Summary
This review provides an overview of chemical, anatomical and morphological changes in bryophytes in response to changes in light availability and assesses the role of these changes in altering bryophyte canopy performance. As a key chemical change, the concentration of chlorophyll increases in response to reduced light availability. Apart from light, within-canopy patterns in chlorophyll are importantly driven by the age of foliage that increases with decreasing light availability, resulting in reduced foliage chlorophyll contents in lower light. In addition, foliage is less strongly aggregated and the density of plants decreases in lower light resulting in greater efficiency of light interception per unit leaf area formed. There is large species variability in canopy architecture, accompanied by species differences in light gradients. Species also differ in structural acclimation to within-canopy light gradients. The species forming new leaves and branches from lateral buds and extending existing lateral branches, in particular, pleurocarpous mosses, can structurally adapt to reductions in light during moss growth, while non-branching, in particular, acrocarpous mosses, are inherently less plastic in their acclimation to light. The degree of aggregation also depends importantly on moss water content with greater degree of aggregation under low water availability, suggesting that changes in aggregation play a dual role in enhancing light interception under wet conditions and decreasing light harvesting under dry conditions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
Light strongly varies within and along plant communities, and foliage photosynthetic function acclimates to prevailing light conditions resulting in enhanced whole canopy light interception and photosynthetic production (Hirose and Werger 1987; Gutschick and Wiegel 1988; Sands 1995; Niinemets and Anten 2009). So far, studies on plant canopies have focused mainly on distribution of light and resources, and photosynthetic acclimation in vascular plants, but the variation in light availability can be particularly large in bryophytes, many species of which colonize the most deeply shaded understory habitats, and are often particularly densely aggregated.
Dense aggregation of mosses can be explained by their inability for active control of water loss by stomata. Individual bryophyte shoots are especially vulnerable to water loss (Dilks and Proctor 1979; Proctor 1984; Green and Lange 1994; Chap. 4). Aggregation of moss foliage elements and shoots to form dense moss cushions significantly increases the moss boundary layer resistance, and reduces the evaporation rate and improved hydration status of entire moss clumps (Proctor 1984; Rice et al. 2001; Rice and Schneider 2004; Chap. 4). Higher degree of moss shoot packing also increases moss water storage capacity and enhances water transport by generating capillary spaces among stems, modifications that altogether significantly extend the period of photosynthetic activity (Proctor 1990; Pedersen et al. 2001).
The trade-off of formation of a “canopy” is a strong reduction of light availability within the moss cushions (Skré et al. 1983; van der Hoeven et al. 1993; Zotz and Kahler 2007; Tobias and Niinemets 2010). In fact, extremely high stem densities and leaf area indices have been reported for mosses in general (Simon 1987; van der Hoeven et al. 1993; Waite and Sack 2010). On the other hand, there is large interspecific variability in moss architecture, leaf area supported and pigment content (Simon 1987; van der Hoeven et al. 1993; Waite and Sack 2010), and the question is how such variation is reflected in within-canopy light gradients. In fact, studies do demonstrate interspecific variation in light gradients among moss species (Skré et al. 1983; van der Hoeven et al. 1993), but the underlying functional traits have not been routinely explored.
Given the extensive light gradients and capacity of many moss species to colonize a range of understory light environments, it is also pertinent to ask to what extent moss foliage and canopy architecture are able to acclimate to such huge variations in light. A number of recent studies demonstrate important variations in moss structural, chemical and photosynthetic characteristics within the moss canopy (Tobias and Niinemets 2005, 2010; Zotz and Kahler 2007; Rice et al. 2011). Although light gradients in moss canopies strongly interact with gradients in leaf senescence, limiting acclimation of foliage to low light and thereby partly deviating from patterns in vascular plants, leaves in the lower canopy still possess a certain photosynthetic activity (Zotz and Kahler 2007; Tobias and Niinemets 2010). This photosynthetic activity likely moderately contributes to carbon gain of the entire canopy when upper canopy leaves are fully active and lower canopy receives extremely low light, but it can be fully employed during dry periods when upper canopy photosynthetic activity is reduced, and leaves become rolled around stem, resulting in enhanced penetration of light deeper into the moister canopy interior (Davey and Ellis-Evans 1996). Such variations in moss water status can occur during the day (Hamerlynck et al. 2000), among the days and during the season (Vitt 1990; Harris 2008) Thus, understanding of within-canopy variations in moss functional traits and dynamics in canopy architecture can play a major role in estimating moss carbon gain over days to seasons.
In this review, we first analyze basic functional attributes determining moss light interception, then summarize the variation patterns in moss canopy traits, study within-canopy variation in light, and how these patterns are related to plant architectural traits and pigment contents, and finally analyze within canopy variation in pigments and photosynthetic activity. Overall, this review demonstrates large variation in moss functional traits, emphasizes the richness of within-canopy variation patterns and suggests that consideration of basic modes of acclimation in different functional traits is needed to scale from moss leaves to canopies.
II. Light Interception in Mosses
A. Basics of Light Interception
Quantum flux density within plant community (Q) decreases from canopy top to bottom with increasing cumulative leaf area index (L C). Traditionally, the Lambert-Beer law has been applied to simulate reduction of light intensity within vegetation (Monsi and Saeki 1953). For direct beam solar radiation,
where Q 0 is the quantum flux density incident to the vegetation, θ is the solar zenith angle and k(θ) is the canopy extinction coefficient that depends on leaf inclination angle distribution (Ross 1981). As Q 0 varies during and between days, it may be often more convenient to analyze variation in relative incident light intensity (transmittance to given position in the canopy), R Q = Q/Q 0, especially when studying implications of differences in canopy structure on light harvesting.
There are two important assumptions in Eq. 9.1: (1), foliage is randomly dispersed, and (2), leaves are optically black. As regards to the assumption of random dispersion of foliage elements, foliage in real canopies tends to be often aggregated (clumped). Foliage aggregation can occur at shoot, branch and canopy scales, and reduces light interception at given L C. Markov models or negative binomial models have been employed to simulate light interception by aggregated foliage elements (Nilson 1971; Baldocchi and Bowling 2003; Cescatti and Niinemets 2004; Niinemets and Anten 2009). In simple Markov approximation, the transmittance of light is given as:
where Ω is the clumping index, and for aggregated canopies 1 > Ω ≥ 0. Thus, aggregated canopies with given foliage inclination angle distribution and L C transmit more light than random canopies (Fig. 9.1). In fact, in canopies with large leaf area indices, random dispersion would result in too strong light interception and very dark lower canopies. From Eq. 9.1 it follows that a canopy with random dispersion of foliage and uniform leaf inclination angle distribution (no preferential foliage orientation in space, k = 0.5) is absorbing 95 % of light for a L C of ca. 6 m2 m−2. Natural plant stands, including moss canopies (Table 9.1), often support much higher leaf area indices (Niinemets 2010; Waite and Sack 2010), further underscoring that spatial aggregation is common in nature (Asner and Wessman 1997; Cescatti and Niinemets 2004; Duursma et al. 2012).
Theoretical dependencies of relative incident quantum flux density at different canopy layers (canopy transmittance) in dependence on cumulative leaf area index (L C) from canopy top to bottom for (a) hypothetical canopies with clumped, random and regular dispersion, and (b) for clumped canopies with varying leaf absorptance (ζ). The simulations were conducted for diffuse light conditions using uniformly overcast sky model. Leaf inclination angle distribution was considered spherical (no preferential orientation in space). For clumped canopy in (a) and in (b), the clumping index, Ω (Eqs. 9.2 and 9.3), was taken as 0.5, while Ω = 1.5 was used for regular dispersion. Leaf absorptance was taken as 0.3 in the main panel of (a) assumed to correspond to typical values in mosses (see section “Moss pigment content and light harvesting”). In the inset of (a) ζ = 0.85, corresponding to typical values in vascular plants (Vogelmann et al. 1996b). For further details in simulating canopy light profiles see Cescatti and Niinemets (2004). The insets in (a) demonstrate shoot silhouettes for moss Rhodobryum roseum that has clumped foliage, and in liverwort Plagiochila deflexa that has regularly dispersed foliage. The insets in (b) show Rhodobryum roseum shoots of varying ζ (greenness).
On the other hand, foliage can also be regularly dispersed, especially in understory where foliage of low-light acclimated plants is filling up the gaps among neighboring foliage elements, resulting in planar canopies with very high efficiency of light interception (Niinemets 2010 for a review). Regular dispersions can be fitted by a positive binomial model or with the Markov model (Nilson 1971; Cescatti and Niinemets 2004). In the case of the Markov model for regular canopies, Ω in Eq. 9.2 is >1, implying that canopies with regular dispersion of foliage transmit less light relative to canopies with randomly dispersed leaves (Fig. 9.1). Overall, we conclude that random dispersion of foliage elements (Eq. 9.1) is frequently not the case in natural canopies, signifying that it is highly relevant to consider foliage aggregation in modeling canopy light climate.
The assumption of optically black leaves that is applied by default in vegetation models (Eq. 9.1) rests on the assumption that leaf absorptance (ζ) for Q is generally so large that transmitted and reflected radiation fluxes (scattering) are relatively small. Precise consideration of scattered radiation fluxes requires complex models (Brakke 1994; Nilson and Ross 1997; Cescatti and Niinemets 2004), but a simplified way of consideration of scattering effects has been suggested by Goudriaan (1977). According to Goudriaan (1977), the effect of scattering scales with \( \sqrt{\zeta } \), and thus, Eq. 9.2 becomes
This form of canopy transmittance is currently widely used in modeling light transmission of natural stands as a simple approximation including both variation in foliage aggregation and light scattering (Anten and Hirose 1999, 2003; Aan et al. 2006). Typically, leaves of vascular plants have leaf absorptances (ζ) between 0.8 and 0.9, and thus, the scattering correction, \( \sqrt{\zeta } \), is relatively small. However, for thin moss leaves, often consisting of only single-layered cells, the scattering effect can be substantial (Fig. 9.1), and need to be included in simulating light climate in moss canopies.
B. Moss Leaf Area Index
A key player in radiative transfer models is the leaf area index (L, Eqs. 9.1, 9.2, and 9.3), but moss leaf area indices are not routinely determined. The situation with L is simple in thalloid liverworts such as e.g., Marchantia polymorpha, Conocephalum conicum or Monoclea forsteri, where L is close to 1 m2 m−2 or somewhat higher (Green and Lange 1994). However, due to small size of foliage elements, determination of L in non-thalloid mosses and liverworts with more complex canopies is not a trivial task, requiring microscopy techniques (Fig. 9.2, Simon 1987).
Illustration of estimation of leaf area index (L) in the moss Pleurozium schreberi. Leaf area index is estimated from the measurements of branch (AL,B) and stem (AL,S) leaf area, number, branch length and stem density as shown in the figure (Adapted from Tobias and Niinemets 2005).
In mosses, leaf area index can be expressed as the product of leaf area on the shoot (A S) and the number of shoots per sampled area (shoot density, N S):
In the case of non-branched mosses, the shoot leaf area is given as the product of average leaf area, \( {\overline{A}}_{\text{L}}\) and the average number of leaves on the stem \( {\overline{F}}_{\text{N}}\):
The situation is more complex for branched mosses, where both stems and branches support the leaves, especially given that stem and branch leaves can differ in size (Fig. 9.2). Thus, for branched mosses, A S is the sum of the leaves carried by stems and branches (Fig. 9.2 for calculation of L in branched moss Pleurozium schreberi).
In practice, for studying light vs. L C relationships, L has to be determined separately for leaf layers of 0.2–1 cm depth, depending on the size of the moss canopy, thereby significantly increasing the effort needed to characterize moss canopy architecture.
To our knowledge, L for non-thalloid mosses has been so far determined only for 17 species (Table 9.1). Overall, compared with vascular plants (Niinemets 2010; Waite and Sack 2010), L is large in mosses, with most published values between 10 and 20 m2 m−2 (Table 9.1). However, the published values of L differ largely from values as high as 129 m2 m−2 in Ceratodon purpureus (Simon 1987) to as low as 4.1 m2 m−2 in Fissidens pacificus (Waite and Sack 2010). The question is whether this large range of variation among published values of L corresponds to true variation in L or reflects some artifacts in measurement protocols. In particular, the extreme values in the study of Simon (1987) have not been confirmed by other studies. Although these values are widely used to emphasize the high L values in mosses (Waite and Sack 2010), they reflect extreme density of the moss cushions studied by the author (Simon 1987). For example, in Ceratodon purpureus, the average number of stems per 1 cm2 was 118, and corresponding value was 44 for Tortula ruralis (Simon 1987). To our knowledge this represents the upper end of moss stem densities ever reported. However, only 1 cm2 patches of moss canopies were studied, but there is always some microheterogeneity in moss stem density in moss cover. Thus, more representative estimates might be obtained by using larger areas for estimating moss density.
A complication with some moss species can be the presence of unique morphological non-chlorophyll containing features including hyaline (“hairy”) leaf tips of the awns, alar cells at the leaf base and costa, hyalocysts at the leaf base etc. (Crandall-Stotler and Bartholomew-Began 2007; Glime 2007). To our knowledge, the contribution of these almost transparent leaf parts to total L has been assessed only in the study of Vitt (1990). In Drummondia prorepens, it was estimated that non-green parts of foliage contribute to ca. 25 % of L (Vitt 1990), indicating that the presence of these structures can importantly affect L estimates. We suggest that in future studies, the contributions of hyaline and green leaf parts should be estimated for species possessing such unique foliar features.
Another potential caveat in estimating L in mosses is the distinction between dead and live biomass. From physiological perspective, only live, photosynthetically active tissue is relevant, and thus, L should only include live plant biomass. This is commonly achieved by separating green and non-green parts of the moss cushions, but the distinction on the basis of tissue greenness is not always clear-cut (Fig. 9.3), and there is a certain physiological activity deep in the canopy until almost all chlorophyll is depleted (section “Gradients of “leaf” traits in moss canopies: acclimation or senescence?”). Furthermore, greenness vs. “deadness” can vary among species (Davey et al. 2009), complicating separation between active and non-active moss parts. Such difficulties in separating between physiologically active and dead biomass can also partly contribute to the large range of L estimates across the studies (Table 9.1). C.
C. Moss Shoot Area Index
Shoot area index (S), i.e., the area of stem and attached branches has been determined in several studies (Table 9.1) (van der Hoeven et al. 1993; Bond-Lamberty and Gower 2007; Rice et al. 2011). Sometimes, S has been misleadingly called “leaf area index” (Bond-Lamberty and Gower 2007), but it is important to recognize that L is the product of S and shoot leaf area to shoot area ratio. Determination of shoot area index is less time-consuming, and can be achieved either by photo-electric planimeters (van der Hoeven et al. 1993; Rice et al. 2011) or by microscopy techniques (Bond-Lamberty and Gower 2007). In the case of planimetric methods, S has been always defined on the basis of projected area, while in the case of microscopy techniques, S for species with cylindrical shoots has been defined on the basis of half-of the total area by multiplying the shoot silhouette area by π/2 (Bond-Lamberty and Gower 2007).
Although determination of S is much faster, and it can be argued that moss branches and stems represent the fundamental units of light interception (Rice et al. 2011), there can be potential acclimation modifications in moss branch and stem architecture in response to environment (see section “Acclimation of moss light harvesting across understory light environments”), altering light harvesting efficiency of shoots. Little research on such acclimation changes has been carried out in mosses, but in many vascular plants, in particular in conifers, shoot architecture – distribution of foliage inclination angles, spatial aggregation and leaf area density – strongly acclimate to local light environment within the canopy (Niinemets et al. 2006, Niinemets 2007). Also, shoot leaf area to shoot area ratio is expected to vary, for instance due to changes in moss water content (section “Controls of light interception in mosses by structure”). L will not be affected by changes in water content (although its efficiency for light interception changes, see section “Controls of light interception in mosses by structure”), but S will inevitably track such changes.
As with L, significant variation exists in the estimates of S among mosses (Table 9.1). Estimates of 1–5 m2 m−2 were reported for Pleurozium schreberi (Rice et al. 2011), while values as high as 8.8–23.6 m2 m−2 were observed in three moss species studied by van der Hoeven et al. (1993). Given that leaf area to shoot silhouette area ratio is ca. 3–5 m2 m−2 for typical feather mosses (Tobias and Niinemets, unpublished data), S values for Pleurozium schreberi in Rice et al. (2011) are broadly comparable with L estimates for this species, and on average in mosses (Table 9.1). However, the values in van der Hoeven et al. (1993), if converted to L, represent the higher end of L estimates in mosses (Table 9.1).
Typically, L and S are defined for moss patches with full moss cover, and moreover, for areas with the cover of the same moss species. In practice, moss species may often grow in intermixed stands (Skré et al. 1983), and in vegetation there are patches with mosses and free of mosses. Thus, in Bond-Lamberty and Gower (2007), S values were scaled to whole stands including areas with and without mosses, overall resulting in low values of S. Such estimates certainly provide useful information for understanding the moss role in vegetation stands in general, for example in overall stand carbon balance. However, these estimates are not comparable with other studies on moss S. Another reason for low S values in Bond-Lamberty and Gower (2007) may also be the arbitrary distinction between dead and live biomass based on fixed depths of moss cushions. For example, in Aulacomnium palustre, Tomentypnum nitens, Pleurozium schreberi, Ptilium crista-castrensis only the upper 1.5 cm part, and in Sphagnum fuscum only the upper 0.5 cm part were considered alive (Bond-Lamberty and Gower 2007). These values are smaller than have been estimated for some of these species in previous studies (Tobias and Niinemets 2005, 2010; Rice et al. 2011), and again underscore the importance for accurate separation between dead and alive tissue in mosses.
D. Controls of Light Interception in Mosses by Structure
The large variability in L and S values among moss species has major implications for light harvesting. Apart from the large variability, the take home message from sections “Gradients in photosynthetic activity” and “Moss shoot area index” is that mosses in general tend to support very high leaf area indices. As discussed in section “Basics of light interception”, such high L values would result in extreme reduction in light within the moss canopies unless foliage is clumped (Eq. 9.2, Fig. 9.1). In fact, canopies of many moss species tend to be clumped, and canopies of acrocarpous non-branching mosses tend to be especially strongly clumped (Fig. 9.4a–c). Nevertheless, even in branching mosses such as Rhytidiadelphus or Calliergonella, the apical extended parts of the moss shoots are strongly aggregated, whereas the foliage dispersion in deeper more strongly branched canopy becomes increasingly random (Fig. 9.4d, e). Finally, foliage dispersion in strongly branched canopies such as in typical feather mosses is essentially random (Fig. 9.4f), and in thalloid or planar mosses and liverworts foliage dispersion becomes increasingly regular.
Representative photographs of moss canopies demonstrating various degrees of aggregation of foliage on stems and branches: (a) – Rhodobryum roseum; (b) – Plagiomnium undulatum; (c) – Tortula ruralis; (d), (e) – Rhytidiadelphus triquetrus side view (d) and top view (e); (f) – Brachythecium rutabulum. (a)–(c) represent strongly aggregated canopies formed of rosette-like moss stems. (d) and (e) demonstrate aggregation in upper canopy and increased randomness in lower canopy, and (f) represents a characteristic canopy with random dispersion of foliage elements.
Comparisons of light gradients in moss species with varying structure are scarce (Fig. 9.5). In van der Hoeven et al. (1993), light gradient was steeper in the canopy of Ctenidium molluscum than in Rhytidiadelphus squarrosus and Calliergonella cuspidata (Fig. 9.5). In fact, the apparent extinction coefficient derived by fitting the simple Lambert-Beer relationship (Eq. 9.1) to R Q vs. cumulative shoot area index (S c) relationships was also larger in C. molluscum than in the other two species (van der Hoeven et al. 1993). Given that C. molluscum grows more strongly appressed to substrate and has more randomly dispersed foliage than either R. squarrosus and C. cuspidata, in which foliage is more strongly aggregated, this difference is in line with theoretical predictions (Eq. 9.2).
Sample light gradients in canopies of four moss species. Data for (a) are from Tobias and Niinemets (2010), and data for the three other species are from van der Hoeven et al. (1993). In the latter study, the measurements were conducted in September (filled symbols) and in December (open symbols). In both studies, the measurements were conducted in the lab under diffuse light.
The importance of foliage aggregation is further emphasized by dynamic changes in moss light interception efficiency in response to alterations in the degree of moss hydration (Fig. 9.6). In many moss species, foliage becomes more strongly adhered to stems with decreasing moss water content (Bayfield 1973; Smith 1982; Seel et al. 1992; Barker et al. 2005). From the radiative transfer point of view, this implies stronger clumping, resulting in enhanced light transmission (Fig. 9.6, Davey and Ellis-Evans 1996; Zotz and Kahler 2007). Analogously, numerous studies demonstrate that reflectance of moss canopies in photosynthetically active spectral region increases with decreasing moss water status (Vogelmann and Moss 1993; Bryant and Baird 2003; Van Gaalen et al. 2007), compatible with enhanced clumping and reduced exposure of chlorophyll packed in appressed leaves. The capacity for leaf rolling and unrolling around the stem in response to changes in water availability appears to be larger in more xerophytic species (in particular in acrocarpous xerophytic species) than in less xerophytic species (Glime 2007). In fact, leaf rolling not only reduces light interception and probability for photoinhibition, but also reduces water evaporation, being thus, an important adaptive feature in mosses regularly undergoing cycles of desiccation and rehydration (Bayfield 1973; Glime 2007).
Light gradients in the canopies of fully hydrated (water content 950 %) and dry (27 %) moss Tortula ruralis (Modified from Zotz and Kahler 2007).
Changes in light gradients within moss canopies have been also observed during the season (Fig. 9.5). These changes were associated with changes in moss S C values, and may also ultimately reflect changes in the degree of foliage aggregation, i.e., increased S C being compatible with reduced degree of foliage aggregation (leaves less strongly appressed to stem resulting in greater stem surface area) (van der Hoeven et al. 1993). This suggestion is supported by reduced shoot area per shoot mass in this study in September relative to December (van der Hoeven et al. 1993). We suggest that such seasonal changes in shoot area may be ultimately associated with seasonal changes in moss water content.
In light of the evidence of the importance of canopy architecture in determining light harvesting of mosses, clearly more quantitative information on moss canopy architecture is needed. New methods based on laser scanning (Rice et al. 2005) or stereo photogrammetry (Krumnikl et al. 2008) may provide a vehicle for rapid screening of moss canopies for relevant architectural traits such as the degree of foliage aggregation.
E. Moss Pigment Content and Light Harvesting
In addition to architecture, moss light interception characteristics are importantly driven by moss foliage optical properties. Unfortunately, data on moss leaf optics are scarce, and studies mainly report information on surface reflectance of moss cushions (Vogelmann and Moss 1993; Bubier et al. 1997; Hamerlynck et al. 2000; Lovelock and Robinson 2002; Arkimaa et al. 2009; Hallik et al. 2009b). However, surface reflectance is driven both by pigment contents and canopy structure and thus does not provide information on optical properties of single moss shoots or leaves. The problem with direct assessment of reflectance, transmittance and absorptance of moss foliage elements is their miniature size such that moss foliage elements, either single leaves, entire shoots or even stems, do not generally fill up the ports of integrated spheres routinely used in assessing vegetation optical characteristics. For small-sized objects such as conifer needles, methods have been developed to determine the optical properties considering the gap fraction of needles in the sample (e.g., Mesarch et al. 1999). However, certain non-uniformity of light field and difficulties in precisely estimating the gap fraction can introduce large errors in derivation of leaf optical characteristics by this method. Attempts have been made to study optical properties of single moss leaves by reducing the field of view with custom-made port covers, but these attempts were not successful due to low sensitivity of available instrumentation (a LI-1800 integrated sphere from Li-Cor, Inc., Lincoln, NE, USA with a Field Spec Pro Spectroradiometer from Analytical Spectral Devices, Inc. Boulder CO, USA; A. Cescatti and Ü. Niinemets, unpublished data 2004).
Given that leaf absorptance is primarily the function of leaf chlorophyll content (Evans 1993b; Vogelmann 1993; Carter and Knapp 2001), leaf absorptance in relation to leaf chlorophyll content per leaf area (χA, μmol m−2) has been described by an empirical equation (Evans 1993b):
This equation has been shown to provide excellent estimates of ζ for a wide range of species, except for hairy or waxy leaves (Evans 1993b; Evans and Poorter 2001).
The use of this equation requires information on chlorophyll content per area, but in mosses, chlorophyll content per dry (χM) or fresh mass is commonly estimated. Furthermore, the estimates generally constitute a weighted sample of moss parts with leaves that contain more chlorophyll and stems that contain less chlorophyll. Using the estimates of moss leaf area per shoot section mass (A M) and chlorophyll content per shoot section mass (χM/A M) (Tobias and Niinemets 2005, 2010), a range of moss leaf chlorophyll contents of 16–39 μmol m−2 is obtained for single-cell-layered leaves of feather moss Pleurozium schreberi. Calculating leaf chlorophyll content as χM/A M does not consider presence of chlorophyll in stem, and thus, χA may be somewhat overestimated. Nevertheless, we suggest that these estimates can still serve as a first approximation of area-based pigment content. From equation (6), the range in chlorophyll contents obtained corresponds to a ζ range of 0.18–0.34. For comparison, typical values of leaf chlorophyll content for vascular plants are 400–800 μmol m−2, corresponding to leaf absorptance of 0.84–0.88 (Gabrielsen 1948; Masoni et al. 1994; Baldini et al. 1997; Carter and Knapp 2001). Thus, these estimates of leaf chlorophyll content and ζ are much lower than characteristic values in vascular plants, indicating that the impact of light scattering (Eq. 9.3) on light profiles within the canopy is of paramount significance in mosses (Fig. 9.1). Furthermore, chloroplast movements that have been shown to be especially significant in shade plants with thinner leaves (Williams et al. 2003) play an important role in mosses as well (Chap. 8 for a review). In particular, chloroplast movement is expected to reduce ζ at given foliage chlorophyll content at the top of the moss canopies, thereby reducing excess light interception (Chap. 8).
Not all mosses have single-cell-layered cells, and may have thickened central leaf parts (costa) as for example in species of Campylopus, Dicranum, Holomitrium, Timmia, Weissia etc., thickened multi-layered leaves such as in Grimmia, plications as in Brachythecium and Drepanocladus, or leaf lamellae as in Polytrichaceae spp. (Crandall-Stotler and Bartholomew-Began 2007; Glime 2007; Waite and Sack 2010). For Polytrichum commune, an average value of leaf area to shoot dry mass of 456 cm2 g−1 was derived for the top 6 cm of the canopy (Tobias and Niinemets 2005 and Tobias and Niinemets unpublished). Given the average chlorophyll content of 6.4 μmol g−1 in this species (Martin and Churchill 1982; Masarovičová and Eliáshš 1987), chlorophyll content per area is 140 μmmol m−2, corresponding to a ζ of 0.65. Compared with vascular plants, these values are still low and are compatible with estimates for young greening leaves, senescing leaves or for chlorotic leaves of chlorophyll-deficient mutants (Gabrielsen 1948; Adams et al. 1990; Masoni et al. 1994; Niinemets et al. 2004). Accordingly, even in relatively thick-leaved moss species, foliage pigment contents importantly alter the light gradients at given L C and degree of foliage aggregation (Fig. 9.1b).
Leaves of many moss species have unique leaf characteristics such as papillae or mamillae on leaf surfaces, and concave or curled leaves (Crandall-Stotler and Bartholomew-Began 2007; Glime 2007). Calculation of leaf absorptance on the basis of leaf chlorophyll content does not consider effects of such anatomical characteristics. In vascular plants, it is known that epidermal thickenings may function as lenses improving light penetration into the leaf (Martin et al. 1991; Myers et al. 1994; Vogelmann et al. 1996a), and it is likely that epidermal modifications in bryophytes also improve light penetration into the chloroplasts.
On the other hand, presence of hyaline hairy leaf tips with high reflectance can dramatically reduce moss light interception, especially in a dry state when leaves are strongly adhered to the stem. Although being non-photosynthetic, hyaline leaf tips can be an important adaptive feature reducing radiation interception during dry periods in xerophytic mosses. Clearly more experimental work is needed to gain quantitative insight into optical characteristics of mosses and to understand how the leaf structural adaptations alter moss light harvesting efficiency.
F. Acclimation of Moss Light Harvesting Across Understory Light Environments
Despite that mosses are commonly considered shade plants, individuals of given moss species can colonize a range of light environments (Frego and Carleton 1995; Thomas et al. 2001; Tobias and Niinemets 2010). The key question is how moss light harvesting characteristics change upon acclimation to different understory light environments. Studies have demonstrated that in higher light, pleurocarpous mosses tend to branch more frequently and achieve higher canopy densities (Rincon and Grime 1989; Rincón 1993; Bergamini and Peintinger 2002). On the other hand, moss foliage chlorophyll contents tend to be larger in lower light (Rincón 1993; Tobias and Niinemets 2010). Thus, the question is how two contrasting trends, increasing foliage density and decreasing chlorophyll content with increasing light, sum up in affecting within-canopy light gradients. In Pleurozium schreberi, within-canopy gradient in light became steeper with increasing understory light availability (Fig. 9.7), suggesting that the variation in light gradients across the habitats was dominated by increases in foliage density. Clearly, further case studies are needed to gain more general insight into the potential variations in the moss light harvesting efficiency. Such studies are also pertinent given the huge range of variation in moss L C values (section “Gradients in photosynthetic activity”) that may not entirely be due to interspecific differences, but also reflect differences in light availability in sampled moss growth habitats, as for instance, has been observed in the study of Waite and Sack (2010).
Apparent extinction coefficient (k depth) for within-canopy relative light (R Q) vs. canopy depth (h) relationships in dependence on incident light availability above the moss layer (Modified from Tobias and Niinemets 2010). R Q vs. h relationships were fitted by \( {R}_{\text{Q}}={e}^{-h{k}_{\text{depth}}}\), and the inset demonstrates variation in canopy light profiles for the range of k depth values observed. The relative incident light availability in different forest habitats was characterized by hemispherical photography and representative hemispherical photographs in low and moderately high light are also demonstrated.
III. Gradients of “Leaf” Traits in Moss Canopies: Acclimation or Senescence?
A. Gradients in Pigments
In simulations in Fig. 9.1, a constant leaf absorptance was assumed for all leaves in the canopy, but light gradients within the canopy are also importantly driven by variation in foliage pigment contents. In vascular plants, contrasting variation patterns in foliage pigment contents across the canopy have been reported (Niinemets 2007; Hallik et al. 2009a). In species that form all foliage throughout the canopy almost simultaneously such as temperate deciduous trees in spring, foliage chlorophyll content per dry mass increases with decreasing light availability from the top to the bottom of the canopy, and this is suggested to be an adaptive feature improving light absorptance per leaf dry mass (for reviews see Niinemets 2007; Niinemets and Anten 2009). However, in herbaceous species and in woody species with rapid growth, lower leaves formed first are continuously overtopped by foliage developing later and thus, light gradients are accompanied by gradients in leaf age and senescence. In herbaceous species, the increase of chlorophyll content per mass with decreased light is either less than in woody species (Evans 1993a, b; Aan et al. 2006; Hallik et al. 2009a, 2012), or chlorophyll content is not related to light at all (Aan et al. 2006; Hallik et al. 2009a) or chlorophyll content decreases with decreasing light availability (Boonman et al. 2007, 2009). This evidence collectively underscores the importance of interacting light and senescence gradients in affecting chlorophyll profiles within the canopy in herbaceous species with rapidly developing canopies.
The situation is similar with mosses where new leaves develop at the top and decompose at the bottom (Fig. 9.3). This pattern of foliage development is reflected in monotonous decreases of chlorophyll content from canopy top to bottom in acrocarpous moss Tortula ruralis, (Zotz and Kahler 2007). However, in pleurocarpous moss Pleurozium schreberi, there is a curvilinear relationship of leaf chlorophyll content with canopy depth and light availability within the canopy, with uppermost foliage having moderately high chlorophyll contents, followed by a maximum and then by monotonous reduction of chlorophyll with further increases in canopy depth (Fig. 9.8a, b, Tobias and Niinemets 2010). This curvilinearity has been attributed to actively growing lateral branches (Fig. 9.8a, b, Tobias and Niinemets 2010), but lower chlorophyll contents at the top of the canopy can also constitute an high light avoidance response (Tobias and Niinemets 2010; Chap. 7).
Within-canopy gradients in chlorophyll and carotenoid contents (a, b), chlorophyll a/b ratio (c, d) and the capacity for photosynthetic electron transport (e, f) in the moss Pleurozium schreberi (Modified from Tobias and Niinemets 2010). Data were fitted by linear and second order polynomial regressions and regressions significant at least at P < 0.05 (r 2 = 0.91 − 0.96) are shown by solid lines and those significant at P < 0.1 (r 2 = 0.83 − 089) are shown by dashed lines.
After the maximum is reached, the reduction in chlorophyll contents with further increases of canopy depth can be suggested to demonstrate age-dependent modifications only. However, chlorophyll a/b ratio monotonically decreases with decreasing light availability in the canopy (Fig. 9.8c, d, Tobias and Niinemets 2010). This reflects increases in light-harvesting pigment-binding complexes, LHC II, relative to the photosystems I and II (Bassi and Caffarri 2000). Such a modification in the stoichiometry of photosynthetic apparatus is a common shade-adaptation response in plants (Anderson et al. 2001; Niinemets 2007), indicating that the foliage in this species has a certain capacity to acclimate to low light. However, differently from Pleurozium schreberi, no changes in chlorophyll a/b ratio were observed in Tortula ruralis, suggesting that the within-canopy change in the stoichiometry of foliage photosynthetic apparatus is not a universal phenomenon in mosses.
The within-canopy variation in carotenoid content is analogous to chlorophyll (Fig. 9.8a, b, Tobias and Niinemets 2010). However, chlorophyll to carotenoid ratio at higher light was smaller than under intermediate light (Tobias and Niinemets 2010), likely indicating enhanced requirement for photoprotective carotenoids in the upper canopy. On the other hand, deeper in the canopy, chlorophyll to carotenoid ratio decreased again, and this may indicate accumulation of carotenoids in plastoglobuli of senescing leaves (Niinemets et al. 2012). Altogether, these data indicate that within-canopy variations in foliage pigment content reflect both overall reduction of pigment content due to senescence, and modifications in pigment stoichiometry being compatible with adaptation to variations in light availability.
B. Gradients in Photosynthetic Activity
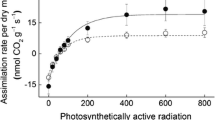
Foliage photosynthesis is another important indicator of within-canopy variation in foliage physiological activity, and it is pertinent to ask whether gradients in photosynthesis accompany light gradients. In vascular plants, foliage photosynthetic capacity strongly increases with increasing light in the canopy, primarily due to enhanced investment of foliage nitrogen in Rubisco and rate-limiting components of photosynthetic electron transport (for reviews see Osmond et al. 1999; Pons and Anten 2004; Niinemets 2007; Niinemets and Anten 2009). As with other important functional traits, studies on moss within-canopy variation in photosynthesis are challenging due to miniature size of mosses, making the use of standard gas-exchange measurement practices difficult (Zotz and Kahler 2007). Nevertheless, gas-exchange measurements have demonstrated that photosynthetic capacity of current-year segments of Hylocomium splendens is much higher than in the canopy segments formed in the previous year (Sonesson et al. 1992). Analogously, subsequently removing 3 mm layers of Tortula ruralis canopy by razor blade, gas-exchange measurements combined with mathematical simulations were used to demonstrate that T. ruralis photosynthesis is strongly reduced from uppermost (layer 1) to lowest (layer 4) studied positions in the canopy (Zotz and Kahler 2007).
Higher spatial resolution data on moss photosynthesis can be obtained by labeling primary photosynthesis products by 14C. According to this method, whole cryptogam cushions are exposed to 14CO2, and after the exposure, the canopy is dissected into layers, and the amount of 14CO2 fixed by each layer is determined. This method was used to gain insight into within-canopy variation of photosynthesis in Sphagnum (Johansson and Linder 1980), in the aquatic moss Drepanocladus exannulatus (Schwartz and Markager 1999) and in the lichen Cladina stellaris (Lechowicz 1983). As with standard gas-exchange measurements, 14C studies have demonstrated large reduction of photosynthetic production with increasing canopy depth (Johansson and Linder 1980; Lechowicz 1983; Schwartz and Markager 1999). However, the disadvantage of the 14C method with entire moss cushions is that there are inherent within-canopy light gradients during the exposure, such that plant parts at different depths in the canopy are exposed to different quantum flux densities. As the result, photosynthetic capacity in canopy interior may be underestimated.
The second key tool for gaining information on small-scale heterogeneity of photosynthetic activity is chlorophyll fluorescence. Portable fluorescence instruments allow measurement of within-canopy photosynthetic profiles with high resolution and as actinic illumination is provided by the fluorescence instrument, light-saturated rates can be estimated at any position in the canopy (Tobias and Niinemets 2010). Chlorophyll fluorescence measurements demonstrate strong depth and light availability dependent variation within the canopy (Fig. 9.8e, f). In fact, significant photosynthetic activity is even observed in leaves with almost complete depletion of chlorophyll (Fig. 9.8). Due to low light, photosynthetic activity of leaves in the canopy interior may be below the observed photosynthetic capacity most of the time. However, during water stress when upper canopy leaves curl and become strongly appressed to the stem, leaves in more humid lower canopy can reach rates close to the theoretical maxima, partly compensating for the reduced activity of the upper canopy layers (Davey and Ellis-Evans 1996). This suggests that in determining moss canopy leaf area index, it is important to consider all foliage that is still physiologically active. Rather than using arbitrary methods, chlorophyll fluorescence may serve as an important tool for rapid and objective separation between alive and “dead” plant parts.
In addition to standard chlorophyll fluorescence techniques that require use of a sample holder (leaf clip), and multiple measurements to cover the whole within-canopy gradient, a new promising method for rapid assessment of spatial patterns in moss photosynthetic activity is imaging fluorescence (Siebke and Weis 1995; Baker et al. 2001; Chaerle et al. 2007). With imaging fluorescence, a whole moss stem can be sampled rapidly, and spatial variations in photosynthetic activity are immediately visualized. Pilot measurements demonstrate that foliage photosynthetic activity is essentially invariable within the shoots of plagiotropic mosses that do not form a canopy (Fig. 9.9a–c), but significant gradients from the top to bottom of the moss stem occur for mosses with orthotropic growth form that create a dense canopy (Fig. 9.9d–f). Overall, the photosynthetic data collectively demonstrate major variations in foliage photosynthetic characteristics from canopy top to bottom, but also that photosynthetic activity is preserved until almost entire depletion of chlorophyll.
Chlorophyll fluorescence images (light adapted maximum fluorescence yield, F m’) in six Hawaiian moss and liverwort species with plagiotropic growth form not forming a dense canopy: (a) – Bazzania sp., (b) – Baldwiniella kealeensis and (c) – Plagiochila deflexa; and with orthotropic growth form forming a canopy (d) – Thuidium cymbifolium (e) – Fissidens pacificus, and (f) – Leucobryum seemannii. The plant material was collected from the leeward side of Koolau mountains in Oahu (Tantalus trail, 21° 20′ N, 157° 48′ W, elevation ca. 400 m, (Peñuelas et al. 2010 for site description)). After sampling, the plants were kept in moist plastic bags, transported to the laboratory and chlorophyll fluorescence was measured with a Walz Imaging-PAM Mini fluorimeter (H. Walz, GmbH, Effeltrich, Germany). Moderately high actinic illumination of 400 μmol m−2 s−1 for 5 min. was used for light-adaptation of the samples. The taxonomy of mosses and liverworts follows (Staples et al. 2004; Staples and Imada 2006). Unpublished data of Ülo Niinemets and Mashuri Waite (2006).
IV. Conclusions
The available evidence demonstrates that most mosses do support high leaf area indices, on average much higher than leaf area indices supported by vascular plants. Such high leaf areas are accompanied by high degree of foliage aggregation reducing the light gradients within the moss stands. In addition, due to thin foliage elements, leaf chlorophyll contents are very low compared with vascular plants, expected to result in low leaf absorptances and much stronger impact of light scattering than in vascular plants. Within-canopy gradients in light are accompanied by gradients in foliage pigment contents and photosynthetic capacity that reflect both senescence in the bottom of the canopy as well as modifications in the stoichiometry of photosynthetic apparatus. Apart from the general patterns, there is high variability in light gradients across moss species driven by differences in the degree of foliage aggregation, canopy density and pigment content per area, but only limited number of species has been studied so far. We suggest that more case studies are needed to gain insight into the determinants of within-canopy light gradients. In particular, further studies are needed on moss leaf area indices and optical characteristics as well as on gradients in moss physiological characteristics.
Abbreviations
- \( {\overline{A}}_{\text{L}}\) :
-
– average leaf area;
- A M :
-
– moss leaf area per shoot section mass;
- A S :
-
– leaf area on the shoot;
- \( {\overline{F}}_{\text{N}}\) :
-
– average number of leaves on the stem;
- h :
-
– depth in the canopy;
- k(θ):
-
– canopy extinction coefficient (Eq. 9.1);
- k depth :
-
– apparent light extinction coefficient characterizing reduction in R Q with canopy depth (h);
- L :
-
– canopy leaf area index;
- L C :
-
– cumulative leaf area index from the canopy top to given location in the canopy;
- N S :
-
– number of shoots per area (shoot density);
- Q :
-
– photosynthetic quantum flux density at given location in the canopy;
- Q 0 :
-
– Q at canopy top;
- R Q :
-
– relative quantum flux density (transmittance of light from canopy top to given position in the canopy, Q/Q 0);
- S :
-
– shoot area index;
- S c :
-
– cumulative shoot area index;
- Ω:
-
– clumping index (Eq. 9.2);
- ζ:
-
– leaf absorptance;
- θ:
-
– solar zenith angle;
- χA :
-
– chlorophyll content per leaf area;
- χM :
-
– chlorophyll content per leaf dry mass
References
Aan A, Hallik L, Kull O (2006) Photon flux partitioning among species along a productivity gradient of an herbaceous plant community. J Ecol 94:1143–1155
Adams WW III, Winter K, Schreiber U, Schramel P (1990) Photosynthesis and chlorophyll fluorescence characteristics in relationship to changes in pigment and element composition of leaves of Platanus occidentalis L. during autumnal leaf senescence. Plant Physiol 93:1184–1190
Anderson JM, Chow WS, Park Y-I, Franklin LA, Robinson SPA, van Hasselt PR (2001) Response of Tradescantia albiflora to growth irradiance: change versus changeability. Photosynth Res 67:103–112
Anten NPR, Hirose T (1999) Interspecific differences in above-ground growth patterns result in spatial and temporal partitioning of light among species in a tall-grass meadow. J Ecol 87:583–597
Anten NPR, Hirose T (2003) Shoot structure, leaf physiology, and daily carbon gain of plant species in a tallgrass meadow. Ecology 84:955–968
Arkimaa H, Laitinen J, Korhonen R, Moisanen M, Hirvasniemi T, Kuosmanen V (2009) Spectral reflectance properties of Sphagnum moss species in Finnish mires. In: 6th EARSeL SIG IS workshop, Imaging Spectroscopy: Innovative tool for scientific and commercial environmental applications, 16–19 March 2009, Tel-Aviv University, Tel-Aviv, Israel, 5 pp
Asner GP, Wessman CA (1997) Scaling PAR absorption from the leaf to landscape level in spatially heterogeneous ecosystems. Ecol Model 103:81–97
Baker NR, Oxborough K, Lawson T, Morison JIL (2001) High resolution imaging of photosynthetic activities of tissues, cells and chloroplasts in leaves. J Exp Bot 52:615–621
Baldini E, Facini O, Nerozzi F, Rossi F, Rotondi A (1997) Leaf characteristics and optical properties of different woody species. Trees Struct Funct 12:73–81
Baldocchi DD, Bowling DR (2003) Modelling the discrimination of 13CO2 above and within a temperate broad-leaved forest canopy on hourly to seasonal time scales. Plant Cell Environ 26:231–244
Barker DH, Stark LR, Zimpfer JF, Mcletchie ND, Smith SD (2005) Evidence of drought-induced stress on biotic crust moss in the Mojave Desert. Plant Cell Environ 28:939–947
Bassi R, Caffarri S (2000) Lhc proteins and the regulation of photosynthetic light harvesting function by xanthophylls. Photosynth Res 64:243–256
Bates JW (1998) Is ‘life-form’ a useful concept in bryophyte ecology? Oikos 82:223–237
Bayfield NG (1973) Notes on water relations of Polytrichum commune Hedw. J Bryol 7:607–617
Bergamini A, Peintinger M (2002) Effects of light and nitrogen on morphological plasticity of the moss Calliergonella cuspidata. Oikos 96:355–363
Bond-Lamberty B, Gower ST (2007) Estimation of stand-level leaf area for boreal bryophytes. Oecologia 151:584–592
Boonman A, Prinsen E, Gilmer F, Schurr U, Peeters AJM, Voesenek LACJ, Pons TL (2007) Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiol 143:1841–1852
Boonman A, Prinsen E, Voesenek LACJ, Pons TL (2009) Redundant roles of photoreceptors and cytokinins in regulating photosynthetic acclimation to canopy density. J Exp Bot 60:1179–1190
Brakke TW (1994) Specular and diffuse components of radiation scattered by leaves. Agric For Meteorol 71:283–295
Bryant RG, Baird AJ (2003) The spectral behaviour of Sphagnum canopies under varying hydrological conditions. Geophys Res Lett 30:1134
Bubier JL, Rock BN, Crill PM (1997) Spectral reflectance measurements of boreal wetland and forest mosses. J Geophys Res – Atm 102:29483–29494
Carter GA, Knapp AK (2001) Leaf optical properties in higher plants: linking spectral characteristics to stress and chlorophyll concentration. Am J Bot 88:677–684
Cescatti A, Niinemets Ü (2004) Sunlight capture. Leaf to landscape. In: Smith WK, Vogelmann TC, Chritchley C (eds) Photosynthetic adaptation: chloroplast to landscape, Ecological Studies, vol 178. Springer, Berlin, pp 42–85
Chaerle L, Leinonen I, Jones HG, Van Der Straeten D (2007) Monitoring and screening plant populations with combined thermal and chlorophyll fluorescence imaging. J Exp Bot 58:773–784
Crandall-Stotler BJ, Bartholomew-Began SE (2007) Morphology of mosses (phylum Bryophyta). In: Zander RH, Eckel PM (eds) Flora of North America north of Mexico (FNA), vol 27. www.efloras.org. Flora of North America Editorial Committee New York, Oxford, pp 3–13
Davey MC, Ellis-Evans JC (1996) The influence of water content on the light climate within Antarctic mosses characterized using an optical microprobe. J Bryol 19:235–242
Davey ML, Nybakken L, Kauserud H, Ohlson M (2009) Fungal biomass associated with the phyllosphere of bryophytes and vascular plants. Mycol Res 113:1254–1260
Dilks TJK, Proctor MCF (1979) Photosynthesis, respiration and water content in bryophytes. New Phytol 82:97–114
Duursma RA, Falster DS, Valladares F, Sterck FJ, Pearcy RW, Lusk CH, Sendall KM, Nordenstahl M, Houter NC, Atwell BJ, Kelly N, Kelly JWG, Liberloo M, Tissue DT, Medlyn BE, Ellsworth DS (2012) Light interception efficiency explained by two simple variables: a test using a diversity of small- to medium-sized woody plants. New Phytol 193:397–408
Evans JR (1993a) Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I. Canopy characteristics. Aust J Plant Physiol 20:55–67
Evans JR (1993b) Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. II. Stability through time and comparison with a theoretical optimum. Aust J Plant Physiol 20:69–82
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Frego KA, Carleton TJ (1995) Microsite tolerance of four bryophytes in a mature black spruce stand: reciprocal transplants. Bryologist 98:452–458
Gabrielsen EK (1948) Effects of different chlorophyll concentrations on photosynthesis in foliage leaves. Physiol Plant 1:5–37
Glime J (2007) Bryophyte ecology. E-book sponsored by Michigan Technological University and the International Association of Bryologists
Goudriaan J (1977) Crop micrometeorology: a simulation study. Pudoc, Wageningen
Green TGA, Lange OL (1994) Photosynthesis in pokilohydric plants: a comparison of lichens and bryophytes. In: Schulze E-D, Caldwell MM (eds) Ecophysiology of photosynthesis, Ecological Studies, vol 100. Springer, Berlin, pp 319–341
Gutschick VP, Wiegel FW (1988) Optimizing the canopy photosynthetic rate by patterns of investment in specific leaf mass. Am Nat 132:67–86
Hallik L, Kull O, Niinemets Ü, Aan A (2009a) Contrasting correlation networks between leaf structure, nitrogen and chlorophyll in herbaceous and woody canopies. Basic Appl Ecol 10:309–318
Hallik L, Kull O, Nilson T, Peñuelas J (2009b) Spectral reflectance of multispecies herbaceous and moss canopies in the boreal forest understory and open field. Can J Remote Sens 35:474–485
Hallik L, Niinemets Ü, Kull O (2012) Photosynthetic acclimation to light in woody and herbaceous species: a comparison of leaf structure, pigment content and chlorophyll fluorescence characteristics measured in the field. Plant Biol 14:88–99
Hamerlynck EP, Tuba Z, Csintalan Z, Nagy Z, Henebry G, Goodin D (2000) Diurnal variation in photochemical dynamics and surface reflectance of the desiccation-tolerant moss, Tortula ruralis. Plant Ecol 151:55–63
Harris A (2008) Spectral reflectance and photosynthetic properties of Sphagnum mosses exposed to progressive drought. Ecohydrology 1:35–42
Hill MO, Preston CD, Bosanquet SDS, Roy DB (2007) BRYOATT: attributes of British and Irish mosses, liverworts and hornworts with information on native status, size, life form, life history, geography and habitat. NERC Centre for Ecology and Hydrology and Countryside Council for Wales, Cambridgeshire
Hirose T, Werger MJA (1987) Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72:520–526
Johansson LG, Linder S (1980) Photosynthesis of Sphagnum in different microhabitats on a subarctic mire. In: Sonesson M (ed) Ecology of a subarctic mire, Ecological Bulletins, vol 30. Swedish Natural Science Research Council, Stockholm, pp 181–190
Krumnikl M, Sojka E, Gaura J, Motyka O (2008) A new method for bryophyte canopy analysis based on 3D surface reconstruction. In: 7th computer information systems and industrial management applications, IEEE, pp 210–211
Lechowicz MJ (1983) Age dependence of photosynthesis in the caribou lichen Cladina stellaris. Plant Physiol 71:893–895
Lovelock CE, Robinson SA (2002) Surface reflectance properties of Antarctic moss and their relationship to plant species, pigment composition and photosynthetic function. Plant Cell Environ 25:1239–1250
Martin CE, Churchill SP (1982) Chlorophyll concentrations and a/b ratios in mosses collected from exposed and shaded habitats in Kansas. J Bryol 12:297–304
Martin G, Myers DA, Vogelmann TC (1991) Characterization of plant epidermal lens effects by a surface replica technique. J Exp Bot 42:581–587
Masarovičová E, Eliáshš P (1987) Some ecophysiological features in woodland mosses in SW Slovakia. 2. Chlorophyll content and photosynthesis. In: Pócs T, Simon T, Tuba Z, Podani J (eds) Proceedings of the IAB conference of bryoecology. Akadémiai Kiadó, Budapest-Vacratot, Hungary, pp 113–123
Masoni A, Ercoli L, Mariotti M, Barberi P (1994) Changes in spectral properties of ageing and senescing maize and sunflower leaves. Physiol Plant 91:334–338
Mesarch MA, Walter-Shea EA, Asner GP, Middleton EM, Chan SS (1999) A revised measurement methodology for conifer needles spectral optical properties: evaluating the influence of gaps between elements. Remote Sens Environ 68:177–192
Monsi M, Saeki T (1953) Über den Lichtfaktor in den Pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Jap J Bot 14:22–52
Myers DA, Vogelmann TC, Bornman JF (1994) Epidermal focussing and effects on light utilization in Oxalis acetosella. Physiol Plant 91:651–656
Niinemets Ü (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30:1052–1071
Niinemets Ü (2010) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol Res 25:693–714
Niinemets Ü, Anten NPR (2009) Packing the photosynthesis machinery: from leaf to canopy. In: Laisk A, Nedbal L, Govindjee (eds) Photosynthesis in silico: understanding complexity from molecules to ecosystems, Advances in photosynthesis and respiration, vol 29. Springer, Berlin, pp 363–399
Niinemets Ü, Kull O, Tenhunen JD (2004) Within canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ 27:293–313
Niinemets Ü, Tobias M, Cescatti A, Sparrow AD (2006) Size-dependent variation in shoot light-harvesting efficiency in shade-intolerant conifers. Int J Plant Sci 167:19–32
Niinemets Ü, García-Plazaola JI, Tosens T (2012) Photosynthesis during leaf development and ageing. In: Flexas J, Loreto F, Medrano H (eds) Terrestrial photosynthesis in a changing environment. The molecular, physiological and ecological bases of photosynthesis driving its response to the environmental changes. Cambridge University Press, Cambridge, pp 357–376
Nilson T (1971) A theoretical analysis of the frequency of gaps in plant stands. Agric Meteorol 8:25–38
Nilson T, Ross J (1997) Modeling radiative transfer through forest canopies: implications for canopy photosynthesis and remote sensing. In: Gholz HL, Nakane K, Shimoda H (eds) The use of remote sensing in the modeling of forest productivity, Forestry sciences, vol 50. Kluwer Academic, Dordrecht, pp 23–60
Osmond CB, Anderson JM, Ball MC, Egerton JG (1999) Compromising efficiency: the molecular ecology of light-resource utilization in plants. In: Press MC, Scholes JD, Barker MG (eds) Physiological plant ecology. The 39th Symposium of the British Ecological Society held at the University of York, 7–9 September 1998. Blackwell Science, Oxford, pp 1–24
Pedersen B, Hanslin HM, Bakken S (2001) Testing for positive density-dependent performance in four bryophyte species. Ecology 82:70–88
Peñuelas J, Sardans J, Llusià J, Owen SM, Carnicer J, Giambelluca TW, Rezende E, Waite M, Niinemets Ü (2010) Faster returns on ‘leaf economics’ and different biogeochemical niche in invasive compared with native plant species. Global Change Biol 16:2171–2185
Pons TL, Anten NPR (2004) Is plasticity in partitioning of photosynthetic resources between and within leaves important for whole-plant carbon gain in canopies? Funct Ecol 18:802–811
Proctor MC (1979) Structure and eco-physiological adaptations in bryophytes. In: Clarke G, Duckett J (eds) Bryophyte systematics. Academic, New York, pp 479–509
Proctor MCF (1984) Structure and ecological adaptation. In: Dyer AF, Duckett JG, Cronshaw J (eds) The experimental biology of bryophytes, Experimental botany: an international series of monographs. Academic, London, pp 9–37
Proctor MCF (1990) The physiological bases for bryophyte production. Bot J Linn Soc 104:61–77
Rice SK, Schneider N (2004) Cushion size, surface roughness, and the control of water balance and carbon flux in the cushion moss Leucobryum glaucum (Leucobryaceae). Am J Bot 91:1164–1172
Rice SK, Collins D, Anderson AM (2001) Functional significance of variation in bryophyte canopy structure. Am J Bot 88(9):1568–1576
Rice SK, Gutman C, Krouglicof N (2005) Laser scanning reveals bryophyte canopy structure. New Phytol 166:695–704
Rice SK, Neal N, Mango J, Black K (2011) Relationships among shoot tissue, canopy and photosynthetic characteristics in the feathermoss Pleurozium schreberi. Bryologist 114:367–378
Rincón E (1993) Growth responses of six bryophyte species to different light intensities. Can J Bot 71:661–665
Rincon E, Grime JP (1989) Plasticity and light interception by six bryophytes of contrasted ecology. J Ecol 77:439–446
Ross J (1981) The radiation regime and architecture of plant stands. Dr. W. Junk, The Hague
Sands PJ (1995) Modelling canopy production. I. Optimal distribution of photosynthetic resources. Aust J Plant Phys 22:593–601
Schwartz A-M, Markager S (1999) Light absorption and photosynthesis of a benthic moss community: importance of spectral quality of light and implications of changing light attenuation in the water column. Freshw Biol 42:609–623
Seel WE, Baker NR, Lee JA (1992) Analysis of the decrease in photosynthesis on desiccation of mosses from xeric and hydric environments. Physiol Plant 86:451–458
Siebke K, Weis E (1995) Imaging of chlorophyll-a-fluorescence in leaves: topography of photosynthetic oscillations in leaves of Glechoma hederacea. Photosynth Res 45:225–237
Simon T (1987) The leaf-area index of three moss species (Tortula ruralis, Ceratodon purpureus, and Hypnum cupressiforme). In: Pócs T, Simon T, Tuba Z, Podani J (eds) Proceedings of the IAB conference of bryoecology, Symposia Biologica Hungarica, 35. Akadémiai Kiadó, Budapest-Vacratot, Hungary, pp 699–706
Skré O, Oechel WC, Miller PM (1983) Moss leaf water content and solar radiation at the moss surface in a mature black spruce forest in central Alaska. Can J For Res 13:860–868
Smith AJE (1982) Bryophyte ecology. Chapman & Hall, London/New York
Sonesson M, Gehrke C, Tjus M (1992) CO2 environment, microclimate and photosynthetic characteristics of the moss Hylocomium splendens in a subarctic habitat. Oecologia 92:23–29
Staples GW, Imada CT (2006) Checklist of Hawaiian Anthocerotes and Hepatics. Trop Bryol 28:15–47
Staples GW, Imada CT, Hoe WJ, Smith CW (2004) A revised checklist of Hawaiian mosses. Trop Bryol 25:35–69
Thomas SC, Liguori DA, Halpern CB (2001) Corticolous bryophytes in managed Douglas-fir forests: habitat differentiation and responses to thinning and fertilization. Can J Bot 79:886–896
Tobias M, Niinemets Ü (2010) Acclimation of moss Pleurozium schreberi photosynthetic characteristics to among-habitat and within-canopy light gradients. Plant Biol 12:743–754
Tobias M, Niinemets Ü (2005) Acclimation to within-canopy light gradient in four moss species of contrasting architecture In: Abstracts. XVII International Botanical Congress, Vienna, Austria, 17–23 July 2005, pp 105–106
van der Hoeven EC, Huynen CIJ, During HJ (1993) Vertical profiles of biomass, light intercepting area and light intensity in chalk grassland mosses. J Hatt Bot Lab 74:261–270
Van Gaalen KE, Flanagan LB, Peddle DR (2007) Photosynthesis, chlorophyll fluorescence and spectral reflectance in Sphagnum moss at varying water content. Oecologia 153:19–28
Vitt DH (1990) Growth and production dynamics of boreal mosses over climatic, chemical and topographic gradients. Biol J Linn Soc 104:35–59
Vogelmann TC (1993) Plant tissue optics. Ann Rev Plant Physiol Plant Mol Biol 44:231–252
Vogelmann JE, Moss DM (1993) Spectral reflectance measurements in the genus Sphagnum. Remote Sens Environ 45:273–279
Vogelmann TC, Bornman JF, Yates DJ (1996a) Focusing of light by leaf epidermal cells. Physiol Plant 98:43–56
Vogelmann TC, Nishio JN, Smith WK (1996b) Leaves and light capture: light propagation and gradients of carbon fixation within leaves. Trends Plant Sci 1:65–70
Waite M, Sack L (2010) How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiin species of contrasting light habitats. New Phytol 185:156–172
Williams WE, Gorton HL, Witiak SM (2003) Chloroplast movements in the field. Plant Cell Environ 26:2005–2014
Zotz G, Kahler H (2007) A moss “canopy” – small-scale differences in microclimate and physiological traits in Tortula ruralis. Flora 202:661–666
Acknowledgements
Authors work on mosses has been supported by the Estonian Ministry of Science and Education (grant IUT 8-3), and the European Commission through European Regional Fund (the Center of Excellence in Environmental Adaptation).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Niinemets, Ü., Tobias, M. (2014). Scaling Light Harvesting from Moss “Leaves” to Canopies. In: Hanson, D., Rice, S. (eds) Photosynthesis in Bryophytes and Early Land Plants. Advances in Photosynthesis and Respiration, vol 37. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6988-5_9
Download citation
DOI: https://doi.org/10.1007/978-94-007-6988-5_9
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6987-8
Online ISBN: 978-94-007-6988-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)