Abstract
H+ ions are remarkably efficient modulators of neuronal excitability. This renders brain functions highly sensitive to small changes in pH which are generated “extrinsically” via mechanisms that regulate the acid–base status of the whole organism; and “intrinsically”, by activity-induced transmembrane fluxes and de novo generation of acid–base equivalents. The effects of pH changes on neuronal excitability are mediated by diverse, largely synergistically-acting mechanisms operating at the level of voltage- and ligand-gated ion channels and gap junctions. In general, alkaline shifts induce an increase in excitability which is often intense enough to trigger epileptiform activity, while acidosis has the opposite effect. Brain pH changes show a wide variability in their spatiotemporal properties, ranging from long-lasting global shifts to fast and highly localized transients that take place in subcellular microdomains. Thirteen catalytically-active mammalian carbonic anhydrase isoforms have been identified, whereof 11 are expressed in the brain. Distinct CA isoforms which have their catalytic sites within brain cells and the interstitial fluid exert a remarkably strong influence on the dynamics of pH shifts and, consequently, on neuronal functions. In this review, we will discuss the various roles of H+ as an intra- and extracellular signaling factor in the brain, focusing on the effects mediated by CAs. Special attention is paid on the developmental expression patterns and actions of the neuronal isoform, CA VII. Studies on the various functions of CAs will shed light on fundamental mechanisms underlying neuronal development, signaling and plasticity; on pathophysiological mechanisms associated with epilepsy and related diseases; and on the modes of action of CA inhibitors used as CNS-targeting drugs.
Susan C. Frost and Robert McKenna (eds.). Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
pH exerts a strong modulatory effect on the central nervous system (CNS) function and excitability. Changes in intracellular or extracellular pH (pHi and pHo, respectively) of 0.5 units or less are often sufficient to trigger or suppress paroxysmal activity and, accordingly, much smaller changes are needed for subtle modulation of neuronal excitability. The physiologically relevant pH range (pH 6.5–8.0) corresponds to a very low free H+ concentration, from 10 to 300 nM. An interesting aspect is that this applies to both the intra- and extracellular compartments. Protons are thus eminently suited to affect H+-sensitive targets both within and outside brain cells. However, studying the physiological and pathophysiological bases of H+-modulation of neuronal functions is not a trivial task, because global and local pH transients are generated by multiple mechanisms operating at various levels of biological organization, from the whole organism to cellular and subcellular microdomains.
At the whole-organism level, the key elements in pH regulation are the lungs which control the partial pressure of CO2 (PCO2) in the blood, and the kidneys which are responsible for the net regulation of other important acid–base species, especially HCO3 − and NH4 +. With the major exception of chemosensitive neurons controlling breathing [1], the excitability of most central neurons and neuronal networks is enhanced by an alkalosis and suppressed by an acidosis. Exogenously-induced respiratory acidosis has a profound suppressing action on neuronal excitability and on seizures [2–5]. Respiratory alkalosis generated by hyperventilation is a standard technique used in the clinic for the precipitation of petit mal-type seizures [6]. Hyperventilation is also involved in the generation of febrile seizures in animal models [4] and most likely in children as well [7]. Metabolic alkalosis associated with renal dysfunction such as seen in the EAST syndrome is known to cause epileptiform activity [8].
The brain is protected by the blood–brain barrier (BBB) which is endowed by acid–base transporter molecules [9] and, as a diffusion barrier, prevents charged acid–base species from having direct access to brain interstitial fluid. Recent work has shown that in addition to this protective role, acid extrusion triggered by birth asphyxia across the BBB can lead to a brain-confined metabolic alkalosis and to consequent seizures [10, 11].
At the cellular level, pHi regulation is based on plasmalemmal transporters of neurons and glia [12, 13]. A fascinating aspect of local H+-signaling within the brain is that fast, robust and often highly localized pH shifts are evoked by electrical activity and by synaptic transmission [13–15]. These intrinsic shifts in pHi and pHo are largely generated by channel or transporter-mediated transmembrane fluxes of acid–base equivalents, and by accumulation of acid end products of energy metabolism such as CO2 and lactate. In the former case, the transmembrane shifts of acid–base species generate pH changes of opposite direction within and outside neurons, while metabolic acidosis implies a fall of pH in both compartments. Distinct neuronal populations show a large heterogeneity in transporter and channel localization and expression [16], and intrinsic pH shifts are therefore likely to generate spatially restricted extra- and intracellular pH-microdomains.
By definition, the H+-sensitive targets involved in pH-dependent modulation of neuronal activity consist of charged groups in proteins which are capable of binding and releasing H+ ions in the physiologically and pathophysiologically relevant pH range. Such interactions affect the conformation and functional properties of a wide variety of membrane proteins involved in neuronal signaling, including voltage gated ion-channels [17, 18], GABAA receptors (GABAARs) [19, 20], N-methyl-D-aspartate receptors (NMDAR) [21, 22], gap junctions [23] and pH-sensing cation channels such as the acid-sensing ion channels [24] and TWIK-related acid-sensitive K+ channels [25]. The pH sensitivity of ion channels and other key proteins that control neuronal excitability does not reflect a property common to all kinds of proteins. Rather, the specific, functionally synergistic patterns of “tuning” of the pKa values of molecules underlying the pH-modulation of neuronal signalling suggests an evolutionary origin for the diverse but largely synergistic roles of H+ as an intercellular and intracellular signalling agent in the brain.
Carbonic anhydrases (CAs) are a family of molecules with a key role in the control of pH at level of the whole organism (e.g. respiratory, energy-metabolic and renal functions), in the BBB, in neurons and glia, and in the interstitial fluid in the brain. For the physiologically ubiquitous CO2/HCO3 − buffering to act in a fast manner, the (de)hydration of CO2 must be catalyzed by CA [26]. The 13 catalytically active CA isoforms identified so far in mammals differ in their tissue distribution, subcellular localization as well as in their enzymatic activity [27] providing a versatile molecular machinery for the modulation of pH. The acid–base equivalents that serve as substrates in the CO2 dehydration-hydration reaction are also engaged in many carrier- and channel-mediated ion movements. In such processes, CA activity is in a key position to modulate transmembrane solute fluxes and their influence on local pH.
Recent findings further suggest that CAs, even if catalytically inactive, can act as ‘proton collecting antennas’ thereby increasing net transmembrane proton flux and suppressing the formation of H+ microdomains [28]. CAs can also affect neuronal function in a manner not dependent on catalytic activity as shown in studies on mice devoid of isoform VIII [29]. Together with isoforms X and XI, isoform VIII belongs to the carbonic anhydrase related proteins (CARPs) that lack catalytic activity [27]. Mice with spontaneous mutation Car8 show changes in e.g. the morphology and function of excitatory synapses in the cerebellum [30, 31].
Currently there are no pharmacological tools available that could be used for isoform-specific inhibition of CAs (see also Chap. 15 in this book). Hence, studies using genetic disruption of distinct CAs have provided much insight into the functional and spatial roles of specific isoforms. As will be discussed, mice devoid of the cytosolic CA II and VII and of the membrane attached isoforms IV and XIV as well as the double knock-outs of CA IV/XIV and CA II/VII have been used in studies focusing on the CA-dependent modulation of neuronal signaling [32–34].
The aim of the present chapter is to provide a general overview of the mechanisms and consequences of pH-mediated signalling in the brain, with an emphasis on the role of various CA isoforms. Despite the obvious, vast potential for elucidating novel physiological and pathophysiological mechanisms involved in of fundamental brain functions such as synaptic transmission and control of neuronal excitability, relatively little work has been done in this field of research. We hope that this review will act as a source of inspiration for further work on the diverse pH-sensitive and CA-dependent mechanisms that operate at the molecular, cellular and neuronal network level in the brain.
2 Generation and Maintenance of the Plasmalemmal pH Gradient
Before discussing the CA-dependent modulation of neuronal excitability in more detail, some basic aspects of pH homeostasis need to be addressed.
Passive equilibration of H+ across the plasma membrane of a cell with a membrane potential at −60 mV and a pHo of 7.3 would drive intracellular pH close to 6.3. However, neuronal pHi (typically around 7.1) is only slightly more acidic than pHo, which implies active regulation of pHi by membrane-located acid–base transporters. Provided that the hydration-dehydration reaction of CO2 and the transmembrane distribution of CO2 are at equilibrium, the transmembrane HCO3 − distribution is set by the pH gradient:
Under these conditions, the equilibrium potential of protons (EH) and bicarbonate (EHCO3) are equal, with a value of −12 mV set by the pHo and pHi given above [35]. The energy required for maintaining the electrochemical gradient (in our example of about −50 mV) is spent on combating intracellular acid loading that is generated by three fundamental mechanisms:
-
(i).
Net transmembrane influx of acid equivalents by transporters working as “acid loaders”, such as the Ca2+/H+ ATPase and the Cl−/HCO3 − exchanger.
-
(ii).
All conductive pathways which are permeable for charged acid–base species. The concentration of H+ ions is very low, and directly-measurable proton conductances have not been described in mammalian neurons [36]. However, a significant acid-loading HCO3 − conductance is provided by GABAARs and by glycine receptors.
-
(iii).
Cellular metabolic processes that lead to de novo production of acid. Here, one should note, however, that weak organic acids such as lactate traverse the membrane in their neutral, H+-bound form, and thus their generation by energy metabolism will not contribute to the long-term cellular acid–base budget.
Perturbations of pHi are not always poised in the acid direction; alkaline loads are also known to take place following e.g. depolarization of the plasma membrane (especially in astrocytes [37]) or sudden removal of an acid load. When a cell is subject to an acid or alkaline load, the rate of pHi change is proportional to the difference between the acid-extrusion and acid-loading rates, and inversely proportional to the total intracellular buffering capacity. A number of cells, including neurons, are equipped with several acid–base transporters which act as acid extruders or loaders. At first sight, such “push-pull” mechanisms look wasteful in terms of energy usage, but their concerted action brings about a much more stable set-point for pHi under physiological conditions where the cell is subject to rapidly alternating acid and alkaline loads [12]. Moreover, a differential distribution of transporters in cells with complex geometry, such as neurons, is likely to bring about pH microdomains within the cell, thus enhancing the spatial precision of H+ ions in intracellular signaling based on pH-sensitive proteins (see Introduction).
In the mammalian CNS, the predominant transporters involved in pHi regulation are the secondary-active transporters that belong to the solute carrier gene families Slc4 and Slc9 [13, 16]. Some studies have reported acid extrusion in the nominal absence of Na+ and CO2/HCO3 − suggesting that a putative H+ pump contributes to neuronal and glial pHi regulation [38–40]. The role of another primary active transporter, the Ca2+/H+-ATPase, has been described in much more detail. In neurons Ca2+/H+-ATPase, a major regulator in intracellular free calcium, works as an acid loader [41–43].
3 pH Buffering and CA Isoforms in Brain Tissue
3.1 pH Buffering Within and Outside Neurons
While transporters are needed for the active extrusion of acid–base equivalents, H+ buffers determine the ability of the cytosol to suppress pHi transients without any contribution by active transport. The total intracellular, cytoplasmic buffering capacity consists of a CO2/HCO3 − -dependent (β CO2) and a non-bicarbonate buffering capacity (β i). The latter mainly arises from phosphates and the imidazole groups of proteins. These buffers cannot cross the plasma membrane and therefore they form a closed buffer system within the cell [12, 44]. The extracellular fluid is practically devoid of non-bicarbonate buffers and thus relies on CO2/HCO3 − -dependent buffering.
In an ideal buffer which is open with respect to CO2, β CO2 is given by β CO2 = 2.3[HCO3 −] [12]. However, attaining this value would require instantaneous equilibration of the system but, in reality, this is not achieved and β CO2 remains much lower than the theoretical maximum in response to fast acid/base perturbations both within and outside cells. For instance, in the hippocampal slice, stimulation-induced changes in pHo indicated an extracellular buffering power that was less than 30 % of the theoretical maximum [45]. Interestingly, despite the presence of extracellular CA (CAo), the amount of CAo activity can also be rate-limiting for effective buffering of pHo changes. Addition of CA II to the perfusion medium has been shown to curtail activity-induced extracellular alkalosis in brain slices [46, 47] and in vivo [48].
3.2 CA Isoforms with an Extracellular Catalytic Site
Since pHo buffering is determined by the CO2/HCO3 − system, CAo is in a key position to govern the kinetics of activity-generated pHo transients. The membrane-bound CA isoforms IV and XIV which have their catalytic site located in the extracellular space are largely responsible for the CAo activity detected in the rodent hippocampus [32, 49]. These isoforms differ in the way they are attached to the membrane and in their cell type-specific expression. CA IV is attached to plasma membrane by a glycosyl-phosphatidyl-inositol anchor [50] of both neurons and glia [51]. The more recently identified CA XIV has a membrane-spanning α-helix and a short intracellular C-terminus [52], and shows neuron-specific expression within the brain [53]. The possible contribution of the other membrane-attached isoforms, CA IX, XII and XV, in the CNS extracellular buffering is unclear as the regional localization of these isoforms has not been determined. The basal expression level of CA XII and IX in the CNS is low, but both isoforms are expressed at higher levels in malignant tumor cells [54, 55]. Their presence can be used as biomarkers for certain tumors with possible further diagnostic implications in the prognosis of malignization [56] (see also Chaps. 10, 11, 12, and 13 in this Book). Pathophysiological conditions such as seizures [57] and asphyxia [58] increase the expression of CA IV and CA XII also in brain cells with no apparent previous pathologies. This is expected to suppress the activity-dependent postsynaptic rise in pHo and consequent NMDAR activation during synchronous neuronal activity, thus acting as a potential neuroprotective mechanism.
A wealth of data has shown that the developmental expression patterns of ion transporters, especially of cation-chloride cotransporters, have a major influence on the fundamental properties of neuronal signalling during brain ontogeny [59]. However, with respect to CAs, data of this kind is largely missing and there are no published reports on developmental changes of CAo activity. Developmental expression patterns of CAo isoforms might shape excitatory and inhibitory transmission in concert with the expression of glutamate as well as GABAAR subunits and the Cl− transporters. For example, the expression of functional NMDARs precedes that of AMPA receptors (AMPARs) during postnatal maturation of rodent cortical structures [60]. Developmentally coinciding upregulation of functional AMPARs and CAo activity would provide control over the NMDAR-modulating pHo transients [21, 22] generated by excitatory transmission.
The contribution of the Cl−-HCO3 − transporting anion exchangers (AEs) in neuronal Cl− regulation has so far gained surprisingly little attention. In embryonic motoneurons the Cl−-HCO3 − exchanger isoform 3 (AE3) acts as an important Cl− uptake mechanism [61]. Since CAo activity has been shown to enhance AE3 mediated Cl−-HCO3 − exchange [49], developmental changes in CAo activity might have a significant influence on GABAergic synaptic signalling.
3.3 CA Isoforms with a Cytoplasmic Catalytic Site
For long the intracellular CA (CAi) activity in the CNS was thought to be mainly restricted to glial cells, endothelium of the capillaries and choroid plexus epithelial cells [62]. Now there is both functional and molecular biological evidence for the presence of intraneuronal CA in the mammalian CNS [63–70]. The first observations on the presence of cytosolic CA activity in CNS neurons, and that this activity promotes GABAAR-mediated net HCO3 − efflux in mammalian CNS neurons were made by us two decades ago [64].
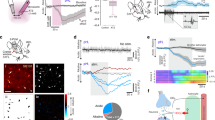
Due to the lack of isoform-specificity of available cytosolic CA inhibitors, previous data on CA VII expression in rat pyramidal neurons [70] did not exclude the possible presence of other neuronal CA isoforms. Using a novel CA VII KO mouse together with a CA II KO and a CA II/VII double KO mouse we demonstrated that there is a sequential expression of two different isoforms in mouse pyramidal neurons (Fig. 14.1a, b) [34]. CA VII fully accounts for the up-regulation of neuronal CA activity detected at around P10 and is the only cytosolic isoform during the time window P10-18. After P18 pyramidal neurons start to express CA II in parallel with CA VII. A notable difference in the cellular expression patterns of the two isoforms was that CA VII is mainly found in the CNS where it localizes only to neurons. The ubiquitous CA II is present in a wide variety of tissues [71], and within the brain parenchyma it is expressed in both glia and neurons.
Cytosolic CA activity in developing mouse CA1 pyramidal neurons is based on sequential expression of isoforms VII and II. (a) Original pHi traces from WT (P5 and P14) and CA VII KO neurons on postnatal day 14 (P14). Replacing the CO2/HCO3 − buffer in the perfusion solution by HEPES (upper horizontal bars) evoked an acetazolamide-sensitive intracellular alkalinization only in the P14 WT neurons, thereby indicating the presence of CAi activity (acetazolamide, AZ, 100 μM). (b) Developmental expression of the CA VII and CA II isoforms. Summary of the results obtained using the cytosolic CA activity detection method shown in a and quantified as the percentage of the cells showing cytosolic CA activity. (c) At P12−16, CA VII is solely responsible for promoting GABAAR-mediated Cl− accumulation and consequent depolarizing GABA responses. Intense stimulation of the interneuronal network evoked a biphasic GABAergic response in CA1 pyramidal neurons in WT slices that was abolished with picrotoxin (PiTX, 80 μM). The GABAA-receptor mediated depolarization was large enough to trigger action potentials in WT but not in the CA VII KO neurons. Figure modified from ref. [34]
A possible explanation for the apparently redundant presence of two cytosolic CAs is that the two isoforms show differences in their biochemical functions other than (de)hydration of CO2, such as esterase/phosphatase activity [72] or as oxygen radical scavengers [73]. The importance of the latter finding is underscored by the fact that a large developmental increase in cerebral oxidative energy metabolism [74] coincides with the upregulation of neuronal CA VII expression. The existing data do not exclude the possibility that CA II and CA VII are located in distinct subcellular microdomains. Formation of isoform-specific metabolons with different acid–base transporters [75–77] would further promote the generation of developmentally and spatially distinct pHi microdomains.
There are very few reports on intracellular CA expression changes after pathophysiological insults [57, 58] and, to our knowledge, none on CA activity changes. The co-operative functions of K-Cl cotransporter KCC2 and CAi in the generation of HCO3 − -dependent depolarizing GABA responses [78] (see also Sect. 5) raise the intriguing question whether changes in CAi expression might take place in parallel to those of KCCs [59]. Parallel down-regulation of KCC2 and CAi would lead to the suppression of excitatory GABAergic response (see also Fig. 14.1c) and, consequently, to suppression of seizures.
Here it is worth recalling that membrane-permeant CA blockers such as acetazolamide have a long history as antiepileptic compounds. The molecular targets and mechanisms of action of these broad-spectrum CA inhibitors at the neuronal network level are still poorly understood [27, 79].
4 Mechanisms and Consequences of Activity-induced H+ Transients in Neurons and Neuronal Networks
Most experiments on activity-induced neuronal pH transients have been conducted using ion-selective electrodes. In mammalian brain tissue practically all data are on pHo because of the obvious difficulties of impaling the neurons and maintaining them functionally intact. When evaluating these data, it is important to note that electrode recordings of pHo are bound to reflect a spatiotemporal average because of the local damage caused by the tip and the relatively slow response time (at best in the range 0.1–1 s) of the electrodes [80].
Fluorescent pH indicators are optimally suited for intracellular recordings, and in this case, the signal-to-noise ratio will largely dictate the data sampling rate and set the temporal resolution. Interestingly, it seems to be possible to obtain useful recordings of pHo transients occurring in the extremely narrow (around 20 nm) extracellular space in brain tissue using pH indicators. Such recordings have also shown that activity-induced pHo changes are, indeed, fast enough to be able to modulate on-going synaptic transmission and neuronal activity [46].
Robust activity-induced (i.e., intrinsic) pH changes have been documented in a large number of studies on the CNS indicating that local pH shifts are a mandatory consequence of both electrical and synaptic signaling [13–15]. These pH transients are of different duration and magnitude, and they arise in the intracellular compartments of both neurons and glia as well as in the interstitial fluid surrounding them. It is evident that, depending on the cellular cytoarchitecture and synaptic connectivity of a given neuronal preparation/recording site and on the stimulation paradigm, the measured pH transients originate from distinct molecular and cellular sources. The activation patterns of multisynaptic circuits with both excitatory and inhibitory connections and the fractional volumes of neurons, glia and the extracellular space are among the key factors that shape local pH shifts. Thus, we have focused on studies made in the rodent hippocampus. Comprehensive overviews on activity-dependent pH-modulation in invertebrates, in other cell types and areas of the CNS are available [13, 81].
Intraneuronal pH measurements in rat hippocampal neurons have shown that both excitatory (glutamatergic) and inhibitory (GABAergic) signaling result in a fall of pHi, but the underlying molecular generation mechanisms are completely different. As will be explained, activation of the anion-selective GABAARs leads to a net efflux of HCO3 − and influx of CO2 which fully explains the GABA-induced intracellular acidosis as depicted in Fig. 14.2b [64, 82]. Because glutamate-gated postsynaptic channels are cation-selective, one might assume that conductive H+ would lead to an intracellular acid load. However, this possibility has been excluded [83]. A key player in this context is the Ca2+/H+-ATPase which is activated by neuronal depolarization leading to an increase in intracellular Ca2+, regardless of whether this is caused by synaptic excitation; application of glutamate agonists; or antidromic stimulation of neurons (Fig. 14.2a) in the continuous presence of blockers of synaptic transmission [83–85]. As might be expected, metabolic acid production is likely to contribute to excitation-linked pH shifts [86].
Extracellular carbonic anhydrase activity (CAo) modulates activity-dependent alkaline shifts. (a) The buffering provided by the CAo-catalyzed CO2/HCO3 system attenuates the rapid alkaline pHo transient generated by neuronal excitation-induced Ca2+ influx (upper panel). Inhibition of CAo compromises CO2/HCO3 buffering by suppressing the rate of CO2 hydration and, hence, boosts the alkalosis. (b) In contrast, CAo activity is needed for the generation of GABAA-receptor mediated alkalosis which is driven by the transmembrane CO2/HCO3 shuttle (upper panel). CAo inhibitors prevent the fast replenishment of CO2 in the extracellular space and largely block the alkalosis (for details, see text). The pHo responses evoked by action potentials (lower panel in a) and stimulation of GABAergic interneurons (lower panel in b) were recorded using ion-sensitive microelectrodes. The small acid shift in the baseline pHo after CAo inhibition is likely due to a decrease in extracellular buffering capacity in face of continuous cellular acid extrusion. The illustrations in the lower panels are modified from ref. [90] (a) and ref. [43] (b)
4.1 CAo-Inhibitors as Tools in Mechanistic Analyses of pHo Transients
In the presence of CA, the CO2/HCO3 − -system efficiently attenuates fast pH changes evoked by H+ fluxes [26] (Fig. 14.2a). On the other hand, if the acid/base disturbance arises from a change in CO2 and HCO3 −, CA activity is instrumental for the generation of a rapid pH shift (Fig. 14.2b). A prime example of the latter case is the GABAAR-mediated CO2/HCO3 − shuttle, which leads to a fall in the CO2 concentration on the extracellular surface of the plasma membrane that is proportionally much higher than the increase in the local HCO3 − concentration. Accordingly, inhibition of CAo activity abolished the GABAAR-mediated alkaline pH transient on the surface of crayfish muscle fibres [87].
Taking advantage of the opposing effects of CAo-activity on the magnitude of pH transients of distinct origin, inhibitors of CAos can be used as diagnostic tools in the analysis of mechanistically heterogeneous pHo shifts triggered by simultaneous excitatory and inhibitory transmission, as will be discussed below. Selective CAo inhibition can be achieved by the poorly-membrane permeable blocker benzolamide or by membrane-impermeant dextrane-bound sulfonamide derivatives [88].
In the extracellular space, activity-induced transmembrane fluxes of acid–base species produce pHo changes which are qualitatively opposite to those seen in pHi recordings. This is because the same molecular mechanisms that are involved in the intraneuronal acidosis contribute to the increase in pHo [87–90]. Thus, a pronounced alkalinization is generally the immediate pHo response to intense neuronal activity (induced by agonist application or electrical stimulation). Using CAo-inhibitors, it was possible to dissect the relative contributions of the H+ shifts caused by glutamatergic transmission and the HCO3 − shifts caused by GABAAR-mediated transmission to heterosynaptic (excitatory and inhibitory) stimulation-evoked pHo responses in hippocampal slices [91]. The results showed that with Schaffer collateral stimulation delivered at low frequencies (5–20 Hz), the activity-evoked extracellular alkalinization has a predominantly glutamatergic origin. However, the dominance is gradually shifted to HCO3 − -dependent, GABAergic alkalinisation by increasing the stimulation frequency to 50–100 Hz. In view of the pH sensitivity of NMDA and GABAARs, this frequency-dependence of the mechanisms underlying activity-induced pHo changes is intriguing. For instance, it is possible that during high-frequency neuronal activity, typically used in paradigms for induction of long-term potentiation (LTP), the intense CO2/HCO3 − shuttle and consequent CAo dependent rise in pHo that will take place in the interstitial fluid close to GABAARs would reduce the efficacy of inhibition, thereby facilitating LTP. In parallel with this, the alkalosis might directly enhance the LTP-inducing, pH-sensitive NMDA current [22, 47, 92–97]. Whether such H+-mediated cross-talk between excitatory and inhibitory mechanisms takes place in the brain is an interesting question to be addressed in future studies. The idea that H+ does act as a modulatory signal in microdomains of the brain extracellular space is supported by findings showing that transporter-mediated acidification of the synaptic microenvironment is sufficient to enhance GABAergic signaling [98].
The initial, fast activity-dependent alkalinization is typically followed by a slower and long-lasting acidification. There is strong evidence that depolarization-dependent activation of Na+- HCO3 − cotransport in glial cells [99], which is likely to be associated with an increase in PCO2, accounts for the slow acid shift [100]. This kind of a mechanism will affect both neuronal pHo and pHi over a large area for a prolonged duration, and is tempting to speculate that it could provide a powerful control over gross network excitability. There is, indeed, evidence that such a feedback mechanism acts as an intrinsic antiepileptic mechanism by limiting the generation and propagation of seizure activity thereby contributing to seizure termination [101, 102].
5 Intraneuronal CAi Activity Promotes Depolarizing and Excitatory GABAergic Transmission
The fact that GABAARs show a substantial permeability to HCO3 − leads to a unique and tight link between the functions of this transmitter system and the pH-regulatory machinery in brain cells and in the extracellular space [35, 103]. To fully understand the role of CA activity in GABAergic signalling and especially how it affects the ‘ionic plasticity’ of inhibitory transmission we need to go back and look at the basic properties of GABAARs.
The transmembrane gradients of Cl− and HCO3 − determine the reversal potential of GABAARs (EGABA-A) [82]. pHi -regulatory transporters maintain E HCO3 at a very positive level, around −10 to −15 mV (see Sect. 2) which means that the current component carried by HCO3 − across GABAARs is always depolarizing. In mature neurons, the K-Cl cotransporter KCC2 extrudes Cl− which keeps E Cl more negative than the resting membrane potential, thus providing the ionic basis for conventional hyperpolarizing IPSPs [104]. Thus, with the relative HCO3 −/Cl− permeability ratio of GABAARs at around 0.2–0.4, E HCO3 > > EGABA-A > ECl [35]. However, especially during intense or prolonged activation of GABAARs, a significant conductive uptake of Cl− takes place [82] which produces a large, activity-dependent depolarizing shift in ECl and consequently, in E GABA-A. In adult mammalian neurons, and especially in their dendrites which have a large surface-to-volume ratio [105], CAi is generally thought to be necessary for maintaining the supply of intracellular HCO3 − that drives GABAergic depolarizing and excitatory responses [34, 78, 106, 107].
In line with the above, intense activation of GABAA channels evokes biphasic GABAergic responses [106–111]. The early hyperpolarization, representing fused individual hyperpolarizing IPSPs, is followed by a prolonged depolarization that is often associated with pronounced spiking. The initial phase of the depolarization is generated by the fast shift in ECl driven by the HCO3 − -dependent net uptake of Cl−. Thereafter, extrusion of the accumulated Cl− via KCC2 leads to a long-lasting increase in extracellular K+ [78] and to a consequent non-synaptically -induced depolarization of both the neurons and the adjacent glial cells.
In the present context, the dual role of KCC2 described above is a very important topic because the depolarizing/excitatory action of GABA in mature pyramidal neurons shows a strict dependence on neuronal CAi. This conclusion is based on the findings that (i) inhibition of intra- but not extracellular CA attenuates the post-tetanic GABAergic depolarization [107] and (ii) the HCO3 − -dependent excitatory effects of GABA parallel the developmental upregulation of cytosolic CA activity [34, 70].
The extent of the activity-dependent rapid shifts in EGABA to more depolarizing values is likely to differ between neuronal subpopulations because of differences in Cl−-HCO3 − homeostasis [59, 61, 112, 113]. There is also experimental data suggesting that excitatory GABAergic transmission might contribute to seizure generation [78, 107, 114].
6 CA VII Contributes to the Generation of Febrile Seizures
We have recently conducted an extensive study on the CA VII KO mouse [34] where we addressed the role of CA VII in HCO3 − -dependent depolarizing GABA responses and in the generation of experimental febrile seizures (eFS) induced by hyperthermia [4]. Given the distinct developmental expression profiles of CA VII and CA II, the novel CA VII KO mouse provided an excellent opportunity to examine how this neuron-specific isoform modulates excitability. Whole cell patch clamp studies on P12-16 WT and CA VII KO hippocampal pyramidal neurons showed that HCO3 − -dependent net uptake of Cl− via GABAARs is strongly facilitated by CA VII activity. Consequently, the high-frequency stimulation –induced, long-lasting GABAergic depolarization was able to induce action potential firing in WT, but not in CA VII KO neurons (Fig. 14.1c). Boosting GABAAR-mediated signalling with diazepam in P14 rat hippocampal slices prolonged the duration of excitatory GABAergic responses and increased the number of action potentials associated with the depolarization. After P35, when neuronal CA II expression has also taken place, both isoforms were equally efficient in promoting HCO3 − -dependent GABAergic depolarization. As expected, in the CA II/CA VII double KO, i.e. in the absence of CAi activity, GABAergic depolarization remained small also at adult stage.
The developmental stage of the rodent brain and especially its cortical structures at P13-P14 is generally thought to be relevant for comparisons to the human situation, where FS are first seen at an age of 6 months [7, 115]. A striking difference in seizure generation was found between WT and CA VII KO mice. Cortical EEG monitoring showed that electrographic seizures were present in WT but not in CA VII KO mice. Importantly, there were no genotype-dependent differences in hyperventilation and the consequent respiratory alkalosis which is a major trigger of eFS [4, 7].
Behavioural experiments on P14 rat pups showed that enhancing GABAAR signalling by a low dose of diazepam facilitated the triggering of eFS without affecting breath rate. At higher concentration diazepam prevented the generation eFS, probably via suppressed breathing. In fact, the suppression of breathing and the consequent block of the FS-promoting respiratory alkalosis might be a major mechanism in the therapeutic actions of diazepam which is routinely given to children with FS.
In humans FS are the most common type of seizures during early childhood [116]. Consistent with a role in FS, exon array analysis showed a prenatal upregulation of CA VII in the human neocortex and hippocampus that precedes the postnatal time period during which FS are most commonly detected [34].
These data as a whole suggest that designing next-generation isoform-specific inhibitors of CA VII has much potential as a novel approach in the treatment of FS and possibly other epileptiform syndromes.
7 Conclusions
In comparison to Ca2+, the multiple and evolutionarily ancient roles of H+ ions in controlling neuronal signaling have received surprisingly little attention. For instance, the strikingly steep pHo dependency of the gating of GABAA and NMDA channels has been recognized for decades, but the amount of work done on the functional impact of activity-evoked pHo transients on synaptic transmission is sparse [46, 47, 96, 97, 117]. What is known about the actions of H+ does indicate that it is one of the most important physiologically-active agents that exert a fundamental modulatory role in neuronal development, plasticity, as well as synaptic and electrical signalling.
Moreover, H+ is an amazingly potent agent in the suppression of seizures [5, 101, 102]. Neuronal pH shifts exert also a strong influence on the outcome from disease states such as stroke and ischemia/anoxia [118]. Observations of this kind are consistent with the multiple physiological roles of H+ signalling, and elucidating the underlying processes is likely to be useful in pre-clinical and clinical work on many other disease states, such as migraine and chronic pain [119]. In the context of pathophysiological mechanisms, strategies that target neuronal pH may turn out to be as, or even more relevant, than those designed for modulation of neuronal Cl− homeostasis [102], an area which has recently attracted extensive attention within the neuroscience community [59]. Here, one should note that in addition to tight Ca2+/H+ interactions at the molecular and cellular level [120], pH and Cl− regulation are closely linked, especially via HCO3 − -dependent mechanisms [121].
The key role of CA isoforms in the suppression, generation and modulation of pH shifts in the brain and other parts of the CNS makes these molecules highly interesting in studies of the fundamental mechanisms underlying neuronal signalling. The developmental profiles of distinct CAs, as well as their strategic localization seen from the level of the whole organism to subcellular microdomains points to a high versatility of their regulatory functions thus providing an exciting subject for molecular, cellular, physiological, medical and pharmacological research [27, 119, 122]. Finally, it is obvious that CAs represent a promising family of targets for CNS drug research and design.
References
Nattie EE (2001) Central chemosensitivity, sleep, and wakefulness. Respir Physiol 129:257–268
Lee J, Taira T, Pihlaja P, Ransom BR, Kaila K (1996) Effects of CO2 on excitatory transmission apparently caused by changes in intracellular pH in the rat hippocampal slice. Brain Res 706:210–216
Dulla CG, Frenguelli BG, Staley KJ, Masino SA (2009) Intracellular acidification causes adenosine release during states of hyperexcitability in the hippocampus. J Neurophysiol 102:1984–1993
Schuchmann S, Schmitz D, Rivera C, Vanhatalo S, Salmen B, Mackie K, Sipilä ST, Voipio J, Kaila K (2006) Experimental febrile seizures are precipitated by a hyperthermia-induced respiratory alkalosis. Nat Med 12:817–823
Tolner EA, Hochman DW, Hassinen P, Otahal J, Gaily E, Haglund MM, Kubova H, Schuchmann S, Vanhatalo S, Kaila K (2011) Five percent CO2 is a potent, fast-acting inhalation anticonvulsant. Epilepsia 52:104–114
Guaranha MS, Garzon E, Buchpiguel CA, Tazima S, Yacubian EM, Sakamoto AC (2005) Hyperventilation revisited: physiological effects and efficacy on focal seizure activation in the era of video-EEG monitoring. Epilepsia 46:69–75
Schuchmann S, Hauck S, Henning S, Gruters-Kieslich A, Vanhatalo S, Schmitz D, Kaila K (2011) Respiratory alkalosis in children with febrile seizures. Epilepsia 52:1949–1955
Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, Van’t Hoff W, Al MO, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R (2009) Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360:1960–1970
Pedersen SF, O’Donnell ME, Anderson SE, Cala PM (2006) Physiology and pathophysiology of Na+/H+ exchange and Na+-K+-2Cl- cotransport in the heart, brain, and blood. Am J Physiol Regul Integr Comp Physiol 291:R1–R25
Helmy MM, Tolner EA, Vanhatalo S, Voipio J, Kaila K (2011) Brain alkalosis causes birth asphyxia seizures, suggesting therapeutic strategy. Ann Neurol 69:493–500
Helmy MM, Ruusuvuori E, Watkins PV, Voipio J, Kanold PO, Kaila K (2012) Acid extrusion via blood–brain barrier causes brain alkalosis and seizures after neonatal asphyxia. Brain 135:3311–3319
Roos A, Boron WF (1981) Intracellular pH. Physiol Rev 61:296–434
Chesler M (2003) Regulation and modulation of pH in the brain. Physiol Rev 83:1183–1221
Ballanyi K, Kaila K (1998) Activity-evoked changes in intracellular pH. In: Kaila K, Ransom BR (eds) pH and brain function. Wiley-Liss, New York, pp 291–308
Kaila K, Chesler M (1998) Activity-evoked changes in extracellular pH. In: Kaila K, Ransom BR (eds) pH and brain function. Wiley-Liss, New York, pp 309–337
Casey JR, Grinstein S, Orlowski J (2010) Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11:50–61
Tombaugh GC, Somjen GG (1996) Effects of extracellular pH on voltage-gated Na+, K+ and Ca2+ currents in isolated rat CA1 neurons. J Physiol 493(Pt 3):719–732
Tombaugh GC, Somjen GG (1997) Differential sensitivity to intracellular pH among high- and low- threshold Ca2+ currents in isolated rat CA1 neurons. J Neurophysiol 77:639–653
Pasternack M, Smirnov S, Kaila K (1996) Proton modulation of functionally distinct GABAA receptors in acutely isolated pyramidal neurons of rat hippocampus. Neuropharmacology 35:1279–1288
Wilkins ME, Hosie AM, Smart TG (2005) Proton modulation of recombinant GABA(A) receptors: influence of GABA concentration and the beta subunit TM2-TM3 domain. J Physiol 567:365–377
Traynelis SF, Cull-Candy SG (1990) Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 345:347–350
Makani S, Chen HY, Esquenazi S, Shah GN, Waheed A, Sly WS, Chesler M (2012) NMDA Receptor-dependent afterdepolarizations are curtailed by carbonic anhydrase 14: regulation of a short-term postsynaptic potentiation. J Neurosci 32:16754–16762
Spray DC, Harris AL, Bennet MVL (1981) Gap junctional conductance is a simple and sensitive function of intracellular pH. Science 211:712–715
Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M (1997) A proton-gated cation channel involved in acid-sensing. Nature 386:173–177
Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M (1997) TASK, a human background K + channel to sense external pH variations near physiological pH. EMBO J 16:5464–5471
Maren TH (1967) Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev 47:595–781
Supuran CT (2008) Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov 7:168–181
Stridh MH, Alt MD, Wittmann S, Heidtmann H, Aggarwal M, Riederer B, Seidler U, Wennemuth G, McKenna R, Deitmer JW, Becker HM (2012) Lactate flux in astrocytes is enhanced by a non-catalytic action of carbonic anhydrase II. J Physiol 590:2333–2351
Jiao Y, Yan J, Zhao Y, Donahue LR, Beamer WG, Li X, Roe BA, LeDoux MS, Gu W (2005) Carbonic anhydrase-related protein VIII deficiency is associated with a distinctive lifelong gait disorder in waddles mice. Genetics 171:1239–1246
Hirasawa M, Xu X, Trask RB, Maddatu TP, Johnson BA, Naggert JK, Nishina PM, Ikeda A (2007) Carbonic anhydrase related protein 8 mutation results in aberrant synaptic morphology and excitatory synaptic function in the cerebellum. Mol Cell Neurosci 35:161–170
Kaya N, Aldhalaan H, Al-Younes B, Colak D, Shuaib T, Al-Mohaileb F, Al-Sugair A, Nester M, Al-Yamani S, Al-Bakheet A, Al-Hashmi N, Al-Sayed M, Meyer B, Jungbluth H, Al-Owain M (2011) Phenotypical spectrum of cerebellar ataxia associated with a novel mutation in the CA8 gene, encoding carbonic anhydrase (CA) VIII. Am J Med Genet B Neuropsychiatr Genet 156B:826–834
Shah GN, Ulmasov B, Waheed A, Becker T, Makani S, Svichar N, Chesler M, Sly WS (2005) Carbonic anhydrase IV and XIV knockout mice: roles of the respective carbonic anhydrases in buffering the extracellular space in brain. Proc Natl Acad Sci U S A 102:16771–16776
Velisek L, Moshe SL, Xu SG, Cammer W (1993) Reduced susceptibility to seizures in carbonic anhydrase II deficient mutant mice. Epilepsy Res 14:115–121
Ruusuvuori E, Huebner AK, Kirilkin I, Yukin A, Blaesse P, Helmy MM, Kang HJ, Muayed M, Hennings JC, Sestan N, Hubner CA, Kaila K (2013) Neuronal carbonic anhydrase VII provides GABAergic excitatory drive to exacerbate febrile seizures. EMBO J 32:2275–2286
Kaila K (1994) Ionic basis of GABA(A) receptor channel function in the nervous system. Prog Neurobiol 42:489–537
Thomas RC, Meech RW (1982) Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature 299:826–828
Deitmer JW, Rose CR (1996) pH regulation and proton signalling by glial cells. Prog Neurobiol 48:73–103
Pappas CA, Ransom BR (1993) A depolarization-stimulated, bafilomycin-inhibitable H+ pump in hippocampal astrocytes. Glia 9:280–291
Bevensee MO, Cummins TR, Haddad GG, Boron WF, Boyarsky G (1996) pH regulation in single CA1 neurons acutely isolated from the hippocampi of immature and mature rats. J Physiol 494:315–328
Yao H, Ma E, Gu XQ, Haddad GG (1999) Intracellular pH regulation of CA1 neurons in Na(+)/H(+) isoform 1 mutant mice. J Clin Invest 104:637–645
Schwiening CJ, Kennedy HJ, Thomas RC (1993) Calcium-hydrogen exchange by the plasma membrane Ca-ATPase of voltage-clamped snail neurons. Proc Biol Sci 253:285–289
Grichtchenko II, Chesler M (1996) Calcium- and barium-dependent extracellular alkaline shifts evoked by electrical activity in rat hippocampal slices. Neuroscience 75:1117–1126
Paalasmaa P, Kaila K (1996) Role of voltage-gated calcium channels in the generation of activity-induced extracellular pH transients in the rat hippocampal slice. J Neurophysiol 75:2354–2360
Burton RF (1978) Intracellular buffering. Respir Physiol 33:51–58
Chesler M, Chen JC, Kraig RP (1994) Determination of extracellular bicarbonate and carbon dioxide concentrations in brain slices using carbonate and pH-selective microelectrodes. J Neurosci Meth 53:129–136
Tong CK, Chen K, Chesler M (2006) Kinetics of activity-evoked pH transients and extracellular pH buffering in rat hippocampal slices. J Neurophysiol 95:3686–3697
Makani S, Chesler M (2007) Endogenous alkaline transients boost postsynaptic NMDA receptor responses in hippocampal CA1 pyramidal neurons. J Neurosci 27:7438–7446
Huang W, Smith SE, Chesler M (1995) Addition of carbonic anhydrase augments extracellular pH buffering in rat cerebral cortex. J Neurophysiol 74:1806–1809
Svichar N, Waheed A, Sly WS, Hennings JC, Hubner CA, Chesler M (2009) Carbonic anhydrases CA4 and CA14 both enhance AE3-mediated Cl-. J Neurosci 29:3252–3258
Zhu XL, Sly WS (1990) Carbonic anhydrase-IV from human lung-purification, characterization, and camparision with membrane carbonic-anhydrase from human kidney. J Biol Chem 265:8795–8801
Svichar N, Chesler M (2003) Surface carbonic anhydrase activity on astrocytes and neurons facilitates lactate transport. Glia 41:415–419
Mori K, Ogawa Y, Ebihara K, Tamura N, Tashiro K, Kuwahara T, Mukoyama M, Sugawara A, Ozaki S, Tanaka I, Nakao K (1999) Isolation and characterization of CA XIV, a novel membrane-bound carbonic anhydrase from mouse kidney. J Biol Chem 274:15701–15705
Parkkila S, Parkkila AK, Rajaniemi H, Shah GN, Grubb JH, Waheed A, Sly WS (2001) Expression of membrane-associated carbonic anhydrase XIV on neurons and axons in mouse and human brain. Proc Natl Acad Sci U S A 98:1918–1923
Tureci O, Sahin U, Vollmar E, Siemer S, Gottert E, Seitz G, Parkkila AK, Shah GN, Grubb JH, Pfreundschuh M, Sly WS (1998) Human carbonic anhydrase XII: cDNA cloning, expression, and chromosomal localization of a carbonic anhydrase gene that is overexpressed in some renal cell cancers. Proc Natl Acad Sci U S A 95:7608–7613
Ivanov S, Liao SY, Ivanova A, Danilkovitch-Miagkova A, Tarasova N, Weirich G, Merrill MJ, Proescholdt MA, Oldfield EH, Lee J, Zavada J, Waheed A, Sly W, Lerman MI, Stanbridge EJ (2001) Expression of hypoxia-lnducible cell-surface transmembrane carbonic anhydrases in human cancer. Am J Pathol 158:905–919
Haapasalo JA, Nordfors KM, Hilvo M, Rantala IJ, Soini Y, Parkkila AK, Pastorekova S, Pastorek J, Parkkila SM, Haapasalo HK (2006) Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res 12:473–477
Halmi P, Parkkila S, Honkaniemi J (2006) Expression of carbonic anhydrases II, IV, VII, VIII and XII in rat brain after kainic acid induced status epilepticus. Neurochem Int 48:24–30
Nogradi A, Domoki F, Degi R, Borda S, Pakaski M, Szabo A, Bari F (2003) Up-regulation of cerebral carbonic anhydrase by anoxic stress in piglets. J Neurochem 85:843–850
Blaesse P, Airaksinen MS, Rivera C, Kaila K (2009) Cation-chloride cotransporters and neuronal function. Neuron 61:820–838
Durand GM, Konnerth A (1996) Long-term potentiation as a mechanism of functional synapse induction in the developing hippocampus. J Physiol Paris 90:313–315
Gonzalez-Islas C, Chub N, Wenner P (2009) NKCC1 and AE3 appear to accumulate chloride in embryonic motoneurons. J Neurophysiol 101:507–518
Ridderstråle Y, Wistrand PJ (1998) Carbonic anhydrase isoforms in the mammalian nervous system. In: Kaila K, Ransom B (eds) pH and brain function. Wiley-Liss, New York, pp 21–44
Nogradi A, Mihaly A (1990) Light microscopic histochemistry of the postnatal-development and localization of carbonic anhydrase activity in glial and neuronal cell-types of the rat central nervous system. Histochemistry 94:441–447
Pasternack M, Voipio J, Kaila K (1993) Intracellular carbonic anhydrase activity and its role in GABA- induced acidosis in isolated rat hippocampal pyramidal neurones. Acta Physiol Scand 148:229–231
Lakkis MM, Bergenhem NCH, OShea KS, Tashian RE (1997) Expression of the acatalytic carbonic anhydrase VIII gene, car8, during mouse embryonic development. Histochem J 29:135–141
Nogradi A, Jonsson N, Walker R, Caddy K, Carter N, Kelly C (1997) Carbonic anhydrase II and carbonic anhydrase-related protein in the cerebellar cortex of normal and lurcher mice. Dev Brain Res 98:91–101
Munsch T, Pape HC (1999) Upregulation of the hyperpolarization-activated cation current in rat thalamic relay neurones by acetazolamide. J Physiol 519(Pt 2):505–514
Wang WG, Bradley SR, Richerson GB (2002) Quantification of the response of rat medullary raphe neurones to independent changes in pH(O) and P-CO2. J Physiol 540:951–970
Willoughby D, Schwiening CJ (2002) Electrically evoked dendritic pH transients in rat cerebellar Purkinje cells. J Physiol-Lond 544:487–499
Ruusuvuori E, Li H, Huttu K, Palva JM, Smirnov S, Rivera C, Kaila K, Voipio J (2004) Carbonic anhydrase isoform VII acts as a molecular switch in the development of synchronous gamma-frequency firing of hippocampal CA1 pyramidal cells. J Neurosci 24:2699–2707
Spicer SS, Stoward PJ, Tashian RE (1979) The immunohistolocalization of carbonic anhydrase in rodent tissues. J Histochem Cytochem 27:820–831
Innocenti A, Scozzafava A, Parkkila S, Puccetti L, De Simone G, Supuran CT (2008) Investigations of the esterase, phosphatase, and sulfatase activities of the cytosolic mammalian carbonic anhydrase isoforms I, II, and XIII with 4-nitrophenyl esters as substrates. Bioorg Med Chem Lett 18:2267–2271
Truppo E, Supuran CT, Sandomenico A, Vullo D, Innocenti A, Di FA, Alterio V, De SG, Monti SM (2012) Carbonic anhydrase VII is S-glutathionylated without loss of catalytic activity and affinity for sulfonamide inhibitors. Bioorg Med Chem Lett 22:1560–1564
Erecinska M, Cherian S, Silver IA (2004) Energy metabolism in mammalian brain during development. Prog Neurobiol 73:397–445
Sterling D, Reithmeier RAF, Casey JR (2001) A transport metabolon. Functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 276:47886–47894
Becker HM, Deitmer JW (2007) Carbonic anhydrase II increases the activity of the human electrogenic Na+/HCO3- cotransporter. J Biol Chem 282:13508–13521
Boron WF (2010) Evaluating the role of carbonic anhydrases in the transport of HCO3–related species. Biochim Biophys Acta 1804:410–421
Viitanen T, Ruusuvuori E, Kaila K, Voipio J (2010) The KCl -cotransporter KCC2 promotes GABAergic excitation in the mature rat hippocampus. J Physiol 588:1527–1540
Thiry A, Dogne J, Supuran CT, Masereel B (2007) Carbonic anhydrase inhibitors as anticonvulsant agents. Curr Top Med Chem 7:855–864
Fedirko N, Svichar N, Chesler M (2006) Fabrication and use of high-speed, concentric H+- and Ca2+-selective microelectrodes suitable for in vitro extracellular recording. J Neurophysiol 96:919–924
Kaila K, Ransom BR (1998) pH and brain function. Wiley-Liss, New York
Kaila K, Voipio J (1987) Postsynaptic fall in intracellular pH induced by GABA-activated bicarbonate conductance. Nature 330:163–165
Paalasmaa P, Taira T, Voipio J, Kaila K (1994) Extracellular alkaline transients mediated by glutamate receptors in the rat hippocampal slice are not due to a proton conductance. J Neurophysiol 72:2031–2033
Trapp S, Luckermann M, Kaila K, Ballanyi K (1996) Acidosis of hippocampal neurones mediated by a plasmalemmal Ca2+/H+ pump. Neuroreport 7:2000–2004
Luckermann M, Trapp S, Ballanyi K (1997) GABA- and glycine-mediated fall of intracellular pH in rat medullary neurons in situ. J Neurophysiol 77:1844–1852
Zhan RZ, Fujiwara N, Tanaka E, Shimoji K (1998) Intracellular acidification induced by membrane depolarization in rat hippocampal slices: roles of intracellular Ca2+ and glycolysis. Brain Res 780:86–89
Kaila K, Saarikoski J, Voipio J (1990) Mechanism of action of GABA on intracellular pH and on surface pH in crayfish muscle fibres. J Physiol 427:241–260
Voipio J, Paalasmaa P, Taira T, Kaila K (1995) Pharmacological characterization of extracellular pH transients evoked by selective synaptic and exogenous activation of AMPA, NMDA, and GABAA receptors in the rat hippocampal slice. J Neurophysiol 74:633–642
Chen JC, Chesler M (1992) Modulation of extracellular pH by glutamate and GABA in rat hippocampal slices. J Neurophysiol 67:29–36
Kaila K, Paalasmaa P, Taira T, Voipio J (1992) pH transients due to monosynaptic activation of GABAA receptors in rat hippocampal slices. Neuroreport 3:105–108
Taira T, Paalasmaa P, Voipio J, Kaila K (1995) Relative contributions of excitatory and inhibitory neuronal activity to alkaline transients evoked by stimulation of Schaffer collaterals in the rat hippocampal slice. J Neurophysiol 74:643–649
Traynelis SF, Hartley M, Heinemann SF (1995) Control of proton sensitivity of the NMDA receptor by RNA splicing and polyamines. Science 268:873–876
Krishek BJ, Amato A, Connolly CN, Moss SJ, Smart TG (1996) Proton sensitivity of the GABA(A) receptor is associated with the receptor subunit composition. J Physiol 492:431–443
Tang CM, Dichter M, Morad M (1990) Modulation of the N-methyl-D-aspartate channel by extracellular H+. Proc Natl Acad Sci U S A 87:6445–6449
Vyklicky LJ, Vlachov V, Krusek J (1990) The effect of external pH changes on responses to excitatory amino acids in mouse hippocampal neurones. J Physiol 430:497–517
Taira T, Smirnov S, Voipio J, Kaila K (1993) Intrinsic proton modulation of excitatory transmission in rat hippocampal slices. Neuroreport 4:93–96
Gottfried JA, Chesler M (1994) Endogenous H + modulation of NMDA receptor-mediated EPSCs revealed by carbonic anhydrase inhibition in rat hippocampus. J Physiol 478(Pt 3):373–378
Dietrich CJ, Morad M (2010) Synaptic acidification enhances GABAA signaling. J Neurosci 30:16044–16052
Pappas CA, Ransom BR (1994) Depolarization-induced alkalinization (DIA) in rat hippocampal astrocytes. J Neurophysiol 72:2816–2826
Voipio J (1998) Diffusion and buffering aspects of H+, HCO3-, and CO2 movements in brain tissue. In: Kaila K, Ransom BR (eds) pH and brain function. Wiley-Liss, New York, pp 45–66
de Curtis M, Manfridi A, Biella G (1998) Activity-dependent pH shifts and periodic recurrence of spontaneous interictal spikes in a model of focal epileptogenesis. J Neurosci 18:7543–7551
Pavlov I, Kaila K, Kullmann DM, Miles R (2013) Cortical inhibition, pH and cell excitability in epilepsy: what are optimal targets for antiepileptic interventions? J Physiol 591:765–774
Farrant M, Kaila K (2007) The cellular, molecular and ionic basis of GABAA receptor signalling. Prog Brain Res 160:59–87
Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K (1999) The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397:251–255
Qian N, Sejnowski TJ (1990) When is an inhibitory synapse effective? Proc Natl Acad Sci U S A 87:8145–8149
Staley KJ, Soldo BL, Proctor WR (1995) Ionic mechanisms of neuronal excitation by inhibitory GABA(A) receptors. Science 269:977–981
Kaila K, Lamsa K, Smirnov S, Taira T, Voipio J (1997) Long-lasting GABA-mediated depolarization evoked by high- frequency stimulation in pyramidal neurons of rat hippocampal slice is attributable to a network-driven, bicarbonate-dependent K+ transient. J Neurosci 17:7662–7672
Alger BE, Nicoll RA (1982) Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol 328:105–123
Alger BE, Nicoll RA (1982) Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J Physiol 328:125–141
Grover LM, Lambert NA, Schwartzkroin PA, Teyler TJ (1993) Role of HCO3- ions in depolarizing GABAA receptor-mediated responses in pyramidal cells of rat hippocampus. J Neurophysiol 69:1541–1555
Smirnov S, Paalasmaa P, Uusisaari M, Voipio J, Kaila K (1999) Pharmacological isolation of the synaptic and nonsynaptic components of the GABA-mediated biphasic response in rat CA1 hippocampal pyramidal cells. J Neurosci 19:9252–9260
Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G (2006) Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311:233–235
Khirug S, Yamada J, Afzalov R, Voipio J, Khiroug L, Kaila K (2008) GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl cotransporter NKCC1. J Neurosci 28:4635–4639
Stasheff SF, Mott DD, Wilson WA (1993) Axon terminal hyperexcitability associated with epileptogenesis in vitro. II. Pharmacological regulation by NMDA and GABAA receptors. J Neurophysiol 70:976–984
Berg AT, Shinnar S (1996) Complex febrile seizures. Epilepsia 37:126–133
Stafstrom CE (2002) The incidence and prevalence of febrile seizures. In: Baram TZ, Shinnar S (eds) Febrile seizures. Academic, San Diego, pp 1–25
Fedirko N, Avshalumov M, Rice ME, Chesler M (2007) Regulation of postsynaptic Ca2+ influx in hippocampal CA1 pyramidal neurons via extracellular carbonic anhydrase. J Neurosci 27:1167–1175
Orlowski P, Chappell M, Park CS, Grau V, Payne S (2011) Modelling of pH dynamics in brain cells after stroke. Interface Focus 1:408–416
Asiedu M, Ossipov MH, Kaila K, Price TJ (2010) Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain 148:302–308
Caldwell L, Harries P, Sydlik S, Schwiening CJ (2012) Presynaptic pH and vesicle fusion in Drosophila larvae neurones. Synapse 67:729–740
Romero MF, Fulton CM, Boron WF (2004) The SLC4 family of HCO3- transporters. Pflugers Arch 447:495–509
Ferrini F, Trang T, Mattioli TA, Laffray S, Del'Guidice T, Lorenzo LE, Castonguay A, Doyon N, Zhang W, Godin AG, Mohr D, Beggs S, Vandal K, Beaulieu JM, Cahill CM, Salter MW, de KY (2013) Morphine hyperalgesia gated through microglia-mediated disruption of neuronal Cl- homeostasis. Nat Neurosci 16:183–192
Acknowledgments
The authors’ original research work has been supported by the Academy of Finland, the Sigrid Jusélius Foundation, the Jane and Aatos Erkko Foundation, and the Letten Foundation. We thank Prof. Juha Voipio for discussions and constructive comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Ruusuvuori, E., Kaila, K. (2014). Carbonic Anhydrases and Brain pH in the Control of Neuronal Excitability. In: Frost, S., McKenna, R. (eds) Carbonic Anhydrase: Mechanism, Regulation, Links to Disease, and Industrial Applications. Subcellular Biochemistry, vol 75. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7359-2_14
Download citation
DOI: https://doi.org/10.1007/978-94-007-7359-2_14
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7358-5
Online ISBN: 978-94-007-7359-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)