Abstract
Reproduction and early life history are central to understanding the biology and ecology of organisms, however such information is limited for solitary corals. Here, we compared the reproductive traits of the solitary coral Heliofungia actiniformis from different latitudinal locations (Singapore, 1°N and the Philippines, 16°N) and examined their early life development, settlement competency, and juvenile growth and survival. A total of 32 corals from Pulau Hantu reefs in Singapore and 102 corals from Bolinao and Anda reefs in the Philippines were studied between 2019 and 2022. Heliofungia actiniformis broadcasts spawned gametes during several nights, generally between 22:00 and 01:00, before and after full moon, from February to May in Singapore and from March to June in the Philippines. Spawning within a month occurred for up to 16 nights in Singapore and 10 nights in the Philippines. Sex change in two individuals between years was observed in the Philippines. The average egg size was smaller in Singapore than that in the Philippines. We determined that eggs were fertilized within 2 h after sperm addition, and developed into swimming larvae within 64 h, which began to settle after 24 h. Larval survival after three mo of culture was 1.72 ± 1.0% and juvenile diameter ranged from 0.33 to 1.30 mm. Asexual buds were first observed in 15 mo old juveniles that were at least 8 mm in diameter. 24 mo old juveniles were observed to detach from their stalk and the empty stalk regenerated polyps. Our results highlight the latitudinal variability in the reproductive traits of solitary corals, serve as a baseline for their early life history, and advance our understanding of their population dynamics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Life history traits, including reproduction and early life development, are central to understanding the biology, ecology, and evolutionary success of organisms. Scleractinian corals present various remarkable life history strategies to maximize their fitness and maintain their populations. For example, they can exhibit asexual, sexual, and mixed reproductive traits (Whitaker 2006; Baird et al. 2009a; Combosch and Vollmer 2013). Asexual reproduction includes budding, fission, fragmentation, polyp bailout, and polyp expulsion, whereas sexual reproduction includes variations in sexuality (i.e., hermaphroditic and gonochoric) and reproductive mode (i.e., broadcasting of gametes and brooding of planulae) (Kramarsky-Winter and Loya 1998; Baird et al. 2009a; Harrison 2011). Most scleractinian corals are hermaphroditic broadcast spawners; however, a few species have mixed sexuality or modes of reproduction (Harrison 2011). Broadcast spawning corals release gametes into the water column where fertilization and development into larvae typically occur within 2 h and 48 h respectively, and then larvae begin settlement at approximately four days after fertilization (Babcock and Heyward 1986). In contrast, brooding corals produce planulae that are competent to settle within a few hours after release (Nozawa and Harrison 2005).
Corals within genera or species may exhibit variability in their reproduction depending on geographical location or environment (Shlesinger and Loya 1985; Baird et al. 2009b). For instance, Coelastrea aspera is a hermaphroditic spawner in the Great Barrier Reef, Australia (Babcock 1984; Babcock and Heyward 1986), a brooder in Palau, and both a spawner and brooder in Okinawa, Japan (Sakai 1997; Nozawa and Harrison 2005). In addition, the timing of spawning differs across locations. Several coral species spawn earlier in the year in low latitudes compared to high latitudes, such as within the Indian Ocean (Mangubhai and Harrison 2008; Baird et al. 2014; Howells et al. 2014) and Japan (Baird et al. 2009b). Spawning months in a given location may also vary between years due to differences in seawater temperatures and timing of lunar cycles as observed in A. downingi, A. hemprichii, Cyphastrea microphthalma, and Platygyra daedalea in the Gulf of Oman (Howells et al. 2014). Moreover, variability in maternal investment within species has been recorded at different latitudinal locations. The egg size and female gonad volumes of A. hyacinthus are larger at lower latitudes (Indonesia, 5°S) than in higher latitudes (Japan, 33°N and 31°N and Taiwan, 23°N, 22°N and 21°N) (Tsounis et al. 2006; Santacruz-Castro 2019) which suggests differential energy allocation to their offspring. Settlement success, growth, and survival of juveniles have also been shown to vary with latitude (Bauman et al. 2015; Nozawa et al. 2021).
Although the reproduction and early life development of corals are well studied in many localities, most reports describe colonial species whereas only a few have examined solitary corals such as those in the family Fungiidae. Similar to colonial corals, fungiids include both spawning (e.g., Fungia scutaria, F. granulosa) and brooding species (e.g., F. fungites; Loya et al. 2009) which release gametes or planulae, respectively, either at night (Krupp 1983; Kramarsky-Winter and Loya 1998) or daytime (Eyal-Shaham et al. 2019, 2020). In addition, variability in sexuality across latitudes has been reported. For example, F. fungites is a gonochoric brooder in Okinawa, Japan, but a broadcast spawner in the Great Barrier Reef, Australia (Loya et al. 2009). One species of solitary corals that is common in many regions is the Heliofungia actiniformis, yet their reproduction and early life development is not well understood.
Heliofungia actiniformis is a large single-polyped coral from the family Fungiidae which is commonly found in reef flats and slopes (Veron 2000; Hoeksema 2012). Its extended fleshy tentacles serve as shelters for various symbionts including fish, shrimp, and molluscs (Hoeksema et al. 2012a; Bos and Hoeksema 2015). As it is susceptible to bleaching caused by thermal stress (Hoeksema et al. 2012b; Cesar et al. 2014) and populations are heavily exploited for the aquarium trade (Green and Shirley 1999; Knittweis 2008), it is listed as ‘vulnerable’ by the IUCN (Hoeksema 2008). In Palau, this species is a brooder and likely hermaphroditic as sperm has been observed to develop before eggs in the same individual (Abe 1937) whereas it has been recorded as a gonochoric broadcast spawner in the Great Barrier Reef, Australia (Babcock and Heyward 1986; Willis et al. 1985; Baird et al. 2009a). Similar with other fungiids, H. actiniformis can also reproduce asexually through budding (Hoeksema 1989; Boschma 1922; Abe 1937; Knittweis et al. 2009b).
To better understand the reproduction and early life history of H. actiniformis, we monitored their reproduction in Singapore and the Philippines, tracked their development from eggs to juveniles, and examined their settlement competency, and juvenile growth and survival. This information is essential for understanding their biology, ecology, and population dynamics at different latitudinal locations as well as in providing insights for their conservation.
Methodology
Study sites and coral collection
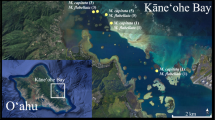
The present study was conducted in Singapore (~ 1°N) and the Philippines (~ 16°N). Heliofungia actiniformis individuals (along with other fungiid species) were collected from Pulau Hantu fringing reefs in Singapore (Fig. 1a and c) during the initial stage of the research in May 2018 (Prasetia et al. 2020). Corals were surveyed in four belt transects (50 \(\times\) 2 m2) that were randomly deployed at the reef crest (2–3 m depth) and reef slope (5–6 m). Heliofungia actiniformis individuals were not abundant (average of 1 individual/100 m2, see Prasetia et al. 2020) at the study sites, and so any individuals encountered during the survey were collected. Corals were individually labelled with a numeric plastic or aluminum tag attached to a nylon fishing cord that was inserted into a small hole drilled at the edge of the coral skeleton using cordless electric drill (following Loya and Sakai 2008), and then corals were returned to their original reef site on the same day of sampling, until collection for spawning observation (Prasetia et al. 2020). Corals (comprising individuals ranging from 67 to 166 mm in diameter) were observed for spawning between March 16 and June 26, 2019 (20 individuals), between October 17 and November 29, 2019 (nine individuals), and between February 8 and March 29, 2020 (19 individuals, 7 from 2019 and 12 new individuals). Spawning observations were conducted at the St John’s Island National Marine Laboratory, Singapore (Fig. 1a and c).
In the Philippines, corals were collected from a reef flat of Bolinao and a subtidal flat of Anda, Pangasinan, Northwestern Philippines and brought to the outdoor hatchery of the Bolinao Marine Laboratory, Marine Science Institute, University of the Philippines (Fig. 1a and b). Heliofungia actiniformis were not abundant in the study sites, therefore individuals were collected as they were encountered along a single belt transect (100 \(\times\) 1 m2) per site. Corals were individually tagged with engraved stainless steel plates attached to a nylon fishing cord that was tied around the coral skeleton while making sure that the mouth was not covered. The corals were observed for spawning (see “Ex situ spawning observation and egg size” below) between 2019 and 2022. In 2019, 33 corals (comprising individuals of various sizes ranging from 49 to 169 mm in diameter) were collected, acclimated in tanks with flow through water (1L/min flow rate) and aeration for 3 d, before spawning observations between March 10 to April 1, 2019. Corals were returned to the reef in 2019. In 2020, 61 corals (diameter: 65–160 mm) were observed. Corals were observed between March 9 and May 16 (15 from 2019 and 28 new individuals), June and July (five from 2019), between August and September (11 new individuals), and between October and December (seven new individuals). In 2021, total of 35 corals (five individuals from 2019, seven individuals from 2020, and 23 new individuals; diameter of individuals: 57–165 mm) were observed for spawning between January 2021 to February 2022. All corals, in both countries, were maintained in tanks with flowing sand-filtered seawater and were exposed to natural light and periodicity. At the end of the observation period, all collected corals were returned to their original reef.
Ex situ spawning observation and egg size
Ex situ spawning observation was conducted by transferring each coral to an individual plastic container (~ 10 to 12 L) where the sex of each individual was recorded by determining the unique shape of the shed gametes and was verified using a microscope (following Loya and Sakai 2008). Observations were conducted every hour, from 16:00 until 09:00 in Singapore, and from 17:00 until 09:00 in the Philippines. Within 1 to 2 h after egg release, the seawater in each plastic container was homogenized by stirring. Three to five subsamples (10–15 ml) of seawater containing eggs were sampled to measure the egg size. The eggs were photographed (n = 37 eggs from three individuals, two to 24 eggs per individual in Singapore; n = 180 eggs from 7 individuals, 5 to 30 eggs per individual in the Philippines) under a compound microscope equipped with a camera. The size of the eggs was estimated by measuring their maximum diameter using ImageJ software (in Singapore) and Motic Image Software (in the Philippines). The eggs used for measurement of egg size in Singapore were obtained during the spawning on March 29, April 24, 27, and 29, 2019; whereas those used in the Philippines were from the spawning on April 13, 2020.
Fertilization, early life development, and settlement on different substrates
Fertilization, early life development, and settlement experiments were conducted in the Philippines using gametes obtained during the spawning on March and April 2020. Within 1 to 2 h after spawning, the eggs from each coral (n = 7 females) were fertilized in containers (12 L) with 600 ml sperm that were collected in equal proportions from three different sperm donors. To measure early life development, we randomly sampled 30 eggs from each container at 2 and 4 h post fertilization (hpf). After 4 hpf, we pooled all fertilized eggs and reared them in three 60 L containers. They were monitored at 12, 17, 24, 36, 48, 60, and 64 hpf, until all individuals have developed to larvae. Early life development stages are based on typical developmental stages in corals (Okubo et al. 2016; Randall et al. 2020; Grinblat et al. 2023) and were quantified and categorized as either (i) intact eggs, (ii) cleaved cells, (iii) gastrula (non-mobile), (iv) gastrula (mobile), or (v) larvae, using a compound microscope (Motic Zeiss) fitted with a Motic camera. In addition, we photographed 10 larvae and measured their longest diameter using Motic Image Software.
To test for settlement on different substrates, ten larvae (64 h old) were transferred to a clear well plate (2 cm in diameter) filled with 4 ml seawater at ambient temperature (29.87 ± 0.01 °C). The seawater used in the experiment was first treated using UV (ultraviolet) light and then filtered (1 µm) to reduce the number of bacteria present. To examine the effect of substrate type on larval settlement, ~ 0.5 cm2 substrate of either (i) coral rubble with crustose coralline algae (CCA), or (ii) coral tiles from dead table corals were added. Both substrate types were collected from shallow reefs and biologically preconditioned in raceways with flowing sand-filtered seawater for at least three weeks. Wells with (iii) no substrate were used as control. Each treatment contained 11 replicate wells. Approximately 50% of UVFSW was changed once per day using a dropper. Proportions of metamorphosed settled larvae were counted daily for eight consecutive days using a dissecting microscope (Motic). The experiment was terminated on day 8, because the number of larvae was insufficient for continuous monitoring, due to mortality.
To measure survival from larvae to juveniles, 200 larvae (64 h old) were allowed to settle on a coral tile (10 × 10 × 1 cm) placed in plastic containers (22 cm length × 16 cm width × 16 cm height, n = 3) filled with 5 L of sand-filtered seawater and with aeration. Approximately 50% of the seawater was changed daily for 30 d before flow-through seawater (0.5 L min−1 flow rate) was provided. After three mo of culture, we counted the number of juveniles using a dissecting microscope (Motic).
The remaining larvae that were not used in the settlement and survival experiments were allowed to settle on 10 fiber cement tiles (10 × 10 × 0.5 cm) with CCA placed in a container (60 L). The container was treated in a similar seawater flow system as in the larval survival experiment. After three mo of culture, juveniles from the 10 tiles were counted and then their survival was followed until 15 mo of culture. Juveniles (n = 30 to 118) were randomly chosen and photographed to measure their maximum diameter at 3, 5, 6, 7, 15 months of culture using a dissecting microscope (Motic) fitted with a Motic camera. The maximum diameter was measured using the Motic image software. In addition, the number of asexual buds around the base of the juvenile stalk was also counted. Detachment of stalk was also monitored up to 24 mo of culture.
Data analyses
To analyze the diel reproductive timing, the number of spawning corals was grouped into three time categories; 19:00 to 22:00, 22:01 to 01:00, and 01:01 AM to 04:00. These categories were chosen because corals spawned as early as 19:00, with notable spawning between 22:00 and 01:00, and spawning was not observed beyond 04:00. Moreover, a 3 h time range for each time category was chosen because major spawning of fungiid corals (i.e., C. echinata and C. crassa) were reported to last within 3 h (Loya et al. 2009) and also some species of colonial corals were reported to have high fertilization success when gametes are combined within 4 h after spawning (Dela Cruz and Harrison 2020). Differences in coral size across sexual groups (male, female, hermaphrodite, non-reproductive) in each country were analyzed using either One-way ANOVA or Kruskal–Wallis test (if data is not normally distributed), whereas egg size of H. actiniformis between the Philippines and Singapore were compared using Mann–Whitney U-test. Generalized Linear Mixed Model was used to analyze the percent settlement of larvae across different substrates through time with substrate type and time as fixed factor and replicate plastic wells as random effect. All statistical analyses were conducted in R version 4.1.1 software using Rstudio version 1.4.1717 (R Core Team 2021).
Results
Reproductive timing
Helifungia actiniformis in Singapore and the Philippines broadcasts spawned gametes. In Singapore, 5 to 50% of individuals released their gametes from February to May whereas 2.9 to 60.6% of individuals released their gametes from March to June in the Philippines (Figs. 2 and 3). Eggs were neutrally buoyant or were distributed throughout the water column for a few hours after spawning, and some eggs sank at the bottom of the container in the following morning.
a Daily and b monthly percentage of spawning Heliofungia actiniformis from Singapore from March to June and October to November 2019 and January to March 2020. Non-spawning days are not shown. The number of corals observed represented by each bar is shown. Dates of full moon were March 21, April 19, May 19, 2019, February 9, March 9, 2020
a Daily and b monthly percentage of spawning Heliofungia actiniformis from the Philippines from March 2019, March to December 2020, January to December 2021, and January to February 2022. Non-spawning days are not shown. The number of corals observed represented by each bar is shown. Dates of full moon were March 21, 2019, March 9, April 8, May 7, June 6, 2020, February 27, March 29, April 27, May 26, and June 25, 2021
Coral individuals in Singapore, spawned between 7 days before and 11 nights after full moon, except for a single individual (1 ind, 5.3%) that spawned at two nights after new moon (16 nights after full moon) in March 2020 (Fig. 2). In the Philippines, corals spawned their gametes between 8 days before and 11 nights after full moon. Additionally, only few individuals spawned between 2 days before and 7 days after new moon (between 13 and 21 days after full moon) in March 2020 (4 individuals, 2.3 to 10%), April 2021 (3 ind, 3.7 to 4.8%), and in May 2021 (1 ind, 5%) (Fig. 3). Spawning events in a given month occurred for up to 16 nights in Singapore, but only up to 10 nights in the Philippines. However, individuals were observed releasing concentrated gametes for two consecutive days only.
Spawning mostly occurs during the night both in Singapore and the Philippines. In the Philippines, major spawning activity generally occurred between 22:00 and 01:00 (79.81%, 87 out of 109 spawning observations), with minor spawning activity occurring earlier after sunset (between 19:00 and 22:00) (19.27%, 21 out 109), and post-midnight (between 01:00 and 04:00) (0.92%, 1 out of 109) (Fig. 4). The exact spawning time was not recorded in Singapore.
Coral sexuality and size
In Singapore, coral individuals either released sperm (37.5%, 12 out of 32, i.e., male gonochoric individuals) or released both gametes sequentially and simultaneously (9.38%, 3 out of 32, i.e., hermaphroditic individuals). No gonochoric females were recorded in either 2019 or 2020 observations (Table 1).
In the Philippines, H. actiniformis released either sperm (26.47%; 27 out of 102 individuals) or eggs (21.57%; 22 out of 102 individuals) (i.e., gonochoric individuals) except for the four individuals (3.92%) that released both gametes (i.e., hermaphroditic individuals) (Table 1). Among the four hermaphroditic individuals, one released both gametes sequentially (i.e., released sperm and eggs at different nights), whereas the other three individuals released both gametes simultaneously.
In addition, two individuals exhibited changes in their sexuality in the Philippines. One displayed bidirectional sex change (i.e., from female in 2019 to male in 2020 and to female in 2021) whereas the other individual changed from male in 2019 to female in 2020; however, its sexuality was not observed in 2021.
The male-to-female ratio of H. actiniformis in the Philippines varied across spawning months at each location with values ranging from 0 to 1, whereas female-to-male-to-hermaphrodite (1:0:0.44) was only observed in April 2020 (Table 2). The male to hermaphrodite ratio in Singapore ranged from 0 to 0.5 (Table 2).
The longest diameter of H. actiniformis varied among coral sexual groups in both Singapore (One-way ANOVA: F = 7.80, df = 2, p < 0.01) and the Philippines (Kruskal–Wallis: \({\chi }^{2}\)= 17.695, df = 3, p < 0.001, Fig. 5). In Singapore, hermaphroditic individuals (150.05 ± 10.70 mm; mean ± SE; range: 130–166, n = 3) and male (150.18 ± 4.46 mm; range: 127–161 mm; n = 7) were similar in size (p > 0.05) and both were significantly larger than the non-reproductive individuals (117.05 ± 7.03 mm, range: 67–142 mm, n = 10) (p < 0.05). In the Philippines, the female (132.21 ± 3.16 mm, range: 92–155 mm; n = 28) and hermaphroditic individuals (134 ± 5.18 mm, range: 124–148 mm; n = 4) were similar in size (p > 0.05) and both were larger than male (110.18 ± 5.12 mm, range: 57–169 mm, n = 40) and non-reproductive individuals (98.97 ± 5.88 mm, range: 49–148 mm, n = 30) (p < 0.05). Male and non-reproductive corals were similar in size (p > 0.05).
Diameter of different sexual groups also showed significant differences between countries (Kruskal–Wallis: \({\chi }^{2}\) = 32.61, df = 6, p < 0.001). Hermaphroditic and female individuals in the Philippines were similar in size to the hermaphroditic individuals in Singapore (p > 0.05). Male individuals were smaller in the Philippines than in Singapore (p < 0.05), whereas non-reproductive individuals were similar in size in the two countries (p < 0.05).
Egg size
The mean (± SE) egg size of H. actiniformis was smaller in Singapore (296.02 ± 7.75 \(\mu\)m; range: 193 to 384 \(\mu\)m; n = 37) compared with the Philippines (342.04 ± 1.65 \(\mu\)m; range: 242 to 410 \(\mu\)m, n = 180) (Mann–Whitney U test: Z = 6.43, p < 0.001).
Fertilization, larval development, and survival
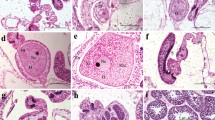
Fertilized eggs (indicated by cell cleavage) was observed within 2 h of post sperm addition. Non-motile and motile gastrula (e.g., swimming in circular pattern) were observed at 9 and 24 h post fertilization (hpf), respectively. The swimming larvae (1096 ± 54.01 \(\mu\)m (mean ± SE); n = 10) were first observed at 60 hpf and all individuals developed to larvae at 64 hpf (Fig. 6). Survival from larvae to juveniles after 3 months of culture was 1.72 ± 1.0% (mean ± SE).
Larval settlement on different substrates
The number of settled larvae was not significantly different among substrates (GLMM: \({\chi }^{2}\) = 0.30, p > 0.05) but it was significantly different across time (GLMM: \({\chi }^{2}\) = 130.45, p < 0.0001), and no interaction between treatment and time (GLMM: \({\chi }^{2}\) = 9.45, p > 0.05) (Fig. 7). A significant number (6.36%) of settled larvae was observed starting at 3 days post hatching (p < 0.05). Most of the larvae (61. 8%) settled at 7 d post hatching.
Percent settlement (mean ± standard error of the means, n = 11) of Heliofungia actiniformis larvae exposed to different substrates for 8 days. Percent settlement was not significantly different across treatments (GLM: \({X}^{2}\) = 0.30, p > 0.05) but showed significant differences across time (GLM: \({X}^{2}\) = 130.45, p < 0.0001), and no interaction between treatment and time (GLM: \({X}^{2}\) = 9.45, p > 0.05)
Juvenile size and survival
Three month old juveniles had a diameter of 0.58 ± 0.04 mm (mean ± SE; range: 0.33 to 1.30 mm, n = 30) whereas 15 mo old juveniles had a 5.44 ± 0.28 mm (mean ± SE; range: 1.0 to 13.0 mm, n = 118) diameter (Fig. 8). Survival of 3 mo old juveniles until 15 mo of culture was 56.12% ± 9.04 (mean ± SE).
Production of asexual buds in juveniles and detachment of stalk
15 month old juveniles with \(\ge 0.\)8 cm in diameter (n = 11) had 1 to up to 6 buds or new polyps around the base of their stalks (Fig. 9a and b). After 24 month of culture, juveniles with 3.1 to 4.3 cm diameter (n = 4) were observed to detach from their stalks (Fig. 9c). One month after detachment, the empty stalks showed regeneration of polyps (Fig. 9d).
Discussion
Reproductive timing
Broadcast spawning of H. actiniformis was observed nights before and after full moon from February to May in Singapore and March to June in the Philippines. Similar spawning timing has been also observed in other fungiid corals such as F. scutaria (1 to 4 d after full moon) in the northern Red Sea (Kramarsky-Winter and Loya 1998) and C. echinata, C. crassa and F. repanda (4 to 8 nights after full moon) in Okinawa, Japan (Loya et al. 2009). Corals (e.g., Acroporidae, Mussidae, Agariciidae, Faviidae, Oculinidae, Merulinida, Poritidae and Pectiniidae) rely on environmental cues including light and temperature to synchronize spawning. Evidently, many colonial corals across different regions, including the Philippines and Singapore broadcast spawn their gametes nights after the full moon during warmer months (Guest et al. 2002; Zayasu and Shinzato 2016; Gomez et al. 2018; Jamodiong et al. 2018a). In addition, our observation of spawning occurring earlier in lower latitudes is similar to previous findings for Japan, where timing of peak reproductive activity is one month earlier for every 2–3° south (Baird et al. 2009b), and for the Indian Ocean, where spawning occurs early in the year in low latitudes (February and March, 12°N) and later in high latitutudes (April and May, 25°N; June to September, 30°N) (Shlesinger and Loya 1985; Mangubhai and Harrison 2008; Baird et al. 2014; Howells et al. 2014).
The major spawning activity of H. actiniformis generally occurred between 22:00 and 01:00 similar to other fungiid corals such as Ctenactis echinata (22:00–23:30) and C. crassa (1:00–2:00) (Loya et al. 2009). However, few individuals spawned earlier at night (from ~ 19:00), similar to F. scutaria (17:00–18:00) (Kramarsky-Winter and Loya 1998), and early in the morning (1:00–4:00), similar to F. repanda (3:00–5:00) (Loya et al. 2009). Synchronous spawning within few hours is common among corals (Babcock et al. 1986), as this facilitates fertilization and larval survival (Loya et al. 2009). For instance, high fertilization rates were achieved in Acropora tenuis, A. millepora, and Favites colemani when their gametes were combined within 4 h after release (Dela Cruz and Harrison 2020).
Spawning of H. actiformis was observed for up to 16 nights, but individuals were observed releasing concentrated gametes for two consecutive days. This is similar to the fungiid coral C. echinata, which spawns for up to four successive nights, but typically releases their gametes for up to two days with a single gamete release per individual being the most common (Loya et al. 2009). The release of gametes in high concentrations could be a strategy to avoid gamete dilution that may delay sperm-egg encounters. Indeed, lower concentrations of sperm yield low fertilization in colonial corals (e.g., Acropora tenuis, A. millepora and Favites colemani) (Dela Cruz and Harrison 2020). Moreover, it has been reported that fertilization success in corals (e.g., A. tenuis, A. digitifera, Coelastrea aspera and Platygyra daedalea) increases as sperm concentration and contact time increase (Buccheri et al. 2023). Spawning duration was longer in Singapore (14 consecutive nights from a single coral and 16 consecutive nights from different corals) than in the Philippines (five consecutive nights from a single coral and 10 consecutive nights from different corals). Releasing gametes for several days is likely a strategy to enhance survival by having more chances of encountering favourable conditions and avoiding exposure of gametes to a single catastrophic event which may result to low fertilization and survival (Baird et al. 2010; Gilmour et al. 2016). For instance, F. scutaria that inhabit shallower areas with high fluctuations in environmental conditions have a prolonged spawning period compared to F. granulosa (1 to 2 nights) that live in calmer and deeper waters (15–20 m) (Kramarsky-Winter and Loya 1998). Moroever, the differences in the day of spawning observed in H. actinformis in this study could also be due to the absence of other natural spawning cues in the experimental set up such as tides and water current (Babcock et al. 1986; Hayashibara et al. 1993).
Reproductive mode and sexuality
Unlike many colonial corals which release buoyant sperm-egg bundles, H. actiniformis broadcast spawn either a cloud of sperm or eggs, similar to F. granulosa (Kramarsky-Winter and Loya 1998), F. repanda, and C. echinata (Loya and Sakai 2008), although individuals that released both gametes were also observed. Individuals that release sperm are smaller in size compared to those that release eggs, suggesting that H. actiniformis is a protandrous hermaphrodite.
Changes in sex were observed in H. actiniformis. To date, sex changes have only been reported in a few species of fungiid corals. For instance, Fungia repanda and F. scruposa exhibit protandrous sex change, Herpolitha limax exhibit bidirectional sex change, whereas C. echinata and C. crassa display both protandrous and bidirectional sex change (Loya and Sakai 2008; Loya et al. 2009; Eyal-Shaham et al. 2019). The observed change in sex from male to female, and female to male to female, in H. actiniformis suggests that the species displays bidirectional sex change.
Reproductive output
The egg size of H. actiniformis (193 to 410 μm) was larger compared to other fungiid species (e.g., C. echinata, C. crassa, F. repanda, F. granulosa), which typically ranges between 100 and 150 μm (Loya et al. 2009), but smaller compared to other colonial corals (e.g., Favidae and Acroporidae) with egg sizes that range between 390 to 700 μm (Maboloc et al. 2016; Jamodiong et al. 2018b; Gomez et al. 2018). Heliofungia actiniformis has relatively larger polyps than the fungiid species mentioned above, hence it is expected that its eggs will be also larger in size as seen in other coral species (Rinkevich and Loya 1987).
The egg size at higher latitudes (the Philippines) was larger compared to lower latitudes (Singapore), contrary to the findings of Santacruz-Castro (2019). This difference in egg size is most likely not a function of colony size since female and hermaphroditic spawning coral individuals between regions have similar sizes. The variability may be due to differences in maternal energy investment during gametogenesis as well as exposure to different environmental conditions (Michalek-Wagner and Willis 2001; Santacruz-Castro 2019). Sedimentation and turbidity in Singapore are relatively high compared to Philippines (Guest et al. 2002), and corals in Singapore may allocate some of their energy to counteract sedimentation, leading to less energy being allocated to reproduction.
Larval development, settlement, and juvenile growth and survival
Heliofungia actiniformis displayed early life development typical of many coral species. Fertilization and larval development occurred within a few hours and settlement began at 24 h after development to larvae; however, some lasted up to several days in the water column. During pre-settlement, H. actiniformis larvae started to migrate towards the bottom of the container, explore the bottom, and exhibit a circular movement before they attached to the substrate and metamorphosed. Coral larvae explore their surroundings to look for suitable substrates for attachment and they settle faster when preferred substrates are available. Previous studies have shown that substrates with CCA are effective settlement inducers in corals (Whitman et al. 2020). However, the larvae of H. actiniformis in this present study, did not show preference for either coral rubble with CCA substrate or the coral tiles, and they even settled and metamorphosed in containers without substrates. In addition, the larvae that did not successfully settle and metamorphose at 8 d post hatching started to degrade. It has been suggested that larvae need to settle and metamorphose before they deplete their energy reserves, otherwise, they will experience slower growth and low survival. Although longer planktonic stage might be beneficial for high dispersal, it also increases larval mortality due to predation. The early settlement (i.e., < 8 d) observed in H. actiniformis which is similar to the report (2–3 d) by Abe (1937) suggests shorter pelagic duration and limited dispersal of larvae; this has implications on their population distribution and genetic structure. Indeed, lack of genetic structuring was found among populations of H. actiniformis in the Spermonde archipelago (~ 30 km distance between populations); however, a large scale genetic differentiation was detected in the Indo-Malay archipelago (Knittweis et al. 2009a, b).
Similar to many other coral species, survival of H. actiniformis larvae to post settled juveniles was extremely low (1.72%). However, 3 month old juvenile had only 54% mortality after 15 month of culture, suggesting that juveniles may have acquired some mechanisms essential for survival (i.e., feeding). The high maintenance and care of juveniles during culture in the hatchery, such as by eliminating predators in the set up and cleaning them of sediments may have also contributed to the high survival during culture. Considering that juveniles had a size of 5.4 ± 0.28 mm they would be prone to predation and even accidental grazing in the natural environment.
Budding, detachment, and regeneration of polyps
Production of asexual buds and detachment of polyps from the stalk has been documented in solitary coral H. actiniformis, but only in the natural environment (Hoeksema 1989; Boschma 1922; Abe 1937; Knittweis et al. 2009b). Here, we showed that juveniles of \(\ge 0.\) 8 cm in diameter (n = 11) can already produce asexual buds whereas 24 mo old juveniles that were 3.1 to 4.3 cm in diameter (n = 4) can detach from their stalks. This finding is similar to Knittweis et al. (2009b) where H. actiniformis polyps detached at around 3–4 cm, and aligns with other species under the same family, including Fungia fungites which detach when polyps reach sizes of around 5 cm (Goffredo and Chadwick-Furman 2003).
Mushroom corals are generally able to reproduce asexually through regeneration of buds after polyp injury or after the coral disc detaches from its stalk (Hoeksema and Yeemin 2011). In the case of the genus Cycloseris, individuals can undergo natural autotomy, where they can divide into wedge-shaped segments repetitively (Hoeksema et al. 2018). Serial budding or detachment of polyp form the stalk is evident in several genera such as Fungia and Heliofungia (Hoeksema and Yeemin 2011; Knittweis et al. 2009b). In this present study, approximately one month after detachment of the polyp, we found scars from empty stalks of H. actiniformis that had regenerated and developed buds with tentacles. This asexual reproductive strategy may help H. actiniformis maintain and increase its population, particularly under natural conditions (e.g., grazing and predation on polyps) and physical disturbances (e.g., detachment due to strong waves). Since H. actiniformis is able to reproduce asexually, there is a possibility that some of the sampled individuals in the present study were clones. We suggest that future studies should examine the effects of fungiid clonality on reproductive traits, such as the timing and extent of reproduction among clones, fertilization success, and juvenile development.
Conclusion
This study highlights the variability in the reproductive traits of the solitary coral H. actiniformis at different latitudinal locations and provides insights into their early life development, settlement competency, and juvenile growth and survival. Heliofungia actiniformis, a broadcast spawner, exhibited similar lunar spawning periodicity in the Philippines (16°N) and Singapore (1°N); however, spawning at higher latitudes was later in the year, and corals displayed a shorter duration of spawning nights and a larger egg size. This variability in reproduction may be due to differences in maternal investment as well as environmental conditions across latitudes. Given that H. actiniformis shares similar development, settlement competency, and post-settlement survival with many coral species that are known to have early life histories that are vulnerable to changing climate and anthropogenic disturbances, it is likely that they are also vulnerable to these stressors. This information is essential for understanding the biology, ecology, and population dynamics of solitary corals across different latitudinal locations. Since populations of H. actiniformis are threatened by the aquarium trade (Knittweis and Wolff 2010), the successful production of H. actiniformis and their capacity to produce asexual buds under hatchery conditions show their potential for aquaculture for coral reef restoration and to reduce pressure on natural stocks caused by the aquarium trade.
Data availability
Data from this study are available from the corresponding author on a reasonable request.
References
Abe N (1937) Post-larval development of the coral Fungia actiniformis var. palawensis Doederlein. Palao Trop Biol Station Stud 1:73–93
Babcock RC (1984) Reproduction and distribution of two species of Goniastrea (Scleractinia) from the Great Barrier Reef Province. Coral Reefs 2:187–195. https://doi.org/10.1007/BF00263572
Babcock RC, Heyward AJ (1986) Larval development of certain gamete-spawning scleractinian corals. Coral Reefs 5:111–116. https://doi.org/10.1007/BF00298178
Babcock RC, Bull GD, Harrison PL, Heyward AJ, Oliver JK, Wallace CC, Willis BL (1986) Synchronous spawnings of 105 scleractinian coral species on the Great Barrier Reef. Mar Biol 90:379–394. https://doi.org/10.1007/BF00428562
Baird AH, Guest JR, Willis BL (2009a) Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst 40:551–571. https://doi.org/10.1146/annurev.ecolsys.110308.120220
Baird AH, Birrel CL, Hughes TP, Mcdonald A, Nojima S, Page CA, Prachett MS, Yamasaki H (2009b) Latitudinal variation in reproductive synchrony in Acropora assemblages: Japan vs. Australia. Galaxea, J Coral Reef Stud 11:101–108. https://doi.org/10.3755/galaxea.11.101
Baird AH, Kospartov MC, Purcell S (2010) Reproductive synchrony in Acropora sssemblages on reefs of New Caledonia. Pac Sci 64:405–412. https://doi.org/10.2984/64.3.405
Baird AH, Abrego D, Howells EJ, Cumbo VR (2014) The reproductive season of Acropora in Socotra, Yemen. F1000Res 3:78
Bauman AG, Guest JR, Dunshea G, Low J, Todd PA, Steinberg PD (2015) Coral settlement on a highly disturbed equatorial reef system. PLoS ONE 10:e0127874. https://doi.org/10.1371/journal.pone.0127874
Bos AR, Hoeksema BW (2015) Cryptobenthic fishes and co-inhabiting shrimps associated with the mushroom coral Heliofungia actiniformis (Fungiidae) in the Davao Gulf, Philippines. Environ Biol Fish 98:1479–1489. https://doi.org/10.1007/s10641-014-0374-0
Boschma H (1922) On budding and coalescence of buds in Fungia fungites and Fungia actiniformis. Proc K Ned Akad Wet 24:257–268
Buccheri E, Ricardo GF, Babcock RC, Mumby PJ, Doropoulos C (2023) Fertilisation kinetics among common Indo-Pacific broadcast spawning corals with distinct and shared functional traits. Coral Reefs. https://doi.org/10.1007/s00338-023-02431-2
Cesar SA, Amoin NB, Dy DT (2014) Thermal stress affects zooxanthellae density and chlorophyll-a concentration of the solitary mushroom coral, Heliofungia actiniformis. Philipp J Sci 143:35–42
Combosch DJ, Vollmer SV (2013) Mixed asexual and sexual reproduction in the Indo-Pacific reef coral Pocillopora damicornis. Ecol Evol. https://doi.org/10.1002/ece3.721
Dela Cruz DW, Harrison PL (2020) Optimising conditions for in vitro fertilization success of Acropora tenuis, A. millepora and Favites colemani corals in northwestern Philippines. J Exp Mar Biol Ecol 524:151286. https://doi.org/10.1016/j.jembe.2019.151286
Eyal-Shaham L, Eyal G, Sakai K, Nozawa Y, Harii S, Sinniger F, Bronstein O, Ben-Zvi O, Shlesinger T, Loya Y (2019) Repetitive sex change in the stony coral Herpolitha limax across a wide geographic range. Sci Rep 9:2936. https://doi.org/10.1038/s41598-018-37619-y
Eyal-Shaham L, Eyal G, Ben-Zvi O, Sakai K, Harii S, Sinniger F, Hirose M, Cabaitan P, Bronstein O, Feldman B, Shlesinger T, Levy O, Loya Y (2020) A unique reproductive strategy in the mushroom coral Fungia fungites. Coral Reefs 39:1793–1804. https://doi.org/10.1007/s00338-020-02004-7
Gilmour J, Speed CW, Babcock R (2016) Coral reproduction in Western Australia. PeerJ 4:e2010. https://doi.org/10.7717/peerj.2010
Goffredo S, Chadwick-Furman N (2003) Comparative demography of mushroom corals (Scleractinia: Fungiidae) at Eilat, northern Red Sea. Mar Biol 142:411–418. https://doi.org/10.1007/s00227-002-0980-9
Gomez EJ, Jamodiong EA, Maboloc EA, Ligson CA, Tabalanza TD, Villanueva RD, Cabaitan PC (2018) Gametogenesis and reproductive pattern of the reef-building coral Acropora millepora in northwestern Philippines. Invertebr Reprod Dev 62:202–208. https://doi.org/10.1080/07924259.2018.1496155
Green E, Shirley F (1999) The global trade in corals. WCMC Biodivers Ser 10:1–74
Grinblat M, Eyal-Shaham L, Eyal G, Ben-Zvi O, Harii S, Morita M, Sakai K, Hirose M, Miller DJ, Loya Y (2023) Energy allocation trade-offs as a function of age in fungiid corals. Front Mar Sci 10:1113987. https://doi.org/10.3389/fmars.2023.1113987
Guest J, Baird A, Goh B, Chou L (2002) Multispecific, synchronous coral spawning in Singapore. Coral Reefs 21:422–423. https://doi.org/10.1007/s00338-002-0263-4
Harrison PL (2011) Sexual reproduction of scleractinian corals. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Netherlands, Dordrecht, pp 59–85
Hayashibara T, Shimoike K, Kimura T, Hosaka S, Heyward A, Harrison P, Kudo K, Omori M (1993) Patterns of coral spawning at Akijima Island, Okinawa, Japan. Mar Ecol Prog Ser 101:253–262
Hoeksema B (1989) Taxonomy, phylogeny and biogeography of mushroom corals (Scleractinia: Fungiidae). Zool Verh 254(1):1–295
Hoeksema BW (2012) Distribution patterns of mushroom corals (Scleractinia: Fungiidae) across the Spermonde Shelf, South Sulawesi. Raffles Bull Zool 60:183–212
Hoeksema BW, Yeemin T (2011) Late detachment conceals serial budding by the free-living coral Fungia fungites in the Inner Gulf of Thailand. Coral Reefs 30:975–975. https://doi.org/10.1007/s00338-011-0784-9
Hoeksema B, Rogers A. Quibilan M 2008. Heliofungia actiniformis. The IUCN Red List of Threatened Species 2008: e.T133269A3663591. Accessed 19 Sep 2023
Hoeksema BW, Van Der Meij SET, Fransen CHJM (2012a) The mushroom coral as a habitat. J Mar Biol Ass 92:647–663. https://doi.org/10.1017/S0025315411001445
Hoeksema BW, Matthews JL, Yeemin T (2012b) The 2010 coral bleaching event and its impact on the mushroom coral fauna of Koh Tao, western Gulf of Thailand. Phuket Mar Biol Cent Res Bull 71:71–81
Hoeksema BW, Bouwmeester J, Range P, Ben-Hamadou R (2018) A large aggregation of self-fragmenting mushroom corals in the Arabian/Persian Gulf. Ecology 99:1236–1238. https://doi.org/10.1002/ecy.2139
Howells EJ, Abrego D, Vaughan GO, Burt JA (2014) Coral spawning in the Gulf of Oman and relationship to latitudinal variation in spawning season in the northwest Indian Ocean. Sci Rep 4:7484. https://doi.org/10.1038/srep07484
Jamodiong EA, Maboloc EA, Leriorato JC, Tañedo MCS, Diaz LA, Tabalanza TD, Cabaitan PC, Villanueva RD (2018a) Coral spawning and spawn-slick observation in the Philippines. Mar Biodivers 48:2187–2192. https://doi.org/10.1007/s12526-017-0680-9
Jamodiong EA, Maboloc EA, Villanueva RD, Cabaitan PC (2018b) Gametogenesis and inter-annual variability in the spawning pattern of Acropora hyacinthus in Northwestern Philippines. Zool Stud. https://doi.org/10.6620/ZS.2018.57-46
Knittweis L (2008) Population demographics and life history characteristics of Heliofungia actiniformis: a fungiid coral species exploited for the live coral aquarium trade in the Spermonde Archipelago, Indonesia. Dissertation, Bremen University
Knittweis L, Wolff M (2010) Live coral trade impacts on the mushroom coral Heliofungia actiniformis in Indonesia: potential future management approaches. Biol Cons 143:2722–2729. https://doi.org/10.1016/j.biocon.2010.07.019
Knittweis L, Kraemer WE, Timm J, Kochzius M (2009a) Genetic structure of Heliofungia actiniformis (Scleractinia: Fungiidae) populations in the Indo-Malay Archipelago: implications for live coral trade management efforts. Conserv Genet 10:241–249. https://doi.org/10.1007/s10592-008-9566-5
Knittweis L, Jompa J, Richter C, Wolff M (2009b) Population dynamics of the mushroom coral Heliofungia actiniformis in the Spermonde Archipelago, South Sulawesi, Indonesia. Coral Reefs 28:793–804. https://doi.org/10.1007/s00338-009-0513-9
Kramarsky-Winter E, Loya Y (1998) Reproductive strategies of two fungiid corals from the northern Red Sea:environmental constraints? Mar Ecol Prog Ser 174:175–182. https://doi.org/10.3354/meps174175
Krupp DA (1983) Sexual reproduction and early development of the solitary coral Fungia scutaria (Anthozoa: Scleractinia). Coral Reefs 2:159–164. https://doi.org/10.1007/BF00336722
Loya Y, Sakai K (2008) Bidirectional sex change in mushroom stony corals. Proc R Soc B 275:2335–2343. https://doi.org/10.1098/rspb.2008.0675
Loya Y, Sakai K, Heyward A (2009) Reproductive patterns of fungiid corals in Okinawa, Japan. Galaxea, J Coral Reef Stud 11:119–129. https://doi.org/10.3755/galaxea.11.119
Maboloc EA, Jamodiong EA, Villanueva RD (2016) Reproductive biology and larval development of the scleractinian corals Favites colemani and F. abdita (Faviidae) in northwestern Philippines. Invertebr Reprod Dev 60:1–11. https://doi.org/10.1080/07924259.2015.1086829
Mangubhai S, Harrison P (2008) Asynchronous coral spawning patterns on equatorial reefs in Kenya. Mar Ecol Prog Ser 360:85–96. https://doi.org/10.3354/meps07385
Michalek-Wagner K, Willis BL (2001) Impacts of bleaching on the soft coral Lobophytum compactum. II. Biochemical changes in adults and their eggs. Coral Reefs 19:240–246. https://doi.org/10.1007/PL00006959
Nozawa Y, Harrison PL (2005) Temporal settlement patterns of larvae of the broadcast spawning reef coral Favites chinensis and the broadcast spawning and brooding reef coral Goniastrea aspera from Okinawa, Japan. Coral Reefs 24:274–282. https://doi.org/10.1007/s00338-005-0476-4
Nozawa Y, Villanueva RD, Munasik M, Roeroe KA, Mezaki T, Kawai T, Guest J, Arakaki S, Suzuki G, Tanangonan JJB, Ang PO, Edmunds PJ (2021) Latitudinal variation in growth and survival of juvenile corals in the West and South Pacific. Coral Reefs 40:1463–1471. https://doi.org/10.1007/s00338-021-02169-9
Okubo N, Hayward DC, Forêt S, Ball E (2016) A comparative view of early development in the corals Favia lizardensis, Ctenactis echinata, and Acropora millepora - morphology, transcriptome, and developmental gene expression. BMC Evol Biol 16:48. https://doi.org/10.1186/s12862-016-0615-2
Prasetia R, Lim ZW, Teo A, Shlesinger T, Loya Y, Todd PA (2020) Population dynamics and growth rates of free-living mushroom corals (Scleractinia: Fungiidae) in the sediment-stressed reefs of Singapore. Advances in Marine Biology. Elsevier, pp 115–140
R Development Core Team (2021) R: a language and environment for statistical omputing. R Foundation for Statistical Computing
Randall CJ, Negri AP, Quigley KM, Foster T et al (2020) Sexual production of corals for reef restoration in the Anthropocene. Mar Ecol Prog Ser 635:203–232. https://doi.org/10.3354/meps13206
Rinkevich B, Loya Y (1987) Variability in the pattern of sexual reproduction of the coral Stylophora pistillata at Eilat, Red Sea: a long-term study. Biol Bull 173:335–344. https://doi.org/10.2307/1541546
Sakai K (1997) Gametogenesis, spawning, and planula brooding by the reef coral Goniastrea aspera (Scleractinia) in Okinawa, Japan. Mar Ecol Prog Ser 151:67–72. https://doi.org/10.3354/meps151067
Santacruz-Castro AM (2019) Regional variability in reproductive traits of the Acropora hyacinthus species complex in the Western Pacific Region. PLoS ONE 14:e0208605. https://doi.org/10.1371/journal.pone.0208605
Shlesinger Y, Loya Y (1985) Coral community reproductive patterns: Red Sea versus the Great Barrier Reef. Science 228:1333–1335. https://doi.org/10.1126/science.228.4705.1333
Tsounis G, Rossi S, Aranguren M, Gili J-M, Arntz W (2006) Effects of spatial variability and colony size on the reproductive output and gonadal development cycle of the Mediterranean red coral (Corallium rubrum L.). Mar Biol 148:513–527. https://doi.org/10.1007/s00227-005-0100-8
Veron JEN (2000) Corals of the world. Australian Institute of Marine Science, Townsville
Whitaker K (2006) Genetic evidence for mixed modes of reproduction in the coral Pocillopora damicornis and its effect on population structure. Mar Ecol Prog Ser 306:115–124. https://doi.org/10.3354/meps306115
Whitman TN, Negri AP, Bourne DG, Randall CJ (2020) Settlement of larvae from four families of corals in response to a crustose coralline alga and its biochemical morphogens. Sci Rep 10:16397. https://doi.org/10.1038/s41598-020-73103-2
Willis BL, Babcock RC, Harrison PL, Oliver JK, Wallace CC (1985) Patterns in the mass spawning of corals on the Great Barrier Reef from 1981 to 1984. In: Proceedings of the Fifth International Coral Reef Congress, Tahiti, French Polynesia, pp 343–348
Zayasu Y, Shinzato C (2016) Hope for coral reef rehabilitation: massive synchronous spawning by outplanted corals in Okinawa, Japan. Coral Reefs 35:1295–1295. https://doi.org/10.1007/s00338-016-1463-7
Acknowledgements
The authors would like to thank Kenith Adolfo, Robert Valenzuela, Ronaldo de Guzman, Renato Uriarte, Renato Adolfo, Fernando Castrence, Charlon Ligson, and Celine Campos of the Bolinao Marine Laboratory for the assistance with the experiment. We thank also Zi Wei Lim and Aaron Teo from the Experimental Marine Ecology Laboratory, National University of Singapore for field assistance in collecting the corals. We acknowledge the St. John’s Island National Marine Laboratory, a National Research Infrastructure under the National Research Foundation Singapore, for providing the facility necessary for conducting the research in Singapore. Sample collection in Singapore was carried out under permit issued by Singapore National Parks Board (Permit No. NP/RP18-042).
Funding
This study was funded in part by the In-House Grant from the Marine Science Institute, UPD to PCC and the National Research Foundation, Prime Minister’s Office, Singapore (NRF) and the Israel Science Foundation (ISF) Joint Research Program No. NRF-ISF-2654/17 to PAT and YL.
Author information
Authors and Affiliations
Contributions
SGS, RP, PT, YL, PCC conceptualized the study. All authors contributed to the collection and preparation of data. SGS, RP did the statistical analyses. SGS prepared the first draft of the manuscript. All authors contributed to the review and writing of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals have been obtained.
Additional information
Responsible Editor: S. Harii.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sayco, S.L.G., Prasetia, R., Todd, P.A. et al. Reproductive biology and early life history of the solitary coral Heliofungia actiniformis from Singapore and the Philippines. Mar Biol 171, 56 (2024). https://doi.org/10.1007/s00227-023-04378-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04378-y