Abstract
The quest for renewable and sustainable energy source is necessary to meet the growing global demand, and to substitute the fast depleting nonrenewable fossil fuels. Biofuel is a viable and proven alternative to petroleum based fuel. Algae are appearing to have the greatest potential to replace the fossil fuel. Continuous research on growing algae, harvesting, oil extraction and conversion to biofuel in large scale is indispensable to identify the practical difficulties involved, and find solutions to manipulate the complications, to operate the system farmer friendly and sustainable. This chapter discusses about the influence of various factors such as light, temperature, nutrients and culture mixing in mass cultivation of microalgal species in open raceway clay ponds with the results on average growth and oil content over a period of five years.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Microalgae
- Raceway pond
- Mixing
- Scenedesmus
- Scale up
- Light
- Temperature
- Carbon Dioxide
- Nutrients
- Harvest
- Extraction
- Algal oil
- Contaminants
1 Introduction
Increasing global demand and environmental concerns have led to a search for alternative and greener sources of fuel and other products. Algae shine as an attractive source with their ability to grow in areas unsuitable for agricultural purposes and thereby do not compete with arable land for food production. However, the production of various products from algae presents several obstacles like the selection of suitable algae, developing suitable growth conditions for optimal lipid yield and prevention of contamination from undesired algal species and other organisms. These obstacles multiply to an even greater magnitude when algal growth is pursued on large-scale outdoor settings where weather and contamination pose a constant threat. Therefore, there exists an exigency for new algal production technologies.
Microalgae are a diverse group of prokaryotic and eukaryotic photosynthetic microorganisms that grow rapidly due to their simple structure. They can potentially be employed for the production of biofuels in an economically effective and environmentally sustainable manner. Microalgae have been investigated for the production of a number of different biofuels including biodiesel , bio-syngas, and bio-hydrogen. Production of these biofuels can be coupled with flue gas CO2 mitigation, wastewater treatment, and the production of high-value chemicals (Li et al. 2008) . Microalgal farming can also be carried out with seawater using marine microalgal species as producers. Developments in microalgal cultivation and downstream processing (e.g., harvesting, drying, and thermochemical processing) are expected to further enhance the cost-effectiveness of the biofuel from microalgae strategy (Table 1).
Microalgae are grown commercially in photo-bioreactors and open ponds . Benemann in 2008 compared the economies of open ponds with closed bioreactors . Growing microalgae in bioreactors is a high yielding technology (0.5–8 g/l), low risk of contamination, but expensive ($ 250,000–1,000,000/ha) with an expected approximate life span of 10–15 years for the bioreactors. The raceways are perceived to be less expensive than photobioreactors , because they cost less to build and operate. Although raceways are low-cost ($ 100,000/ha), they have a low biomass productivity (0.1–0.5 g/l) when compared with photobioreactors (Pulz 2001; Alabi et al. 2009) . Richardson et al. 2012 estimated the total cost of lipid production is $ 12.74/gal and $ 32.57/gal for the open pond and PBR, respectively.
The first attempt to overcome difficulty in managing culture growth in open ponds was by the use of simple plastic covers or greenhouses. This allows for an extension of the growing period, facilitates the provision of carbon dioxide and the maintenance of high temperature at nights result an improvement of the biomass productivities (Richmond 1992) . The open ponds are highly scalable; some commercial pond culture systems such as Earthrise, Cyanotech have been operating profitably for more than 30 years. Culturing species of Chlorella, Scenedesmus sp. , Haematococcus sp. , Spirogyra sp., Dunaliella sp. , Nannochloropsis sp. , Botryococcus sp., in open ponds at commercial scale targeting fuel and food resources have also been successful (Robert Henrikson 1989; Kay 1991; Pauline et al. 2006; Gouveia and Oliveira 2009) . Biomass of Skeletonema sp. , Chaetoceros sp. , Tetraselmis sp. , Isochrysis sp. , Crypthecodinium sp., is popular as feed for juvenile shrimp and fish species (Hoff and Snell 2008). Algae also play great roles in absorbing hazardous materials from wastewater and convert them as useful bio fertilizers (Oswald 1988; Kaushik 1998) .
2 Strain Selection
As mentioned earlier, microalgae have been a potential source of biofuels , and there is an increasing focus for finding appropriate species and exploiting them for various applications. The promising algal species are distributed in fresh, brackish and marine water habitats (Metzger and Largeau 2005; Natrah et al. 2007; Deng et al. 2009) . Many microalgal strains are collected from local freshwaters and are grown in modified Bold Basal and or BG11 medium (Allen and Stanier 1968; Stein 1973; Watanabe et al. 2000) , incubated at 25 ± 1 °C under 100–200 µmoles photon m−2 s−1 irradiance with 12:12 light-dark cycle. Sforza et al. 2012 observed the growth rate of Nannochloropsis culture was optimum at 150 µE/m2/s and declined at 350–1000 µE/m2/s. Solovchenko et al. 2008 reported the optimum growth rate of Parietochloris incisa, a green unicellular micro alga at 400 µE/m2/s on a complete medium. Hence, the growth media and light intensities are adjusted to find an increase in algal productivity . The emerging, faster growing species are isolated; purified by serial dilution, repeated agar plating and antibiotic treatment. The pure culture is subsequently scaled up and transferred to 20 L glass carboys, recurrently subcultured for 10–12 times. Then the cultures are domesticated by treating in higher (~ 33 °C) and lower (~ 10 °C) temperatures, various light intensities (30–1000 µmoles photon/m2/s), and different nutrient formulations to pretend the outdoor weather conditions. The culture is then inoculated into 50 L open mini raceway ponds with inbuilt paddle wheels made of acrylic sheet, are exposed to ambient conditions and subsequently subcultured several times for a period of 10–12 weeks. These cultures are monitored closely for growth, polymorphism, contamination, light and temperature tolerance , nutritional requirement, and oil content. All selected strains undergo this process simultaneously. The effective, robust strains are selected based on faster growth rate (g/m2), higher oil content (% dwt.) and simple to harvest and extraction process . These outdoor experiments enable the researcher to determine the highest productivity peak of a particular strain corresponding to its nutrient composition, light, temperature and mixing velocity requirements.
3 Cultivation in Open Raceways
The mass cultivation ponds are of various shapes from circular, square to rectangular raceways. Raceway ponds for mass culture of microalgae have been used since the 1950s (Burlew 1953) for food production and waste management (Benemann et al. 1987) . Extensive experience exists in the operation and engineering of raceways (Dodd 1986) . The largest raceway-based biomass production facility occupies an area of 440,000 m2 (Pauline et al. 2006) . A raceway pond is a closed loop recirculation channel that is typically about 0.3 m deep, comprises parallel rectangular channels with semi-circular or sufficiently curved channels on either end joining neighboring ends of the parallel rectangular channels to form a continuous channel (Lee 2001) . Ponds involve evenly divided lanes by the width of each lane staying constant throughout the course of the pond (Douglas and Blanca 2012) . The pond bottom is compacted, smooth and even. A small dip, approximately one foot deep is constructed in one corner of the pond to support pumping operations. A foot raise of pond sidewalls prevents the culture from rainwater runoff into the pond and a continuous running paddle wheel circulates the growing culture (Vonshak 1997; Chisti 2007; Chen et al. 2009; James and Boriah 2010) . The smaller ponds are constructed with concrete or fiberglass materials to minimize stress to the freshly inoculated culture from soil borne contaminants and to enable adaptation to the new conditions at the initial stages of the culture cycle. The clay ponds in the pilot site, at Aquatic Energy LLC, range from 30 × 5 to 122 × 14 m to hold a total volume of 2,000,000 L of culture. The depth of the pond culture is maintained maximally up to 25 cm to allow sunlight to pass through the entire water column of the pond. Sheehan et al. 1998 extensively studied the production of microalgal biomass for making biodiesel in raceway ponds .
Several nontoxic acrylic liners may also be used on the sidewalls of the clay pond to avoid soil erosion on the course of rainfall and frequent culture operations. The edges of the lines must be fixed in order to protect from wind (Fig. 1).
The pond size decides the paddle wheel size and number. The smaller ponds are designed with single paddle wheels with four or six paddles and the larger ponds are constructed with two paddle wheels of six paddles each on either side of the pond. The paddle wheel is positioned so that it straddles the median divide and outside wall of the pond, and is able to push the culture to a great distance before the lane curves. Experience has shown that the ground clearance between the paddle and pond bottom less than 0.3 in. is effective to provide maximum contact with the culture. The mixing speed should ensure 90 % of the algae are suspended. Lesser mixing would affect the productivity by ways of biomass sedimentation and decay. The microscopic cell counting, optical density analysis and chlorophyll estimation before and after mixing the culture is employed to detect the suitable mixing velocity. The normal mixing velocity ranges between 12 cm/sec and 18 cm/sec, but the exact value could slightly vary for different strains (Borowitzka 2005) . Generally, for the heavier or larger strains stronger mixing is preferred than the lighter ones. Ugwu and Aoyagi 2012 discussed various designs, operation and application methods in microalgal culture systems.
A shallow depth of < 5 in. in a clay based raceway pond results in large volumetric productivity but it leads to sudden pH rise or fall, CO2 depletion and other negative effects in the culture such as cell stress in hot summer & winter seasons and upwelling of silt affecting light penetration. Thus, a depth of 8 to 10 in. would increase the efficiency of the paddle wheel and minimize dead zones in the pond (Boyd and Tucker 1998) .
3.1 Scale Up and Culture Management
The domesticated pure Scenedesmus cultures are inoculated into smaller outdoor raceway ponds (3 × 1 m and 60 L) with clean water. A nutrient composition is supplied to the raceway pond at about the same time as the diluting step, wherein the nutrient composition comprises urea, phosphate and minerals. The denser cultures are subsequently transferred from smaller to larger ponds (122 × 14 m and 50,000 L). At each stage, the ponds are closely monitored for cell abnormalities and contaminants .
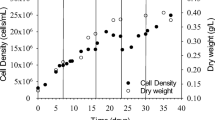
A cooling liquid is added to the raceway pond if a temperature of 33˚C or higher is reached. The pH is maintained < 9 using CO2 dispensers. The paddle wheels are in continuous operation to keep the culture in suspension and to circulate the nutrients throughout the pond area. The culture depth is maintained between 8 and 10 in. in each pond. Samples are collected three times a day to estimate culture density and nutrient residue . The depleting nutrients are added to the culture near the paddle wheels to ensure that the algae are not starving. The cell density is maintained at 5 M cells/ml and harvest or dilution is done when the cell density reaches between 5 M and 7 M cells/ml. Culture density above 7 M cells/ml affects light delivery. The highly efficient chlorophyll antenna systems of microalgae cause mutual shading as cell concentration increases. The chlorophyll absorbs excess light even though they cannot process all the photons absorbed. This affects the light penetration depth and thus the depth of the photic zone (Park and Lee 2001) . As a result, photosynthetic efficiency will be decreased and photoshading results in colony clumping and settling affecting gas exchanges in the pond.
3.2 Light and Water Temperature
Light is a fundamental factor determining the growth of algae . Open cultivation of microalgae is challenging, where the light intensity widely fluctuates from time to time. An increase in respiration rate occurred (Molina Grima et al. 2001) at low light conditions and higher intensities lead to photoinhibition (Lu and Vonshak 1999; Gouveia 2011) affecting productivity. Water temperature is another factor that greatly influences the microalgal growth. Especially in an open pond situation, the temperature fluctuation is inevitable. The high ambient temperatures extremely affect the cell metabolism by interacting with nutrients , cell membrane transport system, enzymes, and production of useful metabolites (Quinn and Williams 1983; Wheeler 1983) . A reduction in cell size at higher temperatures was observed by Rijssel and Gieskes 2002 ; a significant decrease in protein content and an increase in lipids and carbohydrates in Spirulina cultures was noticed (Tomaselli et al. 1988; Oliveira et al. 1999) . Hancke et al. 2008 studied the temperature effects on photosynthesis activities of a few species of diatoms, and observed the photo synthetic rate was strongly stimulated by temperature and the optimum growth occurred between 20 and 25 °C. Chen et al. 2012 evaluated four species of marine microalgae growing at different temperatures for their ability to remove ammonia from intensive marine fish and shrimp culture systems. The growth of Chlorella vulgaris was affected at temperatures above 30 °C and a 17 % decrease in its growth rate at 35 °C. Further increase in temperature led to an abrupt interruption of microalgal growth and resulted in cell death (Converti et al. 2009) . Temperature fluctuation can also bring changes in pond water ionic equilibrium, pH, O2, CO2 solubility; although different species are influenced to differing degrees by this effect (Bouterfas et al. 2002) .
In Aquatic Energy LLC culture facility, as Fig. 2 indicates, the growth reached its maximum of 34 g/m2 when the monthly average temperature ranges between 21 and 27 °C.. There was a decline in growth when the temperature was either above or below the optimum. The highest productivity was achieved in midsummer months and low growth during the winter months, to 22 g/m2 at 10 C.
3.3 Nutrients
Microalgae use CO2 as a basic carbon source for growth. The absence or insufficient availability of CO2 seriously affects productivity in raceway ponds . Based on the average chemical composition of algal biomass, approximately 1.8 t of CO2 are needed to grow 1 t of biomass (Rodolfi et al. 2008) . Natural dissolution of CO2 from the air into the water is not enough. This could be improved by bubbling air through the water but, since air contains CO2 as a trace gas at a concentration of about 0.0383 % per volume, all of the CO2 in about 37,000 m3 air is needed for 1 t of dry algae. Thus an additional supply of CO2 is required to maintain the pond algal productivity . The assimilation and tolerance rate of CO2 in raceway culture is complicated to understand because of the impact of other factors such as light, temperature and nutrients . Maraskolhe et al. 2012 observed a maximum growth of Scenedemus species when the CO2 concentration in the medium was 36 %.
Several species have been tested with flue gas for CO2 tolerance , Maeda et al. 1995 found a strain of Chlorella sp. T-1 which could grow under 100 % CO2, despite the maximum growth rate occurred under a 10 % concentration. Scenedesmus sp. could grow under 80 % CO2 tolerance conditions but the maximum cell mass was observed in 10–20 % CO2 concentrations (Hanagata et al. 1992) . Cyanidium caldarium (Seckbach et al. 1971) and some other species of Cyanidium can grow in pure CO2 (Graham and Wilcox 2000, Table 2).
The carbon content in CO2 is around 27 % and the shortage of carbon in the medium is overcome by supplying carbon source from external CO2 cylinders using micro bubble diffusers (Ben-Amotz 2007; Yun et al. 1997; Sawayama et al. 1995) . Though, higher CO2 dosages result in rapid cell flooding, the dosage between 60 and 63 g/m2 showed a marginal increase in productivity (Fig. 3) but unstable during the course of growth. The CO2 requirement varies between species and the dosage is decided in accordance with other parameters that favors growth (Ono and Cuello 2003) .
It was observed that nitrogen and phosphate fertilizers added in tiny amounts, help to maintain the culture quality and to ward off the competitors. The nitrogen metabolism is affected by environmental changes (Thomas and Krauss 1955; Dohler 1998) ; similar results are observed in open pond cultures at Aquatic Energy LLC pilot facility. For even more addition of nitrogen and phosphate nutrients , during high (> 30 °C) and low (< 15 °C) temperature conditions, no increase in growth is detected. It is suggested that; the nutrients are to be dosed matching the growth rate, temperature and light conditions. Excess or overdosing of nutrients is not necessary. The surplus nutrients in the culture ponds either evaporate by volatilization into the atmosphere or precipitate at higher pH values, increasing the cost of production, further attract undesirable species that contaminate the culture.
3.4 Biomass Harvest
The selection of harvesting technology is crucial to the economic production of microalgal biomass (Schenk et al. 2008) . A factor such as strain selection is an important consideration since certain species are much easier to harvest. Spirulina’s long spiral shape (20–100 mm long) naturally lends itself to the relatively cost-efficient and energy-efficient microscreen harvesting method (Benemann and Oswald 1996) . Thus the selection of harvesting technique is dependent on the characteristics of microalgae e.g. size, density, and value of the target products (Olaizola 2003) . Generally, microalgae bulk harvesting is focused on separation of biomass from the bulk suspension. This will depend on the initial biomass concentration and technologies employed, including flocculation , flotation or gravity sedimentation . Another method is to concentrate the slurry through techniques such as centrifugation , filtration and ultrasonic aggregation, hence, is generally a more energy intensive step than bulk harvesting (Brennan and Owende 2010) .
At the onset of harvest, 1 or 2 in. of the culture is pumped from the growth ponds into a wet well and the mixture is allowed to settle for about 2 h and then pumped into a Dissolved Air Flotation System (Fig. 4a). At this time, nontoxic polymers are added to separate the algal biomass from water. The algal slurry (Fig. 4b) is stored in a holding tank (Fig. 4c) and pressed onto a belt press (Fig. 4d) until it becomes 30 % dry (Fig. 4e). This is further dried in a propane-fueled oven to obtain a 90–95 % dry biomass. The harvested water is evaluated for nutrient residue with an analyzer (Fig. 4f), and pumped back to the culture pond after filtration and UV treatment, replenished with necessary nutrients.
3.5 Extraction of Algal Oil
Microalgae compared with traditional crops, they have a high areal productivity , a relatively high oil and protein content, and do not depend on arable land. Microalgae are theoretically capable of producing much more lipids than any conventional crop, approximately 20 times higher than soybean (Chen et al. 2009; Darzins et al. 2010) and are, therefore, attractive as a potential source of biodiesel . Because lipids from algae are often rich in the long-chain omega-3 fatty acids EPA and DHA , algae may also be a more sustainable source of these fatty acids for use as food or feed compared with fish oil (Lee et al. 2010) .
Lipids have been recovered from microalgae via a multitude of extraction methods (Dunahay et al. 1996; Xu et al. 2004; Hossain et al. 2008; Ryckebosch et al. 2011) . Because of the nature of microalgae , regular extraction methods (used for example as food) may not be applicable. First of all, microalgae are either single celled or colonial, but each cell is surrounded by an individual cell wall. Furthermore, they often contain ‘unusual’ lipid classes and fatty acids differing from those in higher animal and plant organisms (Guschina and Harwood 2006) .
Lipid analysis is performed using both fluorescence and total lipid extraction. Fluorescence test is a quick method using a fluorometer to measure the lipid content in the cells. The harvest process from the larger raceway pond is performed once the lipid concentration of about 25 % of the cell mass is reached. The dye Nile Red is highly fluorescent in the presence of lipids and used to achieve readings (Castro et al. 2005) . A Turner model 1–10 fluorometer with emission filters (420–470 nm) and excitation filters (> 520 nm) are employed. For this procedure, the culture is diluted to 3 mg/l. The dye is then added at a concentration of 1 mg/l. This solution is mixed using a vortex mixer for 5 min and results are read at 5-minute intervals for one hour, and are compared against a standard solution of 1 mg/l triolein with 1 mg/l Nile red.
In a laboratory set up, the total lipid extraction is performed using a modified Bligh and Dyer 1959 method. The algal slurry is dried overnight using a bench-top dehydration unit. The dried algal flakes are ground well using a grinder and then weighed. Chloroform and methanol solvents are used to extract algal oil . The algal power is mixed with the solvents and water using a vortex. After 30 min, the mixture is poured into a separating apparatus, and left for a few hours undisturbed. After the appearance of three distinct layers (Methanol, Chloroform and Water) in the separating flask, the green algal oil layer is collected and filtered. Later evaporating the solvents, the algal oil residue is measured and computed with initial weight to determine the oil content. Testing samples with ethanol as a solvent (Fajardo et al. 2007) for total lipid extraction resulted a low yield (< 10 dwt. %) against Bligh and Dyer method (> 20 dwt. %). A Dionex Accelerated Solvent Extraction unit is also used (Fig. 4g) to extract the oil from algal biomass using different solvents and the lipid results are more or less similar to Bligh and Dyer method.
In a large-scale extraction, the algal sample is placed inside a tubular column with a very fine mesh that separates the algal powder to prevent flowing into the collection apparatus. The hot hexane (70 °C) is poured onto the algal powder and the dripping liquid is collected and drizzled on to the algal powder repeatedly for five times. After this process, the mixture is finally washed with fresh hot hexane. The collected liquid is filtered to remove any foreign material, and heated to evaporate the hexane. The percentage of algal oil is calculated by the initial weight of the sample.
A proportionate relationship is observed (Fig. 5) between the growth and oil content in the cultures of Scenedesmus . In some instances, few of the culture samples are manipulated with low dosages of nitrogen (< 2 mg/l/m2) and phosphate (< 0.5 µg/l/m2), and some culture ponds are left with no nitrogen source, also showed 2–5 (% dwt.) increase in lipid content. In some cases, when the ambient temperature is high, the algal cells are unable to cope with nitrogen starvation leads to photooxidative damage. Several research papers supported that the nitrogen starvation results to an increase in lipid content, but in the open raceway culture facilities, the nitrate and phosphate starvation showed both positive and negative effects in biomass yield and lipid content as well, possibly due to the influence of other environmental factors. The sustained growth of Scenedesmus biomass after the exhaustion of phosphate in phosphorus starvation mode led to a significant increase in biomass yield and was nearly six times more than that nutrient feeding (Yin et al. 2012) . High temperature and different light intensities also impact on the lipid content in microalgae (Pohl and Zurheide 1979; Mayzaud et al. 1989) . Therefore one or a combination of many factors plays a significant role in an open culture system, either increasing or decreasing the lipid content besides nutrient manipulation (Lewin 1962; Tedesco and Duerr 2006) .
After the extraction processes , the resulting microalgal oil is converted into biodiesel through transesterification process. This reaction consists of transforming triglycerides into fatty acid alkyl esters, in the presence of an alcohol, such as ethanol or methanol and a catalyst, such as an alkali or acid with glycerol as a byproduct (Vasudevan and Briggs 2008; Dragone et al. 2010; Khola and Ghazala 2012) .
The algal meal of Aquatic energy’s Scenedesmus strain dry weight comprises 3 % crude fiber, 0.1 % calcium and 39 % protein. The omega-3 fatty acids are obtained as a byproduct during the lipid extraction process by treating the lipids under different temperature processes. The alga yields around 22 % of omega 3, 29 % of PUFAs , 20 % of monounsaturated fat and 27 % of saturated fat. Carbon chains include, but are not limited to, C12–C24 chains in different percentages. Actual lipid profile varied with an increase or decrease in one or more components of the culture system.
4 Management of Contaminants
It is impossible to avoid contamination in open ponds . Bacteria, viruses, fungi, zooplankton, insects, leaves and airborne materials are the common constituents of contaminants . It is important to keep the contamination under acceptable limits, as this should not affect the health of the main culture. In large ponds, the floating contaminants are removed by scooping nets or fixing screens along the water flow.
Close monitoring of pH, dosing the right nutrients at the right time, maintaining the culture at high density and following strict sanitation practices control the contaminants in Scenedesmus culture ponds. Drastic lowering of pH to about 3 by surging CO2 for 2 h is a common practice to control Brachionus infestation (Becker 1994) . In a clay pond, pH above 9 favors the growth of blue green algae (Van der Westhusien and Eloff 1983) and a low pH invite other competing green algae. Availability of nitrogen and phosphate sources in excess may flourish toxic cyanobacteria (Hughes et al. 1958) . Some fungal contaminants such as Phlyctidium scenedesmi , Phlyctidium sp. are identified infecting the Scenedesmus leading to cell death in a short span of time. Fungicides, such as Triton-N (Benderliev et al. 1993) and Funginex are used at different concentrations depending upon the infection rate as a control measure. The infection of Chytridium species is not uncommon in open clay ponds; it is identified by microscopic observation, the parasite harbors between the outer and the inner part of the thallus and produces a residual structure that absorbs the cellular contents of the host. Addition of Mg2+ or K+ at concentrations of 10–2 moles or higher inhibited their growth (Abeliovich and Dikbuck 1977) (Fig. 6).
Contaminants observed in outdoor cultures: a Microcystis sp. (× 450). b Merismopedia sp. (× 450). c Anabaena sp. (× 450). d Oscillatoria sp. (× 450). e Haplosiphon sp. (× 100). f Nodularia sp. (× 450). g Lyngbya sp. (× 100). h Cylindrospermopsis sp. (× 450). i Actinastrum sp. (× 450). j Tetrastrum sp. (× 450). k Coelastrum sp. (× 450). l Umizakia sp. (× 450). m Brachionus sp. (× 450)
Clean contaminant free water is one of the best ways to avoid most of the contaminant sources. Underground bore well water is allowed to pass through a pre-filter followed by ultraviolet exposure to deactivate most of the aquatic contaminants (Whitby and Palmateer 1993) . The UV treated water was filtered before pumping into the pond. The motor and hoses are thoroughly cleaned; chlorine disinfection is done on a weekly basis to ensure cleanliness of the equipment. The poor pond construction and improper mixing may cause many anaerobic zones in the pond affecting productivity.
5 Conclusions
Mass cultivation of microalgae in open raceway pond is economical but limits for a few species only. The effort in successfully growing, harvesting microalgae and extracting for biodiesel and other products have been in practice for decades. Although significant literature exists on microalgal growth and biochemistry, more work needs to be undertaken to understand and potentially manipulate algal lipid metabolism to determine the viability of the various options for large-scale culture. The greatest potential for cost reduction and increased yields most probably lies within open production systems. Knowledge on various contaminants , their sources and controlling techniques are utmost important for a sustainable operation. Apart from bestowing useful products, the large-scale cultivation of microalgae plays an important role in saving the planet earth.
Abbreviations
- Å:
-
Angstrom
- °C:
-
Celsius
- c/ml:
-
Cells per milliliter
- CO2 :
-
Carbon Dioxide
- cm/sec.:
-
Centimeter Per Second
- DAF:
-
Dissolved Air Floatation
- Fig.:
-
Figure
- gm/m2 :
-
Grams per square meter
- K:
-
Potassium
- m:
-
Meter
- M:
-
Million
- Mg:
-
Magnesium
- mM:
-
Millimolar
- µm:
-
Micromolar
- mg/l:
-
Milligram per Liter
- nm:
-
Nanometer
- pH:
-
Decimal logarithm of the reciprocal of the Hydrogen ion Concentration
- ppm:
-
Parts per Million
- PUFA:
-
Poly Unsaturated Fatty Acids.
- UV:
-
Ultra violet
- WHO:
-
World Health Organization
- <:
-
less than
- 10−2 :
-
0.01
- Algal oil:
-
An oil extracted from algae using solvents.
- Algal Powder:
-
Dried and powdered algae.
- Algal slurry:
-
A mixture of algal biomass and water.
- Anaerobic dead zones:
-
Areas with no oxygen.
- Bio fertilizers:
-
A substance that contain living microorganisms used to improve soil fertility.
- Carbon dioxide:
-
A colorless, odorless, incombustible gas.
- Chloroform:
-
An organic compound with the molecular formula CHCl3.
- Circulation:
-
The movement of water and culture in circular direction.
- Clumping:
-
Algal cells stick together as a mass.
- Competitors:
-
Undesired organisms compete for food, light and space.
- Contaminants:
-
Microorganisms infect the algal cells and/or affect the culture quality.
- Culture:
-
Growing a single or group of organisms in a given area.
- Dispensers:
-
Device used to supply CO2 in minimum doses to diffuse in to the liquid.
- Fluorometer:
-
Equipment used to measure lipid content.
- Hexane:
-
A hydrocarbon with chemical formula C6H14, used as solvent in oil extraction.
- Light:
-
Electromagnetic radiation that has a wavelength in the range from 4000 to 7700 Å.
- Mixing:
-
To combine or blend into one.
- Nitrogen:
-
A colorless, odorless and almost inert diatomic gas.
- Nitrate:
-
Polyatomic ion, used as fertilizers.
- Nutrients residue:
-
Quantities of unused nutrients present in the culture.
- Paddle wheel:
-
Blades attached to a central frame and move in circular motion in the pond to push the liquid culture.
- Phosphate:
-
A fertilizer containing phosphorus compounds.
- Photobioreactors:
-
Devices that used to grow micro algae in a controlled environment.
- Photo shading:
-
Shades caused by a high density of algae that prevent light penetration.
- Raceway pond:
-
A pond track resembles the horse raceway track.
- Scale up:
-
Increase proportionally.
- Temperature:
-
A physical property of matter that quantitatively expresses the common notions of hot or cold.
- Tolerance:
-
The maximum capacity to endure.
- Botryococcus braunii :
-
A green microalga widely grown to extract hydrocarbons.
- Chaetoceros sp.:
-
Microalga belonging to diatom group widely used as aquatic feed.
- Chlorella sp.:
-
Single celled, green micro algae grown for high food values.
- Crypthecodinium cohnii :
-
A dinoflagellate alga, with high docosahexaenoic fatty acid content.
- Cylindrotheca sp.:
-
Microalga belonging to the diatom group.
- Chytridium sp.:
-
A fungal parasite infects and kills the microalgae.
- Dunaliella primolecta :
-
An oval shaped green alga grown for beta carotene.
- Isochrysis sp.:
-
Microalgae used in the aquaculture industry.
- Nannochloris sp.:
-
A marine unicellular green alga.
- Nannochloropsis sp.:
-
A green alga with high PUFA content.
- Neochloris oleoabundans :
-
A green alga used for biofuel production.
- Nitzschia sp.:
-
A diatom genus found common in aquatic habitats.
- Phaeodactylum tricornutum :
-
A diatom with high lipid content.
- Phlyctidium scenedesmi :
-
Fungal parasite infects and destroys the mass culture of algae.
- Scenedesmus sp.:
-
Unicellular green alga.
- Skeletonema sp.:
-
A diatom cultured as food for marine shrimp and fish larvae.
- Schizochytrium sp.:
-
Heterotrophic micro alga with high fatty acid content.
- Tetraselmis suceica :
-
A marine green alga, used in aquaculture.
References
Abeliovich A, Dikbuck S (1977) Factors affecting Infection of Scenedesmus obliquus by a Chytridium sp. Sewage Oxidation Ponds. Appl Environ Microbiol 34(6):832–836
Alabi AO, Tampier M, Bibeau E (2009) Microalgae technologies and process for Biofuels/Bio energy production in British Columbia: Current technology, suitability and barriers to implementation. A report submitted to British Columbia innovation Council by Seed science. pp 1–88
Allen MM, Stanier RY (1968) Growth and division of some unicellular blue green algae. J Gen Microbiol 51:199–202
Becker EW (1994) Microalgae. Biotechnology and Microbiology. Cambridge University Press, Cambridge, pp 1–293
Ben-Amotz A (2007) Production of marine unicellular algae of commercial power plant waste: from black coal to green bio-fuel. Presented at the Algae Biomass Summit, San Francisco
Benderliev KM, Pouneva ID, Ivanova NI (1993) Fungicidal effect of Triton-N on Phlyctidium. Biotechnol Tech 7(5):335–338
Benemann JR, Oswald WJ (1996) Systems and economic analysis of microalgae ponds, final report. Pittsburgh Energy Technology Center, Pittsburgh, pp 1–188
Benemann JR, Tillet DM, Weissmann JC (1987) Microalgae biotechnology. Trends Biotechnol 5:47–53
Bligh FH, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Borowitzka MA (2005) Culturing microalgae in open ponds. In: Anderson RA, Phycological Society of America (eds) Algal culturing techniques. Elsevier Academic Press, London, pp 205–218
Boyd CE, Tucker CS (1998) Pond aquaculture water quality management. Kluwer Academic Publishers, The Netherlands, pp 1–700
Bouterfas R, Belkoura M, Dauta A (2002) Light and temperature effects on the growth rate of three fresh water (2pt) algae isolated from a eutrophic lake. Hydrobiologia 489:207–217
Brennan L, Owende P (2010) Biofuels from microalgae-A review of technologies for production, processing, extractions of biofuels and co-products. Renewable Sustainable Energy Reviews 14(2):557–577
Burlew JS (ed) (1953) Algae culture, from laboratory to pilot plant. Carnegic Institution of Washington, Washington, D.C., pp 1–357
Castro GR, Larson BK, Panilaitis B, Kaplan DL (2005) Emulsan quantitation by Nile red quenching fluorescence assay. App Microbiol Biotechnol 67:767–770
Chen P, Min M, Chen Y, Wang L, Li Y, Chen Q, Wang C, Cheng Y, Deng S, Hennessey K, Lin X, Liu Y, Wang Y, Blanca M, Ruan R (2009) Review of the biological and engineering aspects of algae to fuels approach. Int J Agricult Biol Eng 2(4):1–30
Chen SY, Pan LY, Hong MJ, Lee AC (2012) Effect of temperature on the growth of and ammonia uptake by marine microalgae. Bot Stud 53:125–133
Chisti Y (2007) Biodiesel from microalgae. Biotechn Adv 25:294–306
Converti A, Casazza AA, Ortiz EY, Perego PA, Borghi MD (2009) Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. J Chem Eng Process 48:1146–1151
Darzins A, Pienkos P, Edye L (2010) Current status and potential for algal biofuels production. A report to IEA Bioenergy task T39, pp 1–131
Deng X, Li Y, Fel X (2009) Microalgae: a promising feedstock for biodiesel. Afr J Microbiol Res 3(13):1008–1014
Dodd JC (1986) Elements of pond design and construction. In: Richmond A (ed) CRC handbook of Microalgal mass culture. CRC Press, Boca Raton, pp 265–283
Dohler G (1998) Effect of ultraviolet radiation on pigmentation and nitrogen metabolism of Antarctic phytoplankton and ice algae. J Plant Physiol 153(5–6):603–609
Douglas A, Blanca AL (2012) Achieving a green solution: limitations and focus points for sustainable Algal fuels. Energies 5:1613–1647
Dragone D, Fernandes B, Vicente AA, Tsixeira JA (2010) Third generation biofuels from micro algae. In: Mentez-Vilas A (ed) Current research, technology and education topics in applied microbiology and microbial biotechnology. Formatex Microbiol Ser 2:1355–1366
Dunahay TG, Jarvis E, Dais S, Roessler PG (1996) Manipulation of microalgal lipid production using genetic engineering. App Biochem Biotechnol 57–58(1):223–231
Fajardo RM, Cerdán LE, Medina AR, Fernández FGA, Moreno PAG, Grima EM (2007) Lipid extraction from the microalga Phaeodactylum tricornutum. Eur J Lipid Sci Technol 109:120–126
Gouveia L (2011) Microalgae as a Feed stock for Biofuels. Springer briefs in Microbiology, New York, pp 1–68
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Graham LE, Wilcox LW (2000) Algae. Prentice-Hall, Inc. Upper Saddle River, pp 1–640
Guschina IA, Harwood JL (2006) Lipids and Lipid metabolism in Eukaryotic Algae. Process in Lipid Res 45:160–186
Hanagata N, Takeuchi T, Fukuju Y, Barnes DJ, Karube I (1992) Tolerance of microalgae to high CO2 and high temperature. Phytochemistry 31(10):3345–3348
Hancke K, Torunn BH, Olsen LM, Johnsen G (2008) Temperature effects on microalgal photosynthesis-light responses measures by O2 production, pulse-amplitude-modulated fluorescence, and 14C assimilation. J Phycol 44:501–514
Helm MM, Bourne N, Lovatelli A (2004) Hatchery culture of Bivalves: a practical manual, FAO Fisheries technical paper 471, Italy, pp 1–200
Henrikson R (1989) Earth food Spirulina. ISBN: 0-9623111-0-3. Ronore enterprises, Hawaii, pp 1–186
Hoff FH, Snell TW (2008) Plankton culture manual. Florida Aqua farms, Inc. Date City, Florida, pp 1–186
Hossain ABMS, Salleh A, Boyce ANM, Chowdhury P, Naqiuddin M (2008) Biodiesel Fuel Production from Algae as Renewable Energy. Am J Biochem Biotechnol 4(3):250–254
Hughes EO, Gorham PR, Zehnder A (1958) Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol 4:225–36
James SC, Boriah V (2010) Modeling Algae growth in an open-channel raceway. J Comput Biol 17(7):895–906
Kay RA (1991) Microalgae as food and supplement. Crit Rev Food Sci Nutr 30(6):555–73
Kaushik BD (1998) Use of Cyanobacterial Biofertilizers in rice cultivation: a technology improvement. In: Subramanian G, Kaushik B, Venkataraman GS (eds) Cyanobacterial Biotechnology. Oxford & IBH Publishing Co. New Delhi, pp 211–222
Khola G, Ghazala B (2012) Biodiesel production from algae. Pak J Botany 44(1):379–381
Kodama M, Ikemoto H, Miyachi S (1993) A new species of highly CO2-tolerant fast growing marine microalga suitable for high-density culture. J Mar Biotechnol 1:21–25
Lee YK (2001) Microalgal mass culture systems and methods: their limitations and potentials. J App Phycol 13:307–315
Lee JY, Yoo C, Jun SY, Ahn CY, HM Oh (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:875–877
Lewin RA (ed) (1962) Physiology and Biochemistry of Algae. Academic Press, NY, pp 1–929
Li Y, Horsman M, Wu N, Lan CQ D-CN (2008) Biofuels from microalgae. Biotechnol Prog 24(4):815–20
Lu C, Vonshak A (1999) Photoinhibition in outdoor Spirulina platensis cultures assessed by polyphasic chlorophyll fluorescence transcients. J App Phycol 11:355–359
Maeda K, Owada M, Kimura N, Omata K, Karube I (1995) CO2 fixation from the flue gas of coal fired thermal power plant by microalgae. Energy Convers Manag 36:717–720
Maraskolhe VN, Warghat AR, Charan G, Nandhar PB (2012) Carbon sequestration potential of Scenedesmus sp. (Microalgae) under the fresh water ecosystem. Afr J Agricult Res 7(18):2818–2823
Matsumoto H, Shioji N, Hamasaki A, Ikuta Y, Fukuda Y, Sato M, Endo N, Tsukamoto T (1995) Carbon dioxide fixation by microalgae photosynthesis using actual flue gas discharged from a boiler. App Biochem Biotech 51–52(1):681–692
Mayzaud P, Chanut JP, Ackman RG (1989) Seasonal changes of the biochemical composition of marine particulate matter with special reference to fatty acids and sterols. Mar Ecolo Prog Ser 56:189–204
Metzger P, Largeau C (2005) Botryococcus braunii: a rich source of hydrocarbons and related other lipids. App Microbiol Biotechnol 66(5):486–496
Miura Y, Yamada W, Hirata K, Miyamoto K, Kiyohara M (1993) Stimulation of hydrogen production in algal cells grown under high CO2 concentration and low temperature. App Biochem Biotech 39–40:753–761
Miyairi S (1995) CO2 assimilation in a thermophilic cyanobacterium. Energy Convers Manag 36(6–9):763–766
Molina Grima GE, Fernandez J, Acien FG, Chisti Y (2001) Tubular photobioreactor design for algal culture. J Biotech 92:113–131
Natrah FMI, Yusoff FM, Shariff M, Abas F, Mariana NS (2007) Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J App Phycol 19(6):711–718
Nakano Y, Miyatake K, Okuno H, Hamazaki K, Takenaka S, Honami N, Kiyota M, Aiga I, Kondo J (1996) Growth of photosynthetic algae Euglena in high CO2 conditions and its photosynthetic characteristics. Acta Hortic 440:49–54
Nagase H, Eguchi K, Yoshihara K, Hirata K, Miyamoto K (1998) Improvement of microalgal NOx removal in bubble column and air lift reactors. J Ferment Bioeng 86(4):421–423
Olaizola M (2003) Commercial development of microalgal biotechnology: from test tube to the market place. Biomol Eng 20:459–466
Oliveira MAS, Monteiro MP, Robbs PG, Leite SG (1999) Growth and chemical composition of Spirulina maxima and Spirulina platensis biomass at different temperatures. Aquacult Int 7:261–275
Ono E, Cuello JL (2003) Selection of optimal microalgal species for CO2 sequestration. Second annual conference on carbon sequestration, developing and validating the technology base to reduce carbon intensity. VA, pp 1–7
Oswald WJ (1988) Micro-algae and wastewater treatment. In: Borowitzka MA, Borowitzka LJ (eds) Micro-algal biotechnology. Cambridge University press, Cambridge, pp 305–328
Park KH, Lee GC (2001) Effectiveness of flashing light for increasing photosynthetic efficiency of microalgal cultures over a critical density. Biotechnol Bioprocess Eng 6:189–193
Pauline S, Joannis-Cassan C, Duran E, Isambert A (2006) Commercial applications of microalgae. J Biosci Bioeng 101(2):87–96
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. App Microbiol Biotech 57(3):287–293
Pohl P, Zurheide F (1979) Fatty acids and lipids of marine algae and the control of their biosynthesis by environmental factors. In: Hoppe HA, Levring T, Tanaka Y (eds) Marine algae in pharmaceutical science. Walter de Gruyter, Berlin, pp 473–523
Quinn PJ, Williams WP (1983) The structural role of lipids in photosynthetic membranes. Biochim Biophys Acta 737:223–266
Richmond A (1992) Open systems for the mass production of photoautotrophic microalgae outdoors: Physiological principles. J App Phycol 4:281–286
Richardson JW, Johnson MD, Outlaw JL (2012) Economic comparison of open pond raceways to photo bio-reactors for profitable production of algae for transportation fuels in the Southwest. Algal Res 1:93–100
Rijssel VM, Gieskes WWC (2002) Temperature, light, and the dimethylsulfoniopropionate (DMSP) content of Emiliana huxleyi (Prymnesiophyceae). J Sea Res 48(1):17–27
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici NR (2008) Microalgae for Oil: Strain selection, Induction of Lipid Synthesis and Outdoor Mass Cultivation in a low cost Photobioreactor. Biotechnol Bioeng 102(1):100–112
Ryckebouch E, Muylaert K, Foubert I (2011) Optimization of analytical procedure for extraction of lipids from microalgae. J Am Oil Chem Soc 89(2):189–198
Sawayama S, Inoue S, Dote Y, Yokoyama SY (1995) CO2 fixation and oil production through microalga. Energy Convers Manag 36:729–731
Schenk PM, Thomas HCR, Stephens E, Marx UC, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioene Res 1:20–43
Seckbach J, Gross H, Nathan MB (1971) Growth and Photosynthesis of Cyanidium caldarium cultured under pure CO2. Isr J Botany 20:84–90
Sforza E, Simionato D, Giacometti GM, Bertucco A, Morosinotto T (2012) Adjusted light and dark cycles can optimize photosynthetic efficiency in Algae growing in Photobioreactors. PLoS ONE 7(6): e38975. doi:10.1371/journal.pone.0038975:1–10
Sheehan J, Dunahay T, Benemann JR, Roessler PG, Weismann JC (1998) A look back at the U.S. department of energy’s aquatic species program-biodiesel from Algae. A report to National Renewable Energy Laboratory. DOE, Denver, CO, pp 1–328
Solovchenko AE, Goldberg IK, Cohen SD, Cohen Z, Merzlyak MN (2008) Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J App Phycol 20:245–251
Stein J (ed) (1973) Handbook of phycological methods. Culture methods and growth measurements. Cambridge University Press, pp 1–448
Tedesco MA, Duerr EO (2006) Light, temperature and nitrogen starvation effects on the total lipid and fatty acid content and composition of Spirulina platensis UTEX 1928. J App Phycol 1(3):109–201
Thomas WH, Krauss RW (1955) Nitrogen metabolism in Scenedesmus as affected by environmental changes. Plant Physiol 30(2):113–122
Tomaselli L, Geovannetti L, Sacchi A, Bocci F (1988) Effect of temperature on growth and biochemical composition in Spirulina platensis strain M 2. In: Stadler T, Mollion J, Verdus M-C, Karamanos Y, Morvan H, Christien D (eds) Algal Biotechnology. Elsevier Applied Science, London, pp 305–314
Ugwu CU, Aoyaki H (2012) Microalgal culture systems: an insight in to their designs, operation and applications. Biotechnology 11(3):127–132
Van der Westhusian AJ, Eloff JN (1983) Effect of Culture Age and pH of Culture medium on the growth and toxicity of the Blue-Green Alga Microcystis aeruginosa. Z Pflanzenphysiol 110(2):157–163
Vasudevan PT, Briggs M (2008) Biodiesel production, current state of the art and challenges. J Industrial Microbiol Biotech 35:421–430
Vonshak A (ed) (1997) Spirulina platensis (Arthrospira): physiology, cell-biology and biotechnology, 1st edn. Taylor and Francis Inc, USA, pp 1–252
Watanabe MM, Kawachi M, Hiroki M, Kasai F (2000) NIES-Collection list of strains, Microalgae and Protozoa, microbial culture collections, 6th edn 2000. National Institute for Environmental Studies, Tsukuba, pp 1–159
Wheeler PA (1983) Phytoplankton nitrogen metabolism. In: Carpenter EJ, Capone DG (eds) Nitrogen in the marine environment. Academic Press Inc., London, pp 309–334
Whitby GE, Palmateer G (1993) The Effect of UV Transmission, suspended solids and Photoreactivation on microorganisms in waste water treated with UV light. Water Sci Technol 27(3–4):379–386
Xu H, Miao X, Wu Q (2004) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Yin HW, Yin Y, Xin L, Ying HH, Feng ZS (2012) Biomass production of a Scenedesmus sp., under phosphorous starvation cultivation condition. Biores Technol 112:193–198
Yoshihara K, Nagase H, Eguchi K, Hirata K, Miyamoto K (1996) Biological elimination of nitric oxide and carbon dioxide from flue gas by marine microalga NOA-113 cultivation in a long tubular photobioreactor. J Fermentation Bioeng 82(4):351–354
Yun YS, Lee SB, Park JM et al (1997) Carbon dioxide fixation by algal cultivation using waste water nutrients. J Chem Technol Biotechnol 69:451–455
Acknowledgements
I would like to thank all my colleagues at Aquatic Energy LLC for encouraging me continuously with enthusiasm in developing a successful and sustainable microalgal open raceway pond cultivation system for biofuels, food and pharmaceutical products.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Ravikumar, R. (2014). Micro Algae in Open Raceways. In: Bajpai, R., Prokop, A., Zappi, M. (eds) Algal Biorefineries. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7494-0_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-7494-0_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7493-3
Online ISBN: 978-94-007-7494-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)