Abstract
Bile is a fluid that helps us to digest food and its main function is to break down fats in food. Bile is made by the liver and stored in the gall bladder. Bile ducts are tubes that carry bile and they connect the liver and the gall bladder to the duodenum and the small intestine. In people who have had their gall bladders removed, bile flows directly from the liver into the duodenum and the small intestine. The bile ducts and gall bladder are known as the biliary system (Fig. 10.1). Cholangiocarcinoma (CC) is a malignant tumor arising from the bile duct epithelium. They start in mucus glands that line the bile ducts. If cancer starts in the part of the bile ducts within the liver it is known as intra-hepatic. If it starts in bile ducts outside the liver it is known as extra-hepatic. It may arise from the right and left hepatic ducts at or near their junction (hilar cholangiocarcinoma) which are considered as carcinoma of the extrahepatic bile ducts (for a review, please see Refs. [1–8]). Cancers of the biliary system are almost always adenocarcinomas. The incidence of cholangiocarcinoma reveals wide geographic variations: the highest incidence is reported in areas suffering from endemic infestation with liver fluke. The liver flukes, Opisthorchis viverrini and Clonorchis sinensis, which induce cholangiocarcinomas, are common in Africa and Asia, especially in Thailand and Laos in Southeast Asia, and in some parts of China. Intrahepatic cholangiocarcinoma is the second most prevalent intrahepatic primary cancer. Hilar cholangiocarcinoma is the fourth most common gastrointestinal malignancy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Vascular Endothelial Growth Factor
- Epidermal Growth Factor Receptor
- Bile Duct
- Gall Bladder
- Primary Sclerosing Cholangitis
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
10.1 Introduction
Bile is a fluid that helps us to digest food and its main function is to break down fats in food. Bile is made by the liver and stored in the gall bladder. Bile ducts are tubes that carry bile and they connect the liver and the gall bladder to the duodenum and the small intestine. In people who have had their gall bladders removed, bile flows directly from the liver into the duodenum and the small intestine. The bile ducts and gall bladder are known as the biliary system (Fig. 10.1). Cholangiocarcinoma (CC) is a malignant tumor arising from the bile duct epithelium. They start in mucus glands that line the bile ducts. If cancer starts in the part of the bile ducts within the liver it is known as intra-hepatic. If it starts in bile ducts outside the liver it is known as extra-hepatic. It may arise from the right and left hepatic ducts at or near their junction (hilar cholangiocarcinoma) which are considered as carcinoma of the extrahepatic bile ducts (for a review, please see Refs. [1–8]). Cancers of the biliary system are almost always adenocarcinomas. The incidence of cholangiocarcinoma reveals wide geographic variations: the highest incidence is reported in areas suffering from endemic infestation with liver fluke. The liver flukes, Opisthorchis viverrini and Clonorchis sinensis, which induce cholangiocarcinomas, are common in Africa and Asia, especially in Thailand and Laos in Southeast Asia, and in some parts of China. Intrahepatic cholangiocarcinoma is the second most prevalent intrahepatic primary cancer. Hilar cholangiocarcinoma is the fourth most common gastrointestinal malignancy.
10.2 Molecular Carcinogenesis of Cholangiocarcinoma
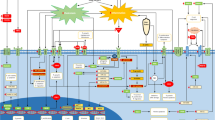
The development of cholangiocarcinoma, similar to other types of cancer, can be divided into at least three stages, namely, Initiation, Promotion and Progression [9, 10]. A molecular scheme of cholangiocarcinoma development, and the various factors that affect the development of cholangiocarcinoma are shown in Fig. 10.2. The etiological factors of cholangiocarcinoma can be broadly divided into genetic/epigenetic factors and environmental factors. The Initiation stage of carcinogenesis involves damages and genetic/epigenetic alterations of the genome. Increased carcinogenic nitroso-compounds as a result of regional dietary factors or environmental contaminants, are thought to produce genetic changes including mutations in DNA of the normal biliary epithelial cells. The mutations are “fixed” in the genome by subsequent rounds of DNA replication or repair, which can occur as the bile duct cells are stimulated to divide and proliferate. This becomes the second step of the carcinogenic process, the Promotion stage, which may proceed further as a result of chronic inflammation of the tissues. At this stage, dysplastic/hyperplastic biliary epithelium may develop from normal epithelial cells. Liver fluke infestation causes chronic inflammation and enhances susceptibility of the bile duct epithelium to carcinogens/free radicals, leading to genetic and epigenetic changes in cells.
A molecular scheme of cholangiocarcinoma development. The various factors that affect the development of cholangiocarcinoma are presented. The etiological factors of cholangiocarcinoma can be divided broadly as genetic/epigenetic factors and environmental factors. The developmental process can be divided into three stages, namely 1st stage: Initiation, 2nd stage: Promotion, and 3rd stage: Progression
Hepatolithiasis, the presence of stones in the bile ducts of the liver, is associated with a high-risk for intrahepatic cholangiocarcinoma because of recurrent bacterial infections and bile stasis. It is more frequently seen in East Asian than in Western countries. Hepatitis virus infection has also been reported as a risk factor for cholangiocarcinoma. Infection by hepatitis virus may contribute to the stage of promotion by inducing chronic inflammation, cell-death and cell-proliferation. However, the relationship between HBV/HCV and cholangiocarcinoma formation is not unequivocally established. Recent reports indicated that hepatitis C and hepatitis B nucleic acids as well as viral proteins are present in intrahepatic cholangiocarcinomas [11–13].
In addition, primary sclerosing cholangitis (PSC), another risk factor, is a chronic liver condition producing progressive inflammation and scarring of the bile ducts of the liver [6]. The inflammation impedes flow of bile to the gut, which can ultimately lead to liver cirrhosis, liver failure and liver cancer. The underlying cause of inflammation is believed to be due to autoimmunity. Patients with primary sclerosing cholangitis have a tendency to develop bile duct carcinoma. Moreover, inflammatory bowel disease (IBD), such as ulcerative colitis, is a chronic inflammatory bowel condition. People with this disease are also at an increased risk of developing cholangiocarcinoma. It is of interest to note that patients with congenital abnormal bile duct diseases, such as choledochal cysts, Caroli’s disease and congenital hepatic fibrosis, are more at risk of developing cholangiocarcinoma. Other genetic/epigenetic defects that may contribute to the development of cholangiocarcinoma include drug detoxification defect (MGMT), DNA repair defect (hMLH1) and excessive production of pro-inflammatory cytokines.
The third stage of development of cholangiocarcinoma is the Progression stage, which involves the transition of dysplastic/hyperplastic biliary epithelium to become carcinoma of the bile-duct. At this stage, many critical genes that have been altered can be detected, especially the proto-oncogenes and tumor suppressor genes, for example, p53 [14–16], p16INK4A [17–22], ErbB-1, erbB-2, VEGF [23–27], K-ras [28–31], cMet, p120, Cadherin and many Cell-cycle genes. Induced serum markers such as ALP, GTT, bilirubin, Ca19-9, CA125, CEA, MUC5AC are found. Cholangiocarcinomas can arise in the absence of any known etiological factors.
10.3 “Yin-Yang” Negative- and Positive-Control Hypothesis of Cholangiocarcinoma Cell Development
Similar to other kinds of cancer including hepatocellular carcinoma [9], the development of cancer cells of the bile-duct epithelium may be considered as Yin-Yang or negative-positive control of cell-growth and cell-death. As shown in Fig. 10.3, the “Yang” factors usually refer to the growth factors, receptors, cellular signal transducers and nuclear transcriptional factors which are mostly proto-oncogenes that promote cellular proliferation and survival. On the other hand the “Yin” factors are molecules that suppress cell-growth and facilitate cell-death including apoptosis. It is the delicate interplay and regulation of expression and action of these positive and negative modulators that result in the control-growth of a normal cell. Mutations and/or altered expression in proto-oncogenes and suppressor genes lead to aberrant functions of proteins, which in turn may induce abnormal growth and differentiation of the cells.
10.4 Molecular Markers of Cholangiocarcinoma
The histology of cholangiocarcinoma with H and E staining is shown in Fig. 10.4a, b. Figure 10.4a shows a typical cholangiocarcinoma (glandular type with numerous fibrous stromal-regions), and Fig. 10.4b shows a papillary type with mucous and intraluminal papillary masses. The expressions of several important molecular markers such as K-ras (Fig. 10.4c), CK19 (Fig. 10.4d, e), and tumor suppressor p16INK4A (Fig. 10.4f), are also shown [21].
The histology and molecular markers of cholangiocarcinoma H and E staining and markers of cholangiocarcinoma are shown. Cholangiocarcionoma (a, b: H&E), (a) is an adenocarcinoma with numerous fibrous stromal regions, and (b) is a papillary cholangiocarcinoma with intraluminal papillary masses. The expressions (immunohistochemical stainings) of K-ras (c), CK19 (d, e), and p16 (f) are also shown [21]
10.5 Tumor Suppressor Gene P53 Mutation
The wild-type p53 plays an important role in the regulation of the cell cycle process, cell growth, and apoptosis in the event of DNA damage. It is also known as the gate-keeper for these important cellular events. P53 encodes a phosphorylated protein with a molecular weight of 53 kD. It is the most commonly mutated tumor suppressor gene associated with human cancer, being abnormal in over 50 % of known human cancers [14–16). The suppressor p53 protein is involved in many pathways by interacting with many gene products including transcription, DNA repair, cell cycling and genomic stability. DNA damages stabilize p53 which binds to p53 control elements in genes and activate transcription. These p53 modulating genes include cell-cycle genes such as p21CIP/WAF1, a cyclin kinase inhibitor, BAX and Fas for apoptosis, and GADD45 for DNA repair. Alternatively, p53 may form protein-protein complexes with proteins of DNA synthesis and repair such as RPA, topoisomerase I and XPD helicase. Mutated p53 is also more stable and render cells to escape from cell-cycle arrest, delay in S-phase synthesis, and apoptosis.
The p53 gene is resided on the short arm of chromosome 17 (17p13.1). Inactivation of the p53 gene by missense or nonsense mutations and by loss of chromosome 17p, induces disruption of critical growth-regulating mechanisms and may have a crucial role in carcinogenesis. The reported incidence of p53 mutation is 11–37 % in intrahepatic cholangiocarcinomas [14]. It has been reported that loss of chromosome 17p was present in 38 % of intrahepatic cholangiocarcinomas [9]. It has also been documented that there are over 90 different types of p53 mutations found in cholangiocarcinoma p53 database, by the International Agency for Research on Cancer (IARC). The codon distribution and mutation pattern is described in Figs. 10.5 and 10.6. The spectrum of mutations for p53 apparently is specific for the populations in different regions and presumably for the carcinogens. Over 50 % of mutated p53 in Thailand were G:C to A:T transitions at CpG sites, while in Korea, it was only 17 % [14]. Alkylating agents such as N-nitroso compounds, tend to induce G:C to A:T transitions in genes via the formation of O-6-methylguanine. Mutation in p53 is apparently dependent on environmental factors and carcinogens exposed, which may vary in different populations and locations. Figure 10.5 shows the mutation distribution of p53 in cholangiocarcinoma. The codon distribution of p53 single base substitutions in cholangiocarcinoma indicates that the mutation hotspots are at codons 175, 179, 245, 248, 273 and 282 respectively [14]. In Fig. 10.6, the mutation pattern of the 92 reported p53 mutations in cholangiocarcinoma is shown. This is the proportion of the different types of p53 mutations as reported, which is the number of mutations of each type divided by the total number of mutations [14]. The most commonly reported type of mutation is at CpG sites (29.3 %), which was found in over 50 % of p53 mutations in Thai patients. Alkylating agents such as N-nitroso compounds tend to induce G:C-A:T transitions in p53 via the formation of O-6-methylguanine [14]. It is apparently dependent on environmental factors including differences in nature or dose of exposure, which vary in different populations.
Tumor suppressor gene P53 mutation distribution in cholangiocarcinoma. Codon distribution of p53 single base substitutions in cholangiocarcinoma. The bar chart shows the proportion of all reported single base substitutions at each codon of p53 in cholangiocarcinoma which is the number of single base substitutions at each codon divided by the total number of single base substitutions [14]
Tumor suppressor gene P53 mutation pattern in cholangiocarcinoma. Mutation pattern of the 92 reported p53 mutations in cholangiocarcinoma. The pie chart is a representation of the proportion of the different types of p53 mutations as reported, which is the number of mutations of each type divided by the total number of mutations [14]
10.6 Tumor Suppressor P16INK4A Alteration and Methylation
p16INK4A is a regulatory protein in the cell cycle and a cyclin-dependent kinase (cdk4/cdk6) inhibitor. The tumor suppressor gene p16 is commonly inactivated in many neoplasms. Three distinct mechanisms of p16 inactivation have been reported in biliary neoplasms: deletion and point mutations of the p16 gene, and hypermethylation of 5′ regulatory regions of p16 [17–22]. As shown in Fig. 10.7, the methylation pattern of the promoter region of p16 shows increased methylation in the tumor tissues as compared to the non-tumor tissues. The increased methylation is a mechanism for down-regulating the expression of the gene. A study of intrahepatic cholangiocarcinomas reports that no p16 gene mutations are present but alterations of p16 gene are frequent: methylation of CpG island is present in the 5′ region of the gene (54 %), allelic loss at the p16 locus on chromosome 9p21 (20 %), and homozygous deletion (5 %). Therefore, the p16 gene may possibly be crucial for intrahepatic biliary carcinogenesis and progression. This is somewhat similar to HCCs as we had reported which contain multiple p16 alternations including deletions and methylations [22].
Tumor suppressor p16INK4A methylation in cholangiocarcinoma. Methylation analysis of p16 promoter region in normal, non cholangiocarcinoma. Methylation specific PCR results are expressed as unmethylated p16 specific bands (U) and methylated bands (M) [21]
10.7 Epidermal Growth Factor Receptor (EGFR) Family ErbB-1 and ErbB-2
This is the family of the avian erythroblastic leukemia viral (v-erb-b) oncogene homolog. They are members of the Epidermal growth factor receptor subfamily (EGFR), which are typeItyrosine kinase receptors, and can bind EGF and TGF-α. ErbB-1 (HER1) and ErbB-2 (HER2) share approximately 40 % homology in their extracellular binding domains. On the other hand, ErbB-2 has no ligand binding domain of its own and therefore cannot bind growth factors. However, it does bind tightly to other ligand-bound EGF receptor family members to form a heterodimer, stabilizing ligand binding and enhancing kinase-mediated activation of downstream signalling pathways. Amplification and overexpression of c-erbB-2 are frequently seen in cancers of the biliary tract [23–26]. It has been reported that a high incidence of cholangiocarcinomas (intrahepatic and extrahepatic) and gallbladder cancers developed in transgenic mice overexpressing ErbB-2. Reported values of the frequency of tumors overexpressing ErbB-2 varies from 0 to 73 %.
In another report, 44 % of intrahepatic cholangiocarcinoma are ErbB-1-positive and that ErbB-1 expression is correlated with grade and proliferative index [26]. Immunohistochemical expression of these molecules was assessed retrospectively in 236 cases of cholangiocarcinoma, as well as the associations between the expression of these molecules and clinicopathological factors or clinical outcome. The proportions of positive cases for EGFR and HER2 overexpression were 27.4, and 0.9 % in intrahepatic cholangiocarcinoma (IHCC), and 19.2 and 8.5 % in extrahepatic cholangiocarcinoma (EHCC), respectively. EGFR overexpression was associated with macroscopic type (P = 0.0120), lymph node metastasis (P = 0.0006), tumor stage (P = 0.0424), lymphatic vessel invasion (P = 0.0371), and perineural invasion (P = 0.0459) in EHCC, and multivariate analysis showed that EGFR expression was a significant prognostic factor [hazard ratio (HR), 2.67; 95 % confidence interval (CI), 1.52–4.69; P = 0.0006] and also a risk factor for tumor recurrence (HR, 1.89; 95 % CI, 1.05–3.39, P = 0.0335) in IHCC. These results strongly indicate that EGFR expression is associated with tumor progression in cholangiocarcinoma. The immunohistochemical staining of EGFR family members in cholangiocarcinoma is shown in Fig. 10.8. Figure 10.8a is EGFR, Fig. 10.8b is HER2, and Fig. 10.8c is VEGF. In addition, Fig. 10.8d shows Epidermal growth factor receptor tends to be expressed in the poorly differentiated component while Fig. 10.8e shows Human epidermal growth factor receptor 2, which is preferentially expressed in more differentiated areas such as the glandular or papillary component [26]. Figure 10.9 shows the EGFR expression and survival in cholangiocarcinoma. Survival curves of EGFR-positive and -negative expression in (Fig. 10.9a), IHCC and (Fig. 10.9b), EHCC. The outcome of EGFR-positive cases was significantly worse than that of EGFR-negative cases in both IHCC and EHCC [26].
EGFR immunohistochemical staining in cholangiocarcinoma. Immunohistochemical staining of (a) EGFR, (b) HER2, and (c) VEGF in cholangiocarcinoma. (d) Epidermal growth factor receptor tends to be expressed in the poorly differentiated component. (e) Human epidermal growth factor receptor 2 is preferentially expressed in more differentiated areas such as the glandular or papillary component [26]
EGFR expression and survival in cholangiocarcinoma. Survival curves of EGFR-positive and -negative expression in (a), IHCC and (b), EHCC. The outcome of EGFR-positive cases was significantly worse than that of EGFR-negative cases in both IHCC and EHCC (26]
10.8 Vascular Endothelial Growth Factor (VEGF)
This gene is a member of the PDGF/VEGF growth factor family and encodes a protein that is often found as a disulfide linked homodimer. This protein is a glycosylated mitogen that specifically acts on endothelial cells and has various effects, including mediating increased vascular permeability, inducing angiogenesis, vasculogenesis and endothelial cell growth, promoting cell migration, and inhibiting apoptosis. VEGF plays an important role in inducing endothelial cell growth and in promoting angiogenesis.
VEGF which has been considered as potential therapeutic targets in cholangiocarcinoma and immunohistochemical expression was assessed retrospectively in 236 cases of cholangiocarcinoma, and the associations between clinicopathological factors or clinical outcome were determined [26]. The proportions of positive cases for VEGF were 53.8 % overexpression in intrahepatic cholangiocarcinoma (IHCC), and 59.2 % in extrahepatic cholangiocarcinoma (EHCC), respectively. Clinicopathologically, VEGF overexpression was related to intrahepatic metastasis (P = 0.0224) in IHCC. These results suggest that VEGF expression may be involved in haematogenic metastasis in cholangiocarcinoma. Another report showed that VEGF A expression was more frequently encountered in peripheral cholangiocarcinoma (69 % vs. 25 %, P < 0.0001) and correlated with increased vascular density [27]. Thus, VEGF is a potentially useful marker in predicting metastasis and angiogenesis in cholangiocarcinoma.
10.9 Proto-Oncogene K-ras Mutation
K-ras is a proto-oncogene of GTP-GDP binding protein family with GTPase activity. The K-ras proto-oncogene is thought to exert control over the mechanisms of cell growth and differentiation. This gene is converted to an active oncogene by point mutations, significantly concentrated in codons 12, 13 or 61, similar to the H-ras mutations in other tumors. The reported rates of K-ras mutations in intrahepatic cholangiocarcinomas vary widely (28–32]. Variations are caused by racial and geographic variations, and the use of different assay techniques, for example, a mutation rate of 50–56 % was found in Japanese patients versus 0–8 % in Thai patients. It has been reported that mutation rates are higher in periductal and spicular-forming tumors than mass-forming ones. The expression of K-ras in cholangiocarcinoma is shown in Fig. 10.4c.
10.10 Reduced Expression of P120 Catenin and Cadherin
P120-catenin is a member of the Armadillo (ARM)/β-catenin gene family and is essential for mesenchymal cadherin-mediated regulation of cell motility and invasiveness. Altered expression of beta-catenin was reported in intrahepatic cholangiocarcinoma [32]. On the other hand, Cadherin, one of the transmembrane cell-cell adhesion receptors involved in development, and morphogenesis of intrahepatic cholangiocarcinoma (ICC), is necessary and sufficient for P120 targeting cell-cell junctions. P120 is to stabilize cadherins at the cell membrane by regulating cadherin turnover and degradation. P120 may stabilize cell junctions or regulate membrane trafficking machinery. Down-regulated expression of E-cadherin and P120 occurs frequently in ICC which may contribute to the progression and development of tumor [33]. Both of them may be valuable biologic markers for predicting tumor invasion, metastasis and patients’ survival, and P120 is an independent prognostic factor for ICC [34]. In Fig. 10.10, reduced E cadherin (A–C) and p120 catenin (D–F) expression by immunohistochemistry in cholangiocarcinoma is shown. Figure. 10.10a, d are the preserved type, while Fig. 10.10b, e are the reduced type, and Fig. 10.10c, f are the complete absent type [33]. Figure 10.11 shows the correlation of survival of patients against the expression of p120 catenin (Fig. 10.11a) and E-cadherin (Fig. 10.11b). Increased survivals were found in the positive cases, vs. the negative cases.
Reduced p120 and cacherin expression in cholangiocarcinoma. Immunostaining of E-cadherin and p120 catenin in intrahepatic cholangiocarcinoma. (a, d) Preserved type, (b, e) reduced type, and (c, f) completely absent type [33]
Correlation of p120 cadherin expression and survival in cholangiocarcinoma. (a) Survival curve of p120-catenin positive and negative cholangiocarcinoma. (b) Survival curve of E-cadherin positive and negative cholangiocarcinoma [33]
10.11 Up-Regulated Expression of the Multi-Functional Receptor Annexin A2 (ANXA2) and its Ligand Tenascin
In one recent study, membrane protein was extracted from four cholangiocarcinoma (CC) cell lines with different tumor forming capabilities [35]. Two-dimensional-PAGE followed by MALDI-TOF-MS was used to identify differentially expressed proteins. Among 20 up-regulated membrane proteins identified in the CC cell lines was ANXA2, a participant in tumor invasion and metastasis in other cancers. ANXA2 expression was verified in human subjects by probing, using monoclonal antibody and a tissue microarray of CC (301 diagnosed cases), where it was found to associate with one of several tumor progression stages as reflected by lymphatic invasion (P = 0.014) and metastasis (P = 0.026). Patients with high expressions of ANXA2 had a significantly shorter survival time (P = 0.011). ANXA2 expression in tumors may be useful for predicting the poor outcome of CC patients. We also had found that the expression of ANXA2 was up-regulated in hepatocellular carcinoma (Chan et al., unpublished data). These results indicated that ANXA2 could be a useful biomarker for different kinds of hepatic malignancies. In addition, one of the ligands of ANXA2, Tenascin, has been shown to express strongly at the invasive front of IHCC which was associated with poor prognosis in intrahepatic cholangiocarcinoma [36]. Figure 10.12 shows the enhanced ANXA2 expression in cholangiocarcinoma. The immunohistochemical staining of Annexin A2 (ANXA2) in normal liver tissue is shown in (Fig. 10.12A), in bile duct hyperplasia tissue (Fig. 10.12B), and in cholangiocarcinoma (CCA) tissues (Fig. 10.12C, D). Annexin A2-positive cells were clustered within bile duct hyperplasia (B) and CCA tissues (C and D), but not detected or expressed at very low levels in stroma, normal liver and bile duct cells (A). Annexin A2 was preferably membranous (D) in location of CCA tissues, although some cytoplasmic staining (C) was observed [35].
Enhanced ANXA2 expression in cholangiocarcinoma. Immunohistochemical staining of Annexin A2 (ANXA2) in normal liver tissue (a), bile duct hyperplasia tissue (b) and cholangiocarcinoma (CCA) tissues (c, d). Annexin A2-positive cells were clustered within bile duct hyperplasia (b) and CCA tissues (c, d), but not detected or expressed at very low levels in stroma, normal liver and bile duct cells (a). Annexin A2 was preferably membranous (d) in location of CCA tissues, although some cytoplasmic staining (c) was observed [35]
10.12 Cytokeratin 19 (CK19)
The keratins are intermediate filament proteins responsible for the structural integrity of epithelial cells. CK19 is also involved in the organization of myofibers and together with KRT8, it helps to link the contractile apparatus to dystrophin at the costameres of striated muscle [37, 38]. Cytokeratin immunostaining forms the bedrock of the immunohistochemical evaluation of tumors. CK19 belongs to a family of keratins, which are normally expressed in the lining of the gastroenteropancreatic and hepatobiliary tracts [37]. CK19 has been shown to be an independent prognostic factor for pancreatic neuroendocrine tumors, especially the insulin-negative tumors. CK19 positive tumors are associated with poor outcomes irrespective of the established pathologic parameters such as size, mitoses, lymphovascular invasion, and necrosis. CK19 is useful in the work-up of pancreatic endocrine tumors. CK19 is also positive in most neuroendocrine tumors occurring in the rest of the GIT, except rectal tumors, which are negative.
In the liver, CK19 is of prognostic value in hepatocellular carcinomas and is of use in distinguishing cholangiocarcinoma from hepatocellular carcinomas. It can also be used to highlight native ductules in the liver and helps separate conditions such as focal nodular hyperplasia from hepatic adenoma. The vast majority of adenocarcinomas in the GIT and pancreas are CK19 positive. In a study of the differences between hepatocellular carcinoma (HCC) and peripheral type of cholangiocarcinoma (CC) using cytokeratin (CK) and carcinoembryonic antigen (CEA) expressions, 50 % of HCCs were positive for CEA, presenting a canalicular staining pattern [38]. For CK7, all but one (which was focally positive), or 80 % of CHCs were diffusely positive, whereas only two HCCs were positive. For CK19, 80 % of CCs were diffusely positive, while all but two HCCs (a moderately and a poorly differentiated tumor) were negative. For CK, 8/18, or 70 % of HCCs were diffusely positive, whereas only 20 % of CHCs were positive. For CK17, 60 % of CHCs were positive, while all HCCs were negative. 80 % of CHCs were positive for AB1 anti-CKs complex, whereas only 50 % of HCCs were positive. Thus, CKs and CEA might be considered helpful, in addition to other diagnostic criteria, for the differential diagnosis of primary carcinomas of the liver, especially in difficult cases.
10.13 Other Molecular Markers
Other markers for cholangiocarcinoma that showed alterations are DNA repair proteins and repair defects such as Methyguanine methyl transferase [39], mismatch repair proteins MSH2, MLH1 [40, 41], and RAD51 associating protein-1 [42], oxo-dihydro-dG [43], nitrative and oxidative DNA damage [44], hTERT mRNA [45], microsatellite instability, stem cell factor and c-Kit [46], Cox-2 and PE2 [47], other epigenetic alteration [48], hedgehog ligand [49], Galectin-3 [50], Maspin and Bax [51], p27 [52], TGF-beta type II receptor [40], angiogenesis and lymphangiogenesis [5]. However, Glycine-N-methyltransferase was shown to be a favorable factor for cholangiocarcinoma [53].
10.14 Chromosomal Alteration
In intrahepatic cholangiocarcinoma, losses of heterozygosity at chromosomal loci 3p13-p21, 5q35-qter, 8p22, 17p13, and 18q have been reported [8]. These chromosomal alterations may contain other unidentified proto-oncogenes or tumor suppressor genes.
10.15 Serum Tumor Markers
For non-invasive diagnostic tests of cholangiocarcinoma, blood tests are probably the best at this juncture in time [54–56]. Serum biochemical tests usually support the clinical suspicion of CC but they are rarely diagnostic. Jaundice occurs if there is obstruction of the right and left hepatic ducts or the common bile duct. In these circumstances, elevation of serum levels of bilirubin and markers of biliary epithelial injury, such as alkaline phosphatase (ALP) and gamma glutamyltransferase (GTT) are common [56, 57]. However, in the presence of unilateral intrahepatic biliary obstruction, elevation of ALP or GTT may be present without any increase in the serum bilirubin level. Other abnormal laboratory findings include hypo-albuminemia and prolonged prothrombin time, which reflect the combination of diminished hepatic synthetic function, cachexia and malabsorption of vitamin.
Other tumor markers may support the diagnosis of CC, although none of them is sensitive enough to be used for screening purposes. The commonly used markers are carbohydrate antigen (CA19-9), carcinoembryonic antigen (CEA) and CA-125. CA19-9 is the most useful of these three [57–61]. CA19-9 is frequently upregulated in pancreatobiliary neoplasia. However, it may also be elevated in patients with jaundice due to biliary obstruction, but in the absence of a tumor, and in other non-hepato-pancreatico-biliary conditions. Thus, these tumor markers are not very specific as they can be elevated in the presence of other malignancies (e.g. pancreas and stomach) and with benign conditions such as cholangitis and hepatolithiasis. Serum CA19-9 levels above 100 U/ml in patients without PSC have a sensitivity of 53 % and a specificity of 75–90 % for the diagnosis of CC. In patients with PSC, serum CA19-9 levels above 100 U/ml have a sensitivity of 75–89 % and a specificity of 80–86 % for the diagnosis of CC. In a recent study the optimal cutoff value for serum CA19-9 in patients with PSC was 20 U/ml which provided a sensitivity of 78 %, a specificity of 67 %, a positive predictive value of 23 % and a negative predictive value of 96 % [60]. Nevertheless, serum CA19-9 combined with either ultrasonography, computed tomography, or magnetic resonance imaging provided a sensitivity of 91, 100 and 96 % respectively for CC diagnosis. The levels of CA19-9 appear to correlate with the stage of the disease. It was reported that the sensitivity of CA19-9 above 100 U/ml for the diagnosis of CC in patients with resectable tumors was 33 % compared to 72 % in patients with unresectable tumors [58]. Using more than one tumor marker for patients with PSC may improve the detection rate of CC. Thus, CA19-9 and CEA are helpful devices in the management of gastrointestinal malignancies and belong to clinical routine in surgical oncology. The validity of these parameters in terms of tumor extension and prognosis of bile duct malignancies still remains unclear. From 1998 to 2008, preoperative CA19-9 and CEA serum levels in 136 patients with hilar cholangiocarcinoma were obtained. In another correlative study, the tumor stage, resectability rate and survival were correlated with preoperative CA19-9 and CEA serum levels. CA19-9 and CEA levels increased significantly with rising tumor stages. Patients with pre-operative serum levels of CA19-9 (>1,000 U/ml) and CEA (>14.4 ng/ml) showed a significant poorer resectability rate and survival than patients with lower CA19-9 and CEA serum levels respectively. CA19-9 and CEA serum levels were associated with the tumor stage. If preoperatively obtained CA19-9 and CEA serum levels were highly elevated patients had an even worse survival and the frequency of irresectability was significantly higher. Several new markers are currently being investigated. The human mucin five, subtypes A and C (MUC5AC) are the most promising for future clinical use with a sensitivity and specificity of 71 and 90 %, respectively [62–64]. MMPs are also potentially useful serum makers for CC [65]. Alpha-fetal-protein is known to be a useful serum marker for HCC, but it can be expressed in intrahepatic cholangiocarcinoma as well [66, 67], which suggests probable cancer stem cell origin [67]. Figure 10.13 shows the correlation of CA19-9 with bilirubin and the sensitivity and specificity. Figure 10.13a is a plot of total bilirubin versus CA19-9 for benign diseases, while Fig. 10.13b is a plot of total bilirubin versus CA19-9 for malignant diseases. Figure 10.13c is a plot of sensitivity versus 1-Specificity for CA19-9 [60]. These data indicate that CA19-9 is positively correlated with bilirubin in benign diseases while it is randomly distributed in malignant diseases.
Serum tumor marker CA19-9 in cholangiocarcinoma. (a) Plot of total bilirubin versus CA19-9 for benign diseases. (b) Plot of total bilirubin versus CA19-9 for malignant diseases. (c) Plot of sensitivity versus 1-Specificity for CA19-9 [60]
10.16 Molecular Markers as Target of Therapy
For therapy of cholangiocarcinoma, complete surgical resection is the only curative approach. This can be accomplished only in a minority of patients, since most of them present with an advanced disease. In addition, those patients who have undergone complete surgical resection experience a high tumor recurrence rate. Non-resectable biliary tract cancer is associated with a poor prognosis due to resistance of the tumor to chemotherapy agents and radiotherapy. It is essential to search for new therapeutical approaches. Clinical study data with molecular therapy are now starting to be available for this tumor [68–73]. Inhibitors of the epidermal growth factor receptor (EGFR) family, such as erlotinib, cetuximab, and lapatinib were recently investigated [68]. Furthermore, bortezomib, an inhibitor of proteasome, imatinib mesylate, an inhibitor of c-kit-R, bevacizumab, an inhibitor of vascular endothelial growth factor (VEGF), and sorafenib (BAY 43-9006), a multiple kinase inhibitor that blocks not only receptor tyrosine kinases but also serine/threonine kinases along the RAS/RAF/MEK/ERK pathway, have been tried. Although early evidence of antitumor activity was seen, the results are still too early and require further investigations. Another report indicated that biliary cancers overexpress epidermal growth factor receptor (EGFR), and angiogenesis has been correlated with a poor outcome. Erlotinib, an EGFR tyrosine kinase inhibitor, and bevacizumab, a vascular endothelial growth factor (VEGF) inhibitor have been shown to have activity in biliary cancer. Patients with advanced cholangiocarcinoma or gallbladder cancer were treated with bevacizumab (5 mg/kg) and erlotinib (150 mg). In 53 eligible patients, 6 had a confirmed partial response while stable disease was documented in another 25 patients (51 %) [69]. The median overall survival (OS) was 9.9 months, and the time to progression (TTP) was 4.4 months. Combination chemotherapy with bevacizumab and erlotinib showed clinical activity with infrequent adverse effects. Thus, the combination of bevacizumab and erlotinib may be a therapeutic alternative in patients with advanced biliary cancer.
10.17 Conclusion
Cholangiocarcinomas are epithelial neoplasms that originate from cholangiocytes. They can occur at any level of the biliary tree and they are classified into intrahepatic tumors, (extrahepatic) hilar tumors and (extrahepatic) distal bile duct tumors. A better understanding of the predispositions, risk factors and the molecular pathways for cholangiocarcinoma development will provide new insights in the management of this cancer. The environmental factors and genetic/epigenetic factors can be eliminated, neutralized or avoided. The diagnosis can be established much earlier and accurately with new molecular markers and improved non-invasive imaging. Advanced cytological and chromosomal analysis may aid early diagnosis. Biological therapy basing on the molecular markers discovered (including proto-oncogenes and tumor suppressor genes) may be very useful for patients with unresectable cholangiocarcinoma. These, together with neoadjuvant chemo-irradiation, can be used as therapeutic alternatives in patients with advanced or recurrent biliary cancers.
References
Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol. 2007;19:615–7.
Malhi H, Gores GJ. Review article: the modern diagnosis and therapy of cholangiocarcinoma. Aliment Pharmacol Ther. 2006;23:1287–96.
Aljiffry M, Walsh MJ, Molinari M. Advances in diagnosis, treatment and palliation of cholangiocarcinoma: 1990–2009. World J Gastroenterol. 2009;15:4240–62.
Meza-Junco J, Montano-Loza AJ, Ma M, et al. Cholangiocarcinoma: has there been any progress? Can J Gastroenterol. 2010;24:52–7.
Thelen A, Scholz A, Weichert W, et al. Tumor-associated angiogenesis and lymphangiogenesis correlate with progression of intrahepatic cholangiocarcinoma. Am J Gastroenterol. 2010;105:1123–32.
Karlsen TH, Schrumpf E, Boberg KM. Genetic epidemiology of primary sclerosing cholangitis. World J Gastroenterol. 2007;13:5421–31.
Shen WF, Zhong W, Xu F, et al. Clinicopathological and prognostic analysis of 429 patients with intrahepatic cholangiocarcinoma. World J Gastroenterol. 2009;15:5976–82.
Rashid A. Cellular and molecular biology of biliary tract cancers. Surg Oncol Clin N Am. 2002;11:995–1009.
Chan JY, Wang Z. Tumor markers. In: Lau WY, editor. Hepatocellular carcinoma. Singapore: World Scientific Publishing Co; 2008. p. 159–82.
Chan JY, Lee KKH, Chui YL, et al. Molecular aspects. In: Lau WY, editor. Hepatocellular carcinoma. Singapore: World Scientific Publishing Co; 2008. p. 243–78.
Perumal V, Wang J, Thuluvath P, et al. Hepatitis C and hepatitis B nucleic acids are present in intrahepatic cholangiocarcinomas from the United States. Hum Pathol. 2006;37:1211–6.
Chen RF, Li ZH, Zou SQ, et al. Effect of hepatitis C virus core protein on modulation of cellular proliferation and apoptosis in hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2005;4:71–4.
Zhou H, Wang H, Zhou D, et al. Hepatitis B virus-associated intrahepatic cholangiocarcinoma and hepatocellular carcinoma may hold common disease process for carcinogenesis. Eur J Cancer. 2010;46:1056–61.
Khan SA, Thomas HC, Toledano MB, et al. p53 Mutations in human cholangiocarcinoma: a review. Liver Int. 2005;25:704–16.
Liu XF, Zhang H, Zhu SG, et al. Correlation of p53 gene mutation and expression of P53 protein in cholangiocarcinoma. World J Gastroenterol. 2006;12:4706–9.
Tullo A, D’Erchia AM, Honda K, et al. New p53 mutations in hilar cholangiocarcinoma. World J Surg. 2004;28:995–1000.
Caca K, Feisthammel J, Klee K, et al. Inactivation of the INK4a/ARF locus and p53 in sporadic extrahepatic bile duct cancers and bile tract cancer cell lines. Int J Cancer. 2002;97:481–8.
Klump B, Hsieh CJ, Dette S, et al. Promoter methylation of INK4a/ARF as detected in bile-significance for the differential diagnosis in biliary disease. Clin Cancer Res. 2003;9:1773–8.
Kang YK, Kim WH, Jang JJ. Expression of G1-S modulators (p53, p16, p27, cyclin D1, Rb) and Smad4/Dpc4 in intrahepatic cholangiocarcinoma. Hum Pathol. 2002;33:877–83.
Taniai M, Higuchi H, Burgart LJ, et al. p16INK4a promoter mutations are frequent in primary sclerosing cholangitis (PSC) and PSC-associated cholangiocarcinoma. Gastroenterology. 2002;123:1090–8.
Tannapfel A, Benicke M, Katalinic A, et al. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut. 2000;47:721–7.
Liew CT, Li HM, Lo KW, et al. High frequency of p16INK4A gene alterations in hepatocellular carcinoma. Oncogene. 1999;18:789–95.
Zheng J, Zhu YM. Expression of c-erbB-2 proto-oncogene in extrahepatic cholangiocarcinoma and its clinical significance. Hepatobiliary Pancreat Dis Int. 2007;6:412–5.
Nakazawa K, Dobashi Y, Suzuki S, et al. Amplification and overexpression of c-erbB-2, epidermal growth factor receptor, and c-met in biliary tract cancers. Hum Pathol. 2003;34:902–10.
Treekitkarnmongkol W, Suthiphongchai T. High expression of ErbB2 contributes to cholangiocarcinoma cell invasion and proliferation through AKT/p70S6K. World J Gastroenterol. 2010;16:4047–54.
Yoshikawa D, Ojima H, Iwasaki M, et al. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–25.
Guedj N, Zhan Q, Perigny M, et al. Comparative protein expression profiles of hilar and peripheral hepatic cholangiocarcinomas. J Hepatol. 2009;51:93–101.
Tada M, Omata M, Ohto M. High incidence of ras gene mutation in intrahepatic cholangiocarcinoma. Cancer. 1992;69:1115–8.
Kiba T, Tsuda H, Pairojkul C, et al. Mutations of the p53 tumor suppressor gene and the ras gene family in intrahepatic cholangiocellular carcinomas in Japan and Thailand. Mol Carcinogen. 1993;8:312–8.
Tsuda H, Satarug S, Bhudhisawasdi V, et al. Cholangiocarcinomas in Japanese and Thai patients: difference in etiology and incidence of point mutation of the c-Ki-ras proto-oncogene. Mol Carcinogen. 1992;6:266–9.
Chen CY, Shiesh SC, Wu SJ. Rapid detection of K-ras mutations in bile by peptide nucleic acid-mediated PCR clamping and melting curve analysis: comparison with restriction fragment length polymorphism analysis. Clin Chem. 2004;50:481–9.
Sugimachi K, Taguchi K, Aishima S, et al. Altered expression of beta-catenin without genetic mutation in intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900–5.
Zhai B, Yan HX, Liu SQ, et al. Reduced expression of P120 catenin in cholangiocarcinoma correlated with tumor clinicopathologic parameters. World J Gastroenterol. 2008;14:3739–44.
Riener MO, Vogetseder A, Pestalozzi BC, et al. Cell adhesion molecules P-cadherin and CD24 are markers for carcinoma and dysplasia in the biliary tract. Hum Pathol. 2010;41:1558–65.
Yonglitthipagon P, Pairojkul C, Chamgramol Y, et al. Up-regulation of annexin A2 in cholangiocarcinoma caused by Opisthorchis viverrini and its implication as a prognostic marker. Int J Parasitol. 2010;40:1203–12.
Aishima S, Taguchi K, Terashi T, et al. Tenascin expression at the invasive front is associated with poor prognosis in intrahepatic cholangiocarcinoma. Mod Pathol. 2003;16:1019–27.
Jain R, Fischer S, Serra S, et al. The use of Cytokeratin 19 (CK19) immunohistochemistry in lesions of the pancreas, gastrointestinal tract, and liver. Appl Immunohistochem Mol Morphol. 2010;18:9–15.
Stroescu C, Herlea V, Dragnea A, et al. The diagnostic value of cytokeratins and carcinoembryonic antigen immunostaining in differentiating hepatocellular carcinomas from intrahepatic cholangiocarcinomas. J Gastrointestin Liver Dis. 2006;15:9–14.
Koga Y, Kitajima Y, Miyoshi A, et al. Tumor progression through epigenetic gene silencing of O6-methylguanine-DNA methyltransferase in human biliary tract cancers. Ann Surg Oncol. 2005;12:354–63.
Nagai M, Kawarada Y, Watanabe M, et al. Analysis of microsatellite instability, TGF-beta type II receptor gene mutations and hMSH2 and hMLH1 allele losses in pancreaticobiliary maljunction-associated biliary tract tumors. Anticancer Res. 1999;19(3A):1765–8.
Obama K, Satoh S, Hamamoto R, et al. Enhanced expression of RAD51 associating protein-1 is involved in the growth of intrahepatic cholangiocarcinoma cells. Clin Cancer Res. 2008;14:1333–9.
Liengswangwong U, Karalak A, Morishita Y, et al. Immunohistochemical expression of mismatch repair genes: a screening tool for predicting mutator phenotype in liver fluke infection-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2006;12:3740–5.
Thanan R, Murata M, Pinlaor S, et al. Urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine in patients with parasite infection and effect of antiparasitic drug in relation to cholangiocarcinogenesis. Cancer Epidemiol Biomarkers Prev. 2008;17:518–24.
Pinlaor S, Sripa B, Ma N, et al. Nitrative and oxidative DNA damage in intrahepatic cholangiocarcinoma patients in relation to tumor invasion. World J Gastroenterol. 2005;11:4644–9.
Leelawat K, Leelawat S, Ratanachu-Ek T, et al. Circulating hTERT mRNA as a tumor marker in cholangiocarcinoma patients. World J Gastroenterol. 2006;12:4195–8.
Mansuroglu T, Ramadori P, Dudás J, et al. Expression of stem cell factor and its receptor c-Kit during the development of intrahepatic cholangiocarcinoma. Lab Invest. 2009;89:562–74.
Han C, Wu T. Cyclooxygenase-2-derived prostaglandin E2 promotes human cholangiocarcinoma cell growth and invasion through EP1 receptor-mediated activation of the epidermal growth factor receptor and Akt. J Biol Chem. 2005;280:24053–63.
Tischoff I, Wittekind C, Tannapfel A. Role of epigenetic alterations in cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2006;13:274–9.
Jung Y, McCall SJ, Li YX, et al. Bile ductules and stromal cells express hedgehog ligands and/or hedgehog target genes in primary biliary cirrhosis. Hepatology. 2007;45:1091–6.
Dechaphunkul A, Kanngurn S, Dechsukhum C, et al. The significance of galectin-3 immunohistochemistry, clinical characteristics and liver imaging in differentiating intrahepatic cholangiocarcinoma from adenocarcinoma liver metastasis. J Med Assoc Thai. 2010;93:523–8.
Romani AA, Soliani P, Desenzani S, et al. The associated expression of Maspin and Bax proteins as a potential prognostic factor in intrahepatic cholangiocarcinoma. BMC Cancer. 2006;6:255–8.
Fiorentino M, Altimari A, D’Errico A, et al. Low p27 expression is an independent predictor of survival for patients with either hilar or peripheral intrahepatic cholangiocarcinoma. Mod Pathol. 2001;14:900–5.
Huang YC, Chen M, Shyr YM, et al. Glycine N-methyltransferase is a favorable prognostic marker for human cholangiocarcinoma. J Gastroenterol Hepatol. 2008;23:1384–9.
Nuzzo G, Giuliante F, Ardito F, et al. Intrahepatic cholangiocarcinoma: prognostic factors after liver resection. Updates Surg. 2010;62:11–9.
Zhang F, Chen XP, Zhang W, et al. Combined hepatocellular cholangiocarcinoma originating from hepatic progenitor cells: immunohistochemical and double-fluorescence immunostaining evidence. Histopathology. 2008;52:224–32.
Charatcharoenwitthaya P, Enders FB, Halling KC, et al. Utility of serum tumor markers, imaging, and biliary cytology for detecting cholangiocarcinoma in primary sclerosing cholangitis. Hepatology. 2008;48:1106–17.
Juntermanns B, Radunz S, Heuer M, et al. Tumor markers as a diagnostic key for hilar cholangiocarcinoma. Eur J Med Res. 2010;20(15):357–61.
Patel AH, Harnois DM, Klee GG, et al. The utility of CA 19-9 in the diagnoses of cholangiocarcinoma in patients without primary sclerosing cholangitis. Am J Gastroenterol. 2000;95:204–7.
Hatzaras I, Schmidt C, Muscarella P, et al. Elevated CA 19-9 portends poor prognosis in patients undergoing resection of biliary malignancies. HPB (Oxford). 2010;12:134–8.
Morris-Stiff G, Teli M, Jardine N, et al. CA19-9 antigen levels can distinguish between benign and malignant pancreaticobiliary disease. Hepatobiliary Pancreat Dis Int. 2009;8:620–6.
Furmanczyk PS, Grieco VS, Agoff SN. Biliary brush cytology and the detection of cholangiocarcinoma in primary sclerosing cholangitis: evaluation of specific cytomorphologic features and CA19-9 levels. Am J Clin Pathol. 2005;124:355–60.
Yuan SF, Li KZ, Wang L, et al. Expression of MUC1 and its significance in hepatocellular and cholangiocarcinoma tissue. World J Gastroenterol. 2005;11:4661–6.
Bamrungphon W, Prempracha N, Bunchu N, et al. A new mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC mucin for the diagnosis of cholangiocarcinoma. Cancer Lett. 2007;247:301–8.
Boonla C, Sripa B, Thuwajit P, et al. MUC1 and MUC5AC mucin expression in liver fluke-associated intrahepatic cholangiocarcinoma. World J Gastroenterol. 2005;11:4939–46.
Leelawat K, Sakchinabut S, Narong S, et al. Detection of serum MMP-7 and MMP-9 in cholangiocarcinoma patients: evaluation of diagnostic accuracy. BMC Gastroenterol. 2009;9:30–3.
Zhou YM, Yang JM, Li B, et al. Clinicopathologic characteristics of intrahepatic cholangiocarcinoma in patients with positive serum a-fetoprotein. World J Gastroenterol. 2008;14:2251–4.
Ishikawa K, Sasaki A, Haraguchi N, et al. A case of an alpha-fetoprotein-producing intrahepatic cholangiocarcinoma suggests probable cancer stem cell origin. Oncologist. 2007;12:320–4.
Wiedmann MW, Mössner J. Molecular targeted therapy of biliary tract cancer–results of the first clinical studies. Curr Drug Targets. 2010;11:834–50.
Lubner SJ, Mahoney MR, Kolesar JL, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol. 2010;28:3491–7.
Qun W, Tao Y. Effective treatment of advanced cholangiocarcinoma by hepatic arterial infusion chemotherapy combination with sorafenib: one case report from China. Hepatogastroenterology. 2010;57:426–9.
Francis H, Alpini G, DeMorrow S. Recent advances in the regulation of cholangiocarcinoma growth. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1–9.
Tonini G, Virzì V, Fratto ME, et al. Targeted therapy in biliary tract cancer: 2009 update. Future Oncol. 2009;5:1675–84.
Zhang Z, Oyesanya RA, Campbell DJ, et al. Preclinical assessment of simultaneous targeting of epidermal growth factor receptor (ErbB1) and ErbB2 as a strategy for cholangiocarcinoma therapy. Hepatology. 2010;52:975–86.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht and People's Medical Publishing House
About this chapter
Cite this chapter
Chan, J.Y.H., Lee, K.K.H., Chui, Y.L. (2013). Molecular Markers of Cholangiocarcinoma. In: Lau, W. (eds) Hilar Cholangiocarcinoma. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-6473-6_10
Download citation
DOI: https://doi.org/10.1007/978-94-007-6473-6_10
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-6472-9
Online ISBN: 978-94-007-6473-6
eBook Packages: MedicineMedicine (R0)