Abstract
Sexual reproductive activity has been demonstrated in all reef-building (zooxanthellate) scleractinian corals examined from Mexico to the equatorial eastern Pacific (Galápagos Islands). Eleven of 13 species spawn gametes, six are gonochoric, three hermaphroditic, and four exhibit significant mixed sexuality (both gonochoric and hermaphroditic). Four or 30.1 %, two species each of Pocillopora and Porites, produce autotrophic ova. Porites panamensis is the only known zooxanthellate brooder. Also sexually active are the azooxanthellate scleractinian Tubastraea coccinea and the zooxanthellate hydrocoral Millepora intricata. Reproductive structures, sex ratios, age at sexual maturity, sexuality, and developmental mode have been determined from largely histological evidence. Agariciid corals, comprising more than one-third of investigated species, exhibit predominantly mixed sexual systems with sequential cosexual hermaphroditic cycles in four species. Mixed sexuality is also minimally exhibited in populations of two dominantly gonochoric species. Several eastern Pacific corals spawn mostly on lunar day 17 and 1–2 days following; however, multispecific spawning has not been observed probably because of seasonal, diel, and variable timing in spawning behavior. Factors contributing to the high fecundity of eastern Pacific corals include (1) seasonally prolonged reproductive activity, (2) small size of mature gametes allowing for production of high numbers, (3) split spawning with bimonthly gamete production in some species, (4) alternation of sex maturation in gamete development, and possibly (5) their low latitudinal location under relatively constant and high thermal conditions. Coral community persistence, reef growth and recovery are highly dependent on both sexual and asexual reproductive processes. Asexual fragmentation by physical and biotic causes is particularly important, especially for branching pocilloporid species and the fungiid coral Diaseris distorta. Asexual propagation in massive and encrusting poritid and agariciid species is also common-place, often the result of bioerosion and colony breakage by foraging reef fishes. Some research areas in need of attention are noted, for example (a) timing of spawning and the behavior of gamete release of several species, (b) life cycles of Pocillopora spp. and Millepora intricata, and (c) effects of anthropogenic stressors on eastern Pacific coral reproduction and recruitment.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
15.1 Introduction
Sexual and asexual reproductive functions are critical life history processes in the maintenance, repopulation and recovery of coral communities. This is especially true for highly dispersed coral populations and for coral reefs subject to frequent disturbances, such as those in the eastern tropical Pacific. The sexual patterns, spawning modes and timing, fecundity, and recruitment are examined in this chapter for several coral species that contribute importantly toward reef-building and coral community structure. In addition, the reproductive biology of eastern Pacific corals will be compared with those of reef corals in other biogeographic regions to underline similarities and differences in relation to environmental conditions.
A central question that emerged early in the study of eastern Pacific coral reefs was how the small and dispersed reefs of this region sustain growth and persist in the face of frequent disturbances (Dana 1975; Richmond 1990). Richmond’s (1982, 1985) initial studies in Panama suggested that sexual reproduction was rare or non-existent in Pocillopora damicornis, the principal reef-building species in most eastern Pacific areas. This result contributed importantly to the hypothesis that eastern Pacific reefs were populated and maintained primarily by asexual processes such as fragmentation, and by initial and perhaps episodic long distance dispersal of larvae from source populations in the central and western Pacific (Dana 1975; Richmond 1987a, b, 1990).
Studies of the reproductive biology and ecology of eastern Pacific corals have continued in the equatorial eastern Pacific (EEP: Panama, Costa Rica, and the Galápagos Islands) from the 1980s to the present, and in Mexico beginning in the early 2000s. As a result, sexual reproduction has been found to be prevalent among the eastern Pacific genera of major reef-building families (Pocilloporidae, Poritidae, Agariciidae). In addition, less structurally important families, such as the zooxanthellate Siderastreidae (Psammocora spp.), Fungiidae (Diaseris), and azooxanthellate Dendrophylliidae (Tubastraea), have also been shown to exhibit frequent sexual activity. New information on the reproduction of Millepora intricata, an abundant zooxanthellate hydrocoral in western Panama (Gulf of Chiriquí), has also shown this species to be sexually active.
Within the present chapter, sexual reproductive processes are first examined, followed by recruitment and the importance of asexual reproduction in conjunction with the effects of physical and biotic (corallivory, fish foraging, bioerosion) factors on coral fragmentation. A discussion of the regionally unique environmental conditions of the eastern Pacific, and potentially stressful anthropogenic effects on coral reproduction, conclude the chapter.
15.2 Patterns of Sexual Reproduction
15.2.1 Gonads, Gametes and Larvae
Coral gonads are not true organs but rather sites of gamete development within or closely associated with the 12 primary mesenteries. Ovaries and testes enlarge, with developing oocytes and spermaries assuming spherical sizes and shapes as gametogenesis proceeds. In order to quantify the pace of gamete maturation, gonads have been assigned to four developmental stages in eastern Pacific studies. Morphological changes during development are briefly summarized for both broadcast spawning and brooding species in Tables 15.1 and 15.2. Color comparisons of gametes that follow are based on histological preparations of Azocarmine G and Aniline Blue (Luna 1968).
15.2.1.1 Oogenesis
Histological preparations of Porites, Pocillopora and Tubastraea provided more detail in oogenic development than those of the genera Pavona, Gardineroseris, Psammocora and Diaseris. Color changes occurring as development progresses were not as evident in those species of the latter group. Oocytes of this group contained homogeneously fine and grainy ooplasm. This is due in part to a less complex condition of the yolk, which was evident throughout development. Stages I and II oocytes of all species generally displayed similar staining characteristics, however, lipid vesicles appeared at one pole, most notably in both species of Porites (P. lobata, P. panamensis). During Stage III, oocytes generally darkened. Pocillopora oocytes transitioned to bright peach, and in Porites oocyte gametogenesis progressed into deeper hues of red. In Tubastraea, oocytes transitioned from gray (Stage I) to light pink (Stage II) to deep pink as yolk differentiated (Stage III). And in the Agariciidae (Pavona, Gardineroseris), some darkening was experienced, but could be light blue, light pink or light peach. Ooplasm remained homogeneously granular in this family. Psammocora oocytes (two species) approximated the characteristics of the Agariciidae, however, they were often of an orange hue or brick red. Color change was not observed in Diaseris oocytes, which remained yellow throughout most of development. Stage IV Diaseris oocytes, however, were frequently mottled with raspberry blotches, as were sometimes agariciid oocytes.
Both Psammocora spp. and Pavona varians contained “satellite nucleoli-like structures” (approximately 2–5). These had the same perfectly round, raspberry or dark pink appearance as the prominent nucleolus, however, they were significantly smaller, located in the ooplasm clustered near, but outside, the nucleus and most noticeably in Stage III oocytes. Their function is unknown; these could be remnants of oocyte fusion that has been observed in at least one species of eastern Pacific corals (Tubastraea coccinea) where nuclear fragments were present.
Stage IV ovum development had the most obvious changes in cell structure and coloration. A subtle to dramatic color change was represented in Stage IV ova of many species, and the nucleus completely migrated to the ovum membrane. In some species where few changes could be identified, i.e., Diaseris distorta, Stage IV ova had an “off center nucleus” that eventually moved to a more peripheral location, but was never observed in preparations to be close to the nuclear membrane. A space often formed between the nucleus and ooplasm in late Stage IV ova of Porites and Pocillopora species. In Pocillopora, Stage IV ova were replete with prominent, colorless lipid vesicles/vacuoles within the peach-colored ooplasm, presumably to aid in buoyancy. This was not apparent in Porites spp., which darkened to maroon and contained dense granular material before being released.
Nuclei in Porites, Pocillopora and Pavona became irregular in shape instead of spherical after approaching the cell membrane. Dome, triangular, and cube shapes were observed. Sickle or crescent shapes were in part due to the portion of the nucleus exposed in the histological section as it lay flattened and parallel, adjacent to the oocyte membrane.
A notch or indentation was observed in late Stage IV ova of Porites and Pocillopora, but was not usually present in ova of other species. This structure had been reported previously in species other than those of the eastern Pacific (Szmant-Froelich et al. 1985).
A border was evident just inside the plasma membrane of developing oocytes of several species. This border was approximately 3 µm wide and was evident in species of Porites, Pavona, Tubastraea and Psammocora. It stained more deeply in color than the rest of the ooplasm, but displayed approximately the same hue. This border was most noticeable in Porites and Tubastraea oocytes and had a granular appearance. In Tubastraea, which has very large oocytes (up to 800 µm), the granularity was due to the detection of numerous minute, barely-visible vesicles. Also present in Pavona spp., this border had a more subtle, granular appearance. Its function is unknown, but could be associated with a cortical layer of vesicles (Harrsion and Wallace 1990) that bursts, causing an elevation of the membrane to prevent polyspermy at the time of fertilization. In brooders , this border has disappeared in early Stage I planulae.
Porites evermanni is now recognized as occurring commonly in the eastern Pacific, but it reproduces chiefly by asexual fragmentation (Boulay et al. 2013). Due to the high level, year round gametogenesis observed in P. lobata, it is likely that few if any P. evermanni were confused in the sampling of P. lobata.
15.2.1.2 Spermatogenesis
Stage I spermaries in eastern Pacific corals are generally similar; however, there are some differences in staining that are characteristic of certain species. Porites Stage I spermaries can be enlarged brown or red cells, while those in the Agariciidae are often gray or bluish. In Tubastraea, the spermaries are grayish-pink to light pink and sometimes beige.
Stage II spermaries contain cells that are of the same size, shape and staining properties as those of Stage I. Towards the end of this phase, the spermatocytes become densely packed, and the prominent nucleus seen earlier has disappeared. Stage II spermaries generally take on more rose-colored hues or may darken if previously pink in color. Cells become more regular in size with less detail. Delineation of developmental stages for each species was based on overall gametogenic characteristics. Therefore, stage delineation was determined per species and is not exactly the same for all.
In Stage III, increased volume is due to dense packing and increased number of mitotic products. This results in a honeycomb appearance of the spermary. Cell coloration is similar to that of late Stage II when mitosis often begins. No nucleus is visible in cells that are smooth and without inclusions. Stage III spermaries are generally dark pink, raspberry, or peach colored, but can be light blue or gray if the de-staining of Azocarmine is overly executed. A lumen forming centrally gives the spermary a raspberry-like morphology.
The most consistent characteristic in spermatogenesis of eastern Pacific corals is the color change to deep purple, which occurs in Stage IV. Meiosis occurs during this phase and the color deepens as divisions increase. The lumen becomes filled with division products as meiosis progresses peripherally within the spermary. Spermaries in this transitional phase can be magenta to purple with a variety of cell sizes present. In the eastern Pacific studies, Szmant’s (1986) Stages IV and V were combined; therefore both divisions and the formation of spermatozoan tails were included in the same stage (IV). Golden tails typically form at the end of this phase (although not observed in Tubastraea histological samples) and purple spermaries assume a teardrop shape with tails aligned at the tapered end, hence the term bouquet. Sperm heads can also occasionally be observed aligned in radial rows.
15.2.1.3 Planula Development
In brooding species , morphological changes during planula development can also be conveniently divided into four stages. Table 15.3 compares planula development in the two eastern Pacific species, Porites panamensis (zooxanthellate) and Tubastraea coccinea (azooxanthellate). Early embryos or Stage I planulae are released into the gastrovascular cavity or break out of the gastrodermis. This early planula stage typically appears as a bubbly amorphous mass. Blastula or gastrula stages were not observed. A thin mesogleal layer is one of the defining features of Stage II planulae, which appears as a (blue) ring towards the periphery of the larva. Interstitial cell formation initiated earlier, exterior to this (ectoderm formation), progresses in consecutive rows towards the mesoglea and differentiation of cell types begins. Stage III planulae are generally elongate with an oral pore opening into the gastrovascular cavity. Differentiation of the ectodermal cell types continues. Formation of Stage IV planulae results in the possession of two or more mesenteries with mesenterial filaments that can be well developed. Cells of the gastrodermis are formed. The planulae of Porites panamensis enlarge as development proceeds, from 200–300 μm in length in early stages to 400–600 μm in length in Stage IV. Tubastraea planulae were 0.5 to 1.5 mm in length when released. Additional developmental changes in yolk color and disposition, appearance of mesoglea and mesenteries, and cellular differentiation are noted in Table 15.3.
15.2.1.4 Histological Documentation
To substantiate the prevalence of sexual reproduction in eastern Pacific corals, following is a collection of photomicrographs of the reproductive products of 11 coral species from Mexico to equatorial locations. [Sampling sites and some relevant oceanographic conditions are noted in Fig. 15.1a, b. The reader is referred to Fiedler and Lavín (Chap. 3) for more details.] Three abundant species in Banderas Bay, Mexico demonstrate the hermaphroditic condition (Pocillopora damicornis, Fig. 15.2a, b), the gonochoric condition with mature ovaries and a spermary with the bouquet arrangement (Porites panamensis, Fig. 15.2c, d), and Stage IV ova and Stage III spermaries with centrally-located lumina (Pavona gigantea, Fig. 15.2e, f) of a species that demonstrates a mixed sexual pattern. Zooxanthellae are visible within a mature ovum of P. panamensis (Fig. 15.2c).
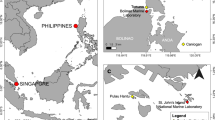
a Locations of reproductive sampling sites and b oceanographic conditions across the eastern tropical Pacific. a MNP, MCP, MSP, Mexico northern (25°N), central (20°N), and southern (15°N) Pacific; MSP includes Entrega Bay, Montosa and Riscalillo sites; Gulf of Chiriquí, Panama includes Uva I and Bahía Honda sites; Gulf of Panama includes Taboga I and Saboga I sites; Galápagos Is include Santa Cruz I, Itabaca Canal, Floreana I and Playa La Picona. BC Baja California; ES El Salvador; CR Costa Rica. Oceanic islands: Rev Revillagigedo Is; Clp Clipperton Atoll; Coc Isla del Coco; Mal Malpelo I; Gal Galápagos Is. Equatorial eastern Pacific (EEP) sites, study areas between 11°N and 2°S, include Costa Rica, Panama, and Galápagos Islands. b Dotted lines off Mexican coast denote limits of frontal boundary between California Current and tropical surface water. CRCC Costa Rican Coastal Current; dashed lines denote positions of Intertropical Convergence Zone (ITCZ) in October and April. Panama Current flows seasonally from the Panama Bight towards the Galápagos Islands from January to mid–April. Bold arrows denote upwelling centers at Cabo Pulmo, Baja California, Gulf of Tehuantepec (Mexico), Gulf of Papagayo (Costa Rica/Nicaragua), Gulf of Panama (Panama), and western Galápagos Islands
Photomicrographs of gonads from corals sampled at Banderas Bay, Mexico. a, b Pocillopora damicornis simultaneous hermaphrodites with ovaries and spermaries. c Porites panamensis Stage IV ovum with zooxanthellae. d Porites panamensis Stage IV spermary. e Pavona gigantea Stage III oocytes. f Pavona gigantea Stage III spermaries. Staining: a, c–f, Azocarmine G and Toluidine Blue (Humason 1967); b, Mallory Heidenhain. ov ovary, sp spermary, n nucleus, nu nucleolus, z zooxanthellae
Stages I-IV ovaries are present in Porites panamensis from Uva Island, Panama (Fig. 15.3a–c). Advanced vitellogenesis is evident in Stage IV ova (Fig. 15.3b, c). A mature ovum is readily identified by its crescent-shaped nucleus that has migrated to the peripheral cell membrane (Fig. 15.3c). Numerous zooxanthellae are also visible in mature ova. All spermary stages (I-IV) are present in Fig. 15.3d–f, including the centrally located lumen in Stage III and a presumed empty spermary (‘x’) after spawning. Early Stage I larvae are typically irregularly shaped and contain abundant lipid stores (Fig. 15.4a). A thin mesogleal layer divides the ciliated ectoderm and gastrodermis in Stage II larvae (Fig. 15.4b). An oral pore, cnidocytes and mesenteries are present in Stage III planulae (Fig. 15.4c). Mesenterial filaments make their appearance in Stage IV planulae (Fig. 15.4d). Zooxanthellae are present in all larval stages with a tendency to aggregate peripherally in the gastrodermis, just inside the mesoglea, as development proceeds.
Photomicrographs of gonads from Porites panamensis, Uva Island study reef, Gulf of Chiriquí, Panama. a Ovary with Stages I, II and III oocytes. b Stage IV ovum. c Stage IV ovum. d Spermary with Stages I and IV spermatocytes. e Stage III spermary. f Stages II and IV spermaries, ‘x’ denotes probable location of spent spermary. Staining: Heidenhain’s Aniline-blue (Luna 1968) and Azocarmine G. sp spermary, n nucleus, l lipid vesicle, z zooxanthellae
Photomicrographs of Porites panamensis planulae, Uva Island reef, Gulf of Chiriquí, Panamá. a–d Stages I-IV planula larvae respectively. Staining: Heidenhain’s Aniline-blue and Azocarmine G. Roman numerals denote planula stages. o oral pore, cl cilia, cn cnidocytes, ec ectoderm, m mesentery, mg mesoglea, s septal filament, z zooxanthellae
The disposition of gonads in advanced stages of development in seven broadcast spawning species is illustrated in Figs. 15.5a–f and 15.6a, b. Numerous Stage IV spermaries are visible in the cross-section of most of the 12 mesenteries surrounding the gastrovascular cavity in many Porites lobata polyps (Fig. 15.5a). Of the agariciid species, Stage IV ova are arranged longitudinally along the mesenteries of Pavona gigantea (Fig. 15.5b). Two clusters of Stage III ovaries are present at the bases of mesenteries in Pavona clavus (Fig. 15.5c). Stage IV spermaries, several with bouquet arrangements of spermatozoa, are shown for Pavona chiriquiensis (Fig. 15.5d) and Pavona varians (Fig. 15.5e, f). Bouquet structures are also shown for Gardineroseris planulata (Fig. 15.6a) and Psammocora stellata (Fig. 15.6b) with free spermatozoa in the former section. An advanced planula of azooxanthellate Tubastraea coccinea, in transition between Stages III and IV, shows clear colorless areas that are gastrodermal anlage and gray granular strings that will form mesenteries (Fig. 15.6c). Asynchronous spermatocyte development (Stages II-IV) and a single Stage II oocyte (with nucleus and nucleolus) illustrate the hermaphroditic condition of T. coccinea (Fig. 15.6d).
Photomicrographs of gonads. a Stage IV spermaries and associated mesenteries in Porites lobata (Santa Cruz I, Galápagos Is). b Pavona gigantea with several Stage IV ova (Itabaca Canal, Galápagos Is). c Pavona clavus (Saboga I, Gulf of Panama) with two clusters of Stage III oocytes. d Pavona chiriquiensis (Uva I, Gulf of Chiriquí) with several Stage IV spermaries. e Pavona varians with numerous Stage IV spermaries (Uva I, Gulf of Chiriquí). f Pavona varians with Stage IV spermaries (Uva I, Gulf of Chiriquí). Staining: Heidenhain’s Aniline-blue and Azocarmine G. m mesentery, msg mesoglea, b bouquet, gvc gastrovascular cavity
Photomicrographs of reproductive structures of scleractinian corals and hydrocorals sampled at various sites in the equatorial eastern Pacific. a Gardineroseris planulata (Caño I, Costa Rica) with Stage IV spermaries. b Psammocora stellata (Floreana I, Galápagos Is) with Stages III and IV spermaries. c Tubastraea coccinea planula, Stage III (Uva I, Gulf of Chiriquí). d Tubastraea coccinea (Uva I, Gulf of Chiriquí) with Stages I, II, III, IV spermaries, and Stage II oocyte. e Millepora intricata (Uva I, Gulf of Chiriquí) with a developing medusa. Parts of two oocytes visible, one with nucleus. f Millepora intricata (Uva I, Gulf of Chiriquí), two medusae within ampullae. Developing oocytes at periphery. Staining: Heidenhain’s Aniline-blue plus Azocarmine G. s spermatozoa, ooc oocytes, ov ovum, f sperm flagella, lip lipid vesicles, c cnidocytes, msg mesoglea, m mesentery, z zooxanthellae, rc medusa ring canal, amp ampulla
Sexual reproduction in the hydrozoan coral Millepora intricata begins in the sessile polypoid generation by the budding off of male and female planktonic medusae (Soong and Cho 1998; Lewis 2006). Evidence of sexual reproduction was difficult to detect in M. intricata, which is restricted in distribution to the Gulf of Chiriquí (Panama) in the eastern Pacific. Histological preparations, however, did demonstrate gametogenesis . A developmentally advanced medusa with two oocytes, one large with a nucleus present and a small portion of one near the ring canal, is shown in Fig. 15.6e. Zooxanthellae are visible in the large oocyte. Two immature medusae within their respective ampullae are shown in Fig. 15.6f. The medusa in the lower left corner contains two developing oocytes. These specimens were collected in January, but due to sparse data, the timing and seasonality of gamete production was not delineated for this species.
15.2.1.5 Sizes of Sexual Products
Critical metrics in assessing the fecundity of corals are gonad, gamete and planula sizes, and their rates of growth and production during the reproductive period. The size ranges of oocytes and spermaries (minimum and maximum diameters), and the mean mature ova sizes and planula volumes are summarized in Tables 15.4 and 15.5, respectively, for the principal reef-building and reef-associated eastern Pacific coral species. It is important to note that size measurements were derived largely from histologically processed samples. These measurements do not take into account shrinkage of cells and tissues that may range from 15 to 30 % (Harriott 1983; Glynn et al. 2008). This issue, and adjustments to compensate for it, is considered later under Fecundity (Sect. 15.4).
Mature ova (Stage IV) in broadcast spawning species commonly ranged from about 50–150 μm in diameter (Table 15.5). Ova in brooding corals were considerably larger, ranging from 300 to 800 μm in diameter. The planulae of brooding species (Porites panamensis and Tubastraea coccinea) were considerably larger than the ova of spawning species. This is a general trend across different coral reef regions (Harrison and Wallace 1990). Mean ovum volume measurements of broadcast spawning species were similar among species and across localities. Mature spermaries (Stage IV) ranged from about 50–250 μm in diameter.
Ovum sizes of nine species at four localities in the EEP demonstrated relatively similar dimensions with mean diameters that ranged from 90 to 117 μm (Table 15.5). Only Tubastraea has larger ova, beyond the range of the others. There are some apparent size differences with the sampled ova of Mexican corals, but whether these are consistently different will require further study. For example, at all EEP sites the mean diameters of Pavona gigantea ova exceeded 100 μm whereas at Banderas Bay, Mexico mean ovum diameter was 71 μm with a maximum diameter of 107 μm (Carpizo-Ituarte et al. 2011). Porites panamensis ova in Mexico, ~140 μm at Banderas Bay and 132–287 μm at Huatulco (unpub. data), overlap the EEP population at Uva Island, Panama with diameters of 60–250 μm (Smith 1991). Planula diameters of P. panamensis in southern Mexico (Huatulco), however, that ranged from 329 to 512 μm were notably larger than those reported from Panama, 170–330 μm (Uva Island) and 174 μm (Gulf of Panama, Table 15.5). Maximum diameters of Stage IV ova of Pocillopora verrucosa and Pocillopora meandrina from Isla Gaviota, Bahía de La Paz, Mexico were 116 µm (Campos-Vázquez et al. 2014), and comparable to the 100 µm ova of equatorial eastern Pacific Pocillopora damicornis and Pocillopora elegans of Glynn et al. (1991). Ovum and planula sizes of Tubastraea coccinea were markedly larger than those of P. panamensis at two localities in Panama. At Uva Island, the azooxanthellate T. coccinea planula volume was two orders of magnitude larger than in P. panamensis (Table 15.5). If some of these differences can be substantiated it would be of interest to explore the possibility of environmental effects, especially in upwelling and nonupwelling areas.
Ovum sizes in broadcast spawning Pocillopora and Porites species in the northern Red Sea (Boumeester et al. 2011) and on the Great Barrier Reef (Schmidt-Roach et al. 2012) are within the same size range as comparable eastern Pacific species, i.e. 100–180 μm in diameter. Eastern Pacific Pavona varians and Pavona chiriquiensis, however, produce mature ova only slightly exceeding 100 μm at all localities. Compared with coral faunas in the Caribbean (Szmant 1986) and Indo-Pacific (Harrison and Wallace 1990; Shlesinger et al. 1998), where egg diameters of diverse genera generally exceed 200–300 μm, eastern Pacific species produce eggs that are small. The planula larvae of Porites panamensis, the only zooxanthellate brooder in the eastern Pacific, are also considerably smaller than the larvae of other brooding species from the Caribbean (2–8 times smaller than in Agaricia) and Great Barrier Reef (3–9 times smaller than in Acropora) listed in Harrison and Wallace (1990).
15.2.1.6 Spermatozoa Morphology
The single study of the ultrastructure of spermatozoa in eastern Pacific corals examined Pavona gigantea and Pocillopora spp. in Costa Rica (Steiner and Cortés 1996). This study revealed inconsistencies with other suggested sexual patterns. Harrison’s survey (1990) and review (2011) have shown that the more primitive conical-shaped sperm heads are mostly associated with gonochoric species, suggesting that gonochorism is an ancestral trait in the Scleractinia. However, Pavona gigantea with conical type sperm demonstrated a mixed sexual pattern of gonochoric and hermaphroditic colonies with hermaphrodites predominant at some localities. Pocillopora damicornis and Pocillopora elegans with bullet-shaped sperm heads and elongated mitochondria are also hermaphrodites but conform closely to acroporid corals. Harrison (1990) suggested that the sperm morphology of hermaphroditic corals may be better suited for swimming, which could reduce rates of self-fertilization in species releasing combined sperm/egg bundles. However, eastern Pacific Pocillopora species, although simultaneous hermaphrodites, most likely do not form gamete bundles, therefore this pattern may not apply in this case.
Molecular phylogenetic analyses suggest that the Scleractinia consist of two large clades, the Robust and Complex corals that diverged early in the evolutionary history of the order (e.g., Romano and Palumbi 1996; Fukami et al. 2008). Pocilloporid and acroporid corals are now recognized as members of the Robust and Complex molecular clades, respectively (Kerr 2005; Fukami et al. 2008). This suggests that sperm morphology is more closely linked to sexual pattern than to phylogenetic relationship.
15.2.2 Sexuality and Mode of Development
Sexuality and mode of development are summarized for 13 scleractinian corals in six families in relation to colony morphology and presence of zooxanthellae in mature ova (Table 15.6). Six species, in the families Poritidae (Porites panamensis, Porites lobata), Siderastreidae (Psammocora spp.), Agariciidae (Pavona clavus) and Fungiidae (Diaseris), are stable gonochoric. Three species were simultaneous hermaphrodites, two in the family Pocilloporidae (Pocillopora damicornis, P. elegans) and one in the family Dendrophylliidae (Tubastraea coccinea). Mixed breeding systems, with some colonies demonstrating both gonochoric and hermaphroditic sexuality, occurred in all five species in the family Agariciidae (Table 15.6). In addition, Pocillopora verrucosa and Pocillopora meandrina in Bahía La Paz, Mexico are simultaneous hermaphrodites (Campos-Vázquez et al. 2014), and Pavona gigantea at four locations off the Oaxaca coast in the southern Mexican Pacific were found to have mixed breeding systems (Santiago-Valentín et al. 2015).
It is unclear if Tubastraea displays a mixed breeding system or is simultaneously hermaphroditic. Each polyp section is dominated by the intense staining and characterisitic large sizes of Stages III and IV oocytes. This makes earlier stages of female gametes and male gametes difficult to detect and less numerous in the section viewed. It appears that this species is simultaneously hermaphroditic with spermaries appearing at certain times of the year, while oocytes are present year round. The life histories of the simultaneous hermaphroditic genera seem to be very different, since one of them, Tubastraea, is a brooder with spermaries identified in only a few polyps, while Pocillopora spp. contain approximately the same number of oocytes and spermaries in many polyps, and are broadcast spawners .
In four of the mixed breeding agariciid species, the proportion of hermaphroditic colonies was relatively high, ranging from 16.0 % (Gardineroseris planulata) to 70.7 % (Pavona chiriquiensis). Sequential cosexual hermaphroditism, or alternating maturation of different sex gametogenesis , has been established in these species (Glynn et al. 1996, 2000). Within this hermaphroditic condition, a cosexual sequential colony alternates maturation of each gender, and therefore, alternates between release of male gametes during one spawning episode and then mature female gametes during the next spawning event. Therefore, cosexual sequential hermaphroditic colonies function gonochorically during breeding season periods.
Two of the stable gonochoric species also displayed a minimal percentage of sequential cosexual polyps. A small proportion (<1 %) of sampled colonies of the agariciid Pavona clavus was hermaphroditic over three locations. Porites lobata (Poritidae) exhibited 14 % hermaphroditic colonies (n = 50) from Costa Rica and stable gonochorism at other localities (Glynn et al. 1994). These species were classified as stable gonochoric as per Harrison (2011); in cases where hermaphrodites are relatively rare, the species can be considered to exhibit stable gonochorism and are classified as gonochoric (Harrison 2011; sensu Giese and Pearse 1974).
Brooders in the eastern Pacific, Tubastraea coccinea and Pavona panamensis, have life history strategies that appear to be very different. Tubastraea coccinea is a cryptic, hermaphroditic, azooxanthellate ahermatype, while P. panamensis is an encrusting, gonochoric, phototrophic species with zooxanthellae present in both ova and planulae. Colonies of T. coccinea contain female gametes of several stages year round, but male gametes were detected in only 3.7 % of tissue samples, and spermaries were significantly smaller than the eggs (Table 15.4). This may be due in part to the small size and faint staining characteristics of Tubastraea spermaries. An equal sex ratio, approximately 1:1, was evident in gonochoric P. panamensis (see Sect. 15.2.3). Spermary maturation in T. coccinea generally followed the sequence of planulae development at sampled locations, and sperm release in this species was observed in Panama and the Galápagos Islands (1–5 nights after full moon). Planula release in both brooding species was greatest at full moon with some new moon activity. Planulae of P. panamensis are much shorter in length (400–500 μm) than those of T. coccinea, which are bright tangerine (same color as adults) and are 0.5–1.5 μm long. Both species seem to settle close to the maternal colonies, however, the dispersal distance of T. coccinea may typically be of shorter range. Tubastraea coccinea populations can be found clustered in mass on reef frameworks or more generally on non-carbonate rock substrata, commonly under subdued light conditions, while P. panamensis can form separate nodular colonies dispersed across reef-rubble substrates. Porites panamensis can recruit to basalt outcrops and contribute to reef coral cover as well. Tubastraea contributes little to reef structures, although it has invaded and recruited to many coral communities and reefs in the Pacific and Atlantic regions (Fenner 2001; Fenner and Banks 2004; Figueira de Paula and Creed 2004). In addition, T. coccinea thrives in the Galápagos Islands, while the zooxanthellate poritid brooder does not occur at this location, but is endemic only to eastern Pacific mainland localities (Glynn and Ault 2000).
In the Indo-Pacific and Caribbean, many major reef building corals form gamete bundles just prior to spawning. Gamete bundle formation requires that polyps be simultaneously hermaphroditic and that both sex gametes are mature and ready for release concurrently. Although simultaneous hermaphroditic maturation exists in the tissues of eastern Pacific Pocillopora damicornis and Pocillopora elegans at and near full moon, no spawning activity has been witnessed in this region, and therefore no reports of gamete bundle formation. No evidence of this trait has been detected in hundreds of tissue samples collected around the suspected time of spawning.
In other regions, broadcast spawning species of Pocillopora have been observed to release gametes of both sexes in a gamete cloud. Pocillopora verrucosa, P. meandrina, P. damicornis, and P. eydouxi on the Great Barrier Reef were observed in aquaria to release gametes separately into the water column (Schmidt-Roach et al. 2012). In Okinawa, Japan and in the central Red Sea, Pocillopora verrucosa was observed to spawn clouds of mixed sex gametes in aquaria and in the field, respectively (Boumeester et al. 2011). Pocillopora eydouxi (also found in the eastern Pacific) was observed to freely spawn both sexes of gametes during the daytime in Okinawa (Kinzie 1993). Therefore, although Pocillopora species are the major reef builders in the eastern Pacific, this genus may likely not bundle gametes.
Hybridization of similar species, which is reported as a result of mass spawning events (Willis et al. 1993, 2006; Richmond 1997), may be possible in eastern Pacific corals. Pocillopora damicornis usually dominates reef flat habitats, but two additional species in this genus (Pocillopora elegans and Pocillopora eydouxi) are also often present in this zone. However, while lunar cycles of gamete maturation in two of these species (P. elegans and P. damicornis) are very similar, interannual monthly activity may vary. The potential for hybridization also exists within the family Agariciidae, but seasonal trends of each of these species at eastern Pacific locations can be very different, making this less likely, especially due to low population densities and dispersed species’ distributions.
Eastern Pacific corals exhibit a preponderance of species with mixed breeding systems. Most agariciid species have developed functional gonochorism within the hermaphroditic mode. Even poritids, whose species generally display stable gonochorism, exhibited some evidence of hermaphroditism (e.g., Porites lobata in Costa Rica). Although this breeding strategy is prevalent in the eastern Pacific, mixed breeding systems and sequential cosexual hermaphroditism have rarely been reported in the scleractinian literature. Guest et al. (2012) noted this condition in Diploastrea heliopora, a broadcast spawner near Singapore with predominantly gonochoric polyps. The colonies contained male, female and a low proportion of cosexual polyps with the most plausible explanation being that polyps switch sexes with oogenic and spermatogenic cycles occasionally overlapping. This condition occurred in corals on equatorial reefs that were chronically impacted near a large urban center.
Other cases of mixed breeding systems have been reported in different biogeographic regions. These are listed in Harrison (2011) with their respective sources. Caribbean agariciids (two species from Curaçao), Porites astreoides in both Puerto Rico and Caribbean Panama, Monomyces rubrum (Flabellidae) from South Africa, and members of the Fungiidae, Fungia scutaria in the Red Sea, Heliofungia actiniformis at Palau and Sandalolitha robusta in Hawaii all have displayed mixed breeding characteristics. In addition, Waller et al. (2005), in Guest et al. (2012), discovered all samples of three Atlantic, deep-sea species of Caryophyllia (Caryophylliidae) to contain gametes of both sexes, but with only one sex viable at a time (sequential cosexuality).
Therefore, the sequential cosexual strategy may be more common than originally considered. There may be a genetic/systematic component to the development of this type of sexual system or this strategy may develop in species adapted to more equatorial environs, to extreme or marginal habitats or perhaps both. Regardless of the conditions that may induce the development of this reproductive pattern, such corals are common in the eastern Pacific region, and numerous populations with mixed sexual systems have survived here. The agariciids with mixed sexual systems comprise more than a third of EEP species.
15.2.3 Sex Ratios and Sex Allocation
The sex ratios of eastern Pacific coral populations are influenced by fragmentation resulting from a variety of disturbances. Sea surface temperature extremes associated with ENSO events, seasonal upwelling, and variable water temperature result in an ecosystem often at the limits of coral habitation. The sex ratio of simultaneous hermaphrodites, such as species of Pocillopora, mature gametes of both sexes at the same time thereby exhibiting colony sex ratios of 1:1. Sequential cosexual hermaphroditism, wherein male and female gamete maturation alternates in each colony, should also display a 1:1 sex ratio. Although the spawning activities and sex ratios of individual colonies have not been tracked in the eastern Pacific, any deviation from 1:1 sex ratios per spawning event would be “balanced” over time for both sexes in alternating cycles of gametogenesis. The sex first matured in each colony may be genetically determined, and therefore the proportion of colonies maturing each sex may not be equal at any given time. Although sex allocation would seem to be approximately equal in simultaneous hermaphrodites (Pocillopora spp.), and sequential cosexual hermaphrodites (agariciid species), sex allocation may become more key in gonochoric and brooding species.
Except for Porites spp., the sex ratios of species that are gonochoric and/or displayed little sequential cosexual hermaphroditism frequently deviated from 1:1 (Table 15.7). (Only poritid samples of sufficiently large size, n ≥ 50 colonies, were tested.) Dominance of a particular sex was not consistent from site to site for any particular species. This could result from frequent disturbances to study (monitored) populations and/or from fragmentation creating clonal populations. For gonochoric species in the eastern Pacific, difficulty in finding a mate could be pronounced, thereby reliance on asexual fragmentation for propagation of these species may be significant.
The burden of generating sufficient energy in gonochoric species appears to be on those colonies that produce eggs, which may require more energy to produce than spermaries (Charnov 1982; Harrison and Wallace 1990). In several EP broadcast spawning species, the sequential hermaphroditic mode of development may allow for efficient allocation of reproductive resources and also offer all colonies of a species the potential to contribute to each spawning event. Two stable gonochoric species, Porites lobata and Pavona clavus, did display some degree of cosexual hermaphroditism. A low incidence of hermaphroditism (2.7 %) was also detected in a population of Porites porites subjected to urban and industrial pollution (eutrophication) in Barbados, West Indies (Tomascik and Sander 1987). Therefore, the reproductive mode in these corals may be more malleable than previously recognized and sequential hermaphroditism in at least some EP coral populations may be a response to local stressors.
Sex ratios, and therefore sex allocation, in eastern Pacific brooding species are very different. The gonochoric brooder, Porites panamensis had approximately equal female to male numbers (1.1:1 Taboga Island, Gulf of Panama and 1:0.5 Caño Island). About the same proportion of colonies sampled contained planulae (sporadic sampling, pooled over several years) (Glynn et al. 1994). Smith (1991) reported a 1:1 sex ratio for this species at Uva Island whose population was probably recruited after the1982–1983 El Niño disturbance. The ratio of female to hermaphrodite colonies of Tubastraea coccinea ranged from about 3:1 to 10:1 (Table 15.7). Spermaries are sparse and very difficult to observe in tissues of this species, and do not exhibit detectable flagella. Planulae were observed in tissues and in the field generally throughout the year, depending on the location. This genus has been reported to develop oocytes spontaneously within the tissues, which may explain egg maturation throughout the year. Allocation to sperm development appears to be very small in comparison to that of the ova. Mature eggs are 300–800 µm and mature spermaries 150–200 µm.
Siderastrea radians, a gonochoric brooder in the Caribbean, exhibits a significant female bias, which Szmant (1986) attributed to its limited capacity to bear large ova and planulae. If this condition were a genetically determined reproductive trait, then Porites panamensis might also be expected to exhibit a preponderance of female colonies, which it does not. Cabral-Tena et al. (2013) have demonstrated a gender bias in P. panamensis at some localities, with significantly greater skeletal extension and calcification rates in male compared with female colonies. If relatively high male growth rates confer some advantage during recruitment and early community succession, this might then bias selection toward a greater proportion of male colonies under certain conditions.
Two species sampled at the Taboga Island site (Pavona gigantea and Pavona varians), located immediately south of the eutrophic waters of Panama Bay (D’Croz and Robertson 1997), exhibited an excess of male colonies. A male bias was also observed in Porites porites populations along a eutrophic gradient in Barbados (Tomascik and Sander 1987). It was hypothesized that this pattern in the latter study (Caribbean) was due to increased turbidity and reduced light levels that could depress zooxanthella photosynthesis and thus deprive oogenesis of essential nutrients. Rinkevich (1989) demonstrated experimentally the contribution of zooxanthella photosynthetic products to coral reproduction. Considering the relatively small sample sizes and the high likelihood of a clonal population structure due to asexual fragmentation (see Sect. 15.5 below, Recruitment and Asexual Fragmentation), in this case it is difficult to demonstrate genetic adaptation to local conditions.
True protandrous sex change, and possible protandrous hermaphroditism from initial male function in small corals to female function in larger corals, has been demonstrated for several solitary fungiid species (Loya and Sakai 2008; Loya et al. 2009; Harrison 2011). The possibility of sex change in Diaseris distorta was examined in a size series collection (n = 24) in the Galápagos Islands (Colley et al. 2000). Only a single female was found (1190 mm2, surface area). Since several smaller (238–1088 mm2) and larger (1260–1980 mm2) males were collected, and two males were the same size as the female, no relationship between size and sex was evident. However, the skewness of the sex ratio (males dominated the population 5.4:1, p < 0.001, chi square) toward males and the strong evidence of fragmentation (only one sexual recruit could be identified) may be keeping individual size small, and therefore, possibly predominantly male, if maleness is related to small size. It is also likely that prolific daughter fragment production is creating a male dominated clonal population. Size series collections of all species are discussed in Sect. 15.2.3 below, Sex Ratios and Sex Allocation.
15.2.4 Age at Sexual Maturity
Size series collections of reproductively active colonies have allowed estimates of the ages to first reproduction of several species. Coral ages can be estimated by relating known species skeletal growth rates to the greatest growth axes of colonies. There are drawbacks to this method however, e.g., (a) biased sampling of larger more easily encountered colonies, (b) highly variable colony growth rates, and (c) fragmentation and fusion that can obscure the true ages of colonies.
Examination of a size series of Diaseris distorta in the Galápagos Islands revealed sexual maturity in small individuals, and likely at an early age (Colley et al. 2000). Both asexual fragments and sexually derived individuals, from 1.8 cm to 2.7 cm in skeletal diameter, were reproductively active, i.e. bearing mature gametes. The ages of these fungiids are not known, but it is possible they approximate two years (Yamashiro and Nishihira 1998). Most adult individuals of D. distorta in the study population ranged from 3.7 to 5.0 cm in diameter (Feingold 1995). Colony growth is critical in early benthic stages vis-à-vis interphyletic competition for space.
At Uva Island, Panama the mean size of colonies of six sampled species with gonads varied substantially (Table 15.8). The smallest colonies bearing gonads belonged to the encrusting species Pavona chiriquiensis with a mean vertical growth axis of 1.6 cm in females and 2.5 cm in male colonies. Applying a mean skeletal growth rate of 3.0 cm year−1, this extrapolation suggests that both sexes can reach reproductive maturity in less than one year. Sexually mature colonies of Pavona varians, also with an encrusting growth habit, were notably larger and possibly much older than P. chiriquiensis, i.e., 10 years in age in both sexes. The remaining agariciid species and Porites lobata possess large, dome-shaped colonies with reproductive ages similar to Pavona varians. The relatively large female colonies of Pavona clavus may be a result of biased sampling. Small colonies of this species were unavailable.
Rapid recovery is especially important in environments with high nutrient and light levels that favor opportunistic algae and other epibenthic taxa (Birkeland 1977; Highsmith 1980). Eastern Pacific reef substrates generally consist of low-lying turf algae, due in large measure to intense fish and echinoid grazing (Birkeland 1997; Fong et al. 2006; Wartian 2006). Ten-cm high coral reproductive colonies would offer an escape in size, generally extending above micro-filamentous algal films or turf substrates. Species with very young maturation ages, such as Pavona chiriquiensis, could have an advantage in population recovery. Presumably they would have more rapid generation times and hence be better poised to adapt to changing environmental conditions. The large age differences in reaching sexual maturity in eastern Pacific agariciid species would offer an ideal opportunity to test for such a life history difference.
15.3 Timing of Reproduction
Interest in coral reproductive schedules is long-standing, from the first systematic studies conducted during the Great Barrier Reef Expedition (1928–29) to the present (e.g., Fadlallah 1983; Shlesinger and Loya 1985; Szmant 1986; Oliver et al. 1988; Harrison and Wallace 1990; Richmond and Hunter 1990; Richmond 1997; Guest et al. 2005; van Woesik 2009; Harrison 2011). Harrison and Wallace (1990) and Harrison (2011) have offered comprehensive reviews of the environmental factors implicated in regulating the timing of coral reproduction. These range from annual (seasonal) to lunar and diel time-scales, and include such proximate controls as sea temperature, day length, lunar cycles, daily light/dark periods, moon light, calm spells, and tidal height. Of the various factors implicated in regulating annual to daily reproductive processes, it is necessary to caution that the proposed causalities have not been unequivocally demonstrated.
Although lunar cycles of eastern Pacific corals may be similar, the seasonal and diel timing, and differences in annual temperature regimes may prevent multiple species from spawning together. Pavona varians and Pavona chiriquiensis have spawned monthly in the Gulf of Chiriquí during the dry season (January–April), but do so 12 h apart. Pavona clavus is reported to mature gametes monthly at Culebra, Costa Rica (Bezy 2009), but in Panama spawning of this species is restricted to the middle of the wet season, i.e. July–October (Glynn et al. 2011). Gardineroseris planulata favors the warmest time of the dry (non-upwelling) season, and Pavona gigantea is most active in the Galápagos Islands during the coolest time of year.
Long-term studies have shown that warmer or cooler years result in a variety of breeding outcomes. In the Galápagos Islands, a warm water pulse of about two months duration in 1991 accompanied extended and year round gamete production in Pavona varians and Pavona chiriquiensis (Colley et al. 2006). Pocilloporid species (Pocillopora elegans, Pocillopora damicornis) are particularly good examples of variable reproductive activity. For example, mature ova and sperm were usually observed in tissues at full moon, but some collections over a span of 20 years have shown a variety of gametogenic seasonal patterns during several months (Glynn et al. 1991; Colley et al. 2006). This is in contrast to the results of Campos-Vázquez et al. (2014) who found other pocilloporid species, namely Pocillopora verrucosa and Pocillopora meandrina in Bahía La Paz, Mexico to have one annual gametogenic cycle from approximately May to September (a 16-month study). Commencement and completion of these gametogenic cycles coincided with seawater temperatures between 23–30 °C.
In addition, although ripe gametes have been found historically in both Pocillopora elegans and Pocillopora damicornis on and just after full moon, field observations have never revealed gamete release in these species. Therefore, it is possible that at least some eastern Pacific species may release gametes on the same lunar days, but generally seasonal months of release can vary between species, due to location, variations in yearly temperature regimes, and presumed sensitivity or insensitivity to temperature (Colley et al. 2006).
Sensitivity to water quality can affect six chemically-mediated steps in the reproductive cycle of broadcast spawning corals: (1) synchronization among conspecific colonies, (2) egg-sperm interactions leading to fertilization, (3) embryological development, (4) larval dispersal and substratum searching, (5) metamorphic induction, and (6) acquisition of zooxanthellae in those species that are lacking vertical, maternal transmission. Pollutants, including freshwater, can result in the failure of one or more of these links, and such interference may result in a cascade effect preventing the replenishment of reef populations (Richmond 1994, 1997).
As the eggs of most coral species float following release, they are subjected to surface waters for varying periods prior to fertilization. As fresh water is less dense than seawater, rainfall and coastal runoff reduce the quality of this upper layer where eggs generally become fertilized. Even moderate reductions in sea surface salinities to about 15 ‰ from 35 or 30 ‰, can result in 50 to 90 % reduction in fertilization rates (Richmond 1994). As eastern Pacific spawning events span the wet season (and peak ENSO thermal anomalies), increasing levels of coastal watershed runoff can lead to reproductive failure following these periods. The addition of sediment and nutrients from runoff (Humphrey et al. 2008) as well as agrochemicals and hydrocarbons further reduce fertilization rates over larger areas. Most eastern Pacific coral species mature gametes during the wet and generally warmer season, which approximates mid–April to mid–December. Eggs of many eastern Pacific coral species are neutral or negatively buoyant as described below (Sect. 15.3.5, Spawning and Gamete Characteristics), and thus may avoid surface water stress effects. Location may also be important since Psammocora spp., which mature gametes in nonupwelling Costa Rica in the wet season, are not reproductively active at the same time of year in the nonupwelling Gulf of Chiriquí, Panama.
15.3.1 Seasonal
Sexual reproductive activity is generally greatest seasonally during warm or non-upwelling periods in the three major eastern Pacific upwelling centers (Chávez-Romo and Reyes-Bonilla 2007; Carpizo-Ituarte et al. 2011; Rodríguez-Troncoso et al. 2011; Glynn et al. 2012). The reproductive activity of Pocillopora damicornis and Pavona gigantea along the Mexican coast is mainly restricted to the summer months (May-August) when sea temperatures are at a maximum (>27–28 °C). This pattern has also been shown in Pocillopora verrucosa and Pocillopora meandrina (Campos-Vázquez et al. 2014). In central and southern Mexico, Porites panamensis is reproductively active from March or May to September, but in seasonally upwelling Gulf of Tehuantepec (Huatulco), P. panamensis is most active after upwelling ceases and later, from March until August (Rodríguez-Troncoso et al. 2011). Curiously, a year-long sampling of P. damicornis off southern Mexico (Huatulco, 15.7°N), including the height of the warm season during 2002–2003, failed to reveal any reproductive activity (Rodríguez-Troncoso 2004).
Further south, near the equator, coral reproduction is also most pronounced during the warmest times of year. At Costa Rica, about 6° latitude farther south, Pavona clavus was active monthly in Culebra Bay (10.5°N) from May to November, during the non-upwelling season. In the Gulf of Chiriquí, Panama (8°N), which is subject to seasonal thermocline shoaling, the reproductive season was more restricted to the warmest months. Similarly, in the equatorially-situated Galápagos Islands, reproductive activity was pronounced but limited to the warmest time of year. Spawning was observed every month from August to October in the Gulf of Panama (Glynn et al. 2012) and within this time frame at Culebra Bay, Costa Rica (Bezy 2009). This is at the height of the wet season (non-upwelling) at both localities.
However, there are exceptions to reproduction occurring in the warmest seasonal period. During seasonal upwelling in the Gulf of Panama (January–April) and the cool season in the Galápagos Islands (June–December), the latter region under the influence of the Oceanic Peru Current, coral reproduction in most species ceases or falls off (Colley et al. 2006). In Galápagos waters, where mean temperatures decline rapidly in May and June (from 25 to 22 °C and lower), Pavona gigantea alone contained mature gametes during much of the cool season. This suggests unusual local adaptation of this species to low thermal conditions (Glynn et al. 1996). In the Gulf of Panama, most species do not initiate gametogenesis during the upwelling months. Pavona gigantea, however, is reproductively active there during both the upwelling and non-upwelling seasons.
In non-upwelling areas in the EEP where mean sea temperatures remain high throughout the year (28–29 °C), gametogenesis is often more prolonged with some species exhibiting gametogenesis year-round (Fig. 15.7). In the Gulf of Chiriquí, Panama, Pocillopora spp. were active year around, with Pocillopora damicornis exhibiting a decline at the beginning and end of the calendar year. Pavona chiriquiensis and Pavona varians also show year-round reproductive activity at this locality and in non-upwelling Costa Rica, but are limited to the warm seasons in the Galápagos Islands and the Gulf of Panama.
Timing of seasonal, lunar and diel reproductive activities in 11 broadcast spawning EEP zooxanthellate corals. Data are primarily from presence of mature gonads in histological preparations and spawning observations at Uva Island reef, Gulf of Chiriquí, Panama. Lunar and diel spawning times supplemented with observations from Gulf of Panama (Pocillopora inflata, Pavona clavus) and Galápagos Islands (Pavona gigantea). Modified after Glynn et al. (2012)
Porites panamensis , a brooder, reproduces year-round in the EEP at most sites (Smith 1991; Glynn et al. 1994), with reproductively active colonies present in the Gulf of Panama from April–May until November, one month before the onset of upwelling. Planula development occurs in the Gulf of Panama only after upwelling ceases, however, in the Gulf of Chiriquí planulae were released at this and other times of the year (Smith 1991). Therefore, it is most likely that P. panamensis has an extended period of larval release in the non-upwelling Gulf of Chiriquí.
Some studies conducted at low latitude reef sites elsewhere, where environmental conditions such as sea temperature and solar radiation are relatively constant year round, reported extended spawning seasons. These extended over several months or during most of the year (Oliver et al. 1988; Richmond and Hunter 1990; Penland et al. 2004; Mangubhai and Harrison 2009) as observed at equatorial locations in the eastern Pacific. Longer spawning seasons are not restricted to lower latitudes, however; for example, in the Solitary Islands, Australia at 30°S latitude, reproductive activity is spread over several months (Wilson and Harrison 2003). And at some equatorial locations (e.g. Singapore) coral species spawn seasonally, mainly during a 2-month period (Guest et al. 2005).
A general decline in the reproductive condition of corals has been documented during severe elevated sea temperature bleaching events in several studies from the Caribbean to central/west Pacific regions (Table 15.9). Whole bleached coral colonies or bleached patches on colonies demonstrate a variety of adverse effects that are often manifest several months after recovery. These range from declines in the percentage of reproductive colonies that survived and recovered from bleaching to lowered rates of fertilization, reduced sperm motility, reductions in egg size and numbers (Table 15.9). From Mexico to the EEP, all major reef-building corals have demonstrated varied stages of decline in reproduction associated with ENSO elevated thermal stress (Glynn et al. 1985; Colley et al. 2006; Carpizo-Ituarte et al. 2011; Rodriguez-Troncoso et al. 2011). A study of the effects of bleaching on the Great Barrier Reef not only demonstrated declines in coral reproductive success during and shortly after the stress event, but also suggested that reproduction was impaired one year later and that bleached corals showed an increased susceptibility to future stress (Ward et al. 2000).
Moderate ENSO warming, however, has been observed to increase gonadal activity in certain eastern Pacific localities where low temperatures often depress or inhibit development. Colley et al. (2006) documented that all monitored EEP coral species reproduced during the 1997–98 bleaching event. At Uva Island from 1982 to 1996, sexual recruitment in Pavona varians was significantly related to maximum monthly positive sea surface temperature (SST) anomalies that occurred in the year preceding recruitment (Glynn et al. 2000). Recruitment failed when SST anomalies exceeded 1.6–1.9 °C during the severe ENSO events of 1982–1983 and 1997–1998, suggesting an elevated temperature threshold for this species. Sexual reproduction in the Gulf of Panama normally ceases during the upwelling season when mean SSTs decline below 24–25 °C. In 1998, when upwelling was suppressed, Porites panamensis, Porites lobata and Pocillopora spp. demonstrated unseasonable reproductive activity (Colley et al. 2006). Cox (2007) has shown that the sexual cycle of Montipora capitata in Hawaii was not disrupted in spite of bleaching. She hypothesized that this species was able to produce sufficient energy stores for reproduction by feeding heterotrophically, a capacity for trophic gains demonstrated by Grottoli et al. (2004, 2006).
15.3.2 Lunar
Compared with coral reef regions with species-rich faunas where multiple and synchronous spawning events are common-place, e.g. in the Caribbean, central and western Pacific, and Indian Ocean (Szmant 1986; Guest et al. 2005; Harrison 2011), lunar spawning schedules in the eastern Pacific are relatively diffuse. Most low latitude eastern Pacific broadcast spawning species produce mature gametes during 3 to 4 day periods before or following full moon (Fig. 15.7). Several species display a post-full moon bias of from one to four days. Six species also show heightened sexual activity (mature gametes) near new moon. Pocillopora inflata spawned gametes on lunar day 24, two days after a waning quarter moon. This record is atypical and may be due to stress associated with its collection. The spawning occurred in a 20 l container, albeit with frequent water renewal, about 30 min after collection.
Therefore it may generally be concluded that eastern Pacific corals spawn on or around the 17th lunar day and 1–2 days following (Fig. 15.7). Pavona varians, Pavona chiriquiensis and Pavona clavus begin gamete release on these days (Glynn et al. 2001a, b, 2011). Pocillopora damicornis and Pocillopora elegans harbor mature gametes on full moon (day 15) and spent gonads were observed in tissue samples 2–3 days later (Glynn et al. 1991). Peak percent colonies of Porites lobata with mature gonads occur at full moon and taper off a few days later.
There are at least eight morphospecies of closely related pocilloporid corals that occur on EEP reefs (but see Chap. 14, Pinzón). Only two of these (Pocillopora elegans and Pocillopora damicornis) have been studied extensively and contain ripe gametes of both sexes around full moon. Although there is evidence of variation in the annual breeding behavior of these Pocillopora species, the potential exists that these, and perhaps a third pocilloporid (Pocillopora eydouxi), spawn synchronously at least occasionally. Species in the Caribbean reef-building Orbicella/Montastraea complex spawn on the same days and times annually during the warmest time of year (Van Veghel 1993). Structurally important Acropora species, as well as many other corals, spawn on the Great Barrier Reef in the highly visible mass spawning event, again in the warm season. However, Pocillopora species, the major contributor to reef framework in the eastern Pacific appear to be more sensitive to thermal conditions than other species of this region, and thus their timing of gamete release may vary annually (Colley et al. 2006).
15.3.3 Diel
Spawning was observed in only six species, including Pocillopora inflata. Spawning in P. inflata could have been stimulated prematurely due to collection (Fig. 15.7). Multiple interspecific spawning is unlikely among several of the eastern Pacific species since species-specific spawning activity has been witnessed during daylight hours, soon after sunset, and just before sunrise. Pavona gigantea was observed spawning during daylight hours in the Galápagos Islands. Several colonies spawned on two different days, during a flooding spring tide beginning at 1700 and lasting for about 30 min. Pocillopora inflata also spawned during daylight hours (0730–1030), but this observation may be atypical as noted above.
Two closely related encrusting species, Pavona chiriquiensis and Pavona varians, spawn gametes on the same lunar days (2–3 days after full moon) during multiple months in the dry season (Glynn et al. 2000, 2001a, b). Gamete release was also associated with peak high-water stands of spring tides. However, P. chiriquiensis spawned about one hour after sunset at Uva Island and P. varians began spawning 1–2 h before sunrise at this location with some colonies continuing to spawn until about 0830. No overlap or hybridization would be expected in these species since their diel timing is approximately 12 h out of phase. The spawned gametes observed after 12 h appeared to lose vitality. This timing is well beyond gamete viability and dilution effects of a few hours for corals on the central Great Barrier Reef (Oliver and Babcock 1992).
Histological and field observations indicated that spawning of Pavona clavus in the Gulf of Chiriquí was centered around full moon, most frequently on lunar day 17 and near sunset (1800 h) (Glynn et al. 2011). In Culebra Bay, Costa Rica, an upwelling site, P. clavus also spawns on the 17th lunar day, 2–3 days after full moon (Bezy 2009).
It is likely that overlap in spawning may occur between species of Pocillopora. Pocillopora damicornis and Pocillopora elegans display very similar gamete development, however, differences in thermal sensitivities may prevent synchronous gametogenic cycles in at least some locations. In Bahía La Paz, Mexico, gamete maturation occurs at the same time of year (September) in Pocillopora verrucosa and Pocillopora meandrina (Campos-Vázquez et al. 2014), but further study is needed to determine if there is any lunar day synchronization. In the Gulf of Chiriquí, gametogenesis occurs year round in the multi-year pooled sample set, but although gamete maturation occurs at full moon, no spawning activity has ever been observed, even after the completion of numerous monitoring exercises in the field.
As reasoned above, the likelihood of spawning overlap of the majority of studied eastern Pacific corals is slight. Some overlap of Pavona varians and Psammocora stellata is possible since both species spawn at night around full moon, but the diel timing of P. stellata is still unknown. According to the seasonal (June, October), lunar (full and new moon), and diel (1800 h) schedules, Pavona clavus and Pavona chiriquiensis could conceivably spawn at the same time. But, oocyte maturation peaks in these two species in different seasons. Colonies of both species occurring in close proximity at Uva Island have been monitored to explore this possibility, but simultaneous interspecific spawning has not been observed.
15.3.4 Length of Gametogenic Cycles
The spawning seasons of eastern Pacific species are generally variable in length. The seasonality and duration of breeding at each study site are species or genus specific and also depend on the local temperature regime. However, gametogenic cycles of different species can overlap, and it appears that several eastern Pacific corals can release gametes multiple times during their respective breeding seasons. Although sampled colonies in the EEP were not tagged, and data from all years’ collections were recorded but pooled, histological collections suggest that gamete release in many eastern Pacific coral species can occur at least monthly during this time. At least four species at Uva Island (Pocillopora elegans, Porites lobata, Pavona varians, Pavona chiriquiensis) produce mature gametes year round (Glynn et al. 2011). Eight of the Uva reef species showed evidence of spawning or loss of mature gametes at or up to four days following full moon, and six of eight species exhibited some evidence of split spawning with possible gamete release occurring a few days around new moon as well.
Observed multiple spawning episodes could indicate population behavior and not necessarily that of individual colonies. That is, individual colonies may not spawn on multiple occasions, but that a population may exhibit multiple spawning behavior due to different colonies spawning at different times. The population of Pavona clavus in the Gulf of Panama spawned in August, September and October, however, in one year with 4-month consecutive observations, the sampled colonies spawned every other month. Spawning behavior of Pavona varians and Pavona chiriquiensis was observed on the Uva Island reef in January and April 1997, and in May of the same year in a flow-through sea water tank at Naos Island (Panama).
Whether the full moon falls at the beginning of a month or near its end can influence in which calendar month lunar synchronized coral species spawn. Since many corals spawn a few days after full moon, if the full moon falls late in the month, spawning can occur within the next calendar month following. For example, in Orbicella annularis, mature gametes were present in August–October in 1983, but only in August and September in 1984 (Szmant 1986). This was associated with the full moon falling a week earlier in 1984 than in 1983. There also was evidence that individual colonies spawned more than once. The association of spawning with the lunar period may represent the fundamental timing mechanism used by corals to achieve synchronized spawning within a population, while factors such as temperature and photoperiod, which have important roles in regulating reproductive cycles, may control initiation of gametogenesis (Szmant 1986). Therefore, spawning episodes of different years may not occur in exactly the same set of months. This could artificially “extend” the breeding season viewed in pooled annual data. However, there is ample evidence that at least some species of eastern Pacific corals release gametes monthly over a period of several months and that individual colonies of at least some species spawn monthly as well.
Thus, the exact cycle of gamete development is still somewhat unclear. In support of the monthly developmental cycle, mature gametes were observed in tissues of several species approximately one month after collections showed no gametogenic development. For example, in the Gulf of Panama, gametes were found in tissues at the beginning of April–May just after upwelling ends, and mature gametes were present in tissues approximately a month later. Histological slide preparations have revealed Stage IV eggs and spermaries present in tissues over several months in the same year. And the sexual activity of agariciid corals, of which most species in the eastern Pacific have colonies that display overlapping male/female/male generations, suggest that monthly completion of gamete development is likely.
15.3.5 Spawning and Gamete Characteristics
Similar spawning characteristics were observed in four agariciid species, two encrusting and two with massive colony morphologies. Of the encrusting colonies, gametes from Pavona varians were positively buoyant with sperm released in a slow-rising diffuse cloud and ova in mucus-bound strings. The gametes, however, did not rise to the surface, but remained in the water column close to the spawning colonies. Spawning in Pavona chiriquiensis (also encrusting) was similar with neutral to negatively buoyant gametes that did not disperse far from the parental colonies. Ova and sperm were emitted slowly from Pavona gigantea, a massive species, in mucus-bound filaments that appeared to be nearly neutrally buoyant. Gametes remained suspended in the water column where they were dispersed by currents, imparting a milky appearance to the water in the immediate spawning area. Gametes released by Pavona clavus (massive colony morphology) were enveloped in mucus-laden strings that adhered to colony surfaces. Ova of P. varians and P. chiriquiensis were beige or white and dark green respectively, the latter possibly cryptic after sunset.
Agariciid ova are generally small and contain very fine lipid granules (see Sect. 15.2.1.1 and Fig. 15.5). Although mature agariciid ova generally stain lightly in contrast to the ova of some other eastern Pacific species, the ova of Psammocora (2 spp.) and Diaseris (1 sp.) also are small and stain lightly. Perhaps a more complex yolk that stains darkly (with Azocarmine), such as that displayed in the broadcast spawners Porites and Pocillopora, is not necessary or would interfere with the non-buoyant type of dispersal described above. Two species of Pocillopora (P. damicornis and P. elegans) possess ova that are laden with large lipid vesicles (Fig. 15.2). This would suggest positive buoyancy and perhaps contributes to the release of gametes, which would rise to the surface and disperse.
Spawning events in the western Pacific are closely associated with neap tidal cycles in several species and as a result tidal rhythms have been proposed to serve as proximate spawning cues. Neap tidal spawning has been hypothesized to promote the retention of sexual products on natal reefs (see Harrison and Wallace 1990). However, the four Pavona species (P. gigantea, P. varians, P. chiriquiensis, P. clavus) that were observed spawning in the eastern Pacific did so during the flooding phase of spring tides. Whether this would help to disperse gametes and larvae off reef with a positive effect on survival or some other life history function, is in need of study (Strathmann et al. 2002).
In summary, the asynchronous spawning observed in the eastern Pacific is similar to that reported in the northern Red Sea (Shlesinger and Loya 1985; Shlesinger et al. 1998; but see Hanafy et al. 2010), in Hawaii (Kolinski and Cox 2003), and other areas in the central and western Pacific (Richmond and Hunter 1990). While different coral assemblages demonstrate asynchronous and more protracted spawning across a wide range of latitudes, these patterns are generally more common on reefs in equatorial locations (Baird et al. 2009; Harrison 2011). Several studies have suggested that mass spawning is adaptive because the large volume of gametes released would satiate predators and allow for relatively high survival. This argument can also be inverted for coral faunas that demonstrate irregular spawning schedules. For example, it might be difficult for potential predators to evolve focused feeding behaviors for diverse species that are often relatively dispersed and that release gametes at different seasons, lunar phases and time of day.
15.4 Fecundity
Ovum and larval production rates (fecundity) are essential processes in coral reproduction, and for eastern Pacific corals the importance of sexual reproductive success may vary since several species appear to depend significantly on asexual fragmentation. As noted above, gonad and gamete development may occur up to a certain stage, but then be interrupted by a stress event such as a rapid elevation of sea temperatures. A reduction in fecundity or cessation of gametogenesis and larval development can then greatly reduce or nullify sexual reproduction, dispersal and recruitment.
Fecundity estimates have been determined for some of the more common eastern Pacific corals, but most comprehensively at three of four equatorial localities (Table 15.10). For the majority of the studied zooxanthellate species, thousands of ova cm−2 are produced annually at several sites although in a few instances this may vary by an order of magnitude. Most species of Pavona and Psammocora have demonstrated mean annual fecundity values of 10–20 × 103 ova cm−2 at non-upwelling sites (Caño and Uva Islands) and in the Galápagos Islands, the latter region characterized by variable and seasonally cool conditions. The large massive species, Porites lobata, Pavona gigantea, and Gardineroseris planulata, exhibited the lowest fecundities, often producing fewer than 5 × 103 ova cm−2 year−1. While Porites lobata produced only 40 to 100 ova cm−2 year−1 in the Galápagos Islands, Pavona gigantea was highly fecund there, exhibiting between 10 to 31 × 103 ova cm−2 year−1. Considering all species at all sites, high fecundities were observed at Uva Island and the Galápagos Islands, each represented by three species in the genera Pavona and Psammocora. Diaseris distorta, sampled only where abundantly present in the Galápagos Islands, exhibited the highest fecundity of all studied species, ranging from 32 to 104 × 103 ova cm−2 year−1, depending on the number of annual gametogenic cycles. The high levels of fecundity are unexpected across sites since temperature regimes differ significantly, being generally stable at Uva Island and highly variable in the Galápagos Islands. Overall, fecundities were lowest in the seasonally upwelling Gulf of Panama where temperatures often reach stressfully low levels during a three and one-half month period at the beginning of each calendar year, and gametogenesis is not initiated until after upwelling ceases.
Eight eastern Pacific species, all broadcast spawners , are clustered around relatively small ovum sizes of ~100 μm, but demonstrate a wide range of fecundities (Fig. 15.8a). Seven of these species (Pavona, 4 species; Psammocora, 2 species; Diaseris distorta) exhibited annual fecundities exceeding 104 ova cm−2. Three species, Pavona gigantea and Pavona clavus, and D. distorta exhibited the highest fecundities at some localities, in excess of 3.0 × 104 ova cm−2 year−1. These occurred in sample sets from the Galápagos Islands. Pavona varians and Pavona chiriquiensis (Pavona spp.) also demonstrated high fecundities, in excess of 103 ova cm−2 year−1. These high fecundities occurred in non-upwelling environments (Caño Island, Uva Island, Galápagos Islands). Porites lobata, the seventh broadcast spawner , produced ova sizes at Caño and Uva Islands that were relatively large with fecundity estimates in excess of 103 cm−2 year−1. The Galápagos fecundity estimates for Porites lobata (40–100 ova cm−2 year−1) are probably uncommonly low due to unfavorable local conditions. As noted above, these values were calculated from a single, presumably mature small ovum. This species was sampled in the Canal de Itabaca (Santa Cruz Island) in a small, shallow embayment with limited circulation. In addition, the colonies were small and generally in poor condition (i.e. with pale/mottled tissue and algal filaments in the gastrovascular cavity). The two brooding species, Tubastraea coccinea and Porites panamensis, produced planulae that were consistently larger than the ova sizes in broadcast spawning species. This trend is consistent with observations in the Indo-Pacific and Caribbean regions (Harrison and Wallace 1990).
Size of mature ova and planulae versus fecundity of scleractinian corals. a Ten equatorial eastern Pacific species sampled at four localities (see Tables 15.1 and 15.6). b Indo-Pacific and Caribbean species (n = 32) slightly modified after Harrison and Wallace (1990). Multiple fecundity values plotted for a specific mean ovum or planula size express the range of observed estimates. To assist in comparing two data sets, horizontal and vertical lines in ‘b’ denote ranges of ova/planula sizes and fecundities of eastern Pacific species illustrated in ‘a’. Ranges of planula diameters (vertical line) in azooxanthellate brooder, and fecundities (horizontal line) in zooxanthellate brooder were very small, respectively, and therefore are not shown. Pavona spp. are P. varians and P. chiriquiensis; since ova diameters were statistically equal they were averaged; fecundities were also averaged due to similarity of annual spawning cycles (Glynn et al. 2000)
Comparing the size of reproductive products and fecundity of corals in the eastern Pacific with other regions, two major differences stand out. The first is the smaller size of eastern Pacific ova, all with mean diameters <200 μm, and brooder larvae ~300 μm in diameter or less (Fig. 15.8a). Ova sizes of Indo-Pacific and Caribbean species cluster between 200 and 600 μm in diameter (Fig. 15.8b), and the planulae from these regions range between 700 and 1500 μm in diameter (Fig. 15.8a, b). However, ova size of species in the families Poritidae, Agariciidae, Pocilloporidae, and Fungiidae from the Indo-Pacific and Caribbean also tends to be small (Harrison and Wallace 1990). Therefore, this pattern may be phylogenetically determined and species with small ova may have a survival advantage in the eastern Pacific. It is also possible that small ova and planulae are a result of a life history strategy that limits time for lipid storage due to frequent and rapid gametogenesis.
The second difference is the notably high fecundity, 103 to 105 ova cm−2 year−1, demonstrated by several eastern Pacific broadcast spawning species. The few observations available indicate that eastern Pacific brooding species are also moderately fecund, but more data are needed to make any meaningful interregional comparisons. Perhaps the development of small ova with less complex yolk allows the generation of more numerous gametes during any given reproductive cycle. Such an opportunistic reproductive trait may be advantageous in an unstable environment such as the eastern Pacific, which is subject to sudden and frequent fluctuations in physico-chemical conditions. Thus, the marginal setting of the eastern Pacific may favor the colonization and persistence of highly fecund coral taxa with small eggs.
15.5 Recruitment and Asexual Fragmentation
15.5.1 Sexual Recruitment
Coral populations are maintained and replenished primarily by sexual larval recruitment and asexual fragmentation (e.g., Highsmith 1982; Harrison and Wallace 1990; Richmond 1997). The asexual regrowth or budding of patches of tissue resulting from partial mortality during disturbance events, such as bleaching, may also be important in perpetuating colony survival (Fong and Glynn 2001; Glynn and Fong 2006). Sexual recruitment of settling larvae has been studied by the deployment and retrieval of artificial surfaces (settling plates) or the appearance of new juveniles on natural reef substrates. The former method focuses on the number of larvae arriving and settling, whereas the latter focuses on the number of juvenile corals surviving until they are visible to the naked eye (i.e. ≥1 cm). Asexual recruitment is inferred from the appearance of colonies according to the criteria noted below in Sect. 15.5.5. The reader is referred to Mundy (2000) for an appraisal of methods employed in sexual recruitment studies, and Baird et al. (2006) for fluorescence, and Edmunds (2010) for aluminum tag detection techniques. All methods described here (Table 15.11) are based on corals counted on natural substrates and artificial surfaces.
Sexually produced planula larvae or genets, which settle onto suitable substrates, are distinguished from ramets that are genetically identical fragments originating from pre-existing colonies. Here a recruit is defined as a new individual introduced to a population whether as a sexual recombinant planula (genet) or an asexual fragment (ramet). Both modes of reproduction offer advantages under different environmental conditions, summarized hypothetically by Pinzón et al. (2012) as follows. Highly clonal species generating ramets would tend to dominate local optimal habitats as asexual reproduction (fragmentation) is important to local persistence. Conversely, sexual recombination increases diversity and would contribute to long-range dispersal and colonization of new habitats and would, therefore, be favored in variable and/or unpredictable environments.
In contrast to central and western Pacific reef areas, notably low rates of sexual recruitment were reported in the first eastern Pacific studies (Birkeland 1977; Wellington 1982; Richmond 1985; Reyes-Bonilla and Calderón-Aguilera 1994). In the Gulf of Panama, no sexual recruitment was observed in the Pearl Islands over an 8-mo period (Wellington 1982) or very low rates of ~1 recruit m−2 over 5 years (Birkeland 1977), and 11 colonies of Pocillopora sp. after 15 months (Richmond 1985). These low rates of sexual recruitment of the major reef-building species were cited as evidence of the maintenance of eastern Pacific reefs by asexual propagation and long distance dispersal from central/western Pacific populations. In more recent studies in Mexico, low rates of sexual recruitment have also been reported for the major branching and massive reef-building species (Medina-Rosas et al. 2005; López-Pérez et al. 2007). However, one study off the southern Mexican coast of Guerrero reported a low level of sexual recruitment of Pocillopora damicornis (García-Ocampo 2005). Recruitment rates at five localities ranged from 0.1 to 0.3 colonies m−2 year−1 to maximum rates of 0.6–0.8 colonies m−2 year−1.
Since the early findings from Panama, continuing studies have added to the data base of coral recruitment for the principal eastern Pacific species at several localities. These data are from colonies that recruited to natural and artificial plots monitored over periods that ranged from <1 to 22 years. In the majority of these studies, no distinction was made between sexual and asexual recruitment. The calculated rate estimates do, however, allow for comparisons among species and sites. Inspection of Table 15.11 indicates that recruitment rates are highly variable and generally low for broadcast spawning species, usually with mean colony densities of <1.0 m−2 year−1. The highest recruitment rates for broadcast spawners (Pocillopora elegans, Pavona varians, Porites lobata), ~1.0 colony m−2 year−1, occurred at non-upwelling sites in Costa Rica and Panama. The sexual recruitment of P. lobata was shown by Boulay et al. (2012), utilizing microsatellite markers, to be the predominant mode of recruitment at Isla del Coco, Costa Rica. Although recruitment rates were not reported, the repopulation of P. lobata by sexual means has contributed importantly to the recovery of Cocos Island reefs during the last two decades (Guzmán and Cortés 2007). Recruitment of Porites panamensis, the single zooxanthellate brooding species, was considerably higher than the broadcast spawners, often exceeding 1.0 colony m−2 year−1, and as high as 20.4 colonies m−2 year−1 at one site in Mexico (Montosa Island, Huatulco).
Millepora intricata, a hydrocoral, has only been found in the eastern Pacific in the Gulf of Chiriquí, Panama. At Uva Island colonies are common on the reef slope (2–5 m depth), but are among the first zooxanthellate species to bleach and die during ENSO warming events (Glynn 1990). This species is opportunistic, among the first to recruit to reef slopes after mortality events, presumably from deeper (8–15 m) refuge populations in cooler water. Although colonies of M. intricata easily fragment, recolonization of the shallow reef slope presumably occurs via sexual recruits (Smith et al. 2014).
15.5.2 Asexual Fragmentation
Of the different kinds of asexual reproduction in corals that give rise to new individuals or colonies, e.g. budding and transverse or longitudinal fission, polyp bail-out, detachment of polyp balls, and asexually produced planulae (Harrison and Wallace 1990; Richmond 1997), asexual fragmentation is the predominant process in the eastern Pacific. Several studies have supported the importance of this reproductive mode (e.g., Glynn et al. 1972; Highsmith 1982; Guzmán and Cortés 1989; Colley et al. 2000; Glynn et al. 1996, 2011; Lirman et al. 2001; López-Pérez et al. 2007; Aranceta-Garza et al. 2012).
Although storms can cause fracturing, especially in ramose species (Jiménez 1998), coral fragmentation often has a biotic provenance, i.e. it is initiated or exacerbated by bioerosion (see Chap. 12, Alvarado et al.) and corallivory (see Chap. 10, Enochs and Glynn). Bioerosion by sponges, bivalve molluscs, and other endolithic organisms weakens coral skeletons, rendering them susceptible to physical damage by waves, storm surge and projectiles. Branching species of Pocillopora are especially susceptible to breakage by nest-building and foraging fishes (Jiménez 1996–1997; Glynn 2008). It may seem counterintuitive, but large massive colonies of Porites, Pavona, and Gardineroseris are also subject to fragmentation. For example, Highsmith (1982) observed that Pavona clavus colonies excavated by burrowing molluscs (Lithophaga spp.) resulted in collapse and breakage with the generation of several new colonies. Massive and encrusting colonies, such as Porites lobata, Porites panamensis and Pavona varians, are commonly excavated by the triggerfish Pseudobalistes naufragium (Fig. 15.9). The triggerfish, in search of boring bivalves, often produces cm-size fragments that can regenerate, giving rise to new colonies if they remain upright on a suitable substrate (Guzmán 1986; see also Guzmán 1988; Guzmán and Cortés 1989). The combination of a relatively weak skeleton and a high density of lithophagine bivalves, up to 100 ind per 0.01 m2, render this coral a frequent target of triggerfish attacks (Scott and Risk 1988). A recent study has shown that morphologically similar massive species of Porites reproduce mainly asexually (Porites evermanni) or sexually (Porites lobata), depending on the interaction of triggerfish predators and their endolithic boring bivalve prey (Boulay et al. 2013). The triggerfish preferentially attack P. evermanni colonies, which are infested with higher abundances of bivalves than is P. lobata.
Porites lobata colony presumably attacked by Pseudobalistes naufragium. Lithophagine boreholes are visible in two broken sections of the skeleton, top and bottom right side of photograph. Several live fragments lay on bottom to left of colony (arrows). Observed on a patch reef at Bahía Honda, Gulf of Chiriquí, Panamá (5 m depth, 14 March 2003, courtesy Tyler Smith)
Highsmith (1982) has reasoned that some successful corals have evolved a predisposition for colony fragmentation into their life histories. As an example he proposed that pocilloporid corals, which are the predominant reef builders in the eastern Pacific, fragment easily and thus contribute importantly to the lateral expansion of reef frameworks. Fragmented branches, if not stabilized by existing colonies and frameworks, tend to move laterally down slope where they continue to grow and contribute to reef progradation (Fig. 15.10). Additional information on frequent storm-related fragmentation in massive Porites colonies can be found in Done and Potts (1992).
Mean percent attached, detached, and loose colonies of Pocillopora damicornis along 9-m long transects (~3-m depth gradient) on three reefs in the Gulf of Chiriquí, Panama. a Generalized profiles of reefs sampled showing locations of reef flat, fore reef slope, and reef base. b Data from 27, 10-m long transects, with total number of observations n = 1350. Modified after Highsmith (1982)
A recent study by Baums et al. (2014) indicates that the densest known aggregration of Pocillopora in the Galápagos Archipeligo is of asexual origin. A large population of 1614 live Pocillopora colonies present on the southern shore of Isabela Island was analyzed for genetic diversity. Multilocus genotyping, capable of resolving individual clones, indicated that this coral stand is monogenotypic, and thus the high density of colonies is a result of asexual reproduction, likely via fragmentation.
Diaseris distorta, free-living fungiid species as adults, exhibits frequent asexual fragmentation, which is aided by the spontaneous formation of sutures and the separation of daughter segments. Morphological evidence indicates this is perhaps the predominant means of propagation in the Galápagos Islands (Colley et al. 2000). This radial asexual fragmentation through natural autonomy is a compelling example of an evolved trait (Yamashiro and Nishihira 1998).
15.5.3 Asexual Recruitment
Data on the abundances of asexually produced recruits in Panama and Mexico indicate that branch fragmentation can potentially contribute substantially to coral community recovery. The asexual recruit densities of Pocillopora damicornis in Panama in 1980, increasing from 0.17 to 0.44 colonies m−2, were observed after censuses of 4.5, 8 and 12 months, respectively (Highsmith 1982). This level of fragmentation was attributed to wave action, bioerosion, and excavation by foraging fishes. The high mean pocilloporid fragment densities reported by Lirman et al. (2001) in southern Mexico (Huatulco), as high as 26.2 fragments m−2 at the Riscalillo sampling site, were observed in 1997, one to two months after three major cyclones passed across the Huatulco reef tract (Table 15.12). These storms caused extensive coral breakage, especially among branching Pocillopora spp. The fragment densities observed by López-Pérez et al. (2007), sampled in 2001–2002 along the same Huatulco reef tract, were overall about 2.5 times significantly lower (mean densities = 6.2 vs. 15.7 colonies m−2, p = 0.012, t test, 1-tailed) than those assessed in 1997. This significant difference was likely due to the sampling performed soon after the storm impacts in 1997. An expected lasting result of coral communities subject to frequent storms would be the formation of clones and low genetic diversity, a trend demonstrated recently in the lower half of the Gulf of California. A molecular genetic study of branching Pocillopora verrucosa colonies revealed a significant relationship between tropical storm activity and the genotypic indexes of richness and diversity (Aranceta-Garza et al. 2012). This analysis offered strong evidence supporting the role of asexual fragmentation in producing clonal populations at localities subject to frequent storms.
The recent study by Baums et al. (2014) at Isabela Island noted above (Sect. 15.5.2) also strongly suggests the asexual origin of this population. All colonies belonged to Pocillopora mitochondrial open reading frame lineage type 3a. Typing of additional samples from three other islands indicated that this large stand of colonies was unique in the Galápagos Islands, representative of the only type 3a population. Colony size distribution, while imperfect, suggested that the population originated from remnant colonies that survived the 1997–98 ENSO event, but may postdate the 1982–83 ENSO.
López-Pérez et al. (2007) underlined the importance of successful and unsuccessful fragmentation in terms of fragment survival. Cementation of pocilloporid fragments can occur rapidly, in 1–2 months, if retained in suitable reef habitats such as stable living or dead reef structures (Lirman et al. 2001). If fragments are deposited in shallow, wave swept zones or in soft sediments, attachment is unlikely and survivorship low (Ayre et al. 1997). Successful cementation was quantified at Huatulco and ranged between 16 and 58 % (López-Pérez et al. 2007). Thus, to determine the efficacy of asexual fragmentation it is necessary to continue monitoring coral survival of past initial fragmentation events.
15.5.4 Other Factors Affecting Recruitment
Recruitment is also chemically mediated in corals. Rather than being a solely stochastic process of random searching and settlement, larvae of some species respond to specific metamorphic inducers, including those associated with certain species of crustose coralline algae, which are hypothesized to enhance survivorship (Golbuu and Richmond 2007). Physical interference with such cues, through sediment and fleshy algal cover, can prevent coral recruitment. Pollutants, including pesticides, may block metamorphic inducers or larval receptors, and prevent settlement and metamorphosis (Peters et al. 1997). However, many eastern Pacific reefs are located at remote island sites; therefore, such chemical pollutants are less of a concern. Instead, sedimentation during the wet season, especially in areas with significant coastal development and deforestation, can be an obvious contributor to stress.
Fleshy and filamentous algae are often present and abundant on eastern Pacific reefs due to nutrient availability from both upwelling and coastal runoff. Benthic algae are a repository for sediments, which can be re-suspended by water motion. Algae can also draw down oxygen and pH levels during periods of photorespiration at night. As such, the combination of benthic fleshy algae and terrigenous sediments interact to reduce both substrate availability and water quality, affecting coral recruitment and survival.
15.5.5 Comparisons/Discussion
In an attempt to evaluate the relative importance of sexual versus asexual recruitment through fragmentation, colonies of several species in study plots in the EEP were enumerated according to their presumed provenance. The following criteria, employed for live colonies from 1 to 10 cm greatest dimension, were: sexual recruits—cemented to substrate, symmetrical colony morphology, no indication of recent or old skeletal fractures; asexual fragments—usually loose or weakly cemented to substrate, colony morphology often irregular, recent or old skeletal fractures evident. Some mis-assignments can be expected since these criteria are not mutually exclusive. The magnitude of this type of error has not been reported.
For most species and localities, sexual recruits greatly outnumbered asexual recruits (Table 15.13). In the Galápagos Islands, Pocillopora spp. sexual recruits predominated at three of four monitoring sites and made up between about 60 to 100 % of all colonies sampled. Only at Playa La Picona, Floreana Island, were asexual recruits (n = 16) more abundant than sexual recruits (n = 14). This site is often exposed to moderate-to-strong wave action. The majority of sexual recruits were present on the tops and sides of basalt boulders, and asexual recruits were usually unattached on coral rubble/sand substrates. At Cabo Pulmo, Gulf of California, Pocillopora fragments were predominant, contributing 59 % to the recruitment pool (Reyes-Bonilla 1993).
Only sexual recruits were observed in the massive colonies of Porites lobata, Pavona gigantea, and Pavona clavus. As noted previously, asexual fragmentation in P. lobata (and especially in Porites evermanni) occurs commonly where this species is present, however, it is relatively rare on the Uva reef. Only seven small colonies (presumably sexual recruits) were found in the monitoring plot at Uva reef-south (Table 15.13). Asexual fragments were encountered in one of three surveys of Pavona varians on the Uva Island reef. This species is sometimes targeted by Pseudobalistes naufragium, which can severely fragment encrusting colonies such as P. varians (Glynn et al. 1972). In four of six surveys, Gardineroseris planulata exhibited only sexual recruits; however, asexual recruitment predominated in two surveys. The preponderance of asexual:sexual (100:22) recruits of G. planulata on the Uva Island reef in 1993 was ostensibly a result of colony breakage by foraging Pseudobalistes naufragium, which were present on the reef at that time. Asexual outnumbered sexual recruits in Psammocora profundacella at a north-end sampling site on the Uva reef in 1995. Data are not available for Porites panamensis at low latitude sites, but at Cabo Pulmo, Gulf of California, asexual fragmentation was noted in only about 8 % of colonies (Reyes-Bonilla and Calderón-Aguilera 1994).
Molecular genetic methods employing high resolution markers can more accurately distinguish between sexually and asexually derived recruits. Such studies have revealed varying contributions of genets and ramets to the population structure of pocilloporid assemblages. In Panama, Pocillopora communities consisted primarily of sexually-recruited colonies (Combosch and Vollmer 2011). Two studies conducted in the Gulf of California have reported dissimilar results. Aranceta-Garza et al. (2012) found that clonal populations of Pocillopora were present at sites frequented by storms, whereas Pinzón et al. (2012) reported clonal populations predominant in protected habitats. Since biotic agents (bioerosion, corallivores, foraging fishes) can cause significant colony fragmentation, it is imperative to consider these potential effects.
In a comparative study of the population biology of Pocillopora damicornis in the central/western and eastern Pacific, Richmond (1985) observed that 100 % of recruits at Enewetak originated from sexually-produced planula larvae whereas nearly 97 % of recruits in Panama resulted from asexual fragmentation. Other minute recruits of P. damicornis in Panama, with 1–12 polyps each, were attributed to ‘polyp bail-out’ as reported by Sammarco (1982). The nearby presumed donor colonies were under attack by the gastropod corallivore Jenneria pustulata. These results indicate marked regional differences in sexual and asexual recruitment among, as well as within, regions for a morphologically similar coral genus.
15.6 Climate Change and Anthropogenic Stressors
Climate change is considered one of the most pressing concerns for the future of coral reefs (e. g., Hughes et al. 2003). Although much discussion in this chapter has included data and other information acquired during a variety of ENSO events, other climate change factors are worthy of mention, although not the major focus of the studies that contributed to this chapter. Elevated seawater temperatures are responsible for mass-bleaching events caused by the loss of symbiotic zooxanthellae, and such stressed corals either die or are energetically compromised with insufficient energetic resources to support normal gamete production (Table 15.9). Elevated levels of pCO2, such as those predicted to occur by the end of the century, have been found to reduce fertilization, growth and recruitment in colonies of Acropora palmata (Albright et al. 2010). While gametogenesis proceeded unabated when ambient seawater pH and aragonite saturation values were experimentally reduced, there is evidence that energy allocated to egg production under such conditions may further reduce calcification and growth rates in gravid colonies (Cohen and Holcomb 2009; Albright 2011).
The energetics of planula larvae in both brooding and spawning species affect dispersal distances and eventual recruitment success (Richmond 1987a, b, 1988). As lipids provide energy reserves, temperature increases associated with climate change are predicted to likewise increase metabolic and energy consumption rates during the planktonic period and reduce overall dispersal potential. This could affect population connectivity among eastern Pacific reefs and recovery trajectories following mortality events.
Reproduction and recruitment can be affected by both natural and anthropogenic stressors, the former often being exacerbated by the latter (e.g., Richmond 1997, 2005; Fabricius 2005; Harrison 2011). The physiological state of a coral will determine the allocation of energy into various functions, including colony growth, tissue repair (when necessary), calcification, and reproduction. Stressed corals may exhibit a reduction in the quantity and quality of gametes, even to the point of reproductive failure under severe conditions, such as elevated temperatures (Table 15.9). In addition to effects on fecundity, reduced water and substratum quality can affect fertilization, embryological development, zooxanthellae community composition (Garren et al. 2006; LaJeunesse et al. 2010), and the metamorphic induction of coral larvae (Richmond 1993).
Eastern Pacific reefs are subject to the common anthropogenic stressors affecting coral reefs elsewhere, including sediment loading and land-based sources of pollution as well as increased competition for space from algae due to overfishing of herbivores. Although the specific effects of these stressors on coral reproduction are unstudied in the eastern Pacific, various potentially disrupting impacts are threatening coral reefs from Mexico (Reyes-Bonilla 2003) to several equatorial eastern Pacific localities (Cortés and Jiménez 2003; Glynn 2003; Maté 2003; Zapata and Vargas-Ángel 2003). Seasonal upwelling is an added source of stress to which reefs from other areas are normally not exposed (Fig. 15.1b), and associated increases in nutrients can alter competition for substrate space in favor of epibenthos other than coral larvae and recruits.
As previously mentioned, high sediment loading onto reefal habitats during the rainy season, due to deforestation of surrounding land forms, decreases visibility and sunlight penetration in many locales. Associated salinity changes are potentially harmful, as well as seasonal low tide exposures that kill tissues on exposed pocilloporid reef flats, leaving behind retracted polyps that can potentially regenerate damaged tissues.
15.7 Conclusions: Reproduction in a Marginal Environment
Environmental physical data, with particular attention to sea temperature and salinity extremes, aragonite saturation state, light penetration, and inorganic nutrient concentration, were analyzed by Kleypas et al. (1999) to identify and classify marginal reef development worldwide. Potentially stressful conditions were listed for several eastern Pacific reef sites and coral communities from the Galápagos Islands to the Gulf of California. Of special note was the low aragonite saturation state of waters across the eastern tropical Pacific region (see also Manzello et al. 2008; Manzello 2010; see Chap. 18, Manzello et al.). The erratic occurrence of ENSO events, warm El Niño and cool La Niña phases (and attendant stressful conditions), also contribute importantly to disturbances in the eastern Pacific (see Chap. 8, Glynn et al.).
Although geographically patchy, reef building has occurred over large parts of the eastern Pacific, at some sites for periods of 100–1000 s of years (see Chap. 6, Toth et al.). It is of interest, therefore, to consider the attributes of coral reproduction that have likely contributed to this success. The following discussion stems largely from Glynn and Ault (2000), who addressed the dispersal potential and regional distribution of corals, and Glynn and Colley (2008), who examined coral survival under diverse conditions of disturbance in the eastern Pacific. These studies were based largely on the possible roles of reproductive traits in larval dispersal and repopulation of devastated coral communities following large scale disturbances.
All eastern Pacific corals with high frame-building potential are regionally widely distributed, and broadcast-spawn gametes: Pocillopora damicornis, Pocillopora elegans, Porites lobata, Pavona gigantea, Pavona clavus, and Gardineroseris planulata. Broadcast spawning species typically produce planktonic larvae capable of long distance dispersal (Harrison and Wallace 1990). Although larval longevity of eastern Pacific corals is not known, recent studies indicate a highly extended and variable duration of planktonic life of western Pacific, non-autotrophic coral larvae (Graham et al. 2008; Connolly and Baird 2010). For example, the larvae of all five species in the Graham et al. (2008) study demonstrated longevities of from 195 to 244 days. The previously reported record, for Acropora valida, was 130 days. This may offer a dispersal advantage over brooding corals. Thus, this reproductive mode would offer an advantage to corals subject to local disturbances and high mortality, for example resulting from extreme thermal stress, with distant source populations capable of supplying larvae that would contribute to the recovery of depleted populations. Porites panamensis, the sole eastern Pacific zooxanthellate brooder with a relatively narrow geographic range, experienced severe reductions on Panamanian reefs during the 1982–83 El Niño bleaching event (Glynn 1984), and was not observed again on monitored reefs for 3–5 years (Glynn et al. 2001a, b).
Most eastern Pacific corals are reproductively active for several months or in some cases year-round. If acute stress events do not completely inhibit gametogenesis or cause gonad reabsorption, sexual reproduction could possibly resume when conditions improve. Some species have continued to be reproductively active during weak to mild ENSO events when sea temperatures are elevated but do not exceed critical thresholds (Colley et al. 2006). For example, in the Galápagos Islands Pocillopora damicornis and Pocillopora elegans both demonstrated enhanced gametogenesis during moderate El Niño warming. Also, Pavona varians in Panama revealed significant increasing sexual recruitment over a 14 year period with increasing sea temperatures up to a monthly maximum SST anomaly of ~1.6 °C (Glynn et al. 2000).
Extended annual reproductive activity contributes to the high fecundity of eastern Pacific corals. Some other traits also adding to the fecundity of agariciid species (Pavona, 3 species; Gardineroseris planulata) are the number of monthly spawning cycles. For example, Pavona spp. in the Galápagos Islands spawn monthly and may even exhibit split bi-monthly spawning (Glynn et al. 2000). The very small size and prodigious numbers of mature ova in most eastern Pacific species contribute directly and importantly to fecundity. Where estimates of the ages at first reproduction have been reported for eastern Pacific agariciid species, these range from 5 (Pavona varians, Pavona chiriquiensis) to 20 (Gardineroseris planulata) years. These rates cannot be compared with other regions due to the lack of studies on broadcast spawning species in this family.
Perhaps more than in any other coral reef region, corallivore fish foraging and bioturbation activities are at a high level in eastern Pacific coral communities (see Chap. 10, Enochs and Glynn). This leads to frequent asexual colony fragmentation in both branching and massive corals. Where corals occur in favorable environments or under conditions that have normalized after a disturbance, asexual propagation can greatly accelerate local recovery. Unfortunately, information is presently too incomplete to offer any general conclusions on the relative contributions of sexual and asexual reproduction in promoting coral community recovery. It is likely that both reproductive modes are effective, with one or the other predominant depending on the type and severity of disturbance and the particular local conditions prevalent during recovery.
The importance of sexual and asexual reproduction is now clearly recognized for several species within the EEP region. Nonetheless, only 13 of 38 valid zooxanthellate species (Reyes Bonilla 2002) have been studied, and numerous areas within the EEP have not been investigated. Several of the unstudied species, in the genera Pocillopora (6 of 8 species), Porites (7 of 9), and the family Agariciidae (8 of 13), contribute significantly to coral communities and reef frameworks in many areas. Since most of our understanding of coral sexuality, mode of development, timing of spawning, and fecundity has been gained from histological studies, numerous questions remain that are best addressed by direct observations in the field.
Both sexual and asexual reproduction contribute importantly to recruitment in the EEP, but their relative contributions with respect to species and environmental conditions need to be clarified and quantified. For example, how might the spatial occurrence of potentially stressful natural impacts affect coral community structure and diversity, reef growth, recruitment, and recovery processes? Coastal Mexico and her offshore islands are subject to frequent hurricane effects; are these critical drivers of asexual propagation compared with other eastern Pacific areas? Upwelling centers occur in several areas off Mexico and in the EEP, and it is known that sexual reproduction in several species is greatly diminished during upwelling activity. Freshwater discharge and sedimentation reach extreme levels off Colombia and northern Ecuador, but no information has been published on how these conditions affect coral reproduction along this coastal corridor. Finally, preliminary evidence shows that the sudden thermal swings that accompany ENSO events are correlated with atypical reproductive activity. Since no two ENSO cycles are identical, spatially or with respect to severity and duration, continuing research is necessary to relate critical stressors to their regional influence on coral reproduction.
While several kinds of anthropogenic stressors have been noted to affect eastern Pacific reefs (Cortés 2003), e.g. deforestation and coastal development, overfishing, and agricultural chemicals, no studies have examined directly how these affect coral reproduction. Past work has been conducted on reefs lacking signs of direct human impact. Therefore, it is urgent that attention be directed toward an assessment of coral reproduction at coastal and island sites in the EEP where human activities are now encroaching on coral populations and coral reefs. The interactive effects of natural and human-induced stresses on coral reproduction are largely unknown. Reproduction is a vital life history function and for coral reef ecosystems to survive into the future it must not be compromised.
References
Albright R (2011) Reviewing the effects of ocean acidification on sexual reproduction and early life history stages of reef-building corals. J Mar Biol. doi:10.1155/2011/473615
Albright R, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral Acropora palmata. Proc Nat Acad Sci 107:20,400–20,404
Aranceta-Garza F, Balart EF, Reyes-Bonilla H, Cruz-Hernández P (2012) Effect of tropical storms on sexual and asexual reproduction in coral Pocillopora verrucosa subpopulations in the Gulf of California. Coral Reefs 31:1157–1167. doi:10.1007/s00338-012-0941-9
Ayre DJ, Hughes TP, Standish TP (1997) Genetic differentiation, reproductive mode, and gene flow in the brooding coral Pocillopora damicornis along the Great Barrier Reef, Australia. Mar Ecol Prog Ser 159:175–187
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baird AH, Salih A, Trevor-Jones A (2006) Fluorescence census techniques for the early detection of coral recruits. Coral Reefs 25:73–76
Baird AH, Guest JR, Willis BL (2009) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40:551–571
Baums IB, Durante MD, Laing AA, Feingold J, Smith T, Bruckner A, Monteiro J (2014) Marginal coral populations: the densest known aggregation of Pocillopora in the Galápagos Archipeligo is of asexual origin. Front Mar Sci 1:59. doi:10.3389/fmars.2014.00059
Bezy MB (2009) Reproducción sexual y reclutamiento del coral masivo, Pavona clavus, en Bahía Culebra, Golfo de Papagayo, Costa Rica. MSc thesis, Univ Costa Rica, San Pedro, Costa Rica, p 151
Birkeland C (1977) The importance of rate of biomass accumulation in early successional stages of benthic communities to the survival of coral recruits. In: Proceedings of 3rd International Coral Reef Symposium, vol 1, Miami, pp 15–21
Birkeland C (1997) Geographic differences in ecological processes on coral reefs. In: Birkeland C (ed) Life and death of coral reefs. Chapman and Hall, New York, pp 273–297
Boulay JN, Cortés J, Nivia-Ruiz J, Baums IB (2012) High genotypic diversity of the reef-building coral Porites lobata (Scleractinia: Poritidae) in Isla del Coco National Park, Costa Rica. Rev Biol Trop (suppl 3):279–292
Boulay JN, Hellberg ME, Cortés J, Baums IB (2013) Unrecognized coral species diversity masks differences in functional ecology. Proc R Soc B 281:20131580. doi:10.1098/rspb.2013.1580
Boumeester J, Berumeni ML, Baird AH (2011) Daytime broadcast spawning of Pocillopora verrucosa on coral reefs of the central Red Sea, Galaxea. J Coral Reef Stud 13:23–24
Cabral-Tena RA, Reyes-Bonilla H, Lluch-Cota S, Paz-García DA, Calderón-Aguilera LE, Norzagaray-López O, Balart EF (2013) Different calcification rates in males and females of the coral Porites panamensis in the Gulf of California. Mar Ecol Prog Ser 476:1–8
Campos-Vázquez RA, Balart EF, Vallejo-Fuerte M, Rodríguez-Jaramillo C (2014). Ciclo reproductivo de los corales Pocillopora verrucosa y Pocillopora meandrina en Isla Gaviota, Bahía de La Paz, Mexico. Congr Nac Oceanogr June 2014. La Paz, Mexico
Carpizo-Ituarte E, Vizcaíno-Ochoa V, Chi-Barragán G, Tapia-Vázquez O, Cupul-Magaña AL, Medina-Rosas P (2011) Evidence of sexual reproduction in the hermatypic corals Pocillopora damicornis, Porites panamensis, and Pavona gigantea in Banderas Bay, Mexican Pacific. Cienc Mar 37:97–112
Charnov EL (1982) The theory of sex allocation. Princeton monographs in population biology 18. Princeton Univ Press, Princeton NJ, p 355
Chávez-Romo E, Reyes-Bonilla H (2007) Sexual reproduction of the coral Pocillopora damicornis in the southern Gulf of California, Mexico. Cienc Mar 33:495–501
Cohen AI, Holcomb M (2009) Why corals care about ocean acidification: uncovering the mechanisms. Oceanogr 22:118–127
Colley SB, Feingold JS, Peña J, Glynn PW (2000) Reproductive ecology of Diaseris distorta (Michelin) (Fungiidae) in the Galápagos Islands, Ecuador. In: Proceedings of 9th International Coral Reef Symposium, vol 1, Bali, pp 373–379
Colley SB, Glynn PW, May AS, Maté JL (2006) Species-dependent reproductive responses of eastern Pacific corals to the 1997–1998 ENSO event. In: Proceedings of 10th International Coral Reef Symposium, vol 1, Okinawa, pp 61–70
Combosch DJ, Vollmer SV (2011) Population genetics of an ecosystem-defining reef coral Pocillopora damicornis in the tropical eastern Pacific. PLoS ONE 6(8):e21200. doi:10.1371/journal.pone.0021200
Connolly SR, Baird AH (2010) Estimating dispersal potential for marine larvae: dynamic models applied to scleractinian corals. Ecology 91:3572–3583
Cortés J, Jiménez C (2003) Corals and coral reefs of the Pacific of Costa Rica: history, research and status. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 361–385
Cox EF (2007) Continuation of sexual reproduction in Montipora capitata following bleaching. Coral Reefs 26:721–724
Dana TF (1975) Development of contemporary eastern Pacific coral reefs. Mar Biol 33:355–374
D’Croz L, Robertson DR (1997) Coastal oceanographic conditions affecting coral reefs on both sides of the Isthmus of Panama. In: Proceedings of 8th International Coral Reef Symposium, vol 2, Panama, pp 2053–2058
Done TJ, Potts DC (1992) Influences of habitat and natural disturbances on contributions of massive Porites corals to reef communities. Mar Biol 114:479–493
Edmunds PJ (2010) Population biology of Porites astreoides and Diploria strigosa on a shallow Caribbean reef. Mar Ecol Prog Ser 418:87–104
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Poll Bull 50:125–146
Fadlallah YH (1983) Sexual reproduction, development and larval biology in scleractinian corals: a review. Coral Reefs 2:129–150
Feingold JS (1995) Effects of elevated water temperature on coral bleaching and survival during El Niño disturbance events. Ph.D. dissert, Univ of Miami, Coral Gables, Florida p 236
Fenner D (2001) Biogeography of three Caribbean corals (Scleractinia) and the invasion of Tubastraea coccinea into the Gulf of Mexico. Bull Mar Sci 69:1175–1189
Fenner D, Banks K (2004) Orange cup coral Tubastraea coccinea invades Florida and the Flower Garden Banks, northwestern Gulf of Mexico. Coral Reefs 23:505–507
Figueira de Paula AF, Creed JC (2004) Two species of the coral Tubastraea (Cnidaria, Scleractinia) in Brazil: a case of accidental introduction. Bull Mar Sci 74:175–183
Fine D, Tehernov D (2007) Scleractinian coral species survive and recover from decalcification. Science 315:1811(single page)
Fong P, Glynn PW (2001) Population abundance and size-structure of an eastern tropical Pacific reef coral after the 1997–98 ENSO: a simulation model predicts field measures. Bull Mar Sci 69:187–202
Fong P, Smith TB, Wartian MJ (2006) Epiphytic cyanobacteria maintain shift to macroalgal dominance on coral reefs following ENSO disturbance. Ecology 87:1162–1168
Fukami H, Chen CA, Budd AF, Collins A, Wallace C, Chuang Y-Y, Chen C, Dai C-F, Iwao K, Sheppard C, Knowlton N (2008) Mitochondrial and nuclear genes suggest that stony corals are monophyletic but most families of stony corals are not (Order Scleractinia, Class Anthozoa, Phylum Cnidaria). PLoS ONE 3:1–9
García-Ocampo MR (2005) Patrones de reclutamiento de las colonias juveniles de coral del género Pocillopora Lamarck 1816 (Anthozoa: Scleractinia) en cinco localidades de Ixtapa-Zihuatanejo, Guerrero, México. Undergrad thesis Mar Biol, Univ del Mar, Pto Ángel, Oaxaca p 56
Garren M, Walsh SM, Caccone A, Knowlton N (2006) Patterns of association between Symbiodinium and members of the Montastraea annularis species complex on spatial scales ranging from within colonies to between geographic regions. Coral Reefs 25:503–512
Giese AC, Pearse JS (1974) Introduction: general principles. In: Giese AC, Pearse JS (eds) Reproduction of marine invertebrates, vol 1. Academic Press, New York, pp 1–49
Glynn PW (1984) Widespread coral mortality and the 1982–83 El Niño warming event. Environ Conserv 11:133–146
Glynn PW (1985) El Niño-associated disturbance to coral reefs and post disturbance mortality by Acanthaster planci. Mar Ecol Prog Ser 26:295–300
Glynn PW (1990) Coral mortality and disturbances to coral reefs in the tropical eastern Pacific. In: Glynn PW (ed) Global ecological consequences of the 1982–83 El Niño-Southern Oscillation. Elsevier Oceanogr Ser 52, Amsterdam, Elsevier, pp 55–126
Glynn PW (2003) Coral communities and coral reefs of Ecuador. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 449–472
Glynn PW (2008) Food-web structure and dynamics of eastern tropical Pacific coral reefs: Panamá and Galápagos Islands. In: McClanahan TR, Branch GM (eds) Food webs and the dynamics of marine reefs. Oxford Univ Press, Oxford, pp 185–208
Glynn PW, Ault JS (2000) A biogeographic analysis and review of the far eastern Pacific coral reef region. Coral Reefs 19:1–23
Glynn PW, Colley SB (2008) Survival of brooding and broadcasting reef corals following large scale disturbances: is there any hope for broadcasting species during global warming? In: Proceedings of 11th International Coral Reef Symposium. Ft Lauderdale (session 11), pp 1–5
Glynn PW, Fong P (2006) Patterns of reef coral recovery by the regrowth of surviving tissues following the 1997–98 El Niño warming and 2000, 2001 upwelling cool events in Panamá, eastern Pacific. In: Proceedings of 10th International Coral Reef Symposium, vol 1, Okinawa, pp 624–630
Glynn PW, Stewart RH, McCosker JE (1972) Pacific coral reefs of Panamá: structure, distribution and predators. Geol Rundsch 61:483–519
Glynn PW, Peters EC, Muscatine L (1985) Coral tissue microstructure and necrosis: relation to catastrophic coral mortality in Panamá. Dis Aquat Org 1:29–37
Glynn PW, Gassman NJ, Eakin CM, Cortés J, Smith DB, Guzmán HM (1991) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador) I. Pocilloporidae. Mar Biol 109:355–368
Glynn PW, Colley SB, Eakin CM, Smith DB, Cortés J, Gassman NJ, Guzmán HM, Del Rosario JB, Feingold JB (1994) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador) II. Poritidae. Mar Biol 118:191–208
Glynn PW, Colley SB, Gassman NJ, Black K, Cortés J, Maté JL (1996) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador). III. Agariciidae (Pavona gigantea and Gardineroseris planulata). Mar Biol 125:579–601
Glynn PW, Colley SB, Ting JH, Maté JL, Guzmán HM (2000) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá, and Galápagos Islands (Ecuador). IV. Agariciidae, recruitment and recovery of Pavona varians and Pavona sp a. Mar Biol 136:785–805
Glynn PW, Maté JL, Stemann TA (2001a) Pavona chiriquiensis, a new species of zooxanthellate scleractinian coral (Cnidaria: Anthozoa: Agariciidae) from the eastern tropical Pacific. Bull Biol Soc Wash 10:210–225
Glynn PW, Maté JL, Baker AC, Calderón MO (2001b) Coral bleaching and mortality in Panamá and Ecuador during the 1997–1998 El Niño-Southern Oscillation event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Glynn PW, Colley SB, Maté JL, Cortés J, Guzmán HM, Bailey RL, Feingold JS, Enochs IC (2008) Reproductive ecology of the azooxanthellate coral Tubastraea coccinea in the equatorial eastern Pacific: Part V. Dendrophylliidae. Mar Biol 153:529–544
Glynn PW, Colley SB, Guzmán HM, Enochs IC, Cortés J, Maté JL, Feingold J (2011) Reef coral reproduction in the eastern Pacific: Costa Rica, Panamá and the Galápagos Islands (Ecuador). VI. Agariciidae, Pavona clavus. Mar Biol 158:1601–1617
Glynn PW, Colley SB, Maté JL, Baums IB, Feingold JS, Cortés J, Guzmán HM, Afflerbach JC, Brandtneris VW, Ault JS (2012) Reef coral reproduction in the equatorial eastern Pacific: Costa Rica, Panamá, and the Galápagos Islands (Ecuador). VII Siderastreidae, Psammocora stellata and Psammocora profundacella. Mar Biol 159:1,917–1,932 doi:10.1007/s00227-012-1979-5
Golbuu Y, Richmond RH (2007) Substratum preferences in planula larvae of two species of scleractinian corals, Goniastraea retiformis and Stylarea punctata. Mar Biol 152:639–644
Graham EM, Baird AH, Connolly SR (2008) Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27:529–539
Grottoli AG, Rodrigues LJ, Juarez C (2004) Lipids and stable carbon isotopes in two species of Hawaiian corals, Porites compressa and Montipora verrucosa, following a bleaching event. Mar Biol 145:621–631
Grottoli AG, Rodrigues LJ, Palardy JE (2006) Heterotrophic plasticity and resilience in bleached corals. Nature 440:1186–1189
Guest JR, Baird AH, Goh BPL, Chou LM (2005) Seasonal reproduction in equatorial coral reefs. Inv Repr Dev 48:207–218
Guest JR, Baird AH, Goh BPL, Chou LM (2012) Sexual systems in scleractinian corals: an unusual pattern in the reef-building species Diploastrea heliopora. Coral Reefs. doi:10.1007/s00338-012-0881-4
Guzmán HM (1986) Estructura de la comunidad arrecifal de la Isla del Caño, Costa Rica, y el efecto de perturbaciones naturales severas. MSc thesis, Univ Costa Rica, San Pedro, Costa Rica, p 179
Guzmán HM (1988) Distribución y abundancia de organismos coralívoros en los arrecifes coralinos de la Isla del Caño, Costa Rica. Rev Biol Trop 36:191–207
Guzmán HM, Cortés J (1989) Coral reef community structure at Caño Island, Pacific Costa Rica. Publ Staz zool Napoli (I: Mar Ecol) 10:23–41
Guzmán HM, Cortés J (2001) Changes in reef community structure after fifteen years of natural disturbances in the eastern Pacific (Costa Rica). Bull Mar Sci 69:133–149
Guzmán HM, Cortés J (2007) Reef recovery 20 years after the 1982–1983 El Niño massive mortality. Mar Biol 151:401–411
Hanafy MH, Aamer MA, Habib M, Rouphael AB, Baird AH (2010) Synchronous reproduction of corals in the Red Sea. Coral Reefs 29:119–124
Harriott VJ (1983) Reproductive ecology of four scleractinian species at Lizard Island, Great Barrier Reef. Coral Reefs 2:9–18
Harrison PL (1990) Sperm morphology and fertilization strategies in scleractinian corals. Adv Invert Reprod 5:299–304
Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Berlin, pp 59–85
Harrison PL, Wallace CC (1990) Reproduction, dispersal and recruitment of scleractinian corals. In: Dubinsky Z (ed) Coral reefs, ecosystems of the world 25. Elsevier, Amsterdam, pp 133–207
Highsmith RC (1980) Geographic patterns of coral bioerosion: a productivity hypothesis. J Exp Mar Biol Ecol 46:177–196
Highsmith RC (1982) Reproduction by fragmentation in corals. Mar Ecol Prog Ser 7:207–226
Hirose M, Hidaka M (2000) Reduced reproductive success in scleractinian corals that survived the 1998 bleaching in Okinawa. Galaxea JCRS 2:17–21
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C, Grosberg R, Hoegh-Guldberg O, Jackson JBC, Kleypas J, Lough J, Marshall P, Nystrom M, Palumbi SR, Pandolphi JM, Rosen B, Roughgarden J (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301:929–933
Humason GL (1967) Animal tissue techniques, 2nd edn. WH Freeman and Co, San Francisco, p 569
Humphrey C, Weber M, Lott C, Cooper T, Fabricius K (2008) Effects of suspended sediments, dissolved inorganic nutrients and salinity on fertilization and embryo development in the coral Acropora millepora (Ehrenberg, 1834). Coral Reefs 27:837–850
Jiménez CE (1996–1997) Coral colony fragmentation by whitetip reef sharks at Coiba Island National Park, Panamá. Rev Biol Trop 44/45:698–700
Jiménez CE (1998) Arrecifes y comunidades coralinas de Bahía Culebra, Pacífico norte de Costa Rica (Golfo de Papagayo). MSc thesis, Univ Costa Rica, San Pedro, Costa Rica, p 218
Kerr AM (2005) Molecular and morphological supertree of stony corals (Anthozoa: Scleractinia) using matrix representation parsimony. Biol Rev 80:543–558
Kinzie RA III (1993) Spawning in the reef corals Pocillopora verrucosa and P. eydouxi at Sesoko Island, Okinawa. Galaxea 11(2):93–105
Kleypas JA, McManus JW, Meñez LAB (1999) Environmental limits to coral reef development: where do we draw the line? Amer Zool 39:146–159
Kolinski SP, Cox EF (2003) An update on modes and timing of gamete and planula release in Hawaiian scleractinian corals with implications for conservation and management. Pac Sci 57:17–27
LaJeunesse TC, Smith R, Walther M, Pinzón J, Pettay DT, McGinley M, Aschaffenburg M, Medina-Rosas P, Cupul-Magaña AL, López Pérez A, Reyes-Bonilla H, Warner ME (2010) Host-symbiont recombination versus natural selection in the response of coral-dinoflagellate symbioses to environmental disturbance. Proc R Soc B 277:2925–2934
Lewis JB (2006) Biology and ecology of the hydrocoral Millepora on coral reefs. Adv Mar Biol 50:1–55
Lirman D, Glynn PW, Baker AC, Leyte Morales GE (2001) Combined effects of three sequential storms on the Huatulco coral reef tract, Mexico. Bull Mar Sci 69:267–278
López-Pérez RA, Mora-Pérez MG, Leyte-Morales GE (2007) Coral (Anthozoa: Scleractinia) recruitment at Bahías de Huatulco, western México: implications for coral community structure and dynamics. Pac Sci 61:355–369
Loya Y, Sakai K (2008) Bidirectional sex change in mushroom stony corals. Proc R Soc B 275:2335–2343
Loya Y, Sakai K, Heyward A (2009) Reproductive patterns of fungoid corals in Okinawa, Japan, Galaxea. J Coral Reef Stud 11:119–129
Luna JG (1968) Manual of histological staining methods of the armed forces institute of pathology. McGraw-Hill, New York
Mangubhai S, Harrison PL (2009) Extended breeding seasons and asynchronous spawning among equatorial reef corals in Kenya. Mar Ecol Prog Ser 374:305–310
Manzello DP (2010) Coral growth with thermal stress and ocean acidification: lessons from the eastern tropical Pacific. Coral Reefs 29:749–758
Manzello DP, Kleypas JA, Budd DA, Eakin CM, Glynn PW, Langdon C (2008) Poorly cemented coral reefs of the eastern tropical Pacific: possible insights into reef development in a high-CO2 world. Proc Nat Acad Sci 105:10,450–10,455
Maté JL (2003) Corals and coral reefs of the Pacific coast of Panamá. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 387–417
Medina-Rosas P, Carriquiry JD, Cupul-Magaña AL (2005) Recruitment of Porites (Scleractinia) on artificial substrate in reefs affected by the 1997–98 El Niño in Banderas Bay, Mexican Pacific. Cienc Mar 31:1–7
Mundy CN (2000) An appraisal of methods used in coral recruitment studies. Coral Reefs 19:124–131
Oliver J, Babcock R (1992) Aspects of the fertilization ecology of broadcast spawning corals: sperm dilution effects and in situ measurements of fertilization. Biol Bull 183:409–417
Oliver JK, Babcock RC, Harrison PL, Willis BL (1988) Geographic extent of mass coral spawning: clues to ultimate causal factors. In: Proceedings of 6th International Coral Reef Symposium, vol 2, Townsville, pp 803–810
Omori M, Fukami H, Kobinata H, Hatta M (2001) Significant drop of fertilization of Acropora corals in 1999: an after effect of heavy coral bleaching? Limnol Oceanogr 46:704–706
Penland L, Kloulechad J, Idip D, van Woesik R (2004) Coral spawning in the western Pacific Ocean is related to solar insolation: evidence of multiple spawning events in Palau. Coral Reefs 23:133–140
Peters EC, Gassman NJ, Firman JC, Richmond RH, Power EA (1997) Ecotoxicology of tropical marine ecosystems. J Environ Tox Chem, Annu Rev 16:12–40
Pinzón JH, Reyes-Bonilla H, Baums IB, LaJeunesse TC (2012) Contrasting clonal structures among Pocillopora (Scleractinia) communities at two environmentally distinct sites in the Gulf of California. Coral Reefs. doi:10.1007/s00338-012-0887-4
Reyes-Bonilla H (1993) Estructura de la comunidad, influencia de la depredación y biología poblacional de corales hermatípicos en el arrecife de Cabo Pulmo, B.C.S. MSc thesis, Centro de Investigación Científica y de Educación Superior de Ensenada, Depart Ecología, p 169
Reyes-Bonilla H (2002) Checklist of valid names and synonyms of stony corals (Anthozoa: Scleractinia) from the eastern Pacific. J Nat Hist 36:1–13
Reyes-Bonilla H (2003) Coral reefs of the Pacific coast of Mexico. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 331–349
Reyes-Bonilla H, Calderón-Aguilera LE (1994) Parámetros poblacionales de Porites panamensis (Anthozoa: Scleractinia), en el arrecife de Cabo Pulmo, México. Rev Biol Trop 42:121–128
Richmond RH (1985) Variations in the population biology of Pocillopora damicornis across the Pacific. In: Proceedings of 5th International Coral Reef Congress, vol 6, Tahiti, pp 101–106
Richmond RH (1987a) Energetics, competency, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar Biol 93:527–533
Richmond RH (1987b) Energetic relationships and biogeographical differences among fecundity, growth and reproduction in the reef coral Pocillopora damicornis. Bull Mar Sci 41:594–604
Richmond RH (1988) Competency and dispersal potential of planula larvae of a spawning versus a brooding coral. In: Proceedings of 6th International Coral Reef Symposium, vol 2, Townsville, pp 827–831
Richmond RH (1990) The effects of the El Niño/Southern Oscillation on the dispersal of corals and other marine organisms. In: Glynn PW (ed) Global ecological consequences of the 1982–83 El Niño-Southern Oscillation. Elsevier Oceanogr Ser 52, Amsterdam, Elsevier, pp 127–140
Richmond RH (1993) Coral reefs: present problems and future concerns resulting from anthropogenic disturbance. Am Zool 33:524–536
Richmond RH (1994) Effects of coastal runoff on coral reproduction. In: Ginsburg RN (compiler) Proceedings of the colloquium on global aspects of coral reefs: health, hazards and history. Rosenstiel School of Marine and Atmospheric Science, Univ Miami, Miami, pp 360–364
Richmond RH (1997) Reproduction and recruitment in corals: critical links in the persistence of reefs. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 175–197
Richmond RH (2005) Coral reproduction and recruitment as tools for studying the ecotoxicology of coral reef ecosystems. In: Ostrander G (ed) Techniques in aquatic toxicology, vol II. CRC Press, Boca Raton, Florida, pp 331–338
Richmond RH, Hunter CL (1990) Reproduction and recruitment of corals: comparisons among the Caribbean, the tropical Pacific, and the Red Sea. Mar Ecol Prog Ser 60:185–203
Rinkevich B (1989) The contribution of photosynthetic products to coral reproduction. Mar Biol 101:259–263
Rodríguez-Troncoso AP (2004) Caracterización del ciclo reproductivo de Pocillopora damicornis (Linnaeus 1758), en el arrecife de la Entrega, Oaxaca, México. Undergrad thesis, Univ del Mar, Pto Ángel, Oaxaca, p 62
Rodríguez-Troncoso AP, Carpizo-Ituarte E, Leyte-Morales GE, Chi-Barragán G, Tapia-Vázquez O (2011) Sexual reproduction of three coral species from the Mexican south Pacific. Mar Biol 158:2673–2683
Romano SL, Palumbi SR (1996) Evolution of scleractinian corals inferred from molecular systematics. Science 271:640–642
Sammarco PW (1982) Polyp bail-out: an escape response to environmental stress and a new means of reproduction in corals. Mar Ecol Prog Ser 10:57–65
Santiago Valentín JD, Rodríguez-Troncoso AP, Carpizo-Ituarte E, Benítez-Villalobos F, Torres-Hernández P, López-Pérez A (2015) Reproductive pattern of the reef building coral Pavona gigantea (Scleractinia: Agariciidae) off southwestern Mexico. Cienc Mar 41:233–246
Schmidt-Roach S, Miller KJ, Woolsey E, Gerlach G, Baird AH (2012) Broadcast spawning by Pocillopora species on the Great Barrier Reef. PLoS ONE 7(12):e50847. doi:10.1371/journal.pone.0050847
Scott PJB, Risk MJ (1988) The effect of Lithophaga (Bivalvia: Mytilidae) boreholes on the strength of the coral Porites lobata. Coral Reefs 7:145–151
Shlesinger Y, Loya J (1985) Coral community reproductive patterns: Red Sea versus the Great Barrier Reef. Science 228:1333–1335
Shlesinger Y, Goulet TL, Loya Y (1998) Reproductive patterns of scleractinian corals in the northern Red Sea. Mar Biol 132:691–701
Smith DB (1991) The reproduction and recruitment of Porites panamensis Verrill at Uva Island, Pacific Panamá. MS thesis, Univ Miami, Coral Gables, Florida, p 64
Smith TB, Glynn PW, Maté JL, Toth LT, Gyory J (2014) A depth refugium from catastrophic coral bleaching prevents regional extinction. Ecology 95:1663–1673. doi:10.1890/13-0468.1
Soong K, Cho LC (1998) Synchronized release of medusa from three species of hydrozoan fire corals. Coral Reefs 17:145–154
Steiner SCC, Cortés J (1996) Spermatozoan ultrastructure of scleractinian corals from the eastern Pacific: Pocilloporidae and Agariciidae. Coral Reefs 15:143–147
Strathmann RR, Hughes TP, Kuris AM, Lindeman KC, Morgan SG, Pandolfi JM, Warner RR (2002) Evolution of local recruitment and its consequences for marine populations. Bull Mar Sci 70(suppl):377–396
Szmant-Froelich A, Reutter M, Riggs L (1985) Sexual reproduction of Favia fragum (Esper): lunar patterns of gametogenesis, embryogenesis and planulation in Puerto Rico. Bull Mar Sci 37:880–892
Szmant AM (1986) Reproductive ecology of Caribbean reef corals. Coral Reefs 5:43–54
Szmant AM, Gassman NL (1990) The effects of prolonged ‘bleaching’ on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8:217–224
Tomascik T, Sander F (1987) Effects of eutrophication on reef-building corals III. Reproduction of the reef-building coral Porites porites. Mar Biol 94:77–94
van Veghel MLJ (1993) Multiple species spawning on Curaçao reefs. Bull Mar Sci 52:1017–1021
van Woesik R (2009) Calm before the spawn: global coral spawning patterns are explained by regional wind fields. Proc R Soc B 277:715–722
Waller RG, Taylor PA, Gage JD (2005) Sexual reproduction in three hermaphroditic deep-sea Caryophyllia species (Anthozoa: Scleractinia) from the NE Atlantic Ocean. Coral Reefs 24:594–602
Ward S, Harrison P, Hoegh-Guldberg O (2000) Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. In: Proceedings of 9th International Coral Reef Symposium, vol 2, Bali, pp 1123–1128
Wartian MJ (2006) Determinants of community structure and resilience on tropical eastern Pacific coral reefs. PhD dissert, Univ Calif, Los Angeles, p 247
Wellington GM (1982) Depth zonation of corals in the Gulf of Panama: control and facilitation by resident reef fishes. Ecol Monogr 52:223–241
Willis BL, Babcock RC, Harrison PL, Wallace CC (1993) Experimental evidence of hybridization in reef corals involved in mass spawning events. In: Proceedings of 7th International Coral Reef Symposium, vol 1, Guam, p 504 (abstract)
Willis BL, van Oppen MJH, Miller DJ, Vollmer SV, Ayre DJ (2006) The role of hybridization in the evolution of reef corals. Annu Rev Ecol Evol Syst 37:489–517
Wilson JR, Harrison PL (2003) Spawning patterns of scleractinian corals at the Solitary Islands—a high latitude coral community in eastern Australia. Mar Ecol Prog Ser 260:115–123
Yamashiro H, Nishihira M (1998) Experimental study of growth and asexual reproduction in Diaseris distorta (Michelin 1843) a free-living fungoid coral. J Exp Mar Biol Ecol 225:253–267
Zapata FA, Vargas-Ángel B (2003) Corals and coral reefs of the Pacific coast of Colombia. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 419–447
Acknowledgments
The many individuals who helped with logistics, permitting, collections, histology, and data generation over the years are too numerous to identify here, but are duly acknowledged in the Glynn, Colley et al. publications referenced below. Special thanks go to Bernadette Bezy for sharing information on coral spawning in Costa Rica. This overview benefitted from critiques offered by Joshua S. Feingold and two anonymous reviewers. Information supplied by Peggy Fong, Sascha Steiner and Herman Wirshing is noted with gratitude. We thank Michael C. Schmale for the use of his microscope and digital camera equipment. Joshua Levy and Alissa Mones kindly assisted in producing figures and tables. Principal financial support was provided by grants from the U.S. National Science Foundation (Biological Oceanography Program), the Office of Forestry, Environment and Natural Resources, Bureau for Science and Technology (USAID), the Darwin Initiative (Conservation International), and the National Geographic Society. Essential in-country support has been offered long-term by the Centro de Investigaciones en Ciencias del Mar y Limnología (Costa Rica), the University of Panama, Instituto Nacional de Recursos Naturales, and Smithsonian Tropical Research Institute (Panama), and the Charles Darwin Research Station, Galápagos National Park Service, and Transporte Aereo Militar de Ecuador (Ecuador).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Glynn, P.W., Colley, S.B., Carpizo-Ituarte, E., Richmond, R.H. (2017). Coral Reproduction in the Eastern Pacific. In: Glynn, P., Manzello, D., Enochs, I. (eds) Coral Reefs of the Eastern Tropical Pacific. Coral Reefs of the World, vol 8. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7499-4_15
Download citation
DOI: https://doi.org/10.1007/978-94-017-7499-4_15
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7498-7
Online ISBN: 978-94-017-7499-4
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)