Tian X, Zhang C (2010) Characteristics of hydrogen production and substrate consumption of Rhodopseudomonas palustris CQK 01 in an immobilized-cell photobioreactor. Bioresour Technol 101:4034–4041
Winkler M, Hemschemeier A, Gotor C, Melis A, Happe T (2002) [Fe]-hydrogenases in green algae: photo-fermentation and hydrogen evolution under sulfur deprivation. Int J Hydrog Energy 27:1431–1439
Wykoff DD, Davies JP, Melis A, Grossman AR (1998) The regulation of photosynthetic electrontransport during nutrient deprivation in Chlamydomonas reinhardtii. Plant Physiol 117:129–139
Zhang L, Huang G, Yu Y (1998) Immobilization of microalgae for biosorption and degradation of butyltin chlorides. Artif Cell Blood Substit 26:399–410
Zhu H, Suzuki T, Asada Y, Miyake J (1999a) Entrapment of Rhodobacter sphaeroides RV in cationic polymer/agar gels for hydrogen production in the presence of NH4 +. J Biosci Bioeng 88:507–512
Zhu H, Suzuki T, Tsygankov A, Asada Y, Miyake J (1999b) Hydrogen production from tofu wastewater by Rhodobacter sphaeroides immobilized on agar gel. Int J Hydrog Energy 24:305–310
Zurrer H, Bachofen R (1985) Production of molecular hydrogen with immobilized cells of Rhodospirillum rubrum. Appl Microbiol Biotechnol 23:15–20
Acknowledgements
This work was supported by Russian Ministry of Science and Education (Agreement 8077).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Tsygankov, A., Kosourov, S. (2014). Immobilization of Photosynthetic Microorganisms for Efficient Hydrogen Production. In: Zannoni, D., De Philippis, R. (eds) Microbial BioEnergy: Hydrogen Production. Advances in Photosynthesis and Respiration, vol 38. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-8554-9_14
Download citation
DOI: https://doi.org/10.1007/978-94-017-8554-9_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-8553-2
Online ISBN: 978-94-017-8554-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)