Abstract
Hydrogen is a promising energy carrier and a replacement for fossil fuels, since it is clean and has high energy and its application does not contribute to the greenhouse effect. Renewable resources, such as lignocellulosic materials and organic wastes, in particular, dark fermentative hydrogen methods, as the feedstock for hydrogen production have great potential for supplying hydrogen.
The development of novel and effective cellulase enzymes, the optimization and improvement of cellulase systems, and engineering approaches for cellulose pretreatment and saccharification to produce biohydrogen have high interest in the scientific community. This chapter gives an introduction to the feedstocks (lignocellulosic materials and organic wastes), primary technologies (physical, chemical, physicochemical, and biological), process of feedstock pretreatments, microorganisms, fermenter types (continuous stirred tank reactors, upflow anaerobic sludge blanket, anaerobic biofilm and granule reactor, membrane bioreactor, etc.), and operational conditions (substrate concentrations, nutrients, pH, temperature, hydraulic retention time, etc.) for producing biohydrogen.
Pretreatment and saccharification are at the heart of producing biological hydrogen from lignocellulosic feedstocks. Efficient production of biohydrogen via lignocellulosis and organic waste depends largely on the fermenter type. Selection of the pretreatment system and fermenter type is the key for economic success of the biohydrogen production plant. This chapter also aims to develop a fundamental understanding of key technologies and variables during biohydrogen production from lignocellulosic raw materials and organic feedstock.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction to Feedstocks
Biofuel sources can be classified into first-, second-, and third-generation feedstocks. The first-generation biofuel feedstocks are traditionally food related such as corn for ethanol, vegetable oil, and animal fats. Second-generation biofuel feedstocks are of the nonfood crop type, wastewaters, lignocellulosic agriculture, and forest residues. However, one issue is that the energy crops required for the biofuel source can compete with land use for agriculture [1]. The key challenge for developing the next generation of biofuels is acquiring an economically viable feedstock. Feedstock costs contribute 80–90 % to the final fuel price for many processes and therefore they are important to the economics of biofuels [2].
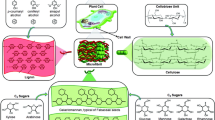
The feedstock is made up of organic compounds such as carbohydrates that can be converted to biohydrogen by biological metabolism [3]. Biological hydrogen is produced via dark fermentation of organic wastes [4]. Fermentative hydrogen production is carried out by anaerobic bacteria that ferment organic compounds to volatile fatty acids (VFAs), alcohols, carbon dioxide, and hydrogen (Fig. 2.1). Many different substrates can be fermented to produce hydrogen [5]. Dark fermentation has advantages, for example, high rate of bacterial growth, low energy requirement, no oxygen limitation problems, low cost [6, 7], and biohydrogen production without light, and various carbon sources can be utilized as the substrate [8].
Waste materials that have been used as substrate for biohydrogen include palm oil mill effluent (POME) [6, 9–17], starch-based materials [18–28], food waste [29–33], and condensed molasses fermentation soluble (CMS) [34–38]. Sugary wastewater [39–42] is a more efficient source of carbohydrates than raw materials for biohydrogen production. Simple sugars, such as sucrose and glucose, can be converted at high temperatures into hydrogen at high conversion efficiencies [43]. Glucose, which is an easily biodegradable carbon source, is present in many industrial effluents and can be obtained abundantly from agricultural wastes [44]. Therefore, sugary wastewater can be considered to be most useful for industrial hydrogen production.
1.1 Organic Wastes
Various organic solid wastes or wastewaters have attracted considerable attention for biohydrogen production due to the advantages of high organic loading possibilities, low nutrient requirements, concurrent wastewater treatment, and positive net energy gain. Table 2.1 shows complex organic wastes that are considered as feedstock, such as kitchen, food processing, mixed, and municipal wastes for biohydrogen production. These organic wastes usually have high concentrations of protein and fat, making their hydrogen conversion efficiencies lower than that of the carbohydrate-based wastewaters. As a matter of fact, previous studies show that the hydrogen production prospective of carbohydrate-based wastes was higher than that of fat- and protein-based wastes by about 20 times [57]. This is partially due to the protein degradation that produces nitrogen that consumes the free hydrogen. In many kinds of wastes, the organic fraction of municipal solid waste (OFMSW) is considered to be quite favorable as a potential raw material for biohydrogen production, because it is able to represent up to 70 % of the total MSW produced, consisting of paper (up to 40 %), food wastes, garden residues, and wood [56]. It should be noted that the process will lead to an extra cost, because an initial selection/separation process would be necessary in order to obtain the suitable substrates. Starchy- and sugary-based biomass and wastes are readily fermented by microorganisms for hydrogen generation. Although lignocellulosic biomass is abundant in agricultural residues, it needs pretreatment to be useable.
1.2 Lignocelluloses
Lignocellulose is the most abundant renewable biomass in the world with an annual quantity of about 220 billion tons (dry weight) per year [58]. To use this feedstock, pretreatment is necessary to decompose the lignin structure and loosen the crystalline cellulose structure to promote enzyme accessibility. Pretreated lignocellulosic materials (e.g., sugarcane bagasse, corncob, wheat straw, cornstalks, grass, energy crops, oil palm trunk, and beer lees [59]) can be used for fermentative hydrogen production. Some studies have focused on biohydrogen and biomethane production from raw or pretreated solid wastes such as olive pulp, household solid waste, and potato waste [60–68]. The feedstock costs are high due to processing (shredding, densifying, pulverizing, and handling), collection, and transportation. Despite the challenges, these second-generation feedstock options are plentiful and produce great amounts of fuel. Lignocellulosic material basically consists of three different types of polymers, namely, cellulose, hemicellulose, and lignin. Agricultural and forestry residues are rich in carbohydrates and the cost of obtaining these residues is negligible. However, these do not contain readily accessible free sugars necessary for efficient fermentation. To convert cellulose, it is necessary to transform the carbohydrate polymers into fermentable sugars through the use of enzymes and change the structure of the cellulosic biomass. Therefore, biotransforming it into hydrogen is a difficult task in most cases. In Table 2.2 different types of residues used as feedstock for hydrogen production are presented, along with the achieved hydrogen yields and rates.
2 Pretreatment of Lignocellulosic Feedstock
Pretreatment is widely accepted to be an essential step for making lignocellulosic biomass accessible to enzymatic attack by breaking the lignin seal, removing hemicellulose, or disrupting the crystalline structure of cellulose [56]. An effective and economical pretreatment should meet the following requirements: (a) delignify feedstock for enzymatic attack, (b) avoid destruction of hemicelluloses and cellulose, (c) avoid formation of possible inhibitors, (d) minimize energy demand, (e) reduce cost for size reduction of feedstock, (f) reduce reactor costs, (g) produce low residues, and (h) decrease chemical costs [82]. Several methods have been introduced for pretreating lignocellulosic materials, namely, prior enzymatic hydrolysis or digestion. These methods are classified into physical, chemical, physicochemical, and biological pretreatments. The main principles of each pretreatment method are illustrated below.
2.1 Physical
The objective of the physical pretreatment is to reduce particle size, pore size, crystallinity of lignocellulosic material, and the degree of polymerization and increase the specific surface [83, 84]. Different types of physical processes such as milling (e.g., ball milling, two-roll milling, hammer milling, colloid milling, and vibro energy milling) and irradiation (e.g., by gamma rays, electron beam, or microwaves) can be used to improve the enzymatic hydrolysis or biodegradability of lignocellulosic waste materials [82].
Milling can be employed to alter the inherent ultrastructure of lignocellulosic material and the degree of crystallinity and consequently make it more amenable to cellulase [85]. Milling and size reduction have been applied prior to enzymatic hydrolysis or even other pretreatment processes such as dilute acid, steam, or ammonia [85, 86]. Among the milling processes, the colloid mill, fibrillator, and dissolver are the only ones suitable for wet materials, e.g., wet paper from domestic waste separation or paper pulps. However, the extruder, roller mill, cryogenic mill, and hammer mill are usually used for dry materials.
Irradiation by gamma rays, electron beam, and microwaves can improve enzymatic hydrolysis of lignocellulosic materials as well. The combination of radiation and other methods such as acid treatment can further accelerate enzymatic hydrolysis [87]. Irradiation has enhanced enzymatic degradation of cellulose into glucose. However, pre-irradiation was found to be more effective in air than in an acid solution [88].
Ultrasound can be used for disintegration of waste-activated sludge and aquacultural effluents [89, 90] due to its advantage on the mechanical properties of sludge hydrolysis. In this method, the sludge is disintegrated and the bacterial cell walls are disrupted [91]. Several factors such as ultrasonic density and intensity, sludge pH, and sludge concentration have an impact on disintegration [92].
2.2 Chemical
Chemical pretreatment for lignocellulosic feedstocks employs different chemicals such as acids, alkalis, and oxidizing agents, e.g., peroxide and ozone [93]. Among these methods, dilute acid pretreatment using H2SO4 is the most widely used method. Depending on the type of chemical used, pretreatment can have different effects on lignocellulose structural components. Alkaline pretreatment, ozonolysis, and peroxide and wet oxidation pretreatments are more effective in lignin removal, whereas dilute acid pretreatment is more efficient in hemicellulose solubilization [94–96].
Alkali pretreatment refers to the application of alkaline solutions such as NaOH, Ca(OH)2 (lime), or ammonia to remove lignin and a part of the hemicellulose. The purpose of alkali pretreatment is to (1) induce swelling of the biomass and lead to an increase of internal surface area, (2) separate cellulose from hemicellulose and lignin, (3) reduce crystallinity of cellulose, (4) disrupt lignin structure, (5) eliminate both hydrolysis and fermentation inhibitors, and (6) improve accessibility of cellulose and hemicellulose toward enzymatic hydrolysis [97, 98]. Alkaline peroxide is an effective method for the pretreatment of biomass. In this method, the lignocellulose is soaked in pH-adjusted water (e.g., to pH 11–12 using NaOH) containing H2O2 at room temperature for a period of time (e.g., 6–24 h). The process can improve the enzymatic hydrolysis after delignification.
Acid pretreatment has received considerable research attention over the years [99]. Dilute sulfuric acid has been added to cellulosic materials for some years to commercially manufacture furfural [100]. Dilute sulfuric acid is mixed with a biomass to hydrolyze hemicellulose to xylose and other sugars and then continue to break xylose down to form furfural. The most widely used and tested approaches are based on dilute sulfuric acid. However, nitric acid, hydrochloric acid, and phosphoric acid have also been studied.
Processing of lignocellulosic biomass with ionic liquids (IL) and other solvents has gained importance in the last decade due to the tunability of the solvent chemistry and hence the ability to dissolve a wide variety of biomass types. Ionic liquids are salts, typically composed of a small anion and a large organic cation, which exist as liquids at room temperature and have very low vapor pressure [101].
2.3 Physicochemical
Pretreatments that combine both chemical and physical methods are referred to as physicochemical processes. Physicochemical pretreatment for lignocellulosic feedstock employs different methods such as steam explosion, steam explosion with addition of SO2, ammonia fiber explosion (AFEX), liquid hot-water pretreatment, and microwave–chemical pretreatment [71, 102–105]. Steam-explosion pretreatment is one of the most commonly used pretreatment options, as it uses both chemical and physical techniques to break the structure of the lignocellulosic material. This hydrothermal pretreatment method subjects the material to high pressures and temperatures for a short duration of time after which it rapidly depressurizes the system, disrupting the structure of the fibrils. The disruption of the fibrils increases the accessibility of the cellulose to the enzymes during hydrolysis. Particle size is a major contributing factor on the effectiveness of the process, and it has been observed that relatively large particle sizes have been able to yield maximum sugar concentrations [106]. The steam-explosion pretreatment process is a proven technique for the pretreatment of different biomass feedstocks as it is able to generate complete sugar recovery while utilizing a low capital investment. Steam-explosion pretreatment also has a low environmental impact in regard to the chemicals being used and the way the process is implemented. Steam-explosion pretreatment method is highly efficient [106].
Acid catalysts have been used within the steam-explosion processes in dilute quantities to improve hemicellulose hydrolysis during the pretreatment stage and cellulose digestibility in later stages of the process. Dilute acids have the ability to decrease retention times and temperatures of the current operating systems and allow for the use of softwoods in this pretreatment technique, where it was originally thought to be uneconomical. By decreasing the retention time and temperature with the addition of this acid catalyst, a reduction of inhibitory compounds formed is observed, nearly all the hemicellulose is removed, and there is an increase rate of hydrolysis later on in the production [107].
2.4 Biological
Biological hydrolysis of cellulose is carried out by cellulolytic microorganisms or by the cellulose enzyme complex [108–111]. In nature, cellulosic materials are degraded by microorganisms, of which brown-white and soft-rot fungi have proven to readily degrade lignin and hemicellulose in waste materials and are used in biological pretreatment processes [112]. A mixed culture [113, 114] comprising of cellulolytic bacterium and a noncellulolytic bacterium could degrade natural cellulosic materials aerobically or anaerobically without sterilization, thereby having a high degree of stability to degrade cellulosic material for long periods of time. The advantages of biological pretreatment include minimal cost, low energy requirement, and mild impact on the environment. However, utilizing these microorganisms and enzymes to process natural cellulosic materials without pretreatment and/or sterilization is difficult and the rate of hydrolysis is also low.
The main aim of pretreatment is to increase accessible surface area, to decrystallize cellulose, and to remove hemicellulose and lignin. The effects of different pretreatments are listed in Table 2.3. Several factors are mentioned to have a positive effect on the overall economy of the process. It is, for example, favorable to avoid the production of inhibitors [115], because the detoxification of the liquid fractions showed to be costly and/or ineffective [116, 117], leaving the lignin with the substrate and removing it after the hydrolysis of the (hemi)cellulose will minimize the overall cost of the process [118], and the use of low concentrations of water, energy, and alkali/acid during pretreatment can be attractive for industrial applications [106].
2.5 Organosolv Pretreatment
It is known that organosolvent (organosolv) pretreatment can be applied with a large number of organic or aqueous–organic solvent systems with or without added catalysts in the temperature range of 100–250 °C [119], while organic acid pretreatment can be applied under mild conditions, even at room temperature [120, 121]. For most organosolv processes, there is no need for acid addition if the pretreatment is conducted at high temperatures (185–210 °C), as it is believed that organic acids act as catalysts for breaking the lignin–carbohydrate complex [122]. However, when acid catalysts are added, the rate of delignification is increased and high yields of xylose are obtained. Mineral acids (hydrochloric acid, sulfuric acid, and phosphoric acid) are good catalysts to accelerate delignification and xylan degradation, while some organic acids such as formic, oxalic, acetylsalicylic, and salicylic acid also can be used as catalysts [123, 124]. Most of the hemicellulose and lignin are solubilized, but the cellulose remains as solid. The organic solvents used in the process need to be recycled to reduce the cost. On the other hand, removal of solvents from the system is necessary because the solvent may be inhibitory to the growth of organisms, enzymatic hydrolysis, and fermentation. Organosolv pretreatment yields three separate fractions: dry lignin, an aqueous hemicellulose stream, and a relatively pure cellulose fraction [122].
Heterogeneous catalysis for lignocellulosic biomass conversion is gaining attention in the literature [125–130]. This type of acid catalyst is a good alternative to concentrated sulfuric acid for hydrolysis reaction. It has numerous advantages over sulfuric acid in terms of activity, selectivity, catalyst lifetime, and reusability. Moreover, the use of solid acid reduces liquid pollutants and cost of wastewater treatment and thus reduces the costs [131–135].
3 Fermentative Hydrogen Production
Biohydrogen production performance is directly determined by operational strategy and key process parameters such as microorganism, fermenter type, substrate concentration, pH, temperature, and hydraulic retention time (HRT).
3.1 Microorganisms
In a previous review paper, the major hydrogen-producing bacteria identified are related strictly to facultative anaerobic genera (Escherichia coli, Enterobacter, Citrobacter), to anaerobic genera (clostridia, methylotrophs, rumen bacteria, methanogenic bacteria, archaea), and to aerobic genera (Alcaligenes, Bacillus). Table 2.4 shows hydrogen-producing bacteria and their characteristics. Dark fermentative hydrogen production is the most practical to be applied among the various biological hydrogen production methods [148, 149] due to its efficient processes to convert organic substrates to energy and electrons. Three types of metabolism to dark fermentative hydrogen production are as follows. The first type is for Escherichia coli and Enterobacteriaceae [150, 151], which has two major enzymes: (1) pyruvate formate lyase (PFL) and (2) formate hydrogen lyase (FHL). Pyruvate formed via the Embden–Meyerhof–Parnas (EMP) pathway is split into acetyl-CoA and formate by PFL under anaerobic conditions. H2 and CO2 are then generated from formate by FHL [152]. The second type typical for Clostridium species [153] includes pyruvate:ferredoxin oxidoreductase (PFOR) and Fd-dependent hydrogenase (HydA) [152]. Pyruvate:ferredoxin oxidoreductase catalyzes the oxidative decarboxylation of pyruvate to form acetyl-CoA and CO2 under anaerobic conditions. The electrons are first transferred to Fdox with a highly negative potential (−420 mV) [154]. The electrons in Fdrd are then transferred to protons to generate hydrogen by HydA. The third type is reported to exist in many thermophilic bacteria and some Clostridium species for utilizing NAD(P)H to form hydrogen. This biochemical reaction is catalyzed by two major enzymes, NAD(P)H:ferredoxin oxidoreductase (NFOR) and HydA [155]. NAD(P)H formed during carbon metabolism by Fdox reaction. This hydrogen-producing reaction is then processed by Fdrd and HydA.
3.2 Fermenter Types
3.2.1 CSTR
Continuous stirred tank reactors (CSTR) are commonly used for continuous biohydrogen production [7, 56, 156–158]. In a CSTR, hydrogen-producing microbes are completely mixed and suspended in the reactor liquor by the mixing pattern. Biomass is well suspended in the mixed liquor, which has the same biomass concentration in the effluent [159]. Under such hydrodynamics, good substrate–microbe contact and mass transfer can be accomplished. On the other hand, the CSTR is unable to maintain high levels of fermentative biomass because of the rapidly mixed operating pattern. Biomass washout may occur at short hydraulic retention times (HRTs) [160]; thus, the hydrogen production rates are considerably restricted [7]. To retain high biomass concentrations in reactors, various techniques have been developed for hydrogen fermentation, including sludge immobilization [35, 157, 158], utilization of the upflow reactor [42], and immobilization on a porous support such as loofah sponges, expanded clays, activated carbons [161], and membrane reactors [162, 163].
3.2.2 UASB
An upflow anaerobic sludge blanket (UASB) process is a widely applied anaerobic treatment system that has high treatment efficiency and a short hydraulic retention time (HRT). UASB hydrogen production systems have been used in granulation enhancement and granule microstructure [164–166]. Numerous works have dealt with hydrogen-producing UASBr, since hydrogen production granule (HPG) formation was first reported by Fang et al. [167], and as mentioned above, this reactor generally demonstrates a high and stable performance. However, for most studies in this field, synthetic wastewater is generally applied as a substrate. Chang and Lin [168] produced hydrogen from sucrose using a UASBr seeded with heat-pretreated sewage sludge. The highest HY (hydrogen yield) and HPR (hydrogen production rate) values were 0.75 mol H2/mol hexose and 0.25 l H2/l∙h, respectively, at a HRT of 8 h. In an effort to decrease the start-up period in the UASBr, Jung et al. [169, 170] inoculated heat-treated sludge to a CSTR, and then the mixed liquor in the CSTR was transferred to the UASBr as a seeding source. As a result, hydrogen production granule with an average size of 1.9 mm was successfully formed in the UASBr after 45 days of operation using coffee drink manufacturing wastewater (CDMW), which was the first report on the formation of HPG from actual wastewater.
3.2.3 Anaerobic Biofilm and Granule Reactor
To overcome biomass washout problems, an addition of immobilized cells into the conventional CSTR for increasing the biomass retention in biohydrogen-producing fermenters has been previously attempted. Biohydrogen-producing fermenters, such as a carrier-induced granular sludge bed reactor (CIGSB) [160] and continuously stirred anaerobic bioreactor (CSABR) used with the silicone immobilized cells and agitated granular sludge bed reactor (AGSBR), have been investigated [35, 171–173]. Attempts to enhance biomass retention by immobilized cells exhibited a better hydrogen production performance than that of conventional CSTR, with HPR ranging from 6 to 360 l/l∙d [160, 172]. Consequently, immobilized cells created by natural or synthetic matrices [174] were often used to allow better retention of hydrogen-producing bacterial cells for stable operations at high feeding rates. Cell immobilization by surface attachment [175] or self-flocculation [35, 173], [168, 176, 177] may have higher feasibility in practical environmental applications. Wu et al. [178] studied the hydrogen production from a sucrose-rich wastewater in a fluidized bed reactor by immobilized cell. Results showed that a stable yield of 182 ml H2/g hexose and a hydrogen production rate of 22.3 l/l∙d were obtained in the fluidized bed reactor.
3.2.4 Membrane Bioreactor
The membrane bioreactor (MBR) has emerged as an effective means of attaining performance improvement in wastewater treatment and has been applied to anaerobic processes due to its capability of increasing biomass retention via membrane separation [179, 180]. Attempts have been made to apply the MBR process to hydrogen production, but relatively little research has been carried out so far. Oh et al. [181] demonstrated that HPR increased by 25 %–0.32 l/l.h due to a 164 % increase in biomass concentration from 3.53 to 5.8 g/l with an increase of the slurry retention time (SRT) from 3.3 to 12 h using an external cross-flow membrane. Membrane fouling is a key process limitation and remains one of the most challenging issues with future MBR development [182].
3.3 Environmental Operational Conditions
3.3.1 Substrate Concentration
Many review reports [61, 65, 183, 184] have summarized the optimum values of substrate concentration, although most studies focus on lab-scale systems. The indexes for identifying high biogas production efficiency are biohydrogen or biomethane production yield (HY or MY, defined as the biohydrogen or biomethane production per unit weight of consumed substrate, mol H2/g COD or mol CH4/g COD) and biohydrogen/biomethane production rate (HPR or MPR, defined as the biohydrogen or biomethane production per unit working volume per day, l/l∙d).
Finding the optimal substrate concentration in continuous operation mode is more meaningful and practical, since the batch mode does not take into consideration the hydrodynamic effect, steady state of the substrate concentration, and pH condition for bacterial growth. The best performance is found to be at 30 g sucrose COD/l with HY of 1.09 mol H2/mol hexose using a CSTR [185]. At inlet substrate concentrations below 20 g COD/l, the HY decreases along with a significant decrease in the n-butyrate/acetate ratio. The appearance of hydrogen-consuming bacteria and decrease of substrate removal efficiency was observed at over 35 g COD/l [185].
High substrate concentration allows more energy-efficient operation but product inhibition is likely to set the upper limit. Certain levels of metabolic products in the dark fermentative hydrogen production reactor may inhibit the hydrogen-producing pathway as well as microbial activity. It is known that butyrate has the highest inhibiting effect on Clostridium sp., among various acids; thus many attempts have been made to alleviate butyrate inhibition, mostly by chemical extraction [186].
Table 2.5 shows the biohydrogen production performance data in continuous operation mode in 2013–2015. Most investigators try to use agriculture waste or the residue from biofuel production processes or food waste to extend the feedstock resources for the biohydrogen production. The study trend for enhancement of hydrogen production rate seems to change the hydrodynamic properties by changing the fermenter type.
To recover more energy from the biomass substrate, researchers have begun to explore two-stage (biohydrogen+biomethane) [192–195] production technology system recently. Two-stage biohydrogen and biomethane production systems can really increase the energy gain from biomass resource by around 8–43 % energy compared with one-stage anaerobic digestion systems [192, 196].
3.3.2 Nutrients and Metals
Excluding the main substrate, carbohydrate materials, dark fermentative hydrogen production (DFHP) requires nutrients for bacterial activity like all biological treatment processes. The nutrients include nitrogen (N), phosphorous (P), ferrous (Fe), and some trace metals. Among the many kinds of nutrients, N is the most essential one for bacterial growth. Optimal C/N ratio is 47 according to Lin and Lay [197]. P and Fe concentrations affect the metabolic pathway of Clostridium sp., and hydrogen production potential decreases when their concentrations are limited.
The effect of iron has been investigated many times in DFHP, since it is an essential component of hydrogenase. Lin and Lay [198] studied the requirement of 11 trace metals in hydrogen fermentation. Magnesium, sodium, zinc, and iron were found to be the important trace metals with magnesium being the most significant one. Hydrogen production is enhanced by 30 % at optimal combined concentrations, 4.8 mg Mg2+/l, 393 mg Na+/l, 0.25 mg Zn2+/l, and 1 mg Fe2+/l.
The effect of metal ions on the fermentative hydrogen production has been widely studied such as Ni [199–201], Fe [199, 202, 203], Cu [201, 204–206], Cr [201, 204], Zn [201, 204, 206], Cd [201], and Pb [201] ions. Hydrogenase enzymes catalyze the reduction of proton to H2. Hydrogenase enzymes are classified into [Ni–Fe] and [Fe–Fe] hydrogenases, according to the metal content at their active site [207]. [Ni–Fe] hydrogenases are extensively distributed among bacteria [208], and both nickel and iron have important effects on fermentative H2 yields [199, 200, 202, 209].
In a biohydrogen production process, electrons are transported via an intramolecular electron transfer chain from the redox partner of the [Ni–Fe] hydrogenases to the active site, and then the protons are reduced by producing biohydrogen [210, 211]. Since nickel is a fundamental component making up the [Ni–Fe] hydrogenases, it plays an important role in fermentative hydrogen production.
Karadag and Puhakka [199] investigated the effect of Fe2+ and Ni2+ on continuous hydrogen production in anaerobic completely stirred tank reactor (ACSTR). They found that hydrogen production increased about by 71 % with the increasing of iron and nickel supplementation, and the highest yields were achieved at the concentrations of 50 mg Fe2+/l and 25 mg Ni2+/l. Wang and Wan [200] reviewed the effects of Fe2+ on anaerobic hydrogen production and reported some inconsistency on the optimal Fe2+ concentration. They also found that increasing Ni2+ concentration up to 0.2 mg/l enhanced the hydrogen production by using batch experiments at 35 °C. Metabolic pathway shifted at different Ni2+ concentrations and higher Ni2+ concentration promoted the growth of hydrogen-producing bacteria. Lee et al. [202] investigated the effect of iron on the efficiency of continuous hydrogen production in a submerged membrane bioreactor system. They found FeSO4 concentration is the key factor affecting the fermentation pathway for hydrogen production with the membrane bioreactor. Both increase in the hydrogen production rate and the hydrogen yield were obtained by adding FeSO4. They indicated that iron sulfate increased hydrogenase activity and hydrogen production in a membrane bioreactor when FeSO4 concentration closes to 10.9 mg/l.
Lin and Shei [204] investigated the effect of Cr, Cu, and Zn ions on biohydrogen production using anaerobic sewage sludge microflora. Cr, Cu, and Zn significantly affect hydrogen-producing microflora enriched from sewage sludge with Zn and Cr being the most and least toxic metals, respectively. The microflora’s hydrogen production activity could be reduced by 50 % for a biomass in contact with 4.5 mg Zn/l, 6.5 mg Cu/l, and 60 mg Cr/l. However, low concentrations of 2 mg Cu/l and 15 mg Cr/l resulted in peak hydrogen production by 20 and 10 %, respectively. Zheng and Yu have reported that the specific hydrogen production rate was enhanced by the dosage of Cu at 50–100 mg/l, but was inhibited by Cu over 200 mg/l from glucose by enriched anaerobic culture [206]. Li and Fang found that Cu strongly inhibited the bioactivity of hydrogen-producing sludge [201]. Han et al. [205] investigated Cu2+ concentration effect in a sucrose-fed CSTR on fermentative hydrogen production by mixed cultures. The result shows that 6.4 mg/L Cu2+ is the optimal concentration for the CSTR at HRT 4 h. In addition, copper causes shift in the metabolic pathway.
Li and Fang [201] studied the inhibition of six heavy metals and found the bioactivity of hydrogen-producing sludge in the following order: Cu (most toxic) > Ni >Zn > Cr > Cd > Pb (least toxic). Hydrogen-producing sludge exhibited in general higher resistance to metal toxicity than methanogenic granular sludge. Furthermore, Han et al. [203] studied the effects of hematite nanoparticle concentration on hydrogen production in batch system. The optimum hematite nanoparticle concentration was 200 mg/l, with the maximum hydrogen yield of 3.21 mol H2/mol sucrose which was 32.64 % higher than the blank test. The slow release of hematite nanoparticles had been verified by transmission electron microscopy (TEM). In addition, TEM analysis indicated that the hematite nanoparticles can increase the length and narrow the width of bacteria.
3.3.3 pH
The control of pH is crucial to fermentative hydrogen and methane production due to its effects on hydrogenase activity and metabolic pathways. When the pH of a fermentation medium is too low, hydrogenase activity and methanogens would be inhibited or there would be a switch in metabolic pathway resulting in cessation of hydrogen and methane generation. Anaerobic hydrogen production process is typical during the exponential growth phase of clostridia [212]. The reactions shift from a hydrogen/acid production phase to a solvent production phase when the population reaches the stationary growth phase. The accumulation of volatile fatty acids such as butyric and propionic acids and hydrogen during the exponential growth phase prompts this shift. Some researchers claimed that this shift occurred when the pH dropped to 4.5 or below [213, 214], while others found that the shift occurred at pH levels above 5.7 due to enzyme synthesis or enzyme activation, which led to solvent production [215]. Thus, it is important to remove excess hydrogen from the system and control the pH at an optimal range to maintain hydrogen production. Otherwise, the biohydrogen production will stop due to the microbial population shift caused by pH uncontrolled in the desired range.
Khanal et al. [216] investigated the effect of pH on biological hydrogen production using sucrose and starch as organic substrates in batch system. Based on the evaluation of maximum hydrogen production rate, the optimum operational pH range was about 5.5–5.7. This result could be applied in continuous-flow processes to maintain a high rate of hydrogen production. Lin and Lay [217] found that phosphate acted as a better buffer source for hydrogen production than carbonate. Its addition enhanced hydrogen production by 1.9 times and decreased the lag period. Cavinato et al. [218] found that recirculation of anaerobic digested sludge after a mild solid separation to control the pH in an optimal hydrogen production range of 5–6 resulted in a stable hydrogen production output. The importance of pH control for continuous hydrogen production has been investigated extensively. The rapid pH depletion could cause a metabolic change of the microorganisms in the hydrogen production process, resulting in the shift of intermediate production pathway and a decrease in hydrogen production or system upset [216].
Reported optimal pH values for different systems or substrates differed substantially from 4.0 to 6.5, but for each specific situation, the optimal pH range was quite narrow within 0.5 [219]. Chu et al. [220] also reported a pH-phased two-stage fermentation process (combining thermophilic hydrogen production and mesophilic methane production) with recirculating digested sludge. These results show that a recirculation of precipitated digester sludge to a hydrogen reactor can maintain the hydrogen reactor pH at an optimal range without adding any reagents. Recently, most researches operated the hydrogen production fermenter at pH 5–5.5 as an optimal condition at mesophilic temperature [54, 69, 221–223].
3.3.4 Temperature
Fermentative hydrogen production via mixed cultures is conducted mostly under mesophilic (20–40 °C) and thermophilic (50–60 °C) conditions with only few studies being carried out under hyperthermophilic (65–75 °C) conditions. Biohydrogen production temperatures within 23–60 °C show that hydrogen production yield and hydrogen production rate increase along with the temperature increment [183].
Vatsala et al. [224] improved hydrogen production from a sugarcane distillery effluent using co-cultures at 100 m3 reactor and found that unsteady hydrogen production was presumably due to temperature variation during daytime (32–39 °C) and nighttime (26–32 °C). Zhang et al. [225] reported biogas production from brown grease at mesophilic temperature (34.3–37.9 °C) in a pilot-scale high-rate anaerobic digester with a methane yield of 0.40–0.77 m3 CH4/kg-VS (higher than a typical range of other food wastes, 0.11–0.42 m3 CH4/kg-VS), a mean methane content of 75 %, and <200 ppm of hydrogen sulfide.
Cheong and Hansen [226] indicate that thermophilic acidogenesis enhances hydrogen production consistent with the biochemical pathway of butyrate fermentation. Under thermophilic temperatures (55 °C), the maximum hydrogen production potential of 134 ml with a specific hydrogen production rate of 25 ml H2/h.g cell can be achieved. Throughout the thermophilic batch experiments, the main intermediate metabolites were acetate, n-butyrate, and ethanol. Propionate formation was suppressed completely during fermentation. Yields of produced hydrogen were correlated with increasing concentrations of n-butyrate, and quantities of ethanol present were significant in the batches producing lower yields of hydrogen.
Zhang et al. [19] studied conversion of starchy wastewater into hydrogen at thermophilic condition (55 °C) with batch experiments. The mixed liquor was composed mostly of acetate (40.2–53.4 %) and butyrate (26.0–40.9 %). Luo et al. [227] evaluated the pretreatment methods on mixed inoculum for both batch and continuous thermophilic biohydrogen production from cassava stillage. They found that butyrate was predominant and accounted for more than 75 % of the total amount of VFA/ethanol except the case of loading-shock pretreated sludge (56 % in this study). Butyrate concentration was observed to be correlated with the hydrogen production. Chen et al. [228] also found highest biohydrogen production was obtained when butyrate was predominate (70–85 % of the total VFA/ethanol).
Lin et al. [229] studied biohydrogen production with mesophilic conditions with a mixed microflora on a pilot scale. They found that the primary soluble microbial products (SMP) were butyrate (iso- and n-butyrate) accounting for 44.4–53.2 % of SMP. Acetate was also produced and accounted for 21.3–26.4 % of SMP. Productions of propionate and ethanol ranged from 7.2–10.6 % to 14.3–22.3 % of SMP, respectively. However, propionate and ethanol are unfavorable metabolites for hydrogen production [158, 172, 230]. The ratios of ethanol/acetate and acetate/butyrate have been used to indicate the performance of hydrogen production [231, 232]. Wu et al. [172] indicated that there might be an optimal acetate/butyrate ratio for hydrogen production, but the ratio is highly dependent on the anaerobic culture or the carbon substrate used.
3.3.5 HRT
Shortening hydraulic retention times (HRTs) is a well-used and effective operation strategy to enhance hydrogen production from organic wastewater and solid wastes because of its ability to exclude methanogens which have longer generation time. The proper HRTs for hydrogen and methane production from organic fractions of municipal solid wastes (OFMSW) are 1–2 days and 10–15 days, respectively. A thermophilic hydrogen production reactor operating at HRT 1.3 days and a mesophilic methane production reactor operating at HRT 5.0 day have been combined to convert OFMSW into a pilot-scale two-phase fermentation system [220]. A pilot-scale two-phase hydrogen/methane fermentation system for food waste was operated at HRT 21 h with a peak hydrogen yield of 1.82 H2 mol/mol glucose. Over 80 % of the methane was produced in the methane fermentation tank with acetic acid as the dominant organic acid. An economic evaluation shows that two-phase hydrogen/methane fermentation has greater potential for recovering energy than that of methane fermentation [233]. Cavinato et al. [218] operated pilot-scale hydrogen and methane fermenters by using HRTs of 3.3 and 12.6 days resulting in a specific hydrogen yield of 66.7 l/kg total volatile solids (TVS) and a specific biogas yield of 0.72 m3/kg TVS respectively.
For most studies on continuously dark fermentative hydrogen production (DFHP), continuous systems are expected to operate at a low HRT 36–12 h [222, 234], very low of HRT 12–2 h [69, 229, 235–239], for obtaining a high biohydrogen production that can be operated at extremely low of HRT 2–0.5 h [35, 157, 240–243] with immobilized cell in the biohydrogen production fermenters. A mixture of food industry wastewater with rice straw hydrolyzate as substrate was conducted in a continuously stirred anaerobic bioreactor (CSABR) at HRT 4 h and found the hydrogen production of 10 l/l∙d [69]. The rice straw hydrolyzate as sole substrate in continuously external circulating bioreactor (CECBR) prevented biomass washout by using a high volumetric flow rate with HRT of 4–2 h. It was found that the value of hydrogen production rate of 16.32 l/l∙d at HRT 4 h was three times more than that at HRT 8 h [4].
4 Conclusions and Future Outlook
In recent years, the goal of biohydrogen systems has been to design economically viable hydrogen production processes. In this chapter, the potential feedstock, pretreatment processes, microorganisms, fermenter types, and operational conditions have been introduced. From this segment, readers will have attained a basic knowledge on biohydrogen production technologies from biomass waste. However, commercial biohydrogen production plants are not yet established because abundant and suitable feedstocks are not easily accessible yet. The economic feasibility of the dark fermentative hydrogen production process is dependent on the availability of cellulosic materials and organic wastes. Enhancements in the hydrogen production rate and yield from these feedstocks are important subjects for metabolic engineering. From an engineering point of view, the easily converted and abundant feedstock should be the first option to supply the biohydrogen production plants. Carbohydrate-rich organic wastes are a promising feedstock for anaerobic biohydrogen production. The sugary wastewater has a big potential for hydrogen fermentation for future industrial applications. The potential economic gains from the internal rate of return (IRR) evaluation result in the motivation to apply this technology. From a global economic perspective, sugary wastewater could have higher profits with biogas energy used on-site for replacing natural gas and also could satisfy the energy requirements of some local areas. The adoption of fermentative hydrogen production from organic wastes will potentially lead to great advancements in energy and the environment. Finally, there are many pilot plants still at work in Spain [244], Taiwan (see Fig. 2.2) [229, 245], Italy [246], and the UK [247]. Toyota already has the first commercial fuel cell car (Mirai) [248] selling in Europe and North America right now. We believe that profitable and sustainable hydrogen production from biomass waste will be achieved in the near future.
References
Thompson W, Meyer S. Second generation biofuels and food crops: co-products or competitors? Glob Food Secur. 2013;2:89–96.
De Souza ACC, Silveira JL. Hydrogen production utilizing glycerol from renewable feedstocks—the case of Brazil. Renew Sustain Energy Rev. 2011;15:1835–50.
Kraemer J, Bagley D. Improving the yield from fermentative hydrogen production. Biotechnol Lett. 2007;29:685–95.
Liu C-M, Wu S-Y, Chu C-Y, Chou Y-P. Biohydrogen production from rice straw hydrolyzate in a continuously external circulating bioreactor. Int J Hydrog Energy. 2014;39:19317–22.
Abo-Hashesh M, Hallenbeck P. Fermentative hydrogen production. In: Hallenbeck PC, editor. Microbial technologies in advanced biofuels production. New York: Springer; 2012. p. 77–92.
Siriporn Yossan SO-T, Prasertsan P. Effect of initial pH, nutrients and temperature on hydrogen production from palm oil mill effluent using thermotolerant consortia and corresponding microbial communities. Int J Hydrog Energy. 2012;37:13806–14.
Show K-Y, Lee D-J, Chang J-S. Bioreactor and process design for biohydrogen production. Bioresour Technol. 2011;102(102):8524–33.
Das D, Veziroglu TN. Advances in biological hydrogen production processes. Int J Hydrog Energy. 2008;33:6046–57.
Rasdi Z, Rahman NAA, Abd-Azi S, Lai-Yee P, Zulkhairi M, Yusoff M, et al. Statistical optimization of biohydrogen production from palm oil mill effluent by natural microflora. Open Biotechnol J. 2009;3:79–86.
Khaleb NA, Jahim JM, Kamal SA. Biohydrogen production using hydrolysates of Palm Oil Mill Effluent (Pome). J Asian Sci Res. 2011;2:705–10.
Kamal SA, Jahim JM, Anuar N, Hassan O, Daud WRW, Mansor MF, et al. Pre-treatment effect of Palm Oil Mill Effluent (POME) during hydrogen production by a local isolate clostridium butyricum. Int J Adv Sci Eng Inf Technol. 2012;2(4):54–60. ISSN: 2088–5334.
Chong M-L, Rahim RA, Shirai Y, Hassan MA. Biohydrogen production by Clostridium butyricum EB6 from palm oil mill effluent. Int J Hydrog Energy. 2009;34:764–71.
Prasertsan P, O-Thong S, N-Kr B. Optimization and microbial community analysis for production of biohydrogen from palm oil mill effluent by thermophilic fermentative process. Int J Hydrog Energy. 2009;34:7448–59.
O-Thong S, Mamimin C, Prasertsan P. Effect of temperature and initial pH on biohydrogen production from palm oil mill effluent: long-term evaluation and microbial community analysis. Microb Biotechnol Electron J Biotechnol. 2011;14(5).
Atif AAY, Fakhru’l-Razi A, Ngan MA, Morimoto M, Iyuke SE, Veziroglu NT. Fed batch production of hydrogen from palm oil mill effluent using anaerobic microflora. Int J Hydrog Energy. 2005;30:1393–7.
O-Thong S, Prasertsana P, Intrasungkha N, Dhamwichukorn S, N-Kr B. Optimization of simultaneous thermophilic fermentative hydrogen production and COD reduction from palm oil mill effluent by Thermoanaerobacterium-rich sludge. Int J Hydrog Energy. 2008;33:1221–31.
O-Thong S, Prasertsan P, Intrasungkha N. Improvement of biohydrogen production and treatment efficiency on palm oil mill effluent with nutrient supplementation at thermophilic condition using an anaerobic sequencing batch reactor. Enzym Microb Technol. 2007;41:583–90.
Akutsu Y, Li Y-Y, Harada H, Yu H-Q. Effects of temperature and substrate concentration on biological hydrogen production from starch. Int J Hydrog Energy. 2009;34:2558–66.
Zhang T, Liu H, Fang HHP. Biohydrogen production from starch in wastewater under thermophilic condition. J Environ Manag. 2003;69:149–56.
Masset J, Calusinska M, Hamilton C, Hiligsmann S, Joris B, Wilmotte A, et al. Fermentative hydrogen production from glucose and starch using pure strains and artificial co-cultures of Clostridium spp. Biotechnol Biofuels. 2012;5:3.
Chen S-D, Lee K-S, Lo Y-C, Chen W-M, Wu J-F, Lin C-Y, et al. Batch and continuous biohydrogen production from starch hydrolysate by Clostridium species. Int J Hydrog Energy. 2008;33:1803–12.
O-Thong S, Hniman A, Prasertsan P, Imai T. Biohydrogen production from cassava starch processing wastewater by thermophilic mixed cultures. Int J Hydrog Energy. 2011;36:3409–16.
Chen S-D, Sheu D-S, Chen W-M, Huang Y-CL-I, Lin C-Y, Chang J-S. Dark hydrogen fermentation from hydrolyzed starch treated with recombinant amylase originating from Caldimonas taiwanensis On1. Biotechnol Prog. 2007;23:1312–20.
Hussy I, Hawkes FR, Dinsdale R, Hawkes DL. Continuous fermentative hydrogen production from a wheat starch co-product by mixed microflora. Biotech Bioeng. 2003;84:619–26.
Liu G, Shen J. Effects of culture and medium conditions on hydrogen production from starch using anaerobic bacteria. J Biosci Bioeng. 2004;98(4):251–6.
Wang C-H, Lu W-B, Chang J-S. Feasibility study on fermentative conversion of raw and hydrolyzed starch to hydrogen using anaerobic mixed microflora. Int J Hydrog Energy. 2007;32:3849–59.
Lee K-S, Hsu Y-F, Lo Y-C, Lin P-J, Lin C-Y, Chang J-S. Exploring optimal environmental factors for fermentative hydrogen production from starch using mixed anaerobic microflora. Int J Hydrog Energy. 2008;33:1565–72.
Arooj MF, Han S-K, Kim S-H, Kim D-H, Shin H-S. Continuous biohydrogen production in a CSTR using starch as a substrate. Int J Hydrog Energy. 2008;33:3289–94.
Zong W, Yu R, Zhang P, Fan M, Zhou Z. Efficient hydrogen gas production from cassava and food waste by a two-step process of dark fermentation and photo-fermentation. Biomass Bioenergy. 2009;33:1458–63.
Kim S-H, Han S-K, Shin H-S. Feasibility of biohydrogen production by anaerobic co-digestion of food waste and sewage sludge. Int J Hydrog Energy. 2004;29:1607–16.
Shin H-S, Youn J-H, Kim S-H. Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int J Hydrog Energy. 2004;29:1355–63.
Reungsang A, Sreela-or C, Plangklang P. Non-sterile bio-hydrogen fermentation from food waste in a continuous stirred tank reactor (CSTR): performance and population analysis. Int J Hydrog Energy. 2013;38:15630–7.
Han S-K, Shin H-S. Biohydrogen production by anaerobic fermentation of food waste. Int J Hydrog Energy. 2004;29:569–77.
Hsiao C-L, Chang J-J, Wu J-H, Chin W-C, We F-S, Huang C-C, et al. Clostridium strain co-cultures for biohydrogen production enhancement from condensed molasses fermentation solubles. Int J Hydrog Energy. 2009;34:7173–81.
Chu C-Y, Wu S-Y, Hsieh C, Lin C-Y. Biohydrogen production from immobilized cells and suspended sludge systems with condensed molasses fermentation solubles. Int J Hydrog Energy. 2011;36:14078–85.
Lay C-H, Wu J-H, Hsiao C-L, Chang J-J, Chen C-C, Lin C-Y. Biohydrogen production from soluble condensed molasses fermentation using anaerobic fermentation. Int J Hydrog Energy. 2010;35:13445–51.
Chen C-C, Wu J-H, Lay C-H, Sen B, Chang J-S. Kinetics of hydrogen production from condensed molasses fermentation solubles using sewage sludge in a continuous stirred tank reactor. Sustain Environ Res. 2011;21(2):117–21.
Chang J-J, Wu J-H, Wen F-S, Hung K-Y, Chen Y-T, Hsiao C-L, et al. Molecular monitoring of microbes in a continuous hydrogen-producing system with different hydraulic retention time. Int J Hydrog Energy. 2008;33:1579–85.
Liu H, Fang HHP. Hydrogen production from wastewater by acidogenic granular sludge. Water Sci Technol. 2002;47(1):153–8.
Oh S, Logan BE. Hydrogen and electricity production from a food processing wastewater using fermentation and microbial fuel cell technologies. Water Res. 2005;39:4673–82.
Ueno Y, Otsuka S, Morimoto M. Hydrogen production from industrial wastewater by anaerobic microflora in chemostat culture. J Ferment Bioeng. 1996;82:194–7.
Yu H, Zhu Z, Hu W, Zhang H. Hydrogen production from rice winery wastewater in an upflow anaerobic reactor by using mixed anaerobic cultures. Int J Hydrog Energy. 2002;27:1359–65.
Ginkel SV, Oh S-E, Logan BE. Biohydrogen gas production from food processing and domestic wastewaters. Int J Hydrog Energy. 2005;30:1535–42.
Kapdan IK, Kargi F. Bio-hydrogen production from waste materials. Enzym Microb Technol. 2006;38:569–82.
Mohan SV, Babu VL, Sarma P. Anaerobic biohydrogen production from dairy wastewater treatment in sequencing batch reactor (AnSBR): effect of organic loading rate. Enzym Microb Technol. 2007;41:506–15.
Ren N, Chua H, Chan S, Tsang Y, Wang Y, Sin N. Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour Technol. 2007;98:1774–80.
Davila-Vazquez G, Alatriste-Mondragón F, de León-Rodríguez A, Razo-Flores E. Fermentative hydrogen production in batch experiments using lactose, cheese whey and glucose: influence of initial substrate concentration and pH. Int J Hydrog Energy. 2008;33:4989–97.
Gadhe A, Sonawane SS, Varma MN. Enhancement effect of hematite and nickel nanoparticles on biohydrogen production from dairy wastewater. Int J Hydrog Energy. 2015;40:4502–11.
Koutrouli EC, Kalfas H, Gavala HN, Skiadas IV, Stamatelatou K, Lyberatos G. Hydrogen and methane production through two-stage mesophilic anaerobic digestion of olive pulp. Bioresour Technol. 2009;100:3718–23.
Ntaikou I, Kourmentza C, Koutrouli E, Stamatelatou K, Zampraka A, Kornaros M, et al. Exploitation of olive oil mill wastewater for combined biohydrogen and biopolymers production. Bioresour Technol. 2009;100:3724–30.
Jung K-W, Kim D-H, Lee M-Y, Shin H-S. Two-stage UASB reactor converting coffee drink manufacturing wastewater to hydrogen and methane. Int J Hydrog Energy. 2012;37:7473–81.
Zhu G, Liu C, Li J, Ren N, Liu L, Huang X. Fermentative hydrogen production from beet sugar factory wastewater treatment in a continuous stirred tank reactor using anaerobic mixed consortia. Front Environ Sci Eng. 2013;7:143–50.
Xie L, Dong N, Wang L, Zhou Q. Thermophilic hydrogen production from starch wastewater using two-phase sequencing batch fermentation coupled with UASB methanogenic effluent recycling. Int J Hydrog Energy. 2014;39:20942–9.
Lay CH, Sen B, Kuo SY, Chen CC, Lin CY. Biohydrogen production from textile wastewater by mixed microflora in an intermittent‐flow, stirred tank reactor: effect of feeding frequency. J Chin Chem Soc. 2014;61:791–6.
Han W, Liu DN, Shi YW, Tang JH, Li YF, Ren NQ. Biohydrogen production from food waste hydrolysate using continuous mixed immobilized sludge reactors. Bioresour Technol. 2015;180:54–8.
Ntaikou I, Antonopoulou G, Lyberatos G. Biohydrogen production from biomass and wastes via dark fermentation: a review. Waste Biomass Valor. 2010;1:21–39.
Lay J-J, Fan K-S. Influence of chemical nature of organic wastes on their conversion to hydrogen by heat-shock digested sludge. Int J Hydrog Energy. 2003;28:1361–7.
Yuan X, Wen B, Ma X, Zhu W, Wang X, Chen S, et al. Enhancing the anaerobic digestion of lignocellulose of municipal solid waste using a microbial pretreatment method. Bioresour Technol. 2014;154:1–9.
Ishola MM, Jahandideh A, Haidarian B, Brandberg T, Taherzadeh MJ. Simultaneous saccharification, filtration and fermentation (SSFF): a novel method for bioethanol production from lignocellulosic biomass. Bioresour Technol. 2013;133:68–73.
Quéméneur M, Bittel M, Trably E, Dumas C, Fourage L, Ravot G, et al. Effect of enzyme addition on fermentative hydrogen production from wheat straw. Int J Hydrog Energy. 2012;37:10639–47.
Zhang M-L, Fan Y-T, Xing Y, Pan C-M, Zhang G-S, Lay J-J. Enhanced biohydrogen production from cornstalk wastes with acidification pretreatment by mixed anaerobic cultures. Biomass Bioenergy. 2007;31:250–4.
Pan C, Fan Y, Hou H. Fermentative production of hydrogen from wheat bran by mixed anaerobic cultures. Ind Eng Chem Res. 2008;47:5812–8.
Zhu H, Parker W, Basnar R, Proracki A, Falletta P, Béland M, et al. Buffer requirements for enhanced hydrogen production in acidogenic digestion of food wastes. Bioresour Technol. 2009;100:5097–102.
Wang X, Y-c Z. A bench scale study of fermentative hydrogen and methane production from food waste in integrated two-stage process. Int J Hydrog Energy. 2009;34:245–54.
Guo XM, Trably E, Latrille E, Carrère H, Steyer J-P. Hydrogen production from agricultural waste by dark fermentation: a review. Int J Hydrog Energy. 2010;35:10660–73.
Yang Z, Guo R, Xu X, Fan X, Li X. Enhanced hydrogen production from lipid-extracted microalgal biomass residues through pretreatment. Int J Hydrog Energy. 2010;35:9618–23.
Carver SM, Hulatt CJ, Thomas DN, Tuovinen OH. Thermophilic, anaerobic co-digestion of microalgal biomass and cellulose for H2 production. Biodegradation. 2011;22:805–14.
Lakaniemi A-M, Hulatt CJ, Thomas DN, Tuovinen OH, Puhakka JA. Biogenic hydrogen and methane production from Chlorella vulgaris and Dunaliella tertiolecta biomass. Biotechnol Biofuels. 2011;4:34.
Liu C-M, Chu C-Y, Lee W-Y, Li Y-C, Wu S-Y, Chou Y-P. Biohydrogen production evaluation from rice straw hydrolysate by concentrated acid pre-treatment in both batch and continuous systems. Int J Hydrog Energy. 2013;38:15823–9.
Levin DB, Islam R, Cicek N, Sparling R. Hydrogen production by Clostridium thermocellum 27405 from cellulosic biomass substrates. Int J Hydrog Energy. 2006;31:1496–503.
Datar R, Huang J, Maness P-C, Mohagheghi A, Czernik S, Chornet E. Hydrogen production from the fermentation of corn stover biomass pretreated with a steam-explosion process. Int J Hydrog Energy. 2007;32:932–9.
Pattra S, Sangyoka S, Boonmee M, Reungsang A. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int J Hydrog Energy. 2008;33:5256–65.
Kyazze G, Dinsdale R, Hawkes FR, Guwy AJ, Premier GC, Donnison I. Direct fermentation of fodder maize, chicory fructans and perennial ryegrass to hydrogen using mixed microflora. Bioresour Technol. 2008;99:8833–9.
Ntaikou I, Gavala H, Kornaros M, Lyberatos G. Hydrogen production from sugars and sweet sorghum biomass using Ruminococcus albus. Int J Hydrog Energy. 2008;33:1153–63.
Ivanova G, Rákhely G, Kovács KL. Thermophilic biohydrogen production from energy plants by Caldicellulosiruptor saccharolyticus and comparison with related studies. Int J Hydrog Energy. 2009;34:3659–70.
Reilly M, Dinsdale R, Guwy A. Mesophilic biohydrogen production from calcium hydroxide treated wheat straw. Int J Hydrog Energy. 2014;39:16891–901.
Patel AK, Debroy A, Sharma S, Saini R, Mathur A, Gupta R, et al. Biohydrogen production from a novel alkalophilic isolate Clostridium sp. IODB-O3. Bioresour Technol. 2015;175:291–7.
Chairattanamanokorn P, Penthamkeerati P, Reungsang A, Lo Y-C, Lu W-B, Chang J-S. Production of biohydrogen from hydrolyzed bagasse with thermally preheated sludge. Int J Hydrog Energy. 2009;34:7612–7.
Cao G, Ren N, Wang A, Lee D-J, Guo W, Liu B, et al. Acid hydrolysis of corn stover for biohydrogen production using Thermoanaerobacterium thermosaccharolyticum W16. Int J Hydrog Energy. 2009;34:7182–8.
Phummala K, Imai T, Reungsang A, Chairattanamanokorn P, Sekine M, Higuchi T, et al. Delignification of disposable wooden chopsticks waste for fermentative hydrogen production by an enriched culture from a hot spring. J Environ Sci. 2014;26:1361–8.
Saratale GD, Kshirsagar SD, Sampange VT, Saratale RG, Oh S-E, Govindwar SP, et al. Cellulolytic enzymes production by utilizing agricultural wastes under solid state fermentation and its application for biohydrogen production. Appl Biochem Biotechnol. 2014;174:2801–17.
Taherzadeh MJ, Karimi K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: a review. Int J Mol Sci. 2008;9:1621–51.
Meng X, Ragauskas AJ. Recent advances in understanding the role of cellulose accessibility in enzymatic hydrolysis of lignocellulosic substrates. Curr Opin Biotechnol. 2014;27:150–8.
Zakaria MR, Fujimoto S, Hirata S, Hassan MA. Ball milling pretreatment of oil palm biomass for enhancing enzymatic hydrolysis. Appl Biochem Biotechnol. 2014;173:1778–89.
Mais U, Esteghlalian AR, Saddler JN, Mansfield SD. Enhancing the enzymatic hydrolysis of cellulosic materials using simultaneous ball milling. Springer; New York, 2002.
Muller CD, Abu-Orf M, Novak JT. Application of mechanical shear in an internal-recycle for the enhancement of mesophilic anaerobic digestion. Water Environ Res. 2007;79:297–304.
Yang C, Shen Z, Yu G, Wang J. Effect and aftereffect of γ radiation pretreatment on enzymatic hydrolysis of wheat straw. Bioresour Technol. 2008;99:6240–5.
Mamar S, Hadjadj A. Radiation pretreatments of cellulose materials for the enhancement of enzymatic hydrolysis. Int J Radiat Appl Instrum Part C Radiat Phys Chem. 1990;35:451–5.
Subhedar PB, Gogate PR. Intensification of enzymatic hydrolysis of lignocellulose using ultrasound for efficient bioethanol production: a review. Ind Eng Chem Res. 2013;52:11816–28.
Jeun J-P, Lee B-M, Lee J-Y, Kang P-H, Park J-K. An irradiation-alkaline pretreatment of kenaf core for improving the sugar yield. Renew Energy. 2015;79:51–5.
Chu CP, Lee DJ, Chang B-V, You CS, Tay JH. “Weak” ultrasonic pre-treatment on anaerobic digestion of flocculated activated biosolids. Water Res. 2002;36:2681–8.
Wang F, Wang Y, Ji M. Mechanisms and kinetics models for ultrasonic waste activated sludge disintegration. J Hazard Mater. 2005;123:145–50.
Talebnia F, Karakashev D, Angelidaki I. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Bioresour Technol. 2010;101:4744–53.
Galbe M, Zacchi G. A review of the production of ethanol from softwood. Appl Microbiol Biotechnol. 2002;59:618–28.
Sanchez OJ, Cardona CA. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour Technol. 2008;99:5270–95.
Tomas-Pejo E, Oliva J, Ballesteros M. Realistic approach for full-scale bioethanol production from lignocellulose: a review. J Sci Ind Res. 2008;67:874.
Chen Y, Stevens MA, Zhu Y, Holmes J, Xu H. Understanding of alkaline pretreatment parameters for corn stover enzymatic saccharification. Biotechnol Biofuels. 2013;6:1–10.
Monlau F, Barakat A, Trably E, Dumas C, Steyer J-P, Carrère H. Lignocellulosic materials into biohydrogen and biomethane: impact of structural features and pretreatment. Crit Rev Environ Sci Technol. 2013;43:260–322.
Mosier N, Wyman C, Dale B, Elander R, Lee Y, Holtzapple M, et al. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour Technol. 2005;96:673–86.
Zeitsch KJ. The chemistry and technology of furfural and its many by-products. Amsterdam: Elsevier; 2000.
Groff D, George A, Sun N, Sathitsuksanoh N, Bokinsky G, Simmons BA, et al. Acid enhanced ionic liquid pretreatment of biomass. Green Chem. 2013;15:1264–7.
Mackie K, Brownell H, West K, Saddler J. Effect of sulphur dioxide and sulphuric acid on steam explosion of aspenwood. J Wood Chem Technol. 1985;5:405–25.
Hu F, Ragauskas A. Pretreatment and lignocellulosic chemistry. Bioenergy Res. 2012;5:1043–66.
Jiang W, Chang S, Li H, Oleskowicz-Popiel P, Xu J. Liquid hot water pretreatment on different parts of cotton stalk to facilitate ethanol production. Bioresour Technol. 2015;176:175–80.
Cheng J, Su H, Zhou J, Song W, Cen K. Microwave-assisted alkali pretreatment of rice straw to promote enzymatic hydrolysis and hydrogen production in dark-and photo-fermentation. Int J Hydrog Energy. 2011;36:2093–101.
Brodeur G, Yau E, Badal K, Collier J, Ramachandran K, Ramakrishnan S. Chemical and physicochemical pretreatment of lignocellulosic biomass: a review. Enzym Res. 2011;2011:787532. p. 1–17.
Qiu W, Chen H. Enhanced the enzymatic hydrolysis efficiency of wheat straw after combined steam explosion and laccase pretreatment. Bioresour Technol. 2012;118:8–12.
Christy PM, Gopinath L, Divya D. A review on anaerobic decomposition and enhancement of biogas production through enzymes and microorganisms. Renew Sustain Energy Rev. 2014;34:167–73.
Dutta S, Wu KC-W. Enzymatic breakdown of biomass: enzyme active sites, immobilization, and biofuel production. Green Chem. 2014;16:4615–26.
Hyeon JE, You SK, Kang DH, Ryu S-H, Kim M, Lee S-S, et al. Enzymatic degradation of lignocellulosic biomass by continuous process using laccase and cellulases with the aid of scaffolding for ethanol production. Process Biochem. 2014;49:1266–73.
Gusakov AV, Salanovich TN, Antonov AI, Ustinov BB, Okunev ON, Burlingame RP, et al. Construction of highly efficient cellulase compositions for enzymatic hydrolysis of cellulose. 2014. US Patents 8,916,363 B2, p. 1–84.
Saratale GD, Chen S-D, Lo Y-C, Saratale RG, Chang J-S. Outlook of biohydrogen production from lignocellulosic feedstock using dark fermentation – a review. J Sci Ind Res. 2008;67:962.
Odom JM, Wall JD. Photoproduction of H2 from cellulose by an anaerobic bacterial coculture. Appl Environ Microbiol. 1983;45:1300–5.
Lewis SM, Montgomery L, Garleb KA, Berger LL, Fahey GC. Effects of alkaline hydrogen peroxide treatment on in vitro degradation of cellulosic substrates by mixed ruminal microorganisms and Bacteroides succinogenes S85. Appl Environ Microbiol. 1988;54:1163–9.
Ramos LP. The chemistry involved in the steam treatment of lignocellulosic materials. Quim Nova. 2003;26:863–71.
Gregg D, Saddler JN. A techno-economic assessment of the pretreatment and fractionation steps of a biomass-to-ethanol process. Seventeenth symposium on biotechnology for fuels and chemicals. Springer; New York, 1996. p. 711–27.
Sivers MV, Zacchi G, Olsson L, Hahn‐Hügerdal B. Cost analysis of ethanol production from willow using recombinant Escherichia coli. Biotechnol Prog. 1994;10:555–60.
Shevchenko SM, Beatson RP, Saddler JN. The nature of lignin from steam explosion/enzymatic hydrolysis of softwood. Appl Biochem Biotechnol. 1999;79:867–76.
Muurinen E. Organosolv pulping: a review and distillation study related to peroxyacid pulping. Finland: Oulun yliopisto; 2000.
Xb Z, Wang L, Liu D. Effect of several factors on peracetic acid pretreatment of sugarcane bagasse for enzymatic hydrolysis. J Chem Technol Biotechnol. 2007;82:1115–21.
Teixeira LC, Linden JC, Schroeder HA. Optimizing peracetic acid pretreatment conditions for improved simultaneous saccharification and co-fermentation (SSCF) of sugar cane bagasse to ethanol fuel. Renew Energy. 1999;16:1070–3.
Duff SJ, Murray WD. Bioconversion of forest products industry waste cellulosics to fuel ethanol: a review. Bioresour Technol. 1996;55:1–33.
Sun Y, Cheng J. Hydrolysis of lignocellulosic materials for ethanol production: a review. Bioresour Technol. 2002;83:1–11.
Ostovareh S, Karimi K, Zamani A. Efficient conversion of sweet sorghum stalks to biogas and ethanol using organosolv pretreatment. Ind Crop Prod. 2015;66:170–7.
Degirmenci V, Hensen EJ. Development of a heterogeneous catalyst for lignocellulosic biomass conversion: glucose dehydration by metal chlorides in a silica‐supported ionic liquid layer. Environ Prog Sustain Energy. 2014;33:657–62.
Grilc M, Likozar B, Levec J. Hydrotreatment of solvolytically liquefied lignocellulosic biomass over NiMo/Al 2 O 3 catalyst: reaction mechanism, hydrodeoxygenation kinetics and mass transfer model based on FTIR. Biomass Bioenergy. 2014;63:300–12.
Larabi C, Al Maksoud W, Szeto KC, Garron A, Arquilliere PP, Walter JJ, et al. Multifunctional heterogeneous catalyst for one step transformation of lignocellulosic biomass into low oxygenated hydrocarbons. Appl Catal A Gen. 2015;495:162–72.
Kobayashi H, Ohta H, Fukuoka A. Conversion of lignocellulose into renewable chemicals by heterogeneous catalysis. Catal Sci Technol. 2012;2:869–83.
Koo B-W, Kim H-Y, Park N, Lee S-M, Yeo H, Choi I-G. Organosolv pretreatment of Liriodendron tulipifera and simultaneous saccharification and fermentation for bioethanol production. Biomass Bioenergy. 2011;35:1833–40.
Hu J, Shen D, Wu S, Zhang H, Xiao R. Composition analysis of organosolv lignin and its catalytic solvolysis in supercritical alcohol. Energy Fuel. 2014;28:4260–6.
Lin Y-C, Huber GW. The critical role of heterogeneous catalysis in lignocellulosic biomass conversion. Energy Environ Sci. 2009;2:68–80.
Lee J-W, Jeffries TW. Efficiencies of acid catalysts in the hydrolysis of lignocellulosic biomass over a range of combined severity factors. Bioresour Technol. 2011;102:5884–90.
Guo F, Fang Z, Xu CC, Smith RL. Solid acid mediated hydrolysis of biomass for producing biofuels. Prog Energy Combust Sci. 2012;38:672–90.
Lanzafame P, Temi D, Perathoner S, Spadaro A, Centi G. Direct conversion of cellulose to glucose and valuable intermediates in mild reaction conditions over solid acid catalysts. Catal Today. 2012;179:178–84.
Feng G, Fang Z. Solid-and nano-catalysts pretreatment and hydrolysis techniques. Pretreatment techniques for biofuels and biorefineries. Berlin: Springer; 2013. p. 339–66.
Kamalaskar LB, Dhakephalkar P, Meher K, Ranade D. High biohydrogen yielding< i> Clostridium sp.</i> DMHC-10 isolated from sludge of distillery waste treatment plant. Int J Hydrog Energy. 2010;35:10639–44.
Chen W-M, Tseng Z-J, Lee K-S, Chang J-S. Fermentative hydrogen production with< i> Clostridium butyricum</i> CGS5 isolated from anaerobic sewage sludge. Int J Hydrog Energy. 2005;30:1063–70.
Baghchehsaraee B, Nakhla G, Karamanev D, Margaritis A, Reid G. The effect of heat pretreatment temperature on fermentative hydrogen production using mixed cultures. Int J Hydrog Energy. 2008;33:4064–73.
Adav SS, Lee D-J, Wang A, Ren N. Functional consortium for hydrogen production from cellobiose: concentration-to-extinction approach. Bioresour Technol. 2009;100:2546–50.
O-Thong S, Prasertsan P, Birkeland N-K. Evaluation of methods for preparing hydrogen-producing seed inocula under thermophilic condition by process performance and microbial community analysis. Bioresour Technol. 2009;100:909–18.
Ren N-Q, Guo W-Q, Wang X-J, Xiang W-S, Liu B-F, Wang X-Z, et al. Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. Int J Hydrog Energy. 2008;33:4318–24.
Liu H, Wang G. Hydrogen production of a salt tolerant strain Bacillus sp. B2 from marine intertidal sludge. World J Microbiol Biotechnol. 2012;28:31–7.
Wu K-J, Saratale GD, Lo Y-C, Chen W-M, Tseng Z-J, Chang M-C, et al. Simultaneous production of 2, 3-butanediol, ethanol and hydrogen with a< i> Klebsiella</i> sp. strain isolated from sewage sludge. Bioresour Technol. 2008;99:7966–70.
Whitman WB, Bowen TL, Boone DR. The methanogenic bacteria. The prokaryotes. Springer; New York, 2006. p. 165–207.
Liu H, Wang G, Zhu D, Pan G. Enrichment of the hydrogen-producing microbial community from marine intertidal sludge by different pretreatment methods. Int J Hydrog Energy. 2009;34:9696–701.
Baghchehsaraee B, Nakhla G, Karamanev D, Margaritis A. Fermentative hydrogen production by diverse microflora. Int J Hydrog Energy. 2010;35:5021–7.
Wong YM, Wu TY, Juan JC. A review of sustainable hydrogen production using seed sludge via dark fermentation. Renew Sustain Energy Rev. 2014;34:471–82.
Levin DB, Pitt L, Love M. Biohydrogen production: prospects and limitations to practical application. Int J Hydrog Energy. 2004;29:173–85.
Brentner LB, Peccia J, Zimmerman JB. Challenges in developing biohydrogen as a sustainable energy source: implications for a research agenda. Environ Sci Technol. 2010;44:2243–54.
Hallenbeck PC, Benemann JR. Biological hydrogen production; fundamentals and limiting processes. Int J Hydrog Energy. 2002;27:1185–93.
Kim S, Seol E, Oh Y-K, Wang G, Park S. Hydrogen production and metabolic flux analysis of metabolically engineered Escherichia coli strains. Int J Hydrog Energy. 2009;34:7417–27.
Hallenbeck P. Fundamentals of the fermentative production of hydrogen. Water Sci Technol. 2005;52:21–9.
Mathews J, Wang G. Metabolic pathway engineering for enhanced biohydrogen production. Int J Hydrog Energy. 2009;34:7404–16.
Chabrière E, Vernède X, Guigliarelli B, Charon M-H, Hatchikian EC, Fontecilla-Camps JC. Crystal structure of the free radical intermediate of pyruvate: ferredoxin oxidoreductase. Science. 2001;294:2559–63.
Wang S, Huang H, Moll J, Thauer RK. NADP+ reduction with reduced ferredoxin and NADP+ reduction with NADH are coupled via an electron-bifurcating enzyme complex in Clostridium kluyveri. J Bacteriol. 2010;192:5115–23.
Ren N-Q, Tang J, Liu B-F, Guo W-Q. Biological hydrogen production in continuous stirred reactor systems with suspended and attached microbial growth. Int J Hydrog Energy. 2010;35:2807–13.
Wu S-Y, Chu C-Y, Yeh W-Z. Aspect ratio effect of bioreactor on fermentative hydrogen production with immobilized sludge. Int J Hydrog Energy. 2013;38:6154–60.
Hawkes FR, Hussy I, Kyazze G, Dinsdale R, Hawkes DL. Continuous dark fermentative hydrogen production by mesophilicmicroflora: principles and progress. Int J Hydrog Energy. 2007;32:172–84.
Wang J, Wan W. Factors influencing fermentative hydrogen production: a review. Int J Hydrog Energy. 2009;3:799–811.
Lee K-S, Lo Y-C, Lin P-J, Chang J-S. Improving biohydrogen production in a carrier-induced granular sludge bed by altering physical configuration and agitation pattern of the bioreactor. Int J Hydrog Energy. 2006;31:1648–57.
Chang J-S, Lee K-S, Lin P-J. Biohydrogen production with fixed-bed bioreactors. Int J Hydrog Energy. 2002;27:1167–74.
Lee K-S, Lin P-J, Fangchiang K, Chang J-S. Continuous hydrogen production by anaerobic mixed microflora using a hollow-fiber microfiltration membrane bioreactor. Int J Hydrog Energy. 2007;32:950–7.
Show KY, Zhang ZP, Lee DJ. Design of bioreactors for biohydrogen production. J Sci Ind Res. 2008;67:941–9.
Xu H, Gong S, Sun Y, Ma H, Zheng M, Wang K. High-rate hydrogenotrophic methanogenesis for biogas upgrading: the role of anaerobic granules. Environ Technol. 2015;36:529–37.
Ab Halim MH, Anuar AN, Azmi SI, Jamal NSA, Wahab NA, Ujang Z, et al. Aerobic sludge granulation at high temperatures for domestic wastewater treatment. Bioresour Technol. 2015;185:445–9.
Lu X, Zhen G, Estrada AL, Chen M, Nic J, Hojo T, et al. Operation performance and granule characterization of upflow anaerobic sludge blanket (UASB) reactor treating wastewater with starch as the sole carbon source. Bioresour Technol. 2015;180:264–73.
Fang HH, Liu H, Zhang T. Characterization of a hydrogen‐producing granular sludge. Biotechnol Bioeng. 2002;78:44–52.
Chang F-Y, Lin C-Y. Biohydrogen production using an up-flow anaerobic sludge blanket reactor. Int J Hydrog Energy. 2004;29:33–9.
Jung K-W, Kim D-H, Shin H-S. A simple method to reduce the start-up period in a H 2-producing UASB reactor. Int J Hydrog Energy. 2011;36:1466–73.
Jung K-W, Kim D-H, Shin H-S. Fermentative hydrogen production from Laminaria japonica and optimization of thermal pretreatment conditions. Bioresour Technol. 2011;102:2745–50.
Wu S-Y, Lin C-N, Chang J-S, Chang J-S. Biohydrogen production with anaerobic sludge immobilized by ethylene-vinyl acetate copolymer. Int J Hydrog Energy. 2005;30:1375–81.
Wu SY, Hung CH, Lin CN, Chen HW, Lee AS, Chang JS. Fermentative hydrogen production and bacterial community structure in high‐rate anaerobic bioreactors containing silicone‐immobilized and self‐flocculated sludge. Biotechnol Bioeng. 2006;93:934–46.
Chu C-Y, Wu S-Y, Wu Y-C, Sen B, Hung C-H, Cheng C-H, et al. Phase holdups and microbial community in high-rate fermentative hydrogen bioreactors. Int J Hydrog Energy. 2011;36:364–73.
Wu SY, Lin CN, Chang JS, Lee KS, Lin PJ. Microbial hydrogen production with immobilized sewage sludge. Biotechnol Prog. 2002;18:921–6.
Lee K-S, Lo Y-S, Lo Y-C, Lin P-J, Chang J-S. H2 production with anaerobic sludge using activated-carbon supported packed-bed bioreactors. Biotechnol Lett. 2003;25:133–8.
Liu H, Fang H. Hydrogen production from wastewater by acidogenic granular sludge. Water Sci Technol. 2003;47:153–8.
Chu CY, Wu SY, Wu YC, Lin CY. Hydrodynamic behaviors in fermentative hydrogen bioreactors by pressure fluctuation analysis. Bioresour Technol. 2011;102:8669–75.
Wu SY, Lin CN, Chang JS. Hydrogen production with immobilized sewage sludge in three‐phase fluidized‐bed bioreactors. Biotechnol Prog. 2003;19:828–32.
Lee D-Y, Li Y-Y, Noike T. Influence of solids retention time on continuous H 2 production using membrane bioreactor. Int J Hydrog Energy. 2010;35:52–60.
Vallero M, Lettinga G, Lens P. Assessment of compatible solutes to overcome salinity stress in thermophilic (55¡ C) methanol-fed sulfate reducing granular sludges. Water Sci Technol. 2003;48:195–202.
Oh SE, Iyer P, Bruns MA, Logan BE. Biological hydrogen production using a membrane bioreactor. Biotechnol Bioeng. 2004;87:119–27.
Yang H, Shao P, Lu T, Shen J, Wang D, Xu Z, et al. Continuous bio-hydrogen production from citric acid wastewater via facultative anaerobic bacteria. Int J Hydrog Energy. 2006;31:1306–13.
Lin C-Y, Lay C-H, Sen B, Chu C-Y, Kumar G, Chen C-C, et al. Fermentative hydrogen production from wastewaters: a review and prognosis. Int J Hydrog Energy. 2012;37:15632–42.
Nissilä ME, Lay C-H, Puhakka JA. Dark fermentative hydrogen production from lignocellulosic hydrolyzates – a review. Biomass Bioenergy. 2014;67:145–59.
Kim S-H, Han S-K, Shin H-S. Effect of substrate concentration on hydrogen production and 16S rDNA-based analysis of the microbial community in a continuous fermenter. Process Biochem. 2006;41:199–207.
Jung K-W, Kim D-H, Kim S-H, Shin H-S. Bioreactor design for continuous dark fermentative hydrogen production. Bioresour Technol. 2011;102:8612–20.
FM Braga A, Júnior NF, Antônio D, Zaiat M. Thermophilic biohydrogen production using a UASB reactor: performance during long-term operation. J Chem Technol Biotechnol. 2015. doi:10.1002/jctb.4665. Article first published online: 5 Mar 2015.
Singh L, Siddiqui MF, Ahmad A, Rahim MHA, Sakinah M, Wahid ZA. Application of polyethylene glycol immobilized Clostridium sp. LS2 for continuous hydrogen production from palm oil mill effluent in upflow anaerobic sludge blanket reactor. Biochem Eng J. 2013;70:158–65.
Lo Y-C, Chen X-J, Huang C-Y, Yuan Y-J, Chang J-S. Dark fermentative hydrogen production with crude glycerol from biodiesel industry using indigenous hydrogen-producing bacteria. Int J Hydrog Energy. 2013;38:15815–22.
Wu X, Lin H, Zhu J. Optimization of continuous hydrogen production from co-fermenting molasses with liquid swine manure in an anaerobic sequencing batch reactor. Bioresour Technol. 2013;136:351–9.
Kumar G, Lin C-Y. Biogenic hydrogen conversion of de-oiled jatropha waste via anaerobic sequencing batch reactor operation: process performance, microbial insights, and reduction efficiency. Sci World J. 2014;2014:946503. p. 1–9.
Massanet-Nicolau J, Dinsdale R, Guwy A, Shipley G. Utilising biohydrogen to increase methane production, energy yields and process efficiency via two stage anaerobic digestion of grass. Bioresour Technol. 2015;189:379–83.
Fradler KR, Kim JR, Shipley G, Massanet-Nicolau J, Dinsdale RM, Guwy AJ, et al. Operation of a bioelectrochemical system as a polishing stage for the effluent from a two-stage biohydrogen and biomethane production process. Biochem Eng J. 2014;85:125–31.
Buitrón G, Kumar G, Martinez-Arce A, Moreno G. Hydrogen and methane production via a two-stage processes (H2-SBR + CH4-UASB) using tequila vinasses. Int J Hydrog Energy. 2014;39:19249–55.
Hsu C-W, Li Y-C, Chu C-Y, Liu C-M, Wu S-Y. Feasibility evaluation of fermentative biomass-derived gas production from condensed molasses in a continuous two-stage system for commercialization. Int J Hydrog Energy. 2014;39:19389–93.
Schievano A, Tenca A, Lonati S, Manzini E, Adani F. Can two-stage instead of one-stage anaerobic digestion really increase energy recovery from biomass? Appl Energy. 2014;124:335–42.
Lin C, Lay C. Carbon/nitrogen-ratio effect on fermentative hydrogen production by mixed microflora. Int J Hydrog Energy. 2004;29:41–5.
Lin C, Lay C. A nutrient formulation for fermentative hydrogen production using anaerobic sewage sludge microflora. Int J Hydrog Energy. 2005;30:285–92.
Karadag D, Puhakka JA. Enhancement of anaerobic hydrogen production by iron and nickel. Int J Hydrog Energy. 2010;35:8554–60.
Wang J, Wan W. Influence of Ni 2+ concentration on biohydrogen production. Bioresour Technol. 2008;99:8864–8.
Li C, Fang HHP. Inhibition of heavy metals on fermentative hydrogen production by granular sludge. Chemosphere. 2007;67:668–73.
Lee D-Y, Li Y-Y, Oh Y-K, Kim M-S, Noike T. Effect of iron concentration on continuous H 2 production using membrane bioreactor. Int J Hydrog Energy. 2009;34:1244–52.
Han H, Cui M, Wei L, Yang H, Shen J. Enhancement effect of hematite nanoparticles on fermentative hydrogen production. Bioresour Technol. 2011;102:7903–9.
Lin C-Y, Shei S-H. Heavy metal effects on fermentative hydrogen production using natural mixed microflora. Int J Hydrog Energy. 2008;33:587–93.
Han H, Jia Q, Wei L, Shen J. Influence of Cu2+ concentration on the biohydrogen production of continuous stirred tank reactor. Int J Hydrog Energy. 2014;39:13437–42.
Zheng X-J, Yu H-Q. Biological hydrogen production by enriched anaerobic cultures in the presence of copper and zinc. J Environ Sci Health A. 2004;39:89–101.
Frey M. Hydrogenases: hydrogen‐activating enzymes. ChemBioChem. 2002;3:153–60.
Casalot L, Rousset M. Maturation of the [NiFe] hydrogenases. Trends Microbiol. 2001;9:228–37.
Yang H, Shen J. Effect of ferrous iron concentration on anaerobic bio-hydrogen production from soluble starch. Int J Hydrog Energy. 2006;31:2137–46.
de Lacey AL, Fernández VM, Rousset M. Native and mutant nickel–iron hydrogenases: unravelling structure and function. Coord Chem Rev. 2005;249:1596–608.
Kim S, Seol E, Raj SM, Park S, Oh Y-K, Ryu DD. Various hydrogenases and formate-dependent hydrogen production in Citrobacter amalonaticus Y19. Int J Hydrog Energy. 2008;33:1509–15.
Minton NP, Clarke DJ. Clostridia. New York: Springer Science & Business Media; 1989.
KIM BH, Zeikus J. Importance of hydrogen metabolism in regulation of solventogenesis by Clostridium acetobutylicum. Dev Ind Microbiol. 1984;26:549–56.
Kim IS, Hwang MH, Jang NJ, Hyun SH, Lee ST. Effect of low pH on the activity of hydrogen utilizing methanogen in bio-hydrogen process. Int J Hydrog Energy. 2004;29:1133–40.
Gottwald M, Gottschalk G. The internal pH of clostridium acetobutylicum and its effect on the shift from acid to solvent formation. Arch Microbiol. 1985;143:42–6.
Khanal SK, Chen WH, Li L, Sung S. Biological hydrogen production: effects of pH and intermediate products. Int J Hydrog Energy. 2004;29:1123–31.
Lin C-Y, Lay C. Effects of carbonate and phosphate concentrations on hydrogen production using anaerobic sewage sludge microflora. Int J Hydrog Energy. 2004;29:275–81.
Cavinato C, Giuliano A, Bolzonella D, Pavan P, Cecchi F. Bio-hythane production from food waste by dark fermentation coupled with anaerobic digestion process: a long-term pilot scale experience. Int J Hydrog Energy. 2012;37:11549–55.
Wu X, Yao W, Zhu J. Effect of pH on continuous biohydrogen production from liquid swine manure with glucose supplement using an anaerobic sequencing batch reactor. Int J Hydrog Energy. 2010;35:6592–9.
Chu C-F, Li Y-Y, Xu K-Q, Ebie Y, Inamori Y, Kong H-N. A pH- and temperature-phased two-stage process for hydrogen and methane production from food waste. Int J Hydrog Energy. 2008;33:4739–46.
Lee C, Lee S, Han S-K, Hwang S. Effect of operational pH on biohydrogen production from food waste using anaerobic batch reactors. Water Sci Technol. 2014;69:1886–93.
Paul JS, Quraishi A, Thakur V, Jadhav S. Effect of ferrous and nitrate ions on biological hydrogen production from dairy effluent with anaerobic waste water treatment process. Asian J Biol Sci. 2014;7:165–71.
Chu C-Y, Tung L, Lin C-Y. Effect of substrate concentration and pH on biohydrogen production kinetics from food industry wastewater by mixed culture. Int J Hydrog Energy. 2013;38:15849–55.
Vatsala TM, Raj SM, Manimaran A. A pilot-scale study of biohydrogen production from distillery effluent using defined bacterial co-culture. Int J Hydrog Energy. 2008;33:5404–15.
Zhang P, Lin C-J, Liu J, Pongprueksa P, Evers SA, Hart P. Biogas production from brown grease using a pilot-scale high-rate anaerobic digester. Renew Energy. 2014;68:304–13.
Cheong D-Y, Hansen CL. Feasibility of hydrogen production in thermophilic mixed fermentation by natural anaerobes. Bioresour Technol. 2007;98:2229–39.
Luo G, Xie L, Zou Z, Wang W, Zhou Q. Evaluation of pretreatment methods on mixed inoculum for both batch and continuous thermophilic biohydrogen production from cassava stillage. Bioresour Technol. 2010;101:959–64.
Chen C-C, Lin C-Y, Lin M-C. Acid–base enrichment enhances anaerobic hydrogen production process. Appl Microbiol Biotechnol. 2002;58:224–8.
Lin C-Y, Wu S-Y, Lin P-J, Chang J-S, Hung C-H, Lee K-S, et al. A pilot-scale high-rate biohydrogen production system with mixed microflora. Int J Hydrog Energy. 2011;36:8758–64.
Li C, Fang HH. Fermentative hydrogen production from wastewater and solid wastes by mixed cultures. Crit Rev Environ Sci Technol. 2007;37:1–39.
Lin C-Y, Hung C-H, Chen C-H, Chung W-T, Cheng L-H. Effects of initial cultivation pH on fermentative hydrogen production from xylose using natural mixed cultures. Process Biochem. 2006;41:1383–90.
Koskinen PE, Beck SR, Örlygsson J, Puhakka JA. Ethanol and hydrogen production by two thermophilic, anaerobic bacteria isolated from Icelandic geothermal areas. Biotechnol Bioeng. 2008;101:679–90.
Lee Y-W, Chung J. Bioproduction of hydrogen from food waste by pilot-scale combined hydrogen/methane fermentation. Int J Hydrog Energy. 2010;35:11746–55.
Fountoulakis M, Dokianakis S, Daskalakis G, Manios T. Fermentative hydrogen production from carob pod: a typical mediterranean forest fruit. Waste Biomass Valor. 2014;5:799–805.
Wu SY, Chu CY, Shen YC. Effect of calcium ions on biohydrogen production performance in a fluidized bed bioreactor with activated carbon-immobilized cells. Int J Hydrog Energy. 2012;37:15496–502.
Ramírez-Morales JE, Torres Zúñiga I, Buitrón G. On-line heuristic optimization strategy to maximize the hydrogen production rate in a continuous stirred tank reactor. Proc Biochem. 2015;50:893–900.
Si B, Li J, Li B, Zhu Z, Shen R, Zhang Y, et al. The role of hydraulic retention time on controlling methanogenesis and homoacetogenesis in biohydrogen production using upflow anaerobic sludge blanket (UASB) reactor and packed bed reactor (PBR). Int J Hydrogen Energy. 2015; 40: 11414–21.
Chookaew T, Sompong O, Prasertsan P. Biohydrogen production from crude glycerol by immobilized Klebsiella sp. TR17 in a UASB reactor and bacterial quantification under non-sterile conditions. Int J Hydrog Energy. 2014;39:9580–7.
Park J-H, Kumar G, Park J-H, Park H-D, Kim S-H. Changes in performance and bacterial communities in response to various process disturbances in a high-rate biohydrogen reactor fed with galactose. Bioresour Technol. 2015;188:109–16.
Chu CY, Wu SY, Shen YC. Biohydrogen production performance in a draft tube bioreactor with immobilized cell. Int J Hydrog Energy. 2012;37:15658–65.
Rosa PRF, Santos SC, Sakamoto IK, Varesche MBA, Silva EL. Hydrogen production from cheese whey with ethanol-type fermentation: effect of hydraulic retention time on the microbial community composition. Bioresour Technol. 2014;161:10–9.
Rosa PRF, Santos SC, Sakamoto IK, Varesche MBA, Silva EL. The effects of seed sludge and hydraulic retention time on the production of hydrogen from a cassava processing wastewater and glucose mixture in an anaerobic fluidized bed reactor. Int J Hydrog Energy. 2014;39:13118–27.
Sivagurunathan P, Sen B, Lin C-Y. High-rate fermentative hydrogen production from beverage wastewater. Appl Energy. 2015;147:1–9.
Tenca A, Perazzolo F, Naldi E, Provolo G, Bodria L, Oberti R. Design and start-up of a two-stage farm-scale pilot plant for biohydrogen and biomethane production. Energy, biomass and biological residues international conference of Agricultural Engineering-CIGR-AgEng 2012: agriculture and engineering for a healthier life, Valencia, 8–12 Jul 2012: CIGR-EurAgEng; 2012. p. C-1068.
Cheng S-S, Chao Y-C, Yang K-H, Bai M-D. Process recovery of biohydrogenation in a pilot plant from methanogens invasion. Int J Hydrog Energy. 2011;36:8779–84.
Environment Park S.p.A. Pilot biomass pretreatment plant, Turin. http://www.envipark.com/en/green-chemistry/plants/. Accessed 1 June 2015.
Richard Dinsdale. Biohydrogen production from food production waste, University of Glamorgan, UK. http://www.ifr.ac.uk/waste/Reports/Biohydrogen_Rep_Glam_Univ.pdf. Accessed 1 June 2015.
Toyota Motor Corporation. Powering the future hydrogen fuel cell vehicles could change mobility forever. http://www.toyota-global.com/innovation/environmental_technology/fuelcell_vehicle/. Accessed 1 June 2015.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Chu, CY., Huang, BS. (2015). Biohydrogen Production via Lignocellulose and Organic Waste Fermentation. In: Fang, Z., Smith, Jr., R., Qi, X. (eds) Production of Hydrogen from Renewable Resources. Biofuels and Biorefineries, vol 5. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7330-0_2
Download citation
DOI: https://doi.org/10.1007/978-94-017-7330-0_2
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7329-4
Online ISBN: 978-94-017-7330-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)