Abstract
Adipose tissue, consisting mainly of adipocytes, functions as a critical organ for energy regulation, inflammation and immune response through intricate signals.
Mature adipocytes were considered to be in the terminal stage of differentiation and stationary, having lost their proliferative ability. Recently, the capability of mature adipocytes to reprogram their gene expression profile and transform into different cytotypes has been demonstrated.
Here, data of both mature and dedifferentiated adipocytes were collected and compared to underline structural and functional features of these cells. In particular, morphology, structure, molecular and immunophenotype markers, and dedifferentiation process of mature isolated adipocytes are analyzed. In addition, molecular and phenotype characterization of dedifferentiated fat cells is described, reporting important results on pluripotent differentiation ability, immunoregulatory and hematopoietic supporting functions of these cells. These findings highlight the concept that adipose lineage cells represent a suitable new cell source for clinical applications in such fields as cell therapy and regenerative medicine.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Brown Adipose Tissue
- Mature Adipocyte
- Dedifferentiated Cell
- Dedifferentiation Process
- Human Mature Adipocyte
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Human adipose tissue is no longer considered simply as a storage depository of lipids, but as a critical organ involved in energy regulation, whole-body insulin action and inflammation, through intricate endocrine, paracrine and autocrine signals (Dandona et al. 2004). Adipose tissue contains adipocytes and non-adipose cells. Mature adipocytes are functionally the most important cell type in adipose tissue, while the stromal vascular fraction contains several cell types and represents a reservoir not only of specific adipocyte precursor cells but also of multipotent stem cells (Gimble and Guilak 2003).
The literature give higher attention to stromal stem cells than to mature adipocytes which demonstrated to have stem cells properties. The stemness features of fat and its in vitro dedifferentiation process is largely unknown, despite the potential clinical applications of both mature adipocytes and dedifferentiated adipocytes in such fields, as regenerative medicine.
White adipose tissue has attracted attention because of its great and reversible capacity for expansion, which appears to be permanent throughout adult life (Planat-Bernard et al. 2004). The process of cellular differentiation in terminally differentiated mammalian cells is thought to be irreversible, but recent data suggest that the mature adipocytes, when under physiological stimuli, are able to reversibly change their phenotype and directly transform into cells with a different morphology and physiology (De Matteis et al. 2009; Poloni et al. 2012a).
Mature adipocytes are functionally the most important cell type in adipose tissue. White adipocytes are spherical cells with a single large lipid droplet formed by triacylglycerols that accounts for >90 % of the cell’s volume (Cinti 2009). They have a variable size that depends mainly on the size of the lipid droplet stored in them. Even on electron microscopy there seems to be no distinct structure separating it from the thin rim of cytoplasm by a non-membranous electron-dense barrier containing functionally important proteins such as perilipin (Blanchette-Mackie et al. 1995). The thin cytoplasm contains the nucleus, characteristically squeezed by the large lipid vacuole, an usually under-developed Golgi apparatus, rough endoplasmic reticulum made up of short, isolated cisternae and rare lysosomes. Mitochondria are thin and elongated, with randomly oriented cristae, and variable in amount: in general, they are less numerous in the larger cells. Several caveolae are found on the outer surface. Numerous pinocytotic vesicles are found at the level of the outer cytoplasmic membrane, where a distinct basal membrane is present. Other organelles are usually poorly represented (Cinti 2009). Mature adipocytes should be studied using their buoyant property (Fig. 5.1).
To obtain isolated mature adipocytes free of stromal-vascular elements, after collagenase digestion, the disrupted tissues were filtered through a 200 μm nylon sieve. The filtered cells should be washed 4–5 times and eventually re-filtered if it is necessary. Only the floating top layer was collected after each washing step (Zhang et al. 2000).
The confocal microscopy analysis of adipogenic cellular fraction isolated is a good and easy way to exclude any contamination coming from other cell types contained in the whole tissue. These cells are large (50–70 μm), unilocular, perilipin-immunoreactive with a peripheral flattened nucleus.
The ceiling culture is the method that uses the buoyant property of adipocytes, allowing them to adhere to the top inner surface of a culture flask which is completely filled with medium (Sugihara et al. 1987). In this way, mature adipocytes adhere to the ceiling surface, then the flask should be reinverted to allow normal observation and the subsequent manipulation of the culture.
The aim of this chapter is to collect and compare the literature data about both mature and dedifferentiated adipocytes to better underline their stem cells properties. In addition to well characterize adipocytes morphologically and structurally, this chapter reports on the pluripotent differentiation ability, immunoregulatory and hematopoietic supporting functions of these cells. These findings open new perspectives on adipose tissue plasticity and highlight the way for cellular therapy and regenerative medicine based on the administration of adipose tissue stem cells.
The Dedifferentiation Process of Mature Adipocytes
Mature adipocytes are generally considered terminally differentiated because they have lost their proliferative abilities, but recent data suggest that the process of cellular differentiation in terminally differentiated mammalian cells is not irreversible.
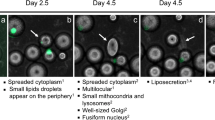
When maintained in culture, mature adipocytes undergo spontaneously a process of dedifferentiation (Matsumoto et al. 2008). Adipocytes lost their intracytoplasmatic lipid droplet, the nuclei became more centralized and the cells became elongated in shape, assuming a fibroblast-like morphology. At this step, the cells enter a proliferative log phase until cellular senescence (Fig. 5.2).
Dedifferentiation process of mature adipocytes. During culturing, the cells attached to the upper surface of the flasks, followed by conversion to fibroblast-like dedifferentiated adipocytes, reached a morphology similar to BM-derived MSCs. (a) Morphological changes at different time points from mature adipocytes to dedifferentiated adipocytes. (b) Mature adipocytes lose their lipid droplets. Cells were stained by toluidine blue. Scale bar in b is equal to 4 μm, in a1 equal to 80 μm, in a2 equal to 40 μm, in a3 equal to 80 μm, in a4 equal to 80 μm, in a5 equal to 120 μm, and in a6 equal to 150 μm
At molecular level, data revealed significant changes in gene expression during the dedifferentiation process (Ono et al. 2011; Poloni et al. 2012a). Adipocytes downregulated many genes that play a role in lipid and fatty acid metabolism, while concomitantly upregulated genes involved in cell proliferation, altered cell morphology, movement and migration of cells, and regulation of differentiation.
As murine adipocytes (De Matteis et al. 2009), recent data demonstrated that also human mature adipocytes expressed genes required for the cell reprogramming process, which include Oct4, Klf4, c-myc and Sox2 (Poloni et al. 2012a). Moreover, these cells expressed transcripts for embryonic stem cell genes that are required for self-renewal and pluripotency. Thus, recent data underline the potential role of mature adipocytes as stem cells (Poloni et al. 2012a; Cinti 2009). The reprogramming of genes in isolated as well as cultivated adipocytes is in line with the plastic properties of these cells.
Immunophenotype Characterization and Pluripotential Properties of Mature and De-differentiated Adipocytes
While dedifferentiated fat cells are well characterized (Matsumoto et al. 2008), there are only few studies that provided the cell-surface antigen profile of human mature adipocytes (Poloni et al. 2012a). The stemness markers expressed by mature adipocytes at the molecular level are also present as surface antigens. These cells are positive stained by some stem cells markers, CD34 (hematopoietic progenitor cell antigen 1, HPCA 1), CD133 (prominin 1), CD90 (Thy-1), CD105 (endoglin), CD271 (NGFR) and CD117 (c-kit). During the dedifferentiation process, the typical mesenchymal stem cells markers are highly preserved at the molecular and antigenic levels, while CD34 and CD133 are lost as antigens.
After the dedifferentiation process, adipocytes are easy to isolate and cultivate, so there are many studies that described their immunophenotype. The cell-surface antigen profile of dedifferentiated cells was analyzed (Matsumoto et al. 2008) and this profile is consistent with previous findings for bone marrow MSCs (Pittenger et al. 1999) with uniformly positive expression of CD13 (aminopeptidase N), CD29 (integrin β1), CD44 (hyaluronate receptor), CD49b (integrin α4-subunit), CD90, CD105, CD271, CD73 (ecto-5′-nuclotidase) and HLA-A, -B, -C, but negative for CD11b (integrin αM), CD31 (platelet endothelial cell adhesion molecule), CD34, CD133, CD45 (leukocyte common antigen), CD106 (vascular cell adhesion molecule-1) and HLA-DR.
These findings suggest that the dedifferentiated adipocytes are an homogeneous population expressing the same markers as bone marrow-derived MSCs and adipose tissue-derived MSCs. Data showed that dedifferentiated adipocytes are a highly homogeneous population of cells respect to adipose-derived stem cells, that contained a variety of cell types: high number of smooth muscle cells (19 %), endothelial cells (3 %) and blood cells (13 %) (Yoshimura et al. 2006). These observations are convincing because dedifferentiated adipocytes originate from a fraction of highly pure mature adipocytes, whereas adipose-derived stem cells are an heterogeneous population.
Electron microscopy (EM) analysis of dedifferentiated adipocytes is shown in Fig. 5.3a, b. The images show most organelles described during the early stages of developing stromal vascular fraction-derived MSCs in primary cultures, i.e., well-developed Golgi complexes, short strands of rough endoplasmic reticulum, small lipid droplets, small mitochondria, lysosomes and small granules of glycogen. Nuclei were fusiform with smooth edges. Thus, the EM features of dedifferentiated adipocytes were very similar to developing stromal vascular fraction-derived MSCs (Fig. 5.3c). The spontaneous dedifferentiation process represents the manifestation of morphological, molecular and functional changes of mature adipocytes, that might be interpreted as a return back to a noncommitted status of the cells.
The methylation status of cells is the most common epigenetic modification of genome in mammalian cells (Bibikova and Fan 2010). Data showed that there are significant difference between the methylation statuses of mature adipocytes and dedifferentiated adipocytes, while there are no difference between dedifferentiated adipocytes and bone marrow-derived MSCs (Poloni et al. 2012a). These findings suggested that during the dedifferentiation process a gene reprogramming event takes place, which leads to changes in cellular epigenetic status. Dedifferentiated adipocytes achieve the DNA methylation status of bone marrow-derived MSCs by this process.
Recent advances in regenerative medicine have created a broad spectrum of stem cell research. Among them, tissue stem cell regulations are important issues to clarify the molecular mechanism of differentiation. Tissue engineering and cell therapy techniques have been developed to reconstitute different tissues. Attention has been focused on cells that might be useful in regenerative medicine (Barrilleaux et al. 2006). However, because stem cells only represent a minor population of the cells in the body, invasive procedures are frequently needed to obtain the amount of stem cells required for cell therapy. Therefore, adult stem cells sources are needed to be easily isolated and expanded with high purity.
Several studies have demonstrated that mesenchymal progenitor cells from various tissues have the potential to differentiate into other cells, suggesting that pre-committed and committed cells have plasticity in cell fate determination (Sudo et al. 2007).
Mature adipocytes are the most abundant cell type in adipose tissue and they can be easily isolated without painful procedures or donor site injury. Recent data demonstrated that mature adipocytes are able to transdifferentiate into another cell type, suggesting a great plasticity of these cells. This process implies an in vivo phenomenon of physiological and reversible reprogramming of genes in mature cells (Cinti 2009). During this process a differentiated cell turns phenotypically and functionally into a differentiated cell of another type without undergoing dedifferentiation. Some other include the additional step of dedifferentiation (Tosh and Slack 2002).
Data showed two important examples of physiological and reversible transdifferentiation. In conditions of chronic cold exposure the amount brown adipose tissue (BAT) in the organ could increase via white-to-brown transdifferentiation and, vice versa, BAT could turn into WAT in case of exposure to an obesogenic diet, to enable greater energy accumulation (Cinti 2011). Moreover, murine mature adipocytes undergo a reversible process of adipocytic/epithelial differentiation during pregnancy and lactation. During pregnancy, adipocytes seem to transform progressively into epithelial cells, forming the lobolo-alveolar part of the mammary glad responsible for milk production. At the end of lactation the lobolo-alveolar component disappears and a new adipocyte population restores the prepregnancy anatomy (Cinti 2011).
While little is known about the potential differentiation of mature adipocytes, more informations are available regarding the dedifferentiated population. Because adipose tissue is abundant and easily accessible tissue at most ages, dedifferentiated cells may be an attractive source of mesenchymal lineages for tissue engineering and other cell-based therapy (Matsumoto et al. 2008). The plastic properties of mature adipocytes are present also in dedifferentiated adipocytes.
In line with these observations, results showed that dedifferentiated adipocytes can be converted into fully differentiated cells, like adipocytes both in vitro and in vivo (Fernyhough et al. 2008), chondrocytes and skeletal myocytes in vitro (Kazama et al. 2008) under the appropriate culture conditions. In particular, dedifferentiated cells may be applicable to bone tissue engineering strategies and cell-based therapies, because they could convert into differentiated osteoblasts in vitro only by transient all-trans retinoic acid stimulation. Thus, dedifferentiated cells can undergo terminal osteoblast differentiation and osteoblast matrix formation, following subcutaneous injection into the peritoneal cavity of mice (Oki et al. 2008).
Moreover, dedifferentiated adipocytes also have the potential to rapidly acquire the endothelial phenotype in vitro and to promote neovascularization in ischemic tissue and vessel-like structure formation, suggesting that cells of endothelial and adipocyte phenotype may have a common precursor (Planat-Benard et al. 2004). These results also highlight the concept that adipose lineage cells represent a suitable new source for therapeutic angiogenesis in ischemic disease.
Data of literature demonstrated that dedifferentiated fat cells can differentiate into smooth muscle cell lineages under specific culture conditions (Sakuma et al. 2009). Green fluorescence protein labelled dedifferentiated fat cells were injected into cryo-injured bladder walls in mice, examining the ability of the fat cells to regenerate smooth muscle tissue after 14 and 30 days after transplantation. Significantly a larger amount of cell expressing α-smooth muscle actin plus green fluorescence protein were observed at the bladder wall injection sites in transplanted mice than in saline injected control mice.
Furthermore, some studies have suggested a close relationship between mature white adipocytes and cardiovascular cells, providing evidence that adipocytes and endothelial cells have a common progenitor cells (Jumabay et al. 2010). Results showed that dedifferentiated adipocytes can act as sources of spontaneously contracting cardiomyocytes in vitro, in which cardiomyocyte phenotype was identified by morphological observations, expression of cardiomyocyte-specific markers and pacemaker activity revealed by electrophysiological studies. These results support a possible link between adipocyte and cardiomyocyte differentiation that might be of importance for pathology and cardiac regeneration.
Moreover, data showed that dedifferentiated adipocytes could differentiated even in cells different from mesenchymal lineages, like neurogenic differentiation. Dedifferentiated cells displayed the capability of forming neurosphere-like structures with significantly increased of nestin expression level respect to non treated control cells (Hermann et al. 2004).
Furthermore, results achieved megakaryocytes and platelets from adipocyte cells with a similar ultrastructures of cells obtained from bone marrow CD34-positive cell. In addition, adipocyte-derived platelets exhibited surface expression of P-selectin and bound fibrinogen upon stimulation with platelet agonists, suggesting that these platelets were functional (Matsubara et al. 2012).
Taken together, the results shown above indicate the capability of the dedifferentiated adipocytes to differentiate into multiple cell lineages. This results might be interpreted as a return back to a noncommitted status for dedifferentiated adipocytes, which was favoured by the culture conditions. These findings suggest that de-differentiated cells have the molecular signature of a reprogrammed cell with features similar to stem cells.
Mature and Dedifferentiated Adipocytes Maintain the Survival and Differentiation of Hematopoietic Stem Cells
Experiments performed in mice demonstrated the presence of hematopoietic progenitors in adipose tissue able to reconstitute hematopoiesis in lethally irradiated animals by systemic infusion of adipose-derived stem cells (Cousin et al. 2003). This beneficial effect could be assigned to the presence of hematopoietic stem cells in the graft, the differentiation of injected mesenchymal stem cells into hematopoietic repopulating cells or the ability of the donor adipose cells to promote the differentiation of residual endogenous hematopoietic progenitors in the host (Corre et al. 2006). In line with this latter hypothesis, results showed that dedifferentiated adipocytes support the complete in vitro differentiation of hematopoietic progenitors without a significant difference in the well-known hematopoietic-supporting capacity of adipose tissue-derived mesenchymal stem cells (Poloni et al. 2012a).
Other data compared the hematopoietic supporting capacity of adipocytes differentiated in culture and adipose-derived MSCs (Ookura et al. 2007; Corre et al. 2006). Umbelical cord blood CD34 + CD38- cells were co-cultured on MSCs or adipocytes and the results showed that the hematopoietic-supporting capacity of MSCs decreased with adipocyte differentiation. However, CD34 + CD38- cells co-cultured with adipocytes preserved the ability to engraft in NOD/SCID mice, suggesting that adipocytes maintain the ability to support transplantable SCID-repopulating cells.
Moreover, a co-culture system of CD34+ cells seeded onto BM-derived adipocytes was studied to investigated their role in supporting hematopoiesis (Corre et al. 2004). These differentiated cells supported complete myeloid and lymphoid differentiation from hematopoietic stem cells, but they not supported the proliferation of immature progenitors. Moreover, the same authors showed that adipocytes differentiated from adipose-derived stem cells secreted cytokines promoting the differentiation of hematopoietic committed progenitors, like IL-6, G-CSF and GM-CSF, and cytokines inhibiting the proliferation of stem cells, like MIP1α (Corre et al. 2006).
Recently data demonstrated that isolated mature adipocytes co-cultured for long time with CD34+ cells has the ability to support the differentiation of hematopoietic stem cells as adipose tissue-derived MSCs (Poloni et al. 2012a, 2013). Because it is abundant and accessible, adipose tissue could be a convenient source of cells for the short-term reconstitution of hematopoiesis.
For many years adipose tissue was regarded as just a heat insulator and store of excess free fatty acids that could be released when needed. Now, it is considered a critical organ involved in energy regulation, inflammation, immune response thought intricate signals and interrelationships with other cells (Kershaw and Flier 2004). The immunoregulatory capacity of mature adipocytes during the dedifferentiation process was analyzed, studying their behaviour in co-cultures with allogeneic lymphocytes. Results showed that the morphological changes of mature adipocytes during the culture time were associated with functional changes. Indeed, dedifferentiated adipocytes were able to inhibit the proliferation of stimulated lymphocytes in direct co-culture, while mature fat cells stimulated their growth. These features may be associated with the ability of adipose tissue to promote inflammation via cytokine production and with the immunoregulatory capacity of MSCs (Gregor and Hotamisligil 2011) derived from different sources such as adipose tissue (Kronsteiner et al. 2011) bone marrow, amniotic fluid and chorionic villi (Krampera et al. 2006; Poloni et al. 2011, 2012b).
References
Barrilleaux B, Phinney DG, Prockop DJ, O’Connor KC (2006) Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng 12(11):3007–3019
Bibikova M, Fan JB (2010) Genome-wide DNA methylation profiling. Wiley Interdiscip Rev Syst Biol Med 2(2):210–223
Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C (1995) Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 36:1211–1226
Cinti S (2011) Between brown and white: novel aspects of adipocyte differentiation. Ann Med 43(2):104–115
Cinti S (2009) Transdifferentiation properties of adipocytes in the adipose organ. Am J Physiol Endocrinol Metab 297(5):E977–E986
Corre J, Barreau C, Cousin B, Chavoin JP, Caton D, Fournial G, Penicaud L, Casteilla L, Laharrague P (2006) Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J Cell Physiol 208(2):282–288
Corre J, Planat-Benard V, Corberand JX, Pénicaud L, Casteilla L, Laharrague P (2004) Human bone marrow adipocytes support complete myeloid and lymphoid differentiation from human CD34 cells. Br J Haematol 127(3):344–347
Cousin B, André M, Arnaud E, Pénicaud L, Casteilla L (2003) Reconstitution of lethally irradiated mice by cells isolated from adipose tissue. Biochem Biophys Res Commun 301(4):1016–1022
Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25(1):4–7
De Matteis R, Zingaretti MC, Murano I, Vitali A, Frontini A, Giannulis I, Barbatelli G, Marcucci F, Bordicchia M, Sarzani R, Raviola E, Cinti S (2009) In vivo physiological transdifferentiation of adult adipose cells. Stem Cells 27(11):2761–2768
Fernyhough ME, Hausman GJ, Guan LL, Okine E, Moore SS, Dodson MV (2008) Mature adipocytes may be a source of stem cells for tissue engineering. Biochem Biophys Res Commun 368(3):455–457
Gimble J, Guilak F (2003) Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5(5):362–369
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Hermann A, Gastl R, Liebau S, Popa MO, Fiedler J, Boehm BO, Maisel M, Lerche H, Schwarz J, Brenner R, Storch A (2004) Efficient generation of neural stem cell-like cells from adult human bone marrow stromal cells. J Cell Sci 117(Pt 19):4411–4422
Jumabay M, Zhang R, Yao Y, Goldhaber JI, Boström KI (2010) Spontaneously beating cardiomyocytes derived from white mature adipocytes. Cardiovasc Res 85(1):17–27
Kazama T, Fujie M, Endo T, Kano K (2008) Mature adipocyte-derived dedifferentiated fat cells can transdifferentiate into skeletal myocytes in vitro. Biochem Biophys Res Commun 377(3):780–785
Kershaw EE, Flier JS (2004) Adipose tissue as an endocrine organ. J Clin Endocrinol Metab 89(6):2548–2556
Krampera M, Cosmi L, Angeli R, Pasini A, Liotta F, Andreini A, Santarlasci V, Mazzinghi B, Pizzolo G, Vinante F, Romagnani P, Maggi E, Romagnani S, Annunziato F (2006) Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells 24(2):386–398
Kronsteiner B, Wolbank S, Peterbauer A, Hackl C, Redl H, van Griensven M, Gabriel C (2011) Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev 20(12):2115–2126
Matsubara Y, Murata M, Ikeda Y (2012) Culture of megakaryocytes and platelets from subcutaneous adipose tissue and a preadipocyte cell line. Methods Mol Biol 788:249–258
Matsumoto T, Kano K, Kondo D, Fukuda N, Iribe Y, Tanaka N, Matsubara Y, Sakuma T, Satomi A, Otaki M, Ryu J, Mugishima H (2008) Mature adipocyte-derived dedifferentiated fat cells exhibit multilineage potential. J Cell Physiol 215(1):210–222
Oki Y, Watanabe S, Endo T, Kano K (2008) Mature adipocyte-derived dedifferentiated fat cells can trans-differentiate into osteoblasts in vitro and in vivo only by all-trans retinoic acid. Cell Struct Funct 33(2):211–222
Ono H, Oki Y, Bono H, Kano K (2011) Gene expression profiling in multipotent DFAT cells derived from mature adipocytes. Biochem Biophys Res Commun 407(3):562–567
Ookura N, Fujimori Y, Nishioka K, Kai S, Hara H, Ogawa H (2007) Adipocyte differentiation of human marrow mesenchymal stem cells reduces the supporting capacity for hematopoietic progenitors but not for severe combined immunodeficiency repopulating cells. Int J Mol Med 19(3):387–392
Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284:143–147
Planat-Benard V, Silvestre JS, Cousin B, André M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Pénicaud L, Casteilla L (2004) Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation 109(5):656–663
Poloni A, Maurizi G, Leoni P, Serrani F, Mancini S, Frontini A, Zingaretti MC, Siquini W, Sarzani R, Cinti S (2012a) Human dedifferentiated adipocytes show similar properties to bone marrow-derived mesenchymal stem cells. Stem Cells 30(5):965–974
Poloni A, Maurizi G, Serrani F, Mancini S, Discepoli G, Tranquilli AL, Bencivenga R, Leoni P (2012b) Human AB serum for generation of mesenchymal stem cells from human chorionic villi: comparison with other source and other media including platelet lysate. Cell Prolif 45(1):66–75
Poloni A, Maurizi G, Serrani F, Mancini S, Zingaretti MC, Frontini A, Cinti S, Olivieri A, Leoni P (2013) Molecular functional characterization of human bone marrow adipocytes. Exp Hematol 41(6):558–566
Poloni A, Maurizi G, Babini L, Serrani F, Berardinelli E, Mancini S, Costantini B, Discepoli G, Leoni P (2011) Human mesenchymal stem cells from chorionic villi and amniotic fluid are not susceptible to transformation after extensive in vitro expansion. Cell Transplant 20(5):643–654
Sakuma T, Matsumoto T, Kano K, Fukuda N, Obinata D, Yamaguchi K, Yoshida T, Takahashi S, Mugishima H (2009) Mature, adipocyte derived, dedifferentiated fat cells can differentiate into smooth muscle-like cells and contribute to bladder tissue regeneration. J Urol 182(1):355–365
Sudo K, Kanno M, Miharada K, Ogawa S, Hiroyama T, Saijo K, Nakamura Y (2007) Mesenchymal progenitors able to differentiate into osteogenic, chondrogenic, and/or adipogenic cells in vitro are present in most primary fibroblast-like cell populations. Stem Cells 25:1610–1617
Sugihara H, Yonemitsu N, Miyabara S, Toda S (1987) Proliferation of unilocular fat cells in the primary culture. J Lipid Res 28(9):1038–1045
Tosh D, Slack JMW (2002) How cells change their phenotype. Nat Rev Mol Cell Biol 3:187–194
Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, Sato K, Inoue K, Nagase T, Koshima I, Gonda K (2006) Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 208:64–76
Zhang HH, Kumar S, Barnett AH, Eggo MC (2000) Ceiling culture of mature human adipocytes: use in studies of adipocyte functions. J Endocrinol 164(2):119–128
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Poloni, A., Maurizi, G. (2014). Molecular and Functional Characterization of Human Adipocytes. In: Hayat, M. (eds) Tumors of the Central Nervous System, Volume 12. Tumors of the Central Nervous System, vol 12. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7217-5_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-7217-5_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7216-8
Online ISBN: 978-94-007-7217-5
eBook Packages: MedicineMedicine (R0)