Abstract
Foundation species facilitate associated communities and provide key ecosystem functions, making anthropogenically driven phase-shifts involving these species critically important. One well-documented such phase-shift has been from coral to algal domination on tropical reefs. On South Pacific coral reefs, the macroalga Turbinaria ornata has expanded its range and habitat but, unlike algae that often dominate after phase-shifts, T. ornata is structurally complex and generally unpalatable to herbivores. Therefore, it may serve a foundational role on coral reefs, such as providing habitat structure to more palatable primary producers and corresponding trophic support to fishes. We predicted increasing T. ornata density would facilitate growth of associated algae, resulting in a positive trophic cascade to herbivorous fish. An experiment manipulating T. ornata densities showed a unimodal relationship between T. ornata and growth of understory algae, with optimal growth occurring at the most frequent natural density. Epiphyte cover also increased with density until the same optimum, but remained high with greater T. ornata densities. Foraging by herbivorous fishes increased linearly with T. ornata density. An herbivore exclusion experiment confirmed T. ornata facilitated epiphytes, but resource use of epiphytes by herbivores, though significant, was not affected by T. ornata density. Therefore, T. ornata performs foundational roles because it provides novel habitat to understory and epiphytic macroalgae and trophic support to consumers, though likely this function is at the expense of the original foundational corals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
We examined the functional role of a macroalga that is expanding on a coral reef.
-
Primary producers were facilitated by increased density of the macroalga.
-
Foraging by fish primary consumers increased due to increased algal resources.
Introduction
Foundation species facilitate associated species and support ecosystem functions through amelioration of harsh conditions, increased trophic support, and/or provision of habitat (sensu Dayton 1972; Stachowicz 2001; Ellison and others 2005). Foundation species often form habitat by providing physical structure for associated organisms to grow on directly or in close proximity. For example, some epiphytes grow directly on foundation species and are important for trophic support across systems (for example, seagrasses, Hughes and others 2004; freshwater macrophytes, Jaschinski and others 2011; oak trees, Angelini and Silliman 2014). In addition, foundation species in many systems can provide canopy that ameliorates harsh conditions (for example, nutrient limitation, photoinhibition, high wind or wave energy) for plants and macroalgae in the understory (for example in terrestrial forests in Gentry and Dodson 1987, Ellison and others 2005; kelp forests in Graham 2004). As the provision of structure, trophic support, and other services by foundation species influences community composition and diversity, we need a better understanding of the potential for species that may be favored by anthropogenic induced phase-shifts to fill foundational roles.
Phase-shifts from one community state to another have been documented in terrestrial, freshwater, and marine systems (Scheffer and others 2001; Folke and others 2004). This includes systems with structurally complex foundation species, such as terrestrial forests and coral reefs. Although the shifted species may occupy the same space, they may not support the same functions as the original foundation species. For example, fire suppression caused historically oak dominated forests to shift to shade-tolerant trees such as maples (Nowacki and Abrams 2008), and increasing human population density and fire frequency turned shrubland into grassland (Talluto and Suding 2008). These shifts in terrestrial foundation species due to anthropogenic influence resulted in drastic changes to ecosystems, such as changing community structure and trophic support, as the species that dominate after a phase-shift often do not support the same associated organisms or ecosystem functions (for example, coral reef examples in McCook 1999; temperate forest examples in Ellison and others 2005). In marine systems, foundation species tend to be structure-forming invertebrates (for example, mussels, Suchanek 1992; corals, Hughes and others 2010) or marine macrophytes (for example, kelp, Graham 2004; seagrasses, Orth and others 2006; rocky shore macroalgae, Korpinen and others 2010) that are also experiencing natural and human-driven phase-shifts (reviewed in deYoung and others 2008). For example, coral reefs are well documented to experience phase-shifts to algal domination due to nutrient enrichment and overfishing (reviewed by Hughes and others 2010). As it is well documented that some ecosystems have been increasingly subjected to phase-shifts (for example, coral reefs; Hughes and others 2010; Dudgeon and others 2010), it is critical to examine the potential for shifted species to perform foundational roles.
Corals are the dominant foundation species in tropical marine systems with hard substrates, while in nutrient-rich temperate waters, fleshy macroalgae often fill this role. In previous experimental studies, phase-shifts on coral reefs involved fast-growing, palatable macroalgal species or multi-species turf algae (for example, multiple species Lewis and Wainwright 1985; Cladophora Smith and others 2005; turf and macroalgae in Smith and others 2010; turf algae in Muthukrishnan and others 2016). Although coral reef macroalgae tend to be smaller, more cryptic, and more ephemeral than temperate macroalgae (reviewed by Fong and Paul 2011), there has been a recent increase in fleshy macroalgae on disturbed coral reefs (Turbinaria in Payri 1984, Martinez and others 2007; Lobophora in Jompa and McCook 2002; Sargassum in Hughes and others 2007). These increases in fleshy macroalgae have been attributed to decreased herbivory for Sargassum (Hughes and others 2007) or a combination of increased nutrient input and decreased herbivory for Turbinaria (Bittick and others 2016) and Lobophora (Jompa and McCook 2002). Whether these novel macroalgal communities that are complex, less palatable, and persistent macroalgae serve foundational roles in tropical reef systems has not been evaluated. Though it is widely acknowledged that algal domination cannot sustain net reef growth because loss of coral results in lower calcification (Gattuso and others 1997), some coral reef macroalgae have been found to have positive impacts on biomass of fish (turf algae, Tootell and Steele 2016), abundance and diversity of invertebrates (Roff and others 2013), and macroalgal richness (Bittick and others 2010). As fleshy macroalgae have increased on many coral reefs, it is important to determine whether they function as foundation species and what ecosystem functions, if any, they may provide.
Our overall objective was to evaluate if Turbinaria ornata, a marine macroalga that is expanding its range and habitat use in the South Pacific (Payri 1984; Martinez and others 2007), provides a foundational role following a phase-shift from coral dominance after disturbance to tropical reefs. Negative impacts of T. ornata on coral have been documented, including inhibiting coral recruits (Brandl and others 2013) and outcompeting coral in high flow conditions (Brown and Carpenter 2014). In Mo’orea, French Polynesia coral populations were recently decimated due to an outbreak of the coralivorous seastar, Acanthaster plancii (Kayal and others 2012), and patches of T. ornata increased in size and dominance on fringing and back reefs (Carpenter 2015; Davis 2016). Further, T. ornata benefits from anthropogenic change as nutrient enrichment cause a strengthening of physical anti-herbivory defenses and therefore reduced herbivory (Bittick and others 2016). However, aggregations of T. ornata benefit understory macroalgae (Bittick and others 2010) by providing a refuge from herbivores thereby increasing species richness and it may protect invertebrates and juvenile fish (personal obs). We predicted that T. ornata would perform roles typically associated with structurally complex foundation species such as provision of habitat for primary producers and trophic support to consumers. We ask: (1) Does T. ornata facilitate epiphytic and understory macroalgae? and (2) Does this facilitation cascade up to herbivorous fish through increased resources?
Methods
Study Site and Survey
The study site was a fringing patch reef at the mouth of Opunohu Bay in Mo’orea, French Polynesia (17°28′59.81″S, 149°50′45.70″W). After the 2006–2010 Acanthaster plancii outbreak, and disturbance by 2010 hurricane Oli, coral cover was lost across much of Mo’orea, and near zero at this site (Kayal and others 2012). Turbinaria ornata requires hard substrate to settle such as dead coral skeletons and often grows in patches, or aggregations, of varying density (see ESM S1, Figure S1). To characterize the aggregations, we constructed a density-frequency distribution from counts of thalli in 0.0625 m2 areas (quadrats were 0.25 m × 0.25 m); we observed this area of aggregations to be the most common on the nearshore reefs during our 2012–2014 study period. This is larger than the median patch size of 0.022 m2 observed by Davis (2016) in a 2012–2015 study. We randomly placed five 30 m transects, selected six random points along each, and counted the number of thalli · 0.0625 m−2 in the nearest aggregation (N = 30). Surveys were conducted in May 2012.
To characterize species distribution and sizes of fish from dominant taxa, we utilized survey data from the Moorea Coral Reef Long Term Ecological Research program (MCR LTER). Four surveys were conducted in August 2012 at two sites on the north shore near our study area. Fish were counted along a 50 m transect 5 m wide and identified to species with an estimate of size to the nearest cm. We calculated the density of fish primary consumer species per 100 m2. We also calculated average length (± SE cm) for the three most abundant species.
Density Manipulation Experiment
To measure the effect of T. ornata density on growth of epiphytic and understory algae and the consequences to herbivore foraging, we thinned existing aggregations of T. ornata (randomly selected, but initially with ≥ 30 thalli · 0.0625 m−2) to create plots of 8 densities: 0, 3, 7, 10, 15, 20, 25, and 30 thalli · 0.0625 m−2 (n = 3). We avoided damselfish territories (family Pomacentridae), although a territory subsequently encroached on a plot of 15 thalli · 0.0625 m−2 (reducing n to 2 for this treatment). Treatments were maintained for 18 days in May 2012, during which we conducted a growth bioassay within the experimental plots using a locally abundant macroalga, Padina boryana. Two grams (standardized wet weight) of P. boryana were placed in window screen cages and attached within the understory of each plot (see Fong and others 2006 for method). Algae were collected after 7 days (17–24 May, 2012), wet weighed, and net growth was calculated as % change from initial wet weight.
At the end of the experiment, three T. ornata thalli (5–12 cm tall) were collected randomly (except for plots where density = 3 where all were collected) from each density plot. Photos were taken of one side of each alga (see Electronic Supplementary Material S1, Figure S2) and percent cover of epiphytes quantified using the point intercept method in ImageJ (U.S. National Institutes of Health). We first measured two-dimensional area in ImageJ using the images. Due to varying image quality and T. ornata thalli size and shape, we used the grid overlay feature scaled for each thalli. The spacing of the grid was limited to whole pixel increments and scaled to produce a minimum of 30 random intersections. Grid overlays were between pixels, so the pixel to the top right was evaluated. Percent epiphyte cover was calculated as 100* the ratio of intersections with epiphytes present over the total intersections within the thalli area.
To determine the relationship between T. ornata density and herbivorous fish, we observed and recorded foraging behavior within density plots. Each plot was observed by the same individual on snorkel three times over the 18 days for 10 min (total 30 min/plot). The observer remained at least 5 m away from the plot and recorded when fish: (1) came within 0.25 m of the plot and (2) took a bite from the canopy, stipe, or understory of the algal aggregation. Only fish from dominant herbivorous taxa were counted in our surveys. However, dominant species and sizes of herbivorous fish in this site were identified in the LTER data (see above). Fish behavior observations of plots did not begin until 72 h after plots were established to allow for stabilization of epiphytes after physical disturbance. All observations were conducted from 14 to 20 May, 2012 and a paired t test comparing frequency of bites by herbivorous fish from the first and last day supports no significant changes in behavior over time (t = 0.85, p = 0.41).
Epiphyte Herbivory Experiment
To determine the influence of T. ornata density and herbivory on epiphyte load, we conducted an in situ 2-factor experiment manipulating T. ornata density (as above) and access to herbivores (± H). The experiment was fully crossed with three replicates of each treatment (n = 48). Herbivore access was limited by exclusion cages (5-sided; 25 × 25 × 30 cm3L × W × H) constructed from hardware cloth with 1 cm openings. Light restriction by caging material was less than 10% with no measurable restriction to water flow in cages constructed of the same material and used at the same site (Clausing and others 2014). Three randomly selected thalli were collected from each plot and photos were taken for analysis of initial percent cover by epiphytes. After 16 days (sensu Bittick and others 2010) during May–June 2014, cages were removed and three thalli were collected from each plot, photographed, and analyzed in ImageJ for final percent cover by epiphytes. Initial epiphyte cover was 61.6 ± 5.6% SEM.
Analysis
All analyses were conducted in R (R Core Team 2015). For all response variables, linear and nonlinear least squares models were fit to the data and compared by Akaike Information Criterion (AICc). We tested whether the relationships between T. ornata density and both epiphytes and understory macroalgae were best explained as either: (1) linear, (2) logistic (that is, positive effects saturate at a certain density), (3) exponential (that is, positive effects increase fastest at lower densities with no saturation) or (4) quadratic (that is, positive effects decline after an optimal density) equations. The model with the lowest AICc value (∆AIC = 0) and highest AICc weight or, if AICs were similar (∆AIC < 3–4), the equation with the lowest number of parameters was chosen by rule of parsimony (Burnham and others 2011) and presented for each data set. Full model comparisons and fit are provided in ESM S3. Further, we expected foraging behavior of herbivorous fish (as bites over a 10-min observation period) would also follow one of these patterns in response to availability of resources. The epiphyte herbivory experiment was analyzed using analysis of covariance (ANCOVA) with caging as the explanatory variable and density as a covariate.
Results
Survey
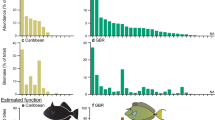
Turbinaria ornata density was normally distributed (Shapiro–Wilk W Test, W = 0.98, p < W = 0.80) ranging from 0 to 40 thalli · 0.0625 m−2. Average density was 19.8 ± 1.9 SEM thalli · 0.0625 m−2 and 83% of the aggregations were 30 thalli or less (Figure 1 a). Approximately 80% of all fish observed approaching and foraging in the density plots were acanthurids (see ESM S2 for distribution). From the MCR LTER 2012 annual survey data, the three most abundant species on the north shore fringing reef were: Chlorulus sordidus (32%), Acanthurus nigrofuscus (26%) and Ctenochaetus striatus (24%) (Figure 1 b, c). The average lengths of these species were 11.4 ± SE 1.9 cm, 10.3 ± SE 9.3 cm, and 11.3 ± SE 2.7 cm, respectively.
Density Manipulation Experiment
There was an increase with density in epiphyte cover on T. ornata thalli until an optimum of 15 thalli · 0.0625 m−2 area (Figure 2 a). Treatments with 3 thalli had about 40% cover by epiphytes, which increased to about 65% cover in the 15 thalli treatments and remained at this level at higher densities; thus, cover saturated in a logistic fit (Figure 2 a; ESM S3). Similarly, macroalgae used as a bioassay for understory macroalgal growth increased in biomass with T. ornata density up to 15 thalli · 0.0625 m−2 (max = 30% growth · 7 days−1; Figure 2 b). After this optimum, growth declined precipitously to nearly zero in treatments with 30 thalli; this was best fit with a quadratic equation (Figure 1 b; ESM S3).
∆AICc selected models for: (A) relationship between T. ornata density and percent epiphyte cover modeled as a logistic fit (y = \( \frac{65.66x}{1.47 + x} \), R2 = 0.45, p < 0.001) (B) Growth of understory macroalgae in response to T. ornata canopy (\( y = - 7.01 + 3.28x - 0.10x^{2} \), R2 = 0.62, p < 0.001) (C) The number of bites by all fish had a positive linear relationship with T. ornata density (\( y = 1.49 + 0.42x \), R2 = 0.30, and p < 0.01).
Foraging behavior measured as bites · 10 min−1 was modeled as a linear increase (Figure 2 c; ESM S3). The relationship between bites · 10 min−1 and T. ornata density was positive, with no evidence of a decline. Of the 408 observed bites, 51% were taken from the canopy, 8% along the algal stipe, and 40% in the understory at the margins of the aggregation.
Epiphyte Herbivory Experiment
T. ornata density facilitated and herbivores reduced abundance of epiphytes. Exclusion of herbivores and increasing T. ornata density both resulted in higher epiphyte cover relative to low density with presence of herbivores. As in the density manipulation experiment in 2012, the 2014 experiment showed a positive effect of T. ornata density on epiphytes; however, this relationship was linear instead of logistic (Figure 3; ESM S3). We found a significant effect of caging (F = 16.92, p = 0.0002) on percent epiphyte coverage, which was further explained by the covariate T. ornata density (F = 36.43, p < 0.0001). However, the accumulation of epiphytes with density (slope) is not significantly different between herbivore treatments (t-test, p = 0.16). The ranges in percent cover by epiphytes in 2012 and 2014 were also comparable across years (28.2–72.6 and 27.2–76.3, respectively).
Discussion
Our results demonstrated Turbinaria ornata performs the role of a foundation species on fringing coral reefs in the South Pacific that have experienced phase-shifts to macroalgae. We suggest this represents a facilitation cascade (for example, Thomsen and others 2010) where T. ornata attaches to hard substrate formed by dead corals after a disturbance, and, once established, performs the key foundational role of facilitating an associated community. One line of evidence for its role as a foundation species is that, up to an optimum, increasing density of T. ornata also increases the abundance of associated primary producer groups such as epiphytes and understory macroalgae that are not typically associated with coral-dominated reefs (Fong and Paul 2011). Other ecosystem functions that have been documented to increase with density of a macroalgal foundation species include more efficient nutrient cycling (Human and others 2015) and reduced photoinhibition (Franklin and others 1996). In addition, the decline in growth of understory macroalgae, but not epiphytes, in our experiment at high T. ornata densities may be attributed to density-dependent increases in intensity of competition for light or nutrients. This relationship has also been found in terrestrial forests where understory species can survive in reduced light up to a critical threshold (Anderson and others 1969) and are positively impacted by tree thinning (Canham and others 1990; Lieffers and others 1999), but canopy-occupying species such as epiphytes benefit from larger trees and denser canopies (Woods and others 2015). Similarly, epiphytes in the “canopy” of T. ornata aggregations may not experience the same reduction in light or nutrients as understory macroalgae. Whatever the mechanism involved, our study demonstrated that T. ornata acts as a foundation species because, once it becomes abundant after a disturbance it facilitates an associated community of primary producers. How this ecosystem function provided by T. ornata compares to those functions provides by the original, coral-dominated foundation species is unknown, but certainly is a critical area for future research as phase-shifts to macroalgal domination have occurred globally (reviewed in Hughes and others 2010).
A second line of evidence that T. ornata is a foundation species is its facilitation of reef consumers through enhanced food resources. Increased densities of T. ornata aggregations caused a facilitation cascade in which more foraging by fish was supported as epiphyte load and macroalgal understory increased. This is consistent with examples in terrestrial and aquatic systems in which trophic support and/or consumer abundance and diversity is negatively impacted by the loss of a foundation species (Hughes and others 2004; Rohr and others 2011; Angelini and Silliman 2014); similarly, in our study reduced density of T. ornata also reduced trophic support. In other systems, primary producers such as macroalgae and understory plants increase trophic support and consumer species diversity (for example, kelp forests, Graham 2004; temperate forests, Gilliam 2007; marshes, Angelini and others 2015). Although the majority of grazing occurred on epiphytes on the surface of the thalli within aggregations, understory macroalgae at the aggregation’s edges provided additional resources to grazers. Taken together these findings suggest higher density T. ornata aggregations provide more food to herbivorous fish than less dense aggregations via increased supplies of epiphytes and understory macroalgae, demonstrating its role as a foundation species through enhanced trophic support. However, while our study compared trophic support across different densities of T. ornata, we were unable to compare these to the ecosystem functions provided by corals as they had been lost to predation. Thus, comparisons between the trophic support provided by corals vs. T. ornata aggregations are needed to fully assess differences in ecosystem functions supported by these alternative communities.
The effects of T. ornata were strongly density-dependent, a phenomenon that has rarely been evaluated in studies examining foundational communities. Rather, most studies assess impacts to associated species in the presence and absence of a focal foundation species (for example, Graham 2004; Angelini and others 2015). However, there are terrestrial studies that showed decreased tree canopy cover, which may be a proxy for density, reduced richness and abundance of associated species (for example, Caners and others 2010; Cach-Pérez and others 2013), suggesting density effects may be important across systems. Further, we found that density effects varied across associated functional groups, with epiphytes responding linearly or logistically and understory macroalgae responding unimodally to T. ornata density. One possible explanation for the macroalgal response is nutrient or light limitation, which may have parallels in terrestrial systems. For example, in forests, canopy cover can have a unimodal effect on understory plant growth and diversity; in this case, nutrient input from the canopy has a positive effect, while growth and diversity are negatively affected by canopy closure, creating a hump-shaped response to canopy cover (reviewed in Gilliam 2007). Thus, facilitation in the case of T. ornata, as in terrestrial forests, is highly density-dependent, and the density of T. ornata that persists after corals is removed by a disturbance can have a profound effect on reef community structure.
In summary, our results demonstrated that T. ornata acts as a foundation species where aggregations facilitate both primary producers and consumers on tropical reefs. Further, we suggest this represents a facilitation cascade (Thomsen and others 2010) where corals form the hard substrate to which T. ornata attaches, and T. ornata provides habitat for epiphytes and increased trophic support for herbivorous fish. Much work is still needed to understand the functional roles of foundation species in many systems, especially when the foundation species dominates as the result of a phase-shift, as with corals and some macroalgae. These phase-shifts are often the result of human impacts that may cause “undesirable” changes to ecosystem functioning (see Ellison and others 2005 for terrestrial examples, coral reefs in Hughes and others 2010). However, in our study, we found that a phase-shift to a different foundation species supports some ecosystem functions, albeit likely very different than those supported by the original coral community. However, even these functions may not be sustainable if T. ornata domination persists at the expense of the original foundational coral community as bioerosion will ultimately break down the reef structure (reviewed in Glynn and Manzello 2015).
References
Anderson RC, Louck OL, Swain AM. 1969. Herbaceous response to canopy cover, light intensity, and throughfall precipitation in coniferous forests. Ecology 50:255–63.
Angelini C, Silliman BR. 2014. Secondary foundation species as drivers of trophic and functional diversity: evidence from a tree-epiphyte system. Ecology 95:185–96.
Angelini C, van der Heide T, Griffin JN, Morton JP, Derksen-Hooijberg M, Lamers LPM, Smolders AJP, Silliman BR. 2015. Foundation species’ overlap enhances biodiversity and multifunctionality from the patch to landscape scale in southeastern United States salt marshes. R Soc Proc B 282:20150421.
Bittick SJ, Bilotti ND, Peterson HA, Stewart HL. 2010. Turbinaria ornata as an herbivory refuge for associate algae. Mar Biol 157:317–23.
Bittick SJ, Clausing RJ, Fong CR, Fong P. 2016. Bolstered physical defences under nutrient-enriched conditions may facilitate a secondary foundational algal species in the South Pacific. Silliman B, editor. J Ecol 104:646–53.
Brandl SJ, Hoey AS, Bellwood DR. 2013. Micro-topography mediates interactions between corals, algae, and herbivorous fishes on coral reefs. Coral Reefs 33:421–30.
Brown AL, Carpenter RC. 2014. Water flow influences the mechanisms and outcomes of interactions between massive Porites and coral reef algae. Mar Biol 162:459–68.
Burnham KP, Anderson DR, Huyvaert KP. 2011. AIC model selection and multimodel inference in behavioral ecology: some background, observations, and comparisons. Behav Ecol Sociobiol 65:23–35.
Cach-Pérez MJ, Andrade JL, Chilpa-Galván N, Tamayo-Chim M, Orellana R, Reyes-García C. 2013. Climatic and structural factors influencing epiphytic bromeliad community assemblage along a gradient of water-limited environments in the Yucatan. Trop Conserv Sci 6:283–302.
Caners RT, Macdonald SE, Belland RJ. 2010. Responses of boreal epiphytic bryophytes to different levels of partial canopy harvest. Botany 88:315–28.
Canham CD, Denslow JS, Platt WJ, Runkle JR, Spies TA, White PS. 1990. Light regimes beneath closed canopies and tree-fall gaps in temperate and tropical forests. Can J For Res 20:620–31.
Carpenter, R. C. 2015. MCR LTER: long-term population and community dynamics: benthic algae and other community components, ongoing since 2005. DOI:http://dx.doi.org/10.6073/pasta/79a6edbcf3aa2380d43deed778856416.
Clausing R, Annunziata C, Baker G, Lee C, Bittick S, Fong P. 2014. Effects of sediment depth on algal turf height are mediated by interactions with fish herbivory on a fringing reef. Mar Ecol Prog Ser 517:121–9.
Davis S. 2016. Mechanisms Underlying Macroalgal Phase Shifts in Coral Reef Ecosystems. Doctoral Dissertation, University of California Santa Barbara. ProQuest Dissertations Publishing. 10194165
Dayton PK. 1972. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound, Antartica. In: Proceedings of the colloquium on conservation problems in Antarctica.
deYoung B, Barange M, Beaugrand G, Harris R, Perry RI, Scheffer M, Werner F. 2008. Regime shifts in marine ecosystems: detection, prediction and management. Trends Ecol Evol 23:402–9.
Dudgeon SR, Aronson RB, Bruno JF, Precht WF. 2010. Phase shifts and stable states on coral reefs. Mar Ecol Prog Ser 413:201–16.
Ellison AM, Bank MS, Clinton BD, Colburn EA, Elliott K, Ford CR, Foster DR, Kloeppel BD, Knoepp JD, Lovett GM, Mohan J, Orwig DA, Rodenhouse NL, Sobczak WV, Stinson KA, Stone JK, Swan CM, Thompson J, Von Holle B, Webster JR. 2005. Loss of foundation species : consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ 3:479–86.
Folke C, Carpenter S, Walker B, Scheffer M, Elmqvist T, Gunderson L, Holling CS. 2004. Regime shifts, resilience, in ecosystem management. Annu Rev Ecol Evol Syst 35:557–81.
Fong P, Paul VJ. 2011. Coral reef algae. (Dubinsky Z, Stambler N, editors.). Dordrecht: Springer Netherlands.
Fong P, Smith TB, Wartian MJ. 2006. Epiphytic cyanobacteria maintain shifts to macroalgal dominance on coral reefs following ENSO disturbance. Ecology 87:1162–8.
Franklin LA, Seaton GGR, Lovelock CE, Larkum AWD. 1996. Photoinhibition of photosynthesis on a coral reef. Plant Cell Environ 19:825–36.
Gattuso JP, Payri CE, Pichon M. 1997. Production, calcification, and air-sea CO2 fluxes of a macroalgal-dominated coral reef community (Moorea, French Polynesia). J Phycol 33:729–38.
Gentry AH, Dodson C. 1987. Contribution of nontrees to species richness of a tropical rain forest. Biotropica 19:149–56.
Gilliam FS. 2007. The ecological significance of the herbaceous layer in temperate forest ecosystems. Bioscience 57:845–58.
Glynn PW, Manzello DP. 2015. Bioerosion and coral reef growth: a dynamic balance. In: Coral reefs in the anthropocene. (Birkeland C, editor.). Dordrecht: Springer.
Graham HM. 2004. Effects of local deforestation on the diversity and structure of Southern California giant kelp forest food webs. Ecosystems 7:341–57.
Hughes AR, Bando KJ, Rodriguez LF, Williams SL. 2004. Relative effects of grazers and nutrients on seagrasses: a meta-analysis approach. Mar Ecol Prog Ser 282:87–99.
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B. 2007. Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–5.
Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS. 2010. Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol 25:633–42.
Human LRD, Snow GC, Adams JB, Bate GC, Yang SC. 2015. The role of submerged macrophytes and macroalgae in nutrient cycling: a budget approach. Estuar Coast Shelf Sci 154:169–78.
Jaschinski S, Brepohl DC, Sommer U. 2011. The trophic importance of epiphytic algae in a freshwater macrophyte system (Potamogeton perfoliatus L.): Stable isotope and fatty acid analyses. Aquat Sci 73:91–101.
Jompa J, McCook LJ. 2002. The effects of nutrients and herbivory on competition between a hard coral (Porites cylindrica) and a brown alga (Lobophora variegata). Limnol Oceanogr 47:527–34.
Kayal M, Vercelloni J, Lison de Loma T, Bosserelle P, Chancerelle Y, Geoffroy S, Stievenart C, Michonneau F, Penin L, Planes S, Adjeroud M. 2012. Predator crown-of-thorns starfish (Acanthaster planci) outbreak, mass mortality of corals, and cascading effects on reef fish and benthic communities. PLoS ONE 7:e47363.
Korpinen S, Jormalainen V, Pettay E. 2010. Nutrient availability modifies species abundance and community structure of fucus-associated littoral benthic fauna. Mar Environ Res 70:283–92.
Lewis SM, Wainwright PC. 1985. Herbivore abundance and grazing intensity on a Caribbean coral reef. J Exp Mar Bio Ecol 87:215–28.
Lieffers VJ, Messier C, Stadt KJ, Gendron F, Comeau PG. 1999. Predicting and managing light in the understory of boreal forests. Can J For Res 29:796–811.
Martinez E, Maamaatuaiahutapu K, Payri C, Ganachaud A. 2007. Turbinaria ornata invasion in the Tuamotu Archipelago, French Polynesia: ocean drift connectivity. Coral Reefs 26:79–86.
McCook LJ. 1999. Macroalgae, nutrients and phase shifts on coral reefs: scientific issues and management consequences for the Great Barrier Reef. Coral Reefs 367:357–67.
Muthukrishnan R, Lloyd-Smith JO, Fong P. 2016. Mechanisms of resilience: empirically quantified positive feedbacks produce alternate stable states dynamics in a model of a tropical reef. Silliman B, editor. J Ecol 104:1662–72.
Nowacki GJ, Abrams MD. 2008. The demise of fire and Mesophication” of forests in the Eastern United States. Bioscience 58:123–38.
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL Jr, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S et al. 2006. A global crisis for seagrass ecosystems. Bioscience 56:987–96.
Payri CE. 1984. The effect of environment on the biology and morphology of Turbinaria ornata (Phaeophyta) from the Tiahura Reef (Moorea Island, French Polynesia). Bot Mar 27:327–33.
R Core Team. 2015. R: A language and environment for statistical computing.
Roff G, Wabnitz CCC, Harborne AR, Mumby PJ. 2013. Macroalgal associations of motile epifaunal invertebrate communities on coral reefs. Mar Ecol 34:409–19.
Rohr NE, Thornber CS, Jones E. 2011. Epiphyte and herbivore interactions impact recruitment in a marine subtidal system. Aquat Ecol 45:213–19.
Scheffer M, Carpenter S, Foley Ja, Folke C, Walker B. 2001. Catastrophic shifts in ecosystems. Nature 413:591–6.
Smith JE, Runcie JW, Smith CM. 2005. Characterization of a large-scale ephemeral bloom of the green alga Cladophora sericea on the coral reefs of West Maui, Hawai’i. Mar Ecol Prog Ser 302:77–91.
Smith JE, Hunter CL, Smith CM. 2010. The effects of top-down versus bottom-up control on benthic coral reef community structure. Oecologia 163:497–507.
Stachowicz JJ. 2001. The structure of ecological communities. Bioscience 51:235–46.
Suchanek TH. 1992. Extreme biodiversity in the marine environment: Mussel bed communities of Mytilus californianus. Northwest Environ J 8:150–2.
Talluto MV, Suding KN. 2008. Historical change in coastal sage scrub in southern California, USA in relation to fire frequency and air pollution. Landsc Ecol 23:803–15.
Thomsen MS, Wernberg T, Altieri A, Tuya F, Gulbransen D, McGlathery KJ, Holmer M, Silliman BR. 2010. Habitat cascades: the conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr Comp Biol 50:158–75.
Tootell JS, Steele MA. 2016. Distribution, behavior, and condition of herbivorous fishes on coral reefs track algal resources. Oecologia 181:13–24.
Woods CL, Cardelús CL, Dewalt SJ. 2015. Microhabitat associations of vascular epiphytes in a wet tropical forest canopy. Piper F, editor. J Ecol 103:421–30.
Acknowledgements
Thank you to the undergraduate students from UCLA’s field courses 2012–2016, and ImageJ processing by Von Phan and Meera Solanki. A special thanks for 2014 field assistance from Briana Fodor and funding from Aquarium of the Pacific. Funding in the field for SJB and PF was provided by UCLA’s OID and the EEB Department; RJC was funded by these sources and the NSF GRFP; and CRF was funded by a Sigma Xi Grant-in-Aid of Research (GIAR). Funding while writing was provided to SJB by the Eugene Cota-Robles Fellowship Program and NSF GRFP. Thank you to the Gump South Pacific Research Station and the French Polynesian Department of Research.
Author information
Authors and Affiliations
Corresponding author
Additional information
Data are available at https://github.com/SJBittick/EcosystemsMoorea
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bittick, S.J., Clausing, R.J., Fong, C.R. et al. A Rapidly Expanding Macroalga Acts as a Foundational Species Providing Trophic Support and Habitat in the South Pacific. Ecosystems 22, 165–173 (2019). https://doi.org/10.1007/s10021-018-0261-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-018-0261-1