Abstract
Neuroendocrine differentiation (ND) is widely observed in prostate cancer (PC). Its role in clinical practice is controversial, but preclinical and clinical evidences underline the association of ND with poor prognosis in PC patients. Neuroendocrine (NE) cells could condition the PC progression, mainly stimulating the PC exocrine neoplastic cells proliferation through the production of paracrine growth factors. Thus, the castrated adapted neoplastic cells are favored to outgrowth through an androgen receptor independent mechanism. Moreover proportion of NE cells in PC increases because of tumor treatment, mainly androgen deprivation therapy, enormously amplifying the promotion of the PC exocrine component growth stimulated by neuroendocrine paracrine growth factors.
This chapter provides an overview of the most relevant clinical studies demonstrating a significant correlation between ND and PC behavior, indicating that ND could represent a prognostic parameter in PC, and strongly suggesting that NE cells in a castrate resistant patients could be targeted through specific treatment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Prostate Cancer
- Androgen Receptor
- Prostate Specific Antigen
- Androgen Deprivation Therapy
- Prostate Cancer Patient
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Distribution and Origin of NE Cells in Normal Gland
The normal human prostate ducts and acini epithelium is composed of two layers cells, including luminal secretory, basal and neuroendocrine (NE) cells (di Sant’Agnese 1992, 1998; Cohen et al. 1994).
Specificity of NE cells has been systematically defined during the 1980s. Thus NE cells have been described as an important component of the prostate (di Sant’Agnese 1992; Berruti et al. 2000).
The origin of these cells in normal prostate is not completely known. In fact different theories about their ontogenesis have been proposed. The most recent studies have postulated that NE, basal and secretory luminal cells originate from a common endodermal pluripotent stem cell (Huss et al. 2004). Thus, it has been hypotized that stem cells in multiple stem cell units (Isaacs and Coffey 1989; Bonkhoff et al. 1994; Bonkhoff 1996) with an unlimited self-renewal ability provide, with an unlimited self- renewal ability, progeny able to differentiate into either transit-amplifying or NE cells (Bonkhoff et al. 1994; Bonkhoff 1996). The luminal secretory cells or basal cells derive subsequently from the transit-amplifying cells. According to this theory, the prostate specific antigen (PSA) has been demonstrated also in NE cells, suggesting the same common precursor of secretory cell (Aprikian et al. 1993). Moreover, the expression of CD44, a marker of lymphocytes and cancer stem cells, has been observed in cells of NE phenotype (Palapattu et al. 2009). On the other hand NE cells could be represent a specific cell lineage of neural crest origin, substantially distinct from urogenital sinus- derived prostate secretory and basal cells (Pearse and Takor 1979).
NE cells are not homogenously distributed in the prostate glands, being consistently found in the periurethral ducts and veru montanum regions. NE cells are generally more numerous in the transition and the peripheral zones than in the central zone (Sant’Agnese 1998).
NE cells are normally not identifiable on standard stained histological sections. They are only recognized by electron microscopy or immunohistochemical staining for NE markers, being chromogranin A (CGA) and synaptophysin the most common.
As scattered or small groups, the distribution of NE cells according to electron microscopy studies could be in two different manner: (i) open, with cells directly extended to gland lumen; and (ii) closed, with cell dendritic-like processes extending between adjacent cells, on the basal lamina and strictly related to stromal nerves (Kamiya et al. 2008).
Intracinar and intraductal prostatic NE cells secrete serotonin and many other neuropeptides. Prostatic NE cells contain dense-core cytoplasmic granules with variable sizes and morphologies, storing peptide hormones and pro-hormones and recognizable by electron microscopy. The products contained in neurosecretory granules are either as a single typology or as mix of different peptides such as CGA, chromogranin B (CGB), somatostatin, neuron-specific enolase (NSE), parathyroid hormone-related protein (PTHrP), bombesin, thyroid-stimulating hormone and calcitonin gene family (calcitonin, katacalcin, and calcitonin gene-related peptide), vasoactive intestinal peptide (VIP), neuropeptide Y, alpha-human chorionic gonadotropin (aHCG), bombesin/gastrin releasing peptide (GRP), thyroid stimulating hormone-like peptide, adrenomedullin, cholecystokinin, and vascular endothelial growth factor (VEGF) (Huang and di Sant’Agnese 2002) (Table 5.1).
Also NE peptides receptors have been identified on NE prostate cells, including receptors for serotonin (5HT1a) (Abdul et al. 1994), bombesin/GRP (GRPR) (Markwalder and Reubi 1999), neurotensin (Seethalakshmi et al. 1997), somatostatin (SSTR1-5) (Dizeyi et al. 2002), cholecytokinin, Neuropeptide Y and calcitonin (Wu et al. 1996). Thus, through a complex network of NE peptides and respective receptors on exocrine cells in prostate gland, NE cells may control the growth, differentiation and secretory activity of the prostatic epithelium through a paracrine mechanism. In addition, the activity of the NE cells activity may be regulated by the neural network.
2 Physiopathology of ND in PC
NE cells are also present in PC. Rarely, NE cells are the only neoplastic component in prostatic carcinoma, such as carcinoid tumors and small cell carcinomas (SCC). A component of SCC or carcinoid could be also associated to conventional adenocarcinoma. More commonly, however, conventional adenocarcinoma are associated by scattered NE cells and distributed as single or grouped elements.
2.1 SCC and Carcinoid Tumors
Pure SCC of the prostate represent no more than 1 % of all prostate carcinomas. They have a very aggressive behavior, being often locally advanced or metastatic at diagnosis (Erasmus et al. 2002). It is occasionally associated with paraneoplastic syndromes (Kawai et al. 2003). Rarely, SCC has been observed in conventional adenocarcinoma patients treated with hormonal therapy (Tanaka et al. 2001). Indeed SCC is more commonly a component of mixed tumors with conventional adenocarcinoma. Histologically, prostate SCC is similar to lung SCC, being constituted both by neoplastic small cells in a solid growth pattern, with fine cromatin and common nuclear crushing. Necrosis and high mitotic index is commonly observed (Yao et al. 2006). Immunohistochemical study can greatly help in diagnosis of this rare tumor. In fact neoplastic cells are commonly positive for one or more neuroendocrine markers, such as chromogranin, synaptophysin, CD56 or NSE. Dot-like positivity for Cytokeratin is commonly observed (Kawai et al. 2003). Differently from conventional prostate adenocarcinoma, prostate SCC does not express Androgen Receptor. Thus hormonal therapy is not adequate in the treatment of such tumors while chemotherapy may be responsible of initial clinical response (Helpap 2002).
Carcinoid is a very rare tumor in prostate. Microscopically all neoplastic cells show complete ND and the organoid pattern of growth is similar to neuroendocrine tumors of other districts. Also carcinoid could be part of mixed tumor with conventional adenocarcinoma. (Ghannoum et al. 2004).
2.2 Focal ND in PC
Focal ND occurs in conventional prostatic adenocarcinomas. As in normal prostate, NE cells are not distinguishable from other cancer cells, unless immunohistochemical staining for NE markers are used. Also in PC CGA is usually considered sensitive and specific. Some NE cells seem to be present in all conventional adenocarcinoma, but only about 5–10 % contain a large number of NE cells (Abrahamsson et al. 1987). They are described in a large spectrum of prostatic neoplasia, from prostatic intraepithelial neoplasia (PIN) (Bostwick et al. 1994) to metastatic disease (Bostwick et al. 2002).
3 The Origin of NE Cells in PC
The biological features of PC NE cells suggest that these cells are different from normal prostate NE cells.
Some observational evidences suggest that the cells are more similar to secretory cells that normal NE cells. As normal NE cells, PC NE cells do not express Androgen Receptor (AR) and PSA, normally present in secretory component (Huang et al. 2006). Also neuron-like processes of normal NE are often not observed in PC NE cells (Xing et al. 2001). Immunohistochemical studies have demonstrated that normally expressed basal cell markers of normal NE cells are not expressed by PC NE cells, such as p63 and cytokeratin 5 (Huang et al. 2006). On the contrary, they are positive for luminal secretory cells markers such as cytokeratin 18 (van Bokhoven et al. 2003). Also alpha-methylacyl-CoA racemase (AMACR), an enzyme involved in the ß-oxidation of fatty acids, expressed in PC non NE cells rather than in normal prostatic secretory cells, has been demonstrated in PC NE cells (Huang et al. 2006). In addition anti-apoptotic Bcl-2 protein is normally expressed by PC NE cells but not by normal NE (Segal et al. 1994). Finally genetic analysis support that PC NE cells are similar to PC secretive cells and not to normal NE (Sauer et al. 2006).
All these considerations have led to the frequent use of the term ‘NE-like’ PC cell to define this neoplastic population (Yuan et al. 2007). Thus the theory that PC NE cells derive from neural crest cells has been finally abandoned (Schron et al. 1984). Two main hypotheses justifying neuroendocrine differentiation in PC are taken into account, transdifferentiation and origin from a common neoplastic stem cell (Bonkhoff et al. 1995).
The transdifferentiation model seems to occur as in response to hormonal and growth factor microenvironmental changes. In fact it has been supported by in vitro experiments on LNCaP cells, an androgen-dependent cell line, developing ND through induction by androgen deprivation or other agents increasing intracellular levels of cAMP such as epinephrine (Cox et al. 2000) interleukin-6, (Deeble et al. 2001) and genistein (Pinski et al. 2006). Moreover, in vivo androgen deprivation can stimulate PC ND either in the animal model or in humans. In fact, an increase percentage of NE cells has been demonstrated in matched tumor samples collected from patients with early PC before and after androgen deprivation therapy (ADT) (Ahlgren et al. 2000). In patients with advanced PC a relative increase of CgA serum levels has been documented after ADT (Berruti et al. 2005). Antineoplastic treatment, not only androgen deprivation, seems to be responsible of ND in PC. In fact, fractionated ionizing radiation (IR) can stimulate ND in vitro in LNCaP (Deng et al. 2008) and docetaxel (DTX) can induce in animal model ND with the same relevance of the androgen deprivation. It has to be emphasized that both therapies are used in the treatment of castration resistant patients (Tang et al. 2009).
Recently, it has been demonstrated that human PC cell lines express the stem cell marker CD44 (Palapattu et al. 2009) as well as human PC tissues were highly positive for CD44 both in secretive and NE component. These evidences could suggest that PC NE cell and PCA secretive cells share the same potential progenitor.
Moreover, the ND in PC is not a stable phenotype. In fact NE trans-differentiation in vitro is a reversible phenomenon, as the neoplastic population could lose its neuroendocrine phenotype after the removal of the inducing agents (Palapattu et al. 2009).
Different signaling pathways are involved in the ND of PC. PI3K-AKT-mTOR pathway seem the main involved pathway (Wu and Huang 2007). The Notch signaling pathway, and specifically of hASH1 (human achaete-scute homolog-1 transcription factor) have been also demonstrated to be as responsible of ND setting (Guillemot et al. 1993). Finally, all treatments responsible of in vitro NE trans-differentiation in LNCaP cells caused deregulated expression of a unique set of genes (Mori et al. 2009).
4 The Function of NE Cells in PC
As NE tumor cells do not express AR, their growth is androgen independent. Therefore, they continue to survive and perform their functions in a milieu devoid of androgens, building a fine network of autocrine and paracrine relationships with the rest of the tumor, providing androgen-independent growth. In fact LNCaP xenografts, being androgen-dependent, cannot normally grow in castrated mice, while they can grow when also NE cells from a mouse NE tumor (NE-10) are transplanted in the castrated hosts (Jin et al. 2004). In vitro studies have demonstrated that NE cells products could favor growth and invasiveness of PC cell lines (Jongsma et al. 2000). Neuropeptides are active on different pathways, up-regulating proteins critical for tumor growth, invasiveness, angiogenesis and metastasis. PC NE cells may promote androgen-independent PC growth through the production of paracrine signals interacting with the secretory PC cells by AR dependent or independent mechanisms. In fact, one of the main pathway activated by neuropeptides is the same activated by androgens. It has been demonstrated that NF-kB pathway activated by neuropeptides could promote cancer growth AR pathway (Jin et al. 2008). Neuropeptides could act on G protein-coupled receptors, overexpressed in PC, and then aberrantly activate AR pathway even in the absence of androgens. The same authors through the development of gastrin related peptide (GRP) overexpressing LNCaP model demonstrated that this cell line showed androgen independent growth and an enhanced motility in vitro. Furthermore, in castrated nude mice, LNCaP-GRP derived tumors were very aggressive, and produced GRP, PSA and nuclear AR. Chromatin immune-precipitation studies of LNCaP-GRP suggested AR recruitment to the cognate promoter also in the absence of androgens (Jin et al. 2008).
The network of protein related to local invasion proteolytic enzyme urokinase-type plasminogen activator (uPA), plasminogen activator inhibitor-1 (PAI-1) and metalloprotease (MMP) are stimulated by bombesin (Festuccia et al. 1998). Bombesin and MMP-9 are highly expressed in high grade carcinoma (Ishimaru et al. 2002). Other MMPs are activated by neuropeptides (Sehgal and Thompson 1999). GRP increases MT1-MMP in androgen independent cell lines DU-145, amplifying their ability of Matrigel invasion (Nagakawa et al. 1999). Finally MMPs are highly expressed by androgen independent respect than androgen dependent cell lines.
Cytokines and their own receptors could contribute to androgen independent PC growth in androgen independent manner. IL-8 is normally overexpressed by PCa NE cells and its receptors CXCR1 has been demonstrated on PCa secretory cells (Huang et al. 2005). IL-8 promotes androgen independent growth and migration of LNCaP cells, a model of the in vivo paracrine mechanism of androgen independent growth and invasion (Lee et al. 2004).
Resistance to apoptosis of PCa is partially conferred by PC NE cells. Survivin is highly expressed by PC NE cells, subsequently more resistant to stress and then to apoptosis (Xing et al. 2001). Bcl-2, the most relevant anti-apoptotic agent, is not expressed by PC NE cells, but it is demonstrated in PC non NE cells, above all in those closest to PC NSE positive NE cells suggesting that apoptosis resistance in PC cells could be induced by NE cells neuropeptides (Segal et al. 1994). Finally bombesin and calcitonin are able to prevent apoptosis in PC cell lines (Salido et al. 2000, 2004; Vilches et al. 2004).
Neovascularization is also promoted by PC NE cells, mainly through VEGF and IL-8 (Chevalier et al. 2002) In particular, VEGF and neovascularization have been significantly reduced in prostate surgical sample of patients previously treated with complete androgen blockade, except in the areas with PC NE cells (Mazzucchelli et al. 2002). Moreover, the number of NE cells are predictor of neovascularization (Grobholz et al. 2000).
Finally VEGF expression seems to be significantly related to microvascular density (MVD), high tumor stage, high Gleason grade and shorter disease free survival (Borre et al. 2000). Some neuropeptides are involved in stimulation of proangiogenic factors VEGF and IL-8 in vitro model. Bombesin activates the expression of such factors in PC-3 cells, probably through NF- kB pathway (Levine et al. 2003). Instead calcitonin appears to stimulate vessel formation by acting directly on endothelial cells. CGA fragment 286–301, representing the C-terminal of pancreastatin, induced the invasive ability of PC-3 and DU-145 PC cell lines. CGA (286–301) also increased the haptotactic migration of these cells and the production of urokinase type plasminogen activator (Nagakawa et al. 1999). Through invasion assay, it has been demonstrated that gastrin-releasing peptide, calcitonin gene-related peptide, and parathyroid hormone-related protein increased invasive ability of PCa cells (Nagakawa et al. 2001).
5 Molecular Mechanisms of ND
NE phenotype is inversely correlated to active AR signaling (Wright et al. 2003). These data could explain increased ND, during inhibition of AR signaling, both in patients with androgen blockade and in LNCaP cell line in androgen-deprived media, through different mechanism, requiring activation of multiple protein, such as ERK (Wu et al. 2006). Thus, androgen deprivation is the main stimulator of ND, but it is not the only one. In fact, each factor determining increase of intracytoplasmic cAMP and then of cAMP-dependent kinase activity could favour ND in vitro model, such as epinephrine and forskolin, IL-6, IL-1 (Albrecht et al. 2004). IL-6 induced ND involving the protein tyrosine kinase pathway (Chung et al. 2000), JAK-STAT signaling, mitogen activated protein kinases (MAPKs), cyclic AMP-dependent protein kinase (PKA) phosphatidylinositol 3-kinase (PI3K) induction of cyclin-dependent kinase (CDK) inhibitor p27 (Kip1) and inhibition of CDKs (Mori et al. 1999). IL-6 induced ND in PC is irreversible respect to epinephrine induced ND and the aggressive phenotype in vitro and in vivo model seems to be related to IL-6 concentration used to treat LNCaP cells (Wang et al. 2004). Also induction of NFkB signaling, as inhibition of proinflammatoy enzyme COX-2 does, could promote ND (Meyer-Siegler 2001). Moreover inhibition of FGF signaling could induce ND in PC cells of transgenic mice promotes ND.
Recently, the activation of phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (PI3K-AKT-mTOR) pathway has been demonstrated as to be essential for ND in PC (Wu et al. 2006). In fact ND of LNCaP is induced also through activation of the PI3K-AKT-mTOR signaling pathways. Moreover, Rapamycin, an inhibitor of mTOR, significantly inhibited the expression of NSE in LNCaP cells induced by androgen withdrawal. Recently, it has been demonstrated that also irradiation of LNCaP can promote ND, through nuclear increasing of CREB, a transcription factor potentially enhancer of ND, and cytoplasmic accumulation of the transcription factor potentially suppressor of NED ATF2 (Deng et al. 2008). Protocadherin-PC, a member of protocadherin gene family, has been shown to be as critical for ND of PC through Wnt signaling activation (Yang et al. 2009).
Heparin binding epidermal growth factor (HB-EGF) activates ND through the involvement of mitogen-activated protein kinase (MAPK) signaling pathway (Kim et al. 2002).
Also autocrine neuropeptides themselves could be directly involved in ND. In fact, it has been documented that VIP, commonly secreted by PC NE cells, induces ND through activation of ERK, PKA and PI-3-kinase pathways (Collado et al. 2004, 2005).
6 NE Differentiation and Hormone-Refractory Prostate Cancer
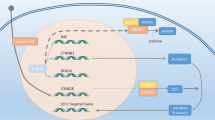
AR is normally present in both prostate stromal and secretory cells and, the androgen, acts in different manner in the two compartment. In fact, on stromal cells, it favors andromedins secretion, supporting the survival and proliferation of luminal secretary epithelial cells (Isaacs 2008), while in luminal secretory cells the androgen suppresses the cell growth through expression of p27Kip1 expression (Waltregny et al. 2001). Clinically, the long-term treatment of patients with androgen deprivation is responsible of the castration-resistant state. This condition has to be partially attributable to the alteration of AR signaling, including AR gene amplification, responsible of a response to low levels of circulating androgens, AR mutations, causing AR pathway activation through antiandrogens or weak androgens, the local synthesis/concentration of androgens, AR activation through growth factors/kinase pathways, and/or changes in AR coregulators (Scher and Sawyers 2005). The most relevant role in the conversion to androgen independent growth of PC seems to be played by ND. Thus, since androgen deprivation depresses the growth of cancer cells growth, translating the normal model to PC model, it can be assumed that the main effect of treatment occurs on the stromal cells, rather than the neoplastic secretory compartment. Neither AR mutation in secretory neoplastic cells, recorded in only 10 % of cases, could be justify a real change of activity in those cells (Buchanan et al. 2001). The paracrine role of stromal cells, sensible to Androgen deprivation, could be gradually substituted by NE cells insensible to androgen deprivation and able to secrete a large variety of neuropeptides and cytokines promoting survival and proliferation of neoplastic epithelial cells (Fig. 5.1).
Neuroendocrine cells in PCa. NE phenotype is inversely correlated to active AR signaling and, therefore, androgen deprivation is the main stimulator of NED that play a pivotal role in conversion to androgen independent growth of Pca. Thus since androgen deprivation depresses the growth of cancer cells, the main effect of treatment occurs on the stromal cells. The paracrine role of stromal cells, sensible to androgen deprivation, could be gradually substituted by NE insensible to androgen deprivation and able to secrete a large variety of neuropeptides and cytokines promoting survival and proliferation of neoplastic epithelial cells. Neuropeptides are active on different pathways, upregulating proteins critical for tumor growth, invasiveness, angiogenesis and metastasis
7 The Diagnosis of Neuroendocrine Prostate Cancer
7.1 Serological Markers
The identification of neuroendocrine markers in the PC patients serum represents a more complete indicator and more objective quantification of ND of tumors, because it corresponds to the entire primary tumor and its associated metastases.
Herein, we list the most relevant serological markers associate to ND:
CGA . This marker is a tumor cell population product, and sometimes, the clue to diagnose special subtypes, such as pure small cell prostate cancer. CGA is an excellent marker for ND in tumors and its measurement is also useful to identify prostatic carcinoma in patients with not elevated PSA. Angelsen and co-workers (1997) showed that the number of CGA-positive NE cells in tumoral tissue significantly related with serum CGA levels in patients with prostatic carcinoma. However elevated serum CGA level could be observed in unrelated tumor conditions, such as in patients with impaired renal function or in those receiving omeprazole treatment for peptic ulcer. Kadmon and co-workers (1991) reported elevated plasma CGA levels in 48 % patients with metastatic PC. Similarly, (Logothetis and Hoosi 1992) found also elevated plasma bombesin levels in 47 % of PC patients treated for androgen-independent growth.
Kadmon observed among advanced stage of PC patients a relative low frequency of high CGA levels (17 %) respect to 33 % of patients with normal conventional PSA and PAP markers, suggesting that high CGA patients was not representative of usual progressive tumor status (Kadmon et al. 1991). The fact that prostatic carcinomas expressed neuropeptide markers (CgA, 17 %; NSE, 15 %) before any endocrine therapy suggests that neuroendocrine products may induce PC progression independently of androgen withdrawal. Data by Deftos et al. (1996), and a more recent by Kimura and co-workers (1997), demonstrated that CGA could be an useful marker in advanced disease. Tarle and Rados (1991) found that elevated plasma NSE levels was observed more frequently in untreated PC patients with localized tumors (28.6 %) than in untreated subjects with disseminated disease (10.7 %).
In a comparison study of serum levels of CGA, pancreastatin, a breakdown product of CGA, c CBG (Angelsen et al. 1997) and chromogranin C (CGC) (Schmid et al. 1994), CGA appears to be the best marker of neuroendocrine prostate tumor activity.
Recently, plasma CGA was assessed by ELISA in 14 patients with Castration resistant PC (CRPC) receiving 3-weekly docetaxel. Increased plasma CgA was observed in 64.3 % of patients. No correlation between baseline CGA and PSA has been observed. Two patients with PSA < 10 ng/ml had elevated CGA. Baseline CGA was not conditioned by clinical parameters such as presence of metastasis, metastasis sites and time to develop CRPC status. Seven patients (50 %) showed PSA-response and five (36 %) CGA-response. In two patients PSA response and CGA response were discordant. Compared to men with normal baseline CGA, a higher proportion of those with elevated baseline CGA had PSA response (55 % vs 40 %), symptomatic response (66 % vs 40 %) and radiological response (55 % vs 20 %). Two patients with symptomatic response had only CGA response. Three patients with disease progression, despite PSA response, had increasing CGA. On the basis of these results, CGA and PSA have been proposed as complementary tumor biomarkers in castration resistant prostate cancer and CGA may be useful to predict the response to therapy with DTX. Increased serum CGA during therapy may be associated with poor prognosis, whereas CGA response is likely to be associated with clinical response (Sarkar et al. 2010). Moreover, in a series of 135 patients with prostatic carcinoma and 28 with benign prostatic hyperplasia plasma CGA, NSE and other neuroendocrine biomarkers have been analyzed and compared to clinical and pathological stages of disease. Particularly elevated levels of CGA were detected in 15 % of patients with PC, before any treatment, but elevation of plasma CGA and NSE levels was observed respectively in 55 and 30 % of the patients during hormone resistant prostate cancer progression. In addition log-rank analysis in stage D3 patients revealed a statistically significant difference between positive and negative CGA groups These data confirmed the role of CGA and, at a lesser extent, of NSE in predicting the prognosis of PC patients even if their role as markers of ND still need additional investigations. Finally, serum NSE and plasma CGA were evaluated in 141 patients with prostatic hyperplasia (BPH), 54 patients with PIN, and 159 patients with Pca; 119 patients were bearing hormone-naive disease and 40 were bearing hormone-refractory disease. Supernormal CGA was observed more frequently in Stage D2 disease patients (45.5 %) compared with Stage D1 (33.3 %), Stage C disease (16.7 %), Stage A/B disease (18.8 %), PIN (25.9 %), and BPH (17.0 %) patients (P < 0.02). Supernormal NSE did not show differences in any of the patient subgroups stages. Elevated CGA was observed in 36.0 % of metastatic patients with hormone-naive disease and in 45.0 % of metastatic patients with hormone-refractory disease, although without statistical significance. Supernormal NSE and CgA values were observed as predictors for poor prognosis in patients with hormone-refractory disease. Significant decreased baseline CgA values has been observed in 1 of 12 patients who received luteinizing hormone-releasing hormone analogs and in 2 of 12 patients receiving chemotherapy. Elevated CGA levels correlated with poor prognosis and were scarcely influenced by either endocrine therapy or chemotherapy (Berruti et al. 2000).
Gastrin-releasing peptide (GRP ), a 27 amino acids neuropeptides, seems to play a role as an autocrine/paracrine growth factor in several cancers. Progastrin-releasing peptide (ProGRP), comprehending three different subtypes of precursors for GRP, has a longer half-life than GRP, with levels similar to GRP itself. The values show excellent sensitivity and correlation with the therapeutic response in neuroendocrine tumors. Indeed ProGRP is mainly used in clinical diagnosis and follow-up of small cell lung cancer. ProGRP levels have also been observed to be elevated in other tumors with neuroendocrine features, such as colorectal, thyroid, and breast cancer. In 60 patients with benign BPH and 200 with PCa, increased ProGRP value was significantly observed in the androgen-independent group (P < 0.0001). In a subset of patients, the ProGRP levels increased transiently when the cancer shifts to became androgen independent status, but remained unchanged or decreased at the androgen-dependent stage. In addition positive ProGRP immunostaining occurred in a different distribution in tumoral tissues when comparing to CGA immunostaining. The clinical results confirm the existence of a regulatory mechanism for GRP, demonstrated in cell lines. These findings suggest that GRP is a growth factor potentially upregulated by androgens but it does not rely principally on androgen modulation (Yashi et al. 2003). Subsequently, serum ProGRP status was determined in 460 men with benign and malignant prostatic diseases, chronic renal failure, and healthy controls. The increased serum of ProGRP was observed in patients with the progression of PC into metastatic and androgen-independent stages. In addition multivariate analysis demonstrated that Performance Status (PS), serum ProGRP, and nadir PSA held an independent predictive value for Progression Free Survival (P < 0.05). Finally Serum ProGRP was the most significant predictor among pre-treatment factors in this model (P = 0.0094) (Yashi et al. 2003).
7.2 Immunohistochemistry
The role of immunohistochemistry in the last decades has been directed to the definition of potential prediction of androgen resistant status related to ND. In this setting, however, no univoque method of interpretation has been used and multiple neuroendocrine markers have been proposed. The methods of evaluation of ND in PC are mainly based of definition of percentage of positive cells (Berruti et al. 2010) The definition of cut off also remain less characterized. An interesting paper defined as how critical for prognosis, the number of NE cells per hot spot area and the pattern of CGA positivity are. In fact, tumors with more than 30 CGA positive cells for hot spot area have a significant worse prognosis than those tumors with less than 30 CGA positive cells for host spot area. In addition, cases with large clusters of NE cells were significantly more aggressive compared to tumors with no cluster or small clusters of NE cells (Grobholz et al. 2005). Although many immunohistochemical markers have been proposed in order to correctly define the ND in PC, CGA remains the most reliable. NE cells were are more common in higher grade and stage disease, but no difference of 5-year survival between patients with NE cell-positive and -negative tumors have been recorded (Allen et al. 1995). However, McWilliam et al. (1997) found that ND correlates with high grade tumor, bone metastasis and shorter patient survival. In addition, Weinstein et al. (1996) reported that ND determined through immunohistochemistry using CGA represented an independent prognostic factor for biochemical progression in clinical organ-confined PC treated by radical prostatectomy. Moreover Kokubo et al. (2005) demonstrated that 22 % of stage D2 PC showed immunohistochemical CGA overexpression and that CGA staining significantly related to shorter time to recurrence after hormone therapy. Finally, Kamiya and co-workers (2008) demonstrated that positive staining for independent CGA and combined CGA with NSE after hormone therapy in stage D2 PC was significantly related to shorter overall survival (OS) representing, also, independent factors in multivariate analysis. Also when the CGA expression was assessed on PC biopsies seems to be a new predictive marker of early resistance to ADT (Berruti et al. 2010).
8 Imaging of Neuroendocrine Prostate Cancer
8.1 PET
Positron emission tomography (PET) is a new imaging modality which has been widely used for the detection of metastasis in various malignancies. F18-fluorodeoxyglucose (FDG), the most common radiotracer, is used for glycolysis evaluation and glucose transporter expression, because most of malignant tumors show increased glucose metabolism. Unfortunately, the use of FDG-PET is not common PCa because of low rate of glycolysis of the tumor cells. In addition, physiologic urinary excretion of FDG does not allow a good visualization of the pelvis (Powles et al. 2007). Indeed Liu et al. found only 4 % sensitivity for identification of primary PCa though FDG-PET (Liu et al. 2001).
But an increased sensitivity for detecting Pca has been obtained through using continuous bladder irrigation (Oyama et al. 2001). FDG-PET could be used to identify local recurrence and distant metastases in patients with increasing PSA after definite local therapy for PCa (Schoder et al. 2005).
In addition, Morris et al. reported that using PSA levels, bone scintigraphy and soft tissue imaging as references, FDG-PET might be a promising outcome measure after chemotherapy in prostate cancer (Morris et al. 2005). Recently, a case was reported showing FDG PET-CT intense uptake in neuroendocrine tumor of the prostate with multiple metastases (Liu 2008). In fact, the cells with ND secrete a variety of factors that can influence growth patterns and metabolic pathways involved in this process and the tumor has different biological behaviour.
8.2 Peptide Imaging (PET or Scintigraphic Detection)
Alternatively, neuroendocrine tumors of the prostate can be imaged through the use of probes of the receptors specifically expressed by prostate cancer cells behaving neuroendocrine phenotype. These receptors bind peptides that play a modulator role also in numerous cancers (Reubi 2003; Reubi et al. 2005). This is the key reason or the use of regulatory peptide receptors in cancer imaging in recent times. The first, and currently best, example of targeted peptide receptors is represented by the somatostatin receptors, discovered to be overexpressed in mot neuroendocrine tumors (Reubi 2003).
Somatostatin. In somatostatin-based cancer imaging, a stable somatostatin analog linked to a chelator that can bind radioactive metals such as 111In, 99mTc, or 68Ga, is injected intravenously. The tracer will selectively bind to somatostatin receptors if the patient cancer contains somatostatin receptors in large amounts. The internalization of ligands lead to a radioactivity accumulation in the tumor, compared with the rest of the organs. Normally, rapid and specific uptake is observed in the tumor, and concomitantly in the kidney and bladder, because of predominant urinary radioligands excretion.
The first commercially available agent was 111 the In- diethylenetriaminepentaacetic acid (DTPA)0-octreotide, but its binding affinity to sst2 is moderate and it is not a suitable chelator for b-emitters such as 90Y and 177Lu. For these radiometals, it is better to use the macrocyclic chelator 1, 4, 7, 10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), because of the formation of stable metal complexes. The most frequently used DOTA-coupled, somatostatin-based radiopeptides are [DOTA0, Tyr3]-octreotide (DOTATOC) and [DOTA0, Tyr3, Thr8]-octreotide (DOTATATE) (Rufini et al. 2006). In order to improve tracer pharmacokinetics coupling octreotide and octreotate has been developed (Schottelius et al. 2004). 6-Hydrazinopyridine-3-carboxylic acid-TATE (HYNIC-TATE), HYNIC-TOC, and N4-TATE were designed for high-specific-activity labeling with 99mTc, demonstrating relevant additional compounds in octreotide backbone clinical practice (Rufini et al. 2006).
Bombesin . Bombesin-based ligands with high affinity for gastrin-releasing peptide (GRP) receptors have been developed. An early report used a 99mTc-based ligand, RP 527, an N3S chelator coupled to bombesin demonstrated identification of primary prostate cancers and their metastases (Van de Wiele et al. 2008). 177Lu-AMBA is a more recently developed analog, with possible diagnostic and therapeutic applications (Lantry et al. 2006). Another bombesin agonist has been developed with high affinity to all 3 bombesin receptors with possibly broader indications (Zhang et al. 2004). 99mTc-demobesin is an interesting compound, that does not internalize significantly into PC-3 tumor cells but ableto label in vivo GRP-R–expressing PC-3 tumors more intensely and for a longer time than the best available GRP-R agonists (Cescato et al. 2008). This extends the paradigm shift on tumor imaging observed earlier with somatostatin antagonists to GRP-R.
Several of these new bombesin-based radiopeptides are conjugated to DOTA and can be labeled with 68Ga. PET studies with a 68Ga-labeled bombesin analog were performed in 11 patients with prostate cancer (Hofmann et al. 2004). Primary tumors were visible in all patients, being the smallest tumor size 5 mm and a plateau of tumor uptake at 15–25 min after injection. Lymph node metastases have been found in three of these patients. However, in four patients a significant nonspecific enrichment has been observed in the upper abdomen, probably due to the pancreas uptake.
9 Therapy of Neuroendocrine-Differentiated Prostate Cancers
9.1 ND and New Treatment Modalities
Despite the initial efficacy of androgen ablation therapies, hormone refractory stage is the normal evolution of PC. Nowadays, a truly hormone-refractory condition is considered when the patient no longer responds to any of the second-line hormonal alternatives. This disease phase often parallels ND in PC. Currently chemotherapy represents the only non-experimental option available. Recently, it was demonstrated that 3-weekly schedule of DTX and prednisone is the standard first-line chemotherapy hormone-refractory PC phase, with a positive impact on OS and time to progression (TTP) (Facchini et al. 2010). But response duration with current chemotherapies is often short. Therefore, novel therapeutic options are needed.
Thus novel approaches currently being tested in early clinical trials include angiogenesis inhibitors, immunological therapies, gene therapy, differentiation therapies and interference in growth-factor-mediated pathways.
9.2 Somatostatin Analogues
Newly developed somatostatin analogues could be useful in the treatment of PC (Hansson and Abrahamsson 2003). Potential mechanisms of antitumor action include the suppression of circulating levels of trophic hormones and growth factors as well as direct effect on tumoral growth, involving autocrine/paracrine mechanisms.
Somatostatin family include regulatory peptides produced by normal neuroendocrine, inflammatory and immune cells, but also by many tumor activated cells.
Exogenously administration of somatostatin induces a wide range of effects on multiple target sites. Thus selective non-peptide agonists have been developed for four of the somatostatin receptors (SSTR) subtypes. Main somatostatin effect is the prevention of cell proliferation through inducing cell cycle arrest and apoptosis. These effects are mediated by SSTR expressed by tumor cells and by non-tumor-cells, secreting hormones and growth factors promoting tumor cell growth. Four SSTRs induce cell cycle arrest via protein tyrosine phosphatase (PTP)-dependent modulation of MAPK, associated with induction of retinoblastoma tumor suppressor protein and p21. In addition SSTR3 triggers apoptosis, through activation of p53 and the pro-apoptotic protein BAX. Thus it seems that somatostatin plays an important role in tumor development and in the future there may be a potential role for somatostatin analogues in the treatment of the PC (Hansson and Abrahamsson 2003). In this view, recently 38 stage D3 PC patients (mean age 71.8 ± 5.9 years) have continued to receive androgen ablation therapy in combination with oral dexamethasone (4 mg daily for the first month of treatment, tapered down to 1 mg daily by the fourth month, with 1 mg daily maintenance dose thereafter) and somatostatin analog (20 mg octreotide i.m. injections every 28 days). Twenty-three of those thirty-eight patients (60.5 %) had partial responses (PR, ≥50 % PSA decline), 9 (21.1 %) had stable disease and 7 (18.4 %) had disease progression. In 47.7 % (18 of 38) of patients, serum PSA levels decreased with treatment but did not return to their respective baselines until the end of follow-up (or death from non-prostate cancer-related causes). All patients reported significant and durable improvement of bone pain and PS (for a median duration of 14 months; 95 % CI, 9–19 months). In addition a statistically significant (P < 0.01) reduction of serum insulin-like growth factor-1 levels was recorded in patients with response to the combination therapy (Koutsilieris et al. 2004). On the basis of this latter study, a randomized controlled clinical trial of 38 stage D3 patients (mean age 72.8 ± 6.8 years) has been performed in order to compare the combination of somatostatin analog (octreotide 20 mg i.m. every 28 days) and oral dexamethasone (4 mg daily for 1 month, gradually reduced to 1 mg daily by the fourth month, with a 1 mg daily maintenance dose thereafter) plus zoledronate (4 mg i.v. every 4 weeks) vs. zoledronate only. All patients in both arms remained in basic androgen blockade. Partial responses (PR, > or =50 % PSA decline) was recorded in 13 out of 20 patients with combination therapy vs. none with zoledronate (Mitsiades et al. 2006).
It was recently proposed as therapy of ND in hormone-independent PC, a combination of oestrogens and somatostatin analogues. The combination of ethinyl estradiol and the somatostatin analogue lanreotide, binding 3/5 SSTRs, showed a favourable toxicity profile and offered objective and symptomatic responses in patients with refractoriness to conventional hormonal therapy strategies. In addition a higher median OS was observed (Sciarra et al. 2003).
9.3 Serotonin Antagonists
NE cells produce and secrete 5-HT, a biogenic amine, neurotransmitter and potent mitogen associated with tumor growth. 5-HT receptors (5-HTR), such as 5-HTR1 and 5-HTR4, are overexpressed in hormone refractory PC tissues and in PC cell lines. Recently promising results have been demonstrated with the use of 5-HTR antagonists (Abrahamsson et al. 1986).
9.4 Bombesin Antagonists
Bombesin produces androgen-dependent growth and invasiveness of PC cells. Bombesin also carries metastatic potential in androgen-insensitive PC. Therefore, bombesin-like antagonists could become an effective treatment option in the future (Hansson and Abrahamsson 2003; Levine et al. 2003).
9.5 Cytokines
Recently, IL-6, an inflammatory cytokine that not only regulates the immune response, but also modulates cancer cell growth, differentiation and survival has been proposed as a possible target in the treatment of androgen resistant PC patients. Recently 53 patients with castration-resistant PC pre- treated with taxane chemotherapy were treated with 6 mg/kg anti-IL-6 antibody, CNTO328 i.v. every 2 weeks for 12 cycles. Two patients (3.8 %; 95 % CI, 0.5–13.0 %) had PSA response. None of the 31 patients with measurable disease had a RECIST (Response Evaluation Criteria in Solid Tumors) response but 7 (23 %) had stable disease. After a median follow-up of 14.8 months, the median progression-free survival (PFS) was 1.6 months (95 % CI, 1.6–1.7) and median OS was 11.6 months (95 % CI, 7.5–19.0). Thirty-two out of thirty-eight patients had C-reactive protein plasma levels decline at 6 weeks. In conclusion, CNTO328 resulted in a PSA response rate of 3.8 % and a RECIST stable disease rate of 23 %, while declining C- reactive protein levels during treatment may reflect biological activity. Despite evidence of CNTO-mediated IL-6 inhibition, elevated baseline IL-6 levels portended a poor prognosis.
In another open-label phase II trial mitoxantrone/prednisone (M/P) with and without CNTO328 was performed in metastatic patients with castration-resistant PC who have had received DTX- based chemotherapy. This trial concluded that while CNTO328 plus M/P appeared well tolerated, improvement in outcomes was not demonstrable (Fizazi et al. 2012).
In conclusion, recent progress in terms of PC research, especially the role of ND in PC has lead to the development of entirely new therapeutic modalities for hormone-refractory PC.
Abbreviations
- ND:
-
Neuroendocrine differentiation
- NE:
-
Neuroendocrine
- PC:
-
Prostate cancer
- PSA:
-
The prostate specific antigen
- CGA:
-
ChromograninA
- NSE:
-
Neuron-specific enolase
- VIP:
-
Vasoactive intestinal peptide
- GRP:
-
Bombesin/gastrin releasing peptide
- aHCG:
-
Alpha-human chorionic gonadotropin
- PTHrP:
-
Parathyroid hormonerelated protein
- VEGF:
-
Vascular endothelial growth factor
- SCC:
-
Small cell carcinomas
- PIN:
-
Prostatic intraepithelial neoplasia
- AMACR:
-
Alpha-methylacyl-CoA racemase
- ADT:
-
Androgen deprivation therapy
- uPA:
-
Urokinase-type plasminogen activator
- PAI-1:
-
Plasminogen activator inhibitor-1
- MMP:
-
Metalloprotease
- MDV:
-
Microvascular density
- MAPKs:
-
Mitogen activated protein kinases
- PKA:
-
Cyclic AMP-dependent protein kinase
- PI3K:
-
Phosphatidylinositol 3-kinase
- CDK:
-
Cyclin-dependent kinase
- CGB:
-
Chromogranin B
- CGC:
-
Chromogranin C
- ProGRP:
-
Progastrin-releasing peptide
- BPH:
-
Prostatic hyperplasia
- PS:
-
Performance status
- PET:
-
Positron emission tomography
- FDG:
-
F18-fluorodeoxyglucose
- DTPA:
-
Diethylenetriaminepentaacetic acid
- DTX:
-
Docetaxel
- OS:
-
Overall survival
- TTP:
-
Time to progression
- DOTA:
-
1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid
- PTP:
-
Protein tyrosine phosphatase
References
Abdul M, Anezinis PE, Logothetis CJ et al (1994) Growth inhibition of human prostatic carcinoma cell lines by serotonin antagonists. Anticancer Res 14:1215–1220
Abrahamsson PA, Wadstrom LB, Alumets J et al (1986) Peptide hormone- and serotonin-immunoreactive cells in normal and hyperplastic prostate glands. Pathol Res Prac 181:675–683
Abrahamsson PA, Wadstrom LB, Alumets J et al (1987) Peptide-hormone and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathol Res Pract 182:298–307
Ahlgren G, Pedersen K, Lundberg S et al (2000) Regressive changes and neuroendocrine differentiation in prostate cancer after neoadjuvant hormonal treatment. Prostate 42:274–279
Albrecht M, Doroszewicz J, Gillen S et al (2004) Proliferation of prostate cancer cells and activity of neutral endopeptidase is regulated by bombesin and IL-1beta with IL-1beta acting as a modulator of cellular differentiation. Prostate 58:82–94
Allen FJ, Van Velden DJ, Heyns CF (1995) Are neuroendocrine cells of practical value as an independent prognostic parameter in prostate cancer? Br J Urol 75:751–754
Angelsen A, Syversen U, Stridsberg M et al (1997) Use of neuroendocrine serum markers in the follow-up of patients with cancer of the prostate. Prostate 31:110–117
Aprikian AG, Cordon-Cardo C, Fair WR et al (1993) Characterization of neuroendocrine differentiation in human benign prostate and prostatic adenocarcinoma. Cancer 71:3952–3965
Berruti A, Dogliotti L, Mosca A et al (2000) Circulating neuroendocrine markers in patients with prostate carcinoma. Cancer 88:2590–2597
Berruti A, Mosca A, Tucci M et al (2005) Independent prognostic role of circulating chromogranin a in prostate cancer patients with hormonerefractory disease. Endocr Relat Cancer 12:109–117
Berruti A, Bollito E, Cracco CM et al (2010) The prognostic role of immunohistochemical chromogranin a expression in prostate cancer patients is significantly modified by androgen-deprivation therapy. Prostate 70:718–726
Bonkhoff H (1996) Role of the basal cells in premalignant changes of the human prostate: a stem cell concept for the development of prostate cancer. Eur Urol 30:201–205
Bonkhoff H, Stein U, Remberger K (1994) Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: simultaneous demonstration of cell-specific epithelial markers. Hum Pathol 25:42–46
Bonkhoff H, Stein U, Remberger K (1995) Endocrine-paracrine cell types in the prostate and prostatic adenocarcinoma are postmitotic cells. Hum Pathol 26:167–170
Borre M, Nerstrom B, Overgaard J (2000) Association between immunohistochemical expression of vascular endothelial growth factor (VEGF), VEGF-expressing neuroendocrine-differentiated tumor cells, and outcome in prostate cancer patients subjected to watchful waiting. Clin Cancer Res 6:1882–1890
Bostwick DG, Dousa MK, Crawford BG et al (1994) Neuroendocrine differentiation in prostatic intraepithelial neoplasia and adenocarcinoma. Am J Surg Pathol 18:1240–1246
Bostwick DG, Qian J, Pacelli A et al (2002) Neuroendocrine expression in node positive prostate cancer: correlation with systemic progression and patient survival. J Urol 168:1204–1211
Buchanan G, Greenberg NM, Scher HI et al (2001) Collocation of androgen receptor gene mutations in prostate cancer. Clin Cancer Res 7:1273–1281
Cescato R, Maina T, Nock B et al (2008) Bombesin receptor antagonists may be preferable to agonists for tumor targeting. J Nucl Med 49:318–326
Chevalier S, Defoy I, Lacoste J et al (2002) Vascular endothelial growth factor and signaling in the prostate: more than angiogenesis. Mol Cell Endocrinol 189:169–179
Chung TD, Yu JJ, Kong TA et al (2000) Interleukin-6 activates phosphatidylinositol-3 kinase, which inhibits apoptosis in human prostate cancer cell lines. Prostate 42:1–7
Cohen MK, Arber DA, Coffield KS et al (1994) Neuroendocrine differentiation in prostatic adenocarcinoma and its relationship to tumor progression. Cancer 74:1899–1903
Collado B, Gutierrez-Canas I, Rodriguez-Henche N et al (2004) Vasoactive intestinal peptide increases vascular endothelial growth factor expression and neuroendocrine differentiation in human prostate cancer LNCaP cells. Regul Pept 119:69–75
Collado B, Sanchez MG, Diaz-Laviada I et al (2005) Vasoactive intestinal peptide (VIP) induces c-fos expression in LNCaP prostate cancer cells through a mechanism that involves Ca2+ signalling. Implications in angiogenesis and neuroendocrine differentiation. Biochim Biophys Acta 1744:224–233
Cox ME, Deeble PD, Bissonette EA et al (2000) Activated 3′,5′-cyclic AMP-dependent protein kinase is sufficient to induce neuroendocrinelike differentiation of the LNCaP prostate tumor cell line. J Biol Chem 275:13812–13818
Deeble PD, Murphy DJ, Parsons SJ et al (2001) Interleukin-6-, cyclic AMP-mediated signalling potentiates neuroendocrine differentiation of LNCaP prostate tumor cells. Mol Cell Biol 21:8471–8482
Deftos LJ, Nakada S, Burton DW et al (1996) Immunoassay and immunohistology studies of chromogranin a as a neuroendocrine marker in patients with carcinoma of the prostate. Urology 48:58–62
Deng X, Liu H, Huang J et al (2008) Ionizing radiation induces prostate cancer neuroendocrine differentiation through interplay of CREB and ATF2: implications for disease progression. Cancer Res 68:9663–9670
di Sant’Agnese PA (1992) Neuroendocrine differentiation in carcinoma of the prostate. Diagnostic, prognostic, and therapeutic implications. Cancer 70:254–268
di Sant’Agnese PA (1998) Neuroendocrine differentiation in prostatic carcinoma: an update. Prostate 36(8):74–79
Dizeyi N, Konrad L, Bjartell A et al (2002) Localization and mRNA expression of somatostatin receptor subtypes in human prostatic tissue and prostate cancer cell lines. Urol Oncol 7:91–98
Erasmus CE, Verhagen WI, Wauters CA et al (2002) Brain metastasis from prostate small cell carcinoma: not to be neglected. Can J Neurol Sci 29:375–377
Facchini G, Caraglia M, Morabito A et al (2010) Metronomic administration of zoledronic acid and taxotere combination in castration resistant prostate cancer patients: phase I ZANTE trial. Cancer Biol Ther 10:543–548
Festuccia C, Guerra F, D’Ascenzo S (1998) In vitro regulation of pericellular proteolysis in prostatic tumor cells treated with bombesin. Int J Cancer 75:418–431
Fizazi K, De Bono JS, Flechon A et al (2012) Randomised phase II study of CNTO328 (CNTO 328), an anti-IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer 48:85–93
Ghannoum JE, DeLellis RA, Shin SJ (2004) Primary carcinoid tumor of the prostate with concurrent adenocarcinoma: a case report. Int J Surg Pathol 12:167–170
Grobholz R, Bohrer MH, Siegsmund M et al (2000) Correlation between neovascularisation and neuroendocrine differentiation in prostatic carcinoma. Pathol Res Pract 196(5):277–284
Grobholz R, Griebe M, Sauer CG et al (2005) Influence of neuroendocrine tumor cells on proliferation in prostatic carcinoma. Hum Pathol 36:562–570
Guillemot F, Lo LC, Johnson JE et al (1993) Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75:463–476
Hansson J, Abrahamsson PA (2003) Neuroendocrine differentiation in prostate carcinoma. Scand J Urol Nephrol 37(Suppl 212):28–36
Helpap B (2002) Morphology and therapeutic strategies for neuroendocrine tumors of the genitourinary tract. Cancer 95:1415–1420
Hofmann M, Machtens S, Stief C et al (2004) Feasibility of Ga-68-DOTABOM PET in prostate carcinoma patients [abstract]. J Nucl Med 45:449P
Huang J, di Sant’'Agnese P (2002) Neuroendocrine differentiation in prostate cancer: an overview. In: Lamberts S (ed) Advances in oncology: the expanding role of octreotide. Bioscientifica Ltd, Bristol, pp 243–262
Huang J, Yao JL, Zhang L et al (2005) Differential expression of interleukin-8 and its receptors in the neuroendocrine and nonneuroendocrine compartments of prostate cancer. Am J Pathol 166:1807–1815
Huang J, Yao JL, Di Sant’agnese PA et al (2006) Immunohistochemical characterization of neuro-endocrine cells in prostate cancer. Prostate 66:1399–1406
Huss WJ, Gray DR, Werdin ES et al (2004) Evidence of pluripotent human prostate stem cells in a human prostate primary xenograft model. Prostate 60:77–90
Isaacs JT (2008) Prostate stem cells and benign prostatic hyperplasia. Prostate 68:1025–1034
Isaacs JT, Coffey DS (1989) Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl 2:33–50
Ishimaru H, Kageyama Y, Hayashi T et al (2002) Expression of matrix metalloproteinase-9 and bombesin/gastrinreleasing peptide in human prostate cancers and their lymph node metastases. Acta Oncol 41:289–296
Jin RJ, Wang Y, Masumori N et al (2004) NE-10 neuroendocrine cancer promotes the LNCaP xenograft growth in castrated mice. Cancer Res 64:5489–5495
Jin RJ, Lho Y, Connelly L et al (2008) The nuclear factor-kappaB pathway controls the progression of prostate cancer to androgen-independent growth. Cancer Res 68:6762–6769
Jongsma J, Oomen MH, Noordzij MA (2000) Androgen-independent growth is induced by neuropeptides in human prostate cancer cell lines. Prostate 42:34–44
Kadmon D, Thompson TC, Lynch GR et al (1991) Elevated plasma chromogranin-a concentrations in prostatic carcinoma. J Urol 146:358–361
Kamiya N, Suzuki H, Kawamura K et al (2008) Neuroendocrine differentiation in stage D2 prostate cancers. Int J Urol 15:423–428
Kawai S, Hiroshima K, Tsukamoto Y et al (2003) Small cell carcinoma of the prostate expressing prostatespecific antigen and showing syndrome of inappropriate secretion of antidiuretic hormone: an autopsy case report. Pathol Int 53:892–896
Kim J, Adam RM, Freeman MR (2002) Activation of the Erk mitogen-activated protein kinase pathway stimulates neuroendocrine differentiation in LNCaP cells independently of cell cycle withdrawal and STAT3phosphorylation. Cancer Res 62:1549–1554
Kimura N, Hoshi S, Takahaski M et al (1997) Plasma chromogranin a in prostatic carcinoma and neuro-endocrine tumors. J Urol 157:565–568
Kokubo H, Yamada Y, Nishio Y et al (2005) Immunohistochemical study of chromogranin a in stage D2 prostate cancer. Urology 66:135–140
Koutsilieris M, Mitsiades CS, Bogdanos J et al (2004) Combination of somatostatin analog, dexamethasone, and standard androgen ablation therapy in stage D3 prostate cancer patients with bone metastases. Clin Cancer Res 10:4398–4405
Lantry LE, Cappelletti E, Maddalena ME et al (2006) 177Lu-AMBA: synthesis and characterization of a selective 177Lu-labeled GRP receptor agonist for systemic radiotherapy of prostate cancer. J Nucl Med 47:1144–1152
Lee LF, Louie MC, Desai SJ et al (2004) Interleukin-8 confers androgen-independent growth and migration of LNCaP: differential effects of tyrosine kinases Src and FAK. Oncogene 23:2197–2205
Levine L, Lucci JA, Pazdrak B et al (2003) Bombesin stimulates nuclear factor kappa B activation and expression of proangiogenic factors in prostate cancer cells. Cancer Res 63:3495–3502
Liu Y (2008) FDG PET-CT demonstration of metastatic neuroendocrine tumor of prostate. World J Surg Oncol 6:64
Liu IJ, Zafar MB, Lai YH (2001) Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology 57:108–115
Logothetis C, Hoosein N (1992) The inhibition of the paracrine progression of prostatic cancer as an approach to early therapy of prostatic carcinoma. J Cell Biochem Suppl 16H:128–134
Markwalder R, Reubi JC (1999) Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res 59:1152–1159
Mazzucchelli R, Lopez-Beltran A, Scarpelli M et al (2002) Predictive factors in prostate needle biopsy. Pathologica 94:331–337
McWilliam LJ, Manson C, George NJ (1997) Neuroendocrine differentiation and prognosis in prostatic adenocarcinoma. Br J Urol 80:287–290
Meyer-Siegler K (2001) COX-2 specific inhibitor, NS-398, increases macrophage migration inhibitory factor expression and induces neuroendocrine differentiation in C4-2b prostate cancer cells. Mol Med 7:850–860
Mitsiades CS, Bogdanos J, Karamanolakis D et al (2006) Randomized controlled clinical trial of a combination of somatostatin analog and dexamethasone plus zoledronate vs. zoledronate in patients with androgen ablation-refractory prostate cancer. Anticancer Res 26:3693–3700
Mori S, Murakami-Mori K, Bonavida B (1999) Interleukin-6 induces G1 arrest through induction of p27(Kip1), a cyclin-dependent kinase inhibitor, and neuron-like morphology in LNCaP prostate tumor cells. Biochem Biophys Res Commun 257:609–614
Mori R, Xiong S, Wang Q et al (2009) Gene profiling and pathway analysis of neuroendocrine transdifferentiated prostate cancer cells. Prostate 69:12–23
Morris MJ, Akhurst T, Larson SM et al (2005) Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res 11:3210–3216
Nagakawa O, Murakami K, Ogasawara M et al (1999) Effect of chromogranin a (pancreastatin) fragment on invasion of prostate cancer cells. Cancer Lett 147:207–213
Nagakawa O, Ogasawara M, Murata J et al (2001) Effect of prostatic neuropeptides on migration of prostate cancer cell lines. Int J Urol 8:65–70
Oyama N, Akino H, Suzuki Y (2001) FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun 22:963–968
Palapattu GS, Wu C, Silvers CR et al (2009) Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer. Prostate 69:787–798
Pearse AG, Takor T (1979) Embryology of the diffuse neuroendocrine and its relationship to the common peptides. Fed Proc 38:2288–2294
Pinski J, Wang Q, Quek ML et al (2006) Genistein-induced neuroendocrine differentiation of prostate cancer cells. Prostate 66:1136–1143
Powles T, Murray I, Brock C (2007) Molecular position emission tomography and PET/CT imaging in urological malignancies. Eur Urol 51:1511–1521
Reubi JC (2003) Peptide receptors as molecular targets for cancer diagnosis and therapy. Endocr Rev 24:389–427
Reubi JC, Macke HR, Krenning EP (2005) Candidates for peptide receptor radiotherapy today and in the future. J Nucl Med 46(suppl 1):67S–75S
Rufini V, Calcagni ML, Baum RP (2006) Imaging of neuroendocrine tumors. Semin Nucl Med 36:228–247
Salido M, Vilches J, Lopez A (2000) Neuropeptides bombesin and calcitonin induce resistance to etoposide induced apoptosis in prostate cancer cell lines. Histol Histopathol 15:729–738
Salido M, Vilches J, Roomans GM (2004) Changes in elemental concentrations in LNCaP cells are associated with a protective effect of neuropeptides on etoposide-induced apoptosis. Cell Biol Int 28:397–402
Sarkar D, Singh SK, Mandal AK et al (2010) Plasma chromogranin a: clinical implications in patients with castrate resistant prostate cancer receiving docetaxel chemotherapy. Cancer Biomark 8:81–87
Sauer CG, Roemer A, Grobholz R (2006) Genetic analysis of neuroendocrine tumor cells in prostatic carcinoma. Prostate 66:227–234
Scher HI, Sawyers CL (2005) Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen receptor signaling axis. J Clin Oncol 23:8253–8261
Schmid KW, Helpap B, Totsch M et al (1994) Immunohisto-chemical localization of chromogranin a and B and secretogranin II in normal, hyperplastic and neoplastic prostate. Histopathology 24:233–239
Schoder H, Herrmann K, Gonen M (2005) 2-[18F]fluoro-2-deoxyglucose positron emission tomography for the detection of disease in patients with prostate-specific antigen relapse after radical prostatectomy. Clin Cancer Res 11:4761–4769
Schottelius M, Poethko T, Herz M et al (2004) First 18F-labeled tracer suitable for routine clinical imaging of sst receptor-expressing tumors using positron emission tomography. Clin Cancer Res 10:3593–3606
Schron DS, Gipson T, Mendelsohn G (1984) The histogenesis of small cell carcinoma of the prostate: an immunohistochemical study. Cancer 53:2478–2480
Sciarra A, Monti S, Gentile V et al (2003) Variation in chromogranin. A serum levels during intermittent versus continuous androgen deprivation therapy for prostate adenocarcinoma. Prostate 55:168–179
Seethalakshmi L, Mitra SP, Dobner PR et al (1997) Neurotensin receptor expression in prostate cancer cell line and growth effect of NT at physiological concentrations. Prostate 31:183–192
Segal NH, Cohen RJ, Haffejee Z et al (1994) BCL-2 proto-oncogene expression in prostate cancer and its relationship to the prostatic neuroendocrine cell. Arch Pathol Lab Med 118:616–618
Sehgal I, Thompson TC (1999) Novel regulation of type IV collagenase (matrix metalloproteinase-9 and −2) activities by transforming growth factor-beta1 in human prostate cancer cell lines. Mol Biol Cell 10:407–416
Tanaka M, Suzuki Y, Takaoka K et al (2001) Progression of prostate cancer to neuroendocrine cell tumor. Int J Urol 8:431–436
Tang Y, Wang L, Goloubeva O et al (2009) The relationship of neuroendocrine carcinomas to anti-tumor therapies in TRAMP mice. Prostate 69:1763–1773
Tarle M, Rados N (1991) Investigation on serum neurone-specific enolase in prostatic cancer diagnosis and monitoring: comparative study of a multiple tumor marker assay. Prostate 19:23–33
van Bokhoven A, Varella-Garcia M, Korch C et al (2003) Molecular characterization of human prostate carcinoma cell lines. Prostate 57:205–225
Van de Wiele C, Phonteyne P, Pauwels P et al (2008) Gastrin-releasing peptide receptor imaging in human breast carcinoma versus immunohistochemistry. J Nucl Med 49:260–264
Vilches J, Salido M, Fernandez-Segura E et al (2004) Neuropeptides, apoptosis and ion changes in prostate cancer. Methods of study and recent developments. Histol Histopathol 19:951–961
Waltregny D, Leav I, Signoretti S et al (2001) Androgen-driven prostate epithelial cell proliferation and differentiation in vivo involve the regulation of p27. Mol Endocrinol 15:765–782
Wang Q, Horiatis D, Pinski J (2004) Interleukin-6 inhibits the growth of prostate cancer xenografts in mice by the process of neuroendocrine differentiation. Int J Cancer 111:508–513
Weinstein MH, Partin AW, Veltri RW et al (1996) Neuroendocrine differentiation in prostate cancer: enhanced prediction of progression after radical prostatectomy. Hum Pathol 27:683–687
Wright ME, Tsai MJ, Aebersold R (2003) Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells. Mol Endocrinol 17:1726–1737
Wu C, Huang J (2007) Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway is essential for neuroendocrine differentiation of prostate cancer. J Biol Chem 282:3571–3583
Wu G, Burzon DT, di Sant’Agnese PA et al (1996) Calcitonin receptor mRNA expression in the human prostate. Urology 47:376–381
Wu C, Zhang L, Bourne PA et al (2006) Protein tyrosine phosphatase PTP1B is involved in neuroendocrine differentiation of prostate cancer. Prostate 66:1125–1135
Xing N, Qian J, Bostwick D et al (2001) Neuroendocrine cells in human prostate over-express the anti-apoptosis protein survivin. Prostate 48:7–15
Yang JC, Ok JH, Busby JE et al (2009) Aberrant activation of androgen receptor in a new neuropeptide-autocrine model of androgen-insensitive prostate cancer. Cancer Res 69:151–160
Yao JL, Madeb R, Bourne P et al (2006) Small cell carcinoma of the prostate: an immunohistochemical study. Am J Surg Pathol 30:705–712
Yashi M, Nukui A, Kurokawa S et al (2003) Elevated serum progastrin-releasing peptide (31–98) level is a predictor of short response duration after hormonal therapy in metastatic prostate cancer. Prostate 56:305–312
Yuan TC, Veeramani S, Lin MF (2007) Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr Relat Cancer 14:531–547
Zhang H, Chen J, Waldherr C et al (2004) Synthesis and evaluation of bombesin derivatives on the basis of pan-bombesin peptides labeled with indium-111, lutetium-177, and yttrium-90 for targeting bombesin receptor-expressing tumors. Cancer Res 64:6707–6715
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Franco, R., Chieffi, P., Perdonà, S., Facchini, G., Caraglia, M. (2013). Neuroendocrine Differentiation in Prostate Cancer. In: Staibano, S. (eds) Prostate Cancer: Shifting from Morphology to Biology. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-7149-9_5
Download citation
DOI: https://doi.org/10.1007/978-94-007-7149-9_5
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-7148-2
Online ISBN: 978-94-007-7149-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)