Abstract
The Metallic nanoparticles synthesis by cyanobacteria presents chronological scientific results on one of the most dynamic topic concerning biogenic production of nanoparticles. After a very short and general presentation of nanoparticles and cyanobacteria, in the context of (bio)nanotechnology, a synopsis on cyanobacteria utilization to produce metallic nanoparticles is presented, focused on their biological implications, advantages and disadvantages, as well as on future prospects.
This paper is dedicated to the memory of my respected master Professor Gheorghe Zarnea (September 22, 1921–June 16, 2012), from the University of Bucharest and the Institute of Biology Bucharest (Romania). His distinction and intelligence impressed and guided his numerous students, including me, to whom his enthusiasm was lastingly transmitted.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Thylakoid Membrane
- Spirulina Platensis
- Magnetotactic Bacterium
- Microcoleus Chthonoplastes
- Cyanobacterium Plectonema Boryanum
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Nanoparticles are materials with different shapes (spherical, triangular, rods etc.) and dimensions between 1 and 100 nm having specific properties which make them very interesting with respect to both fundamental and applicative research. The behaviour of matter at nanoscale is very different from what is familiar and commonly accepted at macroscopic level. Laws relating to physical, chemical, biological, electrical, magnetic and other properties at the nano-scale are different from those that apply at macro scale (Bhat 2003) metals become harder and ceramics become softer, a whole variety of composites and alloys become possible, chemical resistance is increased, interactions with light/other radiations are different (e.g. the absorption of solar radiation in photovoltaic cells is much higher in materials composed of nanoparticles than in thin films of continuous sheets of material, the smaller the particles, the greater the solar absorption) (Bhat 2003; Nalwa 2004, 2011). Furthermore, biologically produced nanoparticles have, sometimes, better applicative properties as compared to not only the bulk material, but also with abiotically produced nanoparticles. For example, the same amount of Pd(0) nanoparticle has a ninefold higher catalytic activity with respect to pollutant removal than commercial Pd(0) powder (De Windt et al. 2005). Nanoparticles studies and applications belong to the Nanotechnology field (Bhattacharya and Gupta 2005), defined as “the understanding and control of matter at dimensions between approximately 1 and 100 nanometers,” and technology that “involves imaging, measuring, modeling, and manipulating matter at this length scale.” (National Nanotechnology Initiative 2009). The commercial value of nanotechnological industry, including the production of nanoparticles, is expected to increase significantly from $91 billion in 2009 to $1 trillion by 2015 and $3 trillion by 2020 (http://www.nanotechproject.org).
The synthesis of Metallic NanoParticles (MNPs) can be performed, essentially, by physical, chemical and biological methods, each method having advantages and disadvantages (see below). The synthesis of MNPs mediated by living matter, so called biogenic synthesis of MNP, is a relatively new scientific topic, reporting the use of bacteria, cyanobacteria and actinomycetes, fungi, lichens, algae and plants extracts in this process (Gericke and Pinches 2006; Prathna et al. 2010; Rai and Duran 2011). These syntheses, usually occur under normal conditions of temperature and pressure, with no toxic chemicals involved in the process, with the exception of metallic ions to be reduced. Thus, this type of protocol is friendly to the environment, with high potential in developing a green technology. However, so far the processes involved in the biogenic synthesis are poorly understood.
MNPs have great potential for applications in the electronic, chemical, mechanical and life sciences industries. In biology and medicine the main applications are in the following directions: fluorescent biological labels; drug and gene delivery; biodetection of pathogens, detection of proteins, probing of DNA structure, tissue engineering, tumor destruction via heating (hyperthermia), separation and purification of biological molecules and cells (Li et al. 2011; Salata 2004; Nalwa 2004, 2011; Brayner et al. 2013). In few years MNPs with improved catalytic activity have become important also for in situ destroying of organic pollutants (Karn et al. 2009; Hennebel et al. 2009, 2011; Mishra and Malik 2013).
Cyanobacteria are the most diversified, ecologically most successful and evolutionary most important group of prokaryotes, clearly defined by the ability to carry out oxygenic photosynthesis in the thylakoid membranes and respiration both in plasma membrane and thylakoid membrane, in some species the major respiratory activity occurring at plasma membrane whereas in other species the main site of aerobic respiration being thylakoid membrane (Peschek 1996). Gleobacter violaceus is the only cyanobacterium found so far which lacks thylakoides, respiration and photosynthesis occurring at the level of cell membrane. Oxygenic photosynthesis, the ability to use the light energy to synthesize glucides from carbon dioxide and water and to evolve oxygen from water molecules is essential for all the other forms of life on earth. Historically, cyanobacteria were the first organisms to perform oxygenic photosynthesis and this metabolic ability of ancient cyanobacteria have converted the early reducing atmosphere of earth (when no free molecular oxygen was available) into an oxidizing one. This process emerged approximately 3.5 billion years and had an essential effect on the evolution of life on our planet. There is a general agreement that the oxic atmosphere allowed the emergence and evolution of aerobic microorganisms, most probably by endosymbiotic association between different types of prokaryotic cells. The ancient cyanobacteria participated to this endosymbiosis thus all photosynthetic organisms on earth have some cyanobacteria as ancestors; together with cyanobacteria all these photosynthetic eukaryotes, including higher plants, contribute today to the synthesis of organic matter and oxygen production, the basis of all life forms here. Cyanobacteria being very versatile microorganisms can live, freely or attached or in symbiosis with different types of organisms, in very different environments with respect to temperature, pH, salinity, light intensity etc., some of them being able to use atmospheric nitrogen for the synthesis of organic compounds (Bryant 1994; Seckbach 2007; Gault and Marler 2009; Peschek et al. 2011; Govindjee and Shevela 2011; Whitton 2012; Srivastava et al. 2013).
The diversity of cyanobacteria, whose systematics and nomenclature is still a matter of debate (Oren 2011) and their involvement in essential processes occurring in nature (primary production, oxygen evolution, nitrogen fixation etc.) allow them to be very important actors in biotechnology. Important reviews on biotechnological applications and potential of cyanobacteria have been published in the last years the most known topics being the photoproduction of molecular hydrogen or electricity, biomass (and related processes, including valuable products), removal of different pollutants (petroleum hydrocarbon, heavy metals, nitrogen and phosphorus etc.) CO2 mitigation, biosensors (Becker 1994; Vonshak 1997; Borowitza and Boroworzka 1988; Ardelean and Zarnea 1998; Kuyucak and Volesky 1989; Richmond 2008; Gault and Marler 2009; Grewe and Pulz 2012; Aryal et al. 2013; chapter “Algal Biotechnology” by Borowitzka, this volume). The presentation of these biotechnological applications is very well widespread in academic literatures, some of the very good contributions in the field being freely accessible on internet/academic site/web pages.
2 Cyanobacteria and Nanotechnology
Cyanobacteria are important for nanotechnology mainly for the following topics: (i) model cells to test biocompatibility/cytotoxicity of nanoparticles, (ii) bioenergetics and (iii) model cells for metallic nanoparticle synthesis. Their use as test organisms for measuring the biocompatibility/cytotoxicity of NP takes the advantage of the occurrence of major metabolic processes at different cellular sites- photosynthesis only at the thylakoid level and respiration both at cell membrane and thylakoid membrane level. It is thus possible to measure the interplay between aerobic respiration or oxygenic photosynthesis and different types (chemical nature, size, shape etc.,) of nanoparticles. Some of the results in this field concerns the toxicity of cerium oxide for Anabaena CPB4337 (Rodea-Palomares et al. 2011), titanium dioxide nanoparticles (nTiO2) against the nitrogen-fixing cyanobacteria Anabaena variabilis (Cherchi et al. 2011), nanosized cadmium sulfide (CdS) against the cyanobacterium Anabaena flos-aquae (Brayner et al. 2011) or of CdSe quantum dots against Synechocystis PCC 6803 in light or in darkness (Ardelean et al. 2011; Sarchizian et al. 2011). The results show structural and functional disorders induced in cyanobacteria by the interactions with these nanoparticles which could be limiting factor for the synthesis of MNP by cyanobacteria (see Biological significance of MNPs synthesis). In the last years there are several reports on using cyanobacteria as model systems to develop biomimetic nanodevices for energy conversion. Grimme et al. (2008) used abiotically synthesized platinum/gold nanoparticles which were coupled directly to isolated cyanobacterial photosystem 1 (PSI) particles; this new structure is able to evolve (for short time) molecular hydrogen. In another approach (Iwuchukwu et al. 2010) -directly related to the ability of cyanobacteria to produce MNP, the topic of this chapter- the reduction of platinum ion (“platinization reactions” according to their terminology) took place in a photobioreactor by incubation of cyt c6, PSI (from Thermosynechococcus. elongatus or Synechocystis PCC 6803) and platinum ions. The self-organized platinization of the photosystem I nanoparticles allows electron transport from sodium ascorbate to photosystem I via cytochrome-c6 and finally to the platinum catalyst, where hydrogen gas is formed. Their system produces hydrogen at temperatures up to 55 °C and is temporally stable for >85 days with no decrease in hydrogen yield when tested intermittently. The maximum yield is 5.5 mmol H2/h/mg chlorophyll and is estimated to be 25-fold greater than current biomass-to-fuel strategies (Iwuchukwu et al. 2010). In another very interesting approach, the thermophilic cyanobacterium Thermosynechococcus elongatus was genetically modified in such a way that their photosystem II (PSII) particles can be immobilized in vitro on abiotically synthesized GNPs by orienting the electron acceptor side to the gold surface. The PSII immobilized on GNP (four to five PSII dimmers are bound to a single GNP) retained O2 evolution activity comparable to that of free PSII. According to the authors, the PSII-GNP conjugate will be a useful nanodevice for the development of artificial systems for light-driven water splitting into O2 and H2 (Noji et al. 2011).
3 Cyanobacteria and Metals
The interaction between metals and (cyano)bacteria is very complex in nature, MNPs synthesis by microorganisms, including cyanobacteria, being only one face of these interactions. Other interactions between microorganisms and metals involves biomining – the use of (micro)organisms to extract metals from ores- and bioremediation- the use of (micro)organisms to remove metals from wastes. All these processes are part of the biogeochemical cycles of metals which involve complex biochemical reaction interacting with pure chemical reactions; these reactions induce changes in the physical and chemical properties of the resulting products (Gadd 2010; Mishra and Malik 2013). The recovery of gold, and other metals, from solutions has been actively studied for industrial purposes to process billions of tons of metal waste every year; however, as the conventional methods for removing metals from ore processing solutions are extremely expensive, alternative methods for metal recovery using living or dead microorganisms have been actively investigated (Lengke et al. 2006a).
For our topic it is important to know that the scientists firstly used microorganism for bioremediation and metal recovery and afterwards the interest for biological synthesis of MNP occurs (Lengke et al. 2006a; Gadd 2010; Mishra and Malik 2013). As pointed out by Lengke et al. 2006a, in the case of cyanobacteria, several laboratory experiments have been conducted to understand the interaction of Au ions with cyanobacteria; it was shown that cyanobacteria were able to accumulate Au from AuCl−4 by a biosorption mechanism, but the morphology of Au particles resulting from this mechanism has not been investigated (Lengke et al. 2006a).
There are several books and reviews concerning the synthesis of MNPs by microorganisms some of them containing excellent results obtained also with cyanobacteria (Mandal et al. 2006; Gericke and Pinches 2006; Rai and Duran 2011; Prathna et al. 2010; Narayanan and Sakthivel 2010; Li et al. 2011; Lengke et al. 2011; Ghorbani et al. 2011; Tikariha et al. 2012; Liesje et al. 2012) but, up to my best knowledge, this is the first review devoted exclusively to the synthesis of MNPs by cyanobacteria.
The present chapter is focused on the use of cyanobacteria for the synthesis of MNPs which is an upcoming topic and has got lot of attention during last few years because of its basic and applied potential.
4 MNP Synthesis by Cyanobacteria
The first experiments on MNPs synthesis by cyanobacteria were done on Plectonema boryanum (Lengke et al. 2006a, b, c). One paper reports two types of experiments to examine the role of cyanobacterium Plectonema boryanum UTEX 485 on the accumulation of Au from aqueous solutions of AuCl− 4 (Lengke et al. 2006b). In type 1, Au solutions (up to 5 mM) were added to the cultures and incubated at different temperature and times (25 °C, 60 °C and 100 °C for up to 1 month, and 200 °C for 1 day). In type 2, Au solutions (up to 5 mM) were added to dead, autoclaved cultures incubated at 25 °C; control experiments were conducted using AuCl− 4 solutions (2 mM) and no cyanobacteria present (Lengke et al. 2006b). In the type 1 experiments, after addition of AuCl−4 all cyanobacteria were killed within several minutes, with concomitant change in color of the culture vessel from (blue) green to purple. Significant increase in precipitation of Au occurred with increase in temperature from 25 to 60–200 °C. In the type 2 experiments, after addition of AuCl− 4 to the dead, autoclaved cyanobacteria the rate of Au precipitation was faster by approximately three times than that in the type 1 experiments at similar temperature indicating that Au precipitation and formation of octahedral platelets were directly related to degradation of cyanobacteria, clearly showing that increased rates of GNP formation can be obtained in the absence of any metabolic activity (Lengke et al. 2006a). The microscopic images from the type 1 experiments show that at 25 °C, the addition of AuCl− 4 to the cyanobacteria killed the cells instantly, but the filaments remained intact. Gold particles of irregular to octahedral habit were precipitated on the bacteria cells. Gold particles were also dispersed throughout the interior of the cells. At 60 °C, the cell structure was distorted and cells became rounded, and the filaments were separated into their constituent cells. At 100 °C, the filaments were distorted and encrusted by Au particles and at 200 °C, the cyanobacteria cells exhibited rounded form, and Au particles were deposited on the individual cells (Lengke et al. 2006b). One can see that, with increasing temperatures, the morphological changes of cyanobacterial filaments become more pronounced; furthermore, in the range of unphysiological temperatures for this strain (above 60 °C), where temperature only can cause cells death, morphology of cells and filaments is far from normality. The authors also showed that the size of Au particles increased with increase in temperature from an approximate diameter of 1.5 μm at 25 °C to 10 μm at 100 °C with a nanometer scale thickness. In the abiotic, chemical control experiments, in the absence of cyanobacteria, the precipitation of Au was observed only at 100–200 °C the pure chemical reaction being promoted by heat. When it comes to the mechanism(s) of gold precipitation both outside and inside the cell, the authors (Lengke et al. 2006b) suggest that Au entered the cells as AuCl− 4 , and then gold ion (Au3+) was reduced to Au° in an unknown manner not directly linked to bacteria metabolism as the cyanobacteria were probably killed by either the gold (III)-chloride or the acidic pH. The results demonstrates two important things: (i) interaction of cyanobacteria with the chemical environment is an important factor controlling the morphology of Au particles and (ii) the reduction of Au(111) is actually two-step, involving an intermediate Au(I)-S phase, with the sulfur being of organic origin (Lengke et al. 2006b).
Another study of this scientific group (Lengke et al. 2006c) was focused on extensive use of laboratory-based cyanobacterial experiments to go deeper in understanding the processes involved in the mechanisms of MNPs formation. The authors clearly stated that the elucidation of the functional groups derived from the cyanobacteria for gold binding is required to understand chemical mechanisms of gold accumulation by cyanobacteria. For the first time, the authors pay a special attention to the effect of interaction between cyanobacteria and the aqueous solutions of gold (III)-chloride on cell viability. Their results showed that at 0.8 mM initial gold chloride concentration the color of the cyanobacteria remained green, but dark purple color was observed in the solution after several hours of reaction. The purple color in the solution is characteristic of formation of colloidal gold. At 1.7 and 7.6 mM initial gold(III)-chloride concentrations change was observed in the color of the cyanobacteria and solutions, from green to reddish brown and yellow to colorless, respectively. Interestingly, the cyanobacteria were killed several minutes after the addition of gold (III)-chloride which argue for a sacrificial gold NP synthesis mechanism (Lengke et al. 2006c). In a larger perspective, the authors discussed their results with respect to geochemical implications for the formation of secondary gold deposits stressing on the fact that the gold concentrations in natural environments are 2–3 orders of magnitude lower than those used in their study, expecting that the reduction of gold (III)-chloride to metallic gold by cyanobacteria is presumably a slow process in nature. The authors put forward a very interesting hypothesis that at low gold concentrations (at ppb level), gold toxicity would not kill all viable bacterial cells, the remaining cells would be able to grow and probably adapt to the presence of gold. To the best of my best knowledge, there are no reports on quantitative relationship between cell viability (either single cells or filamentous cyanobacteria) and gold NP synthesis over a large range of gold chloride concentration. When it comes to the mechanisms involved in gold NP synthesis, the authors stress on the fact that the formation of an intermediate species, gold (I)-sulfide, shows the importance of organic reduced sulfur species (e.g., cysteine, methionine) in gold binding mechanisms by cyanobacteria. Passive gold accumulation via organic sulfur present in the cyanobacterial outer membrane or periplasm may explain the mechanisms of gold bioaccumulation by cyanobacteria as well as by other living (micro) organisms such as algae, fungi and plants (Lengke et al. 2006b, c, 2007c). As Gram-negative bacterium, Plectonema boryanum is able to release vesicles, and this is the case when this cyanobacterium interact with gold ions at high concentration, the interactions being followed by the precipitation of elemental gold (Lengke et al. 2011).
The formation of palladium nanoparticles was investigated by reacting palladium (II) chloride with the filamentous cyanobacterium Plectonema boryanum, strain UTEX 485 (Lengke et al. 2007b). The growth of cyanobacteria and experimental design were as in (Lengke et al. 2006a), with the significant exception that palladium ion PdCl2 was used instead of gold ion. After PdCl2 addition all cyanobacteria were killed within several hours at all temperatures investigated (25–100 °C), probably due to the low pH (1.9) and high temperatures (Lengke et al. 2007b). The interaction of cyanobacteria with PdCl2 solutions at 25, 60, and 100 °C resulted in distinctive morphologies for palladium metal nanoparticles which were precipitated outside the cell (equal or smaller than 30 nm). The authors showed that the reaction of aqueous PdCl2 with cyanobacteria caused the precipitation of dispersed palladium metal (equal or smaller than 30 nm) on the bacterial cells, claiming that the release of organic materials from cyanobacteria during their death plays a role in this process.
The same cyanobacterium produce spherical silver nanoparticles and octahedral silver platelets by a mechanism which could involve metabolic processes from the utilization of nitrate at 25 °C and also organics released from the dead cyanobacteria at 25–100 °C (Lengke et al. 2007a). The papers from this group are pioneering work on MNP synthesis by cyanobacteria, having very important contributions to the correlation between the shape and structure of MNP and physical and chemical parameters during their synthesis.
Another important group in this field demonstrated that three filamentous cyanobacterial strains Anabaena, Calothrix and Leptolyngbya have the capability to reduce Au, Ag, Pd and Pt ions to elemental metal organized as nanoparticles (Brayner et al. 2007). They have reported about the attempts to use cyanobacteria as recyclable bioreactors, showing that the cells recover their full activity (measured as chlorophyll fluorescence and gold nanoparticle production) after a first metal salt addition and reduction (Brayner et al. 2007). These cyanobacteria were cultivated (6–30 days) before the addition of metal as salts at different ions concentration (10−3 10−4 10−6 M) in media without added nitrogen, in order to promote nitrogen fixation activity. These strains have different sensibilities against initial ions concentrations; for example, in Calothrix grown in culture medium at 10−6 M gold the filaments appear in bright field having their normal blue-green color, thus enabling the authors to claim that the cyanobacteria are alive. At higher Au concentrations (10−3 M), the heterocysts color changed from yellow-green to purple-blue and vegetative cells were bleached, this concentration being very close to the lethal dose (Brayner et al. 2007). Contrary to filaments belonging to the genus Calothrix, filaments belonging to the genus Anabaena remain unbleached, both vegetative cells and heterocysts, indicating that, in this strain, the lethal dose was not reached at 10−3 M. The authors put forward the hypothesis that nitrogenase is involved in nanoparticle production, taking into account that most part of these nanoparticles was formed inside the heterocysts where nitrogenase is present. Interesting results were obtained with silver, palladium and platinum salts (Brayner et al. 2007). The authors also reported in vitro enzymatic, nitrogenase mediated, reduction of metallic salts to Au, Ag, Pd, and Pt metallic nanoparticles; the reduction was observed after addition of 10−3 M metallic salts under vigorous stirring at 25 °C during 20 min in the presence of nitrogenase and isolated exopolysaccharides from Calothrix (Brayner et al. 2007). However, there are no details about the way nitrogenase activity was sustained in their in vitro experimental conditions with respect to ATP need. This is the first report concerning screening of cyanobacteria for nanoparticles synthesis over a large metal ion initial concentrations, paying special attention to the structural and functional integrity of cyanobacteria during MNPs synthesis.
Another report (Govindraju et al. 2008) argue the use of the highly structured physical cells of Spirulina platensis for the biosynthesis at room temperature of pure metallic silver, gold as well as Au core/Ag shell nanoparticles. The reduction of aqueous gold and silver ions, in pure solutions or in mixtures, to the corresponding nanoparticles occurs at room temperature and is aided by the polypeptide/proteins of this cyanobacterium; furthermore it is believed that protein might have played an important role in the stabilization of Ag, Au and Au/Ag bimetallic nanoparticles (Govindraju et al. 2008). This is the first report concerning bimetal nanoparticule synthesis by cyanobacteria.
The use of cyanobacteria Lyngbya majuscula, Spirulina subsalsa both for bio-recovery of gold (Au) out of aqueous solution and nanoparticles synthesis is reported (Chakraborty et al. 2009). Interestingly, Au (III) spiked with 198Au was used for the experiment. Gold accumulation by cyanobacterial biomass was 1.93 mg g−1 for L. majuscula and 1.73 mg g−1 for S. subsalsa. It was shown that L. majuscula biomass exposed in HAuCl4 solution produce <20-nm-sized gold particles located both inside as well as on the surface of the cell. As mechanism, the authors put forward that the first step involves the trapping of the metal ions on the surface of the cells possibly via electrostatic interaction between the ions and the negatively charged carboxylate groups present in the cell surface; this first step occurs quickly and is independent of metabolism. Thereafter, the ions are reduced by the enzymes leading to the formation of nuclei, which subsequently grow through the further reduction of metal ions and accumulation of these nuclei. Most probably the reduction of gold particles occurs due to the presence of cellular reductases (Chakraborty et al. 2009). Following the suggestion concerning that the mechanism of bioaccumulation of gold by sulfate reducing bacteria cultured in the presence of gold (I)-thiosulfate complex (Lengke and Southam 2006), these authors (Chakraborty et al. 2009) put forward that in cyanobacteria, (as in sulphate reducing bacteria) localized reducing conditions may be produced by bacterial electron transport chain via energy generating reactions within the cells (for more details Chakraborty et al. 2009). This is the first report concerning the use of mixtures of isotopes for nanoparticule synthesis by cyanobacteria.
The extracellular synthesis of copper oxide nanoparticles by Phormidium cyanobacterium seems to occur by extracellular hydrolysis of the cationic copper by certain metal chelating anionic proteins/reductases secreted by this cyanobacterium under normal growth conditions (aerobic environment, neutral pH and room temperature); the proteins also play a significant role in stabilization of formed nanoparticles at room temperature (Rahman et al. 2009).
Focsan and co workers (2011) aimed to elucidate the interplay between biomineralization and metabolic activities in the case of the cyanobacterium Synechocystis sp. PCC 6803 exposed to gold ions. The authors demonstrated the ability of the cyanobacteria to reduce gold ions, the yield of GNPs synthesis being strongly dependent on the intensity of aerobic respiration and oxygenic photosynthesis. The biosynthesized GNPs (13 ± 2 nm) are localized at the cell wall, plasma membrane and inside the cytoplasm, inclusive at the level thylakoid membranes (Focsan et al. 2011). Interestingly, the authors, alternative to the direct demonstration by TEM of GNP accumulation at the level of thylakoid membranes, surveyed the accumulation of GNPs in cells in real time by making use of a fluorescent signal from chl a molecules at room temperature (Focsan et al. 2011). Indeed, they observed a gradual quenching of fluorescence at 681 nm as a function of GNP production inside cells when cyanobacteria are incubated with gold ions, relative to a reference sample of living cyanobacteria which served as control (cyanobacteria with no gold ions). This quenching of fluorescence can be the result of energy transfer from the excited fluorophore to vicinal GNPs in the cyanobacterial membrane (Focsan et al. 2011). If this quenching of chlorophyll a fluorescence is the mechanism by which the rate of oxygenic photosynthesis (measured as the production of molecular oxygen) decreases during the synthesis of gold nanoparticles is still an open question. To study the interplay between GNP biosynthesis and metabolic activity, the culture of cyanobacteria was divided into four sub-cultures, one for control and the other three were exposed to similar concentrations of gold ion solution and incubated for 50 h in three different external conditions, respectively: (i) in light at 20 °C, (ii) in dark at 20 °C and (iii) in dark at 4 °C. The results show an inhibition, after 16 h of incubation in the presence of gold ions, of aerobic respiratory (oxygen consumption in darkness) and oxygenic photosynthesis (oxygen production in light); the inhibition is stronger in cultures where the synthesis of MNP is higher (in light at 20 °C) and lower in cultures where the synthesis of MNP is slower (in dark at 4 °C). Interestingly, after the first 4 h of incubation there are no differences in the intensity of either aerobic respiration or oxygenic photosynthesis in all sub-cultures, indicating that MNPs synthesis occurred without any inhibition of respiration or photosynthesis. These results argue the involvement of aerobic respiration and oxygen photosynthesis in MNP synthesis by Synechocystis sp. PCC 6803, and the sacrificial nature of this synthesis. When it comes to the electronic mechanisms involved in GNP biosynthesis the authors (Focsan et al. 2011) claim that in their experiments the electron needed for the reduction of gold ions could come from an intracellular electron donor during both photosynthesis and respiration, pointing out the major role of thylakoids in the synthesis of GNPs determined mainly by the photosynthetic electron transport and, to a lesser extent, by the respiratory electron transport occurring both at the cell membrane and at the thylakoid membrane. A significant factor in the synthesis of GNPs by cyanobacteria could be the NADPH2 (nicotinamide adenine dinucleotide phosphate) or NADH-dependent reductases. The reduced cofactor would be oxidized during the reduction of gold ions and recycled via energy generating reactions within the photosynthetic electron transport, respiratory electron transport and redox reaction of the so-called intermediary metabolism, occurring in the cytoplasm (Focsan et al. 2011). The authors do not exclude that, additionally, the reduction of gold ions can also occur due to exported electrons from the molecular ambient by a membrane transporter system or/and by the presence of carboxyl groups and polysaccharides at the cyanobacterial cell membranes, and due to cysteine- or methionine- containing compounds. This is the first paper on cyanobacteria where surface-enhanced Raman scattering, SERS, use biogenic MNP as reporter structures to analysis their own cellular localization, and where the evolution of respiratory oxygen consumption and photosynthetic oxygen production are quantified during (gold) nanoparticule synthesis, thus arguing the involvement of these catabolic and anabolic processes in MNP synthesis by cyanobacteria, in physiological conditions.
Gold chloride reduction by healthy, exponentially growing filaments of the cyanobacterium N. ellipsosporum produce gold nanorod (137–209 nm in length and 33–69 nm in diameter) (Parial et al. 2011). The same group (Parial et al. 2012) further shows that three species of cyanobacteria Phormidium valderianum, P. tenue and Microcoleus chthonoplastes when exposed to hydrogen tetrachloroaurate solution produced gold nanoparticles inside the cell. Interestingly, the shape of NP is dependent on the incubation pH. Phormidium valderianum at pH 9 synthesized mostly spherical nanoparticles (around 13.78 nm) whereas at pH 7 mostly spherical particles (around 7.92 nm) were produced, and at pH 5 gold nanorods (411 nm × 32 nm) together with gold nanospheres 15 nm in diameter (more details on other shapes Parial et al. 2012). Moreover, in all the experimental genera cells were poisoned and died after converting Au (III)- to elemental gold; the reduction of gold is associated with cellular metabolism and presumably involves reducing enzymes or synthesis of other metabolites, the results arguing for a suicidal MNP synthesis (Parial et al. 2012).
S. platensis IPPAS B-256 is able to reduce gold ion from Au(III) to Au(0) by proteins and enzymes on the cell surface, and aggregation of the gold nanoparticles in the solution (Kalabegishvili et al. 2012). Interestingly, the size of GNP is dependent on the extracellular initial gold concentration; at 5 × 10−3 M most of the particles are spherical and their sizes are in the range of 15–40 nm, the majority being in the range of 20–30 nm, with different shapes, whereas at concentration of 10−2 M the size of formed particles is broader, from nanoscale range to the micrometer range (Kalabegishvili et al. 2012).
In the cyanobacterium Anabaena flos-aquae the processes of gold ion incorporation, its intracellular reduction, and Au0 nanoparticle synthesis has been studied, as compared with similar processes occurring in eukaryotic photosynthetic microorganisms (Dahoumane et al. 2012). In Anabaena flos-aquae the synthesized GNP (20 °C, 16 h light/8 h dark) have a disperse location within the bacterial cell, and the dimension depends on the initial concentration of gold ion: at 10−3 M gold ions the mean diameter is 10.0 ± 4.7 and at 10−4 M gold ions the mean diameter is 8.1 ± 2.1. It is important to point out that in the absence of cells, no gold reduction was observed, even when extracted exopolysaccharides (EPS) were present (Dahoumane et al. 2012). One main concern of the authors is the impact of gold reduction on the physiological state of the cells. Before addition of gold salts, the photosynthetic activity of all the microorganisms remains stable during more than 2 months. The addition of gold ion and the interaction of cyanobacterial cells with gold ion at low (10−4 M) or high (10−3 M) concentrations causes the inhibition of photosynthesis as measured by monitoring chlorophyll a fluorescence in vivo. The authors claim that, in Anabaena flos-aquae, all the observed phenomena related to gold colloids occur when cells are damaged, assuming that the diffusion of gold ions and formed nanoparticles is easier and faster, due to the loss of membrane integrity. The experimental results show that the internal and external particle size is different, with larger particles outside the cells, probably involving extracellular and intracellular reduction combined with easy in/out transport through damaged membranes (Dahoumane et al. 2012). Based on their original results, the authors (Dahoumane et al. 2012), in connection with other papers, conclude that the Au(III) species are first in contact with EPS network, where the reduction take place. However, the authors agree that the reduction can also occur directly on the cell surface, as demonstrated by Lengke and Southam 2006, where an intermediate Au(I) species would be involved, which can be stabilized by some cellular products. These two mechanisms are in good agreement with the data showing gold particle formation even with dead cells (Dahoumane et al. 2012). For the particles observed within the cells, the authors (Dahoumane et al. 2012) suggest that, in the case of dead cells, these particles correspond to pre-formed Au(0) particles penetrating damaged membranes. However, for living cells, the specific localization of these particles in the thylakoids strongly suggests that the gold precursor, being either in the form of Au(III) or stabilized Au(I) reach these specific compartments. For the reduction occurring at the level of thylakoides, the authors claim that NAD(P)H can be involved in GNP formation, hydrogenase or nitrate reductase being, in their opinion, the enzyme fueling this co-factor (Dahoumane et al. 2012). They claim that it would be of high interest to perform in vitro experiments with isolated chloroplasts or artificial membranes, involving hydrogenase enzymes or mimicking systems, the immobilization of the biocatalyst within solid supports representing an important step towards the design of “living” bionanoreactor (Dahoumane et al. 2012). This is also the first paper which comparatively discusses about varied effects of different gold ion concentration on photosynthesis in prokaryote and eukaryote microorganisms during GNP formation.
In another report, Spirulina platensis strain PCC 9108 has been shown to convert silver ions to SNP when concentrated biomass from an exponential growth phase is put in contact with 1 mM aqueous AgNO3 solution for 24 h at 25 °C (Mahdieha et al. 2012). The authors claim that, the most probably, the formation of SNPs occurs due to the presence of cellular reductases released by Spirulina platensis into the solution. SNPs are in range of 5–35 nm, the majority of them being within three peaks, with a mean value of the size of 11.6 nm. There are no details concerning the structure and functions of cyanobacteria during and after SNP synthesis.
An interesting proposal concerns the in vitro synthesis of CdS nanoparticles by the use of phycoerythrin extracted from the marine cyanobacterium Phormidium tenue NTDM05 (MubarakAli et al. 2012). This protein was used for the biosynthesis of CdS nanoparticles by simply mixing the phycoerythrin extract with aqueous CdCl2 and Na2S in the concentration 0.25 mM and 1 mM, respectively. The reaction mixture was closely monitored for color change over a period of 5 days. The first step in this reaction sequence of nanoparticles synthesis was the formation of C-PE–Cd 2+ complex, then this complex reacted with Na2S to produce CdS nanoparticles, when the color reaction mixture changed to orange. The TEM results confirmed the spherical shape of nanoparticles at the size of about 5 nm. Essentially, it was found that the pigment is involved in the synthesis but also in the stabilization of CdS nanoparticles (MubarakAli et al. 2012). According to the authors the simplicity of the procedure presented in this study can be useful in commercial scale production of stable CdS nanoparticles.
The screening of cyanobacteria isolated from muthupet mangrove (Aphanothece sp, Oscillatoria sp, Microcoleus sp, Aphanocapsa sp, Phormidium sp, Lyngbya sp, Gleocapsa sp, Synechococcus sp, Spirulina sp) showed that only Microcoleus sp has the ability to reduce silver ions to silver nanoparticles (Sudha et al. 2013). Silver nanoparticles were spherical shaped, with an average size of about 40–80 nm and well distributed without aggregation in solution. These nanoparticles were further characterized with respect to physical properties and antibacterial activity against test pathogenic bacteria (Proteus vulgaris, Salmonella typhi, Vibrio cholera, Streptococcus sp., Bacillus subtilis, Staphylococcus aureus, and Escherichia coli) as compared with classical antibiotics (cephotaxime, ampicillin, tetracycline, cephalexin etc.). The results showed that the synthesized silver nanoparticles are effective antimicrobial agent and proved as an alternative for the development of new antimicrobial agents to combat the problem of resistance to classical antibiotics (Sudha et al. 2013). These results argue the need to select cyanobacterial strains able to reduce metallic ions and to understand the mechanism(s) involved in this ability which could help scientists in understanding more deeply the interplay between metallic ions and cyanobacteria.
MubarakAli et al. (2013) reported the synthesis of gold nanoparticles using photosynthetic microorganisms such as Phormidium sp. The reaction mixture containing cyanobacterium and chloroauric acid (HAuCl4) was incubated for an hour in dark room condition and it was found that nanotriangles (25 nm) were synthesized by a mechanism involving cellular proteins present in Phormidium (MubarakAli et al. 2013).
Rosken et al. (2014) show the ability of the cyanobacterium Anabaena sp. (SAG 12.82) to reduce Au 3+ (at a starting concentration of 0.8 mM HAuCl4•H2O) to Au0 with the subsequent formation of crystalline Au0-nanoparticles. Formation of nanoparticles, recorded by X-ray powder diffraction and transmission electron microscopy over a range of several days, starts within the first minutes at the heterocyst polysaccharide layer. After 4 h, the dominating amount of nanoparticles is found in the vegetative cells. The bioproduced nanoparticles are found in both cell types, mainly located along the thylakoid membranes of the vegetative cells, and have a final average size of 9 nm within the examined timescale of a few days. Interestingly, the authors find that the heterocysts are not the favorite place for nanoparticle formation by their strain of Anabaena sp (SAG 12.82) the most favorable regions, where nanoparticles are recorded, being the thylakoid membranes, inside the vegetative cells (Rosken et al. 2014). The authors stress that another advantage of their experimental system for the production of gold nanoparticles is the absence of anatoxin a production by this strain.
5 Biological Significance of MNP Synthesis by Cyanobacteria
Taking into account the above presented results and different proposed mechanisms of NP synthesis, it seems that the reduction of metal ions to elemental metal organized as nanoparticles when catalyzed/performed by living cyanobacteria is followed by death of cyanobacteria, arguing that MNP bioproduction is detrimental to the cells. However, no experiments are made to check if-at population level- death of some cells during MNP synthesis is related to the chance of other cells to survive because the decrease in metal ion concentration. As suggested by Lengke et al. (2006c) the cyanobacteria reaching low concentration of gold ions could remain active thus having a role in ore formation. Recently, Johnston et al. (2013) has shown that Delftia acidovorans, a gold resident non-photosynthetic bacterium, produces a secondary metabolite, a non ribosomal synthesized peptide, that protects this bacterium from soluble gold through the generation of solid gold forms. This finding is the first demonstration that a secreted metabolite can protect against toxic gold and cause gold biomineralization (Johnston et al. 2013). Theoretically this mechanism could act in other bacteria as well, including cyanobacteria. More experiments are needed in order to better understand the biological signification of MNP synthesis by living cyanobacteria, using not only ion over a large range of concentration, but also different ratio between bacterial biomass and amount of ions, more diverse methods to measure the intensity of cyanobacterial metabolism and their structural and functional integrity, as well as genetically modified cyanobacteria.
6 Advantages and Disadvantages of MNP Production Using Cyanobacteria
The large application of nanoparticles is dependent on the ability to synthesize particles with different chemical composition, shape, size, and monodispersity (Ghorbani et al. 2011). Chemical methods are high energy- consuming and unfriendly to the environment as they use (toxic) chemical reducing agents; however, the rate of synthesis is rather high (Ghorbani et al. 2011). In contrast, biological synthesis use reactions which are carried out in normal conditions thus the energy cost are lower; this topic emerges as an eco-friendly and exciting approach which is still in its infancy (Ghorbani et al. 2011). Furthermore, the growth of cells, the biological catalysts, is not very expensive, and the cells are self regenerable. It was shown that, by controlling the temperature and the pH of the bioreactors, the size and the shape of the synthesized MNP can be changed (Rai and Duran 2011).
Besides the evident (potential) advantages of bioproduced nanoparticles, there are some challenges that need to be addressed such as: long production times as compared to chemical production (Hennebel et al. 2009; Ghorbani et al. 2011) optimization for accurate control of the particle size, proper extraction protocols and the search for strains harboring bioprecipitation potential for other unexplored metals (Hennebel et al. 2009). Furthermore the shape, size, and functionalization of the nanoparticles is defined by the biological system used to produce the nanoparticles, hence for every application a specific biological production process needs to be chosen (Sintubin et al. 2012). On the other hand, biogenic nanoparticles need to compete with chemically produced nanoparticles on the market. Hence, the true challenge for biogenic nanoparticles is finding the balance between scalability, price, and applicability (Sintubin et al. 2012). More fundamental research is essential to obtain MNP produced by cyanobacteria which can be really commercialized on a free market for applications.
7 Further Prospects
Here are some ideas for developing the ability of cyanobacteria to synthesize metallic nanoparticles:
-
1.
Isolation of new cyanobacterial populations, including those living in so called extreme environments (high metal concentrations, elevated temperatures, high salinities and/or pH etc.,) and enrichment of these microorganisms in culture media supplemented with different metal ions (Au, Ag, Pt, Pd, etc.,) over a large ion concentrations.
-
2.
Selection from enriched populations of strains with abilities to produce MNP and isolation in pure culture of representatives of the two ways, sacrificial and non –sacrificial, of MNP synthesis.
-
3.
Optimization of the overall process occurring at laboratory level with respect to: (i) cyanobacterial strain selection and growing conditions before the addition of metal salts; (ii) the nature of metal ion and its initial concentration in the process, and (iii) incubation conditions (pH, temperature, light-dark regime etc.,) during MNP synthesis (in vegetative cells or in heterocysts) at different cellular sites (Table 1), implying different metabolic processes, single enzymatic reaction or pure chemical reaction, having also in mind the need to recover the MNP from the reaction mixtures.
Table 1 Proposed structures and processes that could be involved in MNPs synthesis by (living) cyanobacteria -
4.
Advanced physical and chemical characterization of the MNP produced in each optimized process, in order to understand the interplay between the structure and properties of MNP and biotic and abiotic conditions during their synthesis.
-
5.
Finding of at least one specific utilization for (some of the) produced MNP in order to develop a supply chain production, for scaling up the process and checking its economic viability.
-
6.
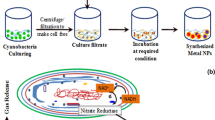
A deeper understanding of the processes involved in MNP synthesis by cyanobacteria – via either sacrificial or non sacrificial way- would help the scientists to genetically modify the selected cyanobacteria in order to: (i) change the structure of their cellular components (intraparietal and extraparietal structures etc.,) involved in biomineralisation, and (ii) facilitate the ions reduction at very specific sites, by specific enzymes involved in respiration, photosynthesis, hydrogen uptake, intermediary metabolism etc. (see Table 1 and Fig. 1).
-
7.
The data reported by Focsan et al. (2011) concerning the use of biologically synthesized MNP as (nano)structures reporter inside the (living) cells, taking the advantages in studying living matter using surface-enhanced Raman scattering, represents a highly increasingly topic within the last years (for more details see Premasiri et al. 2005).
-
8.
The MNP synthesis during detoxification and remediation of toxic metals could be important not only for the utilization of selected microorganisms to produce MNP in laboratory or at industrial scale, but also for the in situ degradation of other pollutants. So far, there are no reports concerning the participation of MNP produced in the environment by cyanobacteria to the catalytic degradation of organic pollutants.
-
9.
Cooperation between scientists working on MNP production by cyanobacteria with those focused on other types of MNP producers such as heterotrophic prokaryotes (e.g. Shewanella, magnetotactic bacteria etc.,) and photosynthetic eukaryotes. So far, the best understood biomineralisation process in magnetotactic bacteria refers to the synthesis of magnetosome, which are bacterial organelle containing magnetic crystals (magnetite or/and greigite) (Bazylinski and Frankel 2004; Faivre and Schüler 2008; Moisescu et al. 2011, 2014; Lefèvre et al. 2013). Furthermore, on this topic there are some data concerning gold and silver trapping by uncultured magnetotactic cocci (Keim and Farina 2005), as well as on the controlled doping of magnetosome (Staniland et al. 2008).
-
10.
Cooperation between scientists working on MNP production by cyanobacteria with those focused on other aspects of cyanobacterial biology, including other types of biomineralisation (calcium, silica etc.,) carried out by some cyanobacteria (more details see Thompson and Ferris 1990; Benning et al. 2002).
References
Ardelean II, Zarnea G (1998) Biosensors with intact cyanobacteria for environmental protection. In: Subramanian G, Kaushik D, Venkataraman GS (eds) Cyanobacterial biotechnology. Publishers M/S Oxford IBH Publishing House, New Delhi, pp 341–346
Ardelean II, Sarchizian I, Manea M, Damian V, Apostol I, Cîrnu M, Armaselu A, Iordache I, Apostol D (2011) CdSe/ZnS quantum dots cytotoxicity against phototrophic and heterotrophic bacteria. In: TANGER Ltd (ed) Proceeding of NANOCON 2011, Brno, Czech Republic, 21–23 Sept 2011, pp 608–617
Aryal UK, Callister SJ, Mishra S, Zhang X, Shitthanandan JI, Angel TE, Shukla AK, Monroe ME, Moore RJ, Koppenaal DW, Smith RD, Sherman L (2013) Proteome analysis of strains ATCC 51142 and PCC 7822 of the diazotrophic cyanobacterium Cyanothece sp. under culture conditions resulting in enhanced H2 production. Appl Environ Microbiol 79:1070–1077
Bazylinski DA, Frankel BR (2004) Magnetosome formation in prokaryotes. Nat Rev Microbiol 2(3):217–230
Becker EW (1994) Microalgae. Biotechnology and microbiology. Cambridge University Press, Cambridge, p 293
Benning LG, Phoenix V, Yee N, Tobin MJ, Konhauser KO, Mountain BW (2002) Molecular characterization of cyanobacterial cells during silicification: a synchrotron-based infrared study. Geochem Earth Surf 6:259–263
Bhat JSA (2003) Heralding a new future –nanotechnology. Curr Sci 85:147–154
Bhattacharya D, Gupta RK (2005) Nanotechnology and potential of microorganisms. Crit Rev Biotechnol 25:199–204
Borowitza MA, Boroworzka LJ (eds) (1988) Microalgal biotechnology. Cambridge University Press, Cambridge
Brayner R, Barberousse H, Hernadi M, Djedjat C, Yepremian C, Coradin T, Livage J, Fievet F, Coute A (2007) Cyanobacteria as bioreactors for the synthesis of Au, Ag, Pd, and Pt nanoparticles via an enzyme-mediated route. J Nanosci Nanotechnol 7:2696–2708
Brayner R, Dahoumane SA, Nguyen JN, Yéprémian C, Djediat C, Couté A, Fiévet F (2011) Ecotoxicological studies of CdS nanoparticles on photosynthetic microorganisms. J Nanosci Nanotechnol 11(3):1852–1858
Brayner R, Fiévet F, Coradin T (ed) (2013) Nanomaterials: a danger or a promise?; A chemical and biological perspective. Springer, London
Bryant DA (ed) (1994) The molecular biology of cyanobacteria. Kluwer Academic Publishers, New York, Boston, Dordrecht, London, Moscow
Chakraborty N, Banerjee A, Lahiri S, Panda A, Ghosh AN, Pal R (2009) Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation – a novel phenomenon. J Appl Phycol 21:145–152
Cherchi C, Chernenko T, Diem M, Gu AZ (2011) Impact of nano titanium dioxide exposure on cellular structure of Anabaena variabilis and evidence of internalization. Environ Toxicol Chem 30(4):861–869
Dahoumane SA, Djediat C, Yepremian C, Coute A, Fievet F, Coradin T, Brayner R (2012) Species selection for the design of gold nanobioreactor by photosynthetic organisms. J Nanopart Res 14:883
De Windt W, Peter A, Willy V (2005) Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol 7:314–325
Faivre D, Schüler D (2008) Magnetotactic bacteria and magnetosomes. Chem Rev 108(11):4875–4898
Focsan M, Ardelean II, Craciun C, Astilean S (2011) Interplay between gold nanoparticle biosynthesis and metabolic activity of cyanobacterium Synechocystis sp. PCC 6803. Nanotechnology 22(48):1–8
Gadd GM (2010) Metals, minerals and microbes: geomicrobiology and bioremediation. Microbiology 156:609–643
Gault PM, Marler HJ (eds) (2009) Handbook on cyanobacteria: biochemistry, biotechnology and applications. Nova, New York
Gericke M, Pinches A (2006) Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132–140
Ghorbani HR, Safekordi AA, Attar H, Sorkhabadib SMR (2011) Biological and non-biological methods for silver nanoparticles synthesis. Chem Biochem Eng Q 25(3):317–326
Govindjee, Shevela D (2011) Adventures with cyanobacteria: a personal perspective. Front Plant Sci 2:1–17
Govindraju K, Basha K, Ganesh S, Kumar V, Singaravelu G (2008) Silver, gold and bimetallic nanoparticles production using single cell protein (Spirulina platensis) Geitler. J Mater Sci 43:5115–5122
Grewe CB, Pulz O (2012) The biotechnology of cyanobacteria. In: Whitton BA (ed) Ecology of cyanobacteria II. Their diversity in space and time. Springer, Dordrecht, pp 707–739
Grimme RA, Lubner CE, Bryant DA, Golbeck JH (2008) Photosystem I/molecular wire/metal nanoparticle bioconjugates for the photocatalytic production of H2. J Am Chem Soc 130:6308–6309
Hennebel T, Gusseme BD, Boon N, Verstraete W (2009) Biogenic metals in advanced water treatment. Trends Biotechnol 27:90–98
Hennebel T, Simoen H, Verhagen P, De Windt W, Dick J, Weise C, Pietschner F, Boon N, Verstraete W (2011) Biocatalytic dechlorination of hexachlorocyclohexane by immobilized bio-Pd in a pilot scale fluidized bed reactor. Environ Chem Lett 9:417–422
Iwuchukwu IJ, Vaughn M, Myers N, O’Neill H, Frymier P, Bruce BD (2010) Self-organized photosynthetic nanoparticle for cell- free hydrogen production. Nat Nanotechnol 5:73–79
Johnston CW, Wyatt MA, Li X, Ibrahim A, Shuster J, Southam J, Margavey NA (2013) Gold biomineralization by a metallophore from a gold-associated microbe. Nat Chem Biol 9:241–243
Kalabegishvili T, Murusidze I, Kirkesali E, Rcheulishvili A, Ginturi E, Gelagutashvili E, Kuchava N, Bagdavadze N, Pataraya D, Gurielidze M, Tsertsvadze G, Gabunia V (2012) Synthesis of gold and silver nanoparticles by some microorganisms. Nano Stud 6:5–14
Karn B, Kuiken T, Otto M (2009) Nanotechnology and in situ remediation: a review of the benefits and potential risks. Environ Health Perspect 117(12):1823–1831
Keim CN, Farina M (2005) Gold and silver entrapment by uncultured magnetotactic cocci. Geomicrobiol J 22:55–63
Kuyucak N, Volesky B (1989) Accumulation of gold by algal biosorbent. Biorecovery 1:189–204
Lefèvre CT, Trubitsyn D, Abreu F, Kolinko S, Jogler C, de Almeida LGP, de Vasconcelos ATR, Kube M, Reinhardt R, Lins U, Pignol D, Schüler D, Bazylinski DA, Ginet N (2013) Comparative genomic analysis of magnetotactic bacteria from the Deltaproteobacteria provides new insights into magnetite and greigite magnetosome genes required for magnetotaxis. Environ Microbiol. doi:10.1111/1462-2920.12128
Lengke MF, Southam G (2006) Bioaccumulation of gold by sulfate-reducing bacteria cultured in the presence of gold (I)-thiosulfate complex. Geochem Cosmochim Acta 70:3646–3661
Lengke MF, Fleet ME, Southam G (2006a) Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold(I)-thiosulfate and gold(III)-chloride complexes. Langmuir 22:2780–2787
Lengke MF, Fleet ME, Southam G (2006b) Bioaccumulation of gold by filamentous cyanobacteria between 25 and 200°C. Geomicrobiol J 23:591–597
Lengke MF, Ravel B, Fleet ME, Wanger G, Gordon RA, Southam G (2006c) Mechanisms of gold bioaccumulation by filamentous cyanobacteria from gold (III)-chloride complex. Environ Sci Technol 40(20):6304–6309
Lengke MF, Fleet ME, Southam G (2007a) Biosynthesis of silver nanoparticles by filamentous cyanobacteria from a silver(I) nitrate complex. Langmuir 23:2694–2699
Lengke MF, Fleet ME, Southam G (2007b) Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial biomass with a palladium(II) chloride complex. Langmuir 23:8982–8987
Lengke MF, Ravel B, Fleet ME, Wanger G, Gordon RA, Southam G (2007c) Precipitation of gold by the reaction of aqueous gold (III) chloride with cyanobacteria at 25–80°C – studied by x-ray absorption spectroscopy. Can J Chem 85(10):651–659
Lengke MF, Sanpawanitchakit C, Southam G (2011) Biosynthesis of gold nanoparticles: a review. In: Rai, Duran N (eds) Metal nanoparticles in microbiology, 1st edn. Springer, New York, pp 37–74
Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 2011:1–16
Liesje S, Willy V, Nico B (2012) Biologically produced nanosilver: current state and future perspectives. Biotechnol Bioeng 10:242
Mahdieha M, Zolanvari A, Azimeea AS, Mahdiehc M (2012) Green biosynthesis of silver nanoparticles by Spirulina platensis. Sci Iran F 19(3):926–929
Mandal D, Bolander ME, Mukhopadhyay D, Sarkar G, Mukherjee P (2006) The use of microorganisms for the formation of metal nanoparticles and their application. Appl Microbiol Biotechnol 69:485–492
Mishra A, Malik A (2013) Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol 43(11):1162–1222
Moisescu C, Bonneville S, Staniland S, Ardelean I, Benning LG (2011) Iron uptake kinetics and magnetosome formation by Magnetospirillum gryphiswaldense as a function of pH, temperature and dissolved iron availability. Geomicrobiol J 28(7):590–600
Moisescu C, Ardelean II, Benning LG (2014) The effect and role of environmental conditions on magnetosome synthesis. Front Microbiol 5:1–12
MubarakAli D, Gopinath V, Rameshbabu N, Thajuddin N (2012) Synthesis and characterization of CdS nanoparticles using C-phycoerythrin from the marine cyanobacteria. Mater Lett 74:8–11
MubarakAli D, Arunkumarb J, Nagc KH, SheikSyedIshackd KA, Baldeva E, Pandiaraje D, Thajuddina N (2013) Gold nanoparticles from Pro and eukaryotic photosynthetic microorganisms—comparative studies on synthesis and its application on biolabelling. Colloids Surf B: Biointerfaces 103:166–173
Nalwa HS. Encyclopedia of nanoscience and nanotechnology. Edited by (vols 1–10, 2004) plus (vols 11–25, 2011). American Scientific Publishers
Narayanan KB, Sakthivel N (2010) Biological synthesis of metal nanoparticles by microbes. Adv Colloid Interface Sci 156:1–13
National Nanotechnology Initiative (2009) What is nanotechnology? http://www.nano.go/html/facts/whatIsNano.html
Noji T, Suzuki H, Gotoh T, Iwai M, Ikeuchi M, Tomo T, Noguchi T (2011) Photosystem II_gold nanoparticle conjugate as a nanodevice for the development of artificial light-driven water-splitting systems. J Phys Chem Lett 2:2448–2452
Oren A (2011) Cyanobacterial systematics and nomenclature as featured in the International Bulletin of Bacteriological Nomenclature and Taxonomy/International Journal of Systematic Bacteriology/International Journal of Systematic and Evolutionary Microbiology. Int J Syst Evol Microbiol 61:10–15
Parial D, Patra HK, Roychoudhury P, Dasgupta AK, Pal R (2011) Gold nanorod production by cyanobacteria – a green chemistry approach. J Appl Phycol 24:55–60
Parial D, Patra HK, Dasgupta AK, Pal R (2012) Screening of different algae for green synthesis of gold nanoparticles. Eur J Phycol 47(1):22–29
Peschek GA (1996) Structure-function relationships in the dual-function photosyntheticrespiratory electron-transport assembly of cyanobacteria (blue-green algae). Biochem Soc Trans 24(3):729–733
Peschek GA, Obinger C, Renger G (eds) (2011) Bioenergetic processes of cyanobacteria: from evolutionary singularity to ecological diversity. Springer, Dordrecht
Prathna TC, Mathew L, Chandrasekaran N, Raichur AM, Mukherjee A (2010) Biomimetic synthesis of nanoparticles: science, technology and applicability. In: Mukherjee A (ed) Biomimetics, learning from nature. Olajnica, Vukovar, Croatia: IN-THE, 1, pp 1–20
Premasiri WR, Moir DT, Klempner MS, Krieger N, Jones G, Ziegler LD (2005) Characterization of the Surface Enhanced Raman Scattering (SERS) of bacteria. J Phys Chem B 109:312–320
Rahman A, Ismail A, Jumbianti D, Magdalena S, Sudrajat H (2009) Synthesis of copper oxide nano particles by using Phormidium cyanobacterium. Ind J Chem 9(3):355–360
Rai M, Duran N (eds) (2011) Metal nanoparticles in microbiology, 1st edn. Springer, New York, pp 37–74
Richmond A (ed) (2008) Mass production of microalgae: photobioreactors. Blackwell Science, Oxford
Rodea-Palomares I, Boltes K, Fernandez-Pinas F, Garcıa-Calvo LF, Santiago J, Rosal R (2011) Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms. Toxicol Sci 119(1):135–145
Rosken LM, Korsten S, Fischer CB, Schonleber A, van Smaalen S, Geimer S, Wehner S (2014) Time-dependent growth of crystalline Au0-nanoparticles in cyanobacteria as self-reproducing bioreactors: 1. Anabaena sp. J Nanopart Res 16:1–14
Salata OV (2004) Applications of nanoparticles in biology and medicine. J Nanobiotechnology 2:3–8
Sarchizian I, Cirnu M, Ardelean II (2011) Isolation of cyanobacteria and quantification of their biotechnological potential with respect to redox properties at single cell level. Rom Biotechnol J 16(6):3–8
Seckbach J (ed) (2007) Algae and cyanobacteria in extreme environments, series: Cellular origin, life in extreme habitats and astrobiology. Springer Netherlands
Sintubin L, Verstraete W, Boon N (2012) Biologically produced nanosilver: current state and future perspectives. Biotechnol Bioeng 109:2422–2436
Srivastava AK, Rai AN, Neilan BA (eds) (2013) Stress biology of cyanobacteria: molecular mechanisms to cellular responses. CRC Press, Taylor & Francis Group, Boca Raton, p 375
Staniland S, Williams W, Telling N, Van der Laan G, Harrison A, Ward B (2008) Controlled cobalt doping of magnetosomes in vivo. Nat Nanotechnol 3:158–162
Sudha SS, Karthic R, Rengaramanujam J (2013) Microalgae mediated synthesis of silver nanoparticles and their antibacterial activity against pathogenic bacteria. Ind J Exp Biol 52:393–399
Thompson JB, Ferris FG (1990) Cyanobacterial precipitation of gypsum, calcite, and magnesite from natural alkaline lake water. Geology 18:995–998
Tikariha S, Singh S, Banerjee AS, Vidyarthi M (2012) Biosynthesis of gold nanoparticles, scope and application: a review. S IJPSR 3(6):1603–1615
Vonshak A (ed) (1997) Spirulina platensis (Arthrospira): physiology, cell-biology and biochemistry. Taylor & Francis, London
Whitton BA (ed) (2012) Ecology of cyanobacteria II. Their diversity in space and time. Springer, Dordrecht, http://www.nanotechproject.org
Acknowledgements
This work was supported by the Romanian Academy (Grant RO1567-IBB05/2013)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Ardelean, I.I. (2015). Metallic Nanoparticle Synthesis by Cyanobacteria: Fundamentals and Applications. In: Sahoo, D., Seckbach, J. (eds) The Algae World. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol 26. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7321-8_16
Download citation
DOI: https://doi.org/10.1007/978-94-017-7321-8_16
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7320-1
Online ISBN: 978-94-017-7321-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)