Abstract
All coral reef organisms are susceptible to diseases, as are terrestrial organisms, but studying these diseases can be more difficult and much remains to be learned. Although health impairments of corals were first recognized only in the early 1970s, increasing numbers of infectious and non-infectious diseases, causing morbidity and mortality in numerous species of tropical marine organisms, have now been identified in diverse species of algae, plants, invertebrates, and vertebrates. Causes of diseases include biotic, as well as abiotic, factors, but identifying a primary pathogen has been reported in only a few cases, and some of those results have been questioned as additional diagnostic tools have been applied. The multitude of stressors affecting reef organisms, particularly along heavily urbanized coastlines, as well as introductions of species to distant reefs by global transport, are contributing to concerns about extinction risks and loss of biodiversity. This chapter presents an overview of diseases of reef organisms, how diseases have adversely affected coral reefs, and new developments in disease diagnoses. The application of concepts from the field of conservation medicine are aiding our understanding of diseases and their impacts on organisms of these shallow to mesophotic ecosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
8.1 Introduction
Most people picture reefs, and their associated fauna and flora, as vigorous, flourishing, and healthy, just as we used to see forests, meadows, and even deserts, despite the differences in organisms and physical conditions. Closely tied to this was the realization that increasing human populations were changing these terrestrial ecosystems with agriculture, the growth of big cities bringing people into closer proximities and encroaching on wildlife, fragmentation of habitat into smaller disconnected parcels, and the development of machines to “improve our lives” by industries that have required the extraction of minerals and oil, deforestation, and release of chemicals foreign to the environment (Deem et al. 2001). Many anthropogenic disturbances of terrestrial resources have similarly been recognized in the oceans and produce medium to high impacts on coral reefs (Richmond 1993; Halpern et al. 2008); these will be discussed in Chaps. 9 and 11.
This chapter will discuss the nature of health impairments now recognized to be affecting reef organisms, which often are the direct or indirect result of anthropogenic disturbances and also contribute to disturbances, as well as affect the recovery of coral reef ecosystems. It has only been since 1970 that we have found corals and other reef organisms to be susceptible to diseases caused by pathogens and parasites, as well as to those conditions caused or aggravated by exposures to anthropogenic pollutants and habitat degradation . Perhaps the demise of tropical reefs was delayed only by the more recent colonization of islands and coastlines in the New World and human populations were kept under control by the profusion of their own and zoonotic disease s —e.g., malaria, smallpox, yellow fever, shigellosis, leishmaniasis, schistosomiasis, onchocerciasis, lymphatic filariasis, trypanosomiasis, cholera, dengue fever—mediated by nutritional deficiencies and travel (Armelagos et al. 2005). Examination of peer-reviewed journal articles indicates a recent increase in disease outbreaks in many reef ecosystems and organisms (Harvell et al. 2004; Ward and Lafferty 2004). However, anthropogenic impacts related to overfishing are thought to have begun hundreds of years ago and the reductions in populations of fish and shellfish species may reduce disease transmission in these organisms while increasing the susceptibility of other organisms to diseases (Jackson et al. 2001; Lafferty 2004; Dinsdale and Rohwer 2011).
In these and other studies on diseases of coral reef organisms, multiple physical and chemical stressors are often identified and teasing out their roles in the development of disease with respect to biotic pathogens can be difficult (Ban et al. 2014). Scientists have been busy documenting “things caused” (Box 8.1)—a variety of health impairments in organisms of reef ecosystems—and are now making advances in identifying the “causes of things,” the etiologic agents of the diseases, lurking amid the myriad and dynamic natural and anthropogenic stressors present in the reef environment.
Box 8.1
“The study of the causes of things must be preceded by the study of things caused.”
J. Hughlings Jackson, British neurologist (1835–1911)
8.2 What Is a Disease?
Disease is defined as any impairment (interruption, cessation, proliferation, or other disorder) of vital body functions, systems, or organs. Diseases have at least two of these features: (1) an identifiable group of signs (observed anomalies indicative of disease in a non-human organism) or symptoms (subjective evidence of disease that a human can explain to another human), and/or (2) a recognized etiologic or causal agent, and/or (3) consistent gross or microscopic structural alterations (e.g., developmental disorders, changes in cellular composition or morphology, tumors). The terms “disease,” and “syndrome” (the latter referring to all the signs or symptoms that comprise the disease) are often included in the name of a recognized functional impairment, and it is not necessary to know the causal agent to use either term when identifying a functional impairment of an organism. The term “health” is defined as the state of an organism when it functions optimally without evidence of disease or abnormality. Although many refer to “ecosystem [coral reef or other] health,” the appropriateness of this is much debated, because ecosystems are dynamic, can exist in multiple stable states, and clear criteria cannot be determined to identify optimal functioning among different ecosystems, which also lack homeostatic mechanisms (Suter 1993; Lancaster 2000; Lackey 2003; Hudson et al. 2006). These are generalized behavioral, physiological, and biochemical responses that may be invoked by an organism over the short or long term, allowing it to adapt to a range of changing conditions while maintaining a preferred state, level (homeostasis), or rate of some process (homeorhesis) (Stebbing, 1981; McNamara and Buchanan 2005; Sokolova et al. 2012). The counteractive capacity of these adaptive responses will allow an organism to maintain its health while being subjected to changing conditions, leading to resistance to the stressor(s).
Any virus, microorganism, or other substance that causes disease is a pathogen , an etiologic agent. Interactions of a pathogen with a host (the organism that may develop disease by being affected by an abiotic pathogen or infected by a biotic pathogen) are both always affected by the environment. This is the paradigm of disease (Work et al. 2008b). Abiotic diseases are those structural and functional body impairments that only result from exposure to abiotic environmental stresses such as changes in physical conditions (salinity , temperature , light intensity or wavelength, sedimentation, oxygen concentrations, currents) or exposures to biotoxins or toxic chemicals (heavy metals, oils, pesticides)—the “other substance” referred to in the above definition of a pathogen (Rougee et al. 2006; Downs et al. 2012). Biotic diseases are those in which the etiologic agent is a living organism such as a microbial, unicellular, or metazoan parasite . If a parasite causes disease and death of the host, then it is known as a pathogen. A variety of organisms normally live in interspecific associations known as symbioses on or within the tissues of other organisms (Amadjian and Paracer 1986). Such associations can range from mutualistic symbioses (beneficial to both organism and host) to parasitic symbioses where the organism derives a nutritional benefit from the host. For example, some symbioses of microorganisms enable their hosts to live in potentially toxic environments or to subsist on nutritionally limited diets. The totality of these associations is now referred to as the holobiont (Rohwer et al. 2002). Infectious agents, those that are spread from host to host, include viruses, bacteria, fungi , protozoans (also known as microparasites), and metazoans such as helminths and arthropods (macroparasites). Zoonotic diseases are those that normally affect animals but can spread to humans. Infectious agents can exist in other organisms (reservoir hosts or vectors) or elsewhere in the environment, to be transmitted to the species they can adversely affect. The interactions of pathogens with hosts within the environments they inhabit and potential outcomes are illustrated in Fig. 8.1.
In the “optimum envelope” of health (center oval), exposure to stressors is limited to pre-adapted levels in which the organism can retain optimal functioning through its homeostatic mechanisms and immune system; exposure to higher or lower levels of stressors than it is adapted can lead to the development of non-infectious disease or increase its susceptibility to invasion by an infectious agent and subsequent illness; death of cells, tissues, or the diseased host can occur as the result of irreversible damage to its vital functions, organs, or systems caused by the primary pathogen (s) or associated secondary pathogens
Exposure to potentially pathogenic levels of abiotic and biotic agents—levels higher or lower than in the range that supports optimal function—may not result in disease . Organisms, including plants, possess a variety of defenses for protection from invasion (non-self recognition) and cellular damage, including innate and (in the case of vertebrates) adaptive, immune systems. These defenses include a surface epithelium of tightly joined cells (e.g., epidermis), the secretion of mucus , ciliary action, production of antibiotic compounds or noxious chemicals that repel or kill parasites, a variety of amoeboid cells that engulf or surround parasites and produce toxicants to destroy them, and in addition to these in vertebrates, the lymphocytes (T, B, and null cells). The degree of vulnerability or susceptibility of an animal to penetration by a pathogen or successful establishment of a parasite resulting in disease may vary between and within species and individuals based on their genetics or may be altered as a result of changes in environmental conditions, nutritional state, developmental stage, and other factors—anything that might impair the optimal functioning of those cells or the products of those cells (Martin et al. 2010). Resistance to infection is characterized by those physiological alterations or responses that occur naturally or develop in the course of invasion by pathogens.
Pathogens cause diseases by acting on the molecules of the organism’s cells and tissues, producing microscopic or grossly visible morphological changes (structural) that indicate biochemical changes (functional). Key metabolic processes are attacked, such as aerobic respiration, cell membrane integrity, and the synthesis of proteins, nucleic acids, lipids, and carbohydrates necessary for the organism. A pathologic change in tissue—not normal in structure or function—either external or internal, detected grossly or microscopically, is known as a lesion, a sign of disease . The mechanisms by which changing environmental conditions, toxicants, toxins, or microorganisms cause disease are varied (see any general pathology textbook) and will also differ with the species and individual affected. Many interactions between pathogens and their hosts occur without clinical signs of disease, until there is a change in host-parasite ecology (Daszak et al. 2001). Pathogens may cause reversible injuries in organisms, with recovery achieved by metabolic responses limiting damage and restoring normal function (homeostasis). Other associations induce changes in host behavior that may enhance transmission of the parasite. The butterflyfish Chaetodon multicinctus preferentially feeds on the “pink pimples” of calcified, pigmented lesions induced in the polyps of Porites spp. by the encysted metacercarial stage of the trematode Podocotyloides stenometra. The polyps cannot retract into the altered skeleton so the fish consume more coral tissue than when feeding on normal polyps; however, few infections of the juvenile trematodes developed in the fish and measurable effects on host condition and liver energy reserves were not detected, despite the large numbers of metacercarial cysts ingested (Aeby 2002). In other cases they may damage the host’s reproductive capabilities (parasitic castration) or seriously affect the functioning of vital organs. Infectious host-specific diseases caused by microbial pathogens may weaken or disable individuals so they are more susceptible to predation or stressful environmental conditions.
However, diseases may also occur as epizootics (similar to epidemics in humans), causing disease and mortalities in large numbers of organisms of a single species, due to introducing a new pathogen into a susceptible population, increasing numbers or virulence of pathogenic microorganisms, or lowering the resistance of the host population. Susceptibility and the relative resistance of the host to a biotic pathogen can also change with the size of the population and the genetic constitution of the microorganisms present, but little is known about the regulation of symbiont populations by the host. Furthermore, as parasites and other pathogens influence the abundance of host populations, they exert strong selective pressures on the genetically-based variability of an individual host’s resistance or its ability to recover from infection within the population. Thus, the nature of the association may be altered over time (Tompkins et al. 2011). In order to adapt, the host will expend energy for survival, growth, and reproduction. However, as the number of stressors and/or their level of intensity increases, energy expenditures will increase but growth and reproduction will slow or cease. The ability of the organism to deal with stress decreases or disappears as the result of exhaustion of critical biochemical and physiological functions, until finally, disease appears. Death of the organism will result if vital functions are destroyed, i.e., the condition is irreversible.
Although the causal agent of a disease in a tropical marine organism may appear to be either biotic or abiotic , both types of diseases are often closely interrelated. For example, some cases of coral bleaching are caused by certain species of bacteria only when water temperatures are elevated (Kushmaro et al. 2001). Therefore, determining the primary cause of a disease may be difficult. In some cases, a pathogenic microorganism that has infected a host may not harm its host unless the host is stressed by some other biotic or abiotic disease factor (a “stress-provoked latent infection”). Conversely, an abiotic disease can become complicated by secondary infections from normally harmless microorganisms. Lesser et al. (2007) proposed that corals were primarily adversely affected by “opportunistic bacterial infections secondary to exposure to physiological stress that resulted in reduced host resistance and unchecked growth of bacteria normally benign and non-pathogenic.” In the tropics, opportunistic pathogen s may replicate rapidly and reach the peak of their growth curve in only a few hours. Some studies have identified specific primary pathogens, particularly when biotic diseases develop in specific hosts, such as with viruses. However, the interactions of physiological environmental stressors with host homeostatic mechanisms and immunity complicate the interpretation of causal agents and more data are needed, particularly experiments to demonstrate pathogenesis of agents on hosts, rather than just identifications of associations (Work et al. 2008b). Koch’s Postulates or other strength-of-evidence analyses (the application of causal considerations) can be used to help identify the roles of biotic and abiotic stressors in diseases (Susser 1991; USEPA 2000).
Multiple disciplines and tools are needed to identify the pathogen (s) causing a health impairment. Detecting disease in organisms who can’t communicate with us requires careful surveillance to evaluate appearance and behavior to understand when they are ADR (“ain’t doin’ right”), and assess whether changes may be within the normal limits of an organism’s life cycle (related to hormonal, seasonal, reproductive, aging, or nutritional factors). Examination of ecological factors is important, what are the abiotic stressors to which the organism(s) has(have) been exposed, key features of the reef where disease is being found, possible microhabitat distinctions, any fluctuations in conditions? Although motile organisms may be able to avoid or limit their contact with pathogens, toxic agents, or adverse physicochemical conditions, sedentary invertebrates generally cannot, but they may produce planktonic larvae to escape. Thus, determining where the organism might have been (e.g., in a crevice, seagrass bed, mangrove forest, marina) is also valuable. After forming a preliminary diagnosis, collection of appropriate samples, both from the affected organism (whole or tissues) and its environment (water, sediment , food, adjacent organisms) and laboratory analyses (behavior, biochemistry, histology, microbiology, immune system responses) are performed to confirm that diagnosis. Timing of sampling to detect the causal agent is critical, because the primary pathogen’s presence and effects on the host can be subsequently missed as other bacteria and fungi , for example, multiply and degrade already damaged tissue. After evaluating these results, additional, modified examinations or tests may be necessary, until a final diagnosis can be made (Woebeser 2007). Histology, the study of microscopic anatomical structure from tissue samples using light or electron microscopy, can provide a wealth of information about the functioning of an organism and should be applied in every disease study. Pathological cell and tissue changes have been recognized in several categories (degeneration: necrosis or bleaching ; growth: atrophy, hypertrophy, proliferation; inflammation: defense, repair, infectious agents, parasites). But morphologic changes may not completely identify an etiologic agent, requiring the application of methods from microbiology, molecular biology, analytical chemistry, biochemistry, or physiology, to succeed (e.g., Work et al. 2008b; Pollock et al. 2011; Work and Meteyer 2014).
In summary, diseases occur as the result of interactions between a susceptible host, a virulent pathogen , and prevailing environmental conditions. Diseases caused by infectious microorganisms, parasites, and non-infectious (nutritional, environmental, or genetic) disorders have been reported from most phyla of marine plants and animals. However, most of our information on diseases of marine organisms has come from studies of commercially important temperate fish and shellfish species. These studies have received extensive funding and were conducted by multidisciplinary pathobiology teams. For tropical species, many reports in the literature are descriptions of “parasites” where the true nature of the organism’s association with the host has not been experimentally determined. There are also a number of reports where the etiologic agent of mass mortalities has not been identified because the disease was not recognized until most of the population was affected and there were few survivors available for study (e.g., the Diadema antillarum mass mortalities of 1983).
Among the arguments for using the term “health” in relation to an ecosystem, Rapport et al. (1985) and Rapport (1999) noted that a “healthy ecosystem” would possess the features of relatively rapid recovery when stressed; disturbances or stressors that are present support the maintenance of the ecosystem; maximum biodiversity of native species, productivity , and size of dominant species; sustainable reproduction rates of the native species; minimal pathology among the species; and genetic diversity . Such ecosystems might exist in the time frames of minutes to millennia. What we do know is that ecosystems can change from one type to another and during the period of change we will see alterations in processes and functions at different levels of biological organization as the “optimum envelope” of levels of biotic and abiotic factors shift; diseases may occur. Humans have been responsible for ecosystem degradation that directly and indirectly affects the health of organisms—including humans, particularly with zoonotic disease s . The presence of pathology (diseases) in multiple organisms in an ecosystem indicates it is unstable. What we don’t know is the direction of the change, for better or worse.
8.3 Survey of Reef Organism Diseases
This chapter presents some of the most noteworthy diseases of coral-reef organisms that have been studied during the last four decades, but it is not an extensive review, since research, particularly on coral diseases, has increased greatly. As noted in Fig. 8.2, diseases in reef-associated flora and fauna have increased during this time. Anecdotal reports and empirical evidence from long-term surveys on reefs around the world (e.g., Williams and Bunkley-Williams 2000; Santavy et al. 2001; Porter et al. 2002; Lang 2003; Bruno et al. 2007) indicate that this increase is real and that more organisms are being affected by each disease . We remain uncertain, however, about how long some diseases might have been present on reefs but not observed. For example, awareness of sea turtle fibropapillomatosis increased in the mid-1980s as scientists and veterinarians found many affected turtles and began conducting focused studies to determine the prevalence and cause of the disease, especially in the Hawaiian Islands and Caribbean Sea. However, researchers discovered a publication describing this disease in a green turtle in the New York Aquarium as well as three out of 200 green turtles examined at the turtle fishery docks in Key West, Florida, in the late 1930s (Davidson 2001). Another potential factor is the increase in the number of scientists and veterinarians studying these diseases. The reader should consult recent publications and Web sites to further understand the nature and etiology of diseases in tropical marine organisms in the Anthropocene.
8.3.1 Reef Plants
Little is known about biotic and abiotic diseases of marine algae and seagrasses in tropical waters. The turtle grass, Thalassia testudinum, in the environmentally stressed Florida Bay, has been affected by a marine slime mold, genus Labyrinthula since the 1980s (seagrass wasting disease ). This pathogen caused blackened, necrotic lesions on the seagrass blades, reduced the photosynthetic production of oxygen in the plant (Durako and Kuss 1994), and resulted in massive die-offs of this important species (Robblee et al. 1991; Thayer et al. 1994); however, low prevalences of the infections occur and multiple environmental factors, but not predation, may be controlling the disease (Bowles and Bell 2004).
Littler and Littler (1994, 1995) reported the appearance, first in 1992, of coralline lethal orange disease (CLOD), affecting encrusting coralline algae on reefs in the Cook Islands, Fiji, Solomon Islands, and Papua New Guinea. The pathogen was bright orange and spread across the algal surface, leaving behind the bleached carbonate skeletal remains of the coralline algae. When the pathogen reached the margin of the algal thallus, it formed upright filaments and globules, similar to those formed by terrestrial slime molds. Microscopic examination revealed motile gliding rods of a colonial bacterium in a mucilaginous matrix. Experimental studies confirmed that the pathogen globules were highly infectious to a variety of coralline algal species. Littler and Littler (1998) discovered a black fungus covering living thallus tissue of encrusting coralline algae on reefs of American Samoa, coralline fungal disease (CFD). In the Caribbean as well as the Indo-Pacific, crustose coralline algae have been observed to lose the pink thallus tissue, with a band of bare carbonate a few millimeters wide remaining next to the thallus; this tissue loss has been named coralline lethal disease, CLD (Goreau et al. 1998). Diaz-Pulido (2000) discovered loss of tissue from three species of the crustose red alga Peyssonelia on reefs of the Caribbean and Great Barrier Reef, Peyssonnelia yellow-band syndrome (PYBS). A distinct yellow microbial mat composed of gliding filaments of a procaryotic microorganism moved across the surface of the algal thallus, followed by a white mat of the gliding bacterium Beggiatoa sp., destroying the tissue. Weil (2004) reported coralline white band syndrome (CWBS=CLD) causing tissue loss in three species of coralline algae in the Caribbean. An investigation of the condition of crustose coralline algae on reefs of U.S.-affiliated Pacific islands by Vargas-Ángel (2010) revealed five categories of grossly visual health impairments in coralline algae: CLOD, CWBS, CFD, coralline target phenomena (CTP), and coralline cyanophyte disease (CCD=PYBS?). Usually found in low prevalences or not detected at most sites, higher prevalences were seen on some reefs with higher human population densities and development . These diseases are also reported from the Caribbean, but little is known about the pathogens that seem to be causing them, although Williams et al. (2014) has identified the CFD etiologic agent as an unculturable fungus in the subphylum Ustilaginomycetes (phylum Basidiomycota).
These diseases can affect the structure and function of many reef sites, since the dead corallines no longer contribute to productivity and carbonate accretion and cementation processes. More importantly, the coralline algae no longer secrete chemicals that attract coral larvae to settle, and fleshy algae overgrow the dead coralline algae, further inhibiting the settlement and growth of reef-building corals.
8.3.2 Reef Invertebrates
Some of the more prominent members of the coral-reef community and associated tropical marine habitats are the sponges, scleractinian or stony corals, soft corals (alcyonaceans), sea fans and sea whips (gorgonaceans), polychaete worms, a wide variety of bivalve and gastropod molluscs, octopus and squid, spiny lobsters and crabs, sea urchins , sea stars, sea cucumbers, crinoids, and brittlestars. Many species are cryptic, living within spaces of the reef framework, burrowing into the calcium carbonate substrate, or present on or even within other organisms in commensal or mutualistic symbiotic relationships. While we understand much about their ecological roles (see excellent review by Glynn and Enochs 2011), studies of the nature and effects of diseases on these organisms are relatively recent, with reports few or unknown for some phyla (e.g., Annelida), and far from completion.
8.3.2.1 Sponges
While scleractinian corals are usually the most noticeable members of the reef community, at least in size if not numbers, species in the phylum Porifera are numerous and also important mediators of reef productivity (Wulff 2006a). Observations on diseases affecting these organisms had been limited until recently. Primarily the commercial species of the genera Spongia and Hippospongia were affected by widespread mortalities in the Caribbean in the late 1930s. The timing and distribution of these mortalities followed the major current patterns. Commercial sponge fisheries were effectively eliminated, although some sponges did recover. Affected sponges exhibited “bald patches” followed by “rotting” of tissue beneath the patches, with the entire sponge degenerating within 1 week. The lesions always contained long slender aseptate (without interior walls) filaments that were believed to be a fungus. Studies suggested that bacteria and changes in water temperature might be responsible, but these observations were never confirmed (Peters 1993).
Healthy sponges contain a variety of mutualistic symbiotic bacteria (Webster and Taylor 2012) that may provide nutrition for their sponge hosts or that use metabolic wastes produced by the host. Thus, investigations of the causal agent(s) of diseases in sponges may be complicated by the presence of these microorganisms or by secondary invasions from seawater populations of microorganisms. Sponges possess a variety of cellular defense mechanisms and many sponges can also produce antimicrobial compounds to control pathogenic microorganisms; however, the relationships of sponge-dwelling bacteria and other micro- and macro-organisms with host metabolism and health are poorly understood. Rützler (1988) was the first to report a disease in the mangrove demosponge Geodia papyracea from Belize. Apparently, the normal cyanobacterial symbionts of this sponge multiplied out of control, resulting in the destruction of the host sponge tissue. Aplysina red band syndrome (ARBS) is another cyanobacterial disease of the Caribbean sponge A. cauliformis (Gochfeld et al. 2012), in which a filamentous cyanobacterium (identity still unknown) forms a band around the sponge and spreads along it, killing the live sponge tissue.
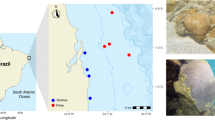
Of more concern has been a sponge disease that has affected many Xestospongia muta, the giant Caribbean barrel sponges (Fig. 8.3). Anecdotal reports of paling pigmentation, complete loss of pigmentation and crumbling of the lower portions of these normally rock-hard sponges began to appear on the Coral Health and Monitoring Program Coral-List listserver in the late 1990s. Nagelkerken (2000) described the death of the giant barrel sponge off Curaçao, which had been a tourist attraction for years because its diameter of almost 2.5 m could surround a diver. The same disease signs appeared on this sponge and complete mortality occurred within a few weeks. He noted that the frequent visits and touching by divers might have contributed to spreading a pathogenic microorganism or damaging the sponge and leaving it more susceptible to disease. Paling of the entire sponge and patchy bleaching has also been reported, even when corals were not bleaching. Sampling and microbial community analyses of this sponge orange band (SOB) disease and apparently healthy X. muta in the upper Florida Keys and Bahamas by Angermeier et al. (2011) revealed a shift from the stable cyanobacterial consortium to a more heterogeneous mix of cyanobacteria, but experimental infection with diseased sample plugs was unsuccessful. They concluded that a specific pathogen was not responsible for the disease.
Bacterial pathogens (Pseudomonas and Bacillus spp.) were isolated from Ianthella basta, in Kimbe Bay, west New Britain, Papua New Guinea. These sponges were observed dying between 1996 and 2000 at only three sites within 16–20 km of shore and these bacteria were not isolated from healthy sponges, moreover, when inoculated into healthy sponges the same disease signs appeared. Cervino et al. (2006) noted that the closest relatives of these bacteria included terrestrial pathogens used against insects and fungi , and proposed that applications of similar bacteria to agricultural land onshore may have contributed virulence factors to potentially pathogenic marine bacteria strains at these sites near river mouths off palm oil plantations. However, Luter et al. (2010) could not find any shifts in the microbial communities of apparently healthy sponges and diseased I. basta collected from sites in the central Torres Strait, but could not completely rule out an infectious agent that might be in low abundance or the high virulence of an existing microbial symbiont.
Bleaching has also occurred in tropical marine sponges that contain photosynthesizing symbionts in their tissues, particularly in the Caribbean during the recent coral bleaching events (Chap. 5). Other sponge diseases have been reported from Indo-Pacific reefs, as well as the Caribbean, with partial or complete loss of pigmentation and tissue breakdown (Harvell et al. 1999; Wulff 2006b; Webster 2007; Angermeier et al. 2011; Webster and Taylor 2012). Study of these lesions is urgently needed to determine what is happening and why.
8.3.2.2 Corals
The tropical zooxanthellate reef-building scleractinian corals not only form the topography of a habitat, but also contribute to its productivity , supporting diverse organisms and protecting land masses as well. Since diseases of corals were first recognized in the 1970s, research has increased greatly as new diseases have been recognized and the number of corals afflicted or killed has also increased. Most of the literature is concerned with scleractinian (hard coral) diseases, but diseases of octocorals (soft or horny corals), particularly the Caribbean sea fan’s mass mortalities since the mid-1990s caused by the fungus Aspergillus sydowii, have also been studied (e.g., Slattery et al. 2013; Nagelkerken et al. 1997; Kim et al. 2000; Toledo-Hernández et al. 2008). It is now impossible to review all of the developments in this area within the space limitations of this chapter. In addition, new discoveries are being made at a rapid pace. This section will present the current status of a few of these diseases. The reader is directed to books (e.g., Porter 2001; Rosenberg and Loya 2004; Raymundo et al. 2008; Woodley et al. 2015), special issues of journals (e.g., “Diseases of Aquatic Organisms” Volume 69), reviews (e.g., Richardson 1998; Green and Bruckner 2000; Richardson and Aronson 2000; Weil et al. 2000; Williams and Bunkley-Williams 2000; Bruckner 2002; Sutherland et al. 2004; Weil and Rogers 2011), and Web sites, for example, the Global Coral Disease Database (United Nations Environment Program-World Conservation Monitoring Centre: www.coraldisease.org) and the National Oceanic and Atmospheric Administration (NOAA)’s Coral Reef Information System (www.coris.noaa.gov). The literature cited within those sources will also provide a more complete and current understanding of coral diseases.
Contributing to the interest in and research on coral diseases was the organization of the Coral Disease and Health Consortium (CDHC) in 2001, under the direction of Dr. Cheryl Woodley as an activity of the U.S. Coral Reef Task Force, with a workshop held in Charleston, South Carolina, for reef scientists and managers, coral biologists, ecologists, statisticians, toxicologists, biochemists, information technologists, invertebrate pathologists, veterinarians, and medical doctors, to improve the communication and understanding of diseases by interactions among all these disciplines. Committees were formed, plans and procedures were developed (http://www.cdhc.noaa.gov/). Coral Disease and Health: A National Research Plan (Woodley et al. 2003) is available online (http://cdhc.noaa.gov/_docs/FInal%20CDHC%20plan%2011_07%20correc.pdf) and several more workshops and training sessions have been held to coordinate and inform additional field and laboratory research efforts. The Coral Reef Targeted Research & Capacity Building for Management (CRTR) Program, a partnership between the Global Environmental Facility, the World Bank, and NOAA, organized a Coral Disease Working Group and provided funding to support research by teams of scientists on coral diseases in several localities from 2004 to 2009 (http://www.gefcoral.org/). One of the outcomes of these efforts has been the realization that gross signs of disease in a coral observed in the field fall into one (or more) of these four categories: microbial mat-associated tissue loss, tissue loss, tissue discoloration, or growth anomaly (abnormal skeletal deposition pattern or rate of deposition that may include changes in the number of polyps). Any of these may include abnormal polyp behavior. Tissue loss is not required for a coral to be functionally impaired.
Black-band disease (BBD) was the first microbial mat disease of corals observed on reefs off Belize and Bermuda, but has since been found throughout the Caribbean as well as the Indo-Pacific and Red Sea (Rützler et al. 1983; Antonius 1985; Ravindran et al. 1999; Dinsdale 2000; Barneah et al. 2007). BBD has also been reported on milleporinids (fire corals) and gorgonaceans. Not all coral species appear to be susceptible to this disease. Massive brain corals (Pseudodiploria formerly Diploria, Colpophyllia, Platygyra, Goniastrea) and star corals ( Orbicella formerly Montastraea spp.) are the most commonly affected members of the families Mussidae and Merulinidae (formerly Faviidae) (Budd et al. 2012), while elkhorn, staghorn, and pillar corals in the Caribbean resist natural infections. Acroporid, poritid, and pocilloporid species are also affected on the Great Barrier Reef (Page and Willis 2006).
Figure 8.4 shows the characteristic appearance of this disease . BBD results from the invasion of coral tissue by a microbial consortium. This consortium is a black mat a few millimeters to centimeters wide, composed of fine cyanobacterial filaments pigmented by phycoerythrin that also contains sulfate-reducing bacteria, sulfide-oxidizing bacteria, other bacteria, and sometimes fungi and protozoans. These microorganisms produce anoxia deep in the band next to the tissue and hydrogen sulfide, as well as microcystins (Richardson et al. 2007; Stanić et al. 2011), which kill the coral tissue and allows the microorganisms to use the organic compounds released by the dying coral cells for their own growth and reproduction (Richardson et al. 1997). The band or mat moves across the surface of the coral at the rate of a few millimeters per day, leaving behind bare coral skeleton that is eventually colonized by filamentous algae. The cyanobacterium was identified as Phormidium corallyticum, but recent molecular studies indicate that more than one species of cyanobacterium might be involved among affected colonies within the Caribbean Sea and between the Caribbean and Indo-Pacific Seas (Frias-Lopez et al. 2004) and the Red Sea (Barneah et al. 2007). Different species of cyanobacteria, with greater amounts of red pigmentation, may form the microbial mats in red-band disease of scleractinian corals and sea fans; a yellow-band disease reported from the Arabian Gulf (Korrubel and Riegl 1998) may have been BBD in sulfur-saturated seawater.
Black-band disease destroying living coral tissue on a colony of star coral, Orbicella faveolata, at Looe Key, Florida Keys National Marine Sanctuary, during the mid-1980s outbreak; the diver on the left is using a suction device to remove the microbial mat from the coral to reduce tissue loss; spreading underwater epoxy along damaged tissue margins and shading affected colonies are more recent treatments (Photo courtesy of H.H. Hudson)
Healthy corals can become infected with BBD when in contact with an infected colony, but injured colonies are most susceptible. Aeby and Santavy (2006) found that a coral-feeding butterflyfish could spread the disease during predation or even when the coral was protected from predation in aquarium studies. Most studies have found that less than 2 % of Caribbean and Great Barrier Reef corals are infected with BBD on any given reef area, although there have been outbreaks at several locations, including Looe Key reef in Florida in the mid-1980s, when most corals had lesions and pieces of microbial mats were dispersed through the water. In addition, other stressors, such as nutrients, light levels, higher water temperatures, and bleaching , may increase the susceptibility of corals to infection with the microbial mats (Kuta and Richardson 2002). A BBD epizootic affecting 10 % of Montipora spp. colonies with mean percent tissue loss of 40 % at Pelorus Island, Great Barrier Reef, was followed beginning in summer 2006 for 2.7 years. It was linked to seasonal increases in water temperatures and light levels; previous Australian surveys had reported background levels <1 % of susceptible coral populations (Sato et al. 2009).
About the same time that BBD was reported, acroporid (elkhorn and staghorn) corals off St. Croix, U.S. Virgin Islands, exhibited tissue sloughing, which started at the base of the branches and moved toward the branch tip at the rate of a few millimeters per day. In contrast to BBD, however, no consistent assemblage of microorganisms could be found at the junction separating the sloughing brown-pigmented tissue from bare coral skeleton. This disease was termed white-band disease (WBD), because the sloughing left a broad band of bare skeleton up to several centimeters wide on the colony that was eventually colonized by filamentous algae (Fig. 8.5). These disease signs can be distinguished from predator damage (e.g., fish, gastropod, or worm feeding scars) and have since been observed on acroporid species throughout the Caribbean, the Red Sea, and off the Philippines. Acute tissue loss on many species from the bases or in patches has also been observed on reefs around the world, and variably named WBD or white plague (WP); irregularly shaped patchy tissue loss from elkhorn coral is termed white pox, patchy necrosis, or white patch disease (WPD); acute tissue loss from tabular acroporids in the Indo-Pacific in a wedge-shaped or central pattern is called white syndrome (WS); and other variations in tissue loss based on pattern, species affected, and rate of tissue loss (from 0.5 to 10 cm per day linear), resulting in partial to complete mortality , have been recognized: shut-down reaction, WBD type II, white plague types II and III, rapid tissue loss, stress-related necrosis (Dustan 1977; Patterson et al. 2002; Bythell et al. 2004; Williams and Miller 2005; Ainsworth et al. 2007b). Inconsistent application of the names and descriptive terminology has led to confusion in the literature (Rogers 2010), and research on etiologies has not consistently linked particular signs with pathogens.
Elkhorn coral, Acropora palmata, afflicted with the characteristic basal tissue sloughing of white-band disease at Grecian Rocks, Florida Keys National Marine Sanctuary (Reproduced from Couch and Fournie 1993)
Unusual aggregates of Gram-negative rod-shaped bacteria were found scattered in the calicoblastic (skeleton-producing) epidermis that lined the gastrovascular canals of the porous skeleton in WBD-affected acroporids from St. Croix and Bonaire, Netherlands Antilles (Peters et al. 1983). The bacterial aggregates were also found in apparently healthy colonies at St. Croix. Five years later, up to 95 % of the elkhorn corals there had died. The role of this microorganism in the development of disease has not been determined; bacterial cultures or other isolation procedures were not conducted. Bythell et al. (2002) did not find them in their samples, and additional histopathological examinations noted that they occurred in apparently healthy samples and were not always found in diseased samples. Polson (2007) identified Gram-negative Pseudomonas spp. bacteria in aggregates from some—but, again, not all—acroporid samples taken during a tissue loss outbreak in the Florida Keys in summer 2003. Ritchie and Smith (1998) discovered Vibrio carchariae associated with the bleaching margin found on diseased staghorn coral with WBD type II, which was tested experimentally in the field using cultures of the Gram-negative V. (carchariae) harveyi by Gil-Agudelo et al. (2006), almost all of Koch’s postulates were satisfied. Both of these papers also noted that WBD type II could turn into WBD type I and vice versa, lacking the bleaching tissue along the tissue loss margin at times. Another Gram-negative bacterium was identified as the cause of WPD in field experiments by Patterson et al. 2002, Serratia marscesans. Extensive work revealed that isolates from infected Acropora palmata were identical to a strain found in human wastewater and caused the same tissue loss in laboratory challenge experiments (Sutherland et al. 2011). In WP, a new genus and species of Gram-negative rod-shaped bacterium was identified as the causal agent (Richardson et al. 1998; Denner et al. 2003). Bythell et al. (2002), however, found a coccoid bacterium associated with patchy loss of tissue on Montastraea (now Orbicella ) annularis.
Other reports have not found the same microbial communities in WBD- and WPD-affected corals of the same species (Kline and Vollmer 2011; Sweet and Bythell 2012; Lesser and Jarrett 2014), but some comparisons of diseased and apparently healthy colonies of the same species’ microbiomes indicate differences that may lead to discovery of the pathogen (Cook et al. 2013; Roder et al. 2013). The causal agent of acroporid white syndrome has been much debated, with some scientists finding tissue loss only due to apoptosis (Ainsworth et al. 2007b) and others identifying vibrio bacteria in the affected corals (Sussman et al. 2008) or ciliates (Work and Aeby 2011; Sweet and Bythell 2012). Cases of acute tissue loss on Red Sea corals that appeared to have the WP disease signs had cyanobacterial mat involvement deep in corallites (Ainsworth et al. 2007a). Work and Aeby (2011) and Work et al. (2012) documented several different types of potential causal agents and host responses to them in Acropora and Montipora white syndromes using histopathological examinations, including ciliates, helminthes, and fungi , along with fragmentation and necrosis; Ushijima et al. (2012) discovered that Vibrio owensi initiated the tissue loss lesions in the latter disease. Casas et al. (2004) sampled apparently healthy and WBD-affected staghorn corals on Panama reefs and identified, using molecular techniques only, a Gram-negative Rickettsiales-like bacterium (90 % similarity to uncultured Rickettsiales with BLASTN based on cloning and sequencing of bacterial 16S rDNAs). But since it was in all of these samples, as well as in other coral species, they concluded it was not the pathogen of WBD type I. Miller et al. (2014) reported that histopathological examinations revealed the presence of a suspect rickettsia-like microorganism infecting and killing mucocytes of staghorn corals, which might be a chronic primary infection reducing their ability to resist infections by other microorganisms or increasing their susceptibility to other secondary abiotic pathogens that explain the variable patterns and rates of tissue loss. Other scientists have detected virus-like particles in corals affected by tissue loss (e.g., Vega Thurber and Correa 2011; Soffer et al. 2013; Lawrence et al. 2014), but their role in disease is unknown. Much remains to be learned about the nature of tissue sloughing in corals, and how many conditions caused by different pathogens or environmental stresses may actually be represented by the same disease sign of rapid tissue loss.
Tissue discolorations, either a loss of color or a change in color, focally or diffusely throughout a coral colony, indicate that the symbiotic association of the dinoflagellate algae, or zooxanthellae, may be impaired or that other microorganisms may be present (Fig. 8.6). Bleaching, the loss of the algae that normally give the coral tissue a brownish coloration, and/or the loss of their photosynthetic pigments, indicates that this important food resource of the tropical corals has been reduced. Chronic partial or wide-spread loss of zooxanthellae, for whatever reason, signals a disturbance in the normal metabolism of the coral host and can lead to delayed or reduced reproduction, tissue degeneration, reduced growth, and death of the affected tissue or entire colony (Chaps. 5 and 11; Williams and Bunkley-Williams 2000; Weis 2008; Rogers and Muller 2012). Bleaching of corals, gorgonaceans, alcyonaceans, and anemones has been attributed to exposure to high light levels, increased solar ultraviolet radiation, high turbidity and sedimentation resulting in reduced light levels, temperature and salinity extremes, and other factors. The nature and extent of bleaching vary between individuals and among species at the same location during a bleaching event and have been attributed to different physiological tolerances of the strains (or species, clades) of zooxanthellae and the coral hosts (Rowan et al. 1997; Jones et al. 2008). Discoveries by scientists in Israel include new species of vibrio bacteria that enter coral cells when water temperatures exceed 25 °C and cause lysis of the algae in temperate and tropical corals (Kushmaro et al. 2001; Ben-Haim et al. 2003). A coccidian (Phylum Apicomplexa), a protozoan known to cause disease in other animals, has also been associated with patchy bleaching in corals (Upton and Peters 1986).
Bleaching of tissue on a colony of Orbicella faveolata, Panama, 1996; translucent tissue still covers the skeleton but the zooxanthellae or their pigments are no longer present to color the tissue brown; diverse patterns of bleaching from gradual systemic paling or multifocal acute to chronic discoloration may occur and be accompanied by tissue loss due to many biotic or abiotic factors or combinations of such stressors
Another discoloration condition that is widespread throughout the Caribbean and causing partial mortalities of star corals, Montastraea (now Orbicella ) spp., and other massive framework-building species is known as Caribbean yellow band disease (CYBD), with high prevalences recorded in Panama, Mona Island, the Netherlands Antilles, and the Florida Keys. Signs of this disease are focal to multifocal pale irregularly shaped patches of yellowish lightened tissue or a margin of yellowish lightened tissue a few cm wide adjacent to sediment patches on the colony (Santavy et al. 1999; Weil et al. 2000; Garzón-Ferreira et al. 2001). Similar lesions have been found on several Indo-Pacific coral species, especially Fungia. Microscopically, fewer zooxanthellae, with less pigment, reduced mitotic indices, and obvious cellular damage, were present within the lightened tissue compared to normally pigmented areas on the same colony (Cervino et al. 2001); microbiologically, several Vibrio spp. have been found in all of these lesions (Cervino et al. 2008).
Instead of showing paling of tissue, several species of corals, particularly the Caribbean Siderastrea, Stephanocoenia, and Orbicella spp., develop darker and often differently colored patches or marginal bands of tissue, which are referred to as dark spots disease or syndrome (DSD or DSS) (Sutherland et al. 2004). Affected areas may stop accreting skeleton with the result that they form depressions on the colony surface, and the dark tissue dies, causing partial mortality in all species. DSS may be a significant source of mortality in some Orbicella and Stephanocoenia colonies; however, the lesions can disappear in Siderastrea siderea colonies over time, suggesting they might be a temporary stress response (see review in Porter et al. 2011). Gross and histopathological observations indicated that in addition to zooxanthellae degeneration, endolithic organisms were present in S. siderea skeletons where the darkened tissue lesions occurred (Galloway et al. 2007; Renegar et al. 2008). Microbial communities from apparently healthy colonies and lesions have been examined using cultured and non-cultured (molecular) analyses, with varying results (reviewed in Kellogg et al. 2014). Although vibrios were associated with the lesions, they were also present in healthy tissue; cyanobacteria and an unclassified vibrio were present only in diseased tissue, but they concluded that their data did not show it to be a bacterial disease; they could not identify an isolated suspect fungal sequence. Sweet et al. (2013) had similar results in a study of Stephanocoenia DSS, but also identified a fungus similar to a plant pathogen (Rhytisma acerinum). Neither of these studies included histopathological examinations. A preliminary look at the histopathology of Orbicella DSS, indicates that suspect thraustochytrids on the colony’s epidermis (Kramarsky-Winter et al. 2006) are being killed, then the darker tissue of the coral is seen, and the surface body wall atrophies (EC Peters, unpubl observ); but molecular and other analyses still need to be done.
Corals also harbor a variety of protozoan and metazoan microorganisms, some of which may be parasites. One relationship has been examined in Hawaiian corals (Porites compressa) containing the metacercarial stage of a digenetic trematode, Podocotyloides stenometra. The host for the final stage of this parasite is a coral-feeding butterflyfish, Chaetodon multicinctus (Aeby 2002). Parasite-infected coral polyps develop into pink, swollen calcified nodules, reducing the ability of the polyps to retract into their calices. Parasite encystment resulted in reduced growth rates of parasitized corals. Fish fed preferentially on infected polyps, and as a result the altered polyp appearance provided both an enhancement of the parasite’s transmission rate and parasite removal from the coral. Healthy polyps then grew back over the feeding scars. Thus, this phenomenon may act as a host strategy of parasite defense.
Anomalous calcification patterns in scleractinian corals may be caused by parasites or commensals. Other examples of enlarged corallites or tumors in the exoskeleton have been attributed to cellular proliferative disorders, including neoplasia (reviewed in Peters et al. 1986). Whitened protuberant calcified tumors have been found on branching acroporid corals in the Caribbean and Indo-Pacific. These skeletal masses have proliferating gastrovascular canals and associated calicodermis (calicoblastic epidermis), the calicoblasts drive the deposition of the aragonite exoskeleton of the coral. As the calicoblastic epithelioma grows, porous skeleton formed by proliferation of gastrovascular canals lined by basal body wall tissue is formed more rapidly than it is in the surrounding tissue, resulting in degeneration of normal polyp structures and loss of zooxanthellae from gastrodermal cells. Mucocytes normally in the epidermis of the coral disappear from the epidermis covering the tumor as the tumor mass grows larger. Having lost the mucous secretory capabilities of the epidermis, the coral is unable to shed sediments and the tissue becomes ulcerated and invaded with filamentous algae. Branches having tumors also exhibit reduced skeletal accretion and growth. Both genetic and environmental factors appear to affect the distribution of tumor-bearing colonies (Peters et al. 1986; Coles and Seapy 1998; Yamashiro et al. 2000).
Additional cases of cellular proliferative disorders generally termed growth anomalies (GAs) have been discovered in coral species worldwide, often with bizarre development of polyps as well as skeletal morphologies (e.g., Gateño et al. 2003; Work et al. 2008a; Burns et al. 2011; Couch et al. 2014). Aeby et al. (2011a) used 937 quantitative coral disease surveys from around the tropical Pacific Ocean and evaluated the prevalence of GAs in relation to several environmental parameters. Strongest correlation of GAs in Acropora and Porites spp. was with coral colony densities and higher regional human population sizes. Suspected pathogens may include nutrient pollution , fungi (Domart-Coulon et al. 2006), or an infectious agent. Kaczmarsky and Richardson (2007) reported transmission of growth anomaly lesions at the point of contact on two apparently healthy small colonies of Porites (lutea or lobata) studied in aquaria in the Philippines, and one arising without contact that was in an aquarium with an affected coral.
Despite the wealth of data accumulating about all of the coral diseases, it is clear that many questions remain. Multidisciplinary research needs to be conducted on the same samples collected at the same time from the same locations, and this must include epizootiology (epidemiology, distribution and abiotic factors), histopathology (light and electron microscopy), microbiology, molecular biology, biochemistry, and analytical chemistry of environmental and tissue samples. Microbiological or molecular studies of suspected pathogens are not of much value unless histopathological examinations are performed at the same time to provide the necessary “phenotypic anchoring” to describe the condition (= structure) of the host’s tissue, detect the presence of microorganisms and microparasites, evaluate how well the host was functioning at the time of sample collection, and aid in understanding pathogenesis and immunity.
8.3.2.3 Molluscs
Diverse species of molluscs live on coral reefs or in adjacent seagrass beds, but few studies have been conducted on these animals, except for the commercially important giant and fluted clams (Tridacna spp.), queen conch (Strombus gigas), and pearl oysters (Pinctada spp.). Diseases of temperate molluscs are caused by viruses, bacteria, fungi , and protozoan and metazoan parasites; nutritional, developmental, and neoplastic disorders are also known. Thus, it is probable that similar diseases are present in tropical molluscs, particularly where reefs are also stressed by abiotic factors.
Giant clams from the Great Barrier Reef were found to contain large numbers of the protozoan Perkinsus sp. (Fig. 8.7). It has been suggested that this microorganism, in conjunction with cooler water temperatures, was responsible for observed mortalities affecting up to a third of the giant clams at Lizard Island from 1984 to 1987. However, the protozoan was also found at low levels of infection and not associated with mortalities in other bivalves on the reef (84 species from the families Spondylidae, Arcidae, and Chamidae, as well as the Tridacnidae were examined). These results indicate that there may be species-specific host susceptibilities among the bivalves, several different species of Perkinsus, or variations in the prevalence of pathogenic strains of Perkinsus (Goggin and Lester 1987). A temperate relative of this protozoan, Perkinsus marinus, has been responsible for extensive mortalities of oysters along the east coast of the United States. The relationship of this pathogen to changes in environmental conditions, including salinity and temperature , has been investigated, and careful monitoring and control measures have been undertaken to protect these food resources (Fisher 1988). Further histological studies of the giant clam mortalities off Lizard Island revealed the presence of an unidentified unicellular organism in some of the clams (Alder and Braley 1989), but many questions remain about the nature of this epizootic . Perkinsus olseni and an exotic species of Perkinsus have been associated with mortalities in ornamental Tridacna crocea shipped to the United States for aquaria (Sheppard and Phillips 2008; Sheppard and Dungan 2009). Studies of cultured T. gigas revealed that their newly settled veliger larvae are also susceptible to known pathogenic marine bacteria, particularly Aeromonas, Plesiomonas, Vibrio, Pseudomonas, Alteromonas, and Alcaligenes spp., with variability in species/strains for initiating mortality (Sutton and Garrick 1993).
A mass mortality of wild queen conch was reported from the Hol Chan Marine Reserve, Ambergris Cay, Belize, mid-September to mid-November 1991, but the cause was not identified (Williams and Bunkley-Williams 2000). Cárdenas et al. (2005, 2007) discovered an apicomplexan infecting the digestive gland of S. gigas at Alacranes Reef, Mexico, and San Andres Island, Colombia, incidentally during a study on their reproduction, collecting 30 specimens monthly for 1 year at each site. Heavy infections were characterized by 75–100 % of digestive tubule cryptic and secretory epithelial cells showing trophozoites, sporocysts, and gamonts containing macrogametes or microgametes and discharged sporocysts present in stomachs. Both populations were affected, the intensity of infections varied from heavy to few infected tubules with the month sampled, but no trend was evident. Although morbidity was not observed, the infections were present in conch throughout the year, and reduced fecundity was associated with the infections.
The commercial market for mother-of-pearl oysters, Pinctada margaritifera, in French Polynesia was severely damaged in 1995 by a virus that killed up to a million oysters and culture of Pinctada spp. in the Indo-Pacific has been affected by several other diseases associated with viruses, Perkinsus sp., or other protistan, bacterial, and metazoan agents (Jones 2007). Larval trematodes of the family Bucephalidae cause parasitic castration or destruction of gonadal tissue in marine bivalves; such infections occur in Pinctada spp. and in a burrowing tridacnid clam from the Great Barrier Reef (Shelley et al. 1988). Bott et al. (2005) found digenean trematode parasites from the families Bucephalidae, Gorgoderidae, and Monorchiidae (in low prevalences, overall 2.3 %) in 12 of 47 species of bivalves from Queensland, Australia, waters, including Heron Island and Lizard Island on the Great Barrier Reef some were new host records. Molluscs possess cellular and humoral defense mechanisms that help to control pathogens but investigations of these immune responses have not been performed on their counterparts in subtropical and tropical marine habitats.
8.3.2.4 Crustaceans
Most reef crustaceans are small and inconspicuous or cryptic. Examples include banded coral shrimps (Stenopus spp.), cleaner shrimps (Periclimenes spp.) associated with anemones, burrowing mantis shrimps (Gonodactylus spp., Callianassa spp.), snapping shrimps that hide in corals or sponges (Alpheus spp.), and a variety of decorator crabs, coral crabs, hermit crabs, and the arrow crab (Stenorhynchus seticornis). The spiny lobsters (Panulirus spp.) and slipper or Spanish lobsters (Scyllarides spp.) are the objects of important subsistence and commercial fisheries in tropical marine waters. However, reports of disease in most of these species are lacking.
Temperate lobsters (Homarus spp.), penaeid shrimp, and various edible crabs are known to be susceptible to a variety of pathogens and parasites, as well as abiotic diseases related to poor nutrition and water quality (Sindermann 1990; Stentiford 2011, introduction to special issue on crustacean diseases). Exposure to pollutants and other environmental stressors are known to cause damage to gills (black gill disease ) and exoskeletons (shell disease) and reduce the quality of lobster and crab fisheries. Both of these diseases involve ulcerations of tissue with necrosis and bacterial invasion. In shell disease, chitinoclastic microorganisms are responsible for eroding the shell, which may have been damaged by mechanical, chemical, or microbial action, followed by secondary infection of the underlying tissue by facultative pathogens (Shields 2011). Again, most of the research has been performed on commercial species, particularly those held in culture facilities, with a few reports of shell disease, pathogenic microorganisms, and parasites in tropical commercially fished crustaceans.
Crustaceans possess fixed and mobile phagocytic cells in the gills, the pericardial sinus, and at the bases of appendages. They produce bactericidins, agglutinins, and lysins to deal with pathogens and parasites. Bactericidins of the West Indian spiny lobster (Panulirus argus) have been examined (see review in Sindermann 1990), and found to be partially nonspecific. Bactericidin activity was enhanced against other Gram-negative bacteria following injections of formalin-killed bacteria. A major concern of the global cultured penaeid shrimp industry has been viruses, particularly white spot syndrome virus (WSSV), and the possibility that some viruses from shrimp pond culture have been released into wild populations through effluent discharges or viruses from wild crustaceans have been introduced into ponds through untreated influent. A survey using sensitive nested PCR screening by Chakraborty et al. (2002) found that multiple species of apparently healthy wild marine shrimps, crabs, and squilla harbored WSSV off the east and west coasts of India, although whether the virus causes mortalities in the wild crustaceans has not been reported.
Shields and Behringer (2004) discovered a viral disease with mean prevalence of 6–17 % of sampled juvenile P. argus from sites surveyed in western Florida Bay and along the reef tract in the Florida Keys during different seasons and years (1999–2000). Affected spiny lobsters were lethargic or suffered tremors, could not right themselves, were shunned by other lobsters, and had milky hemolymph that did not clot. Hemocytes (hyalinocytes and semigranulocytes) and connective tissue cells had emarginated condensed chromatin, hypertrophied nuclei, and faint eosinophilic Cowdry-type A inclusions. The inclusions were found to be composed of unenveloped, nonoccluded, herpes-like DNA virus (HLV-PA, PaV1) virions. Injections of raw hemolymph from infected animals into healthy lobsters resulted in morbidity and mortality within 80 days post injection; the virus has now been found in lobsters from the U.S. Virgin Islands, Mexico, and Belize and juveniles are most susceptible (Shields 2011). This disease is of great concern to the commercial fishery for spiny lobster in the Caribbean. Behringer et al. (2012) also discovered that traps baited with sick lobsters attracted fewer lobsters than those baited with apparently healthy lobsters (11 % of the latter tested positive for the virus). Shields (2011) noted that spiny lobsters should be screened for this virus and other infectious agents (bacteria, fungi , protozoans, helminthes, and even other crustaceans) to help prevent their spread as culture of this species increases.
8.3.2.5 Echinoderms
The echinoderms are represented by such diverse animals as crinoids, sea cucumbers, sea stars, sea urchins , and brittle stars, and all are found in tropical marine habitats. Their influence on the structure and function of coral reef ecosystems can be substantial, particularly in food webs and bioerosion (Birkeland 1989). While bacterial and protozoal diseases of temperate sea urchins and sea stars have received much attention from invertebrate pathobiologists, there have been few studies on the etiologies of diseases and mass mortalities in tropical echinoderms, including the most extensive epizootic ever reported for a marine invertebrate.
Two species of echinoids were observed dying in 1981 around Hawaii. Echinothrix calamaris displayed drooping spines, loss of spines, and sloughing of the tissue covering the test, beginning on the island of Hawaii, then a few months later at Molokai, Maui, Oahu, and Kauai. Similar signs were observed on Diadema paucispinum on Hawaii, but not on other sea urchin species (reviewed in Birkeland 1989). The first mortalities of the long-spined sea urchin Diadema antillarum were seen on reefs off the Caribbean coast of Panama in January 1983. Mortalities of only this species subsequently occurred at other sites around the Caribbean and Bermuda for 1 year, in a pattern that followed major water currents from west to east, with a few exceptions (Lessios 1988). Diseased urchins were initially recognized by an accumulation of sediment on their spines and sloughing of the spine epidermis, accompanied by unusual behavior, moving out from their normal hiding places in the reef into the open during daylight, where they were preyed upon by fish. Pigment in the skin covering the spine muscles, peristome, and anal cone then disappeared, and the spines broke off. The tube feet that normally hold the urchin to the sea bed weakened and could not fully retract. Finally, patches of skin and spines sloughed off and the test disintegrated (Fig. 8.8). Diseased urchins died within 4 days to 6 weeks, depending on locality, although some urchins apparently survived the disease and recovered from the broken spines and skin lesions. Overall, adult populations of this urchin were reduced by 85–100 % at sites throughout the tropical western Atlantic Ocean, with juveniles being rarely affected. Localized mortality events were reported affecting the urchins Astropyga magnifica and Eucidaris tribuloides, in the mid-1980s, in shallow waters of Puerto Rico.
Although numerous studies were conducted on the ecology of the long-spined urchin die-offs in 1983 and on later isolated mortalities of remaining populations of D. antillarum off St. Croix, Grand Cayman, and Jamaica , few samples were obtained for histological or microbiological investigations. Gram-positive anaerobic spore-forming rods of the bacterial genus Clostridium were isolated from two urchins showing similar signs of the disease that died while in a flow-through seawater aquarium in Miami in 1983. Laboratory experiments with cultures of these bacteria caused death of healthy urchins in 10 h to 6 days, depending on water temperature . Microscopic examination of fixed tissues from apparently healthy and diseased St. Croix urchins collected post-1983 revealed Gram-positive micrococci in mucoid cells of the glandular crypts of the esophagus and in connective tissue and muscle bundles of the peristome, spines, and ampullae. However, bacterial samples were not taken in this study (see Peters 1993 for review). A mass mortality of large D. antillarum that occurred in the Florida Keys in April 1991 (Forcucci 1994) provided samples during the event for bacteriological, virological, and histopathological studies, but results proved inconclusive and differed from the earlier observations; the Lower Keys population densities decreased by 88–100 % 6 months later and Middle Keys and Upper Keys reef sites had densities <0.01/m2 by the summer of 1991, the same as after the 1983 event.
A species-specific waterborne pathogen , perhaps introduced to the Caribbean from the ballast water of ships traversing the Panama Canal and discharged at the Caribbean entrance and at Barbados, was suspected to be the causal agent of the D. antillarum epizootic in 1983, since no other species were affected and no adverse changes in environmental conditions were noted at any of the sites (Lessios 1988; Jackson et al. 2014). Perhaps this pathogen is still present and affecting the recovery of the species, although some sites report increasing densities, at others larval recruits disappear and few, scattered adult urchins are found, which probably affects their gamete fertilization success (Edmunds and Carpenter 2001; Miller et al. 2003). Re-occurring outbreaks of disease in other echinoid species have been noted. Echinoderms possess cellular and humoral defense mechanisms in the coelomic fluid and associated tissues that can protect them from invasion by potential pathogens. However, they also usually contain mutualistic bacteria in the gut or other organs. Recent research on immune responses of D. antillarum, Echinometra lucunter, E. viridis, and T. ventricosus on reefs of St. Croix, USVI, revealed that long-spined urchins released lower levels of humoral immune molecules when challenged with lipopolysaccharide or several bacteria species compared with the other urchin species (Beck et al. 2009). This suggests that D. antillarum may have some molecular, gene-mediated defect in its immunity that contributed to its susceptibility to the suspect waterborne, perhaps bacterial, pathogen; however, more research is needed on its populations throughout the Caribbean to determine the significance of these data .
The coral-eating Indo-Pacific crown-of-thorns seastar Acanthaster planci has an unusual bacterium living in its body wall, mucus secretions, and pyloric caecum. When the sea star is held in aquaria, these bacteria become facultative pathogens and leave the animal vulnerable to secondary infections by Vibrio spp. and other bacteria. Mass mortalities of juvenile A. planci that occurred near Fiji over 3 years were not caused by bacteria, however, but apparently resulted from sporozoan parasites infecting the digestive tract (reviewed in Birkeland and Lucas 1990). Similarly, a wide variety of metazoans have been observed to associate with echinoderms, usually without harming their hosts. In tropical marine species, however, Emson et al. (1985) reported severe damage to brittlestars that were heavily parasitized by copepods. Williams and Wolf-Waters (1990) observed emaciation and lack of gonad development in a Caribbean basketstar in which the stomach was heavily infected by a normally ectoparasitic copepod. Additional investigations of these organisms may reveal new biotic pathogens.
8.3.3 Reef Vertebrates
Although numerous examples of viral and bacterial diseases and epizootics have been reported in temperate marine fishes, particularly commercially gathered species and those species used in aquaculture, few studies have examined their counterparts in tropical species. Besides fishes, sea turtles are globally important tropical marine vertebrates and sea snakes may also be encountered on Indo-Pacific reefs. Some information on the normal physiology, biochemistry, behavior, and diseases has accumulated from research on those tropical marine species popular in aquariums and oceanariums (Stoskopf 1993) and in aquaculture (Glazebrook and Campbell 1990a). Studies on captive fishes and turtles have confirmed their sensitivity to adverse changes in environmental conditions and the importance of appropriate water quality and proper nutrition in maintaining their health (Roberts 1989). Diseases which occur under these artificial conditions tell us little about what occurs on coral reefs and there have been few published field observations.
8.3.3.1 Fishes
Panek (2005) provided a detailed review of the literature for coral reef fish species in the tropical western Atlantic Ocean and Gulf of Mexico, but noted that much remains to be learned about distributions of parasites and pathogens in relation to their hosts, the roles of cleaner fish in limiting parasite harm, mechanisms of pathogen transmission, and environmental issues.
Injured or weakened reef species, such as damselfishes, squirrelfishes, soldierfishes, and angelfishes, may become infected with marine bacteria. Gram-negative Photobacterium spp., Vibrio alginolyticus, V. anguillarum, and Gram-positive Streptococcus spp. have been isolated from fishes with skin ulcerations, septicemias, exophthalmias or popeye, and other lesions. Thousands of demersal reef fish washed ashore on beaches of Barbados, Grenada, Saint Vincent, the Grenadines, and Tobago from July to September 1999; the bacterium Streptococcus iniae was isolated from a few moribund fish (Ferguson et al. 2000). Keirstead et al. (2013) isolated S. iniae from moribund and dead fishes found off St. Kitts and Nevis, West Indies, in January–February 2000. Species affected included snappers (Lutjanus campechanus, Ocyurus chrysurus), parrotfish (Sparisoma aurofrenatum, Scarus taeniopterus) and red hind (Epinephelus guttatus). Panek (2005) noted that mycobacteriosis of tropical marine fishes has only been reported from the Red Sea, affecting cage-reared and nearby wild rabbitfish (Siganus rivulatus). Lymphocystis disease , caused by an iridovirus, is characterized by giant cell “tumors” and has been reported from Australia, Hawaii, the Pacific coast of Panama, Indochina, the South Pacific, and the Caribbean. Other viral diseases, including viral erythrocytic necrosis (VEN) caused by another apparent iridovirus, an infectious pancreatic necrosis-like virus (IPN), and a rhabdovirus infection, have been found in captive tropical marine species such as angelfishes, wrasses, and blennies. However, little work has been done on viruses isolated from these fishes (Stoskopf 1993). Captive tropical marine fishes are also susceptible to fungal infections such as Ichthyophonus hoferi.
A wide variety of protozoan and metazoan parasites are known from examination of field-caught and captive tropical marine fishes. They include ectoparasitic flagellates (Amyloodinium and Crepidoodinium pathogens of gills and skin), trypanosomes, hemoflagellates of the genus Trypanoplasma, various genera of ciliates, sporozoans (phylum Apicomplexa), microsporidians, myxosporidians (genera Ceratomyxa, Myxidium, or Leptotheca have been found in gallbladders of marine tropical fish), turbellarians, nematodes, digenetic and monogenetic trematodes, aspidogastrids, cestodes, leeches, copepods, and isopods. The trematodes, cestodes, and nematodes require one or more intermediate hosts to complete their life cycle . Many of these parasites do not cause overt disease or mortalities among wild fish (e.g., Bunkley-Williams and Williams 1994; Aeby 2002; Work et al. 2004b) although they may be found in potentially pathogenic numbers during mass mortalities ; however, losses of captive fish have been attributed to ectoparasitic infestations, especially “coral fish disease” or “velvet disease” caused by Amyloodinium spp. The cleaner fishes and shrimps in tropical marine habitats apparently keep the levels of most pathogens and parasites quite low in wild fish populations, although this has not been demonstrated experimentally.
A few tumors (neoplasms) have been reported in tropical fishes, including hemangiosarcoma, iridophoroma, fibroma, nasal papilloma, reticulum cell sarcoma, and fibropapilloma (Panek 2005). Of particular interest are the neurofibromas, neurofibrosarcomas, and chromatophoromas of the bicolor damselfish, Pomacentrus partitus (Schmale 1991; Schmale et al. 2002). Damselfish neurofibromatosis (DNF), which is similar to the disease in humans known as von Recklinghausen neurofibromatosis, is characterized by the appearance of conspicuous hyperpigmented spots on the skin and fins that arise from multicentric peripheral nerve-sheath (Schwann cell) tumors that vary in degree of pigmentation (due to chromatophores, including melanophores). The tumors (Fig. 8.9) were highly malignant, spreading throughout the animal and eventually causing its death as the result of destruction of vital organs or as secondary infections develop. Affected bicolor damselfish have been found only on reefs off South Florida at prevalences of up to 23.8 %. Disease rates remained relatively stable for each reef site examined over a 9-year period. The distribution of tumorous fish and the results of laboratory transmission experiments suggested that an infectious agent, such as a virus, is involved. The virus could be transmitted during the frequent aggressive interactions that occur among neighboring individuals defending their territories. After much work, Schmale et al. (2002) reported identifying an unusual virus characterized by extrachromosomal DNAs (eDNA)—damselfish virus-like agent (DVLA)—from cell lines derived from tumors. Infected cells produced tumors when injected into healthy fish; a similar pattern of eDNA was found in those tumors as well as tumors from spontaneously diseased fish, thus, DVLA is probably the etiologic agent of this disease. Damselfish neurofibromatosis is being developed as an animal model to study neurofibromatosis and possible treatments for humans. Other species of damselfish and other tropical reef species such as snappers in the Florida Keys have also been found to be afflicted with neurofibromas or neurofibrosarcomas (Panek 2005) and chromatophoromas were found in two species of Hawaiian butterflyfish (Okihiro 1988).
The limited observations of disease in fishes in the wild may be the result of predation. Any condition that weakens a fish or changes its normal behavior, or causes its death, would make it susceptible prey. Isolated cases of diseased fish may thus be overlooked. However, there have been mass mortalities of tropical marine fish, with records from the tropical western Atlantic Ocean in 1946; some of these may have been due to exposure to red tide toxins (Landsberg 1995). Mass mortalities of mullet in the mid-1970s near Miami, Florida, were believed to result from infection of their brains with a newly described bacterium that caused twirling and other signs of neurologic disease (Udey et al. 1977). A Caribbean-wide massive fish kill occurred in August and September of 1980, following Hurricane Allen. Tons of dead and dying fishes washed onto beaches. Wild and captive fish exhibited odd behavior suggesting that fishes surviving the mortalities were “sick” for several months. The cause was never identified. Millions of herrings (Harengula spp.) died at eight locations around the Caribbean during the 1980s (Williams and Buckley-Williams 1990). These mass mortalities were disjunct over time and geographic location, and examinations of moribund fish did not reveal any bacterial infections or other conditions that might have been responsible. Landsberg (1995) reported results of histopathologic examinations of several affected herbivorous reef angelfish, identifying a ciliate parasite , Brooklynella hostilis, as well as bacteria and other microorganisms, as possible etiologic agents of this slime blotch disease first noticed in 1993 along the Florida reef tract. She speculated that biotoxins from dinoflagellates in angelfish food might have contributed to immunosuppression and infections, leading to the mortalities. Williams and Bunkley-Williams (2000) noted that further work linked the ciliate to the disease and it might have caused other moralities of reef fish in the Caribbean.
8.3.3.2 Sea Snakes
Little is known about diseases of the poisonous sea snakes. External parasites include ticks on the skin of laticaudids and a turbellarian on Pelamis sp. from the Gulf of Panama. Foraminiferans, hydrozoans, serpulid polychaetes, bivalve molluscs, bryozoans, and barnacles have been recorded as fouling organisms on sea snakes. Most external symbionts are probably dislodged, however, as the result of frequent shedding of the skin and knotting behavior in these organisms (Zann et al. 1975). Endoparasites include chigger mites in the lungs of the semiterrestrial Laticauda spp., nematodes, and trematodes (Heatwole 1987; see also Culotta and Pickwell 1993).
8.3.3.3 Sea Turtles
The green (Chelonia mydas), loggerhead (Caretta caretta), and hawksbill (Eretmochelys inbricata) turtles are frequently found on coral reefs and associated habitats, where they feed on seagrasses, macroinvertebrates, and sponges, respectively. Most observations of diseases in sea turtles have also been conducted on oceanarium- and aquaculture-reared animals, with reports consisting primarily of systemic bacterial diseases, metazoan parasites, nutritional disorders, and skin tumors. In one survey of 22 wild turtles obtained from the Torres Strait off Townsville, Queensland, Australia, Glazebrook and Campbell (1990b) found a great number and diversity of parasites. Two species of flukes (Class Digenea: Order Spirorchiidae) were found in the heart and major associated arteries and their eggs were found in other organs and tissues. The presence of the flukes was associated with clinical signs of disease , and muscle wasting, bronchopneumonia, and septicemia-toxemia were present in some of the afflicted turtles. Cardiovascular flukes have also been found in wild turtles in the United States, India, Puerto Rico, and elsewhere in Australia. Seven species of flukes were found in the gastrointestinal tracts of nine wild turtles, but there were no signs of pathological changes. The green sea turtle leech can extensively damage the tissues of its host, but Bunkley-Williams et al. (2008) identified it as infecting a posthatchling juvenile hawksbill sea turtle, and also described sea turtle leech erosion disease caused by superinfection of the loggerhead sea turtle leech in a hawksbill sea turtle.
The most studied disease of sea turtles affects wild green, hawksbill, loggerhead, and the other species of sea turtles from the tropical Atlantic and Pacific Oceans (Balazs and Pooley 1991; Williams et al. 1994). Sea turtle fibropapillomatosis (Fig. 8.10) appears as irregular lobulated tumors (neoplasms), up to 30 cm or more in diameter, on the skin, scales, scutes, eyes, and surrounding tissues, and fibromas also develop internally (Flint et al. 2010). The tumors may interfere with vision, breathing, feeding, and swimming. In the 1980s and 1990s, the condition seemed to be afflicting increasing numbers of turtles and spread geographically. The eggs of parasitic trematode worms occur often, but not always, in dermal capillaries within the tumors. A herpesvirus has been identified in the tumor tissue; however, culture of these virus isolates has not been successful (Lackovich et al. 1999; Lu et al. 2000a). One study was able to cause the tumors by inoculation of cell-free filtrates of tumor homogenates, but their protected status made further experimental evaluation of their etiology difficult. A biotoxin, okadaic acid, produced by dinoflagellates that could be present on turtle grass might also promote the development of these tumors (Landsberg et al. 1999; Davidson 2001). Kang et al. (2008) used in-situ hybridization on tissue sections from fibropapillomas and fibromas of Puerto Rican green turtles to show that the fibropapilloma-associated turtle herpesvirus (FPTHV) was present only in the nuclei of epithelial cells of the tumors, not the underlying dermis fibroblasts. Although the ability of the tumors to cause debilitation in the turtles varies, immunosuppression, with development of systemic bacterial infections consisting primarily of vibrios, probably leads to turtle morbidity and mortality (Work et al. 2004a). The geographic distribution of affected turtles suggests that a combination of abiotic and biotic factors are necessary for the development of this disease.
Other novel viruses have been detected in diseased sea turtles, including herpesviruses (Stacy et al. 2008), papillomaviruses (Manire et al. 2008), and a tornovirus (Ng et al. 2009). The application of new molecular techniques to obtain and sequence DNA or RNA from a variety of cells may lead to the discovery of other pathogens in the sea turtles.
8.4 Ecological Implications
Early investigations of the structure and function of coral reefs and associated soft-bottom habitats (mangrove and seagrass communities) generally failed to consider the role of pathogens and parasites in population and community development and alterations. Until the 1970s, diseases of tropical marine organisms were virtually unknown. However, whether caused by abiotic stressors or parasites, they are normal in nature. In a review on this topic, Hudson et al. (2006) noted that research has shown parasites and biotic pathogens comprise perhaps half of the biomass of ecosystems, shape community structure and promote ecosystem functioning, and drive biodiversity and productivity . Revisiting the host-pathogen -environment paradigm, we understand that parasites will be affected by anthropogenic changes as well as their hosts, but perhaps in different ways, some surviving better than others, some having the ability to infect new hosts rather than die out, and some better able to change and adapt to new conditions at different stages in their life cycles . We consider them to be problems, and yet host population control is essential so organisms do not exceed the carrying capacity of the ecosystem. The difference is that enzootic (or endemic) levels of disease agents, producing low levels of morbidity or mortality , are not alarming, whereas epizootic (epidemic) levels or outbreaks get our attention. In areas where organisms are adapted to changing conditions, such as temperate zones, the autumnal reduction in light and temperature causes deciduous plants to stop photosynthesis, and their leaves become susceptible to infections with viruses, bacteria, and fungi that will break down the tissue, recycling it into nutrients on the forest floor, while protecting the plant from winter storm (wind and snow) effects. When such infections occur during the spring or summer, damaging the leaves so they cannot support the plant, then the agent is causing disease. Despite our aversion to pathogens, developed from long observations of morbidities and mortalities caused by them and dislike of suffering (our own or others), they are necessary for proper ecosystem functioning (Gómez et al. 2012).
Improvements in recognizing and diagnosing diseases and identifying pathogens have probably contributed to the increase in reports of emerging infectious diseases (EIDs) in terrestrial and aquatic ecosystems (Gire et al. 2012), because disease agents likely have been circulating undetected in ecosystems for years as subclinical infections. With continuing human population growth, urban development , deforestation, point and nonpoint source pollution , and increasing carbon dioxide concentrations in the atmosphere, we have witnessed ecosystem alterations and increasing loss of organisms to abiotic and biotic stressors impairing their health (Vicente 1989; Harvell et al. 1999, 2004, 2007; Selig et al. 2006; Altizer et al. 2013; Ban et al. 2014). Other chapters in this book examine the changes occurring when populations of reef organisms are altered as a result of predation or exposure to adverse environmental conditions. The same principles and effects apply when populations are affected by biotic diseases. This section will review some of the impacts that diseases have on the tropical marine environment, as aptly summarized by the German marine ecologist Otto Kinne in Box 8.2, and additional concerns.
Box 8.2
“Diseases affect basic phenomena of life in oceans and coastal waters: for example, life span, life cycle , abundance, distribution, metabolic performance, nutritional requirements, growth, reproduction, competition, evolution, as well as organismic tolerances to natural and man-made environmental stress. In short, diseases are a major denominator of population dynamics.” (Kinne 1980, p. 1)
As discussed in other chapters, the demise of even one species of coral-reef organism may have serious repercussions for the structure, composition, processes, and function of a particular community and the reef ecosystem. Diseases of scleractinian corals and coralline algae are perhaps the most important factor in changing the structure and function of coral reef communities because loss of live tissue cover not only reduces the number of polyps producing gametes for potential new recruits, but also opens up new hard substratum space for settlement of benthic organisms. However, Kuta and Richardson (1997) found that, in agreement with other studies, BBD caused extensive tissue loss in reef framework coral species, but few benthic fauna colonized the bared skeletal areas in 5 years of observations, and of those, only one was a reef-framework coral species, which could contribute to a shift in community structure over time. Edmunds (2000) followed tagged coral colonies for 11 years on a shallow reef off St. John, U.S. Virgin Islands and reported similar, but very low, rates of recruitment by scleractinians to coral skeletons remaining after being killed by BBD or Hurricane Hugo. This study was undertaken in a bay with a fully protected watershed and relatively pristine reefs, and the only increase in infection rates and loss of tissue occurred during late summer when seawater temperatures were at their peak.
The other coral tissue loss diseases have affected large areas of reef throughout the Caribbean and Florida Keys and now threaten corals in the Red Sea and Indo-Pacific oceans. The population reductions in branching acroporid corals as a result of WBD, with bioerosion of the remaining exoskeletons, changed reef structure (Gladfelter 1982). Aronson and Precht (2001) reported that WBD caused significant mortality of acroporid corals throughout the Caribbean during the past three decades, resulting in changes to the framework of these reefs. The mass mortality of A. cervicornis from disease or other causes in the central shelf lagoon of the Belize Barrier Reef during a 10-year period around 1990 was discovered by coring into the substratum. It was the first widespread change in species composition in this region for more than 3,000 years. Agaricia tenuifolia replaced the staghorn, but bleached and then was replaced by Porites porites. These species do not provide the same high relief habitat and framework as acroporids, and the associated organisms differ as well. In the Florida Keys, changes in corals and other organisms have been documented (Porter et al. 2002; Alevizon and Porter 2014; and others), some associated with disease outbreaks and loss of acroporids, but with high variability among reef sites and uncertainties in understanding the causes (Brandt et al. 2012; Miller et al. 2014). Similar structural modifications are occurring on Indo-Pacific reefs with the loss of the large table acroporids (Hobbs and Frisch 2010) and montiporids (Work et al. 2012) to WS, montiporids to atramentous necrosis (Jones et al. 2004), and many other partial to complete mortalities resulting from diverse diseases in numerous species (see regional reviews by multiple authors in Rosenberg and Loya 2004). Which species are lost before others will affect community structure and function as well (Ostfeld and LoGiudice 2003).
Although community alterations have been observed more often on coral reefs, diseases may also affect a variety of relationships and processes in soft-bottom benthic communities on reefs and in seagrass beds and mangroves. In addition to predation and competition, bioturbation, sedimentation, primary and secondary production , and other phenomena may be changed with the loss of one or more micro- or macro-organisms to biotic or abiotic diseases. During the mass mortality of turtlegrass Thalassia testudinum in Florida Bay, more than 23,000 ha were affected, with 4,000 ha of seagrass beds completely lost, especially in protected basins. Many reef organisms are associated with seagrass habitats. They are used by larvae and juveniles as refugia from predators. Soft-bottom invertebrates used as prey may suffer from the loss of the seagrass and they can also be affected by pathogens and parasites, as documented in a review by Sousa (1991). Elevated water temperature , the recent local decline in frequency of hurricanes, elevated salinity , and chronic sediment hypoxia were noted as other factors that may have contributed to the Florida Bay die-off (Roblee et al. 1991), stressors that could also have directly or indirectly affected the other organisms living in this ecosystem.
Parasites and their hosts are embedded in food webs (Hudson et al. 2006). Not only may these organisms affect host responses to environmental stresses, thereby altering population size and geographic distribution, but they can also influence biodiversity and productivity and alter intra- and interspecific interactions of species (Rohde 1993). The dramatic phase shift that occurred following the year-long mass mortality of Diadema antillarum adversely affected reefs throughout the tropical western Atlantic, as macroalgae grew with reduced herbivore control, preventing recruitment of corals and other benthic organisms (Lessios et al. 1988). Acroporid corals damaged by Hurricane Allen off Jamaica initially survived as fragments, then apparently succumbed to WBD and macroalgal growth after the D. antillarum mass mortality, changing the pattern of predation by coral snails and shifting the roles of other predators there (Knowlton et al. 1990). Marked reductions in multiple species of sponges (51.3 % of species and 42.6 % of total sponge volume), as documented on a shallow reef at Bocas del Toro, Panama, over 14 years and possibly occurring elsewhere in the Caribbean and the Florida Keys, resulted in the loss of several essential ecosystem services , particularly water clarity, nutrient regeneration, and shelter from predators for many species (Wulff 2006b).
Extensive loss of topographic relief has affected fish populations as protective niches and important habitats have been altered. The decline of coral reef fisheries among western Atlantic coral reefs may be related to such structural changes from the loss of acroporids and other coral species, although overfishing has probably contributed to losses of fish species diversity and numbers as well (Rogers 1985; Jackson et al. 2001) argued that human impacts on marine ecosystems, including the phase shift s experienced during the last 20 years on coral reefs in the Caribbean and community changes on other reefs, can be traced back to the continuous fishing activities of humans. Overfishing of large vertebrates and shellfish, to the extent that they are now missing from most coastal ecosystems, resulted from aboriginal, then European, colonization and exploitation, leading to disease in lower trophic level s. Populations of their prey either became so dense that transmission of pathogenic microorganisms was facilitated (e.g., in Diadema), or predators of microbes (e.g., suspension feeder s) have been removed, facilitating their population explosion now as eutrophication of coastal marine ecosystems increases. Jackson et al. (2014) noted that limiting the blame to climate change as the culprit of disease outbreaks will not lead to appropriate remedies and advocated protection for herbivorous fishes to deal with the macroalgae biomass on many reefs, which has been shown to contribute to coral diseases and reef degradation since the 1980s.
Dinsdale and Rohwer (2011) examined this situation on many degraded coral reefs, where top predators have been “fished down the food chain,” and observed that with increased nutrients supporting phytoplankton and macroalgae, the primary productivity that would normally be transferred through herbivores to the predators through the trophic cascade has instead accumulated as particulate and dissolved organic matter, supporting a different, enriched heterotrophic microbial community. Although the top predators’ prey are more abundant, the prey’s food sources have increased in abundance and cannot be controlled as before. Potentially pathogenic microbes have been identified on algae (Nugues et al. 2004), as well as in the water column, supporting more bioeroders and sponges that may serve as reservoirs of microorganisms implicated in coral diseases (Ein-Gil et al. 2009; Negandhi et al. 2010; reviewed in Webster and Taylor 2012). Bowles and Bell (2004) noted that overfishing of sea turtles was not related to seagrass wasting disease . The numerous other reef organisms that are now being “fished” for food, construction materials, home aquaria, home decorations, and other commercial or socioeconomic reasons are also contributing to the defaunation of coral reef ecosystems, loss of habitat, and disturbances to marine food webs, which will change parasite -host relationships and reduce the ability of organisms to tolerate infections. Another aspect of sorting out the impacts of population reductions on diseases brings us back to the food webs. Diseases can alter the reproductive potential of a population by direct effects on gonad development (e.g., parasitic castration), or indirectly by altering male-to-female ratios and mating or spawning behavior, not to mention reducing population densities and the distribution of individuals that will impair successful reproduction (e.g., Allee effect) (McCallum et al. 2004). What has not been explored are indirect effects on nutrition by reduced intake and absorption of nutrients due to loss of prey or zooxanthellae (contributing nutrients to corals, sponges, anemones) that would also reduce gamete production. And in turn, reducing seasonal releases of gametes and larvae that served as food for other reef organisms. When 90–100 % of Diadema antillarum died, how did their loss change food webs that had been dependent on their spawning?
Porter and Tougas (2001, Figure 5 and text) proposed a model of coral disease in which changing environmental conditions are responsible for altering the interactions between hosts and pathogens, as immune systems become compromised because of various stressors. They noted that inclusion of environmental quality was necessary to explain the simultaneous increase in the diseases, species affected, and rates of mortality over large geographic areas, and hypothesized that the incidence of disease would be higher near polluted population centers. This has certainly been shown in widely separated studies on coral diseases, such as in the U.S. Virgin Islands (Kaczmarsky 2005), the Philippines (Kaczmarsky 2006), Abrolhos Bank, Brazil (Francini-Filho et al. 2008), southeast Florida (Dustan et al. 2008), Hawaiian Islands (Aeby et al. 2011a; Couch et al. 2014), and the Great Barrier Reef (Lamb and Willis 2011), as well as coralline algae diseases of Indo-Pacific reefs (Vargas-Angel 2010) at sites where domestic sewage, agricultural, industrial, and stormwater discharges flow onto reefs. In other cases, diseases do not correlate with the level of human influence (Quéré et al. 2014, coralline algae diseases in Curaçao; Gochfeld et al. 2012, Aplysina red band syndrome not altered with nutrient enrichment), but these seem to be rare exceptions. Field and laboratory experiments have demonstrated pollution impacts on coral reef organisms. For example, nutrients, organic matter, crude oil, copper sulfate, dextrose, potassium phosphate , and sediment have increased morbidity and mortality in exposed corals, but the addition of antibiotics (in lab experiments) has prevented disease (e.g., Mitchell and Chet 1975; Hodgson 1990; Kuta and Richardson 2002; Bruno et al. 2003; Kline et al. 2006; Voss and Richardson 2006; Brandt et al. 2013), indicating that microbial population control, and its potential for producing anoxia and toxins, is important in addition to the toxicity of the contaminant. In addition, hosts and parasites may have different responses to altered environmental conditions. Increased water temperatures have been found to support growth rates for microbes while damaging plants or animals, with increases in disease outbreaks (e.g., Ben-Haim et al. 2003; Selig et al. 2006; Vargas-Ángel 2010; Muller and van Woesik 2014), but more work is needed on synergistic effects of stressors. For example, Williams et al. (2014) found that the fungal destruction of thallus tissue in CFD was reduced when pCO2 and water temperature were elevated together, although the coralline algae calcification was reduced by these stressors, adversely affecting resistance to micro-boring organisms.
The first response of many aquatic organisms, particularly corals, when exposed to an irritant is increased production of mucus to form a protective barrier and limit the exposure. The mucus in turn is used by bacteria and other microorganisms for food. The ensuing breakdown of this organic matter can lead to the release of bacterial toxins and anoxia, resulting in disease if the affected organism cannot escape. Different species of corals can produce different kinds and quantities of antimicrobial compounds to control different kinds of surface bacteria (Koh 1997). Sea fans use chemicals to inhibit tissue infection by fungi and cellular defenses to sequester the hyphae (Kim et al. 2000; Petes et al. 2003). Energy for production of mucus or antimicrobial compounds that support a protective microbial community (mechanisms reviewed in Krediet et al. 2014), as well as cellular and humoral immune responses, can be limited in animals when nutritional sources are scarce or metabolic processes are altered by exposure to temperature extremes or pollutants, leaving them susceptible to attack by latent, facultative, or opportunistic pathogen s (Hayes and Goreau 1998; Lesser et al. 2007; Mao-Jones et al. 2010). Furthermore, secretion of mucus is suppressed following prolonged exposure to elevated temperatures or pollutants. Metabolism, production of detoxification enzymes, and other physiological and biochemical operations are also altered by exposures to anthropogenic pollutants and changes in water quality (Couch and Fournie 1993), which may also affect prey, resulting in structural and functional changes in tissues leading to morbidity and mortality of resident invertebrate and fish populations.
A study of corals and octocorals from Biscayne National Park, off southeast Florida (USA) in the 1970s revealed high levels of organochlorine pesticides and heavy metals, similar to those levels used in toxicity tests that led to bleaching and mortality of the same reef-building species of corals in the laboratory. One-third of coral colonies sampled from this site exhibited lesions and possibly pathogenic microorganisms were found in their tissues, although the presence of the lesions could not be linked to contaminants in this study (Glynn et al. 1989). Similar studies of corals off Australia (Lewis et al. 2009) and elsewhere have detected uptake of pesticides and heavy metals into tissues; however, the presence of biotic or abiotic diseases in contaminated corals from these sites has not been documented. High tissue burdens of such chemicals resulting from chronic exposures may increase the susceptibility of the organisms to biotic disease agents when additional physical or chemical stresses are encountered. Fish diseases are also increasing in tropical bays and mangrove areas where man-made solid and other wastes are disposed. Observations on commercial fish catches in Biscayne Bay, off Miami, Florida, over 10 years (1970–1982) revealed a variety of surficial lesions and abnormalities, including ulcerations, fin and integumental hemorrhages, fin erosion, eye abnormalities, scoliosis, scale disorientation, parasitoses, emaciation, and tumors, especially in bottom-feeding fishes. Excessive nutrients from sewage; petroleum hydrocarbons from marinas, shipping, port facilities, and industries; and toxic chemicals, pesticides, and polychlorinated biphenyls leaching from landfills, rivers, and canals contributed to the decline in water quality of Biscayne Bay (Skinner and Kandrashoff 1988). Because many young stages of reef fishes use inshore waters that may contain toxic chemicals and high levels of nutrients from sewage disposal and stormwater runoff, as well as heavy sedimentation and turbidity, they may also be susceptible to diseases caused by microbial pathogens and parasites. The role of these diseases in limiting reef fish populations, however, is unknown because appropriate studies have not been conducted. Infectious diseases have decreased in some fish populations as intense fishing has reduced host numbers (Ward and Lafferty 2004), limiting contact and exposure and making disease transmission inefficient. This is the “host-density threshold,” another important aspect of disease ecology.
Fishing top predators can indirectly favor disease transmission in prey populations, however, as prey end up in denser aggregations (Lafferty 2004). In 1982, D. antillarum populations were thick on Caribbean reefs, improving the likelihood of infecting another individual. Perhaps their predators were reduced for some reason and perhaps they were starving, which could have further compromised their weak immune systems (McNamara and Buchanan 2005). However, in communities with high biodiversity of potential parasite hosts, a dilution effect has been noted, reducing the risk of vector -borne diseases (Ostfeld and LoGiudice 2003). Loss of biodiversity increases disease transmission by changing the abundance of the host or vector; by changing the behavior of the host, vector, or parasite; or by changing the condition of the host or vector, although more biodiverse communities may contribute novel pathogens (Aeby 2002; Keesing et al. 2010). These concepts have not been explored much in relation to parasites and diseases of coral reef organisms; however, Wood et al. (2014) compared fished and unfished reefs in the Northern Line Islands of the Pacific and found that fishing decreased parasite species, with directly transmitted parasites significantly more affected than those that alternated among hosts, but the effects varied depending on the host species and their sensitivity to fishing impacts. Aeby et al. (2011b) compared Acropora WS-affected corals on a reef in the species-poor northwestern Hawaiian Islands with species-rich reefs off American Samoa. They observed high mortality in the monospecific stand of Acropora on the Hawaiian reef, whereas the disease could not easily spread among different Acropora spp. on the American Samoa reefs, because they differed in their apparent susceptibility to infection and disease severity. Perhaps that was true also of the formerly magnificent monospecific thickets of Caribbean acroporids, facilitating transmission of the agent(s) responsible for WBD, particularly where thickets had been formed by fragmentation of a highly susceptible genotype. Bruno et al. (2007) found that high (>50 %) coral cover on Australian reefs correlated with increased prevalence of WS following exposure to warm temperatures.
Differences in susceptibility to certain diseases as a result of individual or species attributes (genetic or otherwise) are also influential in changing the composition of reef communities (e.g., Koh 1997; Dinsdale 2000; Weil et al. 2000). For example, at some locations, diseased corals show a clumped distribution (Bruckner et al. 1997; Richardson 1998; Sato et al. 2009; Porter et al. 2011), whereas in other areas, diseases have a random distribution (e.g., Edmunds 1991), and exhibit localized adaptation in host infectivity (Grosholz and Ruiz 1997). Muller and Woesik (2012) examined the spatial patterns of yellow-band disease , dark-spot disease, and white-plague disease and suggested that when diseases are not clustered they do not follow a contagious model, rather environmental thresholds are first exceeded that increase corals’ susceptibility or the environmental changes create a favorable environment for already present biotic pathogens and increase their virulence (e.g., Remily and Richardson 2006). García et al. (2002) found differences in disease susceptibility not only among coral species in the Parque Nacional Archipiélago de los Roques off Venezuela, but also among different size classes. For example, WP and CYBD was more likely to affect large (older) colonies (0.9–10.8 m2) and BBD small colonies (0.01–0.09 m2). Muller and van Woesik (2014) found that white pox was more likely to adversely affect older A. palmata colonies after temperature stress. An analysis of constitutive levels of immune-related processes against prevalence and number of diseases affecting different Caribbean coral species revealed that those species studied belonging to older lineages (Siderastreidae, Poritidae, Meandrinidae) tended to resist diseases better than recently divergent lineages (Montastraeidae and Merulinidae, but not Mussidae) (Pinzon et al. 2014), with similar indications in Indo-Pacific species (Palmer et al. 2010). Whether vectors are involved in the spread of pathogenic microorganisms, how they interact with tolerant or susceptible hosts, and whether their own genetics and physiological mechanisms make them susceptible or tolerant to environmental stressors or biotic pathogens, probably also influences the prevalence and intensity of disease outbreaks (Lu et al. 2000b; Panek 2005; Williams and Miller 2005; Aeby and Santavy 2006; Råberg 2014), as well as each organism’s holobiont (Mydlarz et al. 2010).
If the complexity of disease ecology within an ocean basin were not enough, another concern is that organisms are being transported out of their home habitat and introduced into similar or new habitats many miles away, often in different regions or even oceans, as the result of global shipping, oceanarium, aquaculture, marine laboratory, and tropical aquarium trade activities. In addition to attaching to the hull of a vessel, larval organisms and potential microbial pathogens can be picked up with ballast water and deposited offshore or in another harbor. Commercial shipments of live organisms contain not only the desired (or target) organisms or their eggs and larvae, but also other organisms that may be parasites or pathogens in the water or seaweed in which they were originally packed and within the target organisms. If placed in flow-through systems, without proper quarantine or treatment of the discharged water, these animals will be released to the sea. Releases of unwanted fish or other animals by tropical aquarists have also led to introductions in foreign lands. If conditions are suitable for survival and reproduction, the species may become established at the new site. Some introduced species have positive effects in their new environment. But the vast majority of documented introductions have adversely affected existing commercial and recreational fisheries as the result of competition, predation, and the introduction of parasites and pathogens (e.g., Carlton and Geller 1993; Chakraborty et al. 2002; Padilla and Williams 2004; Sheppard and Dungan 2009), a phenomenon termed “pathogen pollution .” A number of countries developed regulations and procedures to restrict such introductions, including permit and certification requirements; culture using filtered, recirculating sea-water; quarantine procedures; and destruction of diseased stocks (DeVoe 1992), as well as new ballast-water regulations (Gollasch et al. 2007). Controlling individual behavior can be difficult (e.g., the aquarist who dumps unwanted organisms in the ocean; Padilla and Williams 2004). However, Jackson et al. (2014) surmised that the massive losses of Caribbean acroporids and long-spined urchins may have resulted from the “enormous increases in ballast water discharge from bulk carrier shipping since the 1960s” (p. 21) and the fact that millions of years of isolation from the Indo-Pacific may have made them particularly vulnerable to introductions of novel pathogenic microorganisms or ones that had developed increased virulence. For already introduced organisms, such as two species of lionfish (Pterois) that are now established (since 1985) and multiplying in the Caribbean Sea and tropical western Atlantic Ocean where they are consuming native fishes (Schofield 2009), perhaps native pathogens and parasites will eventually control them (Albins and Hixon 2013).
Pathogen, nutrient, and toxicant pollution can also be dispersed globally by air. The numerous diseases observed throughout the Caribbean caused scientists at the U.S. Geological Survey to wonder whether disease incidence might be related to dust from the Sahara/Sahel Region of Africa that blows across the Atlantic and is increasing due to drought conditions there. Studies of air samples revealed the presence of viable bacteria and molds apparently shielded from damaging UV radiation by the dust particles. One species of soil fungus found in samples is Aspergillis sydowii, identified as the causal agent of the disease aspergillosis in sea fans. Other potentially pathogenic microorganisms have been found in the dust; human cases of respiratory diseases increase on Caribbean islands and elsewhere when dust sweeps into the area (Shinn et al. 2000; Griffin 2007). Furthermore, Hayes et al. (2001) speculated that increased deposition of the iron -rich dust throughout the region since the 1970s, along with local and global climate change s and pollution loading, has probably altered the ability of coastal ecosystems to limit the multiplication of potentially pathogenic microorganisms, resulting in disease outbreaks in marine organisms in these nutrient-enriched areas. Examining multiple environmental and anthropogenic factors on a small scale (e.g., microhabitat) to large scale (e.g., satellite data of sea surface temperatures) will be important in research on diseases of reef organisms (Bruckner 2002).
The complexity of disease , as it is now understood four decades after the first reports of coral diseases, has incorporated a larger role for anthropogenic alterations in the environment, particularly climate change as shallow-water ocean temperatures have warmed and elevated pCO2 is altering ocean pH. When culture techniques were developed for bacteria, Robert Koch established criteria (Koch’s postulates) more than 100 years ago for determining the causal agents of human diseases. Associations of suspected pathogens have been reported in the literature, and some scientists have claimed that they have identified a microorganism or polymicrobial infectious agent by applying Koch’s postulates, but experimental tests of Koch’s postulates are challenging. Some agents cannot be cultured outside of the host and viruses require special cell lines in which to grow them so they have not been cultured in the laboratory (e.g., herpesvirus of sea turtle fibropapillomatosis , Rickettsiales-like organism of staghorn coral). They may also require interactions with other biotic or environmental factors to become virulent (Lackovich et al. 1999; USEPA 2000). Thus, the procedures used to identify pathogenic microorganisms are changing. Molecular tools can now be used to examine DNA sequences and tease out potentially pathogenic microorganisms in comparison to unaffected hosts (e.g., Ritchie et al. 2001; Lesser et al. 2007; Work et al. 2008b; Sutherland et al. 2011; Wilson et al. 2012; Roder et al. 2013; Sweet et al. 2013, 2014; Williams et al. 2014). However, diseases can also result from abiotic , nutritional, or genetic factors. While molecular approaches are providing key insights into the microbiomes of affected species, it is crucial that this research be done in conjunction with histopathological examinations and physiological tests to understand which target cells or organs are being damaged and how pathogenesis occurs. In addition, analytical chemistry is needed to determine whether affected species contain critical body residues of chemical contaminants or biotoxins, which may actually be the pathogen (s) and cause the microbiome to shift to cellular degraders using molecules from the dying cells for their substrates. And, of course, numerous other environmental variables must be considered. The use of tools that integrate stressor exposures and effects, such as biomarkers, may help to identify causal mechanisms for subcellular damage underlying observed effects at the population or community levels (Woodley et al. 2000; Morgan et al. 2001; Richardson et al. 2001); however, the normal ranges for the various parameters must be carefully examined first and laboratory models using cell cultures or whole organisms are needed (Adams 1990; Huggett et al. 1992; Scully et al. 2001). Knowledge of the variability of normal and abnormal structure and function in most tropical marine organisms, both within and between individuals and species, is still lacking. Comparisons that would be useful indicators of environmental stress are few. The study of bleaching in corals and other organisms containing photosynthetic symbionts has increased to the point that algal densities and photosynthetic pigment fluorescence may be useful indicators, as well as molecular biomarkers.
In addition, scientists in tropical marine environments should be aware of the possibility that diseases and mortalities observed locally may actually be major epizootics or mass mortalities that cover large areas of coral reefs or coastal habitats or even regions. D. Faulkner describes a “healthy” patch reef near Eleuthera in the Bahamas as having numerous D. antillarum and other species of urchins , but surprisingly noticed a patch reef not more than 100 m away that appeared “unhealthy” even “bleak,” with large dead colonies and algae present, completely lacking urchins. He also could not find D. antillarum on Hen and Chickens Reef in the upper Florida Keys reef tract in 1975, and it, too, had dead and dying corals. Although the cause of the reef demise in Florida was suspected to be cold water one winter, he wondered if the lack of urchins had prevented the corals from coming back, as well as whether something had killed the urchins first, resulting in the dying reefs—this was prior to the mass mortality event (Faulkner and Chesher 1979). Could a waterborne pathogen have been present in the Caribbean prior to 1982? What has prevented their return in many areas, particularly the Florida Keys, and where they have returned, are those urchins still susceptible to whatever killed them before (Edmunds and Carpenter 2001; Beck et al. 2009)? Of course, other abiotic and biotic factors may have affected the urchins at these reefs and the sequence of events is unknown. Experience thus far suggests that more extensive networks of scientists need to be developed to track such events and to locate expertise to investigate the role of biotic and abiotic diseases. Both observers in the field and specialists in laboratories are required to respond quickly and gather samples and essential data .
To promote the transdisciplinary and collaborative investigations of the linkages among the health of humans, other animals, and plants (ecosystem health) with environmental changes, the field of conservation medicine developed during the 1990s–2000s (Aguirre et al. 2012). Drawing on data obtained from innovative monitoring strategies, ecological, chemical, physiological, microbiological, histopathological, social science, and other types of research, patterns of shared causes, interrelationships, and the significance of the diseases or mass mortality events can be established to address the conservation of biodiversity . A similar early effort was the Caribbean Aquatic Animal Health Project at the University of Puerto Rico to develop the Marine Ecological Disturbance Information Center (MEDIC) to track reports of major marine ecological disasters (MMEDs) affecting Caribbean aquatic organisms and to alert appropriate experts when field and laboratory studies were required (Williams and Buckley-Williams 1990). Building on this effort, we have seen increased collaborations among experts in different disciplines (CDHC and Targeted Coral Reef Research), more consistency in disease investigations, stakeholder notifications about morbidity and mortality events, incorporation of quarantine or some sort of treatment protocol to prevent spread of disease—particularly from a culture facility to field and vice versa—McCallum et al. 2004), and the realization that human health can be compromised not only by impacts of disease on ecosystem services , but also by direct exposures to zoonotic pathogens. For example, Keirstead et al. (2013) determined antibiotic susceptibility (minimal inhibitory concentrations to various antibiotics) of S. iniae to treat fish handlers if they were infected with the bacterium during the 2008 mass mortality of wild reef fish off St. Kitts, Nevis. Other pathogenic microorganisms in tropical marine waters could also infect humans, indicating the need to develop a One Health approach as advocated in conservation medicine. Terrestrial and aquatic ecosystem health linkages must be included (the watersheds, ridges-to-reefs concept).
In summary, investigations of the nature and role of diseases in coral reef organisms have identified diverse agents responsible for morbidities and mortalities. Based on observations of commercially important invertebrates and vertebrates from temperate seas, more diseases and diverse parasites no doubt remain to be discovered, and will also need to be studied. Long-term changes in water quality, such as elevated levels of nutrients, are of particular concern for attached or sedentary invertebrates, since they cannot escape. Loss of important members of a food web will ultimately affect higher predators, as will the loss of those organisms that perform important functions in an ecosystem, such as decomposition, bioturbation, or nutrient recycling, and the loss of organisms that provide protection and specialized habitats. As water conditions and habitats change due to human actions and globalization, with intense mixing and introductions of species to new locations driving exposures and responses to potentially pathogenic agents, multiple factors and scenarios need to be considered. Population losses thought to be tied to overfishing or natural predation may have been the result of disease ; diseases may result from population losses that reduce biodiversity and increase malnutrition. Pathogens and parasites exert tremendous pressures on individuals, populations, and communities in their interactions with the host and environmental conditions, and must be taken into consideration in any discussion of the dynamics of coral reefs and associated tropical marine habitats, and in the conservation of reef biodiversity. The contributions of humans to the stressors experienced by reef organisms is unprecedented in this unique period of earth’s history, and the Anthropocene is not only changing ecosystems, but how we must think about them.
References
Adams SM (ed) (1990) Biological indicators of stress in fish. American Fisheries Society symposium series 8. American Fisheries Society, Bethesda
Aeby GS (2002) Trade-offs for the butterflyfish, Chaetodon multicinctus, when feeding on coral prey infected with trematode metacercariae. Behav Ecol Sociobiol 52:158–165
Aeby GS, Santavy DL (2006) Factors affecting susceptibility of the coral Montastraea faveolata to black-band disease. Mar Ecol Prog Ser 318:103–110
Aeby GS, Williams GJ, Franklin EC et al (2011a) Growth anomalies on the coral genera Acropora and Porites are strongly associated with host density and human population size across the Indo-Pacific. PLoS One 6(2):e16887. doi:10.1371/journal.pone.0016887
Aeby GS, Williams GJ, Franklin EC et al (2011b) Patterns of coral disease across the Hawaiian Archipelago: relating disease to environment. PLoS One 6(5):e20370. doi:10.1371/journal.pone.0020370
Aguirre AA, Tabor GM, Ostfeld RS (2012) Conservation medicine: ontogeny of an emerging discipline. In: Aguirre AA, Ostfeld RS, Daszak P (eds) New directions in conservation medicine: applied cases of ecological health. Oxford University Press, Oxford
Ainsworth TD, Kramasky-Winter E, Loya Y et al (2007a) Coral disease diagnostics: what’s between a plague and a band? Appl Environ Microbiol 73(3):981–992
Ainsworth TD, Kvennefors EC, Blackall LL et al (2007b) Disease and cell death in white syndrome of acroporid corals on the Great Barrier Reef. Mar Biol 151:19–29
Albins MA, Hixon MA (2013) Worst case scenario: potential long-term effects of invasive predatory lionfish (Pterois volitans) on Atlantic and Caribbean coral-reef communities. Environ Biol Fish 96:1151–1157
Alder J, Braley R (1989) Serious mortality in populations of giant clams on reefs surrounding Lizard Island, Great Barrier Reef. Aust J Mar Freshwat Res 40:205–213
Alevizon WS, Porter JW (2014) Coral loss and fish guild stability on a Caribbean coral reef: 1974–2000. Environ Biol Fish. doi:10.1007/s10641-014-0337-5
Altizer S, Ostfeld RS, Johnson PTJ et al (2013) Climate change and infectious diseases: from evidence to a predictive framework. Science 341:514–519
Amadjian V, Paracer S (1986) Symbiosis: an introduction to biological associations. University Press of New England, Hanover
Angermeier H, Kamke J, Abdelmohsen UR et al (2011) The pathology of sponge orange band disease affecting the Caribbean barrel sponge Xestospongia muta. FEMS Microbiol Ecol 75(2):218–230
Antonius A (1985) Coral diseases in the Indo-Pacific: a first record. Mar Ecol 6(3):197–218
Armelagos GJ, Brown PJ, Turner B (2005) Evolutionary, historical and political economic perspectives on health and disease. Soc Sci Med 61:755–765
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38
Balazs GH, Pooley SG (eds) (1991) Research plan for marine turtle fibropapilloma. Nat Mar Fish Serv Tech Mem, NOAA-TM-NMFS-SWFSC-156. US Dept Comm, NOAA, Washington
Ban SS, Graham NAJ, Connolly SR (2014) Evidence for multiple stressor interactions and effects on coral reefs. Glob Chang Biol 20:681–697
Barneah O, Ben-Dov E, Kramarsky-Winter E et al (2007) Characterization of black band disease in Red Sea stony corals. Environ Microbiol 9(8):1995–2006
Beck G, Miller R, Ebersole J (2009) Significance of immunological responses in the blackspined sea urchin, Diadema antillarum, to Caribbean-wide mass mortality. Proceedings of 11th International Coral Reef Symposium, pp 221–225
Behringer DC, Butler MJ IV, Moss M et al (2012) PaV1 infection in the Florida spiny lobster (Panulirus argus) fishery and its effects on trap function and disease transmission. Can J Fish Aquat Sci 69:136–144
Ben-Haim Y, Zicherman-Keren M, Rosenberg E (2003) Temperature-regulated bleaching and lysis of the coral Pocillopora damicornis by the novel pathogen Vibrio coralliilyticus. Appl Environ Microbiol 69:4236–4242
Birkeland C (1989) The influence of echinoderms on coral-reef communities. In: Jangoux M, Lawrence JM (eds) Echinoderm studies. AA Balkema, Rotterdam, pp 1–79
Birkeland C, Lucas JS (1990) Acanthaster planci: major management problem of coral reefs. CRC Press, Boca Raton
Bott NJ, Healy JM, Cribb TH (2005) Patterns of digenean parasitism of bivalves from the Great Barrier Reef and associated waters. Mar Freshw Res 56(4):387–394
Bowles JW, Bell SS (2004) Simulated herbivory and the dynamics of disease in Thalassia testudinum. Mar Ecol Prog Ser 283:127–132
Brandt ME, Ruttenberg BI, Waara R et al (2012) Dynamics of an acute coral disease outbreak associated with the macroalgae Dictyota spp. in Dry Tortugas National Park, Florida, USA. Bull Mar Sci 88(4):1035–1050
Brandt ME, Smith TB, Correa AMS, Vega-Thurber R (2013) Disturbance driven colony fragmentation as a driver of a coral disease outbreak. PLoS ONE 8(2), e57164. doi:10.1371/journal.pone.0057164
Bruckner AW (2002) Priorities for effective management of coral diseases. NOAA Technical Memorandum NMFS-OPR-22. US Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service, Silver Spring
Bruckner AW, Bruckner RJ, Williams EH Jr (1997) Spread of a black-band disease epizootic through the coral reef system in St. Ann’s Bay, Jamaica. Bull Mar Sci 61:918–928
Bruno JF, Petes LE, Harvell CD et al (2003) Nutrient enrichment can increase the severity of coral diseases. Ecol Lett 6:1056–1061
Bruno JF, Selig ER, Casey KS et al (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol 5(6):e124. doi:10.1371/journal.pbio.0050124
Budd AF, Fukami H, Smith ND et al (2012) Taxonomic classification of the reef coral family Mussidae (Cnidaria:Anthozoa:Scleractinia). Zool J Linn Soc-Lond 166:465–529
Bunkley-Williams L, Williams EH Jr (1994) Diseases caused by Trichodina spheroidesi and Cryptocaryon irritans (Ciliophora) in wild coral reef fishes. J Aquat Anim Health 6(4):360–361
Bunkley-Williams L, Williams EH Jr, Horrocks JA et al (2008) New leeches and diseases for the hawksbill sea turtle and the West Indies. Comp Parasitol 75(2):263–270
Burns JHR, Rozet NK, Takabayashi M (2011) Morphology, severity, and distribution of growth anomalies in the coral, Montipora capitata, at Wai‘ōpae, Hawai‘i. Coral Reefs 30:819–826
Bythell JC, Barer MR, Cooney RP et al (2002) Histopathological methods for the investigation of microbial communities associated with disease lesions in reef corals. Lett Appl Microbiol 34:359–364
Bythell JC, Pantos O, Richardson L (2004) White plague, white band, and other “white” diseases. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer-Verlag, Berlin, pp 351–365
Cárdenas EB, Frenkiel L, Aranda DA (2005) One more threat for the queen conch Strombus gigas? Coccidian (Apicomplexa) infection of the S. gigas digestive gland: preliminary results. Gulf Caribb Fish Inst 58:421–426
Cárdenas EB, Frenkiel L, Zarate AZ et al (2007) Coccidian (Apicomplexa) infecting Strombus gigas Linné 1758 digestive gland. J Shellfish Res 26(2):319–321
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82
Casas V, Kline DI, Wegley L et al (2004) Widespread association of a Rickettsiales-like bacterium with reef-building corals. Environ Microbiol 6:1137–1148
Cervino J, Goreau TJ, Nagelkerken I et al (2001) Yellow band and dark spot syndromes in Caribbean corals: distribution, rate of spread, cytology, and effects on abundance and division rate of zooxanthellae. Hydrobiologia 460:53–63
Cervino JM, Winiarski-Cervino K, Polson SW (2006) Identification of bacteria associated with a disease affecting the marine sponge, Ianthella basta, in New Britain, Papua New Guinea. Mar Ecol Prog Ser 324:139–150
Cervino JM, Thompson FL, Gomez-Gil B et al (2008) The Vibrio core group induces yellow band disease in Caribbean and Indo-Pacific reef-building corals. J Appl Microbiol 105(5):1658–1671
Chakraborty A, Otta SK, Joseph B et al (2002) Prevalence of white spot syndrome virus in wild crustaceans along the coast of India. Curr Sci 82(11):1392–1397
Coles SL, Seapy DG (1998) Ultra-violet absorbing compounds and tumorous growths on acroporid corals from Bandar Khayran, Gulf of Oman, Indian Ocean. Coral Reefs 17:195–198
Cook GM, Rothenberger JP, Sikaroodi M et al (2013) A comparison of culture-dependent and culture-independent techniques used to characterize bacterial communities on healthy and white plague-diseased corals of the Montastraea annularis species complex. Coral Reefs 32:375–388
Couch JA, Fournie JW (eds) (1993) Pathobiology of marine and estuarine organisms. CRC Press, Boca Raton
Couch CS, Garriques JD, Barnett C, Preskitt L, Cotton S, Giddens J, Walsh W (2014) Spatial and temporal patterns of coral health and disease along leeward Hawai’I Island. Coral Reefs 33(3):693–704
Culotta WA, Pickwell GV (1993) The venomous sea snakes: a comprehensive bibliography. Krieger Publishing Company, Malabar
Daszak P, Cunningham AA, Hyatt AD (2001) Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop 78:103–116
Davidson OG (2001) Fire in the turtle house: the green sea turtle and the fate of the ocean. Public Affairs, New York
Deem SL, Karesh WB, Weisman W (2001) Putting theory into practice: wildlife health in conservation. Conserv Biol 15(5):1224–1233
Denner EBM, Smith G, Busse H-J et al (2003) Aurantimonas coralicida gen. nov. sp. nov. the causative agent of white plague type II on Caribbean scleractinian corals. Int J Syst Evol Microbiol 53:1155–1163
Devoe MR (1992) Introductions and transfers of marine species: achieving a balance between economic development and resource protection. South Carolina Sea Grant Consortium, Charleston
Diaz-Pulido G (2000) Microbial degradation of the crustose red alga Peyssonnelia spp. on reefs of the Caribbean and Great Barrier Reef. Proc 9th Int Coral Reef Symp 2:1257–1260
Dinsdale EA (2000) Abundance of black-band disease on corals from one location on the Great Barrier Reef: a comparison with abundance in the Caribbean region. Proc 9th Int Coral Reef Symp 2:1239–1243
Dinsdale EA, Rohwer F (2011) Fish or germs? Microbial dynamics associated with changing trophic structures on coral reefs. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 231–240
Domart-Coulon IJ, Traylor-Knowles N, Peters E et al (2006) Comprehensive characterization of skeletal tissue growth anomalies of the finger coral Porites compressa. Coral Reefs 25(4):531–543
Downs CE, Ostrander GK, Rougee L et al (2012) The use of cellular diagnostics for identifying sub-lethal stress in reef corals. Ecotoxicology 21(3):768–782
Durako MJ, Kuss KM (1994) Effects of Labyrinthula infection on the photosynthetic capacity of Thalassia testudinum. Bull Mar Sci 54(3):727–732
Dustan P (1977) Vitality of reef coral populations off Key Largo, Florida: recruitment and mortality. Environ Geol 2:51–58
Dustan P, Fauth JE, Pante E et al (2008) Using cellular diagnostics to link land-based sources of pollution with coral reef degradation in South Florida. Proc 11th Int Coral Reef Symp, pp 495–499
Edmunds PJ (1991) Extent and effect of black band disease on a Caribbean reef. Coral Reefs 10:l61–l65
Edmunds P (2000) Recruitment of scleractinians onto the skeletons of corals killed by black band disease. Coral Reefs 19:69–74
Edmunds P, Carpenter R (2001) Recovery of Diadema antillarum reduces macroalgal cover and increases abundance of juvenile corals on a Caribbean reef. Proc Natl Acad Sci 98:5067–5072
Ein-Gil N, Ilan M, Carmeli S et al (2009) Presence of Aspergillus sydowii, a pathogen of gorgonian sea fans in the marine sponge Spongia obscura. ISME J 3:752–755
Emson RH, Mladenov PV, Wilkie IC (1985) Studies of the biology of the West Indian copepod Ophiopsyllis reductus (Siphonostomatoida: Cancerillidae) parasitic upon the brittlestar Ophiocomella ophiactoides. J Nat Hist 19:151–l7
Faulkner D, Chesher R (1979) Living corals. Clarkson N Potter, New York
Ferguson HW, St. John VS, Roach CJ et al (2000) Caribbean reef fish mortality associated with Streptococcus iniae. Vet Rec 147:662–664
Fisher WS (ed) (1988) Disease processes in marine bivalve molluscs. Am Fish Soc Spec Pub 18
Flint M, Limpus CJ, Patterson-Kane JC, Murray PJ, Mills PC (2010) Corneal fibropapillomatosis in green sea turtles (Chelonia mydas) in Australia. J Comp Pathol 142:341–346
Forcucci D (1994) Population density, recruitment and 1991 mortality event of Diadema antillarum in the Florida Keys. Bull Mar Sci 54(3):917–928
Francini-Filho RB, Moura RL, Thompson FL et al (2008) Diseases leading to accelerated decline of reef corals in the largest South Atlantic reef complex (Abrolhos Bank, eastern Brazil). Mar Pollut Bull 56:1008–1014
Frias-Lopez J, Klaus JS, Bonheyo GT, Fouke BW (2004) Bacterial community associated with black band disease in corals. Appl Environ Microbiol 70(10):5955–5962
Galloway SB, Work TM, Bochsler VS et al (2007) Coral disease and health workshop: coral histopathology II. NOAA Tech Mem NOS NCCOS 56 and CRCP 4. National Oceanic and Atmospheric Administration, Silver Spring
García A, Cróquer A, Pauls SM (2002) Relacíon entre la incidencia de enfermedades y la estructura de tallas y especies en corales del Parque Nacional y Archipielago de los Roques, Venezuela. Interciencia 27(9):448–453
Garzón-Ferreira J, Gil-Agudelo DL, Barrios LM et al (2001) Stony coral diseases observed in southwestern Caribbean reefs. Hydrobiologia 460:65–69
Gateño D, León A, Barki Y et al (2003) Skeletal tumor formations in the massive coral Pavona clavus. Mar Ecol Prog Ser 258:97–108
Gil-Agudelo DL, Smith GW, Weil E (2006) The white band disease type II pathogen in Puerto Rico. Rev Biol Trop 54:59–67
Gire SK, Stremlau M, Andersen KG et al (2012) Emerging disease or diagnosis? Science 338:750–752
Gladfelter WG (1982) White-band disease in Acropora palmata: implications for the structure and growth of shallow reefs. Bull Mar Sci 32:639–643
Glazebrook JS, Campbell RSF (1990a) A survey of the diseases of marine turtles in northern Australia. 1. Farmed turtles. Dis Aquat Org 9:83–95
Glazebrook JS, Campbell RSF (1990b) A survey of the diseases of marine turtles in northern Australia. 11. Oceanarium-reared and wild turtles. Dis Aquat Org 9:97–104
Glynn PW, Enochs IC (2011) Invertebrates and their roles in coral reef ecosystems. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 273–325
Glynn PW, Szmant AM, Corcoran EF et al (1989) Condition of coral reef cnidarians from the northern Florida reef tract: pesticides, heavy metals, and histopathological examination. Mar Pollut Bull 20:568–576
Gochfeld DJ, Easson CG, Freeman CJ et al (2012) Disease and nutrient enrichment as potential stressors on the Caribbean sponge Aplysina cauliformis and its bacterial symbionts. Mar Ecol Prog Ser 456:101–111
Goggin CL, Lester RJG (1987) Occurrence of Perkinsus species (Protozoa, Apicomplexa) in bivalves from the Great Barrier Reef. Dis Aquat Org 3:113–117
Gollasch S, David M, Voigt M, Dragsund E, Hewitt C, Fukuyo Y (2007) Critical review of the IMO international convention on the management of ships’ ballast water and sediments. Harmful Algae 6:585–600
Gómez A, Nichols ES, Perkins SL (2012) Parasite conservation, conservation medicine, and ecosystem health. In: Aguirre AA, Ostfeld RS, Daszak P (eds) New directions in conservation medicine: applied cases of ecological health. Oxford University Press, Oxford, pp 67–81
Goreau TJ, Cervino J, Goreau M et al (1998) Rapid spread of diseases in Caribbean coral reefs. Rev Trop Biol 46(Suppl 5):157–171
Green EP, Bruckner AW (2000) The significance of coral disease epizootiology for coral reef conservation. Biol Conserv 96:347–361
Griffin DW (2007) Atmospheric movement of microorganisms in clouds of desert dust and implications for human health. Clin Microbiol Rev 20(3):459–477
Grosholz ED, Ruiz GM (1997) Evidence for regional adaptation of black band disease at Carrie Bow Cay, Belize. Proc 8th Int Coral Reef Symp 1:579–582
Halpern BS, Walbridge S, Selkoe KA et al (2008) A global map of human impact on marine ecosystems. Science 319:948–952
Harvell CD, Kim K, Burkholder JM et al (1999) Emerging marine diseases – climate links and anthropogenic factors. Science 285:1505–1510
Harvell CD, Aronson R, Baron N et al (2004) The rising tide of ocean diseases: unsolved problems and research priorities. Front Ecol Environ 2(7):375–382
Harvell D, Jordán-Dahlgren E, Merkel S et al (2007) Coral diseases, environmental drivers, and the balance between coral and microbial associates. Oceanography 20(1):172–195
Hayes RL, Goreau NI (1998) The significance of emerging diseases in the tropical coral reef ecosystem. Rev Trop Biol 46(Suppl 5):173–185
Hayes ML, Bonaventura J, Mitchell TP et al (2001) How are climate and marine biological outbreaks functionally linked? Hydrobiologia 460:213–220
Heatwole H (1987) Sea snakes. The New South Wales University Press, Sydney
Hobbs JP, Frisch AJ (2010) Coral disease in the Indian Ocean: taxonomic susceptibility, spatial distribution and the role of host density on the prevalence of white syndrome. Dis Aquat Org 89(1):1–8
Hodgson G (1990) Tetracycline reduces sedimentation damage to corals. Mar Biol 104:493–496
Hudson PJ, Dobson AP, Lafferty KD (2006) Is a healthy ecosystem rich in parasites? Trends Ecol Evol 21(7):381–385
Huggett RJ, Immerses RA, Mehrle PM Jr et al (1992) Biomarkers: biochemical, physiological, and histological markers of anthropogenic stress. Lewis Publishers, Boca Raton
Jackson JBC, Kirby MX, Berger WH et al (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Jackson JBC, Donovan M, Cramer K et al (eds) (2014) Status and trends of Caribbean coral reefs: 1970–2012. Global Coral Reef Monitoring Network, IUCN, Gland
Jones JB (2007) Review of pearl oyster mortalities and disease problems. In: Bondad-Reantaso MG, McGladdery SE, Berthe FCJ (eds) Pearl oyster health management: a manual. FAO Fish Technical Paper, No. 503, Rome, pp 61–70
Jones RJ, Bowyer J, Hoegh-Guldberg O et al (2004) Dynamics of a temperature-related coral disease outbreak. Mar Ecol Prog Ser 281:63–77
Jones AM, Berkelmans R, van Oppen MJH et al (2008) A community change in the algal endosymbionts of a scleractinian coral following a natural bleaching event: field evidence of acclimatization. Proc R Soc B 275(1641):1359–1365
Kaczmarsky LT (2005) Is there a relationship between proximity to sewage effluent and the prevalence of coral disease? Carib J Sci 41(1):124–137
Kaczmarsky LT (2006) Coral disease dynamics in the central Philippines. Dis Aquat Org 69:9–21
Kaczmarsky L, Richardson LL (2007) Transmission of growth anomalies between Indo-Pacific Porites corals. J Invertebr Pathol 94(3):218–221
Kang KI, Torres-Velez FJ, Zhang J et al (2008) Localization of fibropapilloma-associated turtle herpesvirus in green turtles (Chelonia mydas) by in-situ hybridization. J Comp Pathol 139:218–225
Keesing F, Belden LK, Daszak P et al (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468:647–652
Keirstead ND, Brake JW, Griffin MJ et al (2013) Fatal septicemia caused by the zoonotic bacterium Streptococcus iniae during an outbreak in Caribbean reef fish. Vet Pathol. doi:10.1177/030098 5813505876
Kellogg CA, Piceno YM, Tom LM et al (2014) Comparing bacterial community composition of healthy and dark spot-affected Siderastrea siderea in Florida and the Caribbean. PLoS One 9(10):e108767. doi:10.1371/journal.pone.0108767
Kim K, Kim PD, Alker AP (2000) Chemical resistance of gorgonian corals against fungal infections. Mar Biol 137:393–403
Kinne O (1980) Introduction to the treatise and to volume I. In: Kinne O (ed) Diseases of marine animals, vol I. Wiley, Chichester, pp 1–11
Kline DI, Vollmer SV (2011) White band disease (type I) of Caribbean acroporid corals is caused by pathogenic bacteria. Sci Rep 1:1. doi:10.1038/srep7
Kline DI, Kuntz NM, Breitbart M et al (2006) Role of elevated organic carbon levels and microbial activity in coral mortality. Mar Ecol Prog Ser 314:119–125
Knowlton N, Lang JC, Keller BD (1990) Case study of natural population collapse: post-hurricane predation on Jamaican staghorn corals. Smithson Contrib Mar Sci 31:1–25
Koh EGL (1997) Do scleractinian corals engage in chemical warfare against microbes? J Chem Ecol 23(2):379–398
Korrubel JL, Riegl BR (1998) A new disease from the southern Arabian Gulf. Coral Reefs 17:22
Kramarsky-Winter E, Harel M, Siboni N et al (2006) Identification of a protist-coral association and its possible ecological role. Mar Ecol Prog Ser 317:67–73
Krediet CJ, Ritchie KB, Paul VJ et al (2014) Coral-associated micro-organisms and their roles in promoting coral health and thwarting diseases. Proc R Soc B 280(1755):20122328. doi:10.1098/rspb.2012.2328
Kushmaro A, Banin E, Loya Y et al (2001) Vibrio shiloi sp. nov., the causative agent of bleaching of the coral Oculina patagonica. Int J Syst Evol Microbiol 51:1383–1388
Kuta KG, Richardson LL (1997) Black band disease and the fate of diseased coral colonies in the Florida Keys. Proc 8th Int Coral Reef Symp 1:575–578
Kuta KG, Richardson LL (2002) Ecological aspects of black band disease of corals: relationships between disease incidence and environmental factors. Coral Reefs 21:393–398
Lackey RT (2003) Appropriate use of ecosystem health and normative science in ecological policy. In: Rapport DJ, Lasley WL, Rolston DE et al (eds) Managing for healthy ecosystems. Lewis Publishers, Boca Raton, pp 175–186
Lackovich JK, Brown DR, Homer BL et al (1999) Association of herpesvirus with fibropapillomatosis of the green turtle Chelonia mydas and the loggerhead turtle Caretta caretta in Florida. Dis Aquat Org 37:89–97
Lafferty KD (2004) Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol Appl 14(5):1566–1573
Lamb JB, Willis BL (2011) Using coral disease prevalence to assess the effects of concentrating tourism activities on offshore reefs in a tropical marine park. Conserv Biol 25(5):1044–1052
Lancaster J (2000) The ridiculous notion of assessing ecological health and identifying the useful concepts underneath. Hum Ecol Risk Assess 6(2):213–222
Landsberg JH (1995) Tropical reef-fish disease outbreaks and mass mortalities in Florida, USA: what is the role of dietary biological toxins. Dis Aquat Org 22:83–100
Landsberg JH, Balazs GH, Steidinger KA et al (1999) The potential role of natural tumour promotors in marine turtle fibropapillomatosis. J Aquat Anim Health 11:199–210
Lang JC (ed) (2003) Status of coral reefs in the Western Atlantic: results of initial surveys, Atlantic and Gulf Rapid Reef Assessment (AGRRA) Program. Atoll Res Bull 496:1–630
Lawrence SA, Davey JE, Aeby GS et al (2014) Quantification of virus-like particles suggests viral infection in corals affected by Porites tissue loss. Coral Reefs 33:687–691
Lesser MP, Jarett JK (2014) Culture-dependent and culture-independent analyses reveal no prokaryotic community shifts or recovery of Serratia marcescens in Acropora palmata with white pox disease. FEMS Microbiol Ecol 88(3):457–467
Lesser MP, Bythell JC, Gates RD et al (2007) Are infectious diseases really killing corals? Alternative interpretations of the experimental and ecological data. J Exp Mar Biol Ecol 346:36–44
Lessios H (1988) Mass mortality of Diadema antillarum in the Caribbean: what have we learned? Ann Rev Ecol Syst 19:371–393
Lewis SE, Brodie JE, Bainbridge ZT et al (2009) Herbicides: a new threat to the Great Barrier Reef. Environ Pollut 157:2470–2484
Littler MM, Littler DS (1994) A pathogen of reef-building coralline algae discovered in the South Pacific. Coral Reefs 13:202
Littler MM, Littler DS (1995) Impact of CLOD pathogen on Pacific coral reefs. Science 267:1356–1360
Littler MM, Littler DS (1998) An undescribed fungal pathogen of reef-forming crustose coralline algae discovered in American Samoa. Coral Reefs 17:144
Lu Y, Wang Y, Yu Q (2000a) Detection of herpesviral sequences in tissues of green turtles with fibropapilloma by polymerase chain reaction. Arch Virol 145:1885–1893
Lu Y, Yu Q, Zamzow JP, Wang Y et al (2000b) Detection of green turtle herpesviral sequence in saddleback wrasse Thalossoma duperrey: a possible mode of transmission of green turtle fibropapilloma. J Aquat Anim Health 12:58–63
Luter HM, Whalan S, Webster NS (2010) Exploring the role of microorganisms in the disease-like syndrome affecting the sponge Ianthella basta. Appl Environ Microbiol 76(17):5736–5744
Manire CA, Stacy BA, Kinsel MJ et al (2008) Proliferative dermatitis in a loggerhead turtle, Caretta caretta, and a green turtle, Chelonia mydas, associated with novel papillomaviruses. Vet Microbiol 130:227–237
Mao-Jones J, Ritchie KB, Jones LE et al (2010) How microbial community composition regulates coral disease development. PLoS Biol 8(3):e1000345. doi:10.1371/journal.pbio.1000345
Martin LB, Hopkins WA, Mydlarz LD et al (2010) The effects of anthropogenic global changes on immune functions and disease resistance. Ann NY Acad Sci 1195:129–148
McCallum HI, Kuris A, Harvell CD et al (2004) Does terrestrial epidemiology apply to marine systems? Trends Ecol Evol 19(11):586–591
McNamara JM, Buchanan KL (2005) Stress, resource allocation, and mortality. Behav Ecol 16:1008–1017
Miller R, Adams A, Ogden N et al (2003) Diadema antillarum 17 years after mass mortality: is recovery beginning on St. Croix? Coral Reefs 22:181–187
Miller MW, Lohr KE, Cameron CM et al (2014) Disease dynamics and potential mitigation among restored and wild staghorn coral, Acropora cervicornis. PeerJ 2:e541. doi:10.7717/peerj.541
Mitchell R, Chet I (1975) Bacterial attack of corals in polluted seawater. Microb Ecol 2:227–233
Morgan MB, Vogelien DL, Snell TW (2001) Assessing coral stress responses using molecular biomarkers of gene transcription. Environ Toxicol Chem 20:537–543
Muller E, van Woesik R (2012) Caribbean coral diseases: primary transmission or secondary infection? Glob Chang Biol 18:3529–3535
Muller E, van Woesik R (2014) Genetic susceptibility, colony size, and water temperature drive white-pox disease on the coral Acropora palmata. PLoS One 9(11):e110759. doi:10.1371/journal.pone.0110759
Mydlarz L, McGinty ES, Harvell CD (2010) What are the physiological and immunological responses of coral to climate warming and disease? J Exp Biol 213:934–945
Nagelkerken I (2000) Barrel sponge bows out. Reef Encount 28:14–15
Nagelkerken I, Buchan K, Smith GW et al (1997) Widespread disease in Caribbean sea fans: II. Patterns of infection and tissue loss. Mar Ecol Prog Ser 160:255–263
Negandhi K, Blackwelder PL, Ereskovsky AV et al (2010) Florida reef sponges harbor coral disease-associated microbes. Symbiosis 51:117–129
Ng TFF, Manire C, Borrowman K et al (2009) Discovery of a novel single-stranded DNA virus from a sea turtle fibropapilloma by using viral metagenomics. J Virol 83(6):2500–2509
Nugues MM, Smith GW, van Hooidonk RJ et al (2004) Algal contact as a trigger for coral disease. Ecol Lett 7:919–923
Okihiro MS (1988) Chromatophoromas in two species of Hawaiian butterflyfish, Chaetodon multicinctus and C. miliaris. Vet Pathol 25:422–431
Ostfeld RS, LoGiudice KL (2003) Community disassembly, biodiversity, and the erosion of an ecosystem service. Ecology 81:1421–1427
Padilla DK, Williams SL (2004) Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front Ecol Environ 2:131–138
Page C, Willis B (2006) Distribution, host range and large-scale spatial variability in black band disease prevalence on the Great Barrier Reef, Australia. Dis Aquat Org 69:41–51
Palmer CV, Bythell JC, Willis BL (2010) Levels of immunity parameters underpin bleaching and disease susceptibility of reef corals. FASEB J 24:1935–1946
Panek FM (2005) Epizootics and disease of coral reef fish in the tropical western Atlantic and Gulf of Mexico. Rev Fish Sci 13(1):1–21
Patterson KL, Porter JW, Ritchie KB et al (2002) The etiology of white pox, a lethal disease of the Caribbean elkhorn coral, Acropora palmata. Proc Natl Acad Sci U S A 99(13):8725–8730
Peters EC (1993) Diseases of other invertebrate phyla: Porifera, Cnidaria, Ctenophora, Annelida, Echinodermata. In: Couch JA, Fournie JW (eds) Pathobiology of marine and estuarine organisms. CRC Press, Boca Raton, pp 393–449
Peters EC, Yevich PP, Oprandy JJ (1983) Possible causal agent of “white band disease” in Caribbean acroporid corals. J Invertebr Pathol 41:394–396
Peters EC, Halas JC, McCarty HB (1986) Neoplasia in Acropora palmata, with a review of reports on anomalies of growth and form in the stony corals. J Natl Cancer Inst 76:895–912
Petes LE, Harvell CD, Peters EC et al (2003) Pathogens compromise reproduction and induce melanization in Caribbean sea fans. Mar Ecol Prog Ser 264:167–171
Pinzón CJH, Beach-Letendre J, Weil E et al (2014) Relationship between phylogeny and immunity suggests older Caribbean coral lineages are more resistant to disease. PLoS One 9(8):e104787. doi:10.1371/journal.pone.0104787
Pollock FJ, Morris PJ, Willis BL, Bourne DG (2011) The urgent need for robust coral disease diagnostics. PLoS Pathog 7(10):e1002183. doi:10.1371/journal.ppat.1002183
Polson SW (2007) Comparative analysis of microbial community structure associated with acroporid corals during a disease outbreak in the Florida Reef Tract. PhD dissertation, Medical University of South Carolina, Charleston
Porter JW (ed) (2001) The ecology and etiology of newly emerging marine diseases. Kluwer Academic Publishers, Dordrecht
Porter JW, Tougas JI (2001) Reef ecosystems: threats to their biodiversity. In: Levin SA (ed) Encyclopedia of biodiversity, vol 5. Academic Press, New York, pp 73–95
Porter JW, Kosmynin V, Patterson KL et al (2002) Detection of coral reef change by the Florida Keys Coral Reef Monitoring Project. In: Porter JW, Porter KG (eds) The Everglades, Florida Bay, and coral reefs of the Florida Keys: an ecosystem sourcebook. CRC Press, Boca Raton, pp 749–769
Porter JW, Torres C, Sutherland KP et al (2011) Prevalence, severity, lethality, and recovery of dark spots syndrome among three Floridian reef-building corals. J Exp Mar Biol Ecol 408:79–87
Quéré G, Steneck RS, Nugues MM (2014) Spatiotemporal and species-specific patterns of diseases affecting crustose coralline algae in Curaçao. Coral Reefs. doi:10.1007/s00338-014-1225-3
Råberg L (2014) How to live with the enemy: understanding tolerance to parasites. PLoS Biol 12(11):e1001989. doi:10.1371/journal.pbio.1001989
Rapport DJ (1999) Epidemiology and ecosystem health: natural bridges. Ecosyst Health 3:174–180
Rapport DJ, Regier HA, Hutchinson TC (1985) Ecosystem behavior under stress. Am Nat 125(5):617–640
Ravindran J, Raghukumar C, Raghukumar S (1999) Disease and stress-induced mortality of corals in Indian Reefs and observations on bleaching of corals in the Andamans. Curr Sci 76(2):233–237
Raymundo L, Couch CS, Harvell CD (2008) Coral disease handbook: guidelines for assessment, monitoring & management. Coral Reef Targeted Research and Capacity Building for Management Program. University of Queensland, St. Lucia
Remily ER, Richardson LL (2006) Ecological physiology of a coral pathogen and the coral reef environment. Microb Ecol 51:345–352
Renegar DA, Blackwelder PL, Miller JD et al (2008) Ultrastructural and histological analysis of dark spot syndrome in Siderastrea siderea and Agaricia agaricites. Proc 11th Int Coral Reef Symp, p 185–189
Richardson LL (1998) Coral diseases: what is really known? Trends Ecol Evol 13:438–443
Richardson LL, Aronson RB (2000) Infectious diseases of reef corals. Proc 9th Int Coral Reef Symp 2:1225–1230
Richardson LL, Kuta KG, Schnell S et al (1997) Ecology of the black band disease microbial consortium. Proc 8th Int Coral Reef Symp 1:597–600
Richardson LL, Goldberg W, Kuta KG et al (1998) Florida’s mystery coral-killer identified. Nature 392:557–558
Richardson LL, Smith GW, Ritchie KB et al (2001) Integrating microbiological, microsensor, molecular, and physiologic techniques in the study of coral disease pathogenesis. Hydrobiologia 460:71–89
Richardson LL, Sekar R, Myers JL et al (2007) The presence of the cyanobacterial toxin microcystin in the black band disease of corals. FEMS Microbiol Lett. doi:10.1111/j.1574-6968.2007.00751.x
Richmond RH (1993) Coral reefs: present problems and future concerns resulting from anthropogenic disturbance. Am Zool 33(6):524–536
Ritchie KB, Smith GW (1998) Type II white-band disease. Rev Trop Biol 46(Suppl 5):199–203
Ritchie KB, Polson SW, Smith GW (2001) Microbial disease causation in marine invertebrates: problems, practices, and future prospects. Hydrobiologia 460:131–139
Robblee MB, Barber TR, Carlson PR Jr et al (1991) Mass mortality of the tropical seagrass Thalassia testudinum in Florida Bay (USA). Mar Ecol Prog Ser 71(3):297–299
Roberts RJ (ed) (1989) Fish pathology. Bailliere Tindall, London
Roder C, Arif C, Bayer T et al (2013) Bacterial profiling of white plague disease in a comparative coral species framework. ISMEJ 8:31–39 doi:10.1038/ismej.2013.127
Rogers CS (1985) Degradation of Caribbean and western Atlantic coral reefs and decline of associated fisheries. Proc 5th Int Coral Reef Cong 6:491–496
Rogers CS (2010) Words matter: recommendations for clarifying coral disease nomenclature and terminology. Dis Aquat Org 91:167–175
Rogers CS, Muller E (2012) Bleaching, disease and recovery in the threatened scleractinian coral Acropora palmata in St. John, US Virgin Islands: 2003–2010. Coral Reefs 31(3):807–819
Rohde K (1993) Ecology of marine parasites. CAB International, Wallingford
Rohwer F, Seguritan V, Azam F et al (2002) Diversity and distribution of coral-associated bacteria. Mar Ecol Prog Ser 243:1–10
Rosenberg E, Loya Y (eds) (2004) Coral health and disease. Springer, Berlin
Rougee L, Downs CA, Richmond RH et al (2006) Alteration of normal cellular profiles in the scleractinian coral (Pocillopora damicornis) following laboratory exposure to fuel oil. Environ Toxicol Chem 25:3181–3187
Rowan R, Knowlton N, Baker A et al (1997) Landscape ecology of algal symbionts creates variation in episodes of coral bleaching. Nature 388:285–289
Rützler K (1988) Mangrove sponge disease induced by cyanobacterial symbionts: failure of a primitive immune system? Dis Aquat Org 5:143–149
Rützler K, Santavy DL, Antonius A (1983) The black band disease of Atlantic reef corals III. Distribution, ecology and development. PSZNI Mar Ecol 4:329–335
Santavy DL, Peters EC, Quirolo C et al (1999) Yellow-blotch disease outbreak on reefs of the San Blas Islands, Panama. Coral Reefs 18:97
Santavy DL, Mueller E, Peters EC et al (2001) Quantitative assessment of coral diseases in the Florida Keys: strategy and methodology. Hydrobiologia 460:39–52
Sato Y, Bourne DG, Willis BL (2009) Dynamics of seasonal outbreaks of black band disease in an assemblage of Montipora species at Pelorus Island (Great Barrier Reef, Australia). Proc R Soc B 276:2795–2803
Schmale MC (1991) Prevalence and distribution patterns of tumors in bicolor damselfish (Pomacentrus partitus) on South Florida reefs. Mar Biol 109:203–212
Schmale MC, Gibbs PDL, Campbell CE (2002) A virus-like agent associated with neurofibromatosis in damselfish. Dis Aquat Org 49:107–115
Schofield PJ (2009) Geographic extent and chronology of the invasion of non-native lionfish (Pterois volitans and P. miles) in the Western North Atlantic and Caribbean Sea. Aquat Invasions 4:473–479
Scully EP, Prappas J, Ostrander GK (2001) Laboratory models for the study of coral pathologies. Hydrobiologia 460:91–95
Selig ER, Harvell CD, Bruno JF et al (2006) Analyzing the relationship between ocean temperature anomalies and coral disease outbreaks at broad spatial scales. Coast Estuar Stud 61:111–128
Shelley CC, Glazebrook JS, Turak E et al (1988) Trematode (Digenea: Bucephalidae) infection in the burrowing clam Tridacna crocea from the Great Barrier Reef. Dis Aquat Org 4:143–147
Sheppard BJ, Dungan CF (2009) Exotic Perkinsus sp. protozoa in an imported Vietnamese ornamental clam (Tridacna crocea) maintained in a home aquarium. J Zoo Wildl Med 40(1):140–146
Sheppard BJ, Phillips AC (2008) Perkinsus olseni detected in Vietnamese aquacultured reef clams Tridacna crocea imported to the USA, following a mortality event. Dis Aquat Org 79(3):229–235
Shields JD (2011) Diseases of spiny lobsters: a review. J Invertebr Pathol 106(1):79–91
Shields JD, Behringer DC Jr (2004) A new pathogenic virus in the Caribbean spiny lobster Panulirus argus from the Florida Keys. Dis Aquat Org 59:109–118
Shinn EA, Smith GW, Prospero JM et al (2000) African dust and the demise of Caribbean coral reefs. Geophys Res Lett 27:3029–3032
Sindermann CJ (1990) Principal diseases of marine fish and shellfish, vol. 2: diseases of marine shellfish. Academic, San Diego
Skinner RH, Kandrashoff W (1988) Abnormalities and diseases observed in commercial fish catches from Biscayne Bay, Florida. Water Resour Bull 24(5):961–966
Slattery M, Renegar DA, Gochfeld DJ (2013) Direct and indirect effects of a new disease of alcyonacean soft corals. Coral Reefs 32(3):879–889
Soffer N, Brandt ME, Correa AMS et al (2013) Potential role of viruses in white plague coral disease. ISMEJ 8(2):271–283
Sokolova IM, Frederich M, Bagwe R et al (2012) Energy homeostasis as an integrative tool for assessing the limits of stress tolerance in aquatic invertebrates. Mar Environ Res 79:1–15
Sousa WP (1991) Can models of soft-sediment community structure be complete without parasites? Am Zool 31:821–830
Stacy BA, Wellehan JFX, Foley AM et al (2008) Two herpesviruses associated with disease in wild loggerhead sea turtles (Caretta caretta). Vet Microbiol 126:63–73
Stanić D, Oehrle S, Gantar M et al (2011) Microcystin production and ecological physiology of black band disease cyanobacteria. Environ Microbiol 13(4):900–910
Stebbing ARD (1981) Stress, health and homeostasis. Mar Pollut Bull 12:326–329
Stentiford GD (2011) Diseases of commercially exploited crustaceans: cross-cutting issues for global fisheries and aquaculture. J Invertebr Pathol 106:3–5
Stoskopf MK (1993) Fish medicine. WB Saunders Co, Philadelphia
Susser M (1991) What is cause and how do we know one? A grammar for pragmatic epidemiology. Am J Epidemiol 133:635–648
Sussman M, Willis BL, Victor S et al (2008) Coral pathogens identified for white syndrome (WS) epizootics in the Indo-Pacific. PLoS One 3(6):e2393. doi:10.1371/journal.pone.0002393
Suter GW (1993) A critique of ecosystem health concepts and indices. Environ Toxicol Chem 12:1533–1539
Sutherland KP, Porter JW, Torres C (2004) Disease and immunity in Caribbean and Indo-Pacific zooxanthellate corals. Mar Ecol Prog Ser 266:273–302
Sutherland KP, Shaban S, Joyner JL et al (2011) Human pathogen shown to cause disease in the threatened elkhorn coral Acropora palmata. PLoS One 6(8):e23468. doi:10.1371/journal.pone.0023468
Sutton DC, Garrick R (1993) Bacterial disease of cultured giant clam Tridacnas gigas larvae. Dis Aquat Org 16(1):47–53
Sweet M, Bythell J (2012) Ciliate and bacterial communities associated with white syndrome and brown band disease in reef-building corals. Environ Microbiol 14(8):2184–2199
Sweet M, Burn D, Croquer A et al (2013) Characterization of the bacterial and fungal communities associated with different lesion sizes of dark spot syndrome occurring in the coral Stephanocoenia intersepta. PLoS One 8(4):e62580. doi:10.1371/journal.pone.0062580
Sweet MJ, Croquer A, Bythell JC (2014) Experimental antibiotic treatment identifies potential pathogens of white band disease in the endangered Caribbean coral Acropora cervicornis. Proc R Soc B 281:20140094
Thayer GW, Murphey PL, LaCroix MW (1994) Responses of plant communities in western Florida Bay to the die-off of seagrasses. Bull Mar Sci 54(3):718–726
Toledo-Hernández C, Zuluaga-Montero A, Bones-González A et al (2008) Fungi in healthy and diseased sea fans (Gorgonia ventalina): is Aspergillus sydowii always the pathogen? Coral Reefs 27(3):707–714
Tompkins DM, Dunn AM, Smith MJ et al (2011) Wildlife diseases: from individuals to ecosystems. J Anim Ecol 80:19–38
Udey L, Young RE, Sallman B (1977) Isolation and characterization of an anaerobic bacterium, Eubacterium tarantella spp. nov., associated with striped mullet (Mugilcephalus) mortality in Biscayne Bay, Florida. J Fish Res Board Can 34:402–409
Upton SJ, Peters EC (1986) A new and unusual species of Coccidium (Apicomplexa, Agamococcidiorida) from Caribbean scleractinian corals. J Invertebr Pathol 47:184–193
USEPA (2000) Stressor identification guidance document. EPA/822/B-00/025. US Environmental Protection Agency, Office of Water and Office of Research and Development, Washington, DC
Ushijima B, Smith A, Aeby GS et al (2012) Vibrio owensii induces the tissue loss disease Montipora white syndrome in the Hawaiian reef coral Montipora capitata. PLoS One 7(10):e46717. doi:10.1371/journal.pone.0046717
Vargas-Ángel B (2010) Crustose coralline algal diseases in the U.S.-affiliated Pacific Islands. Coral Reefs 29:943–956
Vega Thurber RL, Correa AMS (2011) Viruses of reef-building scleractinian corals. J Exp Mar Biol Ecol 408(1):102–113
Vicente VP (1989) Regional commercial sponge extinctions in the West Indies: are recent climatic changes responsible? Mar Ecol 10(2):179–191
Voss JD, Richardson LL (2006) Nutrient enrichment enhances black band disease progression in corals. Coral Reefs 25:569–576
Ward JR, Lafferty KD (2004) The elusive baseline of marine disease: are diseases in ocean ecosystems increasing? PLoS Biol 2(4):e120. doi:10.1371/journal.pbio.0020120
Webster NS (2007) Sponge disease: a global threat? Environ Microbiol 9(6):1363–1375
Webster NS, Taylor MW (2012) Marine sponges and their microbial symbionts: love and other relationships. Environ Microbiol 14(2):335–346
Weil E (2004) Coral reef diseases in the wider Caribbean. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, Berlin, pp 35–68
Weil E, Rogers CS (2011) Coral reef diseases in the Atlantic-Caribbean. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer, Dordrecht, pp 465–491
Weil E, Urreiztieta I, Garzón-Ferreira J (2000) Geographic variability in the incidence of coral and octocoral diseases in the wider Caribbean. Proc 9th Int Coral Reef Symp 2:1231–1237
Weis V (2008) Cellular mechanisms of cnidarian bleaching: stress causes the collapse of symbiosis. J Exp Biol 211:3059–3066
Williams EH Jr, Buckley-Williams L (1990) The world-wide coral reef bleaching cycle and related sources of coral mortality. Atoll Res Bull 335:1–71
Williams EH Jr, Bunkley-Williams L (2000) Marine major ecological disturbances of the Caribbean. Infect Dis Rev 2(3):110–127
Williams DE, Miller MM (2005) Coral disease outbreak: pattern, prevalence and transmission in Acropora cervicornis. Mar Ecol Prog Ser 301:119–128
Williams EH Jr, Wolf-Waters TJ (1990) An abnormal incidence of the commensal copepods, Doridocola astrophyticus Humes, associated with injury of its host, the basketstar, Astrophyton muricatum (Lamarck). Crustaceans 59(3):302
Williams EH Jr, Bunkley-Williams L, Peters EC et al (1994) An epizootic of cutaneous fibropapillomas in green turtles Chelonia mydas of the Caribbean: part of a panzootic? J Aquat Anim Health 6:70–78
Williams GJ, Price NN, Ushijima B et al (2014) Ocean warming and acidification have complex interactive effects on the dynamics of a marine fungal disease. Proc R Soc B 281(1778):20133069. doi:10.1098/rspb.2013.3069
Wilson B, Aeby GS, Work TM et al (2012) Bacterial communities associated with healthy and Acropora white syndrome-affected corals from American Samoa. FEMS Microb Ecol 80:509–520
Wobeser GA (2007) Disease in wild animals, investigation and management. Springer, Heidelberg
Wood CL, Sandin SA, Zgliczynski B et al (2014) Fishing drives declines in fish parasite diversity and has variable effects on parasite abundance. Ecology 95(7):1929–1946
Woodley CM, Downs CA, Fauth JE et al (2000) A novel diagnostic system to assess the physiological status of corals. Proc 9th Int Coral Reef Symp 2:1267–1272
Woodley CM, Bruckner AW, Galloway SB et al (2003) Coral disease and health: a national research plan. National Oceanic and Atmospheric Administration. Silver Spring, Maryland
Woodley CM, Downs CA, Bruckner A et al (eds) (2015) Diseases of corals. Wiley-Blackwell, Hoboken, in press
Work TM, Aeby GS (2011) Pathology of tissue loss (white syndrome) in Acropora sp. corals from the Central Pacific. J Invertebr Pathol 107(2):127–131
Work TM, Meteyer C (2014) To understand coral disease, look at coral cells. Ecohealth. doi:10.1007/s10393-014-0931-1
Work TM, Balazs GH, Rameyer RA et al (2004a) Retrospective pathology survey of green turtles Chelonia mydas with fibropapillomatosis in the Hawaiian Islands, 1993–2003. Dis Aquat Org 62:163–176
Work TM, Rameyer RA, Takata G (2004b) Protozoal and epitheliocystis-like infections in the introduced bluestripe snapper Lutjanus kasmira in Hawaii. Dis Aquat Org 57:59–66
Work TM, Aeby GS, Coles SL (2008a) Distribution and morphology of growth anomalies in Acropora from the Indo-Pacific. Dis Aquat Org 78:255–264
Work TM, Richardson LL, Reynolds TL et al (2008b) Biomedical and veterinary science can increase our understanding of coral disease. J Exp Mar Biol Ecol 362(2):63–70
Work TM, Russell R, Aeby GS (2012) Tissue loss (white syndrome) in the coral Montipora capitata is a dynamic disease with multiple host responses and potential causes. Proc R Soc B 279(1746):4334–4341
Wulff J (2006a) Ecological interactions of marine sponges. Can J Zool 84:146–166
Wulff J (2006b) Rapid diversity and abundance decline in a Caribbean coral reef sponge community. Biol Conserv 127:167–176
Yamashiro H, Oku H, Onaga K, Iwasaki H, Takara K (2000) Coral tumors store reduced level of lipds. J Exp Mar Biol Ecol 265:171–179
Zann LP, Cuffey RJ, Kropach C (1975) Fouling organisms and parasite associated with the skin of sea snakes. In: Dunson WA (ed) The biology of sea snakes. University Park Press, Baltimore, pp 151–265
Acknowledgements
I thank the many people who have supported and contributed to the study of diseases of coral reef organisms, particularly Paul P. Yevich and his graduate students and colleagues who fostered my developing curiosity about pathology, and all those who continue efforts to understand the nature of diseases and their impacts on the coral reef ecosystems of the world.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Peters, E.C. (2015). Diseases of Coral Reef Organisms. In: Birkeland, C. (eds) Coral Reefs in the Anthropocene. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7249-5_8
Download citation
DOI: https://doi.org/10.1007/978-94-017-7249-5_8
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7248-8
Online ISBN: 978-94-017-7249-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)