Abstract
The ikaite columns in the Ikka Fjord, SW Greenland, constitute a cold (4 °C), alkaline (pH 10.4), and low-salinity (0.9 %) environment, and they harbor a microbial community adapted to this polyextreme environment. 16S rRNA gene sequence analyses show that the community is rich in new species and that Proteobacteria are dominating. So far, three new species have been characterized and described in detail: Rhodonellum psychrophilum, Arsukibacterium ikkense, and Alkalilactibacillus ikkensis. The bacteria produce a diversity of enzymes, including proteases, lipases, β-galactosidases, and phosphatases. These enzymes are adapted to the polyextreme environment and have application potentials in industrial and biotechnological processes. In this chapter, the research on the microbial population of the ikaite columns that has been conducted over the past decade will be reviewed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
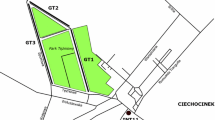
A very unusual alkaline and cold natural environment is found in the southwestern part of Greenland (61°11′N; 48°01′W), the ikaite columns of the Ikka Fjord (Fig. 1). The columns constitute a cold (4 °C), alkaline (pH 10.4), and low-salinity (0.9 %) environment, and they harbor a microbial community adapted to this polyextreme environment.
Location of the Ikka Fjord in southwest Greenland and the ikaite columns in Ikka Bund (Redrawn and modified from Seaman and Buchardt, 2006).
There are more than 600 columns of various sizes with a maximum age of ∼10,000 years, although most of them are probably much younger (Seaman and Buchardt, 2006). Being visible from the water surface, the ikaite columns were known to the early settlers in Greenland and have been mentioned as far back as in old Inuit legends (Krogh, 1982; Rink, 1866). This fascinating environment was declared an officially protected area by the Government of Greenland in 2000 (Seaman and Buchardt, 2006).
Geological investigations of the columns were initiated in 1962 (Pauly, 1963) and resumed in 1995, whereas scientific analyses of the microbial community living in this extreme environment have been carried out since 2002. Focus has been on diversity studies and description of novel bacterial species as well as on the biotechnological potential of enzymes adapted to this environment (Schmidt and Stougaard, 2010; Schmidt et al., 2006a, b, 2007, 2010, 2012; Stougaard et al., 2002).
In this chapter, research on the microbial diversity in this polyextreme environment will be reviewed.
2 Geochemical Characteristics
2.1 The Ikka Fjord
The Ikka Fjord was formed around 8,000 B.C. by transgression of seawater and deglaciation (Seaman and Buchardt, 2006; Kelly, 1977). It is a glacial valley surrounded by steep, 500 m high mountains dominated by lower Proterozoic (Ketilidian) gneisses. The inner fjord is cut in a NW-SE trending belt approximately 3 km wide by alkaline magma intrusions belonging to the Grønnedal-Ika igneous complex (Emeleus, 1964; Allaart, 1976). The Ikka Fjord is 13 km long, 170 m deep, up to 1.6 km wide, and characterized by two distinct parts: a deep outer fjord and a shallow inner fjord area called Ikka Bund. Between the two areas is a shallow sill called “Snævringen” (The Narrowing) that prevents most of the larger icebergs from the Davis Strait and the outer Ikka Fjord from entering Ikka Bund. The ikaite columns are located in Ikka Bund, and the area has been described as Ikka Column Garden (Fig. 1) (Buchardt et al., 1997, 2001; Seaman and Buchardt, 2006).
The Ikka Bund is 30 m deep and the uppermost 1–2 m is composed of freshwater runoff. Below the freshwater layer, the water is marine with a salinity of 33 ‰ and a temperature permanently below 6 °C. In contrast, the freshwater above the halocline can reach a temperature of 12 °C (Buchardt et al., 2001). From November to May, frozen freshwater covers the Ikka Fjord (Seaman and Buchardt, 2006).
2.2 The Ikaite Columns
In an area of 0.5 × 2.5 km in the Ikka Bund, more than 600 individual ikaite columns can be found in different shapes and sizes. Column heights vary from a few cm to more than 18 m and cross sections of up to 15 m have been reported (Buchardt et al., 1997, 2001; Seaman and Buchardt, 2006). Some columns appear as great towers only limited by the halocline, whereas others seem almost needlelike and very fragile. The term “unique” has been used to describe the columns, largely due to the geological factors that allow the columns to exist (Seaman and Buchardt, 2006).
The major mineral in the columns was named ikaite by the discoverer Hans Pauly in 1963 (Pauly, 1963). Ikaite is calcium carbonate hexahydrate (CaCO3·6H2O) a metastable cold-water mineral that forms when alkaline groundwater rich in carbonate ions mixes with calcium-rich seawater in the Ikka Bund (Table 1). Formation of ikaite is closely related to the Grønnedal-Ika complex as the alkalinity of the groundwater is proposed to originate from dissolution of secondary sodium carbonate minerals in the intrusion. This theory was supported by 14C analyses of newly precipitated ikaite that showed high contribution of inorganic carbon from the Precambrian intrusive carbonatitic rocks (Buchardt et al., 2001; Seaman and Buchardt, 2006). Phosphate from the carbonatite also dissolves in the precipitate that runs from the top of the intrusion to the ground beneath the Ikka Bund. High hydraulic head forces the water out under the Ikka Fjord, resulting in submarine springs penetrating an impermeable glaciomarine clay layer under the fjord bottom. Ikaite has a solubility of one to two orders of magnitude larger than that of calcite and aragonite, which are more common carbonate minerals. However, ikaite growth is favored by the low temperature in the fjord (2–6 °C) and the high phosphate concentration in the springwater, which inhibits the nucleation of calcite and aragonite (Bischoff et al., 1993; Brooks et al., 1950). Ikaite will decompose into calcite and water within hours when kept at room temperature (Seaman and Buchardt, 2006; Pauly, 1963).
The columns have been observed to grow directly from the mud that covers the flat areas of the fjord bed with upward growth at the tip of the columns (Buchardt et al., 2001; Seaman and Buchardt, 2006). Vertical growth is facilitated by buoyancy of the springwater due to the lower density compared to seawater in Ikka Bund, which has a salinity of up to 33 ‰ (Seaman and Buchardt, 2006). Furthermore, growth is promoted by the permeable framework of the monoclinic ikaite crystals, which ensure that the columns act as conduits for the springwater (Buchardt et al., 1997; Seaman and Buchardt, 2006). Buchardt and coworkers (2001) investigated the growth rate of the columns and found the increase in height to be 25–50 cm year−1 for a column with 15 cm diameter and estimated porosity of 50 %. Column mergers have been observed when columns grow adjacent to each other and damage to the columns due to boring organisms or mechanical influence initiates ikaite growth at the point of damage resulting in a large diversity of column shapes (Buchardt et al., 2001).
3 Biological Diversity Covering the Ikaite Columns and in the Ikka Fjord
The ikaite columns have a rich fauna of marine eukaryotic organisms living on the outside, which give the columns an appearance resembling coral reefs found in warmer waters (Fig. 2). This fauna is dominant on older columns, whereas newly formed ikaite columns as well as active growth zones show no or little attachment of biota (Dahl and Buchardt, 2006). The eukaryotic organisms have been studied and described in detail by Thorbjorn and Petersen (2003), and a few examples will be highlighted in this section.
The organisms found on the ikaite columns are similar to organisms found on offshore fishing banks, wave beaten shores, and narrow channels, and the environment is sheltered and without any high-energy water movement. In general, diversity increases when moving inwards in the fjord and with increasing depth (Thorbjorn and Petersen, 2003). Several of the organisms found on the columns and in the Ikka Fjord have been reported as new to this part of Greenland, including polychaetes, ascidians, and a copepod parasite (Thorbjorn and Petersen, 2003). Two of the most abundant species found encrusting the ikaite columns are the coralline red algae Clathromorphum and Lithothamnion. Both can be found on the columns all the way down to the bottom, due to the good light penetration in the Ikka Fjord. Buchardt et al. (1997) suggested that they help stabilize the columns (Fig. 2).
3.1 Eukaryotes and Archaea Inside the Columns
So far, only a small number of eukaryotic organisms have been identified in the interior of the ikaite columns. Kristiansen and Kristiansen (1999) described a new species of Chroomonas, C. ikaitensis, and Sørensen and Kristensen (2000) reported a new species of rotifer, Notholca ikaitophila, in the ikaite columns. However, DNA sequencing of 18S rRNA genes from DNA extracted from one column indicated the presence of numerous other, previously unidentified, eukaryotic species (Stougaard et al., 2002). The results suggested the presence of ascomycetes, annelid worms, diatoms, green algae, ciliates, dinoflagellates, and Mesomycetozoa, although all with low similarity to know sequences in databases (<80–96 %). All attempts to amplify archaeal 16S rRNA gene sequences have failed, suggesting a limited presence of Archaea in the ikaite columns. These analyses were conducted on new material from the top of columns, thereby likely only identifying a fraction of the total diversity (Stougaard et al., 2002).
4 Microbial Diversity Associated with the Ikaite Columns
The microbial community inhabiting the cold and alkaline environment of the ikaite columns has been studied for more than a decade. So far three new bacterial species and genera have been described (Schmidt et al., 2006b, 2007, 2012). Both cultivation-dependent and cultivation-independent methods have been applied to establish information on the bacteria adapted to this rare polyextreme environment.
4.1 Total Bacterial Diversity
Phylogenetic analyses based on the 16S rRNA gene were carried out on DNA isolated directly from the ikaite columns. These analyses showed that ∼50 % of the phylotypes are similar (90–99 %) to known meso- or thermophilic alkaliphiles, whereas the remaining ∼50 % showed less than 90 % identity when compared to available 16S rRNA gene sequences (Stougaard et al., 2002). Furthermore, Schmidt et al. (2006a) established a 16S rRNA gene library where 33 % of the clones showed less than 97 % identity to known sequences, suggesting that these may represent new species. It is therefore reasonable to assume that the alkaline, cold, and low-saline environment of the ikaite columns harbor a microbial community rich in novel species.
Recently, a metagenomic analysis of the total bacterial diversity on a pool of six different ikaite columns was conducted by pyrosequencing of the V3 and V4 hypervariable regions of the 16S rRNA gene. The RDP database was used to assign phylogenetic relationships (http://rdp.cme.msu.edu), and the analysis revealed that the most dominant phyla were Proteobacteria (57 %), Cyanobacteria (13 %), Firmicutes (8 %), Actinobacteria (5 %), and Bacteroidetes (5 %) (Table 2) (unpublished data).
Among the Proteobacteria, Beta- (44 %) and Alphaproteobacteria (31 %) were the dominant groups followed by Gammaproteobacteria (12 %). The pyrosequencing results, which show a dominance of Proteobacteria, are in agreement with earlier investigations of cultured isolates and 16S rRNA gene libraries (Stougaard et al., 2002; Schmidt et al., 2006a).
Terminal-restriction fragment length polymorphism (T-RFLP) analysis on three different ikaite columns indicated that each column harbors a distinct microbial community (Schmidt et al., 2006a). The community structure within a column also differs at varying depths, a difference not caused by the presence of other bacteria, but by a varying local abundance of individual species. These results were obtained on material collected in 2002 (Schmidt et al., 2006a) and confirmed on material collected in 2006 (Pedersen, 2007).
Alkaliphilic bacteria from the ikaite columns have been shown to be unique to the columns and not present in the surrounding sea water (Aarup, 2006; Schmidt et al., 2006a; Pedersen, 2007).
4.2 Cultured Bacteria
The first attempts to culture bacteria from ikaite columns were performed by Stougaard et al. (2002). Working on material that had been stored at −18 °C for several years, they were able to cultivate ten different isolates with three phylogenetic affiliations: Firmicutes (6), Proteobacteria (3), and Bacteroidetes (1). All isolates were able to grow at 5 °C with an optimal growth around 13 °C, and three of the isolates had optimal growth at high pH (9–10).
Using ikaite material that had been preserved at 5 °C or at −20 °C in 20 % glycerol, Schmidt et al. (2006a) successfully obtained more than 200 isolates, which were further characterized with respect to pH and temperature tolerance. Five of the cultured isolates were true psychrophiles, whereas the remaining were psychrotolerant. Nine of the isolates were only able to grow at pH 10 and 15 isolates only at pH 9 and pH 10, while the majority of the isolates were able to grow at pH 8–10.
Phylogenetic analysis on 67 of the cultured isolates showed that 54 were affiliated with Proteobacteria: Gamma- (27), Alpha- (26), and Betaproteobacteria (1). Sixteen of the cultured Alphaproteobacteria isolates showed 98 % sequence identity to Loktanella vestfoldensis, a psychrotolerant bacterium isolated in Vestfold Hills, Antarctica (Van et al., 2004), and nine isolates showed 92–99 % sequence identity to Rhodobaca bogoriensis, an alkaliphilic bacterium isolated from a soda lake in Africa (Milford et al., 2000). Most of the Gammaproteobacteria belonged to the Pseudomonadaceae family, eight of which showed 99 % sequence identity to Pseudomonas antarctica, a psychrophilic bacteria from Antarctica (Reddy et al., 2004). Four of the isolates analyzed were related to the Gram-positive Bacilli in the phylum Firmicutes, one being 97 % identical to Bacillus alkaliphilus.
T-RFLP analysis demonstrated that when bacteria from ikaite material are cultivated, the diversity drops and a community of Gamma- and Alphaproteobacteria, Bacilli, and Clostridium is established. Attempts to develop cultivation media resembling ikaite conditions showed no effect in terms of increased diversity (Aarup, 2006).
As in the metagenomic analysis presented in the previous section, Proteobacteria also dominate the cultivable part of the bacteria found in the cold and alkaline ikaite columns.
4.2.1 New Species from the Ikaite Columns
Phylogenetic analyses of cultured isolates and 16S rRNA gene libraries indicated that approximately one third of the isolates may represent new species. So far, three novel species have been described.
Rhodonellum psychrophilum is a red-pigmented bacterium of the phylum Bacteroidetes and family Flexibacteraceae (Schmidt et al., 2006b). It is a strictly aerobic psychrophile with optimal growth at 5–10 °C and a growth range from 0 to 22 °C. R. psychrophilum has a pH range from 7.5 to 10.7 and a growth optimum at 0.6 % NaCl (w/v). Based on 16S rRNA gene sequence analysis and DNA-DNA hybridization, the closest relative is Belliella baltica, a pink-colored bacterium with a growth optimum at 25 °C isolated from surface water in the Baltic Sea (Brettar et al., 2004). The cell wall of R. psychrophilum has a very high amount of branched and unsaturated fatty acids (97 %), which increases membrane fluidity and thereby cellular activity and transport processes, a characteristic adaption for bacteria living at low temperatures and high pH (Schmidt et al., 2006b). Weak biofilm production was observed at 5 °C.
Arsukibacterium ikkense is a nonpigmented, psychrotolerant Gammaproteobacterium (Schmidt et al., 2007). The growth range is from 0 to 30 °C with an optimum at 15 °C. The pH growth range was reported from 7.5 to 10.0 with optimum at 9.2–10.0 and a salinity optimum at 3 %. The closest related species is Rheinheimera baltica, a blue-colored bacterium isolated from the Baltic Sea with a growth optimum at 20–25 °C (Brettar et al., 2002). Only 60 % of the total fatty acids in A. ikkense are branched and unsaturated, which is low compared to R. psychrophilum. This shows that not all bacteria present in the ikaite columns exhibit the typical features of cold adaption (Schmidt et al., 2007). A. ikkense show biofilm formation and alkali-stable and cold-active amylase and protease activity (Sect. 5).
Alkalilactibacillus ikkensis is a nonpigmented psychrotolerant bacterium belonging to the phylum Firmicutes (Schmidt et al., 2012). It is able to grow from 0 to 28 °C and at pH from 8.5 to at least 11.5. Based on 16S rRNA gene sequencing, fatty acid composition, and DNA-DNA hybridization analyses, the closest related species is Halolactibacillus xiariensis, a halophilic and moderately alkaliphilic bacterium isolated from a soda lake in Inner Mongolia (Cao et al., 2008). A. ikkensis was isolated based on its β-galactosidase activity (Schmidt and Stougaard, 2010; Schmidt et al., 2012), but it also shows α-amylase, β-glucuronidase, α-galactosidase, and β-1,3-glucanase activity at low temperatures (Sect. 5) (Schmidt et al., 2012).
4.3 Bacteria from Similar Environments
Natural alkaline environments are relatively rare, and the most intensively studied are soda lakes. Like ikaite columns, they are very alkaline (pH 8 to >12), but unlike ikaite columns, they are highly saline and often found in temperate or subtropical regions with temperatures in the range from 20 to 44 °C (Jones et al., 1998; Duckworth et al., 1996; Grant and Heaphy, 2010). Analyses of microbial diversity in known soda lakes show that Gammaproteobacteria dominate the Gram negative, while Gram-positive bacteria are often related to Bacillus. Archaea are also common and are related to the genera Natronococcus and Natronobacterium (Jones et al., 1998; Duckworth et al., 1996).
More similar to ikaite columns is the Lost City Hydrothermal Field, which is alkaline (pH 9–11) and has low salinity. However, the temperature ranges from 40 to 93 °C (Schrenk et al., 2004). Analyses by Schrenk and coworkers (2004) showed that the microorganisms inhabiting the carbonate columns in Lost City often are associated with dense biofilm. Total cell counts were relatively high (2.0 × 106–3.1 × 108 cells gdw−1), but diversity was low and dominated by Archaea of the order Methanosarcinales (Schrenk et al., 2004). This is in contrast to ikaite columns, where Archaea have not been identified despite several attempts (Stougaard et al., 2002). Bacteria related to Proteobacteria and Firmicutes were also identified in the Lost City Hydrothermal Field (Schrenk et al., 2004).
The ground water of the Portuguese City Cabeço de Vide constitutes a natural environment with a pH of 11.4, a low salinity, and a temperature of 20.5 °C (Tiago et al., 2004). An analysis of the bacterial diversity showed that total bacterial counts were low (3.4–7.2 × 103 cells L−1), as was the diversity of the cultivable community. The majority were associated to Actinobacteria and some to Firmicutes. In contrast to ikaite columns, where Proteobacteria are abundant, only very few Proteobacteria were observed in the groundwater of Cabeço de Vide (Tiago et al., 2004).
The results from environments with some of the characteristics of ikaite columns show that the microbial community found in ikaite columns is unique and very different from previously described environments (Table 3).
5 Enzymes
The springwater seeping through the columns is characterized by a very low content of nutrients (Buchardt et al., 2001). Therefore, it has been hypothesized that the bacteria living in the columns grow on organic matter derived from degradation of animals, algae, and bacteria that either inhabit the columns or have been trapped during the process of ikaite column formation (Schmidt et al., 2006a, b). This theory is supported by the metagenomic analyses conducted on ikaite material, where heterotrophic bacteria are dominant.
Extracellular enzymes produced by the bacteria are the key factors in nutrient degradation outside the cell and they are, like the bacteria, well adapted to the alkaline and cold environment. Intracellular enzymes of alkaliphilic bacteria are often adapted to the neutral or slightly alkaline pH in the cytosol (pH 8.0–9.3) (Yumoto, 2002). Cold-active and alkaline-stable enzymes have considerable application potentials in industrial and biotechnological processes, particularly in the detergent industry, and enzymes that are cold active at neutral pH may be applied to numerous processes where low operating temperatures are preferred, e.g., in food processing, for medical purposes, the chemical industry, waste treatment, and biotechnology. The large number of new bacterial species isolated from the ikaite columns implies that there is a high possibility of finding enzymes with novel properties in this environment. So far, several reports have described ikaite bacteria that produce cold-active and/or alkaline-stable extracellular enzyme activities and two cold-active enzymes, a β-galactosidase and a lipase, have been characterized in detail (Schmidt and Stougaard, 2010; Schmidt et al., 2006b, 2007, 2010, 2012).
5.1 Enzyme Diversity
A collection of 672 bacterial isolates derived from ikaite material plated on R2A medium (pH 10) was screened for enzyme activity (Pedersen, 2007). Nine different enzyme activities were examined by screening on substrates linked to azurine (AZCL linked) or to 5-bromo-4-chloro-indolyl (X linked). Intracellular enzymes included β-galactosidase, β-glucosidase, β-glucuronidase, and phosphatase, and extracellular enzymes were protease, α-amylase, cellulase, xylanase, and mannanase. The results demonstrated that out of the 672 isolates, 450 (67 %) exhibited enzymatic activity on one or more substrates and up to five different enzyme activities could be produced by the same isolate. In total, 53 % of the positive isolates produced β-glucosidase or β-glucuronidase, 30 % phosphatase, 27 % β-galactosidase, 25 % α-amylase, 23 % protease, 11 % mannanase, 9 % cellulase, and 2 % produced xylanase (Table 4).
α-Amylase and cellulase producing isolates were further analyzed at different pH values ranging from pH 7 to 10. Enzyme activity associated with growth was observed: all isolates showed enzymatic activity at pH 9 and 10, whereas only a few showed activities at pH 8 and no activity was observed at pH 7.
Temperature and pH analysis on extracts containing extracellular α-amylases from four isolates showed that the enzymes were active at pH 5–10 and at temperatures ranging from 5 to 50 °C (Pedersen, 2007).
5.1.1 Enzymes of Novel Species
A total of four enzyme-producing bacterial isolates from ikaite columns have so far been described.
Schmidt et al. (2006b) characterized the psychrophilic Rhodonellum psychrophilum which produces a substantial range of enzymes including alkaline phosphatases, esterases, proteases, and galactosidases.
Protease, amylase, and intracellular phosphatase activity was described for the novel Gammaproteobacterium Arsukibacterium ikkense (Schmidt et al., 2007). Preliminary bioinformatic analyses of the genome sequence have revealed a high number of extracellular proteases and α-amylases (unpublished data).
The isolate Alkalilactibacillus ikkensis produces α-amylase, α-galactosidase, β-galactosidase, and β-glucuronidase (Schmidt et al., 2012). The intracellular β-galactosidase showed 57 % identity to the closest related β-galactosidase from Bacillus megaterium (Schmidt and Stougaard, 2010), and the amino acid composition of the protein showed features of cold-active enzymes. The number of Arg and Pro residues and the Arg/(Arg+Lys) ratio were decreased compared to Escherichia coli β-galactosidase, indicating fewer hydrogen and ionic bonds. The A. ikkensis β-galactosidase was successfully expressed in E. coli. The enzyme showed maximum activity at pH 8 and at 20–30 °C, with 90 % activity remaining at pH 9 and ∼60 % at pH 7. The enzyme retained 60 % of the maximum activity at 0 °C, which is the highest activity reported for a recombinantly produced cold-active β-galactosidase (Schmidt and Stougaard, 2010). Furthermore, when compared to a commercially available β-galactosidase, the enzyme showed a twofold higher conversion rate at temperatures between 0 and 20 °C (Schmidt and Stougaard, 2010). The β-galactosidase of A. ikkensis could be irreversibly inactivated by heating to 50 °C for 5 min or 40 °C for 10 min.
An uncharacterized Gammaproteobacterium with close relationship to an uncharacterized Nitrincola sp. E-048 (96 % 16S rRNA gene identity) was isolated due its ability to produce lipase (Schmidt et al., 2010). A novel triacylglycerol lipase with similarity to a lipase from Rhodoferax ferrireducens (51 % identity at the amino acid level) was identified. The lipase showed enzymatic activity from 5 to 80 °C. Maximum activity was observed at 55 °C, which is not typical for cold-adapted proteins. The ability to remain active at high temperatures was reflected in the amino acid composition of the protein. The lipase contained similar amounts of the polar hydrogen-binding residues (Ser, Thr, Asn, and Glu) as a thermophilic lipase produced by Bacillus stearothermophilus P1. However, in silico analysis of the secondary and tertiary structure of the lipase indicated larger loop sizes, which is often associated with cold-adapted enzymes (Schmidt et al., 2010; Gianese et al., 2001; Roy and Sengupta, 2007). The pH optimum was observed at pH 8 and 70 % of the maximum activity remained at pH 9 and 40 % at pH 7. No activity was found at pH 6 or below. It was not possible to measure activity at pH 10 due to instability of the screening substrate.
6 Biofilm
The ikaite columns are rich in biofilm, which has been visualized by cryo-SEM when analyzing the ikaite crystals (Buchardt et al., 2001). The biofilm is often associated with sedentary diatoms, known to secrete large amounts of biofilm, but bacterial production is also likely to occur, as several of the bacteria isolated from ikaite columns are confirmed producers of biofilm, including R. psychrophilum (Schmidt et al., 2006b) and A. ikkense (Schmidt et al., 2007). The biofilm has been proposed to protect the ikaite crystals and stabilize the columns (Seaman and Buchardt, 2006) and may be involved in establishing a protective environment for the bacteria by nutrient sequestering, protection against grazers, and attachment to the columns (Jefferson, 2004; Nichols et al., 2005). The natural substrate for the β-galactosidase activity found in A. ikkensis could be polymers from, e.g., algal cell walls, but it is also possible that the β-galactosidase is able to degrade complex galactose-containing sugars in the biofilm, in which case the biofilm acts as a nutrient in itself (Schmidt and Stougaard, 2010).
In a preliminary study, 250 strains isolated from ikaite columns were analyzed for biofilm production (Aarup, 2006). Under the experimental conditions, 19 of the strains were categorized as excellent (11) or good (8) producers of biofilm, while the remaining showed little (96) or no (135) biofilm production. The best producers of biofilm were all closely related to A. ikkense as determined by 16S rRNA gene sequencing, except one, which was a Firmicutes related to the Clostridium family.
7 Concluding Remarks
The unique environment of the Ikka Fjord was first described in 1962, but further investigations were not initiated until 1995. Here we have reviewed the research conducted on the nature of the ikaite columns and the bacteria surviving in this polyextreme environment.
The geochemical data suggest that the Ikka columns are a unique phenomenon and phylogenetic and metagenomic analyses indicate that the bacterial diversity differs from other known alkaline environments. Three novel species have been described, and two novel enzymes with industrial potential have been characterized. Additionally, screenings of cultured bacteria have shown a wide range of enzymatic activities. The data derived so far describes a unique biological environment with a good prospect of finding novel species and enzymes for industrial applications. Presently, more thorough metagenomic and genomic analyses are being conducted in order to establish more information on the microbial population in the ikaite columns.
References
Aarup C (2006) Identification of biofilm-producing bacteria in ikaite tufa columns. Master thesis, University of Copenhagen
Allaart JH (1976) Ketilidian mobile belt in South Greenland. In: Escher A, Watt WS (eds) Geology of Greenland. Grønlands Geologiske undersøgelse, København, pp 120–151
Bischoff JL, Fitzpatrick JA, Rosenbauer RJ (1993) The solubility and stabilization of Ikaite (CaCO3.6H2O) from 0 °C to 25 °C – environmental and paleoclimatic implications for Thinolite tufa. J Geol 101:21–33
Brettar I, Christen R, Hofle MG (2002) Rheinheimera baltica gen. nov., sp. nov., a blue-coloured bacterium isolated from the central Baltic Sea. Int J Syst Evol Microbiol 52:1851–1857
Brettar I, Christen R, Hofle MG (2004) Belliella baltica gen. nov., sp. nov., a novel marine bacterium of the Cytophaga-Flavobacterium-Bacteroides group isolated from surface water of the central Baltic Sea. Int J Syst Evol Microbiol 54:65–70
Brooks R, Clark LM, Thurston EF (1950) Calcium carbonate and its hydrates. Philos Trans R Soc Lond Ser A 243:145–167
Buchardt B, Seaman P, Stockmann G, Vous M, Wilken U, Duwel L, Kristiansen A, Jenner C, Whiticar MJ (1997) Submarine columns of ikaite tufa. Nature 390:129–130
Buchardt B, Israelson C, Seaman P, Stockmann G (2001) Ikaite tufa towers in Ikka Fjord, southwest Greenland: their formation by mixing of seawater and alkaline spring water. J Sediment Res 71:176–189
Cao SJ, Qu JH, Yang JS, Sun Q, Yuan HL (2008) Halolactibacillus alkaliphilus sp. nov., a moderately alkaliphilic and halophilic bacterium isolated from a soda lake in Inner Mongolia, China, and emended description of the genus Halolactibacillus. Int J Syst Evol Microbiol 58:2169–2173
Dahl K, Buchardt B (2006) Monohydrocalcite in the Arctic Ikka fjord, SW Greenland: first reported marine occurrence. J Sediment Res 76:460–471
Duckworth AW, Grant WD, Jones BE, van Steenbergen R (1996) Phylogenetic diversity of soda lake alkaliphiles. FEMS Microbiol Ecol 19:181–191
Emeleus CH (1964) The Grønnedal-Ika alkaline complex, South Greenland. The structure and geological history of the complex. Grønlands Geologiske Undersøgelse, København
Gianese G, Argos P, Pascarella S (2001) Structural adaptation of enzymes to low temperatures. Protein Eng 14:141–148
Grant WD, Heaphy S (2010) Metagenomics and recovery of enzyme genes from alkaline saline environments. Environ Technol 31:1135–1143
Jefferson KK (2004) What drives bacteria to produce a biofilm? FEMS Microbiol Lett 236:163–173
Jones BE, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2:191–200
Kelly M (1977) Quaternary geology of the Ivigtut-Nunarssuit region, South-West and South Greenland. Rapport Grønlands Geologiske Undersøgelse 85:64–67
Kristiansen J, Kristiansen A (1999) A new species of Chroomonas (Cryptophyceae) living inside the submarine ikaite columns in the Ikkafjord, Southwest Greenland, with remarks on its ultrastructure and ecology. Nordic J Bot 19:747–758
Krogh KJ (1982) Erik den Rødes Grønland; Saga texts = Qallunaatsiaaqarfik Grønland. Nationalmuseets Forlag, Copenhagen
Milford AD, Achenbach LA, Jung DO, Madigan MT (2000) Rhodobaca bogoriensis gen. nov. and sp. nov., an alkaliphilic purple nonsulfur bacterium from African Rift Valley soda lakes. Arch Microbiol 174:18–27
Nichols CA, Guezennec J, Bowman JP (2005) Bacterial exopolysaccharides from extreme marine environments with special consideration of the southern ocean, sea ice, and deep-sea hydrothermal vents: a review. Mar Biotechnol (NY) 7:253–271
Pauly H (1963) “Ikaite”, a new mineral from Greenland. Arctic 16:263–264
Pedersen R (2007) Bacteria from ikaite tufa columns: enzymes and microbial diversity. Master thesis, University of Copenhagen
Reddy GS, Matsumoto GI, Schumann P, Stackebrandt E, Shivaji S (2004) Psychrophilic pseudomonads from Antarctica: Pseudomonas antarctica sp. nov., Pseudomonas meridiana sp. nov. and Pseudomonas proteolytica sp. nov. Int J Syst Evol Microbiol 54:713–719
Rink H (1866) Eskimoiske Eventyr og Sagn. Reitzel, København
Roy D, Sengupta S (2007) Structural features of a cold-adapted Alaskan bacterial lipase. J Biomol Struct Dyn 24:463–470
Schmidt M, Stougaard P (2010) Identification, cloning and expression of a cold-active beta-galactosidase from a novel Arctic bacterium, Alkalilactibacillus ikkense. Environ Technol 31:1107–1114
Schmidt M, Prieme A, Stougaard P (2006a) Bacterial diversity in permanently cold and alkaline ikaite columns from Greenland. Extremophiles 10:551–562
Schmidt M, Prieme A, Stougaard P (2006b) Rhodonellum psychrophilum gen. nov., sp. nov., a novel psychrophilic and alkaliphilic bacterium of the phylum Bacteroidetes isolated from Greenland. Int J Syst Evol Microbiol 56:2887–2892
Schmidt M, Prieme A, Stougaard P (2007) Arsukibacterium ikkense gen. nov., sp. nov, a novel alkaliphilic, enzyme-producing γ-Proteobacterium isolated from a cold and alkaline environment in Greenland. Syst Appl Microbiol 30:197–201
Schmidt M, Larsen DM, Stougaard P (2010) A lipase with broad temperature range from an alkaliphilic γ-proteobacterium isolated in Greenland. Environ Technol 31:1091–1100
Schmidt M, Prieme A, Johansen A, Stougaard P (2012) Alkalilactibacillus ikkensis, gen. nov., sp. nov., a novel enzyme-producing bacterium from a cold and alkaline environment in Greenland. Extremophiles 16:297–305
Schrenk MO, Kelley DS, Bolton SA, Baross JA (2004) Low archaeal diversity linked to subseafloor geochemical processes at the Lost City Hydrothermal Field, Mid-Atlantic Ridge. Environ Microbiol 6:1086–1095
Seaman P, Buchardt B (2006) The columns of ikaite tufa in Ikka Fjord, Greenland. Monographs on Greenland. Geoscience 44:1–39
Sørensen MV, Kristensen RM (2000) Marine Rotifera from Ikka Fjord, SW Greenland. Monographs on Greenland. Bioscience 51:1–46
Stougaard P, Jorgensen F, Johnsen MG, Hansen OC (2002) Microbial diversity in ikaite tufa columns: an alkaline, cold ecological niche in Greenland. Environ Microbiol 4:487–493
Thorbjorn L, Petersen GH (2003) The epifauna on the carbonate reefs in the Arctic Ikka Fjord, SW Greenland. Ophelia 57:177–201
Tiago I, Chung AP, Verissimo A (2004) Bacterial diversity in a nonsaline alkaline environment: heterotrophic aerobic populations. Appl Environ Microbiol 70:7378–7387
Van TS, Mergaert J, Swings J (2004) Loktanella salsilacus gen. nov., sp. nov., Loktanella fryxellensis sp. nov. and Loktanella vestfoldensis sp. nov., new members of the Rhodobacter group, isolated from microbial mats in Antarctic lakes. Int J Syst Evol Microbiol 54:1263–1269
Yumoto I (2002) Bioenergetics of alkaliphilic Bacillus spp. J Biosci Bioeng 93:342–353
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Vester, J.K., Lylloff, J.E., Glaring, M.A., Stougaard, P. (2013). Microbial Diversity and Enzymes in Ikaite Columns: A Cold and Alkaline Environment in Greenland. In: Seckbach, J., Oren, A., Stan-Lotter, H. (eds) Polyextremophiles. Cellular Origin, Life in Extreme Habitats and Astrobiology, vol 27. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6488-0_15
Download citation
DOI: https://doi.org/10.1007/978-94-007-6488-0_15
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6487-3
Online ISBN: 978-94-007-6488-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)