Summary
Plant canopies are characterized by extensive gradients in light availability that importantly alter the photosynthetic productivity of leaves in different canopy layers and result in acclimatory changes in leaf structural, chemical and physiological traits. These within-canopy variations are further importantly driven by species functional type and ecological characteristics such as shade tolerance (ecological controls). This chapter explores the within-canopy variations in key functional traits among different plant functional types and in species with different ecological potentials using a simple methodology to separate the importance of different leaf-level traits in foliage photosynthetic acclimation. As a major acclimatory change, foliage photosynthetic capacity per leaf area (A max A) increases with increasing long-term average integrated quantum flux density (Q int) in the canopy. Within-canopy variation in A max A results in a greater whole canopy carbon gain than having A max A constant through the canopy. The increase in A max A with Q int can potentially result from increases in leaf dry mass per unit area (M A), nitrogen content per unit dry mass (N M) and nitrogen allocation to rate-limiting photosynthetic proteins. This analysis indicates that the importance of these three key factors varies among plant functional types. In species with relatively low rates of canopy expansion and leaf turnover such as woody evergreens and woody deciduous species, within-canopy variation in A max A is primarily determined by M A, while in herbaceous species with high rates of canopy growth and leaf turnover, the variation is mainly driven by changes in N M and nitrogen allocation to rate-limiting proteins of photosynthetic machinery. Furthermore, there are large within-canopy modifications in structural traits such as leaf angles and spatial aggregation modulating light harvesting and light avoidance, and in chemical traits such as xanthophyll cycle carotenoid content and isoprene emission contributing to abiotic stress resistance. As the result of light-dependent alterations in these traits, lower canopy leaves have a greater light harvesting efficiency, while upper canopy leaves a greater capacity for excess radiation dissipation and resistance to abiotic stress. Plasticity for foliar modifications varies among woody species of different ecological potentials with shade-intolerant species tending to have a greater photosynthetic plasticity, while shade-tolerant species greater leaf areas and higher canopy light interception. This review emphasizes the overall large within-canopy variation in key foliage functional traits and underscores the important differences among plant functional types and in species with different ecological potentials in their acclimation to within-canopy environment.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acclimation
- Chlorophyll content
- Carotenoids
- Dry mass per unit area
- Isoprene emission
- Leaf age
- Leaf life span
- Leaf morphology
- Nitrogen content
- Nitrogen partitioning
- Optimization
- Photosynthetic capacity
- Shade tolerance
1 I. Introduction
Variation in light availability is one of the most conspicuous features of plant canopies. Daily integrated average light flux varies often more than 50-fold between canopy top and bottom in dense plant canopies (Fig. 4.1, Hirose et al. 1988; Koike et al. 2001; Valladares 2003; Niinemets and Anten 2009; Chap. 9, Hikosaka et al. 2016b), but unexpectedly, the gradient is still 10–20-fold in relatively open canopies (Hirose et al. 1988; Werger and Hirose 1988; Rambal 2001; Joffre et al. 2007). Even in free-standing plants, foliage is importantly aggregated within the canopy envelope, and leaves at the top shade the leaves positioned lower in the canopy, resulting in major light gradients (e.g., Le Roux et al. 1999; Chap. 11, Disney 2016).
Illustration of within-canopy variation in incident seasonal average integrated quantum flux density (Q int) in a temperate evergreen conifer Pinus sylvestris canopy in Ahunapalu (58°19′ N, 27°17′ E, elevation ca. 60 m). Niinemets et al. (2001) provides further details of the stand. Hemispherical photographs taken from the upper part of the canopy (height of 18 m, Q int = 31.2 mol m−2 d−1) and lower part of the canopy within the shrub layer (height of 1.5 m, Q int = 3.85 mol m−2 d−1) are also demonstrated. Error bars show ± SD of average Q int at each height level. Q int is defined as the average daily integrated quantum flux density during foliage growth and development. Hemispherical photo analysis is a classic method for obtaining the relative potential penetrating quantum flux density in different locations of the canopy (e.g., Anderson 1964). The obtained relative potential values of incident diffuse and direct light availability need to be calibrated by long-term quantum flux density measurements to estimate Q int for each canopy location (e.g., Niinemets et al. 1998a). Note that for illustrative purposes, the y-axis crosses with the x-axis at the highest Q int value
Foliage photosynthetic capacities acclimate to these extensive long-term light gradients through plant canopies such that whole canopy photosynthetic response cannot be predicted from “an average leaf response”, but is the integrated response of leaves in different canopy positions with different physiological potentials tuned to their specific light environment (Hirose and Werger 1987b; Ellsworth and Reich 1993; Anten 1997; Pons and Anten 2004; Niinemets and Anten 2009; Chap. 5, Pons 2016). In fact, multiple leaf structural and chemical traits vary between canopy top and bottom, including leaf dry mass per unit area, leaf nitrogen content and nitrogen partitioning among proteins of photosynthetic machinery (Hirose and Werger 1987b; Ellsworth and Reich 1993; Anten 1997; Pons and Anten 2004; Niinemets and Anten 2009; Chap. 5, Pons 2016). Variations in these key functional traits ultimately drive within-canopy photosynthetic acclimation . Various structural and chemical traits have inherently different plasticities to within-canopy light conditions in different plant life forms and in species with different ecological potentials, leading to a spectrum of within-canopy photosynthetic acclimation responses across species (Niinemets and Anten 2009 for a review).
In addition to long-term variations in light availability, the environmental setting in plant canopies is much more complex. Light is a highly dynamic environmental factor that varies strongly during the day and among the days and seasons. Despite photosynthetic acclimation , leaves at the top of the canopy can be exposed to excess irradiance on clear days, resulting in photoinhibition and oxidative stress (Osmond et al. 1999; Werner et al. 2001b; Demmig-Adams and Adams 2006). Photoinhibition can become particularly pronounced when photosynthesis rates are reduced due to other abiotic stress factors such as soil drought (Ramalho et al. 2000; Werner et al. 2002; Valladares et al. 2005; Niinemets and Keenan 2014). There are major within-canopy gradients in the leaf capacity to adjust to dynamically changing light conditions (Niinemets et al. 2003; García-Plazaola et al. 2004), indicating that coping with excess light can be importantly determined by past leaf light regime.
In addition to light, air temperatures increase from canopy bottom that receives less radiation toward canopy top that is exposed to greater radiation (Niinemets et al. 1999b; Zhang et al. 2010; Krédl et al. 2012; Pinheiro Prado et al. 2013; Zhang et al. 2013). These gradients in temperature are also associated with gradients in relative air humidity (Chiariello 1984; Krédl et al. 2012; Zhang et al. 2013). Lower humidity coupled with greater temperature and radiation input leads to greater evaporative demands and potentially greater water stress in the upper canopy (Niinemets et al. 1999c; Hubbard et al. 2002; Aasamaa et al. 2004; Niinemets et al. 2004d; Sellin and Kupper 2004). In fact, the hydraulic conductivity of stem and branches can limit water transport to upper canopy in clear days with high radiation input (Joyce and Steiner 1995; Brodribb et al. 2005; Renninger et al. 2006; Ewers et al. 2007; Peltoniemi et al. 2012; Chap. 7, Woodruff et al. 2016). Thus, upper canopy leaves may become water-stressed even in situations with ample soil water supply (Joyce and Steiner 1995; Lemoine et al. 2002; Ewers et al. 2007). Stronger water stress in turn can lead to more severe photoinhibition and oxidative stress in leaves in the upper canopy.
This evidence suggests that, apart from changes in photosynthetic capacity, acclimation to within-canopy light gradients also involves structural and chemical adjustments to avoid excess light interception and increase the resistance to photoinhibition and oxidative stress in the upper canopy leaves (Rasmuson et al. 1994; Hikosaka and Hirose 1997; During 1999; Ishida et al. 1999a; James and Bell 2000; Werner et al. 2001b; Niinemets et al. 2003; García-Plazaola et al. 2004). It has been even suggested that interactions among environmental drivers and co-occurrence of multiple stresses can constrain the photosynthetic acclimation to within-canopy light environment, and as the result, “full acclimation” to within-canopy light is principally not possible (Niinemets and Valladares 2004; Niinemets 2012; Peltoniemi et al. 2012).
This chapter describes within-canopy variations in leaf photosynthetic rates and analyzes underlying sources of variation due to modifications in leaf structural, chemical and physiological characteristics. First, a methodology to separate structural, chemical and allocational controls on the variations in foliage photosynthetic rates within plant canopies is introduced. Then a short meta-analyses in broad-leaved evergreen species Quercus ilex is carried out to highlight the many facets of within-canopy foliage structural, chemical and physiological acclimation . The compiled dataset of Q. ilex foliar characteristics is unique in that it covers variations in all key leaf functional traits including diffusion conductances from ambient air to chloroplasts . The comprehensive analysis of the variation patterns in Q. ilex with high leaf longevity is used as a baseline to compare within-canopy acclimation responses in other plant functional type s with higher leaf turnover.
This review also analyses the overall significance of variations in photosynthetic capacity in altering the whole canopy carbon gain and considers the possible structural and chemical constraints on the acclimation of photosynthetic capacity. This chapter further focuses on structural traits determining efficient light harvesting in the lower canopy and avoidance of excess radiation interception in the upper canopy, and on chemical traits responsible for safe dissipation of excess light and increasing resistance to enhanced oxidative stress in the upper canopy. The review emphasizes that there is an important within-canopy variation in how the stress resistance traits respond to dynamic alterations in light availability. Finally, this chapter analyses the variations in plasticity in whole-canopy acclimation among species with different shade tolerance that characteristically colonize habitats with varying light availability. This review emphasizes the strong within-canopy acclimation in key leaf traits and outlines the richness of responses in different plant functional type s and in species with different shade tolerance.
2 II. Evaluation of the Role of Different Leaf Functional Traits Involved in Variation of Photosynthesis Through Plant Canopies
Variation in environmental drivers through plant canopies, in particular, variation in average daily incident integrated quantum flux density during foliage growth and development (Q int) alters a plethora of foliage structural, chemical and photosynthetic traits (Terashima and Hikosaka 1995; Anten et al. 1996, 1998; Koike et al. 2001; Lemoine et al. 2002; Meir et al. 2002; Wright et al. 2006; Niinemets 2007). As a key change, foliage photosynthetic capacity (A max) typically increases with increasing Q int from canopy bottom to top (Terashima and Hikosaka 1995; Anten et al. 1998; Koike et al. 2001; Lemoine et al. 2002; Meir et al. 2002; Wright et al. 2006; Niinemets 2007). Apart from A max that determines foliage assimilation rate at high light, foliage light harvesting efficiency importantly drives photosynthesis at lower light intensities. The initial quantum yield of photosynthesis also often varies, although the within-canopy variation in quantum yield is less than in A max, at least under non-stressed conditions (Cartechini and Palliotti 1995; Sands 1996; Niinemets and Kull 2001; Werner et al. 2001b).
To gain mechanistic insight into sources of within-canopy variation in A max and the initial quantum yield of photosynthesis , the steady-state photosynthesis model of Farquhar et al. (1980) is typically used (Chap. 3, Hikosaka et al. 2016a). According to Farquhar et al. (1980) photosynthesis model, A max is determined by the biochemical potentials of photosynthesis, the maximum carboxylase activity of Rubisco (V cmax) and the capacity for photosynthetic electron transport (J max), and by the stomatal (g s) and mesophyll diffusion (g m) conductances for photosynthesis, while the initial quantum yield of photosynthesis is mainly determined by the initial quantum yield for photosynthetic electron transport (α).
Within-canopy acclimation of J max, V cmax and α results from changes in multiple underlying traits . In the following, I define the modeling framework to evaluate the role of different leaf traits responsible for variations in J max, V cmax and α. The modeling framework will be used further through the chapter to gain insight into the importance of within-canopy variations in leaf structure and chemistry.
2.1 A. Determinants of Foliage Biochemical Potentials
Changes in biochemical photosynthesis potentials are determined by modifications in leaf structural and chemical traits , and the key question is to what extent different traits control variations in V cmax and J max. To separate among the effects of various structural and chemical traits on foliage biochemical potentials, V cmax and J max can be expressed as composites of several independent characteristics. For V cmax:
where V cr is the specific activity of Rubisco , i.e., the maximum rate of ribulose-1,5-bisphosphate carboxylation per unit Rubisco protein (μmol g−1 s−1), M A is the leaf dry mass per unit area (g m−2), F R is the fraction of leaf nitrogen in Rubisco, N M is the leaf nitrogen content per unit dry mass (g g−1) and 6.25 (g g−1) is the nitrogen content of Rubisco protein (Niinemets and Tenhunen 1997). Analogously, J max is given as:
where J mc is the capacity for photosynthetic electron transport per unit cytochrome f, F B is the fraction of nitrogen in rate-limiting proteins of photosynthetic electron transport, and the factor 8.06 considers the nitrogen content of proteins and molar stoichiometry relative to cytochrome f (Niinemets and Tenhunen 1997). Implicit in this expression is that the capacity for linear electron transport rate is determined by electron carriers between photosystems I and II (Niinemets and Tenhunen 1997 for a discussion).
Estimates of J max and V cmax are typically obtained from net assimilation vs. CO2 response curves, ideally from net assimilation (A) vs. chloroplastic CO2 concentration (C c) response curves. In the past, C c was not routinely estimated due to difficulties with estimation of mesophyll diffusion conductance (C c = C i – A/g m, where C i is the CO2 concentration in the intercellular air space). Thus, in the majority of past studies, V cmax and J max estimates were derived from A vs. C i response curves assuming that g m is infinite. However, recent work has demonstrated that g m is finite, and that it varies among species and can limit photosynthesis as significantly as stomatal conductance (Flexas et al. 2012 for a review). Thus, estimates of foliage biochemical potentials from A vs. C i response curves provide apparent, underestimated, values of V cmax and J max, and accordingly F R and F B according to equations 4.1 and 4.2 are also apparent fractions of N in rate-limiting proteins.
Apart from CO2 response curves, inverse modeling techniques can be used to estimate J max and V cmax from light response curves of photosynthesis (e.g., Niinemets and Tenhunen 1997; Niinemets et al. 1999d; Patrick et al. 2009) and estimate V cmax from the light-saturated net assimilation rate (e.g., Niinemets 1999). However, for inverse modeling, one needs an estimate of CO2 concentration in the chloroplasts or at least an estimate of C i. Alternatively, many studies have calculated the photosynthetic nitrogen use efficiency (E N), the ratio of A max to foliage N content (Hirose and Werger 1987a, 1994; Hikosaka et al. 1998; Hirose and Bazzaz 1998; Yasumura et al. 2002; Escudero and Mediavilla 2003; Pons and Westbeek 2004). Photosynthetic nitrogen use efficiency provides another estimate of the allocation of N to rate-limiting components of photosynthesis, but differently from F R that is standardized for variations in g s and g m (C c-based estimate of F R) or g s (C i-based apparent F R), within-canopy and species differences in E N can be affected by differences in diffusion conductances.
2.2 B. Traits Affecting Light Harvesting and Initial Quantum Yield
Classic studies have demonstrated that the initial quantum yield of photosynthesis for an absorbed light measured at a given chloroplastic CO2 and oxygen concentration and temperature (αp,a) is remarkably constant among C3 plants (Ehleringer and Björkman 1977; Leverenz 1987, 1988, 1994). However, quantum yields for an incident light (αp,i) importantly vary due to differences in leaf absorptance (ξ) that modifies the amount of light intercepted at a given incident light intensity, thereby altering the quantum yield (αp,i = ξαp,a) (Leverenz 1987, 1988, 1994; Long et al. 1993; Oberhuber et al. 1993).
Leaf absorptance is primarily a function of leaf chlorophyll content per unit area (χA, mmol m−2) (Evans 1993a; Evans and Poorter 2001), except for hairy or waxy leaves that often have enhanced reflectance (Ehleringer and Björkman 1978; Evans and Poorter 2001; Cescatti and Niinemets 2004). For leaves without such highly reflective epidermal characteristics, Evans (1993a) developed an empirical relationship between ξ and χA that describes well variations in ξ for a broad range of species with differing foliage architectures (Evans and Poorter 2001):
where 0.076 mmol m−2 is an empirical constant.
Leaf chlorophyll (Chl) and chlorophyll-binding proteins contain a large fraction of foliar nitrogen , and therefore, it is pertinent to express leaf chlorophyll content in nitrogen equivalents (Niinemets and Tenhunen 1997) as:
where F L is the fraction of leaf nitrogen invested in light harvesting , and B C (mmol Chl (g N)−1) is the chlorophyll binding defined as the amount of chlorophyll per unit nitrogen invested in light harvesting. It depends on the nitrogen cost of chlorophyll and specific chlorophyll-binding proteins, on the number of chlorophyll-binding sites in each chlorophyll-binding protein and on the stoichiometry of light-harvesting pigment -binding protein complexes (Hikosaka and Terashima 1996; Niinemets and Tenhunen 1997; Bassi and Caffarri 2000). In particular, B C increases with increasing the share of chlorophyll associated with light-harvesting complex of photosystem I I (LHC II) that binds more chlorophyll than the centers of photosystems I and II (PS I and PS II) (Bassi and Caffarri 2000; Jackowski et al. 2001; Paulsen 2001). Since the bulk of chlorophyll b is associated with LHC II and minor light harvesting complexes of PS II (Bassi and Caffarri 2000), increases in B C are also associated with decreases in chlorophyll a/b ratio.
The chlorophyll binding is normally about 2.1–2.5 mmol (g N)−1 in vascular plants (Niinemets and Tenhunen 1997; Niinemets et al. 1998b), and it increases and chlorophyll a/b ratio decreases with decreasing light availability in the canopy (e.g., Evans 1993a, b; Niinemets and Tenhunen 1997; Niinemets et al. 1998b; Pons and Anten 2004), reflecting increases in the amount of chlorophyll associated with LHC II relative to that contained in PS I and PS II. This is an important acclimatory modification as it reduces the N cost of light harvesting (Hikosaka and Terashima 1995). While values of B C are not routinely reported in the literature, chlorophyll a/b ratio is characteristically assessed in studies investigating light acclimation , and can be used as a proxy of light-driven modifications in thylakoid stoichiometry.
3 III. Light-Dependent Variations in Photosynthesis and Underlying Traits Across Plant Canopies
Equations 4.1, 4.2, 4.3, and 4.4 provide a simple means to analyze the effects of variations in foliage structure, nitrogen content and nitrogen partitioning on foliage photosynthetic potentials and initial quantum yield . In this section, I analyze how leaf structural and chemical traits vary in plant canopies and what are the implications for foliage photosynthetic potentials. As realized net assimilation rates are importantly driven by CO2 diffusion conductances from ambient atmosphere to chloroplasts , I also consider within-canopy variations in stomatal and mesophyll conductance s .
This section provides first a meta-analysis of within-canopy variations in leaf traits in the Mediterranean evergreen sclerophyll Quercus ilex. This species grows in water-limited open ecosystems where the variation in light availability as a source for foliage functional differentiation has been traditionally neglected. This meta-analysis serves to identify the basic scaling relationships between key foliage traits and irradiance in the canopy and make the general point that even in species growing in open ecosystems, there can be major within-canopy variations in foliage characteristics. This meta-analysis also serves as an example demonstrating how fragmentary information present in multiple studies can be summarized to gain insight into within-species variability. Overall, there is less data available for broad-leaved evergreen woody species than for herbaceous species and needle-leaved evergreen and winter-deciduous woody species (Niinemets and Anten 2009 for a review), making this analysis particularly pertinent. Furthermore, the data summarized in Q. ilex include all key functional leaf-level traits covering structural, biochemical and diffusional limits of photosynthesis , making the analysis truly comprehensive. In particular, within-canopy variation in mesophyll diffusion conductance has not been routinely studied with a few exceptions (Niinemets et al. 2006a; Cano et al. 2013; Niinemets 2015).
Although the meta-analysis in Q. ilex highlights the basic within-canopy leaf trait variation patterns, evergreens such as Q. ilex support multiple age cohorts. This is significant as in evergreens, older foliage becomes gradually shaded with canopy expansion and formation of new leaves. Accordingly, within-canopy trait patterns of older leaves are importantly driven by the capacity of older foliage to reacclimate to new light conditions. Thus, in the following, I analyze the within-canopy trait variations in older leaf age classes in evergreens primarily focusing on modifications in the overall plastic variations and on the strength of trait vs. light climate relationships.
After highlighting the basic within-canopy variation patterns in evergreens , I further ask how do the within-canopy gradients in foliage traits vary among different plant functional type s ? Different plant functional types are characterized by varying rates of foliage and canopy growth and turnover and such differences in the rates of canopy expansion and leaf longevity can alter the gradients of light through the canopy, leaf lifetime intercepted light integral and the extent of variation in light availability during leaf lifetime (Schulze 1981; Jarvis and Leverenz 1983; Woodward et al. 1994; Niinemets et al. 2012; Niinemets and Keenan 2012). This may significantly alter the degree of within-canopy variation in different leaf-level traits in different plant functional types.
Finally, I analyze the overall significance of within-canopy variations in photosynthetic potentials for whole-canopy net assimilation rates using a simple modeling approach. This model -based analysis further underscores the importance of within-canopy trait variation and emphasizes the need to include phenotypic plasticity in large-scale photosynthesis models .
3.1 A. A Meta-Analysis of Within-Canopy Variations in the Mediterranean Evergreen Quercus ilex
3.1.1 1. Data and Methods
A thorough literature survey identified eight studies that provided information on within-canopy variation in light vs. foliage traits in Q. ilex (Eckardt et al. 1975; Rambal 1992; Sala et al. 1994; Rambal et al. 1996; Niinemets et al. 2002b, 2006a; Davi et al. 2008; Vaz et al. 2011). For these studies, average seasonal average incident integrated quantum flux density for 50 days after bud burst (Q int) was used as an estimate of light availability. In studies reporting leaf dry mass per unit area (M A) in relation to directly measured cumulative leaf area index , relative quantum flux density was derived according to Lambert-Beer’s law using an extinction coefficient of 0.5 (Sala et al. 1994), while for optical leaf area index obtained by LAI -2000 instrument (Rambal et al. 1996), an extinction coefficient of 0.8 (Niinemets et al. 2010) was used. The above-canopy Q int was derived for the year of foliage sampling using global radiation databases as in Niinemets and Keenan (2012). Q int vs. foliage trait relationships were fitted by non-linear regressions in the form y = ax b and y = aLn(x) + b. As leaf dry mass per unit area is strongly correlated with within-canopy variations in Q int (e.g., Fig. 4.2a and Meir et al. 2002; Niinemets 2007; Niinemets and Anten 2009), M A was used as a substitute of light for studies explicitly investigating variations in foliage chemistry within the canopy light gradients, but lacking direct light measurements. This analysis only included mature fully-expanded current year foliage. The relationships in older leaf age classes are analyzed in Sect. III.B.
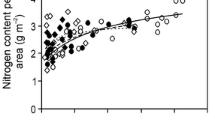
Effects of within-canopy variation in average integrated quantum flux density (Q int) on (a) leaf dry mass per unit area (M A), (b) maximum carboxylase activity of Rubisco (V cmax) and capacity for photosynthetic electron transport (J max), (c) stomatal conductance to water vapor (g s) and mesophyll diffusion conductance (g m) and (d) CO2 drawdown from ambient air to chloroplasts (C a-C i) and from intercellular air space to chloroplasts (C i-C c) in current-year leaves of Mediterranean broad-leaved evergreen sclerophyll Quercus ilex. The inset in (b) demonstrates the correlations of V cmax and J max with leaf dry mass per unit area. The data were fitted by linear (panel inset and C i-C c in d) and by non-linear regressions in the form y = ax b and y = aLn(x) + b, whichever of the two provided a higher r 2 (P < 0.01 for all regressions). Data sources in panel a as indicated, all other data are from Niinemets et al. (2006a). The sampling locations were: 41.73°N, 3.58°E, elevation 270 m (Davi et al. 2008), 41.25°N, 1°E, elevation 700 m (valley) and 975 m (ridge) (Sala et al. 1994), 43.74°N, 3.59°E, elevation 270 m (Rambal et al. 1996) and 45.88°N, 10.87°E, elevation 300 m (Niinemets et al. 2006a). Q int corresponds to average daily integrated incident quantum flux density for 50 days after bud burst
3.1.2 2. Variations in key Functional Traits
Analysis of all published within-canopy patterns of foliage traits in Q. ilex highlights several broad trends in plastic modifications in foliage structural, chemical and photosynthetic characteristics. First of all, M A strongly increased with increasing average quantum flux density during leaf growth, 1.5–2.4-fold between canopy top and bottom (Q int, Fig. 4.2a). Nitrogen content per unit area (N A) also increased, 1.7–2.7-fold, with increasing Q int (r 2 = 0.77–0.93, P < 0.001), but nitrogen content per unit dry mass (N M) varied little within the canopy (r 2 = 0.00–0.12, P > 0.2, average ± SD = 1.57 % ± 0.25 % across the studies analyzed). Foliage photosynthetic capacity per unit area (A max A, r 2 = 0.64, P < 0.001 for the data of Niinemets et al. 2006a) and foliage photosynthetic potentials, the maximum carboxylase activity of Rubisco (V cmax A) and the capacity for photosynthetic electron transport per unit area (J max A, Fig. 4.2b) scaled positively with Q int (ca. 2.5-fold change of foliage photosynthetic potentials between canopy top and bottom for the data in Fig. 4.2b and 1.8-fold change in Vaz et al. 2011), but mass-based photosynthetic characteristics varied little within the canopy (r 2 = 0.00–0.06 for these three traits). Furthermore, the fractions of nitrogen in Rubisco (Eq. 4.1, average ± SD = 0.154 ± 0.025 for the data of Niinemets et al. 2006a) and in bioenergetics (Eq. 4.2, 0.039 ± 0.007 for the data of Niinemets et al. 2006a and 0.036 ± 0.009 for the data of Rambal et al. 1996) were independent of Q int (P > 0.1 for both variables and both datasets). Given the invariability of nitrogen allocation and considering that the area-based traits are the products of mass-based traits and M A, within-canopy variation in N A and foliage photosynthetic potentials was mainly driven by light-dependent variations in M A (Fig. 4.2b inset).
Both stomatal and mesophyll conductance s were greater in the upper canopy (Fig. 4.2c). The CO2 drawdown from ambient air (C a) to intercellular air space (C i) was independent of Q int (r 2 = 0.01), indicating that stomata limited photosynthesis similarly through the canopy. However, the CO2 drawdown from intercellular air space to chloroplasts (C c, C i-C c), and the overall drawdown (C a-C c) increased with increasing Q int (Fig. 4.2d), demonstrating that g m limited photosynthesis more in the upper canopy. Thus, increases in M A were not only associated with stacking of photosynthesizing biomass per unit area, but increased foliage robustness also resulted in reduced efficiency of use of resources invested in photosynthetic machinery. Such enhanced diffusion limitations might reflect increases in cell wall thickness, an acclimation response contributing to withstanding low leaf water potential s in the upper canopy (see Sect. I), but also reducing CO2 diffusion rate through leaf liquid phase (e.g., Terashima et al. 2011; Tosens et al. 2012a, b; Tomás et al. 2013).
As light measurements were not available in studies investigating within-canopy variation in chlorophyll contents, M A was used as a proxy of within-canopy light conditions. Across these studies, foliage chlorophyll content per unit dry mass (χM) scaled negatively with M A (Fig. 4.3a). Given that N M was not correlated with within-canopy variation in light, this result also suggests that N in light harvesting (F L, Eq. 4.4) increases with increasing shading in the canopy. Nevertheless, in this species, within-canopy variation in M A was greater than the variation in χM such that leaf chlorophyll content per unit area was positively correlated with M A (Fig. 4.3b).
Correlations of leaf chlorophyll content per unit leaf dry mass (a) and per unit leaf area (b) with leaf dry mass per unit area in current-year leaves of Quercus ilex. Variations in dry mass per unit area are due to within-canopy differences in light environment (Fig. 4.2a). Data from multiple studies investigating within-canopy variation in leaf traits (Gratani and Fiorentino 1986, Table 1 for site locations; Gratani et al. 1989, 1992; Gratani 1993, 1997) were pooled and fitted by a non-linear regression in the form y = ax b (a) and with a linear regression (b). P < 0.001 for both regressions
3.2 B. Leaf Age-Dependent Variations in Foliage Plasticity in Evergreens
3.2.1 1. Why Should Plasticity Depend on Leaf Age?
Evergreen species support multiple leaf age cohorts, e.g., Q. ilex supports leaves up to 6 years old (Niinemets et al. 2005a), and several conifers can support leaves more than 10 years old (Ewers and Schmid 1981; Schoettle 1989; Schoettle and Fahey 1994; Niinemets and Lukjanova 2003). Increases in leaf age are characteristically associated with increases in leaf dry mass per unit area and in reductions in N M and photosynthetic capacity (Teskey et al. 1984; Brooks et al. 1994, 1996; Niinemets 1997b; Niinemets et al. 2005a). On the other hand, older foliage initially developed at higher light becomes gradually shaded by new foliage and the key question is to what extent the older foliage can reacclimate to the modified light conditions. Although there is some secondary leaf growth at least in conifers (Ewers 1982; Gilmore et al. 1995), rigidification of cell walls after foliage maturation strongly curbs further foliage expansion growth. Thus, foliage structural reacclimation to modified light conditions is inherently limited. However, foliage may reacclimate to altered light conditions by changing nitrogen content and nitrogen allocation among the components of photosynthetic machinery within the leaf (Brooks et al. 1996; Niinemets 1997b; Escudero and Mediavilla 2003; Oguchi et al. 2005, 2006; Muller et al. 2009). Given the structural constraints, it is plausible that foliage photosynthetic plasticity to light is decreasing with increasing foliage age.
3.2.2 2. Analyzing Plasticity Changes
To compare plastic changes in foliage traits of leaves of different age, I calculated the relative light-dependent change (R c) of a given trait as (Niinemets et al. 2015):
where V i is the trait value at a seasonal average quantum flux density of i (Q int,i) and V i+x is the trait value at Q int,i + x (Q int,i+x). R c is normalized with respect to the average trait value across the given light range, (V i+x + V i)/2, to account for age-dependent changes in absolute trait values. The plasticity to within-canopy variations in light increases with increasing the R c value. In the following analysis, R c was calculated with Q int,i = 6 mol m−2 d−1 and Q int,i+x = 12 mol m−2 d−1. Foliage trait vs. Q int relationships are curvilinear (Fig. 4.2), and this is a moderately high light range positioned in the strongly increasing part of foliage trait vs. Q int relationships. In the following, age-dependent changes in plasticity are analyzed in three species, Q. ilex and conifers Abies amabilis and Pinus contorta.
3.2.3 3. Experimental Evidence of Plasticity Modifications
Examination of R c values in leaves of different age indicated that the plasticity in N A (Fig. 4.4a), M A (data not shown) and A max A (Fig. 4.4b) decreased with increasing leaf age in the three species analyzed. The reduction in plasticity was also associated with reduction in the degree of explained variance (Fig. 4.4c, d), indicating that the relationships became weaker and more scattered with increasing leaf age.
However, the age-dependent reduction in the plasticity in A max A was less than in N A and M A (cf. Fig. 4.4a, b). This suggests that differently from structural traits and total nitrogen content, foliage photosynthetic traits of older shaded foliage can adapt to modified light regime (Brooks et al. 1994; Niinemets et al. 2006a). In fact, in Q. ilex, photosynthetic capacity of 1-year-old foliage was even more plastic that photosynthetic capacity of current-year foliage (Fig. 4.4b). This was associated with within-leaf changes in nitrogen allocation among proteins limiting photosynthetic capacity. Differently from current-year leaves (Sect. III.A.2), both F R (Eq. 4.1, r 2 = 0.29, P < 0.05) and F B (Eq. 4.2, r 2 = 0.45, P < 0.01 for the data of Niinemets et al. 2006a) increased with Q int in 1-year-old leaves of Q. ilex. Nevertheless, in these leaves, the within-canopy variation in nitrogen allocation, F R and F B, 1.4–1.5-fold for the whole canopy, was still less than for nitrogen content and leaf dry mass per unit area.
Modifications in relative light-dependent changes in foliage nitrogen content per unit area (a) and photosynthetic capacity per unit area (b) with leaf age and concomitant changes in the explained variance (r 2, c and d) in the temperate evergreen conifers Abies amabilis (Data of Brooks et al. 1996) and Pinus contorta (Data of Schoettle and Smith 1999) and in the Mediterranean evergreen broad-leaved species Quercus ilex (Data of Niinemets et al. 2006a). Relative light-dependent change (R c) of a given trait is defined by Eq. 4.5 and characterizes the light-dependent plasticity normalized with respect to the average trait value to directly compare plasticities in species with different average trait values. The plasticity increases with increasing the R c value. Here R c was calculated for a moderately high light range (Q int) of 6–12 mol m−2 d−1 (Fig. 4.2 for the full light responses). Q int is defined as in Fig. 4.2
In Abies amabilis, it has been further demonstrated that reacclimation to reduced light conditions results in increased nitrogen allocation to light harvesting (Brooks et al. 1994, 1996). This evidence collectively indicates that older foliage of evergreens can reacclimate to altered light conditions primarily due to modifications in nitrogen allocation within leaves, but also that the overall photosynthetic plasticity to light is lower for older leaves (Fig. 4.4). Thus, the modifications in nitrogen allocation cannot fully compensate for the structural inadequacy of shaded older foliage morphologically acclimated to higher past irradiance .
This analysis indicates that in evergreens , foliage photosynthetic characteristics of any canopy layer depend both on the structural, chemical and physiological acclimation to growth light conditions as well as on the reacclimation capacity. Interactions of leaf age with light availability and limited reacclimation capacity can clearly blur light vs. foliage structure and physiological activity relationships (Niinemets et al. 2006a). For example, such a confounding variation in leaf age and within-canopy light regime might explain why the correlations of leaf structural characteristics with light were weak for Australian broad-leaved evergreens (Wright et al. 2006) and in conifer Pinus pinaster (Warren and Adams 2001) when all leaves of different age were analyzed together.
3.3 C. Qualitative Differences among Trait Relationships between Plant Functional Types
3.3.1 1. Species with Low to Moderately High Leaf Turnover
The meta-analysis in the broad-leaved evergreen sclerophyll Q. ilex underscores the strong within-canopy variation in foliage structural, chemical and photosynthetic characteristics (Figs. 4.2 and 4.3) as is typical in plant canopies (Hirose and Werger 1987a, b; Ellsworth and Reich 1993; Pons et al. 1994; Meir et al. 2002; Niinemets 2007). The overall range of variation in M A between canopy top and bottom in Q. ilex was 1.5–2.4 in the eight studies analyzed (Fig. 4.2a). The within-canopy variation in M A was associated with similar variations in area-based contents of nitrogen and chlorophyll (Fig. 4.3), mass-based photosynthetic potentials (Fig. 4.2b) and stomatal conductance (Fig. 4.2c). In contrast, mass-based nitrogen content and photosynthetic potentials were not associated with light availability and chlorophyll content per unit dry mass even decreased with increasing light availability. Overall, this evidence demonstrates that within-canopy increase in area-based characteristics in Q. ilex primarily reflected accumulation of tissue with similar chemical composition and physiological activity per unit leaf area (most traits ) or that the stacking trend dominated over the trend of dilution of the chemicals (chlorophyll).
These observations in Q. ilex are in a broad agreement with past observations in other broad-leaved evergreens . Differently from Q. ilex, a moderate increase in N M with Q int has been observed in temperate evergreen Ilex aquifolium (Aranda et al. 2004) and in several temperate Nothofagus species (Niinemets et al. 2004b), and in tropical species Ficus insipida (Posada et al. 2009). Analogously, photosynthetic capacity per unit dry mass (A max M) (Chazdon and Field 1987; Ishida et al. 1999b; Posada et al. 2009) and nitrogen partitioning coefficients, F R and F B (Evans and Poorter 2001), can either moderately decrease or increase in different evergreen species. Nevertheless, all these studies emphasize that the light-dependent increase in M A is the key factor responsible for within-canopy increases in N A and A max A in broad-leaved evergreens.
The relationships are analogous in evergreen needle-leaved conifers. In conifers, the variations in foliage nitrogen content and photosynthetic capacity per unit area are also dominated by M A (Sprugel et al. 1996; Niinemets 1997a; Stenberg et al. 1999; Palmroth and Hari 2001; Han et al. 2003; Leal and Thomas 2003; Han et al. 2004, 2006). Furthermore, similar relationships have been demonstrated in other species with needle-like assimilative organs such as cladodes in the angiosperm Casuarina (Niinemets et al. 2005b). However, an increase in N M with increasing Q int has been observed in some conifers, and this was associated with increased mesophyll volume fraction and enhanced photosynthetic capacity per leaf dry mass at higher Q int (Niinemets et al. 2007). So far, light-dependent modifications in tissue fractional composition have been studied only in a few conifers (e.g., Aussenac 1973; Kovalyev 1980; Niinemets et al. 2007), and clearly more studies on three-dimensional needle anatomy are called for. Furthermore, in conifers with complex three-dimensional leaf cross-section, foliage photosynthetic capacity per unit projected area also depends on modifications in total to projected leaf area ratio (S T/S P, Sect. IV.A).
The relationships of leaf traits with Q int are qualitatively similar in broad-leaved deciduous species that form all leaves simultaneously in the beginning of growing season, and thus, are characterized by a relatively high leaf longevity (Ellsworth and Reich 1993; Tjoelker et al. 1995; Niinemets and Kull 1998; Niinemets et al. 1998b; Koike et al. 2001; Meir et al. 2002; Iio et al. 2005). Although in some species, N M (Niinemets et al. 1998b), A max M (Niinemets et al. 1998b) and nitrogen partitioning in photosynthetic machinery, F B and F R (Niinemets et al. 1998b, 2010) increase with increasing Q int, in other species, N M (Ellsworth and Reich 1993; Niinemets 1995; Fleck et al. 2003), A max M (Ducrey 1981; Ellsworth and Reich 1993; Niinemets et al. 1998b), and nitrogen partitioning coefficients (Niinemets et al. 2010) can also moderately decrease with increasing Q int. Thus, again the overall photosynthetic response to within-canopy variations in Q int primarily results from modifications in M A. Nevertheless, upon sudden changes in irradiance , woody deciduous species can significantly change foliage photosynthetic capacity through changes in nitrogen partitioning (Naidu and DeLucia 1997; Niinemets et al. 2003; Oguchi et al. 2005, 2006), albeit the acclimation is limited due to anatomical constraints as in evergreen species (Sect. III.B, Oguchi et al. 2005, 2006) and can be relatively time-consuming (Naidu and DeLucia 1997; Kull and Kruijt 1999; Niinemets et al. 2003).
3.3.2 2. Species with High Leaf Turnover
The situation is qualitatively different for broad-leaved deciduous woody species with continuous canopy expansion such as in fast-growing dense young Salix stands or coppice plantations. In such stands, foliage developed earlier becomes shaded by newly developed foliage analogously to different-aged foliage in evergreen canopies (Sect. III.B). Thus, there are strong leaf age and light gradients within the fast-expanding canopies of deciduous species. In fact, in such canopies, most leaves could have been exposed to high light during their development at the top of the canopy. As the result, M A is relatively invariable in fast-growing woody canopies, and the within-canopy variation in N A is primarily driven by a strong gradient in N M (Vapaavuori et al. 1989; Vapaavuori and Vuorinen 1989; Noormets et al. 1996; Kull et al. 1998), while the within-canopy variation in A max A is driven by increases of A max M with Q int (Vapaavuori et al. 1989; Vapaavuori and Vuorinen 1989).
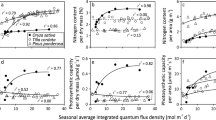
The situation is similar in the canopies of herbaceous species where the entire canopy is formed during a single growing season and there is a continuous canopy growth until the onset of inflorescence formation. Examination of leaf trait vs. Q int relationships in representative grass (Phragmites australis) and herb (Solidago altissima) species (Data of Hirose and Werger 1987a, 1994, 1995, Werger and Hirose 1988) demonstrates that although M A does increase with increasing Q int (Fig. 4.5a), the increase is much less than the corresponding change of N M through the canopy (Fig. 4.5b) such that the increase of N A (Fig. 4.5c) is mainly dependent on within-canopy gradient in N M. In addition to N A, the light-dependent increase of A max A (Fig. 4.5f) is determined by increases in nitrogen allocation (photosynthetic nitrogen use efficiency , E N, Fig. 4.5d), i.e., A max A = E N N A. Increases in both N M and photosynthetic nitrogen use efficiency are responsible for the strong increase of A max M with Q int (Fig. 4.5e; A max M = E N N M). Given further that A max A = E N N M M A, this evidence collectively indicates that nitrogen reallocation among the leaves and modification in nitrogen partitioning within the leaves are the primary mechanisms determining acclimation of herbaceous canopies to within-canopy light gradients, while changes in M A play a less important role.
Light-dependent variations in leaf dry mass per unit area (a), nitrogen content per unit dry mass (b) and area (c), photosynthetic nitrogen use efficiency (photosynthetic capacity per unit nitrogen, (d), and light-saturated net assimilation rate at ambient CO2 concentration (photosynthetic capacity) per unit dry mass (e) and area (f) in the grass Phragmites australis (Data of Hirose and Werger 1994, 1995) and in the herb Solidago altissima (Data of Hirose and Werger 1987a, b; Werger and Hirose 1988). Data were fitted by non-linear regressions in the form of y = aLn(x) + b (all regressions are significant at least at P < 0.02). Seasonal average integrated quantum flux density (Q int) is defined as in Fig. 4.2
Overall, the strong gradients in N M, photosynthetic nitrogen use efficiency and A max M in species with high leaf turnover partly reflect reacclimation to modified light conditions, but also greater leaf turnover and senescence of older leaves at the bottom of plant canopy (Vapaavuori et al. 1989; Vapaavuori and Vuorinen 1989; Pons and Pearcy 1994; Hikosaka 1996; Anten et al. 1998; Weih 2009). In fact, in species with short leaf life-span and fast leaf turnover, it has been demonstrated that shading , especially shading of individual leaves, can introduce programmed cell death, leading to rapid reductions of leaf photosynthetic capacity and leaf abscission (Burkey and Wells 1991; Pons and Pearcy 1994; Ackerly and Bazzaz 1995; Ono et al. 2001; Vos and van der Putten 2001; Boonman et al. 2006). On the other hand, compared with species with low rate of leaf turnover, photosynthetic capacity in species with high leaf turnover can relatively rapidly respond to increases in light availability (Pons and Pearcy 1994; Boonman et al. 2006).
3.4 D. Variations in Photosynthetic Plasticity Among Plant Functional Types
In Q. ilex, the relationships of M A and photosynthetic potentials with Q int were strongly curvilinear, with most of the change in foliage characteristics occurring over the light range of 2–12 mol m−2 d−1 (Fig. 4.2). In the case of M A, clear site differences were evident at the saturating part of M A vs. Q int relationships, at Q int values higher than ca. 12 mol m−2 d−1 (Fig. 4.2a). Although extensive, the ranges of variation in M A, N A and photosynthetic potentials in broad-leaved evergreens are somewhat smaller than the within-canopy variations in these traits of two- to four-fold in the canopies of winter-deciduous forest trees (see Sect. V; e.g., Ellsworth and Reich 1993; Niinemets and Kull 1998; Iio et al. 2005; Niinemets and Anten 2009; Niinemets et al. 2015). In fact, in several deciduous broad-leaved species, there is only a moderate curvilinearity in leaf trait vs. Q int relationships (Niinemets and Kull 1998; Meir et al. 2002; Aranda et al. 2004; Niinemets et al. 2015). The range of variation in trait vs. Q int relationships is also high, more than two- to four-fold in several evergreen shade -tolerant conifers from genera Abies and Picea (Niinemets 1997a; Stenberg et al. 1998; Cescatti and Zorer 2003). However, there was a low within-canopy plasticity of 1.3–1.7-fold in two Picea species in the study of Ishii et al. (2003), and in Pseudotsuga menziesii and Tsuga heterophylla in the study of Bond et al. (1999). Low foliage plasticity 1.3–2-fold has been reported for several Pinus species (Bond et al. 1999; Niinemets et al. 2001, 2002a).
In the case of herbaceous species, high photosynthetic plasticity, typically two- to four-fold (Fig. 4.5f; Hirose and Werger 1994; Anten et al. 1995b; Niinemets et al. 2015), in exceptional cases close to or even more than an order of magnitude (Pons et al. 1993; Hirose and Werger 1994; Anten et al. 1995b, Chap. 5, Pons 2016) has been reported. This high plasticity is associated with moderate changes in leaf dry mass per unit area (Fig. 4.5a) and nitrogen allocation (Fig. 4.5d) and moderate to extensive changes in leaf nitrogen content per unit dry mass (Fig. 4.5b; Hirose et al. 1989; Lemaire et al. 1991; Evans 1993a, b; Hirose and Werger 1994; Niinemets et al. 2015; Chap. 5, Pons 2016).
Overall, there is evidence of greater photosynthetic plasticity in leaves with shorter life-span. The differences among evergreen and deciduous woody species mainly result from the circumstance that evergreens can reduce their M A when growing in shade conditions less than deciduous species, resulting in correspondingly narrower range in photosynthetic potentials in evergreens. High photosynthetic plasticity in herbaceous canopies is mainly associated with moderate to high gradients in all three determinants of photosynthetic capacity (Eqs. 4.1 and 4.2): M A, nitrogen allocation and partitioning. In particular, gradients in nitrogen allocation in herbaceous species reflect the inherent strategy of resource remobilization from shaded leaves undergoing senescence to young developing leaves at the top of the canopy (Werger and Hirose 1988; Hikosaka et al. 1994; Hirose and Werger 1994; Hikosaka 1996; Franklin and Ågren 2002, Chap. 5, Pons 2016).
3.5 E. Importance of Within-Canopy Biochemical Modifications in Whole Canopy Photosynthesis
Within-canopy variation in key leaf traits allows for investment of photosynthesizing biomass in environments where the pay-back is higher, and has therefore been considered as an adaptive feature. Several studies have explored the quantitative benefits of trait variation using either numerical integration or optimality analyses (Field 1983; Hirose and Werger 1987b; Gutschick and Wiegel 1988; Farquhar 1989; Sands 1995; Anten 2005; Peltoniemi et al. 2012; Hikosaka 2014; Chap. 13, Anten 2016). There can be several target variables for optimization of canopy photosynthesis : maximization of canopy photosynthesis for given biomass investment in leaves (Gutschick and Wiegel 1988), maximization of canopy photosynthesis for given total canopy nitrogen content (Field 1983; Hirose and Werger 1987b; Farquhar 1989; Anten 2005; Chap. 13, Anten 2016) or maximization of canopy photosynthesis with given nitrogen and water use (Buckley et al. 2002; Farquhar et al. 2002; Peltoniemi et al. 2012). Overall, all optimality analyses have suggested that foliage photosynthetic capacity and nitrogen content should increase with average quantum flux density in the canopy and that such “optimal ” distribution of resources results in a higher carbon gain than a constant photosynthetic capacity for all leaves in the canopy (Fig. 4.6; Niinemets 2012 for a review).
Simulated whole canopy daily integrated photosynthesis (a, b) in dependence on canopy leaf area index (L) for hypothetical canopies with constant foliage biochemical potentials (canopy photosynthesis, A c,con) and in canopies with light-dependent variation in foliage biochemical potentials (A c,var), and (c) relative differences in daily canopy photosynthesis among canopies with constant and variable biochemistry, (A c,var – A c,con)/A c,con, in relation to L. The simulations were conducted for canopies with high initial quantum yield for photosynthetic electron transport for an incident light of 0.248 mol mol−1 (a) and in canopies with a low quantum yield 0.15 mol mol−1. The high quantum yield scenario corresponds to non-photoinhibited leaves with moderately high leaf absorptance of 0.85, while the low quantum yield scenario corresponds to photoinhibited and/or highly reflective leaves. Foliage net assimilation rates were modeled according to Farquhar et al. (1980) photosynthesis model for constant values of leaf temperature of 25 °C and CO2 concentration in chloroplasts (C c) of 280 μmol mol−1 and using the Rubisco kinetic characteristics as in Niinemets and Tenhunen (1997). In the case of the simulation with constant biochemistry, the maximum carboxylase activity of Rubisco was set at a value of 20 μmol m−2 s−1 and the capacity for photosynthetic electron transport was scaled as 2.5V cmax, and non-photorespiratory respiration rate as 0.015V cmax (see Niinemets et al. 1998b). A sine function with a maximum quantum flux density (Q) of 1,400 μmol m−2 s−1 was used to approximate the diurnal variations in above-canopy Q (Q 0). Variation in Q through the canopy was simulated according to a simple Lambert-Beer model assuming that foliage is randomly dispersed (the clumping index Ω = 1.0): Q = Q 0e−kΩL, where k is the extinction coefficient (k = 0.5 in this simulation). In the case of variable biochemistry, V cmax vs. daily integrated Q (Q int) relationships were fitted for canopies with different L by linear regressions such that the ratio of the values of V cmax at the top of the canopy (V cmax,t) and at the bottom (V cmax,b) was 2.6 (moderately high within-canopy variation in foliage biochemical potentials) and the whole-canopy leaf area-weighted average V cmax was 20 μmol m−2 s−1. All other characteristics of Farquhar et al. (1980) photosynthesis model were varied with V cmax as in the simulations with the constant biochemistry
Comparisons of predicted and observed canopy gradients, however, indicate that unconstrained optimality analyses predict too strong gradients in nitrogen content and foliage photosynthetic capacity (Niinemets and Anten 2009 and Chap. 13, Anten 2016 for reviews). Various hypotheses have been put forward to explain the discrepancies from full optimality. First of all, the condition of optimality can differ depending on the time scale and light characteristics, e.g., for diffuse and direct light (Hikosaka 2014). Thus, definition of the pertinent light (diffuse vs. direct, incident vs. absorbed, instantaneous vs. integrated) driving within-canopy acclimation can importantly modify the predicted optimal distribution. It has further been hypothesized that changes in foliage traits from canopy top to bottom are not only driven by light, but also by other co-varying environmental characteristics (Sect. I), in particular, by variations in evaporative demand (e.g., Niinemets and Valladares 2004). Meeting the needs for hydraulic and structural adjustment to ensure water flux to photosynthetically more active leaves and cope with potentially enhanced water availability limitations in the upper canopy can compromise full photosynthetic acclimation to high light (Peltoniemi et al. 2012; Chap. 7, Woodruff et al. 2016).
There are also biophysical limitations on the minimum and maximum thickness of leaves and their N content per unit dry mass, constraining leaf M A and N A values and ultimately leaf photosynthetic capacity in both high and low light (Gutschick and Wiegel 1988; Dewar et al. 2012; Niinemets 2012). Clearly, including constraints on M A and N A has resulted in more realistic predictions of within-canopy gradients in M A, N A and photosynthetic capacity (Gutschick and Wiegel 1988; Dewar et al. 2012) than assuming unconstrained variation in leaf traits (e.g., Farquhar 1989; Sands 1995).
Using either constrained or unconstrained optimization algorithms, it is possible to analyze what is the possible significance of within-canopy variation in foliage traits in canopies of different leaf area index (L) and structure (Fig. 4.6; Anten et al. 1995a; Anten 2005; Chap. 13, Anten 2016). In the case of constrained optimization, the within-canopy gradient in A max A was fixed at a moderately high level of 2.6-fold between canopy top and bottom. In the case of unconstrained optimization, A max A was set directly proportional to Q int. In all simulations, the whole-canopy leaf area-weighted average A max A was a given fixed constant value (A max,c A). Thus, the “unconstrained” optimization used the greatest gradient to yield the given A max,c A value.
Independent of the way of modeling , these analyses suggest that the possible benefits of foliage acclimation to Q int are greater for canopies with stronger light gradients, i.e., in canopies with a larger leaf area index (Fig. 4.6a, b) and in canopies with higher light extinction coefficient (data not shown, see Chap. 9, Hikosaka et al. 2016b for gradients of cumulative L and light). In the case of the constrained optimization , the optimal distribution was expected to increase whole canopy photosynthesis between 1.5 and 21 % compared with all leaves having a constant photosynthetic capacity equal to A max,c A. The effect of considering within-canopy variation in leaf traits increased with increasing L (Fig. 4.6c). Of course, the stronger the within-canopy gradient in photosynthetic characteristics, the greater is the overall whole-canopy photosynthetic benefit. In the case of “unconstrained” optimization of A max A, whole-canopy photosynthetic rate was predicted to be ca. 50 % greater than in the simulation with a constant A max A (data not shown).
The photosynthetic benefit might seem relatively small for open canopies (Fig. 4.6a), especially when the whole-canopy gradient in A max A is moderate as for instance in the Mediterranean evergreen Q. ilex (Fig. 4.2). Nevertheless, even a moderate improvement of long-term carbon gain can importantly benefit the plant in highly stressful environments where the annual carbon gain is significantly reduced due to soil drought. Furthermore, drought stress often leads to photoinhibition, importantly reducing the initial quantum yield s of photosynthetic electron transport and carbon assimilation (Niinemets and Keenan 2014 for a review). The implication of such a reduction in the initial quantum yields is that the light saturation point of photosynthesis is shifted to higher quantum flux densities, and thus higher quantum flux densities appear limiting to photosynthesis. The overall effect in terms of whole-canopy photosynthesis is that the canopy photosynthesis decreases with a reduction of the quantum yield (cf. Fig. 4.6a, b). However, photosynthesis of canopies with low to moderate L, becomes much more sensitive to within-canopy variations in A max A (Fig. 4.6b, c). Thus, within-canopy differences in photosynthetic capacity can importantly benefit photosynthesis in relatively open canopies as well, especially under conditions leading to reduction of quantum yields of photosynthesis such as drought and photoinhibition stresses.
4 IV. Variations in Traits Improving Light Harvesting and Protecting from Excess Light
Apart from the major within-canopy modifications in foliage functional traits that result in alterations in foliage photosynthetic potentials, variations in a number of leaf traits also alter leaf light harvesting efficiency and/or play a role in avoidance of excess light harvesting. Given the interaction of light with other environmental drivers (Sect. I), there are also significant within-canopy gradients in abiotic stress. In particular, leaves exposed to high irradiances can be severely heat- and drought-stressed, especially in conditions of soil drought, while in the lower canopy, the photosynthetic productivity is still most severely limited by light availability. The interactive effects of environmental drivers are further complicated by highly dynamic nature of light in plant canopies. In this section, I analyze variations in structural and chemical traits responsible for alterations in light harvesting and abiotic stress tolerance, and further consider the dynamic responses of leaf traits to rapid changes in light availability.
4.1 A. Structural Traits as Determinants of Light Harvesting and Avoidance
Section III.A indicated that acclimation to low light availability in the bottom of a plant canopy is associated with enhanced investment of nitrogen in chlorophyll and pigment -binding complexes (see Fig. 4.3), and analogous relationships have been observed in a number of species (Niinemets and Anten 2009 for a review). Such enhanced investment of nitrogen in light harvesting within the leaf enhances light harvesting per unit mass, i.e., increases light availability of an average mesophyll cell (e.g., Niinemets 2007). Although researchers seldom think of light harvesting as a mass-based phenomenon, mass basis characterizes the cost of light harvesting in terms of resource investment.
Differently from the mass basis, area-based chlorophyll contents may increase (Fig. 4.3b), be invariable or decrease with increasing Q int (e.g., Hallik et al. 2009a). Nevertheless, due to non-linear dependence of leaf absorptance on leaf chlorophyll content (Eq. 4.3), effects of such changes in area-based chlorophyll content generally result in minor within-canopy modifications in leaf absorptance (e.g., St-Jacques et al. 1991; St-Jacques and Bellefleur 1993; Poorter et al. 1995). Given this, major reductions in M A in woody species in low light constitute an important acclimation response leading to greater light intercepting surface area, and thus, enhanced light interception with given biomass investment in leaves.
Light harvesting efficiency in needle-leaved species can also be enhanced by changes in total to projected leaf area ratio (S T/S P). S T/S P decreases strongly with decreasing Q int in some shade -tolerant conifers such as in Picea and Abies (Niinemets and Kull 1995; Sprugel et al. 1996; Cescatti and Zorer 2003), increasing the light harvesting surface area at the given total surface area in lower light. However, minor modifications or invariable S T/S P have been observed in intolerant conifers from the genus Pinus (Niinemets et al. 2001, 2002a) and in the angiosperm Casuarina with needle-like cladodes (Niinemets et al. 2005b).
Furthermore, at the shoot scale, the degree of foliage spatial aggregation in shoots decreases with decreasing Q int, implying reduction of within-shoot shading (Stenberg 1996, 1998; Smolander and Stenberg 2001; Cescatti and Zorer 2003; Niinemets et al. 2006b). In addition, foliage inclination angle distributions within shoots become more horizontal in the lower canopy in several shade-tolerant conifers, thereby improving interception of light from vertical inclination angles that constitute a more prevalent source of both diffuse and direct radiation in the lower canopy (Stenberg 1996, 1998; Cescatti and Zorer 2003; Niinemets et al. 2006b). On the other hand, greater foliage aggregation and more vertical foliage inclination angles at higher irradiances reduce mean irradiance on leaf surface, and thus reduce the degree of foliage photoinhibition and severity of heat stress (Cescatti and Zorer 2003; Niinemets et al. 2006b). This implies that modifications in needle and shoot structure play a dual role, improving light harvesting in low light and avoiding excess radiation interception at high light.
In broad-leaved species, there are also classic changes in leaf inclination angle distributions analogous to conifers (Fig. 4.7a; for reviews see Niinemets 2010; Chap. 2, Goudriaan 2016). In addition to changes in average leaf inclination angle from vertical to horizontal with decreasing light availability in the canopy, there are also important modifications in the degree of lamina flatness in several broad-leaved species. In particular, leaves tend to be increasingly rolled at the top of plant canopies (Fig. 4.7b). Such increases in the degree of foliage rolling can strongly reduce leaf light interception and also change the share of light interception by leaf lower and upper surface (Fleck et al. 2003). Consideration of both the within-canopy changes in leaf inclination angle and degree of leaf rolling indicates that the overall efficiency of light interception may vary more than two-fold within the canopy of broad-leaved species due to modifications in these structural traits (Fig. 4.7c). Thus, modifications in inclination angles and degree of rolling play a major role in altering the balance between light interception and avoidance. Overall, these case studies suggest that avoidance of excess light interception leads to a more uniform illumination of foliage in the canopy, i.e., greater penetration of light into deeper canopy layers. Simulations studies indicate that more uniform light distribution strongly benefits the whole canopy carbon gain (Ryel et al. 1994; Hikosaka and Hirose 1997; Werner et al. 2001a; Cescatti and Niinemets 2004; Valladares and Niinemets 2007), and thus, “optimization ” of canopy structure constitutes an important means to maximize canopy carbon gain.
Effects of seasonal average daily integrated quantum flux density (Q int) on (a) the absolute lamina inclination angle, i.e., the average angle between the normal to the leaf plane and the vertical direction (|ϕ L|, inset in (a) for the definition), (b) lamina cross-sectional angle (θ, inset in (b) for the definition) and (c) lamina projected to total area ratio in a dominant (filled circles) and a sub-dominant tree (open circles) of the temperate deciduous species Fagus sylvatica. Inset in (a) also demonstrates the definition of inclination angles of petiole (φP) and leaf lamina at leaf fall-line (φF). Data fitting as in Fig. 4.5 (P < 0.001 for all). In (a), the slopes and intercepts of |ϕ L| vs. LnQ int relationships did not differ among the trees according to covariation analyses, and thus, the data for both trees were fitted by a common regression. Modified from Fleck et al. (2003)
4.2 B. Chemical Traits Improving Abiotic Stress Tolerance
Excess light intercepted during midday on clear days can result in severe photooxidative damage of photosynthetic apparatus compromising photosynthetic activity in the morning and evening periods and on overcast days when light intensities are lower. Temporal exceeding of leaf heat stress limits during lighflecks and upon sustained exposure to high radiation loads can further result in heat damage of photosynthetic apparatus. All such adverse effects are expected to be more significant in the upper canopy due to greater radiation loads (Sect. I).
Plants cope with excess energy by increasing the capacity for non-photochemical quenching (non-radiative dissipation of excess light energy), in particular, through xanthophyll cycle . In the xanthophyll cycle, the xanthophyll violaxanthin is converted into xanthophylls antheraxanthin and zeaxanthin under strong light by violaxanthin deepoxidaze enzyme (Demmig-Adams and Adams 1994, 1996b, 2006). This process is activated by acidification of chloroplast lumen when photosynthetic electron transport exceeds the capacity for electron use in dark reactions of photosynthesis , and ultimately, zeaxanthin formation together with acidification result in thylakoid conformational changes that lead to enhanced non-radiative dissipation of excess light (Demmig-Adams and Adams 1994, 1996, b, 2006; Gilmore et al. 1994; Arnoux et al. 2009). The capacity for non-radiative energy dissipation depends on the pool size of xanthophyll cycle carotenoids , violaxanthin, antheraxanthin and zeaxanthin (VAZ) (Demmig-Adams and Adams 1996a; Demmig-Adams et al. 1998; Logan et al. 1996).
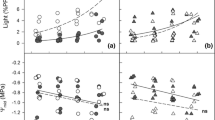
Acclimation to high irradiance typically results in increases in VAZ pool size (Fig. 4.8a, b; Demmig-Adams et al. 1999; Demmig-Adams and Adams 2006). The within-canopy range of variation in leaf area-based VAZ pool size is often three- to four-fold (Fig. 4.8a). Since VAZ content per unit dry mass also increases with increasing light availability (Fig. 4.8b), this increase does reflect greater VAZ content and higher capacity for safe dissipation of excess excitation energy of single mesophyll cells at higher light (e.g., Niinemets et al. 1998a, 2003). However, differently from foliage photosynthetic capacity and nitrogen allocation that are relatively invariable during growing season in temperate trees (Niinemets et al. 2004c; Grassi and Magnani 2005; Grassi et al. 2005), the adjustment in VAZ pool size to changed light conditions is much faster, occurring typically in a few days (Sect. IV.C). The pool sizes of other carotenoids , for example β-carotene and lutein pools, can also change along the light gradients, but the changes are typically only moderate compared with modifications in VAZ. In fact, VAZ to carotenoid ratio also increases with increasing light availability in the canopy (e.g., Niinemets et al. 1998a, 1999a, 2003; Hansen et al. 2002).
Correlations of xanthophyll cycle carotenoids (VAZ) (a, b) and total tocopherol (c, d) contents per unit area (a, c) and dry mass (b, d) with seasonal average integrated incident quantum flux density (Q int) in the canopy of the temperate deciduous tree Populus tremula. VAZ is the sum of contents of violaxanthin , antheraxanthin and zeaxanthin (Data of Niinemets et al. 2003), and total tocopherol content is the sum of contents of α-, β, δ- and γ-tocopherol (Data of García-Plazaola et al. 2004). Data were fitted by non-linear regressions as in Fig. 4.5 (P < 0.001 for all)
Free non-protein-bound, VAZ, in particular zeaxanthin , has also been implicated in direct protection against photooxidative stress (Havaux and Niyogi 1999). In fact, plants have multiple antioxidant systems to cope with oxidative stress , including ascorbate and glutathione in leaf liquid phase and tocopherols in leaf lipid phase (Barclay et al. 1997; Noctor and Foyer 1998; Havaux and Niyogi 1999). In addition to VAZ, the contents of these specific liquid- and lipid-phase antioxidants increase with increasing light in the canopy (Garcia-Plazaola and Becerril 2001; Hansen et al. 2002, 2003; García-Plazaola et al. 2004). However, the within-canopy variation seems to be larger for liphophilic antioxidants than for water-soluble antioxidants (García-Plazaola et al. 2004). For instance, in Populus tremula, total tocopherol content per unit area varied more than three-fold within the canopy (Fig. 4.8c), while total ascorbate and glutathione contents per leaf area varied only ca. 1.5-fold within the canopy (García-Plazaola et al. 2004). In fact, the within-canopy variation of total tocopherol content per unit dry mass was more than two-fold (Fig. 4.8d), while no strong canopy gradient was evident for liquid-soluble antioxidants expressed on a dry mass basis (García-Plazaola et al. 2004).
Some of the lipid-phase antioxidant systems have been implicated in heat stress resistance as well. In particular, zeaxanthin has been demonstrated to play an important role in maintaining membrane integrity in heat-stressed leaves (Havaux et al. 1996; Havaux and Tardy 1997). Furthermore, constitutive isoprene emissions have been demonstrated to improve foliage heat stress resistance in isoprene-emitting species (Sharkey and Singsaas 1995; Singsaas et al. 1997). Improvement of heat resistance by isoprene has been suggested to be due to direct involvement of isoprene in stabilization of membranes at higher temperatures or/and due to antioxidative properties of isoprene that avoids peroxidation of membrane lipids in heat-stressed leaves (Sharkey et al. 2008; Vickers et al. 2009; Possell and Loreto 2013). Although isoprene is emitted constitutively only in a few emitting species (Kesselmeier and Staudt 1999; Fineschi et al. 2013), in the emitting species, there are extensive within-canopy gradients in isoprene emission rate (Harley et al. 1996, 1997; Funk et al. 2006; Niinemets et al. 2010). For example, in deciduous broad-leaved trees, the variation between canopy top and bottom was 27-fold for isoprene emission rate per leaf area (Fig. 4.9a) and 17-fold for isoprene emission rate per leaf dry mass (Fig. 4.9b). Furthermore, the fraction of photosynthetic carbon used for isoprene emission varied 12-fold (Fig. 4.9c), indicating that the plasticity in isoprene emission rate was more than a magnitude larger than the plasticity in net assimilation rate.
Light-dependent variations in isoprene emission rate per unit leaf area (a) and dry mass (b) and the percentage of photosynthetic carbon used for isoprene emission (c) within the canopies of four temperate deciduous species. Data fitting as in Fig. 4.2 (P < 0.001 for all relationships). Measurements of isoprene emission and net assimilation rates were conducted at an ambient CO2 concentration of 380 μmol mol−1, light intensity of 1000 μmol m−2 s−1 and leaf temperature of 25 °C. Modified from Niinemets et al. (2010)
Taken together, the evidence summarized here demonstrates presence of major gradients in photoprotective pigment and antioxidant pools and isoprene emissions in plant canopies. These gradients in protective chemicals likely play key roles in coping with excess irradiance and recurrent heat stress events, whereas the protective capacity is particularly high at the top of plant canopies where the abiotic stress is often the greatest. Presence of such an extensive array of defenses plays a major role in preserving the integrity of foliage photosynthetic capacity through stress periods and allows for rapid onset of photosynthesis when the stress is relieved.
4.3 C. Dynamics in Protective Traits After Rapid Changes in Light Availability
As mentioned in Sect. IV.B, VAZ pool size adjusts to changes in light regime much faster than leaf structure, nitrogen content and allocation and photosynthetic capacity, although the rate of change in photosynthetic traits depends on plant growth form (Sect. III.C). In fact, it seems that the acclimation to potentially damaging high irradiances is governed by adjustments in foliar photosynthetic capacity in long-term, while the safe dissipation of excess light energy is accomplished by changes in xanthophyll cycle pool size during short-term weather fluctuations. However, as discussed in the Sect. III.C, acclimation to altered light conditions not always occurs, and either shading or exposure to excessive light can result in a continuous time-dependent decline in foliage photosynthetic rates and pigment contents, and ultimately leaf abscission.
Provided leaves do acclimate to the modified conditions, the key questions are what is the overall capacity for adjustment of VAZ content and antioxidant pools to changes in light conditions and whether the rate of acclimation varies within the canopy? Field data can to some extent be used to study the speed of xanthophyll cycle acclimation in ecosystems with significant day-to-day variations in quantum flux densities. For instance, in temperate humid forests, clear days are commonly intervened with overcast days such that day-to-day variation in above-canopy irradiance is several-fold (Niinemets et al. 2004c). Averaging quantum flux density over various number of days preceding measurement of physiological and chemical characteristics and using these various estimates of average integrated light can be used to test the strength of correlations of integrated light vs. leaf trait relationships in dependence on the length of light integration period (Ögren and Sjöström 1990; Niinemets et al. 1998a, 1999a; Geron et al. 2000; Werner et al. 2001b).
Using such an approach, it was observed that integrated light for 3 days preceding foliage sampling best explained the within-canopy variation in VAZ pool size in a temperate deciduous tree canopy (Niinemets et al. 1998a, 1999a). This result indicates that VAZ pool size can rapidly adjust to day-to-day variations in light conditions, thereby quickly regulating the capacity for non-photochemical quenching of excess light to match the changed light conditions. However, using illumination with extra light, it was further demonstrated that the degree of acclimation in VAZ pool size varies through the canopy and that there are different response kinetics in leaves developed at different light availabilities in the canopy (Niinemets et al. 2003). In particular, VAZ pool size was less responsive in the lower canopy species Tilia cordata than in the upper canopy species Populus tremula, and the initial increase in VAZ pool size tended to be faster in the upper canopy of Populus tremula (Fig. 4.10a–c). Furthermore, the ratio of VAZ to chlorophyll content was more responsive to extra illumination in the upper canopy leaves in both species (Niinemets et al. 2003; Fig. 4.10d–f), reflecting within-canopy differences in the response of chlorophyll contents to extra illumination (Niinemets et al. 2003, Fig. 4.10g–i). Foliage tocopherol contents responded even stronger to extra illumination than leaf pigments , and the rate of increase of tocopherol content was greater in upper canopy leaves (García-Plazaola et al. 2004). These results together demonstrate that the overall degree of adjustment in pigment pools and foliage antioxidative capacity after light changes can importantly depend on leaf past acclimation status.
Effects of illumination with extra light of ca. 40–50 mol m−2 d−1 upon foliage pigment contents at different positions in the tall tree canopy: time-dependent changes in xanthophyll cycle carotenoid (VAZ) content pear unit area (a–c), ratio of VAZ to chlorophyll (Chl) content (VAZ/Chl, d–f) and chlorophyll content (g–i) for representative control and illuminated leaves of temperate deciduous trees Populus tremula from the upper (a, d, g) and the mid-canopy (b, e, h) and Tilia cordata from the lower canopy (c, f, i). In addition, the inset in (d) demonstrates the variation in VAZ/Chl ratio in relation to cumulative extra irradiance in P. tremula (the control treatment corresponds to leaves without extra light sampled at the same time as the treated leaves). The data were fitted by non-linear (a, h, i) or linear (all others) regressions. The non-significant regression (P > 0.8) in (c) is drawn by a dashed line. In the upper-canopy leaves of P. tremula (height 23 m), the seasonal average natural integrated irradiance (Q int) was 34.4 mol m−2 d−1 for both the control and the treated leaf, and the extra irradiance was 40.8 mol m−2 d−1. In the mid-canopy leaves of P. tremula (height 19 m), Q int was 22.5 mol m−2 d−1 for the control and 18 mol m−2 d−1 for the treated leaf, and the extra irradiance was 50.6 mol m−2 d−1. In the lower canopy leaves of T. cordata (height 17 m), Q int was 6.03 mol m−2 d−1 for the control and 6.10 mol m−2 d−1 for the treated leaf, and the extra irradiance of the illuminated leaf was 48.0 mol m−2 d−1. Data of Niinemets et al. (2003)
Although pigment and antioxidant pool sizes dynamically respond to variations in light input among days, the experiment with extra illumination in the canopies of deciduous trees demonstrated that full acclimation was not reached even after 11 days of exposure to extra light (Fig. 4.10). This delayed response is in agreement with several other experimental studies that have indicated that the response to stepwise increase in light in the field conditions may not even be fully completed in 17 days after start of exposure to enhanced illumination (Logan et al. 1998a, b). This differs from experiments in growth chambers under constant environmental conditions where changes in VAZ and antioxidant pools were completed in 5–7 days after stepwise increases in irradiance (Demmig-Adams et al. 1989; Bilger et al. 1995; Eskling and Åkerlund 1998). This suggests that in natural plant canopies under strongly fluctuating light, temperature and humidity conditions, pigment and antioxidant systems are inherently in non-steady-state conditions. Although pigment pools do rapidly adjust to environmental changes, the period of environmental fluctuations is often shorter than is needed to reach the steady-state pigment and antioxidant pool sizes. Furthermore, the rate of response to altered conditions significantly varies through the canopy, being likely an important factor determining leaf abiotic stress resistance in the canopy.
5 V. Photosynthetic Acclimation in Relation to Species Shade Tolerance
The term “economics spectrum” characterizes the covariation of foliage traits associated with superior performance in low resource environments such as structurally more robust foliage, and traits that improve fitness in high resource environments such as enhanced photosynthetic capacity (e.g., Wright et al. 2004, 2005). However, shading is associated both with reduced foliage robustness and reduced photosynthetic capacity, especially within single species, but also for several plant functional type s (Lusk et al. 2008; Hallik et al. 2009b; Niinemets and Anten 2009). Thus, compared with other stresses, shading constitutes an outlying low resource environment. Here I analyzed within-canopy patterns in key foliage functional traits from the perspective of the leaf economics spectrum with main focus on Northern hemisphere temperate species.
5.1 A. Evidence from the Case Studies
Section III demonstrated that there are important differences in the within-canopy variations in different leaf traits among plant functional type s and that these differences are associated with differences in leaf turnover. Apart from differences among plant functional types, plant stands are often composed of species with different ecological potentials. Characteristically, species in the lower canopy layers and in dense late-successional communities have greater shade tolerance than species in the upper canopy positions and in more open early-successional communities (Valladares and Niinemets 2008). Differences in community position among shade-tolerant and intolerant species are also associated with differences in leaf trait vs. Q int relationships. In broad-leaved deciduous trees, M A often responds less plastically to Q int in more shade-tolerant than in less tolerant species (Fig. 4.11, Kull and Niinemets 1998; Niinemets et al. 1998b; Valladares and Niinemets 2008). However, such a pattern is not always observed (e.g., very intolerant Populus tremula in Fig. 4.11a vs. very tolerant Fagus sylvatica in Fig. 4.11e). Although shade-intolerant species can maintain leaves at low light availabilities when grown in monocultures (Nygren and Kellomäki 1983; Niinemets et al. 2004a), the situation is different in multispecies canopies where shade-tolerant species gradually grow into the upper canopy, and the foliage of intolerant species competes for light availability with foliage of tolerant species in the lower and mid-canopy. Thus, the apparent low plasticity in P. tremula in Fig. 4.11a might reflect the general circumstance that in multispecies canopies intermixed with shade-tolerant species, the intolerant species may not simply be able to maintain leaves below a certain Q int value (ca. 7–8 mol m−2 d−1 for P. tremula in Fig. 4.11a).
Comparison of light-dependent variations in leaf dry mass per unit area within the canopies of five temperate deciduous woody species of contrasting shade tolerance . The insets demonstrate variations in leaf photosynthetic capacity with Q int. Data fitting as in Fig. 4.5 (all relationships are significant at P < 0.001). Species are ranked according to shade tolerance score (SC, Niinemets and Valladares 2006) from the least tolerant to the most tolerant as: Populus tremula (SC = 2.3), Fraxinus excelsior (2.9), Corylus avellana (3.5), Tilia cordata (4.2) and Fagus sylvatica (4.6). Shade tolerance score is a relative measure varying from 1 (least tolerant) to 5 (most tolerant) that characterizes species capacity to endure shade relative to other species (Niinemets and Valladares 2006 for a detailed definition; Valladares and Niinemets 2008. Data for Fagus sylvatica are from Niinemets (1995) and for the four other species from Niinemets et al. (1998b)
As in broad-leaved deciduous woody species, within-canopy plasticity in foliage photosynthetic potentials is mainly driven by changes in M A that result in stacking of rate-limiting photosynthetic proteins per unit leaf area (Sect. III.C), lower within-canopy plasticity in M A is also associated with lower photosynthetic plasticity in less shade -tolerant species (Kull and Niinemets 1998; Niinemets et al. 1998b). It has further been demonstrated that the sensitivity to photoinhibition is greater in more shade-tolerant species (Lovelock et al. 1994; Chazdon et al. 1996; Naidu and DeLucia 1997) that also generally possess lower photosynthetic capacities (Bazzaz 1979; Lovelock et al. 1994).
On the other hand, shade -tolerant temperate species can support foliage at lower Q int than intolerant species and have both smaller minimum and maximum M A values (Fig. 4.11). Thus, despite lower photosynthetic plasticity, shade-tolerant species can form a greater leaf area with given foliage biomass in leaves, improving light interception of the canopy. Such a greater light interception capacity not only improves the whole canopy carbon gain, but the shading by more extensive canopy itself can serve as an important competitive attribute constraining the survival of seedlings and saplings of less shade-tolerant competitors and ultimately leading to dominance of shade-tolerant late-successional plants in the canopy (Küppers 1985; Schieving and Poorter 1999; Anten 2002).
Much less data are available for within-canopy gradients in tropical and southern hemisphere temperate evergreen species. In three tropical species, within-canopy plasticity in M A, N A and A max A was greater in shade -intolerant evergreen species Ficus insipida than in more tolerant Luehea seemannii, whereas the highest plasticity was observed in the shade-intolerant drought-deciduous species Castilla elastica (Posada et al. 2009). Among the tropical Piper species of varying shade tolerance , the within-canopy plasticity in M A, N A and A max A of shade tolerant P. aequale and P. lapathifolium was less than in moderately tolerant P. hispidum and intolerant P. auritum and P. umbellatum (Chazdon and Field 1987). Among southern hemisphere temperate evergreen Nothofagus species, more shade tolerant Nothofagus solandri var. cliffortoides had a greater within-canopy plasticity in M A than less tolerant N. fusca, but the plasticity in N A did not differ among species (Niinemets et al. 2004b). In another study in temperate southern hemisphere evergreens , more tolerant Nothofagus solandri var. cliffortoides had a greater plasticity in M A than very intolerant species Kunzea ericoides (White and Scott 2006). However, in this study, other moderately shade-tolerant species had a similar within-canopy plasticity in M A as the very intolerant K. ericoides (White and Scott 2006). Clearly more comparative studies are needed to gain conclusive insight into the controls of foliage plasticity by shade tolerance in tropics and in southern hemisphere temperate ecosystems.
5.2 B. Generalizing the Patterns
The conclusions drawn from the case studies in temperate deciduous broad-leaved species seem to be valid more widely. Broad-scale analyses of structural, chemical and physiological variation in high-light-developed leaves across Northern hemisphere temperate woody flora indicate that M A decreases with increasing species shade -tolerance also in broad-leaved and needle-leaved evergreen species (Fig. 4.12a). Thus, formation of an extensive leaf area is also the key competitive strategy in shade-tolerant evergreen species (Niinemets 2010; Warren et al. 2012). However, in temperate evergreens , greater canopy leaf area in more tolerant species is also importantly driven by enhanced leaf longevity (Hallik et al. 2009b; Niinemets 2010).
Correlations of leaf dry mass per unit area (a), nitrogen content per unit area (b) and light-saturated net assimilation rate per unit area (photosynthetic capacity, (c) with species shade tolerance score in Northern hemisphere broad-leaved deciduous (BD), broad-leaved evergreen (BE) and needle-leaved evergreen (NE) trees and shrubs (n = 341, modified from Hallik et al. 2009b). Species shade tolerance score defined as in Fig. 4.11. Data were fitted by standardized major axis regressions using SMATR 2.0 (Warton et al. 2006) and non-significant regressions (P > 0.05) are shown by dashed lines
Differently from M A, nitrogen content per unit area (Fig. 4.12b) and photosynthetic capacity per unit area (Fig. 4.12c) were not correlated with species shade tolerance in temperate evergreens . However, the patterns developed for leaves exposed to high light do not necessarily provide insight into variations in within-canopy plasticity in foliage structure and photosynthetic potentials. Given the higher leaf longevity in shade-tolerant species and reduction in leaf-level plasticity with leaf age (Fig. 4.4), canopy-level photosynthetic plasticity across leaves of different age might be lower in more shade-tolerant species.
Differently from Northern hemisphere temperate species, M A of tropical evergreens and southern hemisphere temperate evergreens is typically higher in more shade tolerant species than in less tolerant species (Chazdon 1992; Kitajima 1994; Lusk 2004; Lusk et al. 2008; Houter and Pons 2012). Thus, higher biomass investment is needed for a given plastic change in M A in more shade tolerant species, implying that the high initial M A may limit both structural and photosynthetic plasticity in shade tolerators. Further studies are needed to generalize within-canopy plastic changes in evergreens of different shade tolerance in both temperate and tropical ecosystems.
6 VI. Conclusions
This review highlights major within-canopy modifications in foliage photosynthetic capacity that improve the whole canopy carbon gain compared with a hypothetical canopy with constant photosynthetic capacity for all leaves in the canopy. While scaling of photosynthetic capacity with long-term average integrated quantum flux density is a ubiquitous response in plants, there are important plant functional type differences in the scaling of key foliage functional traits with light availability. The rate of foliage turnover increases in the sequence evergreens < deciduous woody species with deterministic growth < deciduous woody species with indeterminate growth < herbaceous species. The evidence summarized here indicates that plant functional type differences in canopy growth phenology and differences in leaf turnover importantly alter the significance of various leaf traits in determining the within-canopy acclimation of foliage photosynthetic capacity.
This chapter also emphasizes that environmental gradients are typically complex in plant canopies. Leaves at the top of plant canopies often can suffer from more sever water, photoinhibition and oxidative stress es that can constrain photosynthetic acclimation to canopy light regime. Furthermore, coping with such interacting stresses typically leads to structural adaptations reducing excess light interception in the upper canopy, and chemical modifications improving the stress resistance . In fact, within-canopy variations in protective traits can be much larger than in photosynthetic characteristics, and there are further significant within-canopy variations in the degree and rate of adjustment of the pools of protective chemicals to dynamically changing light conditions.
There is still a limited understanding of species differences in foliage plasticity to within-canopy environmental gradients, but the evidence summarized suggests that shade tolerance is an important driver of species plasticity. Shade -tolerant species growing in understory and in late-successional communities characteristically form a greater foliage area and have superior light harvesting capacity, but their photosynthetic capacity and plasticity seem to be lower than in less tolerant species growing in more open communities and in early-successional habitats where rapid carbon gain capacity is the primary attribute of competition . Further studies are needed to gain insight into the generality of suggested variation patterns of foliage plasticity with species ecological potentials. Understanding such plastic variations is not only fundamentally important, but would allow construction of more realistic carbon gain models capable of simulating ecosystem carbon gain through ecosystem development.
Abbreviations
- A :
-
Net assimilation rate
- A c,con :
-
Canopy photosynthesis for constant leaf biochemical potentials
- A c,var :
-
Canopy photosynthesis for variable leaf biochemical potentials
- A max :
-
Photosynthetic capacity
- A max A :
-
Photosynthetic capacity per unit area
- A max M :
-
Photosynthetic capacity per unit dry mass
- B C :
-
Chlorophyll binding (amount of chlorophyll per unit nitrogen invested in light harvesting Eq. 4.4)
- Chl:
-
Chlorophyll
- C a :
-
Ambient CO2 concentration
- C c :
-
Chloroplastic CO2 concentration
- C i :
-
CO2 concentration in the intercellular air space
- E N :
-
Photosynthetic nitrogen use efficiency
- F B :
-
Fraction of leaf nitrogen in rate-limiting proteins of photosynthetic electron transport
- F L :
-
Fraction of leaf nitrogen in light harvesting
- F R :
-
Fraction of leaf nitrogen in Rubisco
- g m :
-
Mesophyll diffusion conductance
- g s :
-
Stomatal conductance
- J max :
-
Capacity for photosynthetic electron transport
- J max A :
-
Capacity for photosynthetic electron transport per unit area
- J mc :
-
Capacity for photosynthetic electron transport per unit cytochrome f
- k :
-
Light extinction coefficient
- L :
-
Leaf area index
- LHC II:
-
Light harvesting complex II
- M A :
-
Leaf dry mass per unit area
- N A :
-
Nitrogen content per unit area
- N M :
-
Leaf nitrogen content per unit dry mass
- PS I:
-
Photosystem I
- PS II:
-
Photosystem I I
- Q :
-
Photosynthetic quantum flux density
- Q 0 :
-
Above-canopy Q
- Q int :
-
Incident integrated Q during leaf growth and development
- R c :
-
Light-dependent change of a given trait (Eq. 4.5)
- SC:
-
Shade tolerance score
- S T/S P :
-
Total to projected leaf area ratio
- VAZ:
-
Xanthophyll cycle carotenoids (violaxanthin antheraxanthin and zeaxanthin )
- V cmax :
-
Maximum carboxylase activity of Rubisco
- V cmax,b :
-
V cmax at the bottom of the canopy
- V cmax,t :
-
V cmax at the top of the canopy
- V cmax A :
-
Maximum carboxylase activity of Rubisco per unit area
- V cr :
-
Specific activity of Rubisco (maximum rate of ribulose-1,5-bisphosphate carboxylation per unit Rubisco protein)
- V i :
-
Trait value at a seasonal average quantum flux density of i (Q int,i)
- α:
-
Initial quantum yield for photosynthetic electron transport
- αp,a :
-
Initial quantum yield of photosynthesis for an absorbed light
- αp,i :
-
Initial quantum yield of photosynthesis for an incident light
- θ:
-
Lamina cross-sectional angle
- ξ :
-
Leaf absorptance
- χA :
-
Chlorophyll content per unit area
- χM :
-
Chlorophyll content per unit dry mass
- φF :
-
Leaf lamina inclination angle at leaf fall-line
- |ϕ L|:
-
Absolute lamina inclination angle (average angle between the normal to the leaf plane and the vertical direction)
- φP :
-
Petiole inclination angle
- Ω:
-
Leaf clumping index
References
Aasamaa K, Sõber A, Hartung W, Niinemets Ü (2004) Drought acclimation of two deciduous tree species of different layers in a temperate forest canopy. Trees 18:93–101
Ackerly DD, Bazzaz FA (1995) Leaf dynamics, self-shading and carbon gain in seedlings of a tropical pioneer tree. Oecologia 101:289–298
Anderson MC (1964) Studies of the woodland light climate. I. The photographic computation of light conditions. J Ecol 52:27–41
Anten NPR (1997) Modelling canopy photosynthesis using parameters determined from simple non-destructive measurements. Ecol Res 12:77–88
Anten NPR (2002) Evolutionarily stable leaf area production in plant populations. J Theor Biol 217:15–32
Anten NPR (2005) Optimal photosynthetic characteristics of individual plants in vegetation stands and implications for species coexistence. Ann Bot 95:495–506
Anten NPR (2016) Optimization and game theory in canopy models. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy Photosynthesis: From Basics to Applications. Springer, Berlin, pp 355–377
Anten NPR, Schieving F, Medina E, Werger MJA, Schuffelen P (1995a) Optimal leaf area indices in C3 and C4 mono- and dicotyledonous species at low and high nitrogen availability. Physiol Plant 95:541–550
Anten NPR, Schieving F, Werger MJA (1995b) Patterns of light and nitrogen distribution in relation to whole canopy carbon gain in C3 and C4 mono- and dicotyledonous species. Oecologia 101:504–513
Anten NPR, Hernandez R, Medina EM (1996) The photosynthetic capacity and leaf nitrogen concentration as related to light regime in shade leaves of a montane tropical forest tree, Tetrorchidium rubrivenium. Funct Ecol 10:491–500
Anten NPR, Miyazawa K, Hikosaka K, Nagashima H, Hirose T (1998) Leaf nitrogen distribution in relation to leaf age and photon flux density in dominant and subordinate plants in dense stands of a dicotyledonous herb. Oecologia 113:314–324
Aranda I, Pardo F, Gil L, Pardos JA (2004) Anatomical basis of the change in leaf mass per area and nitrogen investment with relative irradiance within the canopy of eight temperate tree species. Acta Oecol 25:187–195
Arnoux P, Morosinotto T, Saga G, Bassi R, Pignola D (2009) A structural basis for the pH-dependent xanthophyll cycle in Arabidopsis thaliana. Plant Cell 21:2036–2044
Aussenac G (1973) Effets de conditions microclimatiques différentes sur la morphologie et la structure anatomique des aiguilles de quelques résineux. (Effect of some different microclimatic conditions on morphology and anatomic structure of needles). Ann Sci For 30:375–392
Barclay LRC, Antunes F, Egawa Y, McAllister KL, Mukai K, Nishi T, Vinqvist MR (1997) The efficiency of antioxidants delivered by liposomal transfer. Biochimica et Biophysica Acta - Biomembranes 1328:1–12
Bassi R, Caffarri S (2000) Lhc proteins and the regulation of photosynthetic light harvesting function by xanthophylls. Photosynth Res 64:243–256
Bazzaz FA (1979) The physiological ecology of plant succession. Annu Rev Ecol Syst 10:351–371
Bilger W, Fisahn J, Brummet W, Kossmann J, Willmitzer L (1995) Violaxanthin cycle pigment contents in potato and tobacco plants with genetically reduced photosynthetic capacity. Plant Physiol 108:1479–1486
Bond BJ, Farnsworth BT, Coulombe RA, Winner WE (1999) Foliage physiology and biochemistry in response to light gradients in conifers with varying shade tolerance. Oecologia 120:183–192
Boonman A, Anten NPR, Dueck TA, Jordi WJRM, van der Werf A, Voesenek LACJ, Pons TL (2006) Functional significance of shade-induced leaf senescence in dense canopies: an experimental test using transgenic tobacco. Am Nat 168:597–607
Brodribb TJ, Holbrook NM, Zwieniecki MA, Palma B (2005) Leaf hydraulic capacity in ferns, conifers and angiosperms: impacts on photosynthetic maxima. New Phytol 165:839–846
Brooks JR, Hinckley TM, Sprugel DG (1994) Acclimation responses of mature Abies amabilis sun foliage to shading. Oecologia 100:316–324
Brooks JR, Sprugel DG, Hinckley TM (1996) The effects of light acclimation during and after foliage expansion on photosynthesis of Abies amabilis foliage within the canopy. Oecologia 107:21–32
Buckley TN, Farquhar GD, Miller JM (2002) The mathematics of linked optimisation for water and nitrogen use in a canopy. Silva Fenn 36:639–669
Burkey KO, Wells R (1991) Response of soybean photosynthesis and chloroplast membrane function to canopy development and mutual shading. Plant Physiol 97:245–252
Cano FJ, Sánchez-Gómez D, Rodríguez-Calcerrada J, Warren CR, Gil L, Aranda I (2013) Effects of drought on mesophyll conductance and photosynthetic limitations at different tree canopy layers. Plant Cell Environ 36:1961–1980
Cartechini A, Palliotti A (1995) Effect of shading on vine morphology and productivity and leaf gas exchange characteristics in grapevines in the field. Am J Enol Vitic 46:227–234
Cescatti A, Niinemets Ü (2004) Sunlight capture. Leaf to landscape. In: Smith WK (ed) Photosynthetic Adaptation. Chloroplast to Landscape. Springer, Berlin, pp 42–85
Cescatti A, Zorer R (2003) Structural acclimation and radiation regime of silver fir (Abies alba Mill.) shoots along a light gradient. Plant Cell Environ 26:429–442
Chazdon RL (1992) Photosynthetic plasticity of two rain forest shrubs across natural gap transects. Oecologia 92:586–595
Chazdon RL, Field CB (1987) Determinants of photosynthetic capacity in six rainforest Piper species. Oecologia 73:222–230
Chazdon RL, Pearcy RW, Lee DW, Fetcher N (1996) Photosynthetic responses of tropical forest plants to contrasting light environments. In: Mulkey SR (ed) Tropical Forest Plant Ecophysiology. Chapman & Hall, New York, pp 5–55
Chiariello N (1984) Leaf energy balance in the wet lowland tropics. In: Medina E (ed) Physiological Ecology of Plants of the Wet Tropics Proceedings of an International Symposium Held in Oxatepec and Los Tuxtlas, Mexico, June 29 to July 6, 1983. Dr. W. Junk Publishers, The Hague, pp 85–98
Davi H, Barbaroux C, Dufrêne E, François C, Montpied P, Bréda N, Badeck F (2008) Modelling leaf mass per area in forest canopy as affected by prevailing radiation conditions. Ecol Model 211:339–349
Demmig-Adams B, Adams WW III (1994) Light stress and photoprotection related to the xanthophyll cycle. In: Foyer CH, Mullineaux PM (eds) Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants. CRC Press, Boca Raton, pp 105–126
Demmig-Adams B, Adams WW (1996a) Chlorophyll and carotenoid composition in leaves of Euonymus kiautschovicus acclimated to different degrees of light stress in the field. Aust J Plant Physiol 23:649–659
Demmig-Adams B, Adams WW III (1996b) Xanthophyll cycle and light stress in nature: uniform response to excess direct sunlight among higher plant species. Planta 198:460–470
Demmig-Adams B, Adams WW III (2006) Photoprotection in an ecological context: the remarkable complexity of thermal energy dissipation: Tansley review. New Phytol 172:11–21
Demmig-Adams B, Winter K, Winkelmann E, Krüger A, Czygan FC (1989) Photosynthetic characteristics and the ratios of chlorophyll, ß-carotene, and the components of the xanthophyll cycle upon a sudden increase in growth light regime in several plant species. Bot Acta 102:319–325
Demmig-Adams B, Moeller DL, Logan BA, Adams WW III (1998) Positive correlation between levels of retained zeaxanthin + antheraxanthin and degree of photoinhibition in shade leaves of Schefflera arboricola (Hayata) Merrill. Planta 205:367–374
Demmig-Adams B, Adams WW, Ebbert V, Logan BA (1999) Ecophysiology of the xanthophyll cycle. In: Photochemistry of Carotenoids. Kluwer Academic Publishers, Dordrecht, pp 245–269
Dewar RC, Tarvainen L, Parker K, Wallin G, McMurtrie RE (2012) Why does leaf nitrogen decline within tree canopies less rapidly than light? An explanation from optimization subject to a lower bound on leaf mass per area. Tree Physiol 32:520–534
Disney M (2016) Remote sensing of vegetation: potentials, limitations, developments and applications. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy Photosynthesis: From Basics to Applications. Springer, Berlin, pp 289–331
Ducrey M (1981) Étude bioclimatique d’une futaie feuillue (Fagus silvatica L. et Quercus sessiliflora Salisb.) de l’Est de la France. III. Potentialités photosynthétiques des feuilles à différentes hauteurs dans le peuplement. (Bioclimatological studies in a broad leaf high stand (Fagus silvatica L. and Quercus sessiliflora Salisb.). III. Photosynthetic rates of leaves from various levels in the stand). Ann Sci For 38:71–86
During H (1999) High light and water stress in grapevines: photoinhibition and photoprotection. In: Proceedings of the First ISHS Workshop on Water Relations of Grapevines. International Society of Horticultural Science, Louvain, pp 45–54
Eckardt F, Heim G, Methy M, Sauvezon R (1975) Interception de l’énergie rayonnante échanges gazeux et croissance dans une forèt méditerranéenne à feuillage persistant (Quercetum ilicis). Photosynthetica 9:145–156
Ehleringer J, Björkman O (1977) Quantum yields for CO2 uptake in C3 and C4 plants. Dependence on temperature, CO2 and O2 concentration. Plant Physiol 59:86–90
Ehleringer JR, Björkman O (1978) Pubescence and leaf spectral characteristics in a desert shrub, Encelia farinosa. Oecologia 36:151–162
Ellsworth DS, Reich PB (1993) Canopy structure and vertical patterns of photosynthesis and related leaf traits in a deciduous forest. Oecologia 96:169–178
Escudero A, Mediavilla S (2003) Decline in photosynthetic nitrogen use efficiency with leaf age and nitrogen resorption as determinants of leaf life span. J Ecol 91:880–889
Eskling M, Åkerlund H-E (1998) Changes in the quantities of violaxanthin de-epoxidase, xanthophylls and ascorbate in spinach upon shift from low to high light. Photosynth Res 57:41–50
Evans JR (1993a) Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. II. Stability through time and comparison with a theoretical optimum. Aust J Plant Physiol 20:69–82
Evans JR (1993b) Photosynthetic acclimation and nitrogen partitioning within a lucerne canopy. I. Canopy characteristics. Aust J Plant Physiol 20:55–67
Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24:755–767
Ewers FW (1982) Secondary growth in needle leaves of Pinus longaeva (bristlecone pine) and other conifers: quantitative data. Am J Bot 69:1552–1559
Ewers FW, Schmid R (1981) Longevity of needle fascicles of Pinus longaeva (bristlecone pine) and other North American pines. Oecologia 51:107–115
Ewers BE, Oren R, Kim H-S, Bohrer G, Lai C-T (2007) Effects of hydraulic architecture and spatial variation in light on mean stomatal conductance of tree branches and crowns. Plant Cell Environ 30:483–496
Farquhar GD (1989) Models of integrated photosynthesis of cells and leaves. Philos Trans R Soc Lond Ser B-Biol Sci 323:357–367
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Farquhar GD, Buckley TN, Miller JM (2002) Optimal stomatal control in relation to leaf area and nitrogen content. Silva Fenn 36:625–637
Field C (1983) Allocating leaf nitrogen for the maximization of carbon gain: leaf age as a control on the allocation program. Oecologia 56:341–347
Fineschi S, Loreto F, Staudt M, Peñuelas J (2013) Diversification of volatile isoprenoid emissions from trees: evolutionary and ecological perspectives In: Niinemets Ü and Monson RK (eds) Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Springer, Berlin, pp 1–20
Fleck S, Niinemets Ü, Cescatti A, Tenhunen JD (2003) Three-dimensional lamina architecture alters light harvesting efficiency in Fagus: a leaf-scale analysis. Tree Physiol 23:577–589
Flexas J, Barbour MM, Brendel O, Cabrera HM, Carriquí M, Diaz-Espejo A, Douthe C, …, Warren CR (2012) Mesophyll diffusion conductance to CO2: an unappreciated central player in photosynthesis. Plant Sci 193–194:70–84
Franklin O, Ågren GI (2002) Leaf senescence and resorption as mechanisms of maximizing photosynthetic production during canopy development at N limitation. Funct Ecol 16:727–733
Funk JL, Giardina CP, Knohl A, Lerdau MT (2006) Influence of nutrient availability, stand age, and canopy structure on isoprene flux in a Eucalyptus saligna experimental forest. J Geophys Res Biogeosci 111: G02012
Garcia-Plazaola JI, Becerril JM (2001) Seasonal changes in photosynthetic pigments and antioxidants in beech (Fagus sylvatica) in a Mediterranean climate: implications for tree decline diagnosis. Aust J Plant Physiol 28:225–232
García-Plazaola JI, Becerril JM, Hernández A, Niinemets Ü, Kollist H (2004) Acclimation of antioxidant pools to the light environment in a natural forest canopy. New Phytol 163:87–97
Geron C, Guenther A, Sharkey T, Arnts RR (2000) Temporal variability in basal isoprene emission factor. Tree Physiol 20:799–805
Gilmore AM, Mohanty N, Yamamoto HY (1994) Epoxidation of zeaxanthin and antheraxanthin reverses non-photochemical quenching of photosystem II chlorophyll a fluorescence in the presence of trans-thylakoid deltapH. FEBS Lett 350:271–274
Gilmore DW, Seymour RS, Halteman WA, Greenwood MS (1995) Canopy dynamics and the morphological development of Abies balsamea: effects of foliage age on specific leaf area and secondary vascular development. Tree Physiol 15:47–55
Goudriaan J (2016) Light distribution. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy Photosynthesis: From Basics to Applications. Springer, Berlin, pp 3–22
Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28:834–849
Grassi G, Vicinelli E, Ponti F, Cantoni L, Magnani F (2005) Seasonal and interannual variability of photosynthetic capacity in relation to leaf nitrogen in a deciduous forest plantation in northern Italy. Tree Physiol 25:349–360
Gratani L (1993) Response to microclimate of morphological leaf attributes, photosynthetic and water relations of evergreen sclerophyllous shrub species. Photosynthetica 29:573–582
Gratani L (1997) Canopy structure, vertical radiation profile and photosynthetic function in a Quercus ilex evergreen forest. Photosynthetica 33:139–149
Gratani L, Fiorentino E (1986) Relationship between chlorophyll content and leaf biomass for Quercus ilex in a Mediterranean maquis stand. Photosynthetica 20:267–273
Gratani L, Fiorentino E, Kubová A, Marzi P (1989) Effect of microclimate on ecophysiological features of some sclerophyllous species. Photosynthetica 23:230–233
Gratani L, Marzi P, Crescente MF (1992) Morphological adaptions of Quercus ilex leaves in the Castelporziano forest. Vegetatio 100:155–161
Gutschick VP, Wiegel FW (1988) Optimizing the canopy photosynthetic rate by patterns of investment in specific leaf mass. Am Nat 132:67–86
Hallik L, Kull O, Niinemets Ü, Aan A (2009a) Contrasting correlation networks between leaf structure, nitrogen and chlorophyll in herbaceous and woody canopies. Basic Appl Ecol 10:309–318
Hallik L, Niinemets Ü, Wright IJ (2009b) Are species shade and drought tolerance reflected in leaf-level structural and functional differentiation in Northern Hemisphere temperate woody flora? New Phytol 184:257–274
Han Q, Kawasaki T, Katahata S, Mukai Y, Chiba Y (2003) Horizontal and vertical variations in photosynthetic capacity in a Pinus densiflora crown in relation to leaf nitrogen allocation and acclimation to irradiance. Tree Physiol 23:851–857
Han Q, Kawasaki T, Nakano T, Chiba Y (2004) Spatial and seasonal variability of temperature responses of biochemical photosynthesis parameters and leaf nitrogen content within a Pinus densiflora crown. Tree Physiol 25:737–744
Han Q, Araki M, Chiba Y (2006) Acclimation to irradiance of leaf photosynthesis and associated nitrogen reallocation in photosynthetic apparatus in the year following thinning of a young stand of Chamaecyparis obtusa. Photosynthetica 44:523–529
Hansen U, Fiedler B, Rank B (2002) Variation of pigment composition and antioxidative systems along the canopy light gradient in a mixed beech/oak forest: a comparative study on deciduous tree species differing in shade tolerance. Trees 16:354–364
Hansen U, Schneiderheinze J, Stadelmann S, Rank B (2003) The α-tocopherol content of leaves of pedunculate oak (Quercus robur L.) – variation over the growing season and along the vertical light gradient in the canopy. J Plant Physiol 160:91–96
Harley P, Guenther A, Zimmerman P (1996) Effects of light, temperature and canopy position on net photosynthesis and isoprene emission from sweetgum (Liquidambar styraciflua) leaves. Tree Physiol 16:25–32
Harley P, Guenther A, Zimmerman P (1997) Environmental controls over isoprene emission in deciduous oak canopies. Tree Physiol 17:705–714
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci U S A 96:8762–8767
Havaux M, Tardy F (1997) Photoacoustically monitored thermal energy dissipation and xanthophyll cycle carotenoids in higher plant leaves. J Photochem Photobiol B: Biol 40:68–75
Havaux M, Tardy F, Ravenel J, Chanu D, Parot P (1996) Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: influence of the xanthophyll content. Plant Cell Environ 19:1359–1368
Hikosaka K (1996) Effects of leaf age, nitrogen nutrition and photon flux density on the organization of the photosynthetic apparatus in leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Planta 198:144–150
Hikosaka K (2014) Optimal nitrogen distribution within a leaf canopy under direct and diffuse light. Plant Cell Environ 37:2077–2085
Hikosaka K, Hirose T (1997) Leaf angle as a strategy for light competition: optimal and evolutionary stable light extinction coefficient within a leaf canopy. Écoscience 4:501–507
Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18:605–618
Hikosaka K, Terashima I (1996) Nitrogen partitioning among photosynthetic components and its consequence in sun and shade plants. Funct Ecol 10:335–343
Hikosaka K, Terashima I, Katoh S (1994) Effects of leaf age, nitrogen nutrition and photon flux density on the distribution of nitrogen among leaves of a vine (Ipomoea tricolor Cav.) grown horizontally to avoid mutual shading of leaves. Oecologia 97:451–457
Hikosaka K, Hanba YT, Hirose T, Terashima I (1998) Photosynthetic nitrogen-use efficiency in leaves of woody and herbaceous species. Funct Ecol 12:896–905
Hikosaka K, Noguchi K, Terashima I (2016a) Modeling leaf gas exchange. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy Photosynthesis: From Basics to Applications. Springer, Berlin, pp 61–100
Hikosaka K, Kumagai T, Ito A (2016b) Modeling canopy photosynthesis. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy Photosynthesis: From Basics to Applications. Springer, Berlin, pp 239–268
Hirose T, Bazzaz FA (1998) Trade-off between light- and nitrogen-use efficiency in canopy photosynthesis. Ann Bot 82:195–202
Hirose T, Werger MJA (1987a) Nitrogen use efficiency in istantaneous and daily photosynthesis of leaves in the canopy of a Solidago altissima stand. Physiol Plant 70:215–222
Hirose T, Werger MJA (1987b) Maximizing daily canopy photosynthesis with respect to the leaf nitrogen allocation pattern in the canopy. Oecologia 72:520–526
Hirose T, Werger MJA (1994) Photosynthetic capacity and nitrogen partitioning among species in the canopy of a herbaceous plant community. Oecologia 100:203–212
Hirose T, Werger MJA (1995) Canopy structure and photon flux partitioning among species in a herbaceous plant community. Ecology 76:466–474
Hirose T, Werger MJA, Pons TL, van Rheenen JWA (1988) Canopy structure and leaf nitrogen distribution in a stand of Lysimachia vulgaris L. as influenced by stand density. Oecologia 77:145–150
Hirose T, Werger MJA, van Rheenen JWA (1989) Canopy development and leaf nitrogen distribution in a stand of Carex acutiformis. Ecology 70:1610–1618
Houter NC, Pons TL (2012) Ontogenetic changes in leaf traits of tropical rainforest trees differing in juvenile light requirement. Oecologia 169:33–45
Hubbard RM, Bond BJ, Senock RS, Ryan MG (2002) Effects of branch height on leaf gas exchange, branch hydraulic conductance and branch sap flux in open-grown ponderosa pine. Tree Physiol 22:575–581
Iio A, Fukasawa H, Nose Y, Kato S, Kakubari Y (2005) Vertical, horizontal and azimuthal variations in leaf photosynthetic characteristics within a Fagus crenata crown in relation to light acclimation. Tree Physiol 25:525–536
Ishida A, Toma T, Marjenah (1999a) Leaf gas exchange and chlorophyll fluorescence in relation to leaf angle, azimuth, and canopy position in the tropical pioneer tree, Macaranga conifera. Tree Physiol 19:117–124
Ishida A, Uemura A, Koike N, Matsumoto Y, Hoe AL (1999b) Interactive effects of leaf age and self-shading on leaf structure, photosynthetic capacity and chlorophyll fluorescence in the rain forest tree, Dryobalanops aromatica. Tree Physiol 19:741–747
Ishii H, Ooishi M, Maruyama Y, Koike T (2003) Acclimation of shoot and needle morphology and photosynthesis of two Picea species to differences in soil nutrient availability. Tree Physiol 23:453–461
Jackowski G, Kacprzak K, Jansson S (2001) Identification of Lhcb1/Lhcb2/Lhcb3 heterotrimers of the main light-harvesting chlorophyll a/b-protein complex of photosystem II (LHC II). Biochim Biophys Acta 1504:340–345
James SA, Bell DT (2000) Leaf orientation, light interception and stomatal conductance of Eucalyptus globulus ssp. globulus leaves. Tree Physiol 20:815–823
Jarvis PG, Leverenz JW (1983) Productivity of temperate, deciduous and evergreen forests. In: Lange OL (ed) Physiological Plant Ecology. Springer, Berlin, pp 233–280
Joffre R, Rambal S, Damesin C (2007) Functional attributes of Mediterranean-type ecosystems. In: Pugnaire FI, Valladares F (eds) Handbook of Functional Plant Ecology. CRC Press, Boca Raton, pp 285–312
Joyce BJ, Steiner KC (1995) Systematic variation in xylem hydraulic capacity within the crown of white ash (Fraxinus americana). Tree Physiol 15:649–656
Kesselmeier J, Staudt M (1999) Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. J Atmos Chem 33:23–88
Kitajima K (1994) Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia 98:419–428
Koike T, Kitao M, Maruyama Y, Mori S, Lei TT (2001) Leaf morphology and photosynthetic adjustments among deciduous broad-leaved trees within the vertical canopy profile. Tree Physiol 21:951–958
Kovalyev AG (1980) Vozrast dereva i anatomo-morfologicheskoye stroyeniye hvoi sosny obyknovennoi. (The age of a tree and anatomo-morphological structure of needles in Pinus sylvestris L.). Lesovedeniye 6:30–35
Krédl Z, Středa T, Pokorný R, Kmoch M, Brotan J (2012) Microclimate in the vertical profile of wheat, rape and maize canopies. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis 60:79–90
Kull O, Kruijt B (1999) Acclimation of photosynthesis to light: a mechanistic approach. Funct Ecol 13:24–36
Kull O, Niinemets Ü (1998) Distribution of leaf photosynthetic properties in tree canopies: comparison of species with different shade tolerance. Funct Ecol 12:472–479
Kull O, Koppel A, Noormets A (1998) Seasonal changes in leaf nitrogen pools in two Salix species. Tree Physiol 18:45–51
Küppers M (1985) Carbon relations and competition between woody species in a Central European hedgerow. IV. Growth form and partitioning. Oecologia 66:343–352
Le Roux X, Sinoquet H, Vandame M (1999) Spatial distribution of leaf dry weight per area and leaf nitrogen concentration in relation to local radiation regime within an isolated tree crown. Tree Physiol 19:181–188
Leal DB, Thomas SC (2003) Vertical gradients and tree-to-tree variation in shoot morphology and foliar nitrogen in an oldgrowth Pinus strobus stand. Can J For Res 33:1304–1314
Lemaire G, Onillon B, Gosse G, Chartier M, Allirand JM (1991) Nitrogen distribution within a lucerne canopy during regrowth: relation with light distribution. Ann Bot 68:483–488
Lemoine D, Cochard H, Granier A (2002) Within crown variation in hydraulic architecture in beech (Fagus sylvatica L.): evidence for a stomatal control of xylem embolism. Ann For Sci 59:19–27
Leverenz JW (1987) Chlorophyll content and the light response curve of shade-adapted conifer needles. Physiol Plant 71:20–29
Leverenz JW (1988) The effects of illumination sequence, CO2 concentration, temperature and acclimation on the convexity of the photosynthetic light response curve. Physiol Plant 74:332–341
Leverenz JW (1994) Factors determining the nature of the light dosage response curve of leaves. In: Baker NR, Bowyer JR (eds) Photoinhibition of Photosynthesis: From Molecular Mechanisms to the Field. Bios Scientific Publishers Ltd, Oxford, pp 239–254
Logan BA, Barker DH, Demmig-Adams B, Adams WW III (1996) Acclimation of leaf carotenoid composition and ascorbate levels to gradients in the light environment within an Australian rainforest. Plant Cell Environ 19:1083–1090
Logan BA, Demmig-Adams B, Adams WW III (1998a) Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. upon a sudden increase in growth PPFD in the field. J Exp Bot 49:1881–1888
Logan BA, Demmig-Adams B, Adams WW III, Grace SC (1998b) Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. acclimated to four growth PPFDs in the field. J Exp Bot 49:1869–1879
Long SP, Postl WF, Bolhár-Nordenkampf HR (1993) Quantum yields for uptake of carbon dioxide in C3 vascular plants of contrasting habitats and taxonomic groupings. Planta 189:226–234
Lovelock CE, Jebb M, Osmond CB (1994) Photoinhibition and recovery in tropical plant species: response to disturbance. Oecologia 97:297–307
Lusk CH (2004) Leaf area and growth of juvenile temperate evergreens in low light: species of contrasting shade tolerance change rank during ontogeny. Funct Ecol 18:820–828
Lusk CH, Reich PB, Montgomery RA, Ackerly DD, Cavender-Bares J (2008) Why are evergreen leaves so contrary about shade? Trends Ecol Evol 23:299–303
Meir P, Kruijt B, Broadmeadow M, Barbosa E, Kull O, Carswell F, Nobre A, Jarvis PG (2002) Acclimation of photosynthetic capacity to irradiance in tree canopies in relation to leaf nitrogen concentration and leaf mass per unit area. Plant Cell Environ 25:343–357
Muller O, Oguchi R, Hirose T, Werger MJ, Hikosaka K (2009) The leaf anatomy of a broad-leaved evergreen allows an increase in leaf nitrogen content in winter. Physiol Plant 136:299–309
Naidu SL, DeLucia EH (1997) Acclimation of shade-developed leaves on saplings exposed to late-season canopy gaps. Tree Physiol 17:367–376
Niinemets Ü (1995) Distribution of foliar carbon and nitrogen across the canopy of Fagus sylvatica: adaptation to a vertical light gradient. Acta Oecol 16:525–541
Niinemets Ü (1997a) Distribution patterns of foliar carbon and nitrogen as affected by tree dimensions and relative light conditions in the canopy of Picea abies. Trees 11:144–154
Niinemets Ü (1997b) Acclimation to low irradiance in Picea abies: influences of past and present light climate on foliage structure and function. Tree Physiol 17:723–732
Niinemets Ü (1999) Research review. Components of leaf dry mass per area – thickness and density – alter leaf photosynthetic capacity in reverse directions in woody plants. New Phytol 144:35–47
Niinemets Ü (2007) Photosynthesis and resource distribution through plant canopies. Plant Cell Environ 30:1052–1071
Niinemets Ü (2010) A review of light interception in plant stands from leaf to canopy in different plant functional types and in species with varying shade tolerance. Ecol Res 25:693–714
Niinemets Ü (2012) Commentary. Optimization of foliage photosynthetic capacity in tree canopies: towards identifying missing constraints. Tree Physiol 32:505–509
Niinemets Ü (2015) Is there a species spectrum within the world-wide leaf economics spectrum? Major variations in leaf functional traits in the Mediterranean sclerophyll Quercus ilex. New Phytol 205:79–96
Niinemets Ü, Anten NPR (2009) Packing the photosynthesis machinery: from leaf to canopy. In: Laisk A (ed) Photosynthesis in Silico: Understanding Complexity from Molecules to Ecosystems. Springer, Berlin, pp 363–399
Niinemets Ü, Keenan TF (2012) Measures of light in studies on light-driven plant plasticity in artificial environments. Front Plant Sci 3:156
Niinemets Ü, Keenan TF (2014) Photosynthetic responses to stress in Mediterranean evergreens: mechanisms and models. Environ Exp Bot 103:24–41
Niinemets Ü, Kull O (1995) Effects of light availability and tree size on the architecture of assimilative surface in the canopy of Picea abies: variation in shoot structure. Tree Physiol 15:791–798
Niinemets Ü, Kull O (1998) Stoichiometry of foliar carbon constituents varies along light gradients in temperate woody canopies: implications for foliage morphological plasticity. Tree Physiol 18:467–479
Niinemets Ü, Kull O (2001) Sensitivity to photoinhibition of photosynthetic electron transport in a temperate deciduous forest canopy: Photosystem II centre openness, non-radiative energy dissipation and excess irradiance under field conditions. Tree Physiol 21:899–914
Niinemets Ü, Lukjanova A (2003) Total foliar area and average leaf age may be more strongly associated with branching frequency than with leaf longevity in temperate conifers. New Phytol 158:75–89
Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade-tolerant species Acer saccharum. Plant Cell Environ 20:845–866
Niinemets Ü, Valladares F (2004) Photosynthetic acclimation to simultaneous and interacting environmental stresses along natural light gradients: optimality and constraints. Plant Biol 6:254–268
Niinemets Ü, Valladares F (2006) Tolerance to shade, drought and waterlogging in the temperate dendroflora of the Northern hemisphere: tradeoffs, phylogenetic signal and implications for niche differentiation. Ecol Monogr 76:521–547
Niinemets Ü, Bilger W, Kull O, Tenhunen JD (1998a) Acclimation to high irradiance in temperate deciduous trees in the field: changes in xanthophyll cycle pool size and in photosynthetic capacity along a canopy light gradient. Plant Cell Environ 21:1205–1218
Niinemets Ü, Kull O, Tenhunen JD (1998b) An analysis of light effects on foliar morphology, physiology, and light interception in temperate deciduous woody species of contrasting shade tolerance. Tree Physiol 18:681–696
Niinemets Ü, Bilger W, Kull O, Tenhunen JD (1999a) Responses of foliar photosynthetic electron transport, pigment stoichiometry, and stomatal conductance to interacting environmental factors in a mixed species forest canopy. Tree Physiol 19:839–852
Niinemets Ü, Oja V, Kull O (1999b) Shape of leaf photosynthetic electron transport versus temperature response curve is not constant along canopy light gradients in temperate deciduous trees. Plant Cell Environ 22:1497–1514
Niinemets Ü, Sõber A, Kull O, Hartung W, Tenhunen JD (1999c) Apparent controls on leaf conductance by soil water availability and via light-acclimation of foliage structural and physiological properties in a mixed deciduous, temperate forest. Int J Plant Sci 160:707–721
Niinemets Ü, Tenhunen JD, Canta NR, Chaves MM, Faria T, Pereira JS, Reynolds JF (1999d) Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak, Quercus suber, to elevated atmospheric CO2 concentrations. Global Change Biol 5:455–470
Niinemets Ü, Ellsworth DS, Lukjanova A, Tobias M (2001) Site fertility and the morphological and photosynthetic acclimation of Pinus sylvestris needles to light. Tree Physiol 21:1231–1244
Niinemets Ü, Ellsworth DS, Lukjanova A, Tobias M (2002a) Dependence of needle architecture and chemical composition on canopy light availability in three North American Pinus species with contrasting needle length. Tree Physiol 22:747–761
Niinemets Ü, Hauff K, Bertin N, Tenhunen JD, Steinbrecher R, Seufert G (2002b) Monoterpene emissions in relation to foliar photosynthetic and structural variables in Mediterranean evergreen Quercus species. New Phytol 153:243–256
Niinemets Ü, Kollist H, García-Plazaola JI, Hernández A, Becerril JM (2003) Do the capacity and kinetics for modification of xanthophyll cycle pool size depend on growth irradiance in temperate trees? Plant Cell Environ 26:1787–1801
Niinemets Ü, Al Afas N, Cescatti A, Pellis A, Ceulemans R (2004a) Petiole length and biomass investment in support modify light-interception efficiency in dense poplar plantations. Tree Physiol 24:141–154
Niinemets Ü, Cescatti A, Christian R (2004b) Constraints on light interception efficiency due to shoot architecture in broad-leaved Nothofagus species. Tree Physiol 24:617–630
Niinemets Ü, Kull O, Tenhunen JD (2004c) Within canopy variation in the rate of development of photosynthetic capacity is proportional to integrated quantum flux density in temperate deciduous trees. Plant Cell Environ 27:293–313
Niinemets Ü, Sonninen E, Tobias M (2004d) Canopy gradients in leaf intercellular CO2 mole fractions revisited: interactions between leaf irradiance and water stress need consideration. Plant Cell Environ 27:569–583
Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T (2005a) Leaf internal diffusion conductance limits photosynthesis more strongly in older leaves of Mediterranean evergreen broad-leaved species. Plant Cell Environ 28:1552–1566
Niinemets Ü, Lukjanova A, Sparrrow AD, Turnbull MH (2005b) Light-acclimation of cladode photosynthetic potentials in Casuarina glauca: trade-offs between physiological and structural investments. Funct Plant Biol 32:571–582
Niinemets Ü, Cescatti A, Rodeghiero M, Tosens T (2006a) Complex adjustments of photosynthetic capacity and internal mesophyll conductance to current and previous light availabilities and leaf age in Mediterranean evergreen species Quercus ilex. Plant Cell Environ 29:1159–1178
Niinemets Ü, Tobias M, Cescatti A, Sparrow AD (2006b) Size-dependent variation in shoot light-harvesting efficiency in shade-intolerant conifers. Int J Plant Sci 167:19–32
Niinemets Ü, Lukjanova A, Turnbull MH, Sparrow AD (2007) Plasticity in mesophyll volume fraction governs the light-acclimation in needle photosynthesis in two pines. Tree Physiol 27:1137–1151
Niinemets Ü, Copolovici L, Hüve K (2010) High within-canopy variation in isoprene emission potentials in temperate trees: implications for predicting canopy-scale isoprene fluxes. J Geophys Res Biogeosci 115: G04029
Niinemets Ü, García-Plazaola JI, Tosens T (2012) Photosynthesis during leaf development and ageing. In: Flexas J (ed) Terrestrial Photosynthesis in a Changing Environment. A Molecular, Physiological and Ecological Approach. Cambridge University Press, Cambridge, pp 353–372
Niinemets Ü, Keenan T, Hallik L (2015) Tansley review. A worldwide analysis of within-canopy variations in leaf structural, chemical and physiological traits across plant functional types. New Phytol 205: 973–993
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol 49:249–279
Noormets A, Kull O, Koppel A (1996) Nitrogen dynamics in Salix leaves during the first production year. In: Perttu K, Koppel A (eds) Short Rotation Willow Coppice for Renewable Energy and Improved Environment. Proceedings of a Joint Swedish-Estonian Seminar on Energy Forestry and Vegetation Filters Held in Tartu 24–26 September 1995. Swedish University of Agricultural Sciences, Uppsala, pp 51–59
Nygren M, Kellomäki S (1983) Effect of shading on leaf structure and photosynthesis in young birches, Betula pendula Roth. and Betula pubescens Ehrh. For Ecol Manage 7:119–132
Oberhuber W, Dai Z-Y, Edwards GE (1993) Light dependence of quantum yields of photosystem II and CO2 fixation in C3 and C4 plants. Photosynth Res 35:265–274
Ögren E, Sjöström M (1990) Estimation of the effect of photoinhibition on the carbon gain in leaves of a willow canopy. Planta 181:560–567
Oguchi R, Hikosaka K, Hirose T (2005) Leaf anatomy as a constraint for photosynthetic acclimation: differential responses in leaf anatomy to increasing growth irradiance among three deciduous trees. Plant Cell Environ 28:916–927
Oguchi R, Hikosaka K, Hiura T, Hirose T (2006) Leaf anatomy and light acclimation in woody seedlings after gap formation in a cool-temperate forest. Oecologia 149:571–582
Ono K, Nishi Y, Watanabe A, Terashima I (2001) Possible mechanisms of adaptive leaf senescence. Plant Biol 3:234–243
Osmond CB, Anderson JM, Ball MC, Egerton JG (1999) Compromising efficiency: the molecular ecology of light-resource utilization in plants. In: Press MC (ed) Physiological Plant Ecology. The 39th Symposium of the British Ecological Society Held at the University of York, 7–9 September 1998. Blackwell Science, Oxford, pp 1–24
Palmroth S, Hari P (2001) Evaluation of the importance of acclimation of needle structure, photosynthesis, and respiration to available photosynthetically active radiation in a Scots pine canopy. Can J For Res 31:1235–1243
Patrick LD, Ogle K, Tissue DT (2009) A hierarchical Bayesian approach for estimation of photosynthetic parameters of C3 plants. Plant Cell Environ 32:1695–1709
Paulsen H (2001) Pigment assembly – transport and ligation. In: Aro E-M, Andersson B (eds) Regulation of Photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 219–233
Peltoniemi M, Duursma R, Medlyn B (2012) Co-optimal distribution of leaf nitrogen and hydraulic conductance in plant canopies. Tree Physiol 32:510–519
Pinheiro Prado M, de Oliveira Filho JA, França S, Márcio Amorim A, Schramm Mielke M (2013) Annual variation in canopy openness, air temperature and humidity in the understory of three forested sites in southern Bahia State, Brazil. Ciência Florestal 23:107–116
Pons TL (2016) Regulation of leaf traits in canopy gradients. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy Photosynthesis: From Basics to Applications. Springer, Berlin, pp 143–168
Pons TL, Anten NPR (2004) Is plasticity in partitioning of photosynthetic resources between and within leaves important for whole-plant carbon gain in canopies? Funct Ecol 18:802–811
Pons TL, Pearcy RW (1994) Nitrogen reallocation and photosynthetic acclimation in response to partial shading in soybean plants. Physiol Plant 92:636–644
Pons TL, Westbeek MHM (2004) Analysis of differences in photosynthetic nitrogen-use efficiency between four contrasting species. Physiol Plant 122:68–78
Pons TL, van Rijnberk H, Scheurwater I, van der Werf A (1993) Importance of the gradient in photosynthetically active radiation in a vegetation stand for leaf nitrogen allocation in two monocotyledons. Oecologia 95:416–424
Pons TL, van der Werf A, Lambers H (1994) Photosynthetic nitrogen use efficiency of inherently slow- and fast-growing species: possible explanations for observed differences. In: Roy J, Garnier E (eds) A Whole Plant Perspective on Carbon-Nitrogen Interactions. SPB Academic Publishing bv, The Hague, pp 61–77
Poorter L, Oberbauer SF, Clark DB (1995) Leaf optical properties along a vertical gradient in a tropical rain forest canopy in Costa Rica. Amer J Bot 82:1257–1263
Posada JM, Lechowicz MJ, Kitajima K (2009) Optimal photosynthetic use of light by tropical tree crowns achieved by adjustment of individual leaf angles and nitrogen content. Ann Bot 103:795–805
Possell M, Loreto F (2013) The role of volatile organic compounds in plant resistance to abiotic stresses: responses and mechanisms. In: Niinemets Ü, Monson RK (eds) Biology, Controls and Models of Tree Volatile Organic Compound Emissions. Springer, Berlin, pp 209–235
Ramalho JC, Lauriano JA, Nunes MA (2000) Changes in photosynthetic performance of Ceratonia siliqua in summer. Photosynthetica 38:393–396
Rambal S (1992) Quercus ilex facing water stress: a functional equilibrium hypothesis. Vegetatio 99–100:147–153
Rambal S (2001) Productivity of Mediterranean-type ecosystems. In: Mooney HA (ed) Terrestrial Global Productivity: Past, Present, and Future. Academic Press Inc, San Diego, pp 315–344
Rambal S, Damesin C, Joffre R, Méthy M, Lo Seen D (1996) Optimization of carbon gain in canopies of Mediterranean evergreen oaks. Ann Sci For 53:547–560
Rasmuson KE, Anderson JE, Huntly N (1994) Coordination of branch orientation and photosynthetic physiology in the Joshua tree (Yucca brevifolia). Great Basin Natur 54:204–211
Renninger HJ, Meinzer FC, Gartner BL (2006) Hydraulic architecture and photosynthetic capacity as constraints on release from suppression in Douglas-fir and western hemlock. Tree Physiol 27:33–42
Ryel RJ, Beyschlag W, Caldwell MM (1994) Light field heterogeneity among tussock grasses: theoretical considerations of light harvesting and seedling establishment in tussocks and uniform tiller distributions. Oecologia 98:241–246
Sala A, Sabaté S, Gracia C, Tenhunen JD (1994) Canopy structure within a Quercus ilex forested watershed: variations due to location, phenological development, and water availability. Trees 8:254–261
Sands PJ (1995) Modelling canopy production. I. Optimal distribution of photosynthetic resources. Aust J Plant Physiol 22:593–601
Sands PJ (1996) Modelling canopy production. III. Canopy light-utilisation efficiency and its sensitivity to physiological and environmental variables. Aust J Plant Physiol 23:103–114
Schieving F, Poorter H (1999) Carbon gain in a multispecies canopy: the role of specific leaf area and photosynthetic nitrogen-use efficiency in the tragedy of the commons. New Phytol 143:201–211
Schoettle AW (1989) Potential effect of premature needle loss on the foliar biomass and nutrient retention of lodgepole pine. In: Olson RK, Lefohn AS (eds) Transactions of Air Pollution on Western Forests. Air & Waste Management Association, Anheim, pp 443–454
Schoettle AW, Fahey TJ (1994) Foliage and fine root longevity of pines. In: Gholz HL (ed) Environmental Constraints on the Structure and Productivity of Pine Forest Ecosystems: A Comparative Analysis. Munksgaard International Booksellers and Publishers, Copenhagen, pp 136–153
Schoettle AW, Smith WK (1999) Interrelationships among light, photosynthesis and nitrogen in the crown of mature Pinus contorta ssp. latifolia. Tree Physiol 19:13–22
Schulze ED (1981) Carbon gain and wood production in trees of deciduous beech (Fagus sylvatica) and trees of evergreen spruce (Picea excelsa). Mitt Forstl Bundesvers Wien 142:105–123
Sellin A, Kupper P (2004) Within-crown variation in leaf conductance in Norway spruce: effects of irradiance, vapour pressure deficit, leaf water status and plant hydraulic constraints. Ann For Sci 61:419–429
Sharkey TD, Singsaas EL (1995) Why plants emit isoprene. Nature 374:769
Sharkey TD, Wiberley AE, Donohue AR (2008) Isoprene emission from plants: why and how. Ann Bot 101:5–18
Singsaas EL, Lerdau M, Winter K, Sharkey TD (1997) Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol 115:1413–1420
Smolander S, Stenberg P (2001) A method for estimating light interception by a conifer shoot. Tree Physiol 21:797–803
Sprugel DG, Brooks JR, Hinckley TM (1996) Effects of light on shoot geometry and needle morphology in Abies amabilis. Tree Physiol 16:91–98
Stenberg P (1996) Simulations of the effects of shoot structure and orientation on vertical gradients in intercepted light by conifer canopies. Tree Physiol 16:99–108
Stenberg P (1998) Implications of shoot structure on the rate of photosynthesis at different levels in a coniferous canopy using a model incorporating grouping and penumbra. Funct Ecol 12:82–91
Stenberg P, Smolander H, Sprugel DG, Smolander S (1998) Shoot structure, light interception, and distribution of nitrogen in an Abies amabilis canopy. Tree Physiol 18:759–767
Stenberg P, Kangas T, Smolander H, Linder S (1999) Shoot structure, canopy openness, and light interception in Norway spruce. Plant Cell Environ 22:1133–1142
St-Jacques C, Bellefleur P (1993) Light requirements of some broadleaf tree seedlings in natural conditions. For Ecol Manage 56:329–341
St-Jacques C, Labrecque M, Bellefleur P (1991) Plasticity of leaf absorptance in some broadleaf tree seedlings. Bot Gaz 152:195–202
Terashima I, Hikosaka K (1995) Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ 18:1111–1128
Terashima I, Hanba YT, Tholen D, Niinemets Ü (2011) Leaf functional anatomy in relation to photosynthesis. Plant Physiol 155:108–116
Teskey RO, Grier CC, Hinckley TM (1984) Change in photosynthesis and water relations with age and season in Abies amabilis. Can J For Res 14:77–84
Tjoelker MG, Volin JC, Oleksyn J, Reich PB (1995) Interaction of ozone pollution and light effects on photosynthesis in a forest canopy experiment. Plant Cell Environ 18:895–905
Tomás M, Flexas J, Copolovici L, Galmés J, Hallik L, Medrano H, Tosens T, …, Niinemets Ü (2013) Importance of leaf anatomy in determining mesophyll diffusion conductance to CO2 across species: quantitative limitations and scaling up by models. J Exp Bot 64:2269–2281
Tosens T, Niinemets Ü, Vislap V, Eichelmann H, Castro-Díez P (2012a) Developmental changes in mesophyll diffusion conductance and photosynthetic capacity under different light and water availabilities in Populus tremula: how structure constrains function. Plant Cell Environ 35:839–856
Tosens T, Niinemets Ü, Westoby M, Wright IJ (2012b) Anatomical basis of variation in mesophyll resistance in eastern Australian sclerophylls: news of a long and winding path. J Exp Bot 63:5105–5119
Valladares F (2003) Light heterogeneity and plants: from ecophysiology to species coexistence and biodiversity. In: Esser K (ed) Progress in Botany. Springer, Berlin, pp 439–471
Valladares F, Niinemets Ü (2007) The architecture of plant crowns: from design rules to light capture and performance. In: Pugnaire FI, Valladares F (eds) Handbook of Functional Plant Ecology. CRC Press, Boca Raton, pp 101–149
Valladares F, Niinemets Ü (2008) Shade tolerance, a key plant feature of complex nature and consequences. Annu Rev Ecol Evol Syst 39:237–257
Valladares F, Dobarro I, Sánchez-Gómez D, Pearcy RW (2005) Photoinhibition and drought in Mediterranean woody saplings: scaling effects and interactions in sun and shade phenotypes. J Exp Bot 56:483–494
Vapaavuori EM, Vuorinen AH (1989) Seasonal variation in the photosynthetic capacity of a willow (Salix cv. Aquatica gigantea) canopy. I. Changes in the activity and amount of ribulose 1,5-bisphosphate carboxylase-oxygenase and the content of nitrogen and chlorophyll at different levels in the canopy. Tree Physiol 5:423–444
Vapaavuori EM, Nurmi A, Vuorinen AH, Kangas T (1989) Seasonal variation in the photosynthetic capacity of a willow (Salix cv. Aquatica gigantea) canopy. 2. Comparison of the structure and function of chloroplasts at different levels in the canopy. Tree Physiol 5:445–457
Vaz M, Maroco J, Ribeiro N, Gazarini LC, Pereira JS, Chaves MM (2011) Leaf-level responses to light in two co-occurring Quercus (Quercus ilex and Quercus suber): leaf structure, chemical composition and photosynthesis. Agrofor Syst 82:173–181
Vickers CE, Gershenzon J, Lerdau MT, Loreto F (2009) A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat Chem Biol 5:283–291
Vos J, van der Putten PEL (2001) Effects of partial shading of the potato plant on photosynthesis of treated leaves, leaf area expansion and allocation of nitrogen and dry matter in component plant parts. Eur J Agron 14:209–220
Warren CR, Adams MA (2001) Distribution of N, Rubisco and photosynthesis in Pinus pinaster and acclimation to light. Plant Cell Environ 24:597–609
Warren CR, García-Plazaola JI, Niinemets Ü (2012) Ecophysiology of photosynthesis in temperate forests. In: Flexas J (ed) Terrestrial Photosynthesis in a Changing Environment a Molecular, Physiological and Ecological Approach. Cambridge University Press, Cambridge, pp 465–487
Warton DI, Wright IJ, Falster DS, Westoby M (2006) Bivariate line-fitting methods for allometry. Biol Rev Camb Philos Soc 81:259–291
Weih M (2009) Genetic and environmental variation in spring and autumn phenology of biomass willows (Salix spp.): effects on shoot growth and nitrogen economy. Tree Physiol 29:1479–1490
Werger MJA, Hirose T (1988) Effects of light climate and nitrogen partitioning on the canopy structure of stands of a dicotyledonous, herbaceous vegetation. In: Werger MJA (ed) Plant Form and Vegetation Structure. Adaptation, Plasticity and Relation to Herbivory. SPB Academic Publishing, The Hague, pp 171–181
Werner C, Ryel RJ, Correia O, Beyschlag W (2001a) Structural and functional variability within the canopy and its relevance for carbon gain and stress avoidance. Acta Oecol 22:129–138
Werner C, Ryel RJ, Correia O, Beyschlag W (2001b) Effects of photoinhibition on whole-plant carbon gain assessed with a photosynthesis model. Plant Cell Environ 24:27–40
Werner C, Correia O, Beyschlag W (2002) Characteristic patterns of chronic and dynamic photoinhibition of different functional groups in a Mediterranean ecosystem. Funct Plant Biol 29:999–1011
White JD, Scott NA (2006) Specific leaf area and nitrogen distribution in New Zealand forests: species independently respond to intercepted light. For Ecol Manage 226:319–329
Woodruff DR, McCulloh KA, Meinzer FC (2016) Forest canopy hydraulics. In: Hikosaka K, Niinemets Ü, Anten N (eds) Canopy Photosynthesis: From Basics to Applications. Springer, Berlin, pp 187–217
Woodward FI, Smith TM, Shugart HH (1994) Defining plant functional types: the end view. In: Smith TM (ed) Plant Functional Types. Their Relevance to Ecosystem Properties and Global Change. Cambridge University Press, Cambridge/New York/Melbourne, pp 355–359
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, …, Villar R (2004) The world-wide leaf economics spectrum. Nature 428:821–827
Wright IJ, Reich PB, Cornelissen JHC, Falster DS, Garnier E, Hikosaka K, Lamont BB, …, Westoby M (2005) Assessing the generality of global leaf trait relationships. New Phytol 166:485–496
Wright IJ, Leishman MR, Read C, Westoby M (2006) Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Funct Plant Biol 33:406–419
Yasumura Y, Hikosaka K, Matsui K, Hirose T (2002) Leaf-level nitrogen-use efficiency of canopy and understorey species in a beech forest. Funct Ecol 16:826–834
Zhang G, Leclerc MY, Karipot A (2010) Local flux-profile relationships of wind speed and temperature in a canopy layer in atmospheric stable conditions. Biogeosciences 7:3625–3636
Zhang J, Guo B, Jiang Qo RH, Wang Z (2013) Study on microclimate characteristics and vertical variation of potential evapotranspiration of the Robinia pseudoacacia forest in the loess plateau of China. Adv Meteorol 2013:748418
Acknowledgements
I thank Drs. Thijs Pons and Kouki Hikosaka for insightful comments on the manuscript. Author’s work on plant acclimation has been financially supported by the Estonian Ministry of Science and Education (institutional grant IUT-8-3) and the European Commission through the European Regional Fund (the Center of Excellence in Environmental Adaptation).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Niinemets, Ü. (2016). Within-Canopy Variations in Functional Leaf Traits: Structural, Chemical and Ecological Controls and Diversity of Responses. In: Hikosaka, K., Niinemets, Ü., Anten, N. (eds) Canopy Photosynthesis: From Basics to Applications. Advances in Photosynthesis and Respiration, vol 42. Springer, Dordrecht. https://doi.org/10.1007/978-94-017-7291-4_4
Download citation
DOI: https://doi.org/10.1007/978-94-017-7291-4_4
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-017-7290-7
Online ISBN: 978-94-017-7291-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)