Abstract
Nitrogen (N) saturation arises when atmospheric inputs of N exceed biological N demand, resulting in loss of NO3 - in streams, accompanied by the loss of nutrients (Ca and Mg) that are essential to forest health. Previous studies have shown that some watersheds the Fernow Experimental Forest (FEF), West Virginia, USA, are among the more N-saturated sites in North America. Research from the Gilliam laboratory at Marshall University (West Virginia, USA) began focusing specifically on N biogeochemistry in 1993 with establishment of plots at FEF to carry out long-term in situ (“buried bag”) incubations in three watersheds: two control (WS4, WS7) and one treatment (WS3). This was done in conjunction with the Fernow Watershed Acidification Study, established by the USDA Forest Service in 1989 to treat an entire watershed (WS3) with aerial applications of 35 kg N ha –1 year –1. The initial period (1993–1995) exhibited increases in rates for all watersheds, but especially in treated WS3. This period has been followed by declines in net nitrification, which is consistent with current declines in stream NO3 – and has been especially pronounced in WS3 since 1998. Also during this time, sampling of the herbaceous layer (vascular plants ≤ 1 m in height) has revealed pronounced changes in response to N treatments on WS3, especially in the increase of the shade-intolerant Rubus spp. Future work will investigate the effects of freezing on soil N dynamics. Preliminary results indicate that freezing exacerbates the symptoms of N saturation already seen in soils at FEF, further increasing already high rates of net nitrification.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Nitrogen (N) has been the focus of extensive basic and applied ecological research, more recently and more specifically by biogeochemists. This extends from the discovery of nitrogen (N) as an element in 1772, to its central place in von Liebig’s Law of Minimum for plant growth articulated in 1827, to the discovery of symbiotic N fixation in 1888, to the development of the Haber-Bosch process in 1913 (initiating of its use as fertilizer in crop production), and finally to the present awareness that excess N in the environment can alter the structure and function of ecosystems (Vitousek et al. 1997; Aber et al. 1998; Fenn et al. 1998). As with any scientific discipline, N biogeochemistry has undergone numerous paradigm shifts over time (Vitousek and Howarth 1991; Schimel and Bennett 2004).
It is becoming increasingly apparent that excess N in the environment can cause notable declines in plant species diversity in a variety of terrestrial and aquatic ecosystems (Bobbink et al. 1998, 2010; Gilliam 2006; Lu et al. 2010; Rabalais 2002). Site-specific variability in plant and soil response to excess N precludes broad generalizations regarding the mechanisms behind these effects in terrestrial ecosystems. For example, in temperate hardwood forests, experimentally-added N has been shown to increase cover/biomass of a few nitrophilous herbaceous species, decreasing the usually much higher numbers of N-limited herb species (Gilliam 2006; Bobbink et al. 2010). By contrast, tropical evergreen broadleaf forests are typically less N-limited and more phosphorus-limited, and additions of N have been shown to decrease herb stratum diversity by eliminating ferns and seedlings of diverse tree species via aluminum mobility, calcium leaching, and enhanced fine root mortality (Lu et al. 2010) .
Biogeochemical research at Fernow Experimental Forest (FEF), West Virginia, USA, has a rich history (Adams et al. 2006). Collection of hydrochemical data began for the long-term reference watershed (WS4) in 1980, with research on silvicultural practices pre-dating that. Research that focused more specifically on N biogeochemistry was initiated via the Fernow Watershed Acidification Study, which began as a now-terminated pilot study in 1987 on a watershed adjacent to Fernow. In 1989, it was established on FEF proper, and remains currently on-going. A distinctive feature of the Watershed Acidification Study is that it involves a whole-watershed application of simulated acidic deposition via three aerial additions of (NH4)2SO4 per year, representing a total N addition of 35 kg N ha −1 year −1. This is applied as solid powder, initially via helicopter and currently via airplane, and remains one of few studies utilizing N manipulations at the scale of an entire watershed (Adams et al. 2006).
In 1991, 15 permanent vegetation plots were established in each of four watersheds of contrasting stand age at FEF, with a particular focus on interactions between the forest canopy and the herbaceous stratum, as well as plant-soil interactions, with a focus on how these interactions might vary with forest succession (Gilliam and Roberts 2003) . A sub-set of seven of these original plots in each of three watersheds—WS3, WS4, and WS7—were selected in 1993 for monthly in situ incubation of soil to examine spatial and temporal patterns of net N mineralization and nitrification . This represented the first work at FEF specifically to address a phenomenon that until that time had been described more in Europe and experimental studies in the northeastern United States—N saturation.
Nitrogen saturation arises when atmospheric inputs of N exceed biological N demand, resulting in loss of NO3 − in streams (Aber et al. 1998). In addition to creating environmental problems for impacted aquatic systems, N saturation is also commonly accompanied by the loss of nutrients (Ca2 + and Mg2 + ) (the mobile anion effect) that are essential to plant growth and forest health. Some of the earlier studies published on N saturation in the US (e.g., Stoddard 1994; Gilliam et al. 1996; Peterjohn et al. 1996, 1999) have identified some watersheds at FEF to be among the more N-saturated sites in North America. Increased mobility and leaching of Ca2 + and Mg2 + has been clearly being linked at FEF to increased N deposition, associated enhanced nitrification and movement of NO3 −, along with evidence of decreases in growth rates of dominant tree species (Peterjohn et al. 1996; Christ et al. 2002; May et al. 2005; Gilliam et al. 2005; Adams et al. 2006). Other problems associated with N saturation include increased production of the greenhouse gas, N2O (Peterjohn et al. 1998; Wallenstein et al. 2006a). Work at FEF has also suggested that N saturation has led to phosphorous limitation in several watersheds (Gress et al. 2007).
Here I summarize the research on N biogeochemistry that has been carried out at FEF by ecologists at Marshall University, Huntington, West Virginia, USA: (1) long-term monitoring of rates of net N mineralization and nitrification using in situ incubations, (2) herbaceous layer dynamics, and (3) effects of freezing on soil N dynamics.
2 Methods
2.1 Study Site
The Fernow Experimental Forest occupies approximately 1,900 ha of the Allegheny Mountain section of the unglaciated Allegheny Plateau near Parsons, West Virginia (39° 03′ N, 79° 49′ W). Precipitation at FEF averages approximately 1,430 mm year −1, being higher during the growing season and increasing with elevation. Concentrations of acidic species in wet deposition (including snow) (H + , SO4 2−, and NO3 −) are among the highest in North America (Gilliam and Adams 1996) . Watershed soils are coarse-textured Inceptisols (loamy-skeletal, mixed mesic Typic Dystrochrept) of the Berks and Calvin series sandy loams derived from sandstone (Gilliam et al. 1994) .
Dominant tree species on FEF watersheds vary with stand age. Early-successional species, such as black birch (Betula lenta L.), black cherry (Prunus serotinaEhrh.), and yellow-poplar (Liriodendron tulipifera L.) are dominant in young stands, whereas late-successional species, such as sugar maple (Acer saccharum Marshall) and northern red oak (Q. rubra L.), are dominant in mature stands. Dominant herbaceous layer species vary less with stand age and include stinging nettle (Laportea canadensis (L.) Wedd.), violets (Viola spp.) and several ferns (Gilliam et al. 2006).
Three watersheds have served as sites for various studies. WS4 supports a > 100 year-old mixed-aged stand, serving as the long-time reference watershed at FEF. WS7 supports an approximately 40 year-old even-age stand, and whereas WS3 supports an approximately 40 year-old even-age stand and serves as the “treatment” watershed, whereas WS4 and WS7 were the controls. WS3 has received three aerial applications of (NH4)2SO4 per year, beginning in 1989. March (or sometimes April) and November applications represent approximately 7.1 kg N ha−1; July applications are approximately 21.2 kg N ha −1. The total amount of N deposited on WS3 (application plus atmospheric deposition) is approximately 54 kg N ha −1 year −1, or about three times ambient inputs (Adams et al. 2006).
2.2 Field Sampling
Mineral soil is collected on an on-going basis on WS3, WS4, and WS7 by hand trowel at five points within each of seven plots per watershed to a depth of 5 cm using methods following Gilliam et al. (1996). These five samples are bulked, thoroughly mixed, and then placed in two polyethylene bags—one brought back to the laboratory for immediate extraction and analysis (see 29.2.3 Laboratory and data analyses below) and the other incubated in situ by burying it 5 cm beneath the mineral soil surface for ~ 30 d during all months of the growing season. This was initiated in 1993 and is reported here up to 2005.
The herbaceous layer is sampled on an on-going basis on WS3 and WS4 within seven circular 0.04-ha sample plots (adjacent to soil plots described previously). Each vascular plant species < 1 m in height is identified and visually estimated for cover (%) within 5 1-m2 circular sub-plots in each sample plot. Sub-plots were located within sample plots using a stratified-random polar coordinates method, which was employed to avoid over-sampling the center region of circular plots (Gilliam et al. 2006). This was initiated in 1991 and is reported here through 2003.
To determine the effects of soil freezing on soil N dynamics, mineral soil was taken at three sites shown by previous investigations to represent a gradient in rates of net nitrification: LN (low nitrification rates), MN (medium rates), and HN (high rates), using methods described in Gilliam et al. (2010). Sub-samples of soil from each plot were extracted for analysis of NH4 + and NO3 − immediately upon return to the laboratory (see 29.2.3 Laboratory and data analysesbelow). Approximately 50 g of each sample was placed into each of three 120-mL sterile polyethylene Whirl-Pac® bags for freezing treatment as follows: 0, − 20, and − 80 °C. The − 80 °C treatment was chosen as an extreme temperature to determine whether − 20 °C may represent a temperature threshold for freezing effects, i.e., if there are no differences between − 20 and − 80 °C. The remaining soil was kept in the original bag and refrigerated at 4 °C as Control. All treated samples were subjected to treatments for 7 d.
2.3 Laboratory and Data Analyses
All mineral soil, including that from in situ incubations and the soil freezing experiment, was extracted with 1N KCl (10:1 volume:weight) and analyzed for NH4 + and NO3 −. From 1993 to 1995 this was done with an Orion 720A pH/ISE meter and NH4 + and NO3 − electrodes. For the 2005 field samples, NH4 + and NO3 − were determined colorimetrically with a TrAACS 2000 continuous flow spectrophotometer. For the soil freezing experiment, NH4 + and NO3 − were determined colorimetrically with an AutoAnalyzer III continuous flow spectrophotometer.
Temporal patterns of in situ net N mineralization and nitrification were assessed with second-order polynomials. Species diversity of the herbaceous layer was calculated using the Shannon-Wiener index with natural log (ln) transformation (Gilliam et al. 2006). Means for herb layer diversity, species richness, and cover were compared between WS3 and WS4 and among years (1991 to 2003) using repeated measures analysis of variance (ANOVA). Mean net N mineralization and nitrification potentials were compared among freezing treatments within sites and among sites within treatments using ANOVA and least significant difference tests .
3 Results and Discussion
3.1 In Situ Incubations
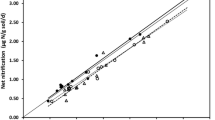
As reported in Gilliam et al. (2001), annual net N mineralization and nitrification increased on all three watersheds during the period 1993–1995, with sharpest increases noted for N-treated WS3. It is thus quite notable that the 2002 and 2005 sampling revealed substantially declining rates for all watersheds since this time, with net nitrification on WS3 currently > 80 % less than the 1995 maximum (Fig. 29.1). Temporal patterns in net nitrification at FEF are consistent with observations of declining concentrations of NO3 − in streams for WS3, WS4, and WS7, and in soil water in WS3 and WS4 (soil solution is not sampled in WS7) (Adams et al. 2006). Furthermore, these patterns of decline are consistent with those at the more synoptic scale of several watersheds throughout the northeastern United States (Goodale et al. 2005) .
Although it is not clear what specific mechanisms are causing such a precipitous decline in net N mineralization and nitrification, several studies have shown that both microbial biomass and composition change drastically with experimental additions of N (Gilbert et al. 1998). Indeed, Schmidt et al. (2004) found that although soil and microbial N pools were higher in N-fertilized plots, both microbial activity and biomass were lower following N fertilization, with negative effects being more profound in the growing season.
Demoling et al. (2008) observed 40 and 30 % reductions in microbial biomass and activity, respectively, from N additions to Swedish coniferous forest soils. They also found profound changes in microbial composition in response to N treatments. More recently, Treseder (2008) performed a meta-analysis of 82 published field studies of the effects of N additions on microbial biomass, estimating that excess N reduced microbial biomass by 15 % across all studies.
Although this is an area requiring further research, increases in N supply to forests clearly have the potential to alter the microbial communities of forest soils and do so at the watershed scale . Wallenstein et al. (2006b) provided clear evidence to indicate that microbial immobilization of N might not be a major mechanism to explain decreases in stream NO3 − observed at FEF (Adams et al. 2006).
Thus, it is possible that excess N-mediated shifts in microbial communities are toward communities that simply process N at much lower rates (i.e., lower net N mineralization and nitrification) rather than those that affect immobilization.
3.2 Herbaceous Layer
The herbaceous layer, defined here as vascular plants ≤ 1 m in height, can represent > 90 % of plant species richness of forest ecosystems (Gilliam 2007). It is also the forest stratum of highest potential sensitivity to changes in resource availability, such as light and, especially, soil N (Gilliam and Roberts 2003). There was an initial period (1991–1994) representing up to a 6 year treatment period during which there were no detectable responses of the herb layer to the N treatment on WS3 (Fig. 29.2). This is in sharp contrast to other field-based N manipulation studies, wherein decreases in herb layer diversity have been observed within a one-year period (see Bobbink et al. 2010 for recent review).
Repeated sampling in 2003 indicated a substantial decline of species diversity of the herbaceous layer in response to aerial N additions, a decrease that appears to be related to loss of species (lower species richness) and increased herb layer cover (Fig. 29.2). Most of this decline appears to have arisen from unexpected increases in the shade-intolerant Rubusspp. (data not shown). Contemporaneously, spatial variability in Rubus spp. cover has decreased on WS3, consistent with declines in spatial variability of soil N (Gilliam et al. 2001), supporting the N homogeneity hypothesis suggested by Gilliam (2006) .
3.3 Effects of Soil Freezing
Paradoxically, global warming is a predicted increase in the probability of soils in north-temperate regions of North America to freeze during the winter, the result of warming-related decreases in snow cover, thus minimizing the insulating effects of snow pack (Groffman et al. 2001). Because of previous observations that net nitrification can increase following soil freezing (see Groffman et al. 2001 for review) and because WS4 exhibits numerous symptoms of N saturation (including generally high net nitrification and NO3 − leaching), an experiment was designed to examine the effects of freezing on net N mineralization and nitrification on soils along a gradient of weathering/nitrification in WS4. The gradient was as follows: LN (highly weathered soils with negligible net nitrification), HN (to less weathered soils with high nitrification), and MN (intermediate weathering and nitrification).
The results of this experiment are reported in full in Gilliam et al. (2010) and are summarized here. Freezing had profound effects on N dynamics in N-saturated soil, with responses varying between temperature treatments and along the gradient (Fig. 29.3).
Effects of freezing on net mineralization and nitrification at three sample sites in WS4, FEF, WV. Means (±1 SE) with the same superscript (x, y, z) are not significantly different (P < 0.05) between sites for a given treatment. Means with the same superscript (a, b, c) are not significantly different (P < 0.05) between treatments for a given site. See text for site abbreviations. C, T0, T-20, T-80 = control, 0°C, -20°C, and -80°C treatments, respectively. Modified from data taken from Gilliam et al. (2010)
Furthermore, lack of significant differences in net N mineralization and nitrification between − 20 and − 80 °C treatments (Fig. 29.3) suggests that − 20 °C may represent a threshold temperature for response of these processes to freezing. In addition, freezing response of N mineralization differed greatly from that of nitrification, suggesting that soil freezing may de-couple two processes of the soil N cycle that are otherwise tightly linked at our site. Results also suggest that soil freezing at temperatures commonly experienced at this site can further increase net nitrification in soils already exhibiting high nitrification from N saturation.
3.4 Future Work
Future research at FEF out of the Gilliam lab at Marshall University includes continuation of all work summarized herein. Through cooperation with the Peterjohn lab at West Virginia University (with funding from the National Science Foundation’s Long Term Research in Environmental Biology program), we are continuing full growing-season in situ incubations to measure net N mineralization and nitrification and mid-growing season sampling of the herbaceous layer. In addition, we will carry out soil freezing experiments to include analyses of microbial communities of soils along the weathering gradient of WS4.
References
Aber, J., McDowell, W., Nadelhoffer, K., Magill, A., Berntson, G., Kamakea, M., McNulty, S., Currie, W., Rustad, L., & Fernandez, I. (1998). Nitrogen saturation in temperate forest ecosystems - Hypotheses revisted. Bioscience, 48, 921–934.
Adams, M. B., DeWalle, D. R., & Hom, J. (Eds.). (2006). The fernow watershed acidification study. New York: Springer.
Bobbink, R., Hornung, M., & Roelofs, J. G. M. (1998). The effects of air-borne nitrogen pollutants on species diversity in natural and semi-natural European vegetation. Journal of Ecology, 86, 717–738.
Bobbink, R., Hicks, K., Galloway, J., Spranger, T., Alkemade, R., Ashmore, M., Bustamante, M., Cinderby, S., Davidson, E., Dentener, F., Emmett, B., Erisman, J.-W., Fenn, M., Gilliam, F., Nordin, A., Pardo, L., & de Vries, W. (2010). Global assessment of nitrogen deposition effects on terrestrial plant diversity: A synthesis. Ecological Applications, 20, 30–59.
Christ, M. J., Peterjohn, W. T., Cumming, J. R., & Adams, M. B. (2002). Nitrification potentials and landscape, soil and vegetation characteristics in two Central Appalachian watersheds differing in NO3 - export. Forest Ecology and Management, 159, 145–158.
Demoling, F., Nilsson, L. O., & Bååth, E. (2008). Bacterial and fungal response to nitrogen fertilization in three coniferous forest soils. Soil Biology and Biochemistry, 40, 370–379.
Fenn, M. E., Poth, M. A., Aber, J. D., Baron, J. S., Bormann, B. T., Johnson, D. W., Lemly, A. D., McNulty, S. G., Ryan, D. F., & Stottlemyer, R. (1998). Nitrogen excess in North American ecosystems: Predisposing factors, ecosystem responses, and management strategies. Ecological Applications, 8, 706–733.
Gilbert, D., Amblard, C., Bourdier, G., & Francez, A.-J. (1998). Short-term effect of nitrogen enrichment on the microbial communities of a peatland. Hydrobiologia, 373/374, 111–119
Gilliam, F. S. (2006). Response of the herbaceous layer of forest ecosystems to excess nitrogen deposition. Journal of Ecology, 94, 1176–1191.
Gilliam, F. S. (2007). The ecological significance of the herbaceous layer in forest ecosystems. Bioscience, 57, 845–858.
Gilliam, F. S., & Adams, M. B. (1996). Wetfall deposition and precipitation chemistry for central Appalachian forest. Journal of the Air and Waste Management Association, 46, 978–984.
Gilliam, F. S., & Roberts, M. R. (Eds.). (2003). The herbaceous layer in forests of Eastern North America. Inc., New York: Oxford University Press.
Gilliam, F. S., Turrill, N. L., Aulick, S. D., Evans, D. K., & Adams, M. B. (1994). Herbaceous layer and soil response to experimental acidification in a central Appalachian hardwood forest. Journal of Environmental Quality, 23, 835–844.
Gilliam, F. S., Adams, M. B., & Yurish, B. M. (1996). Ecosystem nutrient responses to chronic nitrogen inputs at Fernow Experimental Forest, West Virginia. Canadian Journal of Forest Research, 26, 196–205.
Gilliam, F. S., Yurish, B. M., & Adams, M. B. (2001). Temporal and spatial variation of nitrogen transformations in nitrogen-saturated soils of a Central Appalachian hardwood forest. Canadian Journal of Forest Research, 31, 1768–1785.
Gilliam, F. S., Lyttle, N. L., Thomas, A., & Adams, M. B. (2005). Soil variability along a nitrogen mineralization/nitrification gradient in a nitrogen-saturated hardwood forest. Soil Science Society of America Journal, 69, 247–256.
Gilliam, F. S., Hockenberry, A. W., & Adams, M. B. (2006). Effects of atmospheric nitrogen deposition on the herbaceous layer of a central Appalachian hardwood forest. The Journal of the Torrey Botanical Society, 133, 240–254.
Gilliam, F. S., Cook, A., & Lyter, S. (2010). Effects of experimental freezing on soil nitrogen (N) dynamics along a net nitrification gradient in an N-saturated hardwood forest ecosystem. Canadian Journal of Forest Research, 40, 436–444.
Goodale, C. L., Aber, J. D., Vitousek, P. M., & McDowell, W. H. (2005). Long-term decreases in stream nitrate: Successional causes unlikely; possible links to DOC? Ecosystems, 8, 334–337.
Gress, S. E., Nichols, T. D., Northcraft, C. C., & Peterjohn, W. T. (2007). Nutrient limitation in soils exhibiting differing nitrogen availabilities: What lies beyond nitrogen saturation? Ecology, 88, 119–130.
Groffman, P. M., Driscoll, C. T., Fahey, T. J., Hardy, J. P., Fitzhugh, R. D., & Tierney, G. L. (2001). Colder soils in a warmer world: A snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry, 56, 135–150.
Lu, X., Mo, J., Gilliam, F. S., Guoyi, Z., & Fang, Y. (2010). Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Global Change Biology, 16, 2688–2700.
May, J. D., Burdette, E., Gilliam, F. S., & Adams, M. B. (2005). Interspecific divergence in foliar nutrient dynamics and stem growth in a temperate forest in response to chronic nitrogen inputs. Canadian Journal of Forest Research, 35, 1023–1030.
Peterjohn, W. T., Adams, M. B., & Gilliam, F. S. (1996). Symptoms of nitrogen saturation in two central Appalachian hardwood forests. Biogeochemistry, 35, 507–522.
Peterjohn, W. T., McGervey, R. J., Sexstone, A. J., Christ, M. J., Foster, C. J., & Adams, M. B. (1998). Nitrous oxide production in two forested watersheds exhibiting symptoms of nitrogen saturation. Canadian Journal of Forest Research, 28, 1723–1732.
Peterjohn, W. T., Foster, C. J., Christ, M. J., & Adams, M. B. (1999). Patterns of nitrogen availability within a forested watershed exhibiting symptoms of nitrogen saturation. Forest Ecology and Management, 119, 247–257.
Rabalais, N. N. (2002). Nitrogen in aquatic ecosystems. Ambio, 31, 102–112.
Schimel, J. P., & Bennett, J. (2004). Nitrogen mineralization: Challenges of a changing paradigm. Ecology, 85, 591–602.
Schmidt, S. K., Lipson, D. A., Ley, R. E., Fisk, M. C., & West, A. E. (2004). Impacts of chronic nitrogen additions vary seasonally and by microbial functional group in tundra soils. Biogeochemistry, 69, 1–17.
Stoddard, J. L. (1994). Long-term changes in watershed retention of nitrogen: Its causes and aquatic consequences. In L. A. Baker (Ed.), Environmental chemistry of lakes and reservoirs (pp. 223–284). Washington, DC: American Chemical Society.
Treseder, K. K. (2008). Nitrogen additions and microbial biomass: A meta-analysis of ecosystem studies. Ecology Letters, 11, 1111–1120.
Vitousek, P. M., & Howarth, R. W. (1991). Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry, 13, 87–115.
Vitousek, P. M., Aber, J. D., Howarth, R. W., Likens, G. E., Matson, P. A., Schindler, D. W., Schlesinger, W. H., & Tilman, D. G. (1997). Human alteration of the global nitrogen cycle: Sources and consequences. Ecological Applications, 7, 737–750.
Wallenstein, M. D., Peterjohn, W. T., & Schlesinger, W. H. (2006a). N fertilization effects on denitrification and N cycling in an aggrading forest. Ecological Applications, 16, 2168–2176.
Wallenstein, M. D., McNulty, S., Fernandez, I. J., Boggs, J., & Schlesinger, W. H. (2006b). Nitrogen fertilization decreases forest soil fungal and bacterial biomass in three long-term experiments. Forest Ecology and Management, 222, 459–468.
Acknowledgments
I gratefully acknowledge the assistance of Jeff May (Marshall University), Mary Beth Adams (U.S.D.A. Forest Service), Bill Peterjohn (West Virginia University), Forest Service lab and field personnel, and many dedicated undergraduate and graduate students for making this work possible. I thank the International Nitrogen Initiative for support in presenting the paper.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Gilliam, F. (2014). Nitrogen Biogeochemistry Research at Fernow Experimental Forest, West Virginia, USA: Soils, Biodiversity and Climate Change. In: Sutton, M., Mason, K., Sheppard, L., Sverdrup, H., Haeuber, R., Hicks, W. (eds) Nitrogen Deposition, Critical Loads and Biodiversity. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7939-6_29
Download citation
DOI: https://doi.org/10.1007/978-94-007-7939-6_29
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7938-9
Online ISBN: 978-94-007-7939-6
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)