Abstract
The geologic record suggests a diverse array of reef-building corals will survive increasing CO2, but the relative prevalence of different types will shift and the reefs will become degraded and eroded. Although many corals may not go extinct, if pH decreases effectively, reef ecosystem services will deteriorate because bioerosion will accelerate and, for some coral species, net skeletal construction will require more energy when the aragonite saturation state decreases. A similar pattern of many genera of reef-building scleractinian corals surviving, but with relatively little reef accretion, was seen through the roughly 140 million years from the Late Jurassic to the late Paleogene when the calcite seas (Mg/Ca mole ratio <2) and pH <7.8 were unfavorable for aragonitic reef accretion. The geologic record suggests that the corals most vulnerable to extinction were the fast-growing branching species because the traits that provide fast growth have tradeoffs with traits that provide tolerance of stressful environments. Iteroparous animals such as corals are adapted for survival under stressful conditions at the expense of fecundity. Surveys have recorded widespread decreases in living coral cover, but the less visible decrease in fecundity from stress may be more insidious to population recovery. Reduced fecundity and less dense population distribution can act synergistically to produce Allee effects in sessile animals such as corals. Natural coral-reef ecosystems give the appearance of inverted trophic pyramids, but when fished down by about 80 %, recovery has usually not happened, possibly because the larger individuals in the populations were a major source of fecundity. Although biomass of eukaryotes appear to be in inverted trophic pyramids, the turnover and energy is in the form of standard pyramids and although large individuals in the upper trophic levels are especially sensitive to exploitation, subsistence economies can be maintained by harvesting the medium-sized individuals. Large individuals matter more than population biomass because of the distinct roles of large individuals in ecological processes maintaining coral-reef ecosystems and the relatively large reproductive potential of big fishes. The functional traits of both the coral-reef ecosystem and its component animals provide a greater potential for exploitation by globalization in a service-based economy than with an extractive economy, as exemplified by Palau.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 Fecundity and the Loss of Community Resilience
In 1928, a relatively dense population of pearl oysters Pinctada margaritifera was found on Pearl and Hermes Reef in the Northwest Hawaiian Islands. About 150,000 oysters were taken during the next 2 years. A survey was done the following year, 1930, and again in 1994, 2000, and 2003. Although pearl oysters were still present and reproducing, the overall population density was still similar in 2003 to that right after the harvest in 1930 (Keenan et al. 2006). For species in which the adults are sessile, fertilization success decreases when the population density falls below a threshold (Fig. 11.4; Sect. 11.4; Birkeland et al. 2013). Although fecundity may be at normal levels in individuals in the population, the reproductive potential of the population as a whole may become too low to provide enough successful recruitment beyond the cost of mortality of larval and juvenile stages to allow the population to expand and recover to its previous size. Many marine species are “boom and bust” (Uthicke et al. 2009) and episodic abundant recruitment of normally rare species can occasionally allow benthic coral-reef invertebrates such as sponges, corallimorpharians, didemnid tunicates, and Acanthaster planci to swamp the usual rates of larval and juvenile mortality and allow the species to recover to the higher level. Hughes et al. (2000) found that the amount of recruitment to coral populations on the Great Barrier Reef was not associated with the number of adult colonies (living coral cover) but with fecundity of the existing colonies. In corals, fecundity decreases as the colony is stressed (Sect. 12.2.2) and the potential of coral communities to provide increased recruitment that exceeds the normal rates of larval and juvenile mortality and thereby recover from disturbance may decrease with stresses and with added energy demands of climate change , lower ocean pH, and effects of human activities.
Although fishes are motile and therefore not as vulnerable to decreased fertilization when their populations decline, once below a threshold population size, fish are also usually unable to meet the normal rates of larval and juvenile mortality and many populations show no sign of recovery even decades after the fishing pressure was removed (Sect. 12.5; Hutchings and Reynolds 2004). The lower reproductive and recovery potential of motile fish populations may result from the selected harvest of the larger individuals. Fecundity increases exponentially with body size in fish. The fish associations of coral reefs are characterized in their natural state as inverted trophic pyramid s with the top trophic level , piscivores, making up four fifth of the biomass (Friedlander and DeMartini 2002; DeMartini et al. 2008; Sandin et al. 2008; Fenner 2014). Fishing effort focuses primarily on the larger individuals which are especially vulnerable to modern fishing technology (Fenner 2014). Once the larger individuals are gone, the fecundity is greatly reduced. There are “no quick fixes” (Craig 2005) and the inverted trophic pyramids have generally not reappeared (Sect. 12.5). Many coral reefs lost these natural characteristics of coral-reef systems hundreds of years ago (Jackson 1997; Wing and Wing 2001). In the last half century, most populations of large fish have not recovered even after the fishing pressure was removed (Sect. 12.5). Hutchings and Reynolds (2004) analyzed more than 230 populations of fishes with a median decrease in population size of 83 % and found that most exhibit little or no change for at least 15 years after the collapse.
12.2 Natural Selection Favors Survival over Reproduction in Corals
12.2.1 Unreliable Recruitment Leads to Multiple Reproductive Attempts
Lamont C. Cole (1954) explained how age at which reproduction begins is one of the most important characteristics determining the potential of a species to increase rapidly in abundance. Starting reproduction soon, e.g., within a year, gives a species a potential for exponentially greater number of offspring after several years than species that take two or more years to mature. If the offspring of the species are also reproducing when the parents would potentially reproduce a second time, then the selective importance of the parents would be relatively low after the first reproduction because it would be just one of a number of progenitors. For example, a Chinook salmon can produce 15,000 eggs. If it were probable that one percent of its first batch of offspring survived to reproduce, the importance of the parent’s contribution to future generations from a second year’s reproduction would be only 1/150 of the importance of its first reproduction. If the offspring carried genes that do not favor future reproduction and fitness of the elderly, the 150 could have passed on these genes without selection against them. For a second example, if human parents often successfully started families by early to mid-20s, and their offspring were also generally successful in starting families by their early to mid-20s, then the selective pressure to maintain fecundity after their mid-40s or early 50s (i.e., postponing menopause), or avoiding Parkinson’s or Alzheimer’s disease would not be strong. Natural selection for rapid population growth strongly favors reproducing successfully as soon as possible, with little selective pressure towards reproducing after the next generation is reliably established. In contrast to species adapted to rapidly expand populations, most of the dominant animals on coral reefs take longer than a year to mature and then live to reproduce multiple times.
An analysis of data from 251 species of fishes (Longhurst 2002) showed that as recruitment success becomes less reliable, the traits of late maturity, increased longevity, and iteroparity substantially increases (Murphy 1967; Schaffer 1974; Jennings et al. 2001). The number of years of reproductive activity typical of a species might indicate the usual degree of risk of failure to produce successful recruitment and the length of life and number of reproductive attempts to accommodate the risk. In complex coral-reef communities with large standing stocks and relatively low concentrations of nutrient input , intense and ubiquitous predation and competition make recruitment hazardous and unreliable for many species. Since many reef-building corals can live for decades (Fig. 12.1), it seems reasonable that when stressful or disruptive environmental conditions prevail, committing resources towards survival until a time in which conditions for recruitment are more likely to be successful should be favored by natural selection . Coral-reef fishes are also exceptionally long-lived, in contrast to larger, faster-growing, pelagic fishes (Sect. 12.5.3) and the traits of offspring (egg size, egg quality, larval size, larval quality, length of time before dying of starvation) of many species of coral-reef fishes actually increase with the mother’s age (Hixon et al. 2013). Perhaps early reproductive success is not likely, so it is worth “bet-hedging ”.
If settling planulae and juvenile corals are just as vulnerable to stressful conditions as adults, then natural selection might favor adult corals that survive until favorable conditions occur and then reproduce many times. The planulae or juveniles of 1 year may not survive if conditions are stressful. Immature immune systems (Frank et al. 1997) and lack of an adequately developed protective microbial association (Apprill et al. 2009; Sharp et al. 2010) make juvenile corals especially susceptible to harmful microbes (Vermeij et al. 2009; Marhaver et al. 2013). Newly settled coral recruits are especially vulnerable to lower seawater pH (Webster et al. 2013), lower availability of carbonate ions (Kleypas et al. 1999a, b; Albright et al. 2008), reduction of aragonite supersaturation (Cohen and Holcomb 2009), and greater difficulty for the precipitation of calcium carbonate skeletons associated with increased CO2 (Albright et al. 2010). Low pH of seawater can especially affect coral recruits during their first CaCO3 deposition. For certain coral species, crustose coralline algae attract recruits (Morse et al. 1988), but increased CO2 also adversely affects crustose coralline algae (Kuffner et al. 2008), thereby adversely affecting coral recruitment (Doropoulos et al. 2012; Webster et al. 2013).
In fact, planulae settling near adult colonies of their own broadcast-spawning species have lower survival because of harmful microbes that are associated with the adults (Marhaver et al. 2013). Although elegant experiments in both field and laboratory have shown that broadcast-spawning species are vulnerable to microbes associated with adult colonies of their own species (Marhaver et al. 2013), brooding corals may have an advantage of inoculation. The planulae of Heliopora coerulea are brooded on the surface of the adult colony for 6–14 days (Babcock 1990). During these days, the large (3.7 mm long) bright white planulae attract several species of butterflyfishes that prey upon them for extensive periods of time (at least 30 min, Villanueva and Edwards 2010). In the converse of the Janzen-Connell model in which species diversity is favored by seeds and seedlings being less favored to survive near adults of their species because of species-specific predators and pathogens attracted to adults (Marhaver et al. 2013), it is the larval Heliopora that attract predators. The larvae of brooding Pocillopora damicornis have been seen to successfully settle and live next to adult colonies of P. damicornis transplanted on a terra cotta brick into heavily sedimented Ylig Bay, Guam, where the species was not otherwise found (pers. obs.). Likewise, over three hundred spat were observed to have settled on a plastic tray to which a single adult P. damicornis was attached in a water table at the University of Guam Marine Laboratory. The advantages in inoculation of brooding larvae over the vulnerabilities of broadcast-spawning might be analogous to breast-feeding in humans over feeding infants on formulae in bottles.
12.2.2 Tradeoff Between Survival and Fecundity
An often accepted definition of coral-reef “resilience ” is “…the ability of reefs to absorb recurrent disturbances…and rebuild coral-dominated systems” (Hughes et al. 2007). This definition combines two processes, “absorb” (tolerance , acclimatization , adaptation ) and “rebuild” (reproduce, successfully recruit, repair, heal) that often trade off against each other. For example, the decrease of available energy because of turbidity and/or the increased energy expenditure for shedding sedimentation results in a reduction in gametogenesis (Kojis and Quinn 1984). The expenditure for repair of tissue damage can take enough energetic resources to prevent gametogenesis for several years (Lirman 2000). When conditions become stressful and the adult coral requires additional energy to survive, gametogenesis is often reduced to provide the extra energy needed for adult survival (Kojis and Quinn 1985; Van Veghel and Bak 1994). When corals recover from stress that caused bleaching , it can be 4 years before they spawn again (Levitan et al. 2014). The metabolic energy expenditure for recovery of the adult colony from bleaching in response to warm seawater is also paid for by reduction in fecundity (Michalek-Wagner and Willis 2001; Ward et al. 2002), gamete quality (Omori et al. 2001), or gametogenesis the following year (Szmant and Gassman 1990). Likewise, energy spent for survival of the colony in physiological response to elevated levels of nutrients (Ward and Harrison 1997, 2000) results in fewer larvae or eggs per colony. Increased ammonium stopped planulation in the brooding coral Pocillopora damicornis. Planulation resumed 3 months after the ammonium input was halted. Increased ammonium caused a significant reduction in the size of eggs spawned by the coral Montipora capitata (Cox and Ward 2002). There is a general reduction in gametogenesis and larval development along a gradient of increased eutrophication (Tomascik and Sander 1987; Tomascik 1991; Hunte and Wittenberg 1992; Wittenberg and Hunte 1992). Kojis and Quinn (1984) suggested variation in coral fecundity can be used to monitor environmental stress on corals.
Chronic disturbances brought about by increased CO2, such as ocean warming, ocean acidification , and lowering of the aragonite saturation state , have been projected to move continuously outside the historical bounds at any given site on Earth by 2069 (±18 years s.d., Mora et al. 2013). This trend will probably not be reversed for about 1,000 years after the anthropogenic emissions stop (Archer and Brovkin 2008; Solomon et al. 2009). Ultimately, it is a proper combination of survival and gametogenesis that will be favored in surviving corals. But selection for survival responds to current environmental factors that are changing rapidly and may select against the proper combination of characteristics needed in the future. The tradeoff of increased survival at a cost of reduction in fecundity that is frequently observed these days does not appear to accommodate the general increase in the chronic nature of disturbances (Chap. 11). With long term chronic change, survival of adult colonies or clones by acclimatization (physiological adjustment of the colony), does not necessarily enhance adaptation (population genetic adjustment).
12.2.3 Chronic Stress Potentially Leads by Positive Feedback Processes to Decline in Recruitment and Connectivity
If stressful conditions are chronic , a positive feedback system can develop that drives the system into a continuous decrease in corals (Fig. 11.4, Sect. 11.4). Continuous energy loss with physiological stress or repair lowers fecundity which in turn leads to fewer larvae and fewer successful recruits and thereby fewer adult colonies. Fewer adult colonies then leads to a self-reinforcing (positive feedback) sequence because fewer sessile adults diminishes both population fecundity and success in fertilization . The decrease in fertilization success results from fewer adults because a lower number of adult colonies most often lowers population density of adult colonies which, because coral colonies are sessile, leads to a greater average distance between colonies. Field observations of a broadcast-spawning species indicated that if the effective distance between colonies falls below one spawning colony of a species within 100 m2, the probability for fertilization would be nearly zero (Levitan et al. 2004). If only 20–30 % of the corals of a species spawn on a particular night (Levitan et al. 2004), the lowest absolute population density for successful fertilization would be more like 3 or 4 colonies per 100 m2.
Lower fecundity also leads to less connectivity among populations (Fig. 12.2). Although many, perhaps the majority, of coral species have potential larval pelagic duration periods of weeks (Harrigan 1972), some over 200 days (Harrigan 1972; Graham et al. 2008), most of these same species show maximum settlement within the first 3 days (Harrigan 1972). For example, the planulae of Pocillopora damicornis can survive for at least 212 days, but an average of 24 % settle within the first 4–8 h and the majority (70 %) settle within first 24 h (Harrigan 1972). In the near future, the warming of seawater may further shorten substantially the pelagic larval duration of coral planulae. Harrigan (1972) noted the average free-swimming period for P. damicornis planulae in Palau (26–30 °C) is half of that in Hawaii (24–27 °C) and Harrigan’s laboratory records provided evidence for shortened pelagic duration in warmer water.
A large proportion of planulae recruit near their population of origin. As the fecundity of the population is substantially reduced by stress or mortality , the absolute number of larvae that are transported great distances decreases and so stress or mortality also decreases connectivity (Figure is modified from Steneck 2006). The horizontal dotted line represents the number or recruits necessary to meet local mortality, so the source population fecundity must be protected to sustain local populations. Natural selection favors fidelity to the parental population, but incidental “leakage” to distant sites, though selected against, is probably sufficient to spread risks from local disasters among descendants (Strathmann et al. 2002)
Although coral reefs are not completely self-recruiting, genetic studies have indicated that a substantial portion of larvae often recruit back to their population of origin (Sammarco and Andrews 1989; Swearer et al. 1999, 2002; Jones et al. 1999, 2005, 2009; Warner and Cowen 2002; Poulin et al. 2002; Cowen et al. 2000, 2006; Almany et al. 2007; Gerlach et al. 2007; Planes et al. 2009; Underwood et al. 2009; Shanks and Shearman 2009; Harrison et al. 2012; Golbuu et al. 2012; López-Duarte et al. 2012). In addition to the tendency of planulae to settle near home, the rates of mortality of coral larvae are especially high early in life, and so the connectivity at regional scales is less than assumed (Graham et al. 2008). Furthermore, at least in some cases, the probability of successful dispersal declines substantially after 1 km (Buston et al. 2011). The distance of population connectivity is not as reliably predicted by pelagic larval duration as is usually assumed (Weersing and Toonen 2009). Even for species with larvae that have long-pelagic duration, successful long-distance dispersion is a byproduct of large parental populations (Fig. 12.2; Strathmann et al. 2002).
Even when planulae succeed in reaching a disturbed area, survival of coral recruits is severely reduced by unwelcoming conditions such as sedimentation and prevalence of algae (Fabricius 2005). Rates of successful settlement and metamorphosis of corals are nearly zero in areas with sediment . Macroalgae and filamentous turfs preempt available space, trap sediment, intercept light, physically abrade the small coral recruit, transmit coral diseases and allelopathic chemicals, increase the growth of microbes on the surface mucus of corals and potentially affect the small recruits in additional ways (Littler and Littler 1997; Szmant 2002; Nugues et al. 2004; Kuffner and Paul 2004; Fabricius 2005; Smith et al. 2006; Birrell et al. 2008; Kline et al. 2006; Ritson-Williams et al. 2009). But negative settlement clues that trump positive settlement cues may be an adaptation to preempt mortality of coral recruits from sedimentation, algal dominance, or other fatal factors.
Coral communities develop quickly and well on flat basalt with no topographic complexity (Fig. 12.3), a substratum that obviously attracts coral recruits. Roughened plexiglass sometimes attracts more successful coral recruits than does the surrounding CaCO3 reef surface (pers. obs. with Birkeland 1977; Birkeland et al. 1981). Pocillopora damicornis often grows on rope, pilings, cement and other artificial structures in harbors (pers. obs.) and Jokiel (1990) found corals attached to pumice and other floating objects. It may be that planulae are not actually attracted to anthropogenic artificial materials or to basalt or pumice. Rather, these materials lacked negative warning cues associated with factors on disturbed reefs. In experiments with biofilms associated with materials from the Great Barrier Reef (GBR), Prescott et al. (2014) found that Acropora millepora and Pocillopora damicornis settled significantly more on untreated laboratory biofilms than on biofilms that had developed in the presence of particulate organic matter or sediment on the GBR. Her DNA analysis of the microbial communities on the surfaces on which the planulae favored for settlement and those on which they apparently avoided for settlement and metamorphosis indicated that the patterns of association were mostly negative, i.e., the negative associations were strong and determinant while positive association were weak and apparently trumped by the negative associations. Signals of dangers might have more immediate and strong selective value than signals of potentially favorable sites. Passing a potentially favorable site to search for another might often be favored over death. Corals are probably not selectively settling on plexiglass, basalt, rope and pumice, but may be settling because the biofilms are not of the types associated with negative factors on disturbed reefs.
A coral community, mainly composed of Acropora spp., rapidly colonized and became established on a 70,000 m2 lava flow. A volcano covered the site with lava on 9 May 1988. These photographs were taken in November 1994. Tomascik et al. (1996) calculate the tabulate Acropora colonies must have grown by 30 cm y−1 (a radial extension of 15 cm−1). Acropora spp. have no defense to lava, but they can recolonize and grow rapidly, becoming the dominant genus of coral
The effects of coastal runoff on coral reefs have been widely studied and clearly have negative impacts on reefs (see Fabricius 2005; Fabricius et al. 2013 for reviews). These include documented declines in coral larval settlement and metamorphosis (Hodgson 1990; Babcock and Davies 1991; Gilmour 1999; Birrell et al. 2005; Fabricius et al. 2003; Kuffner et al. 2006). The mechanisms that allow avoidance of coral larval settlement under poor water quality conditions are just starting to be investigated. The findings of Prescott et al. (2014) indicate that the spatial distribution of A. millepora and P. damicornis larval settlement and metamorphosis are guided by negative cues rather than positive settlement cues.
In an array of controlled field and laboratory experiments, Dixson et al. (2014) determined that coral larvae from three species of Acropora and larvae from six families of reef fishes all showed significant recruitment preferences for water samples from a marine protected area with abundant coral over water samples from an unprotected area dominated by macroalgae. The larvae of fishes from all six families showed very similar strong preferences for association with Acropora over Porites and over Pocillopora . Whereas Acropora is sensitive to stresses (Sect. 12.3), Porites is renowned for its relative tolerance of conditions of stress, and Pocillopora, although also stress-sensitive, tended to be a rapid colonizer and therefore potentially indicative of recent disturbance. Dixson et al. (2014) interpreted larval behavior as selecting Acropora as an indicator of a healthy undisturbed reef community. The fishes from all six families were also strongly selective for association with some genera of benthic algae over others.
12.2.4 Environmental Stress May Facilitate Reticulate Evolution
The numbers of larvae that potentially self-recruit or recruit to sites farther away are proportional; the absolute numbers are based on proportion times the fecundity of the reproductive source population (Fig. 12.2). The numbers of larvae are also affected by pelagic larval duration and survival. As water temperatures increase, the larvae may develop faster and/or abnormally. During times of environmental stress to corals, the fecundity of corals tends to decrease (Sect. 12.2.2) and the fewer larvae tend to replenish only populations nearer the reproductive source and seed populations at lesser distances from the source. Along with climate change , pollution , sedimentation, and other aspects of environmental stress that reduce fecundity on individual coral colonies, increased patchiness and distance between habitats suitable for successful recruitment brought about by coastal construction and overfishing of herbivores augment the effect of lower fecundity in decreasing connectivity among populations of corals (Steneck 2006).
Veron (1995) proposed that this periodic decrease in connectivity might explain the geographic variations observed in dominant and widespread species of corals that form “species complexes” (Fig. 12.4), such as Orbicella spp. and Acropora spp. Since adult corals are sessile, like plants, the genetic connectivity depends on dispersion of gametes and larvae rather than behavioral coming together of adults. Veron championed the concept of “reticulate evolution” for “species complexes”. When the environment is favorable to corals and fecundity is high in areas of strong water currents, closely related species tend to disperse more widely (Fig. 12.2) and interbreed, bringing about more uniform, genetically integrated species. Alternatively, when conditions are stressful and fecundity is low, populations may tend to become isolated and genetically diverge by genetic drift or by local selective forces. Isolation can be further enhanced by habitat deterioration causing patchiness and reduced pelagic larval development time reducing connectivity.
Veron’s (1995) diagram of the blending together through hybridization and separation by isolation, then further separation by genetic drift or local selective forces, of closely related coral species (in a “species complex”) through time in reticulate (“netlike”) evolution. The blending together might be facilitated by faster large-scale water currents and/or by more benign conditions favoring high fecundity or, separation might be facilitated by warmer waters causing more rapid larval development and thereby less time in the water column, by stressful conditions reducing fecundity (Fig. 12.2) or more stagnant water conditions (© Australian Institute of Marine Science and CRR Pty Ltd [2000]. The Australian Institute of Marine Science does not necessarily endorse any modification to that material)
Molecular genetic evidence and patterns of natural hybridization are consistent with the concept of reticulate evolution (Kenyon 1997; Hatta et al. 1999; Diekmann et al. 2001; van Oppen et al. 2001; Frank and Makody 2002). However, these studies were on Acropora or Madracis which were already known to be “species complexes” so it is not yet known whether metapopulations of broadcast- spawners become more genetically distinct or “structured” under stressful conditions when fecundity is low, appropriate habitat is more patchily separated and scarce, and planulae spend less time in the water column as temperatures rise.
12.2.5 Constraints of Biology on Management
Fundamental forces acting against coral reef ecosystems today, e.g., growth in human populations multiplied by the per capita burden of increased growth in economic demands and needs for natural resources, and the resulting global environmental effects of seawater warming, lowering of seawater pH, and climate change s seem to be considered too daunting to address directly. Coral-reef managers almost always focus actual efforts towards manageable local processes, attempting to control overfishing, sedimentation, and pollution , etc., in hopes that these procedures will increase the local resilience of corals to global changes. Particular areas are selected for protection with the thought that fecundity will be substantial in protected areas and so these populations can replenish downstream disturbed areas.
We cannot rely entirely on improving local situations and setting aside protected areas, depending on sufficiently developed resilience to stress and replenishment of populations by larvae from upstream sources. There are two basic aspects of coral biology that are not supportive of large-scale downstream connectivity . First, although planulae of many coral species can potentially survive for over 200 days and potentially drift thousands of kilometers, it is a characteristic of coral biology that a major portion of the actual surviving larvae replenish local or nearby populations (Sect. 12.2.3). Second, the areas outside the protected areas that are disturbed and have undergone phase shift s can be unfavorable for successful larval recruitment (Sect. 12.2.3; Dixson et al. 2014; Prescott et al. 2014). We must begin to search for effective ways to directly address the deteriorating global conditions. As Ove Hoegh-Guldberg has said, “With all due respect to those contributing effort and funding towards protecting coral reefs, the millions of dollars that are being spent will be of no avail unless there is a concentrated effort to obtain explicit progress in reducing CO2 emissions”.
12.3 Selection Favors Rapid Growth over Survival in Acroporids
12.3.1 Fast Growth Favored by Less Complexity
This section starts by documenting the observation that corals of the genus Acropora have jettisoned their ancestors’ usual mechanisms of defense and tolerance that would have favored survival, possibly in exchange for rapid growth. This seems to have also happened at other times in the Mesozoic, in other scleractinian families (Sect. 12.3.2). Ancestors of Acropora probably included stress-tolerant encrusting acroporid corals such as Astreopora that were capable of surviving the Cretaceous/Paleogene (K-Pg) mass extinction (formerly called the Cretaceous/Tertiary [K-T] mass extinction ), but when environmental conditions again favored reef production, Acropora may have evolved by losing the defenses or tolerances typical of most scleractinians in exchange for rapid growth (Fig. 12.3).
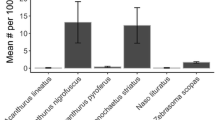
A striking example of the lack of investment in defense by Acropora spp. is their vulnerability to predation. Despite the diversity of corallivores (314 species of invertebrates from 5 phyla or 24 families (Stella et al. 2011) and 128 species of fishes from 11 families (Cole et al. 2008)), only 28 of the genera of reef-building Scleractinia have been recorded as prey, leaving 83 genera that appear to be entirely avoided as prey (Stella et al. 2011). Defense against predation appears to be the norm for corals. Yet nearly all phyla of corallivores easily prey on Acropora spp., and among the important groups of corallivores, Acropora is actually preferred. Acropora species are the preferred prey of the seastar Acanthaster planci, decapod crustaceans, gastropods such as Drupella spp. and Coralliophila spp., the polychaete Hermodice carunculata, the polyclad flatworn Amakusaplana acroporae (Rawlinson and Stella 2012), and fishes such as blennies Exallias brevis, damselfish Stegastes planifrons, and 69 species of butterflyfishes Chaetodon. Considering the magnitude of the effects of predation on Acropora (Fig. 12.5a) it is surprising that natural selection has not favored resistance to predators; but Acropora can grow back rapidly (Fig. 12.5b).
Acropora spp. are also especially susceptible to diseases such as the white-band disease which, since the early 1980s, has substantially reduced the abundance of Acropora palmata and Acropora cervicornis throughout the greater Caribbean. Other reef-building scleractinians are affected by disease, but Acropora spp. are among the most seriously affected (Aronson and Precht 2001). It is possible that being favored prey for several phyla of corallivores facilitates infection by disease and recruitment of bioeroders by increasing the frequency of tissue penetration and the baring of skeleton, respectively.
Nearly all genera of corals are susceptible to bleaching when exposed to extraordinarily warm temperatures, but Acropora is ranked among the most susceptible (Marshall and Baird 2000; McClanahan et al. 2007). The hydrocoral genus Millepora is sometimes the only reef-building genus that is more susceptible than the scleractinian Acropora (Marshall and Baird 2000). Acropora was a dominant genus throughout the Indian Ocean until it suffered major losses in the 1997/1998 bleaching event (McClanahan et al. 2007).
The elevated morphology of branching and tabular Acropora species and their porous skeletons make them particularly susceptible to the strong waves of hurricanes or typhoons (Hughes and Connell 1999; Fig. 12.6a), but they grow back rapidly (Fig. 12.6b). Fast-growing corals were shown by Comeau et al. (2014) to be more sensitive to ocean acidification than were slow-calcifiers.
Even the larval stage of Acropora species appears to be less adapted for survival than the larvae of other coral genera. Graham et al. (2008) quantified the survival of larvae from five genera of broadcast- spawning scleractinians. Although larvae of the representative acroporid Acropora latistella lived as long as 209 days, the median survival was 4 days. The larvae of the other genera also lived a maximum of about 200 days, but their median survivals were substantially longer. For example, the larvae of Goniastrea aspera lived a maximum of 215 days, but the median survival was 138 days.
Denis et al. (2013) found that fast growth was prioritized in Acropora in a tradeoff against regeneration of tissue. The energy and biochemical materials saved by not developing as many effective survival mechanisms against predation, disease , extraordinarily warm seawater, or hurricanes as do most scleractinian corals, perhaps allowed Acropora spp. to allocate more metabolic energy for rapid growth. The tissues of branching corals such as Acropora and pocilloporids average less than a third the thickness of the tissue of massive corals (Loya et al. 2001) and the biomass as measured by ash-free dry weight of Acropora spp. was lower than the biomasses of other genera of corals tested (Thornhill et al. 2011). The Caribbean Acropora spp. show lower metabolic rates , lower rates of protein turnover , and less capacity to acclimatize than some mound corals, but are able to grow substantially more rapidly (Gates and Edmunds 1999). The skeleton of Acropora spp. is extraordinarily porous (Gladfelter 1982) and grows an order of magnitude faster than other genera measured (Gladfelter et al. 1978). Initially, it would be reasonable to surmise that less investment in tissue biomass and repair may facilitate faster growth by allocation of more metabolic energy or nutrients to growth.
Pocillopora spp., to a lesser extent than Acropora spp., are also fast-growing, branching corals with thin tissue (Loya et al. 2001) and substantially lower organic content of tissue than the other coral general tested (Glynn and Krupp 1986). Yet Pocillopora spp. are still preferred prey. How could Acropora and Pocillopora be preferred prey when their tissues are relatively thin and possibly less nutritious? Glynn and Krupp (1986) found that although Pocillopora tissue had low organic content, it provided the highest energetic return to the predator. The tissues of Acropora and Pocillopora are superficially located on the skeleton and therefore may be more accessible, and fewer complex secondary metabolites for defense in the tissue may cause them to be less metabolically resistant.
But what is the resource involved in the tradeoff between survival and fast growth? Light and nutrients are critical resources required by the zooxanthellae that fuel coral growth and calcification . Nevertheless, there is typically a superabundant supply of light in shallow water over coral reefs where Acropora spp. are dominant. Moreover, corals do not respond to changes in nutrients as immediately and strongly as do more simple organisms, i.e., organisms that are less complex physiologically and more distinctly autotrophic or heterotrophic. The growth rates of purely heterotrophic animals, such as mussels, barnacles, bryozoans, and many sponges, and purely autotrophic algae, are strongly associated with the amount of food or nutrients in the water column (Widdows et al. 1979; Ceccherelli and Rossi 1984; Rose and Risk 1985; Page and Hubbard 1987). The growth rates of corals do not respond so quickly and substantially to nutrient input (Kinsey and Davies 1979; Koop et al. 2001). Although adult coral colonies can live well in areas of nutrient input such as upwelling , the recruitment of corals is very difficult in these areas because the recruits of simple heterotrophic animals and algae respond more immediately and substantially to nutrient pulses, grow at substantially greater rates, and can plow away or overgrow recruits of corals which do not respond as quickly and substantially (Birkeland 1977).
Simplicity may be an important a factor in competition for space because it facilitates speed of response to either nutrients or calories from photosynthesis. The symbiotic system of a holobiont (the combination of coral animal, dinoflagellates, and prokaryotes) is too complex to respond as quickly to a pulse of nutrients as do benthic autotrophic algae and sessile heterotrophic animals. Single-celled algae in the phytoplankton can rapidly increase with input of nitrogen and phosphorus, but the density of single-celled algae in the cells of corals is relatively constant within any set of environmental conditions associated with depth. Enhancing the growth rate of zooxanthellae can upset the complex physiological balance in the symbiosis and the corals might dampen the response of the system by expelling the excess zooxanthellae (Muller-Parker and D’Elia 1997).
Simplicity has two aspects. First, complexity requires that metabolic processes have more regulations and time-consuming steps. Second, complexity in terms of additional secondary metabolites requires a greater expense of production. Organisms that are less complex morphologically and/or invest fewer resources into secondary metabolites for defense are generally able to respond more quickly to pulses in resource availability and grow faster than phylogenetically related species that are more complex. The structurally simple green alga Ulva curvata responded to inputs of ammonium with more effective uptake and more rapid growth than did the more structurally complex green alga Codium fragile (Ramus and Venable 1987). Algae with relatively simple morphology generally have higher net productivity per unit biomass and a potential for more rapid response and growth than do algae with more complex structure (Littler and Littler 1980). Diatoms tend to be favored as food for many animals and can grow up to three times as fast as dinoflagellates of the same size under similar conditions (Banse 1982). Although diatoms can produce toxins and are motile with microfibrils, dinoflagellates tend to be more complex, producing secondary compounds that result in toxic red tides and ciguatera, and likely expend more energy on motility driven by two large flagellae. Many dinoflagellates are also more complex because they are mixotrophic (capable of both autotrophy and heterotrophy in the same organism). Mixotrophy requires extra energy to maintain both trophic processes in the same system (Stoeker 1999; Dolan and Pérez 2000). Mixotrophy is advantageous in nutrient-poor environments where solar energy is unlimited (such as shallow-water coral reefs), but organic carbon and dissolved inorganic carbon are limiting (Hallock 1981, 2001). An increase in dissolved inorganic nitrogen not only shifts the advantage to more rapidly growing simple autotrophs and heterotrophs, but it throws the more complex mixotrophic system out of balance and can stimulate bleaching in corals (Wooldridge 2009).
Simple systems are normally advantageous in nutrient-rich environments. In environments with low resource availability or stressful conditions, plants that are favored have slow growth rates and major investments in antiherbivore defenses because they must defend what they already built at such great cost (Coley et al. 1985). In contrast, when resources are abundant or environmental conditions are benevolent, organisms often tend to invest in rapid growth and increased fecundity at the expense of defense. For corals, rapid increase in colony surface area and number of polyps can potentially produce a rapid increase in fecundity and the increased number of early potential progeny might be favored. So while most corals are adapted to survive during periods of stress, corals in the genus Acropora are especially vulnerable to predation, disease , unusually warm seawater, and hurricanes. Yet they have often been the dominant corals because of their rapid growth and elevated morphology (Fig. 12.3). While most corals invest in survival (tolerance ) at the expense of reproduction (recovery ), corals of the genus Acropora invests in rapid growth (recovery) at the expense of survival (tolerance). This is consistent throughout the history of scleractinians since the Triassic.
12.3.2 Fast Growth Favored over Survival in Productive Times
There have been three periods especially favorable for scleractinian reef-building: the Late Triassic, the Middle Jurassic, and the Neogene (Kiessling 2009). These periods of prevalent reef-building are associated with “aragonite seas” (Sect. 2.7, 2.9.3, 2.9.4, and 2.9.5; Stanley and Hardie 1998) and with the prevalence of relatively rapidly growing corals. During mass extinction s, rapidly growing corals did not survive. Survivors were generally massive or mound-shaped corals. Conversely, about 15 million years after the Triassic mass extinction and 30 million years after the Cretaceous-Paleogene mass extinction in aragonite seas, a large number of new scleractinian genera originated and reef-building accelerated while the survivors of the mass extinctions showed substantial rates of elimination. The conditions favoring increased rates of reef-building were beneficial for newly evolved fast-growing corals, while these conditions were detrimental for stress-tolerant, slower-growing survivors of mass extinctions.
On Late Triassic (Norian and Rhaetian) reefs, Retiophyllia spp. may have been somewhat ecologically analogous to Acropora spp. on modern reefs in that it was a “very speciose branching (phaceloid, see Box 12.1 and Fig. 12.7) genus” (Caruthers and Stanley 2008) that was one of the dominant, reef framework-building scleractinians. In the Late Triassic, “Some branching [phaceloid] corals probably grew rapidly, and reached over 10 m high, but bore thin branches only 5–10 mm in diameter” (Wood 1999, p. 104). Retiophyllia spp. were widespread, found in Japan, central and northeast Asia, Indonesia, Russia, the Caucasus, the Alps, Europe, and western North and South America from Alaska, Yukon, Vancouver Island, Oregon, Idaho, Nevada,, northern Chile, and Peru (Caruthers and Stanley 2008). These fast-growing dominant corals fell victim to the mass extinction at the end of the Triassic.
The morphology of the predominant erect corals through the geologic record (Box 12.1)
The corals that survived the Triassic/Jurassic mass extinction were solitary or massive or smaller phaceloid types and did not appear to be fast-growing or dominant. Of the 20 genera that passed from the Triassic to the Jurassic, one (Alakiria) went extinct in the first age that followed (Hettangian). It may have been what Jablonski (2002) called a “dead clade walking”. Another genus, Dimorphastraea, persisted into the Late Cretaceous. The remaining 18 scleractinian genera lived about 15 million years, and each went extinct when conditions became favorable for reef-building corals (Lathuilière and Marchal 2009), beginning in the late Early Jurassic when the Jurassic scleractinians were becoming diverse, with at least 100 new coral genera having originated by the Middle Jurassic (Heckel 1974). The time of greatest rate of origination of scleractinian genera (Kiessling and Barin-Szabo 2004) and greatest rate of reef-building (Kiessling 2009) in the Mesozoic era was in the latter half of the Jurassic period. Corals with the physiological capabilities to survive the Triassic mass extinction were unable to survive in conditions exceptionally favorable to newly evolved scleractinians. One possible explanation is that the usually solitary, massive or encrusting survivors were crowded out by the relatively fast-growing Jurassic corals (Fig. 12.8). However, although competition for space between corals can affect their distributions and community structure (Cox 1986; Pandolfi et al. 2002), I have never documentation of corals driven to extinction by competition.
Box 12.1: Branching Corals
Although erect corals in the Triassic and Jurassic were mostly “phaceloid” (each branch produced by a single polyp), or sometimes “dendroid” (single-polyp branches coming off a common stem), they are sometimes referred to as “branching” or “ramose” corals. “Branching” corals are presently considered more precisely as corals with each branch being continuously covered by a sheet of polyps, i.e., “multiserial erect”. Branching corals become more prevalent abruptly in the late Cretaceous (Turonian Age), but Oculina was the only branching coral that survived the Cretaceous/Paleogene mass extinction . Acropora , Pocillopora , and other extant genera of branching corals originated in the late Paleocene, early Eocene, or more recently.
Although reef-building corals in the Jurassic grew substantially slower than modern corals, averaging about a third the growth rate (Leinfelder 2001), the dominant phaceloid corals were still relatively fast-growing in their time. A main reef-builder throughout the Tethys region in the Middle Jurassic was Thamnastria dendroidea which often formed dense monospecific thickets. “The growth of branches was directed upwards, but also frequently sideward, forming an overtopping morphology…. Thamnasteria dendroidea is therefore interpreted as an “aggressive coral”… Compared with other Mesozoic (Late Jurassic) corals … its growth rate is very high” (Helm and Schȕlke 2000). Leinfelder et al. (2012) found extensive meadows of erect Calamophylliopsis spp. or thickets of Actinastrea crassoramosa in Late Jurassic coral communities. When reef construction is active, “aggressive” erect corals seem to be prevalent.
Near the end of the Jurassic, the increase in volcanism with more rapid seafloor spreading caused high rates of CO2 emissions while magnesium was taken up in the hot basalt, lowering the Mg/Ca ratio in the seawater and shifting the seawater from aragonitic to calcitic (Sect. 2.5, 2.7, Fig. 2.1; Stanley and Hardie 1998). With the shift to calcite seas, there was a substantial drop in production of coral reefs for about 140 million years from the later Jurassic throughout the Cretaceous and through to the early Paleogene.
However, as explained by Kleypas et al. (2001), a diverse coral community may be performing well ecologically even while performing poorly geologically (Fig. 12.3). While the scleractinian fauna in the Cretaceous produced unimpressive reefs compared to those in aragonitic seas, the rudist bivalve s produced volumes of massive calcium carbonate relatively rapidly. These three-dimensional hard substrata above the sediment allowed scleractinian corals to increase in diversity and maintain widespread distribution throughout (Götz 2003). The corals, in turn, helped stabilize the rudists by binding them together (Götz 2003). In modern times, crustose coralline algae (CCA) form algal ridges in areas of consistent wave action and are an agent of binding of coral skeletons while in the Cretaceous, scleractinian corals were more prevalent in areas of consistent wave action and were an agent of binding and stabilization of rudist shells (Götz 2003).
When volcanism decreased and the seafloor spreading slowed, the Mg built up to Mg/Ca > 2 and the oceans became aragonitic during the Oligocene, about 30 million years ago, and reefs began to develop more vigorously. Although Acropora originated in the Later Paleocene – Early Eocene, it did not begin dominating reef frameworks until the Oligocene (Wallace and Rosen 2006) when atmospheric pCO2 dropped below 500 ppm and seawater pH rose to over 8.0 (Pearson and Palmer 200) and reef production became vigorous.
As with 18 of the 20 scleractinian genera that survived the Triassic/Jurassic mass extinction but went extinct in the Middle Jurassic when the scleractinians were greatly increasing in diversity and major reef construction was occurring, the family Actinacididae survived the K-Pg mass extinction and continued for 30–40 million years until the Oligocene when conditions for vigorous reef growth and domination by branching species began to occur, at which time this scleractinian families went extinct. At least 11 scleractinian genera from several families also went extinct in the Oligocene and at least 10 genera went extinct in the Miocene when reef production was increasing and at least 40 new scleractinian genera were originating (Fig. 12.9; Wells 1956).
Geologic history shows that mound or encrusting corals are more frequently seen to be the survivors of mass mortality events, and the dominant, fast-growing, branching species more often go extinct with events of mass extinction , then new genera of branching corals originate in the geologic period following the mass extinction . The data are mainly from Wells 1956. The genera in parentheses are from Ma 1959. Additional genera mentioned by Veron (1995) and Paulay (1997) with question marks are included with question marks and no parentheses. At least a dozen extant survivors from the Cretaceous are not listed because they are deepwater or solitary
Edinger and Risk (1995) attribute extinctions in the Oligocene and Miocene in the greater Caribbean to be caused by a major increase in upwelling , and this is may be true, but some of these genera also went extinct in other oceans at about the same time. For example, Antiguastrea that originated in the Jurassic also went extinct in the Miocene in Australasia, southern Europe and northern Africa (data in corallosphere.com at the British Museum of Natural History). Trochoseris that originated in the Cretaceous also went extinct in the eastern tropical Pacific (López-Pérez 2005). Lamellastraea went extinct in east Asia (corallosphere.com) and Leptomussa in Italy (Wells 1956). Actinacis, Siderofungia, Stylangia, Cyathoseris, and Rhizangia all originated in the Cretaceous and went extinct in Europe or Asia when reef-building was accelerating and new genera were originating (Wells 1956). Budd (2000) listed 21 new genera of scleractinians that appeared in the Caribbean in the Oligocene to the middle Miocene, while 15 genera went extinct. Another six genera originated in the late Miocene.
Scleractinians seem to be improving over time in their abilities to survive mass extinction s in comparison to other marine invertebrates. In contrast to the poor showing in the Triassic/Jurassic mass mortality of scleractinian coral genera (27 % survival, i.e., 20 survived out of 73, Lathuilière and Marchal 2009), 70 ± 4 % of scleractinian genera and over half the species survived the K-Pg mass extinction (Kiessling and Baron-Szabo 2004). The general extinction of marine genera was about 47 % for both the Triassic/Jurassic and K-Pg extinctions (Bambach et al. 2004), so the scleractinians shifted from one of the most vulnerable groups to among the more resilient. Not only did some of the massive and encrusting corals that developed in the Cretaceous survive the K-Pg mass extinction, but also the Paleocene-Eocene Thermal Maximum, the Pliocene-Pleistocene accelerated turnover and extinction in the greater Caribbean (Budd et al. 1996), and they are still prevalent on contemporary coral reefs (Fig. 12.9). Nine of the scleractinian genera of corals that prevailed from the Cretaceous to the present were previously grouped together in the family Faviidae (Wells 1956; Veron 2000), but these nine genera have now been distributed among four families by genetic analyses (Budd et al. 2012). Jackson and McKinney (1991) documented that corals have become more “integrated” in colony morphology with time, but the potential relationship of survival with integrated colonial morphology still needs to be determined. New genera of solitary (Scolymia, Homophyllia, Heterocyathus, some fungiids) and phaceloid (Caulastrea, some Galaxea, some Lobophyllia) corals have originated in the Cenozoic so the less “integrated” corals keep returning anew.
The 54 scleractinian genera from the Cretaceous that are briefly described in Wells (1956) are typically solitary, massive, encrusting, or phaceloid. However, Jackson and McKinney (1991) reported a sudden increase in branching corals in the final 10–15 million years of the Cretaceous; a sudden increase of branching corals to percentages of the coral community without precedent. Nevertheless, the corals surviving the K-Pg mass extinction were generally solitary, massive or encrusting (Fig. 12.9). Oculina might be considered branching or dendroid and Cladocora might be considered phaceloid, but neither counts as a good representative of “aggressive” branching reef-builders and are often without zooxanthellae (Bernecker and Weidlich 2005).
Some massive or encrusting scleractinians that survived the Cretaceous-Paleogene mass extinction (Fig. 12.9) are called “Lazarus taxa” by Wood (1999) and Rosen (2000). The fast-growing, branching corals that have attributes that allow them to dominate the scene today, originated after the mass extinction . They were given the traits that allow them to dominate and live gloriously, but they are the first to die, reminding us of the story of Faust. Van Woesik et al. (2012) noted that dominant and widespread branching corals in the Caribbean in the Pliocene, Stylophora and Pocillopora , became extinct in the Pleistocene , indicating that being abundant and widespread does not protect species from extinction. Likewise, Pandolfi et al. (2001) reported on the extinction of two widespread and dominant corals and the survival of some of the rare species within the recent 82,000 years.
Ultimately, the best “strategy” for selection would be the best combination of survival and reproduction. But natural selection does not act as a “strategy” for the future, but rather works at the individual colony level, favoring traits that provide more successful recruitment in the present. Natural selection on dominant corals during benevolent times seems to favor the increase in rate of growth at the expense of defense and/or tolerance because the more rapid increase in surface area can increase the number of polyps, and thereby increase fecundity . Tabletop morphology of some Acropora allows for increase in diameter of up to 30 cm per year through radial expansion (Tomascik et al. 1996) and the cylindrical branches of arborescent Acropora allow polyps over the entire surface in contrast to encrusting or mound corals can have polyps on only the unattached surface. We are used to considering competition for space to be a major selective force in the evolution of corals, but in the case of Acropora, competitive superiority might be a spinoff of rapid growth and elevated morphology selected for increased fecundity, i.e., the rapid production of greater numbers of potential offspring.
While proximal selection was indirectly successful in making the Acropora species dominant, the ultimate effect of reducing investment in defense and tolerance may result in the dominant species being more susceptible to extinction with change in climate (Birkeland et al. 2013) as it seems to have done with erect, dominant corals, in the Mesozoic. On the other hand, the slower-growing, more tolerant corals that survived the mass extinction s seem to have sometimes continued to live for millions of years only to go extinct when the environment becomes especially favorable for reef-growth and new genera of scleractinians arise. Investments in tolerance may have cost them rapid growth and thereby made them susceptible to being crowded out by new families of more rapid-growing dominants in times of major reef growth. The fast-growing dominant corals go extinct when times are bad (e.g., mass extinctions), while some of the especially tolerant corals go extinct when times get especially good for reef production.
Favoring rapid growth at the expense of survival in Acropora , just as favoring survival at the expense of fecundity in other corals, may have allowed sessile species like corals to survive when disturbances or stressful conditions were acute, but brief. But when stressful conditions become chronic (Chap. 11), as with the influence of overfishing and coastal construction, sedimentation, pollution and increased CO2, resulting from the growth of human populations, the periods of time between successful reproduction and recruitment may become too long for these corals to persist.
12.4 We May Keep the Corals but Lose the Reefs
12.4.1 Many Coral Species May Not Go Extinct
What doesn’t kill you makes you stronger. (Friedrich Nietzsche)
Although coral reefs are likely to continue to deteriorate and become less topographically complex for the foreseeable future (Kleypas et al. 1999a, b; Alvarez-Filip et al. 2009; Fabricius et al. 2011; Kennedy et al. 2013), no longer providing environmental services such as optimal habitat for fish and providing protection against wave action as sea level rises, we need not assume most corals will go extinct. Many studies document the increased tolerance of corals to stressful conditions by acclimatization (physiological or behavioral adjustments of individual colonies) or adaptation (genetic adjustment of populations). In a series of major bleaching events in the eastern tropical Pacific (Glynn et al. 2001; Podestá and Glynn 2001; Jimenez et al. 2001; Cortes and Jimenez 2003; Zapata and Vargas-Ángel 2003), French Polynesia (Adjeroud et al. 2009), Great Barrier Reef (Maynard et al. 2008), and Phuket, Thailand (Dunne and Brown 2001), corals appeared to become more tolerant of warm temperatures with experience (Brown 1997a, b; Coles and Brown 2003; Brown and Cossins 2011; Palumbi et al. 2014; Barshis Chap. 7). Indeed, corals closer to the equator have higher thermal limits than do populations of the same species in subtropical parts of their range (Coles and Jokiel 1978; Oliver and Palumbi 2009, 2011). Berkelmans (2002) determined stress-response thresholds for bleaching from 13 sites on the Great Barrier Reefs across 22° of latitude over 10–12 years and concluded that thermal adaptation had taken place over both small (10s of km) and large (100s–1,000s of km) scales of distance.
The ability of diverse assemblages of corals to survive frequent exposures to high water temperatures is common (Brown 1997a; Berkelmans and Willis 1999). In shallow pools on the small island of Ofu in American Samoa, the water temperature can fluctuate by 6.3 °C daily, reaching 34.5 °C (Craig et al. 2001). Although Millepora spp., Acropora spp., and some corals of other genera occasionally bleach at Ofu, 85 species of coral seem to generally thrive in these pools of fluctuating temperatures. The 85-species assemblage in these pools is comparable to the diversity on the reef front. Coles (1997) observed that a robust assemblage of 24 species of corals occupying 50–75 % of the substrata seemed to survive daily fluctuations of 8.2 °C with no signs of stress. Riegl et al. (2011) documented that in the central region of the Arabian/Persian Gulf, corals did not bleach until exposed to temperatures above 34 °C for 8 weeks, with three of the weeks above 35 °C.
Some of the presently predominant genera of reef-building corals that lived in the Cretaceous (Sect. 12.3.2; Fig. 12.9) probably lived at seawater temperatures 32–36 °C (Poulsen et al. 1999, 2001; Wilson et al. 2002; Schouten et al. 2003; Littler et al. 2011; DeCarlo et al. 2015) or possibly as high as 42 °C (Bice et al. 2006) and also at lower pH, often 7.4–7.6 (Pearson and Palmer 2000; Zeebe 2001; Hönisch et al. 2012) in calcite seas (Mg/Ca mole ratio <2). The source of massive CO2 input was the extensive and chronic volcanism that was producing new seafloor and pushing apart the Americas from Europe and Africa. This was compounded by the volcanism that was producing the Deccan Traps at the end of the Cretaceous and the bolide that hit the Yucatan Peninsula during this time.
Likewise, during the Paleocene and early Eocene, atmospheric pCO2 was 2,000–3,500 ppm and sea surface pH was between 7.4 and 7.6 (Pearson and Palmer 2000) as it was in the Mesozoic (Hönisch et al. 2012). This time, the input of CO2 by volcanism was augmented by the rapid input of about 2 × 1012 metric tons of C into the atmosphere as methane which assisted the rapid lowering of pH and massive dissolution of sea-floor carbonate (Zachos et al. 2005). Seawater temperatures increased 5 °C in the tropics and but reached 24–26 °C near the North Pole (Moran et al. 2006). The increase in seawater temperature was several times greater in polar latitudes than in the tropics and this brought about a lesser latitudinal gradient in seawater temperatures. This suggests that there may be some kind of “ocean thermostat ” that puts limits on tropical seawater temperature rise, at least in some areas (Kleypas et al. 2008). If seawater in much of the tropics in the Cretaceous was 32–36 °C, then the future limits to tropical seawater temperatures in large basins (i.e., oceanic atolls and islands) lie within the range to which modern corals can adapt (Riegl et al. 2011) .
The two dozen or so genera of reef-building scleractinians represent about 17 families that have survived the harsh conditions of the Cretaceous, the Cretaceous-Paleogene mass extinction , the Paleocene-Eocene Thermal Maximum and are still predominant on modern reefs. It seems reasonable to have hope that some descendants will adapt to the “norm” (diverse scleractinians without reefs) and not go extinct. This does not apply to fast-growing species that originate after mass extinctions. They are more prone to extinction (Sect. 12.3.2).
Montastraea is first found in the Upper Jurassic (~150 mya), Diploastrea in the Early Cretaceous (Aptian Age, ~115 mya) (Wells 1956), and Heliopora in the Cenomanian Cretaceous (~95 mya) (Eguchi 1948). Skeletal morphology is not a reliable way to categorize coral genera (Budd et al. 2012), yet they are most likely ancestors to the genera with their names. For example, although Heliopora in the Lower Cretaceous is not morphological distinguishable from Heliopora coerulea in the present (Eguchi 1948; Colgan 1984), it is almost certain that the species has evolved to the extent that if biochemical data were available for comparison, they would be considered different species. Nevertheless, the continuous fossil record indicates that representatives of a number of genera of reef-building scleractinians survived a long history of extensive stressful times and mass extinction s (Wells 1956) and so it seems reasonable to hope that some will survive the present trajectory because their genome may contain substantial relics from their ancestors. This would not necessarily apply to those genera that shed defenses and tolerances when they originated in favorable times (Sect. 12.3.2).
Since corals previously experiencing stress often acclimatize or adapt to become more tolerant (Palumbi et al. 2014), Steve Palumbi and his lab are planning to transplant selected coral colonies that have adjusted to higher temperatures or other stresses to set up “designer reefs”, groups of experienced colonies that are likely to survive where the ordinary corals might not (Mascarelli 2014; van Oppen et al. 2015). Likewise, Hernández-Delgado and Suleimán-Ramos (2014) have also been maintaining a community-based program of coral farming and outplanting for reef rehabilitation in Puerto Rico. Transplanting colonies bypasses the problems of connectivity , i.e., reduced fecundity , reduced fertilization of gametes from sessile adults as disturbance thins their population densities, shortening of development time in warmer water, early and local settlement behavior of many larvae despite potential for long pelagic duration, and reduced acceptability of disturbed habitats for successful recruitment (Sect. 12.2.3). However, paleontology indicates that although corals can adapt physiologically to the effects of CO2 (such as temperature ) and survive, they have not been able to adapt the relative rates of bioerosion and calcification to the effects of low pH and Mg/Ca mole ratio <2 so it seems likely that many genera of corals will survive, but reefs will deteriorate.
12.4.2 Reef Structure Will Deteriorate, as in the Cretaceous and Paleogene
Like obesity, a massive reef accumulation may be the result of remaining stationary for too long under good conditions. (RW Buddemeier and RA Kinzie III (in Kleypas et al. 2001))
If the temperatures of tropical seas become warmer and the pH of shallow seawater becomes lower, most corals may not go extinct in the near future (Sect. 12.4.1), but bioerosion will certainly increase (Chap. 4), accretion by some corals may decrease, and the topographic complexity will decline (Alvarez-Filip et al. 2009; Fabricius et al. 2011), causing many reef ecosystem services to deteriorate. Glynn and Manzello (Chap. 4) explain that coral reefs are in a dynamic and delicate balance between CaCO3 deposition and bioerosion and dissolution. With increased atmospheric pCO2, the uptake of CO2 by the ocean lowers the pH of seawater and accelerates bioerosion while sometimes also reducing the rate of calcification . With decreased pH, rates of bioerosion do increase in low-nutrient waters (Wisshak et al. 2012), but in high-nutrient waters (>1 μM nitrate), bioerosion increases tenfold (DeCarlo et al. 2015). Lower pH decreases the saturation state of aragonite , i.e., decreases the ease by which corals secrete calcium carbonate . Models predict that calcification rates of corals will decrease by 30±18 % in 30–50 years (Kleypas et al. 1999a, b). No matter how successful we will be in abruptly halting local problems such as overfishing, sedimentation, pollution , etc., the increased rates of bioerosion with possibly reduced rates of calcification will lead to severely reduced structural habitat complexity , and ecosystem services of coral reefs. Adapting to ocean acidification by itself is facing an indifferent non-responsive physical factor, but adapting to bioeroders is coevolving in an arms race . There has already been a Caribbean region-wide substantial loss of topographic complexity in the past four decades (Alvarez-Filip et al. 2009), and so although no corals have gone extinct in the Caribbean, the reefs are becoming substantially degraded and less functional ecologically.
Are coral reefs now returning to a “normal” situation in which reef-building is negligible (Kleypas et al. 2001)? The past interglacial period was extraordinarily favorable for scleractinian reefs, and not typical of the 224 million years of scleractinian existence (Hay et al. 1997; Buddemeier and Kinzie 1998; Pearson and Palmer 2000; Berner and Kothavala 2001; Kleypas et al. 2001). The late Jurassic through the late Paleogene was roughly 140 million years in which the Mg/Ca mole ratio of seawater was below 2 (Stanley and Hardie 1998), and so bioerosion increased tremendously (Vermeij 1987) and accretion of coral (aragonite ) reefs became reduced (Stanley and Hardie 1998; Kiessling 2009). Although the diversity of reef-building genera of scleractinians increased (Sepkoski 2002; Kiessling and Szabo 2004), atmospheric concentrations of CO2 were higher than today (may have ranged between 600 and 2,400 ppm), seawater temperatures may have ranged between 32 and 36 °C and seawater pH was usually between 7.4 and 7.6 (Pearson and Palmer 2000), so conditions were not favorable for substantial reef accretion. In the present, as the pH in warm surface ocean waters is likely to drop, bioerosion will certainly increase while accretion of reefs by corals may become more costly energetically.
Kleypas et al. (2001) emphasize that there is not a correlation between how well a coral community is performing biologically and how well it is performing geologically (building reefs). In the calcite seas of the Cretaceous, other creatures such as rudist bivalve s provided three-dimensional substrata that corals could use (Götz 2003). Sepkoski (2002) and Kiessling and Baron-Szabo (2004) found scleractinian diversity to be high in the Cretaceous, and even if there were sources of error in Sepkoski’s compilations, the general pattern of diverse scleractinians with eroded minor reefs was the situation during most of the existence of scleractinians. Now as the atmosphere, and then the oceans, take up more CO2, corals may return to the “norm” of diverse assemblages of scleractinian corals on eroded reefs with minor rates of accretion . The basic message is that we have just been through about 10,000 years of abnormally prolific reef-building, but scleractinians may now return to the pattern in the later Mesozoic. Although a drop in Mg/Ca mole ratio to less than 2 and a drop in pH are very different chemical processes, they may have the same effects of accelerating bioerosion while reducing scleractinian reef accretion.
Corals do quite well without reefs (Fig. 12.3). Corals recruiting to basalt, plexiglass, pumice and many other substrata do as well, and sometimes better, than recruits to coral reefs (Sect. 12.2.3). The past few thousand years have been extraordinarily favorable for reef accretion and preservation (Kleypas et al. 2001) and this favorable period is representative of only 10 % of the past few million years (Hay et al. 1997). Since the origin of scleractinian corals about 224 million years ago, there have been only three periods of major reef construction (Kiessling 2009). However, the greatest diversity of scleractinian corals developed during a time in the later Cretaceous when most of the calcium carbonate structures were being formed by bivalve molluscs (rudists). Reefs are not an extended phenotype of corals like nests are of birds or dams are of beavers (Dawkins 1982), but are a byproduct of long period of good conditions (Buddemeier and Kinzie 1998).
12.5 Importance and Vulnerability of Big Fishes on Coral Reefs
12.5.1 Previous Stocks and Their Failure to Recover
Undisturbed coral reefs can appear to be characterized by inverted trophic biomass pyramids with up to 80 % (DeMartini et al. 2008) or 85 % (Sandin et al. 2008) of the fish biomass in the top level of piscivores and with fishes in all lower trophic level s pooled together constituting only 15–20 %. Although there is controversy as to whether inverted trophic biomass pyramids actually exist (Sect. 1.1), the disparate array of methods for surveying fishes all converge (Rizzari et al. 2014) on the same conclusions that population densities of large fishes are inversely related to the population densities of humans (Friedlander and DeMartini 2002; DeMartini et al. 2008; Sandin et al. 2008; Nadon et al. 2012; Fenner 2014). The apex predators are the initial focus of the fishing activities and the larger fishes are especially vulnerable to modern fishing technology (reviewed in Fenner 2012, 2014). A few fishermen are able to change the trophic structure of a coral-reef ecosystem in very little time with modest effort (Fenner 2014), but the return of large fishes and the recovery of the inverted trophic pyramid usually takes decades if it happens at all (Hutchings and Reynolds 2004; Abesamis et al. 2014). Even subsistence fishing significantly reduced the upper trophic levels and total vertebrate biomass on coral reefs in the Caribbean at least 1,300 years ago (Wing and Wing 2001). By the 1800s, there had been a major decrease in large coral reef and seagrass-associated vertebrates and by the 1950s, the predatory and herbivorous coral-reef fishes of the greater Caribbean were mostly of small size (Jackson 1997).
The Nassau grouper Epinephelus striatus was historically one of the most important food fishes in the western tropical Atlantic. It served as a main source of food and income for shore communities throughout the greater Caribbean (Sadovy 1993). Spawning aggregations consisted of as many as 100,000 individuals (Smith 1972). Now, in the greater Caribbean, some spawning aggregations have disappeared. In Bermuda, the catch of Nassau grouper declined by 93 % in 14 years (Sadovy 1993). Nassau groupers used to reach a weight of 22.7 kg (50 lbs) or more, but now most are taken as juveniles. The World Conservation Union (IUCN) considers the Nassau grouper endangered.
Despite the loss of larger individuals in Caribbean coral-reef fish stocks, as recently as the early 1980s the biomasses of populations of coral-reef fishes were still estimated to be as high as 160–200 metric tons km−2 in the Atlantic (Randall 1963; Munro 1983) and as high as 93–237 metric tons km−2 in the Pacific (Goldman and Talbot 1976; Williams and Hatcher 1983). The mean harvests on reefs of a couple of small islands in the central Philippines over a 5-year period were found to be 11.4 and 16.5 metric tons km−2 year−1 (Alcala and Luchavez 1982). The shoreline fishery of American Samoa has yielded up to 26.6 metric tons km−2 year−1 (Wass 1982). Despite the reduction in stocks of larger fishes on coral reefs, the standing stocks of coral-reef fishes are still about 30–40 times greater than standing stocks on demersal fishing grounds in Southeast Asia, the Mediterranean, or other temperate regions (Russ 1984).
Although the biomasses of fish populations on coral-reefs three or four decades ago remained high in many places, coral-reef fish stocks in many locations dropped by 70–80 % and have remained low since the 1980s. The development of fishing technology made catching fishes easier, so the catch-per-unit-effort (CPUE) often underestimates the actual decrease of reef fish populations in the past three decades. The CPUE of the shoreline fishery of American Samoa dropped 70 % between 1979 and 1994 while the number of common fishes decreased by 75 % and the relative abundances among fishes changed drastically, with a decrease in commercially preferred fishes (Craig et al. 1995). Likewise, in fisheries monitored over a 4-year period in the Philippines, the numbers of adult fishes dropped 80 % and the number of species known to reach adulthood dropped 33 % (McManus et al. 1992). The CPUE on a bank south of Jamaica also declined by 82 % over a 15-year period (Koslow et al. 1988). The Guam nearshore fisheries CPUE decreased 78 % between 1985 and 1997 (Birkeland 1997). Data in Sandin et al. (2008) show that the biomass of reef fishes at Tabuaeran dropped by 68 % and at Kiritimati dropped 75 % in the past two decades. The stocks of coral-reef fishes in the main Hawaiian Islands are at most 20–25 % of what they were a century ago (Shomura 1987; Harman and Katekaru 1988; Smith 1993).
Once the drop in the range of about 80 % in standing stocks of reef fishes occur, the recovery may take decades if at all (Abesamis et al. 2014). The Bolbometopon muricatus were spectacular on Guam in the mid-1970s, but were essentially eliminated by overfishing in the late 1970s and still almost none are found 34 years later. The scenario is similar in American Samoa. Craig (2005) noted that corals recover from hurricanes, Acanthaster, and bleaching , but the fish populations are not recovering. A visiting marine ecologist commented that American Samoan reefs were like a lovely mansion of corals, but the mansion was empty. Where are the fishes? Like many coral reefs around the world, there are actually still many colorful small fishes; it is the big fish es that are gone. Craig (2005) noted there is “no quick fix” and that what we see today is “a shadow of former population abundances”. This pattern is not unique to coral reefs. Hutchings and Reynolds (2004) analyzed data from more than 230 fish populations that had a median breeding population reduction of 83 % from known historical levels and found little or no change in abundance for at least 15 years.
The diverse stocks of fishes on coral reefs are impressive, especially the stocks in relatively untouched areas that appear to have an inverted trophic biomass structure. This unfortunately encourages the investment into fishery-based economies. A journal The Economist stated that commercial trade in large coral-reef fish is a “gold rush ”, a boom and bust, “…an extractive industry that eventually exhausts the resource it exploits” (Anonymous 2000).
12.5.2 Influence of Large Fishes on Community Structure
Coral reefs operate differently than do large-scale pelagic current systems in which concentrated pulses of nutrient input from upwelling act as physical oceanographic drivers of the foodweb. If the pelagic fishes are overharvested, it does not necessarily cause long-term fundamental changes in the system and the populations can replenish themselves with the next upwelling. The relatively low diversity system (two trophic level s between primary production and humans Sect. 1.1) of pelagic fisheries in eastern oceans is relatively simply based on nutrient input from upwelling and are relatively unstable because of the dependency on nutrient input. When there is a major El Niño Southern Oscillation (ENSO ) which dampens upwelling, there is a failure of the anchoveta and tuna fisheries, hundreds of thousands of seabirds and at least hundreds of sea lions and seals die, but the system is resilient and recovers with the return of nutrient input from upwelling. Although the pelagic system is unstable because of its dependence on nutrient input, it is resilient because it does not depend on species interactions to prevent shifting to alternative states.
In the more complex and diverse coral-reef ecosystems, internal biological interactions such as grazing, predation, and competition have dominant roles in community structure. This may provide the communities of coral reefs with general stability because herbivores can potentially control the algae and predators can potentially control outbreaks of herbivores or corallivores. But diverse systems are less resilient because they can fall into alternative stable states. If herbivores are overfished, algae can take over and dominate when a disturbance decreases the cover of living coral and the algal dominance can persist by the effects of algae on coral recruitment (Sect. 12.2.3). There is no alternative steady state in most simple systems. Since coral-reef systems are complex, with species interactions that can maintain the stability of the system, they are less resilient to overfishing or severe disturbances because it is these species interactions that prevent the system from shifting to alternative states.
Long-term studies by McClanahan (2014) on the coast of Kenya found that the piscivores regained their biomass after about 20 years of protection, but the biomass of herbivorous fishes was slow to recover and the functional groups in the coral-reef system had still not fully recovered after 35 years of protection. Even if the biomass of herbivores is recovered, the “functional groups” may not recover unless the large individuals are present. Even at lower trophic level s, the size of the individual fish is of key importance. Parrotfishes below 15–20 cm in length nibble at algae to feed themselves, but those above 15–20 cm in length scape or excavate the substratum, removing algae and creating bare substrata appropriate for successful coral larval recruitment (Bruggemann et al. 1996; Bonaldo and Bellwood 2008; Lokrantz et al. 2008; Ong and Holland 2010).
Hunting the largest individuals not only affects the coral-reef ecosystem, but also has direct and indirect effects on the other fishes associated with targeted fish. Reducing the size and abundance of the predatory fishes led to the fishes at lower trophic level s to increase in average size and longevity (Ruttenberg et al. 2011), and to be in generally better condition. Sex change for protogynous species occurred at a larger body size when there were fewer apex predators (DeMartini et al. 2005). For a given body length, fishes at lower trophic levels had greater body mass, greater liver mass, and more energy reserves on reefs in which the predators at upper trophic levels were less common (Walsh et al. 2012).
The reason for the lower energy reserves and lower body and liver mass per unit length in coral-reef fishes on reefs where the top predators are in their natural abundances is likely to be the high risk of predation restricting the time and distance the potential prey species forages for food away from refuges from predation. Thus the removal of top predators potentially affects the benthic community by allowing the herbivores to forage greater distances from shelter and thereby increasing the distances that barren grounds or haloes separate reefs from seagrass (Randall 1965). Although herbivorous fishes become more predominant where the higher trophic level s have been fished down, this does not mean that the herbivores have higher biomass. The herbivores might also be fished down, but not to the extent of the top predators (Friedlander and DeMartini 2002).
Natural, undisturbed coral reefs are typically characterized by what appears to be an inverted trophic biomass pyramid , where upper trophic level s consist of large predators with relatively slow turnover , and the populations of fishes at lower trophic levels of herbivorous and planktivorous fishes consist of fewer and smaller fishes (DeMartini et al. 2008). The inverse pyramid is sustained because top predators have relatively slow turnover and the lower trophic levels (prey) have relatively rapid turnover. But this makes the system especially vulnerable to overharvesting of the slow-growing species in the upper trophic levels. It is the diversity of consumers and the large number of trophic levels that provide the mechanisms behind the unique structure of coral reefs of large gross primary production and mediocre net production or fisheries yield (Fig. 1.1; Sect. 1.1).
12.5.3 Importance of Large Fishes to Population Sustainability
The number of years of reproductive activity typical of a species indicates the usual degree of risk of failure to produce successful recruitment (Sect. 12.2.1). Analyses of data from 251 species of fishes showed that as recruitment success became more variable, the traits of late maturity, increased longevity, and iteroparity substantially increased (Longhurst 2002). Recruitment to complex coral reefs is hazardous for larval or juvenile fishes. Planktivorous fishes form a “wall of mouth s ” during the day (Hamner et al. 1988) and scleractinian corals, zoanthids, and anemones form a “wall of mouths” at night (Fabricius and Metzner 2004). There have been many studies of the effects of predation by resident fishes on the survival of newly arrived juveniles (Hixon 1991; Hixon and Beets 1993; Hixon and Carr 1997; Holbrook and Schmitt 2002; Hoey and McCormick 2004; Dixson 2011) and on the effects of competition among the recruits (Holt 1984, 1987; Jones 1997; Hixon 1991; Holbrook and Schmitt 2002). The proportion of successfully settled Haemulon favolineatum surviving through the first year was determined to be 0.008 (Ogden 1997). The survival of successfully settled Acanthurus lineatus through their first year has been found to be 0.007 (Craig 1995), but once established as an adult, it can live 42 years (Choat and Robertson 2002).
The hazardous and unreliable successful recruitment on coral reefs is probably the reason that coral-reef fishes are adapted to live long enough to attempt reproduction many times (Sect. 12.2.1). Acanthurids often live to 30 or 40 years (Choat and Robertson 2002) as do some lutjanids (Newman et al. 1996). Individuals of the genera of groupers (Serranidae) such as Epinephalus, Mycteroperca and Cephalopholis live 30–50 years, 33 years and 26 years, respectively. The relatively short-lived Plectropomus spp. still live over a decade (Ferreira and Russ 1994). The holocentrid Myripristis amaena becomes mature after 6 years and lives at least 14 years (Dee and Parrish 1994). Even damselfishes and parrotfishes have lifespans generally between 5 and 20 years, with Stegastes acapulcoensis having a maximum age of 32 years (Meekan et al. 2001). Bolbometopon muricatum takes about 7–8 years to reach sexual maturity at about 60 cm length, with a lifespan of about 40 years (Hamilton et al. 2008; Hamilton and Choat 2012).
The larger individuals among coral-reef fishes are particularly important, not only for maintaining ecosystem structure, but for sustaining their own populations. The fecundity of fishes increases exponentially with body size (Fig. 12.10); and so in natural populations where recruitment is sparse and uncertain and small fishes are at risk of being consumed by predators, the selective pressure strongly favors large body size, long life, and multiple reproductions. When the species that formerly had a refuge in size is now the prey of humans, however, the probability of living long enough to reproduce at a large size becomes small (Fig. 12.11); and selective pressure can shift to favoring rapid growth, early reproduction, and smaller size.
Fecundity increases exponentially with body length in fish. One 61-cm female red snapper (lutjanid) produces the same number of eggs as 212 females at 42 cm each, or 12.5 kg of large snapper produces the equivalent number of eggs as 233 kg of medium-sized snappers. Each smaller snapper is 69 % the length of the larger one (Bohnsack 1998). The reproductive potential of a population is disproportionately affected when fishermen target large individuals. This is especially true for protandrous species
There is a strong selective advantage for a top predator to grow large because fecundity increases exponentially with body size. When the top predator becomes prey to humans, selection turns to favoring early reproduction. Effective reserves protect the intraspecific genetic diversity of populations (Bohnsack 1998)
In contrast, recruitment to fisheries in pelagic systems is relatively reliable with the exception of periods of ENSO every few years and so lifespans of less than a decade should allow the adult fish populations to reproduce relatively reliably in abundance during non-ENSO years. Pelagic fishes such as mahimahi (Coryphaena hippuros) often live 2 years and grow to 14 kg (31 lb) on the In contrast, recruitment to fisheries in pelagic systems is relatively reliable with the exception of periods of ENSO every few years and so lifespans of less than a decade should allow the adult fish populations to reproduce relatively reliably in abundance during non-ENSO years. Pelagic fishes such as mahimahi (Coryphaena hippuros) often live 2 years and grow to 14 kg (31 lb) on the average, and can live a maximum of 4 or 5 years and grow to a maximum of 39.5 kg (87 lb). The pelagic Thunnus albacares (yellowfin tuna) has a lifespan of up to 6.5 years (Lehodey and Leroy 1999) and can grow to over 181 kg (almost 400 lb).
Pelagic fishes possibly grow faster in part because they continually search for dense concentrations of food and sometimes have the opportunity to consume food much more intensely than do coral-reef predatory fishes. When tuna find a mass of fish, they devour their prey intensely, consuming as much as 25 % of their body weight in one day, which partially explains their rapid growth. Coral-reef fishes cannot find prey in such concentrations because the topographic complexity provides refugia for their prey and so they feed on an occasional individual prey that wanders too far from their shelter. The introduced predatory reef fish Cephalopholis argus on the island of Hawaii, where it grows faster than in its native range, is found to consume on the average 0.8 % of its body weight per day (Dierking 2007). Groupers generally grow slowly, rarely over 0.5 kg per year. Yellowfin tuna can average 28 kg per year or 56 times faster growth than coral-reef groupers. When we compare the rate of growth and found to consume on the average 0.8 % of its body weight per day (Dierking 2007). Groupers generally grow slowly, rarely over 0.5 kg per year. Yellowfin tuna can average 28 kg per year or 56 times faster growth than coral-reef groupers. When we compare the rate of growth and potential longevity of coral-reef fishes such as Acanthurus lineatus that can live 42 years (Choat and Robertson 2002) to grow to a maximum size of 2.7 lb (1.24 kg), we can perceive how the ratio of fisheries yield per unit gross primary productivity for the Peruvian upwelling can be about 20–60 times greater than is this ratio for coral reefs (Fig. 1.1; Nixon 1982). There are some exceptions. The larger marlin and bluefin tuna can live 25 and 40 years, respectively. Bluefin tuna take several years to reach maturity and, like large coral-reef fishes, are more vulnerable to overfishing and most populations have decreased over 90 %.
Even the larger individuals of smaller species on coral reefs are important for fecundity . For example, the yellowstripe goatfish Mulloides flavolineatus is a schooling predator of small, soft-bodied invertebrates in the sand. It reaches sexual maturity within 1 year after hatching and has a long period of repeated spawnings each year. The local fishermen on Guam probably do not realize that fishing pressure has been drastically reducing the reproductive potential of the goatfish each year. By 1991, the reproductive potential of M. flavolineatus was only 5 % of its unfished potential (Davis 1992). By harvesting the larger individuals of even the smaller, more rapidly maturing fishes, a greater portion of the breeding stock is taken than is perceived by fishermen.
Although the importance of larger individuals may have been taken into account by Pacific islanders who knew the natural history of their fishes centuries ago, in modern times this is a particular problem because state fishing regulations are often not based on the natural histories of the fishes concerned and therefore allow the taking of important species such as parrotfishes before they have a chance to reproduce for the first time (Howard 2008). Important additional components of this loss of sustainability are the development of technologies such as refrigeration that allowed the switch from local subsistence to a global market economy . New technologies such as scuba and underwater lights provided the means to overharvest large species such as Bolbometopon muricatum while they sleep, eliminating previous natural refuges for breeding stock (Fig. 12.12; Hamilton and Choat 2012).
Westerners’ tendencies to focus harvesting on the largest individuals is as deleterious as the elimination of natural refuges and the switch to a global market. This can reduce the potential for population replenishment by drastically lowering fecundity , shortening the reproductive season, thus preventing the “bet-hedging ” that allow some of the larvae to encounter favorable conditions, and in some cases reducing the average growth rate and endurance strength of larvae (Berkeley et al. 2004a, b; Bobko and Berkely 2004; Hixon et al. 2013). Overharvesting of larger individuals can also lead to the loss of genetic heterogeneity, potentially leading to reduced adaptability (Hauser et al. 2002). In recent decades, selective fishing pressure on the larger (older) individuals has caused a rapid evolution of smaller body size and lower fecundity in some harvested populations (Handford et al. 1977; Ricker 1981; Olsen et al. 2004).
12.5.4 What Does This Mean for Management Practices?
Isolated peoples can be in situations in which they must exploit coral reefs for subsistence. How should fish be taken while still maintaining the integrity of coral reef systems? Fish should be exploited with two forms of moderation: the use of slot limit s on sizes taken and shift the focus from harvesting the capital to harvesting the interest or yield (Fig. 12.13). Slot limits guide the taking of intermediate size classes of a species, but leaving the largest individuals as highly fecund brood stock and leaving the juveniles to manifest their potential for rapid growth.
Traditionally, the optimal harvest was considered to be to the left of the maximum sustainable yield (MSY). Overfishing has sometimes been defined by fishery councils under the MSA as spawning biomass reduced to 20 % of its unfished level. The fishery would be more stable if the same yield is taken from the right of the maximum sustainable yield (Courtesy of Joan Roughgarden. Modified and redrawn from Roughgarden and Smith 1996 with permission Copyright (1996) National Academy of Sciences, USA)
The yellow tang Zebrasoma flavescens is the economically most important aquarium fish in Hawaii and it is compelling to surmise that its spectacular success may be facilitated by effective slot limit s . Well over 200,000 yellow tang are taken from the Kona Coast of Hawaii each year by the aquarium trade (280,125 in 2013 [William Walsh unpubl. data ]). About 89,000 are taken as prey by roi (Dierking 2007), the introduced predatory grouper, Cephalopholis argus. This is an approximate total of 370,000 taken in 2013. Of course, this is an approximation and it must vary from year to year, but it does indicate that the population of yellow tang has been sustaining itself well. On a 217-km continuous reef, the take would be over 1,700 yellow tang per km of coastline per year.
This sustainability has certainly been aided by the establishment of zones in which some of the brood stock of aquarium fishes cannot be taken and there also must be habitat and oceanographic conditions favorable for recruitment . But the yellow tang still stands out as more productive than the other species in these environmental conditions taken for the aquarium trade and taken by predatory fishes. Part of the reason for the sustainability and extremely productive success of this system might be because both roi, the introduced predator, and the aquarium industry favor medium-sized individuals over the larger and the smaller individuals. Meyer and Dierking (2011) documented that Cephalopholis argus on the Kona Coast fed mainly on prey between 3 and 15 cm length (n = 135), while yellow tang grow to 20 cm (Randall 2007). The aquarium trade also finds medium-sized yellow tang (8–15 cm) more marketable for household aquariums than older larger individuals (15–20 cm). By leaving the larger individuals which have the exponentially greater fecundity , and also leaving the smaller individuals which have the greatest proportional growth rates , harvesting the intermediate size classes is the most sustainable (Fig. 12.10).
Of 117 definitions of “overfishing” reviewed by an international panel of experts for the National Marine Fisheries Service, essentially all of them allowed fishing to reduce the stock well below the point of maximum sustainable yield (Darcy and Matlock 1999). For fishery councils operating under the Magnusen-Stevens Act (MSA), it is not uncommon for overfishing to be considered to begin when the spawning biomass is reduced to 20 % of its unfished level. This might not be appropriate for coral-reef fisheries because, as indicated by the longevity and multiple years of spawning typical of reef fishes (Sect. 12.2.1), successful replenishment of the fisheries catch may not be as reliable for coral-reef fisheries as for the target stocks on which theory is based. Roughgarden and Smith (1996) pointed out that when the limits to fishing down the population to the left of MSY (maximum sustainable yield), there is lower reproductive stock and theory indicates that this leads to a very unstable ecological equilibrium, “as difficult to maintain as balancing a marble on top of a dome”. A coral-reef fisheries should be managed to promote stability because of the unreliability of successful recruitment . If the take is limited to the right of MSY, where the reproduction is affected by crowding, the fishery is “as easy to maintain as keeping a marble near the base of a bowl”. Figure 12.13 illustrates how the fisheries yield is potentially the same on either side of MSY, but if we harvest the capital (line A) as in most commercial fisheries, the system is very unstable and difficult to manage. If we harvest the interest (line B) as in many indigenous subsistence fishers, the system is stable with “natural insurance” (Roughgarden and Smith 1996). A caveat for any fisheries theory that refers to MSY is that it is a moving target that is difficult to observe. A quantitative estimate of MSY requires a lot of data repeatedly because it changes frequently. Indigenous subsistence fishers did not need a quantitative estimate of MSY. They could observe if the larger individuals were still present.
Bob Johannes (1998) once pointed out that data -less resource management was the most effective approach to the complex and unpredictable interactions of resource management. For example, to adequately survey Indonesia for fish abundance or biomass would require over 400 man-years and the system being surveyed would change before the survey was complete. Are the quantitative values of abundance or biomass relevant? A hundred kilograms of small goatfish may have an order of magnitude lower fecundity than the same biomass of fewer larger goatfish. A hundred kilograms of parrotfishes <20 cm in length would have relatively little effect on the benthos while the same biomass of fewer larger parrotfishes would be removing algae and clearing substrata for coral recruitment . The information that is necessary to evaluate potential replenishment of marine resources should rely not on statistical analyses of surveys of biomass or population densities of stock or assessments of CPUE, but rather on the straightforward observation of whether the big ones are still there.
12.5.5 Coral Reef Ecosystems Are Not for Export, but Rather for a Service Economy
Although coral reefs have the greatest gross productivity of any ecosystem in the tropical seas, the sustainable fisheries yield and net system productivity are relatively low (Fig. 1.1). Far less than 1 % of the gross primary productivity of a natural coral reef is converted to production that is meaningful for human consumption (Hatcher 1997). The ratio of fisheries yield per unit gross primary productivity for the Peruvian upwelling is about 20–60 times greater than is this ratio for coral reefs (Fig. 1.1; Nixon 1982). The average ratio of gross productivity (P) to respiration (R) is close to 1 (P/R = 1) and the positive excess production, or potential yield from the system, is very close to zero (Sect. 1.1).
The number of trophic level s in food-webs decreases as nutrient input increases. Hallock (1987) explains mechanisms by which chronically low-levels of nutrient input leads to habitat and species diversity . Ryther (1969) assigned one or two steps between phytoplankton and man to upwelling systems and acknowledged that the upwelling regions of the world have the shortest food-webs. Grigg et al. (1984) assigned six trophic levels to coral reefs, probably the most complex of marine systems. Much of the assimilated energy is lost in respiration at each step in the food-web so that it is understandable that there would be less excess production in a system with six trophic levels than in a system with two.
There was a widespread concern in Bermuda that the coral reefs were deteriorating because of commercial fishing, even though the fisheries economy and number of people employed in extractive fisheries was small compared with tourism . Tourism and recreation related in Bermuda grossed more than $9 million in 1988. The hotel owners, charter-boat fishermen, dive and tour-boat operators and other businesses felt the incomes of many were threatened by the activities of a few fishermen. In 1990, considering evidence that the catches were declining substantially and the reef communities were deteriorating, the Government of Bermuda offered payment of up to $75,000 per fishermen, in addition to compensating them for hardware such as pots and winches, in order to close the coral reefs of Bermuda to pot fishing (Butler et al. 1993). This was not popular with commercial fishermen and was considered by some to be draconian. But with the establishment of a number of offshore fish-aggregation devices, some fishermen found alternative livelihoods with recreational charter fishing, offshore longlining for pelagic fishes, and deep-water trapping for crabs and prawns. Unlike fishing on coral reefs which is naturally subsidized by alternative species when the preferred species become scarce, the alternative fisheries each targeted certain types of fish and therefore was relatively self-limiting, requiring healthy stocks in order to have a viable fishery.
The money exchanged in world tourism in 1992 was 1.9 trillion, over 27 times the 70 billion of the world marine fisheries revenue (Weber 1993). In 2012, international tourism alone grossed 1.3 trillion, about 16 times the world marine fisheries which grossed 80 billion. To participate in the world economy , the islands of Oceania can be highly competitive for the high spenders in tourism, while geography (transportation costs for export fishery products), ecology (low sustainable yield and effects of overfishing on ecosystem processes), and biology (life-history characteristics of the species) work against commercial export fisheries from coral reefs. The infrastructure for commercial fisheries (processing, refrigeration, transportation) is more expensive and risky than the commitment to ecotourism. The cost of airfares are paid by the tourists themselves and create local employment, in contrast to the subtraction of air transport costs from the profit on exported fish sales. Analogous to the great diversity and biomass of species supported by reefs in regions of low rates of nutrient input , a greater number of people and jobs can be supported sustainably by coral reefs if economies are based on the natural attributes of the species and ecosystem.
I have been told by fisheries scientists that once a fish is dead, it does not matter whether it is used for subsistence or commercial export. But it takes at least an order of magnitude more fish to support a few commercial fishermen than it does to support a population by subsistence because most of the proceeds from the sale of fishes goes to supporting the marketing and transportation infrastructure. Coral-reef resources can sustain a much greater number of people or careers in the world economy if used in nonexportive enterprises. The nonexportive, service -oriented approaches of Bermuda and Palau (Sect. 12.6.3) are compatible with the attributes of coral-reef ecosystems and species, and with the demands of the growing human populations, growing economies, and urbanization.
Much of the great increases in marine fisheries of the world in the 1950s and 1960s came from the shift in emphasis in fisheries from the predatory fishes to the more plentiful fishes lower in the food-web. The catch of anchoveta (Engraulis ringens) in the Peru upwelling increased from about 200,000 metric tons in 1955 to 12 million tons in 1970, a 60-fold increase (Larkin 1978). In view of these successes, Grigg et al. (1984) suggested a series of important principles of resource management of coral reefs, in which the first was “To maximize efficiency, harvest at levels of sustained yield as low on the food chain as possible. Cropping predators would increase potential yield.” Likewise, at a UNESCO meeting in Sri Lanka, I heard that local fishermen would prefer not having coral reefs because the slower growth of the resident fishes provided less turnover and therefore less sustainable yield, the higher diversity made harvesting and processing less efficient, the residency made replenishment less reliable, and the reefs themselves were a hazard to navigation. If our objective is to feed the growing human population, coral-reef fisheries are too easily overharvested, are too diverse for economically efficient processing, and unlike the pelagic fisheries, overfishing can affect the ecosystem. Although the Palauans and Bermudans agree that coral reefs are susceptible to overfishing and do not provide for a sustainable export economy , they had a rational view that coral reefs actually provide a solid economic basis for globalization with a service -based economy .
12.5.6 Palau, an Exemplary Case of Coral-Reef Management
I believe the evolution of a social system that was built around knowledge has been the true treasure of Palauans. This, in my opinion, is also why Palau has had such great leaders and will continue to benefit for many years to come. (Gerald W. Davis)
Palauans display a more successful insight than any other people of which I am aware into adapting their solid understanding of the natural history of their resources to the changing conditions imposed by globalization of economics. Their policies of resource use appear to be developed by a society with knowledge of how ecosystem processes of coral reefs (Sect. 1.1) and life-history characteristics of animals in coral-reef communities (Sect. 12.5.3) are more sustainable and profitable with a subsistence and service –based economy (tourism ) than with an export-based economy. On only a few hundred meters of reef at Blue Corner and nearby Ngemelis Dropoff, Palau earned about $7,000 per day on the average and up to $14,000 per day in peak season from diving tours back in 1992 (C. Cook, The Nature Conservancy – Palau Office, pers. comm.). A major contribution towards these earnings was a large humphead wrasse that allowed tourist photographers to approach closely. The Palauans were aware that this fish was much more profitable economically as a continuous tourist draw at thousands of dollars per day than it would be as a one-time fisheries yield, and so shooting this individual wrasse for sale in the fish market would have been socially unacceptable. Even dog-tooth tuna and grey reef sharks came close to photographers at Blue Corner. Diving activities by tourists brought in over $12 million to Palau in 1992 (not counting additional expenditures such as hotels, meals, and transportation). In 2010, scuba tourism brought $85 million to Palau (Vianna et al. 2010), and at least 50 % of tourists are scuba divers and close to 90 % go snorkeling or swimming (N. Singeo – Palau Visitors Authority, pers. comm.) (Figs. 12.14 and 12.15).
With its Shark Haven Act of 2009, Palau had the remarkable insight to set up the first major sanctuary for sharks, banning all fishing for sharks throughout its 600,000 km2 exclusive economic zone (EEZ). A study by the Australian Institute of Marine Biology found that 39 % of Palau’s gross domestic product ($218 million) was from scuba-tourism ($85 million) and 21 % ($17.9 million) of diving tourists said their primary purpose for choosing Palau was specifically to see sharks. Vianna et al. (2010) estimated that the main tourist dive sites hosted approximately 100 sharks. Therefore, they concluded an individual live shark brings in about $179,000 in revenue per year in the tourist industry, or a lifetime value of about $1.9 million, compared to $108 as a fisheries catch. The tax revenues to Palau from scuba-diving activity during which the observation of sharks was the major objective was 24 times higher than the total revenues from the fishing industry (Vianna et al. 2010).
Because of their economic and biological insight in developing a service -based economy rather than an extraction-based economy for coral reefs, Palau was given the prestigious Future Policy Award of 2012 for the best ocean policy of all nations. Palau’s intelligent practice of bringing in substantially more revenue with relatively little capital investment, as well as the understanding that the life-history of sharks makes them especially vulnerable to harvest, encouraged similar actions by other nations. In 2010, the year following Palau’s Shark Haven Act, the Maldives established their 90,000 km2 EEZ as a shark sanctuary and Hawaii became the first state in the US to prohibit the sale of shark fins. In 2011, the rest of Micronesia (the Marshalls, Kosrae, Pohnpei, Chuuk, Yap, the Commonwealth of the Northern Marianas, and Guam), Tokelau, Honduras, and the Bahamas established shark sanctuaries throughout their EEZs. French Polynesia (4.7 million km2) and the Cook islands (1.9 million km2) followed suit in 2012 and the British Virgin Islands in 2014. The Bahamas now advertises itself to tourists as the shark-diving capital of the world.
Scuba-diving and snorkeling on coral reefs form a major base of the economies of a several countries. Over 31,000 tourists observe the reefs of Bonaire with scuba annually and the revenue from scuba-related tourism brings in about $23 million each year, about half their gross domestic product. The annual expenditure in reef protection by Bonaire is $0.67 million per year (NOAA Coral Reef Valuation Database), so the return on investment is substantially better than it would be for extractive fisheries. Service-based economies are not only substantially more profitable both in total income and in the ratio of profit to investment, but they are more sustainable for coral reefs because of the life-history characteristics of coral-reef species (Sect. 12.5.3).
Charter-boat sport-fishing of offshore pelagic fishes brings in considerably more money per fish than direct sales of fish. I knew a person who moved to Guam from Florida in hopes of making a living as a commercial fisherman. The expense of ice, diesel fuel, marketing, upkeep of the boat, docking fees, and the cost of living on Guam made it too difficult to compete with fishers in the Philippines who could live and operate on substantially less. Philippine fishers could undersell fishers on Guam despite the added daily cost of air-freighting their fish to the market. Therefore, the person I knew supported his goal of occasionally being a commercial fisherman by actually making his income by chartering his boat for offshore sport-fishing.
In 1980, Japan AID contributed 11 diesel fishing boats and other fishing gear to Palau to promote export of reef fishes. After a year, thousands of dollars were being exchanged in the marketing of reef fishes, but after expenses in operating the boats, marketing and processing the fish, the fishermen were netting only US$ 0.20 per fish. In the export business, fishermen would have to sell about 5 kg of reef fish to purchase 0.2 kg of canned tuna at the supermarket. It was clear that exporting fishes was far less profitable, and well as being in competition with subsistence fishing, recreational fishing, tourism , and selling fishes to local restaurants and hotels.
In view of the more sustainable economic use of resources in subsistence and tourism than in export of biomass, the Government of Palau has passed legislation called the “Marine Protection Act of 1994 ” to gradually phase out the export of coral-reef fishes. “The purpose of this Act is to promote sustainability and develop marine resources of the Republic while also preserving the livelihood of the commercial fishermen of the Republic.” At this time, commercial export of reef-fishes from Palau is illegal during the months of March through July which are the spawning seasons for important reef-fish species. The local fishermen are encouraged to switch completely to other forms of endeavor, e.g., chartering for sport fishing or selling fish locally to hotels and restaurants. The money stays in Palau when the fish are sold locally, the fisherman can make a lot more money per fish by cutting the costs of shipping and middlemen, and the quality of fish in the local hotels and restaurants is greater when they do not have to compete with the export market.
Palau has a strong tradition of a marine tenure system. Resource management regulations may still be put in place by traditional chiefs in each state. A traditional closure is called a “bul”. In support of this tradition, the constitution of the Government of Palau recognizes that each state has the responsibility to manage all activities within their lagoons, inshore areas, and reefs extending 12 nautical miles seaward of the shore. The national government holds an advisory role to the state on all matters of resource management. Access to fish within a state is granted by the traditional leader’s authority.
When a local community establishes restrictions, this helps the Government understand what policies are needed by the local communities. In 1988, before the banning of exports of coral-reef invertebrates (other than those produced by aquaculture) and reef-fishes during their breeding seasons, a “bul” on the export of sea cucumbers was promoted by groups of women who collected them for subsistence (Gerald Davis, pers. comm.). Sea cucumbers are a very popular and commercially valuable food in Asian countries. An Asian entrepreneur came to Palau and began exporting the more valuable species. This quickly depleted the stocks. Women who glean the reefs for subsistence realized how these commercial exports were substantially reducing their abilities to take food home. Their lobbying for a “bul” on the exportation of sea cucumbers made the Government of Palau aware of the need for regulations in cooperation with the local communities (Gerald Davis, pers. comm.). The Marine Protection Act of 1994 was thereby developed from a responsible tradition of cultural regulation of local resources.
Communication between villages and the Government of Palau has been remarkably two-way. Just as the Government pays attention to a local “bul”, the people pay attention to their leaders. The seawater warming of 1997–1998 caused substantial coral mortality . The coral was replaced by algae which inhibited coral recruitment . Tommy Remengesau, Jr., then Vice President of Palau, published advice in the local newspaper to avoid taking herbivorous fishes for food, but rather take predators or omnivores, because the herbivores can control algae and this should facilitate coral recruitment. He also asked people not to step on living corals because in doing so they would damage the brood stock needed for recovery of coral populations. The reefs recovered substantially, so perhaps his advice was heeded. He also initiated the Micronesia Challenge in which Palau and the other nations of Micronesia all pledge to conserve effectively at least 30 % of their nearshore marine resources and 20 % of their terrestrial resources by 2020. In 2014, the President of Palau announced his intention to establish the first nation-wide marine sanctuary that fully protects more than 80 % of its EEZ. Remengesau, now President of Palau, was winner of the Champions of the Earth award by the United Nations for the policies of Palau that promise to sustain economic resilience while maintaining natural resources.
There has also been a two-way communication between Palauan cultural knowledge of the natural history of the coral-reef resources and western science. Bob Johannes (1981) championed the knowledge of Micronesian fishermen and quoted other scientists as well who noted that local fishermen know more about the natural history of their resources than is known in science. The Palauans have special talents to combine traditional culture and economics with science and natural history to get the best results. They accept technical support with an open mind. For example, Noah Idechong received an education in economics, but he grew up knowing Palauan fisheries and was awarded a Pew Fellowship in 1997 to work on marine conservation . National Marine Fisheries Service awarded him a grant to develop a sustainable sport fishery that provided the best use of the inshore fishery resources of Palau.
Noah led the founding of the Palau Conservation Society (PCS), a nongovernmental organization with the goal of solidifying the local communities’ capacities for stewarding their natural resources. PCS takes the comprehensive (marine and terrestrial) ecosystem approach and helped to develop the Protected Areas Network (PAN) in cooperation with the local communities and the national government of Palau. PAN sites are the Palauan commitment towards the Micronesia Challenge. The Palauan Government established a Green Fee, a fee that is paid by anyone leaving Palau. Diving tourists are nearly always happy to pay a coral-reef resource management tax as long as it is directly used for the stated purpose rather than to general government account. The Green Fees of Palau are restricted to a PAN Fund. States, communities or private protected areas may apply for support to develop and manage a PAN site. PCS worked with the government, states and the NOAA Pacific Islands Fisheries Science Center to develop criteria for management plans and monitoring protocols.
The other peoples of the Caroline Islands (Yap, Chuuk, Pohnpei, Kosrae and their numerous associated atolls) also have solid knowledge of their resources. In Yap, they directly manage their terrestrial resources by planting vegetation or raising livestock or poultry to replace what is harvested, but they manage their harvesting of marine resources indirectly by managing their fishing practices because they know they must depend on the whims of natural processes to replace what they harvest. Traditional social organizations of marine tenure , rights of harvest, control of fishing methods vary among the many islands and atolls. However, Palau stands out as leading in adapting management of resources to the changing global economy .
The most important value of coral reefs in the lives of local people is usually not recognized by outsiders. This is the stabilizing effects of reefs on social structure. Fishing is often a cooperative activity in which each of the family members has a clearly recognized role. It has been discerned from interviews of fishermen in Palau that fishing activities help solidify the roles and importance of members of the family. It has been said that reefs may be more important in providing the opportunity for fishing activities than in providing the catch. Fishing and reef-gleaning are often perceived as fun and wholesome. In cases where large developments such as resorts or military bases obstruct access of local people to traditional fishing or reef-gleaning areas, the effects cannot be overcome simply with jobs providing wages by which food can be purchased. As social structure deteriorates, the numbers of suicides and criminal acts increase. The economic costs of such societal maladies are rarely taken into account in the evaluation of coral reefs, but these costs of the deterioration of coral reefs are ultimately paid by all of us.
References
Abesamis RA, Green AL, Russ GR, Jadloc CRL (2014) The intrinsic vulnerability to fishing of coral reef fishes and their differential recovery to fishery closures. Rev Fish Biol Fisheries. doi:10.1007/s11160-014-9362-x
Adjeroud M, Michonneau F, Edmunds PJ, Chancerelle Y, Lison de Loma T, Penin L, Thibaut L, Vidal-Dupiol J, Salvat B, Galzin R (2009) Recurrent disturbances, recovery trajectories, and resilience of coral assemblages on a South Central Pacific reef. Coral Reefs 28:775–780
Albright RA, Mason B, Langdon C (2008) Effect of aragonite saturation state on settlement and post-settlement growth of Porites astreoides larvae. Coral Reefs 27:485–490
Albright RA, Mason B, Miller M, Langdon C (2010) Ocean acidification compromises recruitment success of the threatened Caribbean coral, Acropora palmata. Proc Natl Acad Sci 107:20400–20404
Alcala AC, Luchavez T (1982) Fish yields of the coral reef surrounding Apo Island, Negros Occidental, Central Visayas, Philippines. Proc 4th Internat Coral Reef Sym, Manila l: 69–73
Almany GR, Berumen ML, Thorrold SR, Planes S, Jones GP (2007) Local replenishment of coral reef fish populations in a marine reserve. Science 316:742–744
Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR (2009) Flattening of Caribbeancoral reefs: region-wide declines in architectural complexity. Proc Roy Soc B 276:3019–3025
Anonymous (2000) What price coral? Economist. Nov 4: 87–89
Apprill A, Marlow HQ, Martindale MQ, Rappe MS (2009) The onset of microbial associations in the coral Pocillopora meandrina. ISME J 3:685–699
Archer D, Brovkin V (2008) The millennial atmosphere lifetime of anthropogenic CO2. Clim Change 90:283–297
Aronson RB, Precht WF (2001) White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460:25–38
Babcock R (1990) Reproduction and development of the blue coral Heliopora coerulea (Alcyonaria: Coenothecalia). Mar Biol 104:475–481
Babcock R, Davies P (1991) Effects of sedimentation on settlement of Acropora millepora. Coral Reefs 9:205–208
Bambach RK, Knoll AH, Wang SC (2004) Origination, extinction, and mass depletion of marine diversity. Paleobiology 30:522–542
Banse K (1982) Cell volumes, maximal growth rates of unicellular algae and ciliates, and the role of ciliates in the marine pelagial. Limnol Oceanogr 27:1059–1071
Berkeley SA, Chapman C, Sogard SM (2004a) Maternal age as a determinant of larval growth and survival in a marine fish, Sebastes melanops. Ecology 85:1258–1264
Berkeley SA, Hixon MA, Larson RJ, Love MS (2004b) Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries 29:23–32
Berkelmans R (2002) Time-integrated thermal bleaching thresholds of reefs and their variation on the Great Barrier Reef. Mar Ecol Prog Ser 237:309–310
Berkelmans R, Willis BL (1999) Seasonal and local spatial patterns in the upper thermal limits of corals on the inshore Central Great Barrier Reef. Coral Reefs 18:219–228
Bernecker M, Weidlich O (2005) Azooxanthellate corals in the late Maastrichtian-early Paleocene of the Danish basin: bryozoan and coral mounds in a boreal shelf setting. In: Freiwald A, Roberts JM (eds) Cold-water corals and ecosystems. Springer, Berlin, pp 3–25
Berner RA, Kothavala Z (2001) GEOCARB III: a revised model of atmospheric CO2 over Phanerozoic time. Am J Sci 301:182–204
Bice KL, Birgel D, Meyers PA, Dahl KA, Hinrichs K-U, Norris RD (2006) A multiple proxy and model study of Cretaceous upper ocean temperatures and atmospheric CO2 concentrations. Paleoceanography 21:PA2002. doi:10.1029/2005PA001203
Birkeland C (1977) The importance of rate of biomass accumulation in early successional stages of benthic communities to the survival of coral recruits. Proc 3rd Int Coral Reef Sym, Miami 1: 15–21
Birkeland C (1997) Status of coral reefs in the Marianas. In: Grigg RW, Birkeland C (eds) Status of coral reefs in the Pacific. Univ Hawaii Sea Grant College Program, Honolulu, pp 91–100
Birkeland C, Rowley D, Randall RH (1981) Coral recruitment patterns at Guam. Proc 4th Int Coral Reef Sym, Manila 2: 339–344
Birkeland C, Miller MW, Piniak GA, Eakin CM, Weijerman M, McElhany P, Dunlap M, Brainard RE (2013) Safety in numbers? Abundance may not safeguard corals from increasing carbon dioxide. Bioscience 63:967–974
Birrell CL, McCook JL, Willis BL (2005) Effects of algal turfs and sediment on coral settlement. In: Hutchings PA, Haynes D (eds), Proceedings of catchment to reef: water quality issues in the Great Barrier Reef Region conference. Mar Poll Bull, doi: 10.1016/j.marpolbul.2004.10.022
Birrell CL, McCook LJ, Willis BL, Diaz-Pulido GA (2008) Effects of benthic algae on the replenishment of corals and the implications for the resilience of coral reefs. Oceanogr Mar Biol Ann Rev 46:25–63
Bobko SJ, Berkeley SA (2004) Maturity, ovarian cycle, fecundity, and age-specific parturition of black rockfish (Sebastes melanops). Fish Bull US 80:881–884
Bohnsack JA (1998) Application of marine reserves to reef fisheries management. Aust J Ecol 23:298–304
Bonaldo RM, Bellwood DR (2008) Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar Ecol Prog Ser 360:237–244
Brown BE (1997a) Coral bleaching: causes and consequences. Coral Reefs 16:129–138
Brown BE (1997b) Disturbances to reefs in recent times. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 354–379
Brown BE, Cossins AR (2011) The potential for temperature acclimatization of reefcorals in the face of climate change. In: Dubinsky Z, Stambler N (eds) Coral reefs: an ecosystem in transition. Springer Science, New York, pp 421–433
Bruggemann JH, van Kessel AM, van Rooij JM, Breeman AM (1996) Bioerosion and sediment ingestion by the Caribbean parrotfish Scarus vetula and Sparisoma viride: implications of fish size, feeding mode and habitat use. Mar Ecol Prog Ser 134:59–71
Budd AF (2000) Diversity and extinction in the Cenozoic history of Caribbean reefs. Coral Reefs 19:25–35
Budd AF, Johnson KG, Stemann TA (1996) Plio-Pleistocene turnover and extinctions in the Caribbean reef-coral fauna. In: Jackson JBC, Budd AF, Coates AG (eds) Evolution and environment in tropical America. Univ Chicago Press, Chicago, pp 168–204
Budd AF, Fukami H, Smith ND, Knowlton N (2012) Taxonomic classification of the reef coral family Mussidae (Cnidaria: Anthozoa: Scleractinia). Zool J Linnean Soc 166:465–529
Buddemeier RW, Kinzie RA III (1998) Reef science: asking all the wrong questions in all the wrong places? Reef Encount 23:29–34
Buston PM, Jones GP, Planes S, Thorrold SR (2011) Probability of successful larval dispersal declines fivefold over 1 km in a coral reef fish. Proc Roy Soc Lond B Biol Sci 279:1883–1888
Butler JN, Bulnzett-Blerkes J, Barnes JA, Ward J (1993) The Bermuda fisheries: a tragedy of the commons averted? Environment 35:7–41
Caruthers AH, Stanley GD Jr (2008) Systematic analysis of Upper Triassic silicified scleractinian corals from Wrangellia and the Alexander Terrane, Alaska and British Columbia. J Paleont 82:470–491
Ceccherelli VU, Rossi R (1984) Settlement, growth and production of the mussel Mytilus galloprovincialis. Mar Ecol Progr Ser 16:173–184
Choat JH, Robertson DR (2002) Age-based studies. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic, New York, pp 57–80
Cohen AL, Holcomb M (2009) Why corals care about ocean acidification: uncovering the mechanism. Oceanography 22:118–127
Cole LC (1954) The population consequences of life history phenomena. Q Rev Biol 29:103–137
Cole AJ, Pratchett MS, Jones GP (2008) Diversity and functional importance of coral-feeding fishes on tropical coral reefs. Fish Fish 9:286–307
Coles SL (1997) Reef corals occurring in a highly fluctuating temperature environment at Fahal Island, Gulf of Oman (Indian Ocean). Coral Reefs 16:269–272
Coles SL, Brown BE (2003) Coral bleaching – capacity for acclimatization and adaptation. Adv Mar Biol 46:183–223
Coles SL, Jokiel PL (1978) Synergistic effects of temperature, salinity and light on the hermatypic coral Montipora verrucosa. Mar Biol 49:187–195
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Colgan M (1984) The Cretaceous coral Heliopora (Octocorallia, Coenothecalia) – a common Indo-Pacific reef builder. In: Eldredge N (ed) Living fossils. A casebooks in earth sciences. Springer, Berlin/Heidelberg/New York, pp 266–271
Comeau S, Edmunds PJ, Spindel NB, Carpenter RC (2014) Diel pCO2 oscillations modulate the response of the coral Acropora hyacinthus to ocean acidification. Mar Ecol Prog Ser 501:99–111
Cortés J, Jiménez C (2003) Corals and coral reefs of the Pacific of Costa Rica: history, research and status. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 361–385
Cowen RK, Lwiza KMM, Sponaugle S, Paris CB, Olson DB (2000) Connectivity of marine populations: open or closed? Science 287:857–859
Cowen RK, Paris CB, Srinivasan A (2006) Scaling of connectivity in marine populations. Science 311:522–527
Cox EF (1986) The effects of a selective corallivore on growth rates and competition for space between two species of Hawaiian corals. J Exp Mar Biol Ecol 101:167–174
Cox EF, Ward S (2002) Impact of elevated ammonium on reproduction in two Hawaiian scleractinian corals with different life history patterns. Mar Pollut Bull 44:1230–1235
Craig P (1995) Life history and harvest of the surgeonfish Acanthurus lineatus in American Samoa, Biol Rept Ser No 77. Dept Mar Wildlife Res, American Samoa, 30 pp
Craig P (2005) overfished coral reefs in American Samoa: no quick fix. Reef Encount 33:21–22
Craig P, Green A, Saucerman S (1995) Coral reef troubles in American Samoa. SPC Fish Newsl 72:33–34
Craig P, Birkeland C, Belliveau S (2001) High temperatures tolerated by a diverse assemblage of shallow-water corals in American Samoa. Coral Reefs 20:185–189
Darcy GH, Matlock GC (1999) Application of the precautionary approach in the national standard guidelines for conservation and management of fisheries in the United States. ICES J Mar Sci 56:853–859
Davis GW (1992) Biology of Mulloides flavolineatus. Study 3 in studies of Guam’s recreationally-important fish, Job Progress Report for Project No FW-2R-28, 10 pp
Dawkins R (1982) The extended genotype. Oxford University Press, Oxford, p 307
DeCarlo TM, Cohen AL, Barkley HC, Cobban Q, Young C, Shamberger KE, Brainard RE, Golbuu Y (2015) Coral macrobioerosion is accelerated by ocean acidification and nutrients. Geology 43:7–10
Dee AJ, Parrish JD (1994) Reproductive and trophic ecology of the soldierfish Myripristis amaena in tropical fisheries. Fish Bull 92:516–530
DeMartini EE, Friedlander AM, Holzwarth SR (2005) Size at sex change in protogynous labroids, prey body size distributions, and apex predator densities at NW Hawaiian atolls. Mar Ecol Prog Ser 297:259–271
DeMartini EE, Friedlander AM, Sandin SA, Sala E (2008) Differences in fish-assemblage structure between fished and unfished atolls in the northern Line Islands, central Pacific. Mar Ecol Prog Ser 365:199–215
Denis V, Guillaume MMM, Goutx M, de Palmas S, Debreuil J, Baker AC, Boonstra RK, Bruggemann JH (2013) Fast growth may impair regeneration capacity in the branching coral Acropora muricata. PLoS ONE 8(8):e72618. doi:10.1371/journal.pone.0072618
Diekmann OE, Bak RPM, Stam WT, Olsen JL (2001) Molecular genetic evidence for reticulate speciation in the coral genus Madracis on a Caribbean fringing reef slope. Mar Biol 139:221–233
Dierking J (2007) Effects of the introduced predatory fish Cephalopholis argus on native reef fish populations in Hawaii. PhD dissertation, Univ Hawaii, 115 pp
Dixson DL (2011) Risk assessment by larval fishes during settlement site selection. Coral Reefs 31:255–261
Dixson DL, Abrego D, Hay ME (2014) Chemically mediated behavior of recruiting corals and fishes: a tipping point that may limit reef recovery. Science 345:892–897
Dolan JR, Pérez MT (2000) Costs, benefits and characteristics of mixotrophy in marine oligotrichs. Freshw Biol 45:227–238
Doropoulos C, Ward S, Diaz-Pulido G, Hoegh-Guldberg O, Mumby PJ (2012) Ocean acidification reduces coral. Ecol Lett 15:338–346
Dunne RP, Brown BE (2001) The influence of solar radiation on bleaching of shallow water reef corals in the Andaman Sea, 1993–1998. Coral Reefs 20:201–210
Edinger EN, Risk MJ (1995) Preferential survivorship of brooding corals in a regional extinction. Paleobiology 21:200–219
Eguchi M (1948) Fossil Helioporidae from Japan and the South Sea islands. J Paleontol 22:362–364
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50:125–146
Fabricius KE, Metzner J (2004) Scleractinian walls of mouths: predation on coral larvae by corals. Coral Reefs 23:245–248
Fabricius K, Wild C, Wolanski E, Abele D (2003) Effects of transparent exopolymer particles (TEP) and muddy terrigenous sediments on the survival of hard coral recruits. Estuar Coast Shelf Sci 57:613–621
Fabricius KE, Langdon C, Uthicke S, Humphrey C, Noonan S, De’ath G, Okazaki R, Muehllehner N, Glas MS, Lough JM (2011) Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat Clim Chang 1:165–169. doi:10.1038/NCLIMATE1122
Fabricius KE, Cséke S, Humphrey C, De’ath G (2013) Does trophic status enhance or reduce the thermal tolerance of scleractinian corals? A review, experimental and conceptual framework. PLoS ONE 8(1):e54399. doi:10.1371/journal.pone.0054399
Fenner D (2012) Challenges for managing fisheries on diverse coral reefs. Diversity 4:105–160
Fenner D (2014) Fishing down the largest coral reef fish species. Mar Pollut Bull. doi:10.1016/j.marpolbul.2014.04.049
Ferreira BP, Russ GR (1994) Age validation and estimation of growth rate of the coral trout Plectropomus leopardus (Lacepede 1802) from Lizard Island, Northern Great Barrier Reef. Fish Bull 92:46–57
Frank U, Mokady O (2002) Coral biodiversity and evolution: recent molecular contributions. Can J Zool 80:1723–1734
Frank U, Oren U, Loya Y, Rinkevich B (1997) Alloimmune maturation in the coral Stylophora pistillata is achieved through three distinctive stages, 4 months postmetamorphosis. Proc R Soc B 264:99–104
Friedlander AM, DeMartini EE (2002) Contrasts in density, size, and biomass of reef fishes between the northwestern and the main Hawaiian islands: the effects of fishing down apex predators. Mar Ecol Prog Ser 230:253–264
Gates RD, Edmunds PJ (1999) The physiological mechanisms of acclimatization in tropical reef corals. Am Zool 39:30–43
Gerlach G, Atema J, Kingsford MJ, Black KP, Miller-Sims V (2007) Smelling home can prevent larval dispersal of reef fish larvae. Proc Natl Acad Sci U S A 104:858–863
Gilmour J (1999) Experimental investigation into the effects of suspended sediment on fertilisation, larval survival and settlement in a scleractinian coral. Mar Biol 135:451–462
Gladfelter EH (1982) Skeletal development in Acropora cervicornis: I. Patterns of calcium carbonate accretion in the axial corallite. Coral Reefs 1:45–51
Gladfelter EH, Monahan RK, Gladfelter WB (1978) Growth rates of five reef-building corals in the northeastern Caribbean. Bull Mar Sci 28:728–734
Glynn PW, Krupp DA (1986) Feeding biology of a Hawaiian sea star corallivore, Culcita novaeguineae Muller & Troschel. J Exp Mar Biol Ecol 96:75–96
Glynn PW, Mate JL, Baker AC, Calderon MO (2001) Coral bleaching and mortality in Panama and Ecuador during the 1997–1998 El Niño-Southern Oscillation Event: spatial/temporal patterns and comparisons with the 1982–1983 event. Bull Mar Sci 69:79–109
Golbuu Y, Wolanski E, Idechong JW, Victor S, Isechal AL, Oldiais NW, Richmond RH, van Woesik R (2012) Predicting coral recruitment in Palau’s complex reef archipelago. PLoS ONE 7:e50998. doi:10.1371/journal.pone.0050998
Goldman B, Talbot FH (1976) Aspects of the ecology of coral reef fishes. In: Jones OA, Endean R (eds) Biology and geology of coral reefs. III. Biology 2. Academic, New York, pp 125–254
Götz S (2003) Biotic interaction and synecology in a Late Cretaceous coral-rudist biostrome of southeastern Spain. Palaeogeogr Palaeoclimatol Palaeoecol 193:125–138
Graham EM, Baird AH, Connolly SR (2008) Survival dynamics of scleractinian coral larvae and implications for dispersal. Coral Reefs 27:529–539
Grigg RW, Polovina JJ, Atkinson MJ (1984) Model of a coral reef ecosystem. III. Resource limitation, community regulation, fisheries yield and resource management. Coral Reefs 3:23–27
Hallock P (1981) Algal symbiosis: a mathematical analysis. Mar Biol 62:249–255
Hallock P (1987) Fluctuations in the trophic resource continuum; a factor in global biodiversity cycles. Palaeoceanography 2:457–471
Hallock P (2001) Coral reefs, carbonate sediments, nutrients and global change. In: Stanley GD Jr (ed) The history and sedimentology of ancient reef systems. Kluwer Academic, New York, pp 388–427
Hamilton RJ, Choat JH (2012) Bumphead parrotfish – Bolbometopon muricatum. In: de Mitcheson YS, Colin PL (eds) Reef fish spawning aggregations: biology, research and management. Fish & Fisheries Series 35. Springer, pp 490–496
Hamilton RJ, Adams S, Choat JH (2008) Sexual development and reproductive demography of the green humphead parrot fish (Bolbometopon muricatum) in the Solomon Islands. Coral Reefs 27:153–163
Hamner WM, Jones MS, Carleton JH, Hauri IR, Williams DMB (1988) Zooplankton, planktivorous fish, and water currents on a windward reef face: Great Barrier Reef, Australia. Bull Mar Sci 42:459–479
Handford P, Bell G, Reimchen T (1977) A gillnet fishery considered as an experiment in artificial selection. J Fish Res Board Can 34:954–961
Harman RF, Katekaru AZ (1988) Hawaii Commercial Fishing Survey 1987. Div Aquatic Resources, Dept Land and Natural Resources, State of Hawaii, 71 pp
Harrigan JF (1972) The planula larva of Pocillopora damicornis, lunar periodicity of swimming and substratum selection behavior. PhD University of Hawai’i at Manoa, Honolulu, HI
Harrison HB, Williamson DH, Evans RD, Almany GR, Thorrold SR, Russ GR, Feldheim K, van Herwerden L, Planes S, Srinivasan M, Berumen ML, Jones GP (2012) Larval export from marine reserves and the recruitment benefit for fish and fisheries. Curr Biol 22:1023–1028
Hatcher BG (1997) Organic production and decomposition. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 140–174
Hatta M, Fukami H, Wang W, Omori M, Shimoike K, Hayashibara T, Ina Y, Sugiyama T (1999) Reproductive and genetic evidence for a reticulate evolutionary history of mass-spawning corals. Mol Biol Evol 16:1607–1613
Hauser L, Adcock GJ, Smith PJ, Ramírez JHB, Carvalho GR (2002) Loss of microsatellite diversity and low effective population size in an overexploited population of New Zealand snapper (Pagrus auratus). Proc Natl Acad Sci U S A 99:11742–11747
Hay WW, DeConto RM, Wold CN (1997) Climate: is the past the key to the future? Geol Rundsch 86:471–491
Heckel PH (1974) Carbonate buildups in the geologic record: a review. In: Laporte LF (ed), Reefs in time and space, SEPM Spec. Pub. 18. Society of Economic Paleontologists and Mineralogists, Tulsa, Oklahoma, pp 90–154
Helm C, Schülke I (2000) Contact reactions and fusion of late Jurassic ramose coral Thamnasteria dendroica in a patch reef environment. Coral Reefs 19:89–92
Hernández-Delgado EA, Suleimán-Ramos SE (2014) E.S.A. coral species listing: a roadblock to community-based engagement in coral reef conservation and rehabilitation across the U.S. Caribbean? Reef Encount 29:11–15
Hixon MA (1991) Predation as a process structuring coral reef fish communities. In: Sale PF (ed) The ecology of fishes on coral reefs. Academic, San Diego, pp 437–508
Hixon MA, Beets JP (1993) Predation, prey refuges, and the structure of coral- reef fish assemblages. Ecol Monogr 63:77–101
Hixon MA, Carr MH (1997) Synergistic predation, density dependence, and population regulation in marine fish. Science 277:946–949
Hixon MA, Johnson DW, Sogard SM (2013) BOFFFFs: on the importance of conserving old-growth age structure in fishery populations. ICES J Mar Sci. doi:10.1093/icesjms/fst200
Hodgson G (1990) Sediment and the settlement of larvae of the reef coral Pocillopora damicornis. Coral Reefs 9:41–43
Hoey AS, McCormick MI (2004) Selective predation for low body condition at the larval-juvenile transition of a coral reef fish. Oecologia 139:23–29
Holbrook SJ, Schmitt RJ (2002) Competition for shelter space causes density-dependent predation mortality in damselfishes. Ecology 83:2855–2868
Holt RD (1984) Spatial heterogeneity, indirect interactions, and coexistence of prey species. Am Nat 124:377–406
Holt RD (1987) Prey communities in patchy environments. Oikos 50:276–290
Hönisch B, Ridgwell A, Schmidt DN, Thomas E, Gibbs SJ, Sluijs A, Zeebe R, Kump L, Martindale RC, Greene SE, Kiessling W, Ries J, Zachos JC, Royer DL, Barker S, Marchitto TM, Moyer R, Pelejero C, Ziveri P, Foster GL, Williams B (2012) The geological record of ocean acidification. Science 335:1058–1063
Howard KG (2008) Community structure, life history, and movement patterns of parrotfishes: large protogynous hermaphrodite fishery species. PhD dissertation, Univ Hawaii, 112 pp
Hughes TP, Connell JH (1999) Multiple stressors on coral reefs: a long-term perspective. Limnol Oceanogr 44:932–940
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2000) Supply-side ecology works both ways: the link between benthic adults, fecundity, and larval recruits. Ecology 81:2241–2249
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365
Hunte W, Wittenberg M (1992) Effects of eutrophication and sedimentation on juvenile corals. Mar Biol 114:625–631
Hutchings JA, Reynolds JD (2004) Marine fish population collapses: consequences for recovery and extinction risk. Bioscience 54:297–309
Jablonski D (2002) Survival without recovery after mass extinctions. Proc Natl Acad Sci U S A 99:8139–8144
Jackson JBC (1997) Reefs since Columbus. Proc 8th Int Coral Reef Sym 1: 97–106
Jackson JBC, McKinney FK (1991) Ecological processes and progressive macroevolution of marine clonal benthos. In: Ross RM, Allmon WD (eds) Causes of evolution. Univ Chicago Press, Chicago, pp 173–209
Jennings S, Kaiser KJ, Reynolds JD (2001) Marine fisheries ecology. Blackwell, Oxford, p 417
Jiménez CE, Cortés J, León A, Ruiz E (2001) Coral bleaching and mortality associated with the 1997–98 El Niño in an upwelling environment in the eastern Pacific (Gulf of Papagayo, Costa Rica). Bull Mar Sci 69:151–169
Johannes RE (1981) Words of the lagoon. Univ Cal Berkeley Press, Berkeley, p 245
Johannes RE (1998) The case for data-less marine resource management: example from tropical nearshore fisheries. Trends Ecol Evol 13:243–246
Jokiel PL (1990) Transport of reef corals into the great Barrier Reef. Nature 347:665–667
Jones GP (1997) Relationships between recruitment and postrecruitment processes in lagoonal populations of two coral reef fishes. J Exp Mar Biol Ecol 213:231–246
Jones GP, Milicich MJ, Emslie MJ, Lunow C (1999) Self-recruitment in a coral reef fish population. Nature 402:802–804
Jones GP, Planes S, Thorrold SR (2005) Coral reef fish larvae settle close to home. Curr Biol 15:1314–1318
Jones GP, Almany GR, Russ GR, Sale PF, Steneck RS, van Oppen MJH, Willis BL (2009) Larval retention and connectivity among populations of corals and reef fishes: history, advances and challenges. Coral Reefs 28:307–325
Keenan EE, Brainard RE, Basch LV (2006) Historical and present status of the pearl oyster, Pinctada margaritifera, at Pearl and Hermes Atoll, Northwestern Hawaiian Islands. Atoll Res Bull 543:333–344
Kennedy EV, Perry CT, Halloran PR, Iglesias-Prieto R, Schönberg WM, Form AU, Carricart-Ganivet JP, Fine M, Eakin CM, Mumby PJ (2013) Avoiding coral reef functional collapse requires local and global action. Curr Biol 23:912–918
Kenyon JC (1997) Models of reticulate evolution in the coral genus Acropora based on chromosome numbers: parallels with plants. Evolution 51:756–767
Kiessling W (2009) Geologic and biologic controls on the evolution of reefs. Ann Rev Ecol Evol Syst 40:173–192
Kiessling W, Baron-Szabo RC (2004) Extinction and recovery patterns of scleractinian corals at the Cretaceous-Tertiary boundary. Palaeogeogr Palaeoclimatol Palaeoecol 214:195–223
Kinsey DW, Davies PJ (1979) Effects of elevated nitrogen and phosphorus on coral reef growth. Limnol Oceanogr 2:935–940
Kleypas JA, Buddemeier RW, Archer D, Gattuso J-P, Langdon C, Opdyke BN (1999a) Geochemical consequences of increased atmospheric carbon dioxide on coral reefs. Science 284:118–120
Kleypas JA, McManus JW, Menez LAB (1999b) Environmental limits to coral reef development: where do we draw the line? Am Zool 39:146–159
Kleypas JA, Buddemeier RW, Gattuso J-P (2001) The future of coral reefs in an age of global change. Int J Earth Sci (Geol Rundsch) 90:426–437
Kleypas JA, Danabasoglu G, Lough JM (2008) Potential role of the ocean thermostat in determining regional differences in coral reef bleaching events. Geophysical Research Letters 35:L03613. doi:10.1029/2007GL032257
Kline DI, Kuntz NM, Breitbart M, Knowlton N, Rohwer F (2006) Role of elevated organic carbon levels and microbial activity in coral mortality. Mar Ecol Prog Ser 314:119–125
Kojis BL, Quinn NJ (1984) Seasonal and depth variation in fecundity of Acropora palifera at two reefs in Papua New Guinea. Coral Reefs 3:165–172
Kojis BL, Quinn NJ (1985) Puberty in Goniastrea favulus. Age or size limited? Proc 5th Int Coral Reef Congr, Tahiti 4: 289–293
Koop K, Booth D, Broadbent A, Brodie J, Bucher D, Capone D, Coll J, Dennison W, Erdmann M, Harrison P (2001) ENCORE: the effect of nutrient enrichment on coral reefs. Synthesis of results and conclusions. Mar Pollut Bull 42:91–120
Koslow JA, Hanley F, Wicklund R (1988) Effects of fishing on reef fish communities at Pedro Bank and Port Royal Cays, Jamaica. Mar Ecol Prog Ser 43:201–212
Kuffner IB, Paul VJ (2004) Effects of the benthic cyanobacterium Lyngbya majuscula on larval recruitment of the reef corals Acropora surculosa and Pocillopora damicornis. Coral Reefs 23:455–458
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117
Kuffner IB, Andersson AJ, Jokiel PL, Rodgers KS, Mackenzie FT (2008) Decreased abundance of crustose coralline algae due to ocean acidification. Nat Geosci 1:114–117
Larkin PA (1978) Fisheries management–an essay for ecologists. Ann Rev Ecol Syst 9:57–73
Lathuilière B, Marchal D (2009) Extinction, survival and recovery of corals from the Triassic to Middle Jurassic time. Terra Nova 21:57–66
Lehodey P, Leroy B (1999) Age and growth of yellowfin tuna (Thunnus albacares) from the western and central Pacific Ocean as indicated by daily growth increments and tagging data. Secretariat of the Pacific Community, Noumea, 21 pp
Leinfelder RR (2001) Jurassic reef ecosystems. In: Stanley GD Jr (ed) The history and sedimentology of ancient reef systems. Kluwer Academic/Plenum Publ, New York, pp 251–309
Leinfelder RR, Seemann J, Heiss GA, Struck U (2012) Could ‘ecosystem atavisms’ help reefs to adapt to the Anthropocene? Proc 12th Int Coral Reef Sym, Cairns, Australia 2
Levitan DR, Fukami H, Jara J, Kline D, McGovern TM, McGhee KE, Swanson CA, Knowlton N (2004) Mechanisms of reproductive isolation among sympatric broadcast-spawning corals of the Montastraea annularis species complex. Evolution 58:308–323
Levitan DR, Boudreau W, Jara J, Knowlton N (2014) Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar Ecol Prog Ser 515:1–10
Lirman D (2000) Fragmentation in the branching coral Acropora palmata (Lamarck): growth, survivorship, and reproduction of colonies and fragments. J Exp Mar Biol Ecol 251:41–57
Littler MM, Littler DS (1980) The evolution of thallus form and survival strategies in benthic marine macroalgae: field and laboratory tests of a functional form model. Am Nat 116:25–41
Littler MM, Littler DS (1997) Disease-induced mass mortality of crustose algae on coral reefs provides rationale for the conservation of herbivorous fish stocks. Proc 8th Int Coral Reef Sym 1:719–724
Littler K, Robinson SA, Brown PR, Nederbragt AJ, Pancost RD (2011) High sea-surface temperatures during the early Cretaceous Epoch. Nat Geosci 4:169–172
Lokrantz J, Nyström M, Thyresson M, Johansson C (2008) The non-linear relationship between body size and function in parrotfishes. Coral Reefs 27:967–974
Longhurst A (2002) Murphy’s law revisited: longevity as a factor in recruitment to fish populations. Fish Res 56:125–131
López-Duarte PC, Carson HS, Cook GS, Fodrie FJ, Becker BJ, DiBacco C, Levin LA (2012) What controls connectivity? An empirical, multi-species approach. Integr Comp Biol 52:511–524
López-Pérez RA (2005) The Cenozoic hermatypic corals in the eastern Pacific: history of research. Earth Sci Rev 72:67–87
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik R (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
Ma TYH (1959) Effect of water temperature on growth rate of reef corals. Oceanographica Sinica Special Volume 1, pp 116 +320 plates
Marhaver KL, Vermeij MJA, Rohwer F, Sandin SA (2013) Janzen-Connell effects in a broadcast-spawning Caribbean coral: distance-dependent survival of larvae and settlers. Ecology 94:146–160
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: differential susceptibilities among taxa. Coral Reefs 19:155–163
Mascarelli A (2014) Climate-change adaptation: designer reefs. Nature 508:444–446. doi:10.1038/508444a
Maynard JA, Anthony KRN, Marshall PA, Masiri I (2008) Major bleaching events can lead to increased thermal tolerance in corals. Mar Biol 155:173–182
McClanahan TR (2014) Recovery of functional groups and trophic relationships in tropical fisheries closures. Mar Ecol Prog Ser 497:13–23
McClanahan TR, Ateweberhan M, Graham NAJ, Wilson SK, Sebastian CR, Guillaume MMM, Bruggemann JH (2007) Western Indian Ocean coral communities: bleaching responses and susceptibility to extinction. Mar Ecol Prog Ser 337:1–13
McManus JW, Nañola CL, Reyes RB Jr, Kesner KN (1992) Resource ecology of the Bolinao coral reef system. ICLARM Stud Rev 22:117
Meekan MG, Ackerman JL, Wellington GM (2001) Demography and age structures of coral reef damselfishes in the tropical eastern Pacific Ocean. Mar Ecol Prog Ser 212:223–232
Meyer AL, Dierking J (2011) Elevated size and body condition and altered feeding ecology of the grouper Cephalopholis argus in non-native habitats. Mar Ecol Prog Ser 439: 203–212
Michalek-Wagner K, Willis BL (2001) Impacts of bleaching on the soft coral Lobophytum compactum. I. Fecundity, fertilization and offspring viability. Coral Reefs 19:231–239
Mora C, Frazier AG, Longman RJ, Dacks RS, Walton MM, Tong EJ, Sanchez JJ, Kaiser LR, Stender YO, Anderson JM, Ambrosino CM, Fernandez-Silva I, Giuseffi LM, Giambelluca TW (2013) The projected timing of climate departure from recent variability. Nature 502:183–187
Moran K et al (2006) The Cenozoic palaeoenvironment of the Arctic Ocean. Nature 441:601–605
Morse DE, Hooker N, Morse ANC, Jensen RA (1988) Control of larval metamorphosis and recruitment in sympatric agariciid corals. J Exp Mar Biol Ecol 116:193–217
Muller-Parker G, D’Elia CF (1997) Interactions between corals and their symbiotic algae. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 96–113
Munro JL (ed) (1983) Caribbean coral reef fishery resources. ICLARM Stud Rev 7, 276 pp
Murphy GI (1967) Vital statistics of the California sardine and the population consequences. Ecology 48:731–735
Nadon MO, Baum JK, Williams ID, McPherson JM, Zgliczynski BJ, Richards BL, Schroeder RE, Brainard RE (2012) Re-creating missing population baselines for Pacific reef sharks. Conserv Biol 26:493–503
Newman SJ, Williams DMB, Russ GR (1996) Age validation, growth and mortality rates of the tropical snappers (Pisces: Lutjanidae) Lutjanus adetii (Castelnau, 1873) and L. quinquelineatus (Bloch, 1790) from the central Great Barrier Reef, Australia. Mar Freshw Res 47:575–584
Nixon SW (1982) Nutrient dynamics, primary production and fisheries yields of lagoons. Oceanol Acta 5:357–371
Nugues M, Delvoye L, Bak R (2004) Coral defense against macroalgae: differential effects of mesenterial filaments on the green alga Halimeda opuntia. Mar Ecol Prog Ser 278:103–114
Ogden JC (1997) Ecosystem interactions in the tropical coastal seascape. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 288–297
Oliver TA, Palumbi SR (2009) Distributions of stress-resistant coral symbionts match environmental patterns at local but not regional levels. Mar Ecol Prog Ser 378:93–103
Oliver TA, Palumbi SR (2011) Do fluctuating temperature environments elevate coral thermal tolerance? Coral Reefs 30:429–440
Olsen EM, Heino M, Lilly GR, Morgan MJ, Brattey J, Ernande B, Dieckmann U (2004) Maturation trends indicative of rapid evolution preceded the collapse of northern cod. Nature 428:932–935
Omori M, Fukami H, Kobinata H, Hatta M (2001) Significant drop of fertilization of Acropora corals in 1999: an after-effect of heavy coral bleaching? Limnol Oceanogr 46:704–706
Ong L, Holland KN (2010) Bioerosion of coral reefs by two Hawaiian parrotfishes: species, size differences and fishery implications. Mar Biol. doi:10.1007/s00227-010-1411-y
Page HM, Hubbard DM (1987) Temporal and spatial patterns of growth in mussels Mytilus edulis on an offshore platform: relationships to water temperature and food availability. J Exp Mar Biol Ecol 111:159–179
Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanisms of reef coral resistance to future climate change. Science 344:895–898
Pandolfi JM, Jackson JBC, Geister J (2001) Geologically sudden extinction of two widespread Late Pleistocene Caribbean reef corals. In: Jackson JBC, Lidgard S, McKinney FK (eds) Evolutionary patterns: growth, form, and tempo in the fossil record. Univ Chicago Press, Chicago, pp 120–158
Pandolfi JM, Lovelock CE, Budd AF (2002) Character release following extinction in a Caribbean reef coral species complex. Evolution 56:479–501
Paulay G (1997) Diversity and distribution of reef organisms. In: Birkeland C (ed) Life and death of coral reefs. Chapman & Hall, New York, pp 298–353
Pearson PN, Palmer MR (2000) Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406:695–699
Planes S, Jones GP, Thorrold SR (2009) Larval dispersal connects fish populations in a network of marine protected areas. Proc Natl Acad Sci U S A 106:5693–5697
Podestá GP, Glynn PW (2001) The 1997–98 El Niño event in Panama and Galápagos: an update of thermal stress indices relative to coral bleaching. Bull Mar Sci 69:43–59
Poulin E, Palma AT, Leiva G, Narvaez D, Pacheco R, Navarrete SA, Castilla JC (2002) Avoiding offshore transport of competent larvae during upwelling events: the case of the gastropod Concholepas concholepas in Central Chile. Limnol Oceanogr 47:1248–1255
Poulsen CJ, Barron EJ, Peterson WH, Wilson PA (1999) A reinterpretation of Cretaceous shallow marine temperatures through model-data comparison. Paleoceanography 14:679–697
Poulsen CJ, Barron EJ, Arthur MA, Peterson WH (2001) Response of the mid-Cretaceous global oceanic ocean circulation to tectonic and CO2 forcings. Paleoceanography 16:576–592
Prescott R, Bourne DG, Negri A, Schmidt-Roach S (2014) Negative cues drive settlement distribution of two corals from the Great Barrier Reef: new perspectives from networks (in review)
Ramus J, Venable M (1987) Temporal ammonium patchiness and growth rate in Codium and Ulva (Ulvophyceae). J Phycol 23:518–523
Randall JE (1963) An analysis of the fish populations of artificial and natural reefs in the Virgin Islands. Carib J Sci 3:31–47
Randall JE (1965) Grazing effects on seagrasses by herbivorous reef fishes in the West Indies. Ecology 46:255–260
Randall JE (2007) Reef and shore fishes of the Hawaiian Islands. Sea Grant College Program, Univ Hawaii, 546 pp
Rawlinson KA, Stella JS (2012) Discovery of the corallivorous polyclad flatworm, Amakusaplana acroporae, on the Great Barrier Reef, Australia – the first report from the wild. PLoS ONE. doi:10.1371/journal.pone.0042240
Ricker WE (1981) Changes in the average size and average age of Pacific salmon. Can J Fish Aquat Sci 38:1636–1656
Riegl BM, Purkis SJ, Al-Cibahy AS, Abdel-Moati MA, Hoegh-Guldberg O (2011) Present limits to heat-adaptability in corals and population-level responses to climate extremes. PLoS ONE. doi:10.1371/journal.pone.0024802
Ritson-Williams R, Arnold SN, Fogarty ND, Steneck RS, Vermeij MJA, Paul VJ (2009) New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson Contrib Mar Sci 38:437–457
Rizzari JR, Frisch AJ, Connolly SR (2014) How robust are estimates of coral reef shark depletion? Biol Conserv 176:39–47
Rose CS, Risk MJ (1985) Increase in Ciona deletrix infestation of Montastrea cavernosa heads on an organically polluted portion of the Grand Cayman fringing reef. Mar Ecol 6:345–363
Rosen BR (2000) Algal symbiosis, and the collapse and recovery of reef communities: Lazarus corals across the K–T boundary. In: Culver SJ, Rawson PF (eds) Biotic response to global change: the last 145 million years. Cambridge University Press, Cambridge, pp 164–180
Roughgarden J, Smith F (1996) Why fisheries collapse and what to do about it. Proc Natl Acad Sci U S A 93:5078–5083
Russ GR (1984) A review of coral reef fisheries. UNESCO Rep Mar Sci 27:74–92
Ruttenberg BI, Hamilton SL, Walsh SM, Donovan MK, Friedlander A, DeMartini E, Sala E, Sandin SA (2011) Predator-induced demographic shifts in coral reef fish assemblages. PLoS ONE 6(6):e21062. doi:10.1371/journal.pone.0021062
Ryther JH (1969) Photosynthesis and fish production in the sea. Science 166:72–76
Sadovy Y (1993) The Nassau grouper, endangered or just unlucky? Reef Encount 13:10–12
Sammarco PW, Andrews JC (1989) The Helix experiment; differential localized dispersal and recruitment patterns in Great Barrier Reef corals. Limnol Oceanogr 34:896–912
Sandin SA, Smith JE, DeMartini EE, Dinsdale EA, Donner SD, Friedlander AM, Konotchick T, Malay M, Maragos JE, Obura D, Pantos O, Paulay G, Richie M, Rohwer F, Schroeder RE, Walsh S, Jackson JBC, Knowlton N, Sala E (2008) Baselines and degradation of coral reefs in the northern Line Islands. PLoS ONE 3(2):11, e1548
Schaffer WM (1974) Optimal reproductive effort in fluctuating environments. Am Nat 108:783–790
Schouten S, Hopmans EC, Forster A, van Breugel Y, Kuypers MMM, Damsté JSS (2003) Extremely high sea-surface temperatures at low latitudes during the middle Cretaceous as revealed by archaeal membrane lipids. Geology 32:1069–1072
Sepkoski JJ (2002) A compendium of fossil marine animal genera. In: Jablonski D, Foote M (eds), Bull Am Paleon 363: 1–563
Shanks AL, Shearman RK (2009) Paradigm lost? Cross-shelf distributions of intertidal invertebrate larvae are unaffected by upwelling or downwelling. Mar Ecol Prog Ser 385:189–204
Sharp KH, Ritchie KB, Schupp PJ, Ritson-Williams R, Paul VJ (2010) Bacterial acquisition in juveniles of several broadcast spawning coral species. PLoS ONE 5:e10898
Shomura RS (1987) Hawaii’s marine fishery resources: yesterday (1900) and today (1986). Southwest Fisheries Center Administrative Report H-87-21. 15 pp
Smith CL (1972) A spawning aggregation of Nassau grouper, Epinephelus striatus (Bloch). Trans Am Fish Soc 2:257–261
Smith MK (1993) An ecological perspective on inshore fisheries on the Main Hawaiian Islands. Mar Fish Rev 55:34–49
Smith JE, Shaw M, Edwards RA, Obura D, Pantos O, Sala E, Sandin SA, Smriga S, Hatay M, Rohwer FL (2006) Indirect effects of algae on coral: algae-mediated, microbe-induced coral mortality. Ecol Lett 9:835–845
Solomon S, Plattner G-K, Knuttic R, Friedlingstein P (2009) Irreversible climate change due to carbon dioxide emissions. Proc Natl Acad Sci U S A 106:1704–1709
Stanley SM, Hardie LA (1998) Secular oscillations in the carbonate mineralogy of reef-building and sediment-producing organisms driven by tectonically forced shifts in seawater chemistry. Palaeogeogr Palaeoclimatol Palaeoecol 144:3–19
Stella JS, Pratchett MS, Hutchings PA, Jones GP (2011) Coral-associated invertebrates: diversity, ecological importance and vulnerability to disturbance. Oceanogr Mar Biol Ann Rev 49:43–104
Steneck RS (2006) Staying connected in a turbulent world. Science 311:480–481
Stoeker DK (1999) Mixotrophy among dinoflagellates. J Eukaryot Microbiol 46:397–401
Strathmann RR, Hughes TP, Kuris AM, Lindeman KC, Morgan SG, Pandolfi JM, Warner RR (2002) Evolution of local recruitment and its consequences for marine populations. Bull Mar Sci 70:377–396
Swearer SE, Caselle JE, Lea DW, Warner RR (1999) Larval retention and recruitment in an island population of a coral-reef fish. Nature 402:799–802
Swearer SE, Shima JS, Hellberg ME, Thorrold SR, Jones GP, Robertson DR, Morgan SG, Selkoe KQ, Ruiz GM, Warner RR (2002) Evidence of self-recruitment in demersal marine populations. Bull Mar Sci 70:S251–S271
Szmant AM (2002) Nutrient enrichment on coral reefs: is it a major cause of coral reef decline? Estuar Coasts 25:743–766
Szmant AM, Gassman NJ (1990) The effects of prolonged “bleaching” on the tissue biomass and reproduction of the reef coral Montastrea annularis. Coral Reefs 8:217–224
Thornhill DJ, Rotjan RD, Todd BD, Chilcoat GC, Iglesias-Prieto R et al (2011) A connection between colony biomass and death in Caribbean reef-building corals. PLoS ONE 6:e29535. doi:10.1371/journal.pone.0029535
Tomascik T (1991) Settlement patterns of Caribbean scleractinian corals on artificial substrata along a eutrophication gradient, Barbados, West Indies. Mar Ecol Prog Ser 77:261–269
Tomascik T, Sander F (1987) Effects of eutrophication on reef-building corals. III. Reproduction of the reef-building coral Porites porites. Mar Biol 94:77–94
Tomascik T, van Woesik R, Mah AJ (1996) Rapid coral colonization of a recent lava flow following a volcanic eruption, Banda Islands, Indonesia. Coral Reefs 15:169–175
Underwood JN, Smith LD, van Oppen MJH, Gilmour JP (2009) Ecologically relevant dispersal of corals on isolated reefs: implications for managing resilience. Ecol Appl 19:18–29
Uthicke S, Schaffelke B, Byrne M (2009) A boom-bust phylum? Ecological and evolutionary consequences of density variations in echinoderms. Ecol Monogr 79:3–24
Van Oppen MJH, McDonald BJ, Willis B, Miller DJ (2001) The evolutionary history of the coral genus Acropora (Scleractinia, Cnidaria) based on a mitochondrial and a nuclear marker: reticulation, incomplete lineage sorting, or morphological convergence? Mol Biol Evol 18:1315–1329
Van Oppen MJH, Oliver JK, Putnam HM, Gates RD (2015) Building coral reef resilience through assisted evolution. Proc Nat Acad Sci USA. doi:10.1073/pnas.1422301112
Van Veghel MLJ, Bak RPM (1994) Reproductive characteristics of the polymorphic Caribbean reef building coral Montastrea annularis. III. Reproduction in damaged and regenerating colonies. Mar Ecol Prog Ser 109:229–233
Van Woesik R, Franklin EC, O’Leary J, McClanahan TR, Klaus JS, Budd AF (2012) Hosts of the Plio-Pleistocene past reflect modern-day coral vulnerability. Proc Roy Soc B 279:2448–2456
Vermeij GJ (1987) Evolution and escalation. Princeton Univ Press, Princeton
Vermeij MJA, Smith JE, Smith CM, Thurber RV, Sandin SA (2009) Survival and settlement success of coral planulae: independent and synergistic effects of macroalgae and microbes. Oecologia 159:325–336
Veron JEN (1995) Corals in space and time. Cornell University Press, Ithaca, p 321
Veron JEN (2000) Corals of the world, 3 vols. Australian Institute of Marine Science, Townsville, Australia
Vianna GMS, Meekan MG, Pannel D, Marsh S, Meeuwig JJ (2010) Wanted dead or alive? The relative value of reef sharks as a fishery and an ecotourism asset in Palau. Australian Institute of Marine Science and University of Western Australia, Perth, p 34
Villanueva RD, Edwards AJ (2010) Butterflyfishes feed on externally brooded larvae of the blue coral, Heliopora coerulea. Coral Reefs 29:105
Wallace CC, Rosen BR (2006) Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: implications for the evolution of modern diversity patterns of reef corals. Proc Roy Soc B Bio Sci 273:975–982
Walsh SM, Hamilton SL, Ruttenberg BI, Donovan MK, Sandin SA (2012) Fishing top predators indirectly affects condition and reproduction in a reef-fish community. J Fish Biol 80:519–537
Ward S, Harrison P (1997) The effects of elevated nutrient levels on settlement of coral larvae during the ENCORE experiment, Great Barrier Reef, Australia. Proc 8th Int Coral Reef Sym, Panama 1: 891–896
Ward S, Harrison P (2000) Changes in gametogenesis and fecundity of acroporid corals that were exposed to elevated nitrogen and phosphorus during the ENCORE experiment. J Exp Mar Biol Ecol 246:179–221
Ward S, Harrison P, Hoegh-Guldberg O (2002) Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. Proc 9th Int Coral Reef Sym, Bali 2: 1123–1128
Warner RR, Cowen RK (2002) Local retention of production in marine populations: evidence, mechanisms, and consequences. Bull Mar Sci 70:245–249
Wass RC (1982) The shoreline fishery of American Samoa – past and present. In: Munro JL (ed.), Ecological aspects of coastal zone management. Proc Seminar on Marine and Coastal Processes in the Pacific, Motopore Island Research Center, UNESCO, pp 51–83
Weber P (1993) Reviving coral reefs. In: Brown LR (ed) State of the world. WH Norton, New York, pp 42–60
Webster NS, Uthicke S, Botté ES, Flores F, Negri AP (2013) Ocean acidification reduces induction of coral settlement by crustose coralline algae. Glob Chang Biol 19:303–315
Weersing K, Toonen RJ (2009) Population genetics, larval dispersal, and connectivity in marine systems. Mar Ecol Prog Ser 393:1–12
Wells JW (1956) Scleractinia. In: Moore RC (ed) Treatise on invertebrate paleontology, Part F, Coelenterata, Geological Society of America and University of Kansas Press, Lawrence, KS, pp 328–444
Widdows J, Fieth P, Worrall CM (1979) Relationships between seston, available food and feeding activity in the common mussel Mytilus edulis. Mar Biol 50:195–207
Williams DMB, Hatcher AI (1983) Structure of fish communities on outer slopes of inshore, mid-shelf and outer shelf reefs of the Great Barrier Reef. Mar Ecol Prog Ser 10:239–250
Wilson PA, Norris RD, Cooper MJ (2002) Testing the Cretaceous greenhouse hypothesis using glassy foraminiferal calcite from the core of the Turonian tropics on Demerara Rise. Geology 30:607–610
Wing SR, Wing ES (2001) Prehistoric fisheries in the Caribbean. Coral Reefs 20:1–8
Wisshak M, Schönberg CHL, Form A, Freiwald A (2012) Ocean acidification accelerates reef bioerosion. PLoS ONE 7:e45124
Wittenberg M, Hunte W (1992) Effects of eutrophication and sediment on juvenile corals I. Abundance, mortality and community structure. Mar Biol 112:131–138
Wood R (1999) Reef evolution. Oxford Univ Press, Oxford, p 414
Wooldridge SA (2009) A new conceptual model for the warm-water breakdown of the coral–algae endosymbiosis. Mar Freshw Res 60:483–496
Zachos JC, Röhl U, Schellenberg SA, Sluijs A, Hodell DA, Kelly DC, Thomas E, Nicolo M, Raffi I, Lourens LJ, McCarren H, Kroon D (2005) Rapid acidification of the ocean during the Paleocene-Eocene Thermal Maximum. Science 308:1611–1615
Zapata FA, Vargas-Ángel B (2003) Corals and coral reefs of the Pacific coast of Colombia. In: Cortés J (ed) Latin American coral reefs. Elsevier, Amsterdam, pp 419–447
Zeebe RE (2001) Seawater pH and isotopic paleotemperatures of Cretaceous oceans. Palaeogeogr Palaeoclimatol Palaeoecol 170:49–57
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Birkeland, C. (2015). Biology Trumps Management: Feedbacks and Constraints of Life-History Traits. In: Birkeland, C. (eds) Coral Reefs in the Anthropocene. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7249-5_12
Download citation
DOI: https://doi.org/10.1007/978-94-017-7249-5_12
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7248-8
Online ISBN: 978-94-017-7249-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)