Abstract
Neuroblastoma is a rare sympathetic nervous system neoplasia with a broad spectrum of clinical presentations. Prognosis depends on age, stage, genetic and histological features. However, in spite of favorable prognostic factors, the event-free survival of some neuroblastoma patients with localized disease may be poor. Since the Activated Leukocyte Cell Adhesion Molecule (ALCAM/CD166), involved in nervous system development and neuritis extension, has been linked to tumor progression and metastasis in several tumor types, we studied its expression in neuroblastoma cell lines and primary tumors from patients with localized neuroblastoma. Neuroblastoma cell lines display various levels of ALCAM surface expression, which can be dynamically regulated by metalloprotease-mediated shedding. More importantly, ALCAM is expressed also in neuroblastoma primary tumors and diverse patterns of subcellular localization can be observed. In patients with localized disease and favorable prognostic factors, high levels of ALCAM membrane expression, together with low expression in the cytoplasm and neuropil area, were significantly associated with relapse, suggesting that high ALCAM membrane expression may represent a new negative prognostic factor in these patients. In conclusion, assessment of ALCAM subcellular localization may represent a useful tool to identify patients at high risk of relapse that could benefit from a more careful follow-up.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Epithelial Ovarian Cancer
- Epithelial Ovarian Cancer Cell
- Activate Leukocyte Cell Adhesion Molecule
- Favorable Histology
- Neuropil Area
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Activated Leukocyte Cell Adhesion Molecule (ALCAM/CD166) is a member of the Immunoglobulin gene superfamily belonging to the subgroup with five extracellular immunoglobulin-like domains (VVC2C2C2), which mediates cell-cell clustering through homophile (ALCAM-ALCAM) and heterophile (ALCAM-CD6) interactions (Swart 2002). In adult tissues ALCAM expression is limited to subsets of cells, whereas in several human tumors, including melanoma, prostate, breast, bladder and colorectal cancer, alterations in expression of ALCAM have been reported, as reviewed by Ofori-Acquah and King (2008).
ALCAM role in tumor progression and metastasis has been well documented in several tumor types. In melanoma tumors, high levels of ALCAM membrane expression correlated with the vertical growth phase of tumor progression. Indeed, amino-terminally truncated ALCAM molecules, unable to support homotypic cell clustering, increased spontaneous lung metastasis in a transplant tumor model, indicating that suppression of surface ALCAM adhesive functions was required to mobilize cells from primary tumors, as reviewed in van Kempen et al. (2000). In glioblastoma, Kijima et al. (2011) demonstrated that ALCAM can identify cancer progenitor cells in tumor specimens and that high frequency of ALCAM-expressing cells is a negative prognostic marker. In addition, ALCAM is involved in the regulation of glioblastoma cell motility, as siRNA-mediated down-regulation of ALCAM membrane expression significantly enhanced tumor cell invasion. In epithelial ovarian cancer (EOC) cells, Piazza et al. (2005) showed that ALCAM is expressed at the cell surface and is internalized following soluble ligand engagement. Moreover, Rosso et al. (2007) demonstrated that ALCAM is released from EOC cells by a metalloprotease (ADAM)17/TACE-dependent mechanism leading to the generation of a soluble ALCAM form (sALCAM). Therefore, the perturbation of ALCAM-ligand interaction is relevant to EOC cell motility. Indeed, Mezzanzanica et al. (2008) showed that the loss of EOC cell anchorage is accompanied by a loss of ALCAM expression at the membrane level, and that the decreased/lost membrane expression of ALCAM correlated with a poorer outcome of EOC patients. Tachezy et al. (2011) found that ALCAM expression is an unfavourable prognostic marker also for pancreatic neuroendocrine tumor patients. In patients with colorectal cancer, Weichert et al. (2004) found ALCAM expression both at membrane and cytoplasmic levels; however, only membrane expression significantly correlated with worse patient survival. Conversely, in breast cancer patients, Burkhardt et al. (2006) showed that ALCAM cytoplasmic, rather than membrane, overexpression correlated with disease progression. Taken together, these findings strongly suggest that dynamic changes of ALCAM expression may be relevant for the progression of different tumor cells.
ALCAM has a physiological role in the central nervous system development. Ott et al. (2001) demonstrated that it is fundamental for motor axon growth and guidance to their targets. Afterward, Weiner et al. (2004) showed that ALCAM promotes fasciculation of multiple axonal populations, while Buhusi et al. (2009) demonstrated its pivotal path finding activity during formation of the retinocollicular maps. Moreover, Wade et al. (2012) recently showed that ALCAM enters the axonal retrograde transport route and co-operates with NGF signalling, widening the range of its functions from adhesion molecule to modulator of growth factor signalling in the nervous system.
Neuroblastoma (NB) is a rare sympathetic nervous system neoplasia with a broad spectrum of clinical presentations, varying from aggressive disease (stage 4) to spontaneous maturation and even regression (stage 4S). As reviewed by Maris (2010), prognosis of NB patients depends on age, stage, histological and genetic features, such as MYC-N amplification. As reported by Cohn et al. (2009), patients with localized disease have good prognosis; however, some of them relapse and may eventually die of the disease. Thus, the search for new prognostic markers able to identify patients at risk of relapse is warranted.
Based on the above considerations, and on recent data by Wierzbicki et al. (2008) indicating that ALCAM represents an antigenic target for NB immune recognition, we have investigated ALCAM expression in human neuroblastoma cell lines and primary tumors from patients with localized NB. We also evaluated whether different ALCAM subcellular localization associated with different outcomes of patients with localized NB.
Expression and Localization of Activated Leukocyte Cell Adhesion Molecule in Neuroblastoma Cell Lines
First, we analysed a panel of 13 human neuroblastoma cell lines for cell surface ALCAM expression. ALCAM was expressed at various levels in different cell lines, ranging from very low as GI-LI-N to high levels as GI-ME-N. No relationship was found between cell surface ALCAM and MYC-N amplification or chromosome 1p deletion status. Moreover, surface expression levels were diverse, irrespectively of the relative expression of mRNA, as assessed by semi-quantitative PCR.
Since ALCAM membrane expression may be influenced by its proteolytic cleavage, we then evaluated the expression of the metalloprotease ADAM17/TACE, supposed to generate ALCAM soluble form, as described by Rosso et al. (2007) in ovarian carcinoma cells. ADAM17/TACE was indeed expressed in NB cells both at mRNA and protein levels, and most importantly, it was able to generate the 65 kDa soluble form from the full-length ALCAM molecule. It is interesting to note that in NB cell line conditioned media, as reported in Corrias et al. (2010), ADAM17/TACE generated two soluble ALCAM forms of approximately 95 and 65 kDa, demonstrating that dynamic control of surface ALCAM expression actually occurs also in neuroblastoma cells.
Second, we analysed the pattern of ALCAM distribution in human NB cells following all-trans retinoic acid (ATRA)-induced differentiation. In ATRA-treated NB cell cultures no increase of sALCAM in the conditioned media nor changes in ADAM17/TACE protein expression were detected. However, after ATRA treatment, surface ALCAM expression was evident on the neuritis and dendrites of differentiated NB cells, becoming particularly strong on the neuritis after 7 days of treatment (Fig. 6.1). It is noteworthy that surface ALCAM expression appeared higher on the neuritis than on the cell body membrane at that time point, suggesting that in differentiating NB cells ALCAM re-localized into the growing neuritis. As expected, the acquisition of this ATRA-induced phenotype was independent of ADAM17/TACE activity.
ALCAM re-localization following treatment with retinoic acid. ALCAM expression and localization in differentiated SH-SY-5Y NB cells. Untreated cells (a) and cells treated for 24 (b), 48 (c) hours or 7 days (d) with 10 μM ATRA were stained with the anti-ALCAM I/F8 scFv followed by Alexa488-conjugated goat anti-mouse according to Piazza et al. (2005). Nuclei were counterstained with propidium iodide. Immunofluorescence was visualized by confocal microscopy (original magnification 600×)
Expression and Localization of Activated Leukocyte Cell Adhesion Molecule in Tumor Samples
Since different NB cell lines, that derive from high risk tumors, exhibited various levels of surface ALCAM expression, and because ATRA-treated NB cells, resembling more differentiated low risk tumors, re-localized membrane ALCAM into neuritis, we decided to investigate whether also in primary NB tumors different patterns of ALCAM localization could be observed.
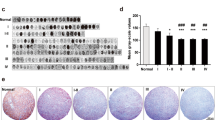
Preliminary immuno histochemical analysis performed on frozen primary NB tumors showed indeed that ALCAM could be expressed at different levels and in different cellular compartments (Fig. 6.2), indicating that a correlation between ALCAM and clinical and pathological characteristics could be explored. We therefore performed immuno histochemical analysis of paraffin embedded archival samples from a cohort of resectable stroma poor NB tumor specimens. As described in Corrias et al. (2010), ALCAM could localize at the body membrane, in the cytoplasm and in the neuropil area formed by non-myelinated neuritis and dendrites.
Expression of ALCAM in cryostat sections from NB primary tumors. Immuno histochemical analysis of ALCAM expression was performed on 5 μm thick cryostat sections from NB tumors. Aceton fixed sections were stained with the anti-ALCAM I/F8 scFv, according to Piazza et al. (2005) and a peroxidase-labelled dextran polymer conjugated anti-mouse antibody. Slides were counterstained with Mayer’s hematoxylin. Magnification 40×
Correlation Between Localization of Activated Leukocyte Cell Adhesion Molecule and Relapse in Patients with Localized Neuroblastoma
Mezzanzanica et al. (2008) showed in EOC tumors that loss of ALCAM membrane expression occurred together with its cytoplasmic re-localization. Moreover, this re-localization correlated with a worse prognosis. Thus, we evaluated whether different subcellular localizations of ALCAM expression were related to different risks of relapse. As demonstrated by Navarro et al. (2006), prognosis of NB patients with localized disease and normal MYC-N copy number is good, unless they present unfavorable histological features. However, among patients with localized disease and favorable histology, few local relapses can be observed. Thus, ALCAM expression was evaluated in 23 tumor specimens from patients with localized disease and favorable histology that had or not had experienced relapse. Precisely, 11 specimens were from patients that relapsed and 12 specimens were from patients that never relapsed. An event-free survival (EFS) analysis was then performed using the pattern of ALCAM expression to stratify the patients. As shown in Fig. 6.3, low expression in the neuropil area significantly associated with worse EFS (P < 0.0001). It is interesting to note that all the patients that showed low ALCAM expression in the neuropil area had high expression in the cell body membrane, suggesting that ALCAM re-localization into neuritis has a protective effect.
In conclusion, NB cell lines, usually derived from highly proliferating tumors, display various levels of ALCAM surface expression, which can be dynamically regulated by ADAM17/TACE metalloprotease activity, which generates two different ALCAM soluble forms in the conditioned media. When NB cell lines were treated with retinoic acid that reduces their proliferation and induces a more differentiated phenotype (see Clagett-Dame et al. 2006 for review), ALCAM re-localized into the growing neuritis. More importantly, ALCAM expression also occurs in NB primary tumors, showing diverse patterns of subcellular localization, involving the membrane, the cytoplasm and the neuritis. In a small but highly uniform cohort of patients with localized NB, favorable histology and normal MYC-N copy number, low ALCAM expression in the neuropil area and high levels in the body cell membrane significantly associated with relapse, suggesting that ALCAM expression may represent a new prognostic factor for these patients.
It could be speculated that the presence of high ALCAM levels in the neuropil area limit NB cell motility through a homophile interaction. Since neuropil is formed by non-myelinated dendrites and neuritis, and ATRA-differentiated NB cell lines showed strong ALCAM expression on neuritis, ALCAM staining in the neuropil identify more differentiated NB tumors, which have better prognosis. Furthermore, elevated ALCAM expression on the cell body of neuroblasts may support local tissue invasion, by acting as path finding molecule. Developmental studies by Ott et al. (2001), Weiner et al. (2004) and Buhusi et al. (2009) have indeed demonstrated that ALCAM favours neuritis outgrowth towards their targets. In this regard it is interesting to note that van Kempen et al. (2000) found that high ALCAM membrane expression in melanoma tumors, which share the same neuro-ectodermal origin as NB, associated with increased tumor progression. Similarly, Weichert et al. (2004) reported that membrane ALCAM overexpression associated with shorter survival time also in colorectal cancer patients.
Apparently conflicting results were reported by Mazzanzanica et al. (2008) and Jezierska et al. (2006) in ovarian and breast cancer patients, respectively. In fact, membrane ALCAM overexpression represented a good prognostic factor in these tumor types. Likely, this contradictory role depends on the fact that in these tumors ALCAM may increase cell to cell interaction rather than act as a path finding molecule. This holds true also in glioblastoma cells, where down-regulation of ALCAM by siRNA resulted in increased tumor cell invasiveness, as shown by Kijima et al. (2011). The function of ALCAM may indeed vary according to the cell type and to stimuli from the microenvironment, spanning from cell-cell adhesion to modulation of signalling in the nervous system, as reported by Wade et al. (2012). In conclusion, assessment of ALCAM subcellular localization may represent an easy and useful tool to identify, in the group of NB patients with localized disease and favorable histology, those at risk of relapse that could benefit from a more careful follow-up.
References
Buhusi M, Demyanenko GP, Jannie KM, Dalal J, Darnell EP, Weiner JA, Maness PF (2009) ALCAM regulates mediolateral retinotopic mapping in the superior colliculus. J Neurosci 29:15630–15641
Burkhardt M, Mayordomo E, Winzer KJ, Fritzsche F, Gansukh T, Pahl S, Weichert W, Denkert C, Guski H, Dietel M, Kristiansen G (2006) Cytoplasmic overexpression of ALCAM is prognostic of disease progression in breast cancer. J Clin Pathol 59:403–409
Clagett-Dame M, McNeill EM, Muley PD (2006) Role of all-trans retinoic acid in neurite outgrowth and axonal elongation. J Neurobiol 66:739–756
Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK, INRG Task Force (2009) The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol 27:289–297
Corrias MV, Gambini C, Gregorio A, Croce M, Barisione G, Cossu C, Rossello A, Ferrini S, Fabbi M (2010) Different subcellular localization of ALCAM molecules in neuroblastoma: association with relapse. Cell Oncol 32:77–86
Jezierska A, Olszewski WP, Pietruszkiewicz J, Olszewski W, Matysiak W, Motyl T (2006) Activated Leukocyte Cell Adhesion Molecule (ALCAM) is associated with suppression of breast cancer cells invasion. Med Sci Monit 12:BR245–BR256
Kijima N, Hosen N, Kagawa N, Hashimoto N, Nakano A, Fujimoto Y, Kinoshita M, Sugiyama H, Yoshimine T (2011) CD166/Activated leukocyte cell adhesion molecule is expressed on glioblastoma progenitor cells and involved in the regulation of tumor cell invasion. Neuro Oncol 14(3):1–11
Maris JM (2010) Recent advances in neuroblastoma. N Engl J Med 362:2202–2211
Mezzanzanica D, Fabbi M, Bagnoli M, Staurengo S, Losa M, Balladore E, Alberti P, Lusa L, Ditto A, Ferrini S, Pierotti MA, Barbareschi M, Pilotti S, Canevari S (2008) Subcellular localization of activated leukocyte cell adhesion molecule is a molecular predictor of survival in ovarian carcinoma patients. Clin Cancer Res 14:1726–1733
Navarro S, Amann G, Beiske K, Cullinane CJ, d’Amore ES, Gambini C, Mosseri V, De Bernardi B, Michon J, Peuchmaur M (2006) Prognostic value of International Neuroblastoma Pathology Classification in localized resectable peripheral neuroblastic tumors: a histopathologic study of localized neuroblastoma European Study Group 94.01 trial and protocol. J Clin Oncol 24:695–699
Ofori-Acquah SF, King JA (2008) Activated leukocyte cell adhesion molecule: a new paradox in cancer. Transl Res 151:122–128
Ott H, Diekmann H, Stuermer CA, Bastmeyer M (2001) Function of Neurolin (DM-GRASP/SC-1) in guidance of motor axons during zebrafish development. Dev Biol 235:86–97
Piazza T, Cha E, Bongarzone I, Canevari S, Bolognesi A, Polito L, Bargellesi A, Sassi F, Ferrini S, Fabbi M (2005) Internalization and recycling of ALCAM/CD166 detected by a fully human single-chain recombinant antibody. J Cell Sci 118:1515–1525
Rosso O, Piazza T, Bongarzone I, Rossello A, Mezzanzanica D, Canevari S, Orengo AM, Puppo A, Ferrini S, Fabbi M (2007) The ALCAM shedding by the metalloprotease ADAM17/TACE is involved in motility of ovarian carcinoma cells. Mol Cancer Res 5:1246–1253
Swart GW (2002) Activated leukocyte cell adhesion molecule (CD166/ALCAM): developmental and mechanistic aspects of cell clustering and cell migration. Eur J Cell Biol 81:313–321
Tachezy M, Zander H, Marx AH, Gebauer F, Rawnaq T, Kaifi JT, Sauter G, Izbicki JR, Bockhorn M (2011) ALCAM (CD166) expression as novel prognostic biomarker for pancreatic neuroendocrine tumor patients. J Surg Res 170:226–232
van Kempen LC, van den Oord JJ, van Muijen GN, Weidle UH, Bloemers HP, Swart GW (2000) Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am J Pathol 156:769–774
Wade A, Thomas C, Kalmar B, Terenzio M, Garin J, Greensmith L, Schiavo G (2012) Activated leukocyte cell adhesion molecule (alcam) modulates neurotrophin signaling. J Neurochem 120(1):7–25
Weichert W, Knosel T, Bellach J, Dietel M, Kristiansen G (2004) ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J Clin Pathol 57:1160–1164
Weiner JA, Koo SJ, Nicolas S, Fraboulet S, Pfaff SL, Pourquie O, Sanes JR (2004) Axon fasciculation defects and retinal dysplasias in mice lacking the immunoglobulin superfamily adhesion molecule BEN/ALCAM/SC1. Mol Cell Neurosci 27:59–69
Wierzbicki A, Gil M, Ciesielski M, Fenstermaker RA, Kaneko Y, Rokita H, Lau JT, Kozbor D (2008) Immunization with a mimotope of GD2 ganglioside induces CD8+ T cells that recognize cell adhesion molecules on tumor cells. J Immunol 181:6644–6653
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Corrias, M.V., Croce, M., Fabbi, M. (2013). Neuroblastoma: Role of Activated Leukocyte Cell Adhesion Molecule. In: Hayat, M. (eds) Pediatric Cancer, Volume 4. Pediatric Cancer, vol 4. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6591-7_6
Download citation
DOI: https://doi.org/10.1007/978-94-007-6591-7_6
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6590-0
Online ISBN: 978-94-007-6591-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)