Abstract
Too many hypotheses in the etiology of atherosclerosis have been proposed. Classically, lipid insudation hypothesis by Virchow and thrombogenic hypothesis by Rokitansky are famous. However, in the recent progress in the area of atherosclerosis, the response-to-injury hypothesis by Ross (Ross R Glomset JA, N Engl J Med 295:369–377, 420–425, 1976; Ross R, Arteriosclerosis 1:293–311, 1981; Ross R, N Engl J Med 314:488–500, 1986; Ross R, Nature 362:801–809, 1993; Ross R, N Engl J Med 340:115–126, 1999) has been the leading one. In this review, however, the author focuses to the recent debate on the role of oxidative modification of atherogenic lipoproteins.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Theories of Atherosclerosis
1.1 LDL Oxidation Hypothesis
Steinberg (1997; Steinberg et al. 1989) suggested that oxidative modification of low density lipoprotein (LDL) is important in the pathogenesis of atherosclerosis. LDL can be modified by incubation with endothelial cells, smooth muscle cells, or monocytes/macrophages in the presence of trace amount of transition metals. This biological modification of LDL is mediated by a free radical-induced peroxidation by copper or iron in the absence of cells. The potential atherogenic effects of oxidized LDL include (1) chemotactic activity, which facilitates the recruitment of circulating monocytes, (2) the inhibition of the migration of macrophages from the arterial wall back to the circulation, (3) the enhanced uptake by macrophages through scavenger receptors, leading to the formation of foam cells, and (4) cytotoxicity to endothelial cells, that may facilitate the accumulation of LDL and monocytes in the early stage, causing endothelial denudation at a later stage.
1.2 Response-to-Retention Hypothesis
Williams and Tabas (1995, 2005) doubted the Steinberg’s LDL oxidation hypothesis, which looks upon the oxidation of LDL as the essential factor in the initiation of atherosclerosis, and proposed the response-to-retention hypothesis. According to their theory, the deposition and retention of lipoprotein particles, particularly LDL, are sufficient for the initiation of atherosclerosis, and the oxidation of LDL is not required for the initial stage of atherosclerosis. LDL accumulated in the arterial intima easily binds to extracellular matrix including proteoglycans, aggregates, and then is taken up by macrophages through scavenger receptors, leading to foam cell formation. Lipolytic enzymes such as lipoprotein lipase and sphingomyelinase may be involved in the intramural retention of lipoproteins. Among the atherogenic lipoproteins, especially lipoprotein(a) is prone to be retained in the intima. Apolipoproteins B100, C-II, and E may also be involved. Among them, apolipoprotein C-II is predisposed to form amyloid, and stimulates inflammatory response of macrophages (Medeiros et al. 2004). In fact, amyloid, macrophages, and apolipoprotein C-II colocalize in human atherosclerotic lesions. Collagen, fibrin, and fibronectin may also be involved in the deposition of lipoproteins.
Although the inflammatory nature of atherosclerosis has been established as will be discussed later, the substances that start inflammation in the artery wall are largely unknown. It is clear that chronic infections yield a higher risk for cardiovascular disease. However, because germ-free animals are also susceptible to atherosclerosis (Wright et al. 2000), it is considered that endogenous substances can also stimulate inflammation. Cholesterol crystal has been reported to be the candidate (Duewell et al. 2010). Small crystals appeared in subendothelial areas that were rich in immune cells as early as 2 weeks after the start of atherogenic diet. A sharp increase in the incidence of cholesterol crystals has been observed in human atherosclerotic lesions as they progress from fatty streaks to more advanced lesions (Rajamäki et al. 2010). Cholesterol crystals activate the NLRP3 inflammasome in phagocytes in vitro in a process that involves phagolysosomal damage. In contrast, cholesteryl esters form droplets rather than crystals and are considered to be a storage form of cholesterol.
1.3 Oxidative Response to Inflammation Hypothesis
In 2004, synthesizing the results obtained from molecular and cellular biology, animal experiments, and clinical and epidemiological studies, Stocker and Keaney (2004) introduced the “oxidative response to inflammation” theory, and proposed that inflammation, but not oxidative stress is the real cause of atherosclerosis, and oxidative stress is merely accompanied as the consequence of inflammation. According to their theory, the primary process of atherosclerosis initiation is not oxidative stress, but inflammation, and oxidative stress is merely a secondary event. Among the evidence related to their theory, atherosclerosis was aggravated in the scavenger receptor deficient animal (de Winther et al. 1999), and the over-expression of scavenger receptor inhibited atherosclerotic process (Whitman et al. 2002). However, the relation between the oxidative stress and inflammation is complex, and it is not easy to differentiate which is the cause and which is the result. Moreover, the view point of atherosclerosis as inflammation is not novel. Indeed, before Ross (1999) proposed the inflammation as the pathogenesis of atherosclerosis, steroidal (Naito et al. 1992) and non-steroidal anti-inflammatory agents (Bailey and Butler 1973) had been reported to inhibit atherosclerosis induced by cholesterol feeding without influencing the plasma cholesterol level.
1.4 Unifying Hypothesis of Atherosclerosis
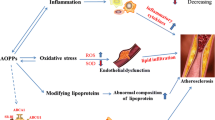
Atherosclerosis is multifactorial pathology, and the author introduced the unifying theory in 2000 (Naito 2000), putting together various aspects of findings, and pointed out the paramount importance of (1) the insudation, deposition, and denaturalization (including oxidative modification) of plasma components, particularly atherogenic lipoproteins such as LDL and fibrinogen into the subendothelial space, (2) the formation of thrombus, fibrinolysis, and the organization, and (3) the inflammatory response to those events (Fig. 8.1).
Unifying hypothesis (Revised from Naito 2000)
Except for the modified version of (inflammatory) response-to-injury hypothesis by Ross, the theories proposed by Steinberg (LDL oxidation hypothesis), Williams and Tabas (response-to-retention hypothesis), and Stocker and Keaney (oxidative response-to-inflammation hypothesis) are all related only to the initiation and early stages of atherosclerosis. After the initiation of atherosclerosis, its development thereafter is explained by Ross’s hypothesis. However, even in the modified version of his hypothesis published only several months before his death (Ross 1999), although the importance of chronic inflammation was emphasized, the role of coagulation and fibrinolysis was not mentioned. There is no sufficient evidence to show the participation of mural thrombosis in the initiation of atherosclerosis, however, this mechanism is essential for the growth and development of the lesion.
2 CRP and Atherosclerosis
C-reactive protein (CRP), an acute-phase protein, binds specifically to phosphorylcholine as a component of capsular polysaccharide of many microorganisms. CRP also binds to apoptotic cells, enhancing their clearance. CRP promotes the clearance of CRP-opsonized particles by binding to FCγ receptors (Bharadwaj et al. 1999; Mold et al. 2001). CRP bound to multivalent ligands also activates a classical complement pathway, enhancing phagocytosis (Mold et al. 1999). CRP binds to oxidized LDL but not native LDL, and the binding to oxidized LDL is mediated through recognition of phosphorylcholine. CRP binds oxidized LDL and apoptotic cells by recognition of a phosphorylcholine moiety that becomes exposed and accessible as a result of oxidation of phosphatidylcholine molecule (Chang et al. 2002). These findings indicate that the main biological function of CRP is a first-line innate immune response to oxidized phosphorylcholine-bearing phospholipids within oxidized LDL and on the plasma membrane of apoptotic cells. However, it was also reported that CRP-bound enzymatically modified LDL did not transform macrophages into foam cells (Singh et al. 2008).
CRP is predominantly synthesized by hepatocytes as an acute-phase reactant and is transcriptionally driven by interleukin-6, with synergistic enhancement by interleukin-1. However, some studies showed that CRP may be produced in atherosclerotic lesion (Yasojima et al. 2001) and by smooth muscle cells (Calabró et al. 2003) and macrophages (Dong and Wright 1996).
CRP is found localized in inflamed tissues, including atherosclerotic lesion (Hatanaka et al. 1995). CRP displays both anti-inflammatory and proinflammatory effects in vitro. The latter includes the ability of ligand-bound CRP to activate the complement system (Volanakis 1982). Activated complements have also been found in human atherosclerotic lesions (Seifert and Kazatchkine 1988).
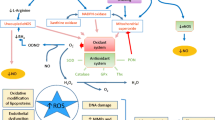
The expression of vascular CRP is closely colocalized with NAD(P)H oxidase, a crucial enzyme for the origin of reactive oxygen species (ROS) in vessel walls (Kobayashi et al. 2003). It is reported that plaque instability was associated with the expression of CRP in directional coronary atherectomy specimens, and the expression of CRP was colocalized with NAD(P)H oxidase p22phox protein. Incubation of cultured coronary artery smooth muscle cells with CRP resulted in enhanced protein expression of NAD(P)H oxidase p22phox and the generation of H2O2. CRP also promotes apoptosis of vascular smooth muscle cells, and may contribute to plaque instability.
CRP increases tissue factor expression in monocytes/macrophages, promotes monocyte chemotaxis and adhesion to endothelial cells, and stimulates the release of ROS, matrix metalloproteinase-1 (MMP-1), CC-chemokine receptor 2 (CCR2), cytokines, and macrophage-colony stimulating factor (M-CSF) (Devaraj et al. 2009). CRP treatment significantly increases the release of MPO from polymorphonuclear cells and monocytes/macrophages and causes nitro-tyrosinylation of LDL (Singh et al. 2009). The colocalization of CRP and macrophages has been demonstrated in atherosclerotic lesions in human (Yasojima et al. 2001) and animals (Fukuchi et al. 2008).
3 Lipid Peroxidation and Atherosclerosis
Lipid hydroperoxide-derived modification of protein may serve as one mechanism for the modification of LDL and subsequent foam cell formation in the atherosclerotic lesion. Oxidation of LDL leads to the loss of ε-amino groups from lysine residues in apolipoprotein B100 due to the covalent adduction of the oxidatively decomposed products of polyunsaturated fatty acid esters.
Lipid hydroperoxides are the initial products of lipid peroxidation, which are subsequently decomposed to a variety of products such as aldehydes. It has been shown that reaction of 13-hydroperoxyoctadienoic acid (13-HPODE) with lysine residues resulted in the formation of two major amide-type adducts, N ε-hexanoyl-lysine (HEL) (Kato et al. 1999) and N ε-azelayl-lysine (AZL) (Kawai et al. 2003).
Aldehydes such as malondialdehyde (MDA) and 4-hydroxy-2-nonenal (HNE), the major lipid peroxidation end-products, are highly reactive to ε-amino groups in proteins. MDA-lysine (Uchida et al. 1997), HNE-lysine (Uchida et al. 1994), and acrolein-lysine adducts (Uchida et al. 1998) are detected in oxidized LDL and atherosclerotic lesion.
In our study, CRP was detected mostly in the macrophage-derived foam cell-rich areas of rabbit fatty lesions. Immunopositive staining of HEL was observed in the foam cell-rich areas, where it almost colocalized with CRP-positive macrophages (Fukuchi et al. 2008). Dityrosine (DY) was also observed in the foam cell-rich areas, essentially similar to the deposition of HEL. HEL and DY were colocalized with CRP-positive macrophages.
HEL is produced in the reaction between linoleic hydroperoxide and lysine moiety, and is an early and stable marker for protein oxidation derived from lipid peroxidation. HEL is shown in oxidized LDL and in human atherosclerotic lesion, using a specific monoclonal antibody to HEL moiety. The hydroperoxide-derived carboxylic adducts, such as AZL, and their esters linked with phospholipids, are also detected in rabbit atherosclerotic lesions (Kawai et al. 2003).
Dityrosine (DY) is formed by the reaction of two tyrosyl radicals and catalyzed by myeloperoxidase in the presence of H2O2, but it can also be generated by metal-catalyzed oxidation. DY has been detected immunochemically in lipofuscin of pyramidal neurons of aged human brains (Kato et al. 1998) and in the atherosclerotic lesions of apolipoprotein E-deficient mice (Kato et al. 2000). NAD(P)H oxidase plays a crucial role in the generation of ROS in vascular cells. NAD(P)H oxidase converts oxygen into superoxide (O2 −), and O2 −then dismutates into H2O2, an oxidizing substrate for myeloperoxidase. Myeloperoxidase, using H2O2 generated by this system, forms a DY cross-link from the tyrosine residue of the target protein (Heinecke 2002). Oxidants derived from the phagocyte NAD(P)H oxidase provide one pathway for generating DY cross-links in vivo. Neutrophils markedly increase their content of protein-bound DY when they are activated in wild-type mice; however, this increase fails to occur in mice that are deficient in phagocyte NAD(P)H oxidase (Bhattacharjee et al. 2001). CRP accumulating in atherosclerotic lesion may mediate the production of DY by increasing NAD(P)H oxidase p22phox expression and ROS generation, since DY was produced in LDL oxidized with tyrosyl radical generated by myeloperoxidase-H2O2 system (Heinecke 2002).
References
Bailey JM, Butler J (1973) Anti-inflammatory drugs in experimental atherosclerosis. I. Relative potencies for inhibiting plaque formation. Atherosclerosis 17:515–522
Bharadwaj D, Stein MP, Volzer M et al (1999) The major receptor for C-reactive protein on leukocytes is Fcγ receptor II. J Exp Med 190:585–590
Bhattacharjee S, Pennathur S, Byun J (2001) NADPH oxidase of neutrophils elevates o, o’-dityrosine cross-links in proteins and urine during inflammation. Arch Biochem Biophys 395:69–77
Calabró P, Willerson JT, Yeh ET (2003) Inflammatory cytokines stimulated C-reactive protein production by human coronary artery smooth muscle cells. Circulation 108:1930–1932
Chang MK, Binder CJ, Torzewski M et al (2002) C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: phosphorylcholine of oxidized phospholipids. Proc Natl Acad Sci U S A 99:13043–13048
de Winther MP, Gijbels MJ, van Dijk KW et al (1999) Scavenger receptor deficiency leads to more complex atherosclerotic lesions in APOE3Leiden transgenic mice. Atherosclerosis 144:315–321
Devaraj S, Singh U, Jialal I (2009) The evolving role of C-reactive protein in atherothrombosis. Clin Chem 55:229–238
Dong Q, Wright JR (1996) Expression of C-reactive protein by alveolar macrophages. J Immunol 156:4815–4820
Duewell P, Kono H, Rayner KJ et al (2010) NLRP3 Inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464:1357–1361
Fukuchi Y, Miura Y, Nabeno Y et al (2008) Immunohistochemical detection of oxidative stress biomarkers, dityrosine and N ε-(hexanoyl)lysine, and C-reactive protein in rabbit atherosclerotic lesions. J Atheroscler Thromb 15:185–192
Hatanaka K, Li XA, Masuda K et al (1995) Immuno histochemical localization of C-reactive protein-binding sites in human atherosclerotic aortic lesions by a modified streptavidin-biotin-staining method. Pathol Int 45:635–641
Heinecke JW (2002) Tyrosyl radical production by myeloperoxidase: a phagocyte pathway for lipid peroxidation and dityrosine cross-linking of proteins. Toxicology 177:11–22
Kato Y, Maruyama W, Naoi M et al (1998) Immunohistochemical detection of dityrosine in lipofuscin pigments in the aged human brain. FEBS Lett 439:231–234
Kato Y, Mori Y, Makino Y et al (1999) Formation of N ε- (hexanonyl)lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J Biol Chem 274:20406–20414
Kato Y, Wu X, Naito M et al (2000) Immunochemical detection of protein dityrosine in atherosclerotic lesion of apo-E-deficient mice using a novel monoclonal antibody. Biochem Biophys Res Commun 275:11–15
Kawai Y, Kato Y, Fujii H et al (2003) Immunochemical detection of a novel lysine adduct using an antibody to linoleic acid hydroperoxide-modified protein. J Lipid Res 44:1124–1131
Kobayashi S, Inoue N, Ohashi Y et al (2003) Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol 23:1398–1404
Medeiros LA, Khan T, El Khoury JB et al (2004) Fibrillar amyloid protein present in atheroma activates CD36 signal transduction. J Biol Chem 279:10643–10648
Mold C, Gewurz H, Du Clos TW (1999) Regulation of complement activation by C-reactive protein. Immunopharmacology 42:23–30
Mold C, Gresham HD, Du Clos TW (2001) Serum amyloid P component and C-reactive protein mediate phagocytosis through murine FcγRs. J Immunol 166:1200–1205
Naito M (2000) Effects of fibrinogen, fibrin and their degradation products on the behaviour of vascular smooth muscle cells. Nihon Ronen Igakkai Zasshi 37:458–463 [Article in Japanese, abstract in English]
Naito M, Yasue M, Asai K et al (1992) Effects of dexamethasone on experimental atherosclerosis in cholesterol-fed rabbits. J Nutr Sci Vitaminol (Tokyo) 38:255–264
Rajamäki K, Lappalainen J, Oörni K et al (2010) Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: a novel link between cholesterol metabolism and inflammation. PLoS One 23(5):e11765
Ross R (1981) George Lyman Duff memorial lecture. Atherosclerosis: a problem of the biology of arterial wall cells and their interactions with blood components. Arteriosclerosis 1:293–311
Ross R (1986) The pathogenesis of atherosclerosis – an update. N Engl J Med 314:488–500
Ross R (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362:801–809
Ross R (1999) Atherosclerosis ― an inflammatory disease. N Engl J Med 340:115–126
Ross R, Glomset JA (1976) The pathogenesis of atherosclerosis. N Engl J Med 295:369–377
Seifert PS, Kazatchkine MD (1988) The complement system in atherosclerosis. Atherosclerosis 73:91–104
Singh SK, Suresh MV, Prayther DC et al (2008) C-reactive protein-bound enzymatically modified low-density lipoprotein does not transform macrophages into foam cells. J Immunol 180:4316–4322
Singh U, Devaraj S, Jialal I (2009) C-reactive protein stimulates myeloperoxidase release from polymorphonuclear cells and monocytes: implications for acute coronary syndromes. Clin Chem 55:361–364
Steinberg D (1997) Lewis A. Conner memorial lecture. Oxidative modification of LDL and atherogenesis. Circulation 95:1062–1071
Steinberg D, Parthasarathy S, Carew TE et al (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med 320:915–924
Stocker R, Keaney JF Jr (2004) Role of oxidative modifications in atherosclerosis. Physiol Rev 84:1381–1478
Uchida K, Toyokuni S, Nishikawa K et al (1994) Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry 33:12487–12494
Uchida K, Sakai K, Itakura K et al (1997) Protein modification by lipid peroxidation products: formation of malondialdehyde-derived N ε- (2-propenol)lysine in proteins. Arch Biochem Biophys 346:45–52
Uchida K, Kanematsu M, Sakai K et al (1998) Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A 95:4882–4887
Volanakis JE (1982) Complement activation by C-reactive protein complexes. Ann N Y Acad Sci 389:235–250
Whitman SC, Rateri DL, Szilvassy SJ et al (2002) Macrophage-specific expression of class A scavenger receptors in LDL receptor(−/−) mice decreases atherosclerosis and changes spleen morphology. J Lipid Res 43:1201–1208
Williams KJ, Tabas I (1995) The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol 15:551–561
Williams KJ, Tabas I (2005) Lipoprotein retention ― and clues for atheroma regression. Arterioscler Thromb Vasc Biol 25:1536–1540
Wright SD, Burton C, Hernandez M et al (2000) Infectious agents are not necessary for murine atherogenesis. J Exp Med 191:1437–1442
Yasojima K, Schwab C, McGeer EG et al (2001) Generation of C-reactive protein and complement components in atherosclerotic plaques. Am J Pathol 158:1039–1051
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Naito, M. (2014). Amide-Adducts in Atherosclerosis. In: Kato, Y. (eds) Lipid Hydroperoxide-Derived Modification of Biomolecules. Subcellular Biochemistry, vol 77. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7920-4_8
Download citation
DOI: https://doi.org/10.1007/978-94-007-7920-4_8
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7919-8
Online ISBN: 978-94-007-7920-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)