Summary
Chloroplast development describes the life cycle of plastids from the proplastid to the mature chloroplast, which is subsequently transformed to a gerontoplast and finally to a necrotic plastid. Similar to any living system, the chloroplast may be defined as an open thermodynamic system far away from equilibrium. It has self-organized dissipative structures, namely, metabolome and genome, which fluctuate with development. The proplastid grows to become a mature chloroplast with self-organizing metabolic networks consisting of core, plastic, and signaling subsystems. The major function of the chloroplast is photosynthesis. Light induces redox reactions resulting finally into the synthesis of sugars. The photoelectron transport systems and sugars are not only two components of the core metabolic network, but these are also elements of signaling subsystems. The signaling regulatory and metabolic networks associated with chloroplast development are complex in nature and therefore are not fully understood. Many experimental data in the area remain to be explained without ambiguity. Examination of chloroplast development with respect to time, structure and fluctuations under the lens of non-equilibrium thermodynamics may contribute to our understanding of the process.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
I. Introduction
The chloroplast is an important organelle in mesophyll tissues of plants. It absorbs solar radiation thereby capturing energy for plants as well as other living systems on the planet Earth. Therefore, the study of structure and function of chloroplasts has become one of the thrust areas of research in plant science. Structure and function of chloroplasts can be well understood through the study of development of the organelle. The transformation of proplastids to chloroplasts and subsequently chloroplasts to gerontoplasts (senescing chloroplasts) are linked to leaf development and senescence (Taylor 1989; Kutik 1998). These transformations during development find their expression in the change in relative concentrations of green and yellow pigments in the organelle and changes in the color of leaves from almost colorless to green to yellow and finally to reddish brown. The colorful panorama of landscapes along with the seasonal changes serves as a feast to the eyes of the beholder (Fig. 2.1). The aesthetic sense associated with an important change on Earth’s surface not only inspires authors and artists but also greatly enhances the inquisitiveness among the researchers to translate the overwhelming emotions into the scientific explanation of the phenomena and their implications on the planet Earth (Fig. 2.2). The importance of this study is reflected in the accumulation of a large number of research papers in the area along with reviews discussing several dimensions of development of chloroplasts. Most of the reviews on organelle development focus on morphology, biochemistry, metabolism, and genetics (see e.g., Thomson and Whatley 1980; Bauer et al. 2001; Biswal et al. 2003; Stern et al. 2004; Biswal 2005; López-Juez and Pyke 2005; Møller 2005; Kessler and Schnell 2009; Inaba and Ito-Inaba 2010; Adam et al. 2011; Nickelsen et al. 2011; Pogson and Albrecht 2011). The tools and databases in the post-genomic era have provided an elaborate and deep insight into the molecular scenario of the development of the organelle from the proplastid to the gerontoplast. Metabolic networks, gene expression, cross-talk between nuclear and plastid genomes and signaling processes are targets of study to understand the developmental process at the molecular level. However, the role of the chloroplast is not limited to photosynthesis and production of metabolites; it further extends to numerous aspects of plant growth and development as a whole (López-Juez and Pyke 2005; López-Juez 2007; Van Doorn and Yoshimoto 2010). Further, development of chloroplasts influences embryogenesis, leaf development, reproductive systems, stress response, and plant-microbe interactions (Inaba and Ito-Inaba 2010). In addition to photosynthesis, the impact of chloroplast development reaches out to the biotic and abiotic systems of the planet Earth. Biological tools are not sufficient to understand the wide pervading effect of chloroplast development. The study of chloroplast development needs a wide angle lens, which can include the entire planetary system. One such discipline is thermodynamics. So far, little effort has been made to review the chloroplast development from the perspective of thermodynamics. An attempt is made in this chapter to consolidate and interpret the reports on chloroplast development in terms of dissipative structures and fluctuations in the thermodynamic time-space.
World map showing the fraction of absorbed photosynthetically active radiation (FAPAR) in April 2006 (a), and September 2005 (c) (From http://fapar.jrc.ec.europa.eu; Gobron et al. 2006). Panorama of deciduous forest landscapes in autumn (b) and in spring (d) (http://en.wikipedia.org/wiki/File:Aerial_View_of_Autumn_Forest_Colors.jpg (b); http://en.wikipedia.org/wiki/File:Spring_Forest_Leaves_in_Texas_Hill_Country.jpg (d)). The green color of the vegetation manifests the green color of chlorophylls in the leaves. The colorful strokes in the landscape reflect the colors of degraded chlorophyll species and dominating carotenoid pigments in senescing or dead leaves.
Representation of Gaia (planet Earth). Energy fluxes, biotic and abiotic components, self-referential heat engines, information and communication processes, and the causal chain of entropic processes along the hierarchy scale of the energetic gradient are depicted (modified after Herrmann-Pillath 2011).
A. Biosystem: A System in Thermodynamics
Living organisms appear to be far away from physical and chemical inanimate systems. However, when we put a biosystem into the frame of thermodynamics, the hard boundary between animate and inanimate worlds melts down. A biosystem is as good as any other physico-chemical system. It is an open thermodynamic system away from the equilibrium. A biosystem exchanges mass and energy with its environment. Hence, thermodynamically it is an open system. Moreover, the fluxes of matter and energy take it to a non-equilibrium region. A system in the non-equilibrium region maintains gradients away from the equilibrium. The system resists and dissipates externally applied gradients; as a result it moves away from the equilibrium. Hence order emerges from disorder in the formation of dissipative structures (Prigogine 1967).
A self-organized structure is supposed to have the following characteristics (Pulselli et al. 2009):
-
1.
A boundary that physically separates a system from its environment. The boundary can generate reactant gradients and flows. Compartmentalization within the plasmamembrane is an important characteristic of higher life. Internal compartments caused by sub-cellular organelles, e.g., mitochondria and plastids, are the main contributors to the characteristic low entropy of living cells (Marín et al. 2009). The entropy associated with the compartmentalization of components in eukaryotic cells, as a function of cell and compartment volumes, and of the concentration of solutes, has been estimated to be approximately −14.4 × 10−14 J K−1 cell−1 (−0.7 J K−1 L−1) in the case of Saccharomyces cerevisiae, a typical eukaryotic cell, and approximately −49.6 × 10−14 J K−1 cell−1 (−1.0 J K−1 L−1) in the more complex cell of Chlamydomonas reinhardtii (Marín et al. 2009). Compartmentalization is believed to be an essential development that significantly decreases the entropy of living cells during biological evolution in comparison to other potential contributing factors, e.g., the informational entropy of DNA and the conformational entropy of proteins (Marín et al. 2009).
-
2.
Inflow-outflow of energy, matter and information: The system must be open and exist along with an environment, far from equilibrium (Prigogine 1978; Pulselli et al. 2009).
-
3.
Other characteristics include non-linear interactions, e.g., feedbacks, autocatalysis or autoinhibiting loops between the elements within the system (Prigogine 1978; Pulselli et al. 2009).
As the system moves away from equilibrium, it takes advantage of all available means to resist externally applied gradients and emerges as a highly ordered system at the cost of increasing disorder at the higher level in the system’s hierarchy (Schneider and Kay 1994).
Boltzman (1886) realized that the solar energy gradient drives the living process and stated:
“The general struggle for existence of animate beings is therefore not a struggle for raw materials –these, for organisms, are air, water and soil, all abundantly available – nor for energy which exists in plenty in anybody in the form of heat (albeit unfortunately not transformable), but a struggle for entropy, which becomes available through the transition of energy from the hot sun to the cold earth.”
Schrödinger (1944) suggested that an organism stays alive by importing energy from the environment and degrading it to maintain the organizational structure of the system:
“Thus the device by which an organism maintains itself stationary at a fairly high level of orderliness (= fairly low level of entropy) really consists in continually sucking orderliness from its environment. …plants can still make use of it. (These, of course have their most powerful supply of ‘negative entropy’ in the sunlight.)”
Biosystems utilize free energy for metabolism and to do work (grow, move, reproduce, etc.). The most common sources of free energy are geologic chemical compounds (chemotrophs), direct sunlight (phototrophs), or organic material (heterotrophs) (Kleidon and Lorentz 2005). Thus biosystems are far from equilibrium dissipative systems and have great potential for reducing radiation gradients on Earth (Kay 1984; Ulanowicz and Hannon 1987).
According to the Gaia concept, the “Earth” system is considered to be an integrated complex system, within which the living subsystem evolves in a way to maintain the biogeochemical, climatic, and other physical conditions necessary for its viability (Lovelock and Margulis 1974; Lovelock 1990). A Gaia system is depicted in Fig. 2.2 with biotic and abiotic components and energy flux. Photosynthesis serves as a major process to funnel energy flux into the Gaia. The original concept suggests that the Gaia system maintains homeostasis at the equilibrium. However, it is observed that Gaia is a non-equilibrium system evolving its own self-organized structures. The Earth system is a non-equilibrium system, which is driven by external energy flux from the solar radiation and by the endogenous processes that have resulted from the emergence of life on Earth (Kleidon 2009, 2010a, b). The non-equilibrium systems follow the path of maximum entropy production (MEP) to reach a steady state. It is the export of entropy that matters, not the status of the exporting system. The entropy of the system decreases at the cost of an increase in the entropy of the environment (Dewar 2003; Kleidon 2010a, b; Herrmann-Pillath 2011). Two entropic time arrows, distinguishable from the general entropic time arrow of the universe have been proposed by Sabater (2009): one for survival of biological systems and the other for aging and evolution. However, there are several reports suggesting the MEP principle, which governs the cosmos, as the doctrine for evolution and survival of the biosystems (Dewar 2003; Kleidon 2010a, b; Herrmann-Pillath 2011). Hence, the time arrows associated with survival, aging and evolution of biosystems are integral parts of cosmological time arrows. Kauffman (2000) wrote:
“Biospheres and the universe create novelty and diversity as fast as they can manage to do so without destroying the accumulated propagating organization that is the basis and nexus from which further novelty is discovered and incorporated into the propagating organization.”
The above statement is referred to as Kauffman’s version of the so-called fourth law of thermodynamics. It explains the accumulation and build up of information to a higher order of complexity as the system evolves.
Life is viewed as the emergence of structures which increase the speed and efficacy of entropy production. This view conforms to non-equilibrium thermodynamics. It also adds to the perspective of information and communication among the elements of the system (Herrmann-Pillath and Salthe 2011). It provides scope to understand the correlation between the dissipation of energy and the accumulation of information.
All systems, biotic or abiotic, evolve through a spontaneous conversion of environmental potential energy into some less available form – “heat”, according to the general principles of thermodynamics (Schrödinger 1944; Kleidon 2004). Such a system is known as heat engine or self-referential heat engine (Herrmann-Pillath 2011). An evolving system and its environment, separated by a permeable interface (membrane in case of a living system) at constant temperature (TS) and pressure (P) constitute a simple self-referential heat engine (Garrett 2011). The interface maintains a Gibb’s energy potential, ΔG (TS, P), so that the system as a whole is able to convert available energy into work (W) within a time frame (t) at a rate w,
where, a is the rate of consumption of energy to do work at a rate w, α is an engine-specific constant coefficient.
The efficiency, ε, is given by
The heat (Q) produced at a rate (a – w) is transferred to the colder surroundings at temperature T. The system with positive feedback loop in which, through work, a and ΔG evolve logarithmically at rate
where, η can be considered as a feedback efficiency or rate of return.
B. The Chloroplast System
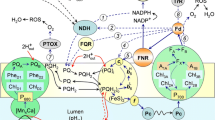
Chloroplasts are responsible for transducing the majority of the energy flux into the Gaia (planet Earth). They absorb light and convert light energy into chemical energy by photoredox reactions. The reactions result in the production of oxygen from water, synthesis of ATP, and reduction of NADP to NADPH. The chemical potential thus stored in these chemical species is utilized to carry out several biological functions: biosynthesis of sugars from carbon dioxide via e.g., Calvin-Benson cycle. A chloroplast has a double membrane envelope as boundary that separates it from its environment (Fig. 2.3). It is an open, non-equilibrium thermodynamic system with high order of self-organization. There is exchange of materials, namely, biomolecules, oxygen, and carbon dioxide across the envelope. Energy in form of heat and light also gets exchanged. The chloroplast has a life cycle that is well programmed and controlled by the plastid genome as well as the nuclear genome. Thus a chloroplast is a living system in miniature.
Diagram depicting a chloroplast as an open thermodynamic system. The double membrane envelope acts as a boundary of the system. The system exchanges mass for example biomolecules, carbon dioxide, oxygen, and water with the environment. Energy exchange with the environment takes place in the form of light and heat predominantly. Metabolic network (self-organization) in the system derives order from disorder and genome is responsible for perpetuating order from order.
II. Chloroplast Development
A. Stages of Chloroplast Development
The changes in the structure and metabolism of the plastid with time are accompanied by changes in the thermodynamic parameters, e.g., free energy and entropy. Appearance of structural patterns, specific signaling systems, selective gene expression, and other biochemical events are associated with chloroplast development. The thermodynamic characteristics of proplastids, mature, and aging chloroplasts, are discussed here. The structural changes are schematically shown in Figs. 2.4 and 2.5. The development of the proplastid to the mature chloroplast is a ‘build up’ process with biosynthesis of membrane structure, organization into grana and lumen, assembly of multimeric protein complexes and onset of well regulated electron transport and metabolic processes (Fig. 2.4) (Adam et al. 2011). On the other hand, transformation of mature chloroplasts to gerontoplasts is basically a dismantling process with disruption of membrane organization, disassembly of protein complexes, changes in the metabolism, and degradation of cellular molecules including pigments, proteins, and lipids.
Diagram of the structural changes during development of self-organized structures in proplastid. Biosynthesis of proteins, pigments, lipids and their organization to orderly structures, namely thylakoid membranes, and metabolic networks, are shown in the figure. The self-organization is perturbed during senescence and the metabolic network disappears upon dissolution.
Schematic representation of the movement of biomass in the form of nutrients from proplastid to chloroplasts. Then, the nutrients are mobilized to gerontoplasts and finally to the seeds. The seeds upon germination give rise to transformation of proplastid to chloroplasts in the seedlings. Seedlings grow into plants with mature chloroplast containing leaves. These leaves undergo senescence and from gerontoplasts nutrients are transported to seeds. Thus, indirectly gerontoplasts transfer mass to a new system, the proplastid, which builds up order from disorder while the gerontoplast itself heads toward complete disorder-death. The cycle of appearance-sustenance-disappearance continues.
B. Characteristics of Well Defined States of Chloroplasts
The development of the chloroplast involves a chain of transformations of the organelle from a proplastid to the photosynthetically active mature chloroplast, then to a gerontoplast, and finally to complete degradation of the plastid. These structurally different plastid forms also have distinct physiology. The above transformations are preprogrammed, well-regulated genetic and metabolic events. However, beyond the stage of the gerontoplast, there is complete degradation with point of no return that results in dissolution of the plastid.
1. Structure and Organization
Proplastids are located in embryonic tissues and meristems. They are small (0.5–1.0 μm in diameter) double membrane compartments, which enclose nucleic acids, proteins, lipids, precursors of internal membrane structures, a few starch grains and plastoglobuli in the stroma (Thomson and Whatley 1980). As noted above, proplastids differentiate into mature plastids.
A mature green plastid, the chloroplast, is structurally and functionally a well organized organelle (Catsky and Sestak 1997). A membrane system, composed of thylakoids, is dispersed in the chloroplast. A fully developed thylakoid membrane system organizes at places into stacked membranous structures called grana. Unstacked thylakoid membranes are called stromal lamellae. There are four major protein complexes constituting the electron transport system located in the thylakoid lipid bilayer (Fig. 2.4). These complexes are PSI, PSII, Cyt b/f complex, and ATP synthase (CF1 CF0). Each complex consists of several protein subunits, some of which are encoded by the nuclear genome and the rest by the plastome (Goldschmidt-Clermont 1998). The carbon dioxide fixing enzyme system is located in the stroma.
A mature chloroplast undergoes structural and functional changes during senescence of green leaves. The changes result in differentiation of a chloroplast into a gerontoplast. Gerontoplasts are smaller in size compared to chloroplasts. Unstacked and degraded thylakoid membranes, loss of photosynthetic pigments, a large number of plastoglobuli with lipophilic materials, a few starch grains, and an intact double layer envelope membrane are structural features of the gerontoplast (Biswal and Biswal 1988; Biswal 2005). A gerontoplast develops from a mature chloroplast without any further growth and it undergoes gradual loss of biosynthetic potential (Thomas et al. 2001, 2003).
A gerontoplast can regenerate to form a chloroplast under certain conditions through a reverse pathway different from the forward one (Smart 1994; Thomas 1994; Zavaleta-Mancera et al. 1999a, b; Van Doorn and Yoshimoto 2010, see also Fulgosi et al., Chap. 26). During development of a gerontoplast the regeneration capability is limited to a threshold of degeneration, the so-called point of no return. The gerontoplast then loses the structural integrity and dissolution of the plastid occurs.
2. Metabolism
Metabolic networks in a biosystem are mathematically described by sets of catalytic elements connected with the substrate fluxes and signals. Each catalytic element belongs to a self-organized set of enzymes (De la Fuente et al. 1999; De la Fuente 2010). These catalytic sets of enzymes are designated as metabolic subsystems. They may exhibit oscillatory and stationary activity patterns (De la Fuente et al. 1999). Global metabolic structures are able to self-organize spontaneously. They are characterized by a set of different active enzymes known as metabolic cores. Other metabolic subsystems exhibit structural plasticity and have conditional dynamic on-off switch states. The global metabolic structure could be present in all living cells (De la Fuente et al. 1999; Almaas et al. 2004, 2005; Almaas 2007; De la Fuente 2010). This may also exhibit emergence of chaotic behaviour within it (De la Fuente et al. 2008, 2009, 2010). Chaotic patterns are sensitive to the initial conditions. A small change in the initial state leads to large changes in the later state of the system. The deviations of the chaotic patterns are dependent on the quantum of perturbation of the initial conditions. The chaos, which has long-term correlations, may be advantageous to the biosystem due to fast and specific responses during the adaptation of the metabolic network to environmental changes (De la Fuente 2010). For example, calcium is associated with the regulation of cell metabolism (Berridge 1993). Cytosolic calcium may exhibit chaotic transitions in response to environmental perturbation (Dixon et al. 1995). It not only exhibits fast and specific metabolic responses during the adaptation to environmental perturbations, but it is also associated with long-term memory properties.
Proplastids import biomolecules including proteins. Synthesis of biomolecules also goes on inside the proplastid. Increase in volume, accumulation of mass and organization of structural components are major biological events that occur in proplastids (Biswal et al. 2003).
Photosynthesis is the major function of the chloroplast. Photosynthesis has evolved as a non-linear system where charge separation between special pair chlorophylls (primary electron donor) and another porphyrin molecule (primary electron acceptor) occurs through a branched pathway with very high efficiency (Juretić and Županović 2003). A special chlorophyll molecule is strategically located close to a primary electron acceptor and a primary electron donor. Similar to an electrical circuit, when the net electron current flows, dissipation occurs and steady state affinity or photocell voltage decreases. In the photosynthetic apparatus, the large majority of photons absorbed by reaction centers are utilized in photoelectron transfer. This implies that a photosynthetic system works far from the chemical equilibrium state for the absorbed and emitted photons (Juretić and Županović 2003, 2005). The MEP and the optimal photochemical yield of the system are estimated to be 19.78 kJ mol−1 s−1 K−1 and 0.946, respectively (Juretić and Županović 2003, 2005). Thus photosynthetic proton pumping and electron transport operate close to the MEP mode, which synthesizes ATP and reduces NADP to NADPH at optimal rates. The evolution of the photosynthetic apparatus is guided by the principle of MEP coupling with the thermodynamic evolution of its surrounding universe (Juretić and Županović 2003). It has become possible to measure thermodynamic parameters of the photosynthetic apparatus by photoacoustic devices (see a review by Hou 2011, and references therein). However, proper selection and preparation of samples for obtaining information of the desired system along with its environment for appropriate interpretation are required. Besides, sustenance of photosynthesis by overcoming variations in environmental parameters also remains an important function of the chloroplast. The fluctuations are overcome by maintaining the redox status through photostasis, the balance between the production of NADPH (and ATP) and carbon dioxide fixation. Mathematically the energy balance state of PSII can be expressed as follows (Falkowski and Chen 2003):
where, σPSII is the effective absorption cross-section of PSII; Ek, the irradiance at which the maximum photosynthetic quantum yield balances the photosynthetic capacity; and τ−1, the rate at which the photosynthetic electrons are utilized by the sink.
The variation is decoded basically by energy imbalance between the source (determined by the redox state of the chloroplast) and the sink (determined primarily by carbon and nitrogen metabolisms) (Ensminger et al. 2006; Wilson et al. 2006). The changes induced by environmental fluctuations create a signaling system for the regulation of gene expression causing modification in cellular metabolism for photosynthetic adaptation (Ensminger et al. 2006; see the review by Biswal et al. 2011). Maintenance of wear and tear, i.e., damage to proteins, pigments, and the membrane system by biosynthesis is a continuous process. Photosynthesis and maintenance of steady states are two important biological processes that occur in a mature chloroplast.
A decline of photosynthesis marks the transformation of chloroplasts to gerontoplasts. Loss of pigments, unstacking of thylakoid membranes, dissociation of protein complexes in thylakoids, loss of Rubisco and other enzymes involved in the fixation of carbon, imbalance in the redox status, and biosynthesis of senescence inducing proteins are the major metabolic phenomena in gerontoplasts. Import of some of the nuclear-encoded proteins into gerontoplasts suggests that during senescence the envelope membrane is capable of active transport (Kawakami and Watanabe 1993). The gerontoplast is an organelle, which exports nutrients, primarily nitrogen containing molecules, to other actively growing and reproductive parts of the plant (see the review by Biswal et al. 2012).
3. Genetics
Genes regulate the processes of a biosystem and its life-cycle including formation, growth, reproduction, aging, and death. Biosystems go through developmental cycles preserving information about the self-organization processes, which have a high probability of success. This is crucial for the continuation of life (Kay 1984). Biosystems are at the most sophisticated end of the complexity scale. When a new biosystem is generated before the death of an earlier one, the information from the latter to the former is transferred to preserve the self-organization processes with a potential to improve its efficiency. The genes are the databases of successful self-organization processes. Genes constrain the process of self-organization to options with high probability of success while self-organization is the mechanism of development (Schneider and Kay 1995). Formation, growth and steady state of a biosystem away from equilibrium can be described as order from disorder while the continuance of developmental cycles from generation to generation is conceived as order from order. The existence of a biosystem is dependent on both the processes order from disorder to generate life and order from order to ensure the continuance of life (Schneider and Kay 1995).
Development of plastids from chloroplasts to gerontoplasts is neither governed by external or internal physicochemical forces, nor it has option to choose the time of occurrence. Rather, physicochemical forces that facilitate the development are controlled by genes through a well programmed genetic expression along a definite time arrow.
The genetic basis of development of the photosynthetic organelle has been investigated in green algae and higher plants (Ryberg and Sundqvist 1991; Barkan et al. 1995; Mache et al. 1997; Leon et al. 1998; Pyke 1999; Rochaix 2011). These studies showed that at the level of gene expression chloroplast development is regulated similarly in all green organisms.
Plastids have semi-autonomous status. They are regulated by both plastid and nuclear genomes. Plastids possess their own genome with a transcriptional machinery (see Kanamaru and Sugita, Chap. 10 in this volume) and are capable of synthesizing some of their own proteins. However, a large number of different plastid proteins are encoded by the nuclear genome. Many multimeric thylakoid complexes and soluble complexes in the stroma are composed of some components encoded by plastid genes and some by nuclear genes (Goldschmidt-Clermont 1998). Down-regulation of these photosynthesis-associated genes (PAGs) in mature chloroplasts parallels the up-regulation of senescence-associated genes (SAGs) (see reviews by Biswal 1999; Biswal et al. 2003).
III. Thermodynamics of Developmental States of the Chloroplast System
A. Thermodynamic Characteristics of Proplastids: A Simple System
The proplastid is an organelle with less ordered subsystems in stroma but has the facilities for active transport across the double membrane envelope. The proplastids also possess the plastid genome and ribosomes for protein synthesis. The metabolic subsystems are yet to be organized. Active transport and potentiality for biosynthesis of proteins, nucleic acids, fatty acids, and pigments suggest that proplastids have a certain level of order, which is much lower than that of the chloroplast (Fig. 2.4). It is a relatively unstable system, which is transformed to a stable chloroplast on receiving specific signals (fluctuations) (Fig. 2.6).
Schematic representation of structure and fluctuation at different developmental states of the plastid system with arbitrary rate of entropy production (dS/dt) in thermodynamic space. The proplastid is at a relatively unstable state and fluctuations are amplified (signal for chloroplast transformation), which drive it to a steady state. Small fluctuations (stress) in steady state are damped by adaptive responses, and the system oscillates about the stable steady state. However, with aging, fluctuations are amplified (senescence signaling) and the system moves to an unstable state – the gerontoplast. A gerontoplast then degrades to a collapsing system, which is at complete equilibrium. Regeneration signals in gerontoplasts may regenerate the steady state of the chloroplast under special circumstances.
B. From Proplastids to Mature Chloroplasts: Toward Order
During transformation of proplastids to chloroplasts, the organelle becomes more and more self-organized with the appearance of characteristic dissipative structures (Fig. 2.6). Biosynthesis of new molecules and their organization in complexes in both membrane and stroma occur in a precise manner, indicating that building up of chloroplasts is associated with a decrease in entropy of the system.
1. Signals for Plastid Differentiation
Proplastids differentiate in mesophyll tissue to chloroplasts in light. Light activates COP9 signalosome through phytochrome. This signaling system associated with gene expression during chloroplast development regulates expression of both nuclear and plastid genes in different signal transduction pathways. Plastid division, biosynthesis of proteins, import of nuclear proteins, intra-organelle protein targeting, pigment biosynthesis, and assembly of functional protein complexes are the major processes that take place during proplastid differentiation (Waters and Pyke 2005).
2. Self-organization of Subsystems and Biological Control
Proteins accumulated in proplastids interact specifically to form different complexes. Formation of functional protein complexes in the stroma or in the membranes does not occur spontaneously; it is rather governed by physico-chemical interactions. These are endergonic processes governed by biological control (genetic programming) leading to self-organization with MEP. A few specific examples of the biological control during chloroplast development are provided below: PSI assembly in thylakoid membrane starts with insertion of PsaB subunit (Rochaix 2011). It is designated as an anchor subunit. Unless the anchor subunit is inserted into the membrane, other subunits do not assemble. Assembly of other subunits occurs in a definite sequence. PsaA subunit binds to PsaB forming the CPI complex in the next step. Only after formation of the CPI complex, the PsaC subunit is assembled. Subsequently, other subunits assemble to form the PSI complex. Synthesis of PsaA is inhibited in the absence of PsaB subunit. PsaA, in the present case, is designated as “control by epistasy of synthesis” (CES) subunit. PsaC is also a CES subunit. Similarly, in PSII, D2 is an anchor subunit and D1 and CP47 are CES subunits (Kanervo et al. 2007). Protein targeting and events associated with the formation of grana structures are other examples of biological control of chloroplast development. Import of nuclear proteins from the cytosol to the stroma through the chloroplast envelope is regulated by Tic and Toc proteins (Vothknecht and Soll 2005; Andrès et al. 2010; Shi and Theg 2010; Strittmatter et al. 2010; Mulo 2011, see also Ling et al., Chap. 12). Targeting of proteins through the thylakoid membrane to the lumen is accomplished by Sec and SRP proteins (Robinson and Mant 2005). Lipid-protein interaction self-organizes to the complex thylakoid structures (Fig. 2.4). Thylakoid membranes stack at places to form more organized grana structures. Membrane appression at grana decreases the excluded volume. In other words, more space is available for the diffusion of proteins into the stroma. This boosts entropy production and acts as a driving force for grana formation (Chow et al. 2005; Kim et al. 2005). Grana formation has many important implications in photosynthesis, e.g., spatial separation of PSI and PSII, light harvesting, regulation of state transition, cyclic ATP synthesis (Chow et al. 2005). Grana formation results in a higher degree of internal compartmentalization. Hence, it may decrease entropy to a great extent (Marín et al. 2009). A proteolytic machinery also operates during the differentiation of plastids in order to degrade the unstable proteins or the assembly of proteins, where one or more subunits or prosthetic groups are missing (Adam 2005). Further, posttranslational modifications, hydrolysis of the peptide tag after targeting or transport, require proteolytic enzymes. The proteolytic machinery is active in mature chloroplasts for regulating protein assembly (Adam 2005). The process of oxidative damage of D1, proteolysis and de novo replacement is a well known example. During senescence, proteolytic and other hydrolytic machineries are obviously active in dismantling of protein assemblies and conversion of macromolecules into smaller molecules. All these phenomena are under biological control and driven by the MEP principle. The metabolic network is also self-organized in the stroma of plastids (Fig. 2.4). Self-organization occurs in proplastids, which undergo transformation to a state of increased order associated with the MEP. The decrease in entropy of the system is achieved primarily at the expense of cytoplasmic energy sources while entropy of the universe is increased.
Biological control of chloroplast development has evolved through “learning” by the system of the most appropriate pathway and storing it into the “memory” subsystem of the genome. Transformation of order from order, evolving the process down to the generations, is a thermodynamic characteristic of a living system (Kauffman 2000).
3. Entropy of the System
The thermodynamic expression in terms of partial molar properties for this transformation, which occurs in an open system, away from the equilibrium, can be written as
where ΔGi, ΔHi and ΔSi are the changes in partial molar free energy (chemical potential), enthalpy, and entropy, respectively; T is the temperature of the system in Kelvin. The free energy term, ΔGi is not equal to zero because the process is away from the equilibrium. Entropy of the process increases as it is spontaneous. The change in entropy of the system is made up of two components: (a) deS, entropy exchange between the system and the surrounding and (b) diS, entropy change due to changes inside the system. The overall change in entropy (dS) of the system is expressed as
diS = 0 for reversible processes and diS > 0 for irreversible processes; however, diS is never less than zero.
In an open system, there is exchange of energy and mass between the system and the environment. The rate of change of entropy is expressed as
where, Jk is the flux or rate of flow and Xk is the force responsible for such flux.
Entropy production is zero, at equilibrium; the flux (Jk) and the forces (Xk) are zero.
where, Lkk > 0, and Lkk is called phenomenological coefficient for a system close to equilibrium. However, in the case of open systems far from equilibrium, where the thermodynamic forces are considerable, the flux is no longer a linear function of the force (Haase 1968; Yon-Kahn and Hervé 2010). During the developmental phase of the chloroplast from the proplastid, dS/dt < 0, as the system becomes more organized. The component diS/dt is always greater than zero, but the other component deS/dt can be less than zero. Hence, with magnitude of deS/dt (deS/dt < 0) being greater than that of diS/dt, the total rate of production of entropy is negative, i.e., entropy within the chloroplast decreases during the development of the chloroplast.
Transportation and targeting biomolecules to specific sites within chloroplasts during development is associated with positive ΔGi quantity as it is an active, precise and directional process. Hence, these processes must be coupled to a process with large negative ΔGi value. Weak interactions and reversible chemical bond formation can provide necessary negative free energy. Phosphorylation, weak polar and hydrophobic interactions, steric compatibility and charge-charge interaction are a few such processes.
C. Mature Chloroplasts: The Order
A fully developed mature chloroplast is characterized by a steady state with respect to its structure and functions. No gross structural change is observed in a mature chloroplast even though the turnover of proteins and other biomolecules may be high. The biological processes of a chloroplast are in a dynamic steady state within the organelle and with the cytoplasmic environment. The major biological processes occurring in a chloroplast are (1) photoelectron transport; (2) synthesis of sugars through the Calvin-Benson cycle; (3) transport of sugars and other metabolites including phosphoglyceric acid (PGA) and dicarboxylic acids across the chloroplast membrane. The mature organelle maintains a stable steady state.
A chloroplast in a steady state exhibits a decrease in the entropy production and the rate of total entropy production is zero.
At steady state, diS/dt decreases to a minimum and \( {\text{d}}_{\text{i}}^{2}\text{S}/{\text{dt}}^{2}=0\).
The steady state of the chloroplast is maintained and it itself does not come out of the state in spite of the perturbation occurring inside the system. Whenever there are changes in external forces, namely temperature, CO2 concentration in the environment, osmotic pressure, and the intensity of light to perturb the system, the flux opposes these changes by the alterations in the redox state of the system, enzyme activity, and stress induced gene expression. These are known as the adaptational responses, which tend to restore the steady state as far as possible (Le Chatelier’s principle). The flux caused by the perturbation may tend to dampen the perturbation so as to bring the system back to the steady state. Therefore, a chloroplast in non-equilibrium steady state, with dS/dt = 0, resists the stress condition by various stress adaptation mechanisms.
D. Aging of Chloroplasts: Breaking Down the Order
Aging chloroplasts exhibit the breaking down of structural organization and loss in photosynthetic function. Their adaptability weakens. Signaling systems induce the genomic information to perturb the steady state, and the chloroplast is moved to an unstable state – the gerontoplast (Fig. 2.6). The self-organized structures in the unstable gerontoplasts degrade. Degradation of macromolecules to small metabolites including amino acids, fatty acids, sugars and their transport to a new site of the growing cell in the plant are the major processes mediated by the gerontoplast (Lim et al. 2007; Biswal et al. 2012) (Fig. 2.5). Consequently, there is an increase in deS/dt, which becomes positive as the dissipative structures of the system collapse. The rate of production of the total entropy dS/dt becomes positive. However, the envelope is intact and active transport occurs in a gerontoplast. Some elements of order still persist with increasing disorder. There is a scope to recover the dissipative structures, i.e., by transformation of gerontoplast back to chloroplast. The gerontoplast may move to a steady state similar to the mature chloroplast (Fig. 2.6) (Smart 1994; Thomas 1994; Zavaleta-Mancera et al. 1999a, b; Van Doorn and Yoshimoto 2010; Parlitz et al. 2011, see also Fulgosi et al., Chap. 26).
E. Dissolution: The Disorder
The collapsing dissipative structures in gerontoplasts drift the system towards equilibrium. A point of no return is reached on the way to the equilibrium state. The process of lysis proceeds. Metabolic activity, active transport across envelope, and genetic regulation disappear. The organelle reaches equilibrium. There is a complete collapse of dissipative structures. The complete disorder with maximum rate of production of total entropy persists.
Thus, the differentiating states of plastids during development appear to have different thermodynamic characteristics.
IV. Chloroplast Development: Recapitulating the Nature of the Living System
The nature of living systems is observed in chloroplast development. Hence, the observations on chloroplast development could be extrapolated to recapitule the nature of living systems or a self-organized dissipative structure in general.
A. Thermodynamics of Manifestation, Sustenance, and Dissolution
Manifestation of self-organized dissipative structures in proplastids, sustenance of self-organization in chloroplasts, and dissolution of self-organization in gerontoplasts constitute the states of development. Before lysis of plastids, the biomass is transported to newly generated proplastids developing into chloroplasts, and finally the biomass is stored in the seeds, which contain dormant proplastids. Germination of seeds gives rise to chloroplasts developing from proplastids in the cotyledons and leaves of seedlings. A seedling grows to a whole new plant which finally decays after producing seeds. Thus the life cycle of chloroplasts along with plant development continues. The life cycle of chloroplasts in a monocarpic plant is depicted in Fig. 2.5.
V. Conclusions
Chloroplast development is similar to the life cycle of any biosystem. It is an open thermodynamic system, far away from equilibrium and, hence, the principle of non-equilibrium thermodynamic systems could be applied to reveal the unknown aspects of the chloroplast system. However, very few reports are available on the study of the thermodynamic parameters of the chloroplast system. Thermodynamics may be useful in investigating metabolic subsystems – core, plastic and signaling networks (Almaas et al. 2005; De la Fuente et al. 2009; De la Fuente 2010). There are several grey areas in chloroplast development (Biswal et al. 2003, 2012). Many questions in this area still remain unanswered. Thermodynamic principles and mathematical approaches may be applied to answer these questions in the future.
The “state of the art” techniques, namely, photoacoustic devices, microarray, and tandem mass spectroscopy (MS-MS) may be applied for accumulation of experimental data on thermodynamic parameters at different states of development of chloroplasts along with changing proteomes and transcriptomes. The techniques are also useful to study self-organized dissipative structures in the chloroplast in the landscape of non-equilibrium thermodynamics.
Abbreviations
- ATP –:
-
Adenosine 5′-triphosphate;
- CES –:
-
Control by epistasy of synthesis;
- CF0 :
-
– Coupling factor intrinsic component;
- CF1 :
-
– Coupling factor extrinsic component;
- Cyt b/f –:
-
Cytochrome b/f complex;
- MEP –:
-
Maximum entropy production;
- MS –:
-
Mass spectroscopy;
- NADPH –:
-
Nicotinamide adenine diphosphate (reduced);
- PSI –:
-
Photosystem I;
- PSII –:
-
Photosystem II;
- Rubisco –:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
References
Adam Z (2005) The chloroplast proteolytic machinery. In: Møller SG (ed) Plastids, annual plant reviews, vol 13. Blackwell, Oxford, pp 214–236
Adam Z, Charuvi D, Tsabari O, Knopf RR, Reich Z (2011) Biogenesis of thylakoid networks in angiosperms: knowns and unknowns. Plant Mol Biol 76:221–234
Almaas E (2007) Biological impacts and context of network theory. J Exp Biol 210:1548–1558
Almaas E, Kovacs B, Vicsek T, Oltvai ZN, Barabási AL (2004) Global organization of metabolic fluxes in the bacterium Escherichia coli. Nature 427:839–843
Almaas E, Oltvai ZN, Barabasi AL (2005) The activity reaction core and plasticity of metabolic networks. PLoS Comput Biol 1(e68):0557–0563
Andrès C, Agne B, Kessler F (2010) The TOC complex: preprotein gateway to the chloroplast. Biochim Biophys Acta 1803:715–723
Barkan A, Voelker R, Mendel-Hartvig J, Johnson D, Walker M (1995) Genetic analysis of chloroplast biogenesis in higher plants. Physiol Plant 93:163–170
Bauer J, Hiltbrunner A, Kessler F (2001) Molecular biology of chloroplast biogenesis: gene expression, protein import and intraorganellar sorting. Cell Mol Life Sci 58:420–433
Berridge MJ (1993) Inositol triphosphate and calcium signalling. Nature 361:315–325
Biswal B (1999) Senescence associated genes of leaves. J Plant Biol 26:43–50
Biswal B (2005) Formation and demolition of chloroplast during leaf ontogeny. In: Pessarakli M (ed) Handbook of photosynthesis. CRC Press, Boca Raton, pp 109–122
Biswal B, Joshi PN, Raval MK, Biswal UC (2011) Photosynthesis, a global sensor of environmental stress in green plants: stress signalling and adaptation. Curr Sci 101:47–56
Biswal B, Mohapatra PK, Biswal UC, Raval MK (2012) Leaf senescence and transformation of chloroplasts to gerontoplasts. In: Eaton Rye JJ, Tripathy BC, Sharkey TD (eds) Photosynthesis: plastid biology, energy conversion and carbon assimilation, advances in photosynthesis and respiration, vol 34. Springer, Berlin/Heidelberg, pp 217–230
Biswal UC, Biswal B (1988) Ultrastructural modifications and biochemical changes during senescence of chloroplasts. Int Rev Cytol 113:271–321
Biswal UC, Biswal B, Raval MK (2003) Chloroplast biogenesis: from proplastid to gerontoplast. Kluwer, Dordrecht, pp 1–380
Boltzmann L (1886) The second law of thermodynamics (a lecture delivered at the Imperial academy of Sciences in Vienna on 29th May, 1886). In: McGinness B (ed) Ludwig Boltzmann, theoretical physics and philosophical problems (1974). Reidel, New York, pp 13–32
Catsky J, Sestak Z (1997) Photosynthesis during leaf development. In: Pessarakli M (ed) Handbook of photosynthesis. Marcel Dekker, New York, pp 633–660
Chow WS, Kim EH, Horton P, Anderson JM (2005) Granal stacking of thylakoid membranes in higher plant chloroplasts: the physicochemical forces at work and the functional consequences that ensue. Photochem Photobiol Sci 4:1081–1090
De la Fuente IM (2010) Quantitative analysis of cellular metabolic dissipative, self-organized structures. Int J Mol Sci 11:3540–3599
De la Fuente IM, Benítez N, Santamaría A, Aguirregabiria JM, Veguillas J (1999) Persistence in metabolic nets. Bull Math Biol 61:573–595
De la Fuente IM, Martínez L, Pérez-Samartín AL, Ormaetxea L, Amezaga C, Vera-López A (2008) Global self-organization of the cellular metabolic structure. PLoS One 3(e3100):1–19
De la Fuente IM, Vadillo F, Pérez-Pinilla MB, Vera-López A, Veguillas J (2009) The number of catalytic elements is crucial for the emergence of metabolic cores. PLoS One 4(e7510):1–11
De la Fuente IM, Vadillo F, Pérez-Samartín AL, Pérez-Pinilla MB, Bidaurrazaga J, Vera-López A (2010) Global self-regulations of the cellular metabolic structure. PLoS One 5(e9484):1–15
Dewar RC (2003) Information theory explanation of the fluctuation theorem, maximum entropy production, and self-organized criticality in non-equilibrium stationary states. J Phys A 36:631–641
Dixon CJ, Cobbold PH, Green AK (1995) Oscillations in cytosolic free Ca2+ induced by ADP and ATP in single rat hepatocytes display differential sensitivity to application of phorbol ester. Biochem J 309:145–149
Ensminger I, Busch F, Huner NPA (2006) Photostasis and cold acclimation: sensing low temperature through photosynthesis. Physiol Plant 126:28–44
Falkowski PG, Chen YB (2003) Photo acclimation of light harvesting system in eukaryotic algae. In: Green BR, Parson WW (eds) Light harvesting antennas in photosynthesis, advances in photosynthesis and respiration, vol 13. Kluwer, Dordrecht/Boston, pp 423–447
Garrett TJ (2011) Are there basic physical constraints on future anthropogenic emissions of carbon dioxide? Clim Change 104:437–455
Gobron N, Pinty B, Aussedat O, Chen JM, Cohen WB, Fensholt R, Gond V, Lavergne T, Mélin F, Privette JL, Sandholt I, Taberner M, Turner DP, Verstraete MM, Widlowski J-L (2006) Evaluation of fraction of absorbed photosynthetically active radiation products for different canopy radiation transfer regimes: methodology and results using joint research center products derived from SeaWiFS against ground-based estimations. J Geophys Res Atmos 111(13):D13110
Goldschmidt-Clermont M (1998) Coordination of nuclear and chloroplast gene expression in plant cells. Int Rev Cytol 177:115–180
Haase R (1968) Thermodynamics of irreversible processes. Addison Wesley, Reading, pp 1–509
Herrmann-Pillath C (2011) Revisiting the Gaia hypothesis: maximum entropy, Kauffman’s “Fourth Law” and physiosemeiosis. Frankfurt School Working Paper Series No. 160, arXiv: 1102.3338, SSRN: http://ssrn.com/abstract=1762603
Herrmann-Pillath C, Salthe SN (2011) Triadic conceptual structure of the maximum entropy approach to evolution. Biosystems 103:315–330
Hou HJM (2011) Enthalpy, entropy, and volume changes of electron transfer reactions in photosynthetic proteins. In: Mizutani T (ed) Application of thermodynamics to biological and materials science. InTech, Rijeka, pp 93–110
Inaba T, Ito-Inaba Y (2010) Versatile roles of plastids in plant growth and development. Plant Cell Physiol 51:1847–1853
Juretić D, Županović P (2003) Photosynthetic models with maximum entropy production in irreversible charge transfer steps. Comput Biol Chem 27:541–553
Juretić D, Županović P (2005) The free-energy transduction and entropy production in initial photosynthetic reactions. In: Kleidon A, Lorentz R (eds) Non-equilibrium thermodynamics and production of entropy: life, earth, and beyond. Springer, Berlin, pp 161–171
Kanervo E, Suorsa M, Aro EM (2007) Assembly of protein complexes in plastids. In: Bock R (ed) Cell and molecular biology of plastids, vol 19. Springer, Berlin, pp 283–314
Kauffman SA (2000) Investigations. Oxford University Press, Oxford, pp 1–308
Kawakami N, Watanabe A (1993) Translatable mRNAs for chloroplast targeted proteins in detached radish cotyledons during senescence in darkness. Plant Cell Physiol 34:697–704
Kay JJ (1984) Self-organization in living systems. PhD thesis, Systems Design Engineering, University of Waterloo, Waterloo
Kessler F, Schnell D (2009) Chloroplast biogenesis: diversity and regulation of the protein import apparatus. Curr Opin Cell Biol 21:494–500
Kim EH, Chow WS, Horton P, Anderson JM (2005) Entropy-assisted stacking of thylakoid membranes. Biochim Biophys Acta 1708:187–195
Kleidon A (2004) Beyond Gaia: thermodynamics of life and earth system functioning. Clim Change 66:271–319
Kleidon A (2009) Non-equilibrium thermodynamics and maximum entropy production in the earth system: applications and implications. Naturwissenschaften 96:653–677
Kleidon A (2010a) Non-equilibrium thermodynamics, maximum entropy production and earth-system evolution. Philos Trans R Soc A 368:181–196
Kleidon A (2010b) Life, hierarchy, and the thermodynamic machinery of planet earth. Phys Life Rev 7:424–460
Kleidon A, Lorentz R (2005) Entropy production by earth system processes. In: Kleidon A, Lorentz R (eds) Non-equilibrium thermodynamics and production of entropy: life, earth, and beyond. Springer, Berlin, pp 1–20
Kutik J (1998) The development of chloroplast structure during leaf ontogeny. Photosynthetica 35:481–505
Leon P, Arroyo A, Mackenzie S (1998) Nuclear control of plastid and mitochondrial development in higher plants. Annu Rev Plant Physiol Plant Mol Biol 49:454–480
Lim PO, Kim HJ, Nam HG (2007) Leaf senescence. Annu Rev Plant Biol 58:115–136
López-Juez E (2007) Plastid biogenesis, between light and shadows. J Exp Bot 58:11–26
López-Juez E, Pyke KA (2005) Plastids unleashed: their development and their integration in plant development. Int J Dev Biol 49:557–577
Lovelock JE (1990) Hands up for the Gaia hypothesis. Nature 344:100–102
Lovelock JE, Margulis L (1974) Atmospheric homeostasis for and by the biosphere: the Gaia hypothesis. Tellus 26:2–10
Mache R, Zhou DX, Lerbs-Mache S, Harrak H, Villain P, Gauvin S (1997) Nuclear control of early plastid development. Plant Physiol Biochem 35:199–203
Marín D, Martín M, Sabater B (2009) Entropy decrease associated to solute compartmentalization in the cell. Biosystems 98:31–36
Møller SD (2005) Plastids, vol 13, Annual plant reviews. Blackwell, Oxford, pp 1–330
Mulo P (2011) Chloroplast-targeted ferredoxin-NADP(+) oxidoreductase (FNR): structure, function and location. Biochim Biophys Acta 1807:927–934
Nickelsen J, Rengstl B, Stengel A, Schottkowski M, Soll J, Ankele E (2011) Biogenesis of the cyanobacterial thylakoid membrane system – an update. FEMS Microbiol Lett 315:1–5
Parlitz S, Kunze R, Mueller-Roeber B, Balazadeh S (2011) Regulation of photosynthesis and transcription factor expression by leaf shading and re-illumination in Arabidopsis thaliana leaves. J Plant Physiol 168:1311–1319
Pogson BJ, Albrecht V (2011) Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiol 155:1545–1551
Prigogine I (1967) Introduction to thermodynamics of irreversible processes, 3rd edn. Interscience Publishers/Wiley, New York, pp 1–147
Prigogine I (1978) Time, structure and fluctuations. Science 201:777–785
Pulselli RM, Simoncini E, Tiezzi E (2009) Self-organization in dissipative structures: a thermodynamic theory for the emergence of prebiotic cells and their epigenetic evolution. Biosystems 96:237–241
Pyke KA (1999) Plastid division and development. Plant Cell 11:549–556
Robinson C, Mant A (2005) Biogenesis of the thylakoid membrane. In: Møller SG (ed) Plastids, annual plant reviews, vol 13. Blackwell, Oxford, pp 180–213
Rochaix JD (2011) Assembly of the photosynthetic apparatus. Plant Physiol 155:1493–1500
Ryberg M, Sundqvist C (1991) Structural and functional significance of pigment-protein complexes of chlorophyll precursors. In: Scheer H (ed) Chlorophylls. CRC Press, Boca Raton, pp 587–612
Sabater B (2009) Time arrows and determinism in biology. Biol Theory 4:174–182
Schneider ED, Kay JJ (1994) Life as a manifestation of the second law of thermodynamics. Math Comput Model 19:25–48
Schneider ED, Kay JJ (1995) Order from disorder: the thermodynamics of complexity in biology. In: Murphy MP, O’Neill LAJ (eds) What is life: the next fifty years reflections on the future of biology. Cambridge University Press, London, pp 161–172
Schrödinger E (1944) What is life? Cambridge University Press, London
Shi LX, Theg SM (2010) A stromal heat shock protein 70 system functions in protein import into chloroplasts in the moss Physcomitrella patens. Plant Cell 22:205–220
Smart CM (1994) Gene expression during leaf senescence. New Phytol 126:419–448
Stern DB, Hanson MR, Barkan A (2004) Genetics and genomics of chloroplast biogenesis: maize as a model system. Trends Plant Sci 9:293–301
Strittmatter P, Soll J, Bölter B (2010) The chloroplast protein import machinery: a review. Methods Mol Biol 619:307–321
Taylor WC (1989) Regulatory interactions between nuclear and plastid genomes. Annu Rev Plant Physiol Plant Mol Biol 40:211–233
Thomas H (1994) Aging in the plant and animal kingdoms – the role of cell death. Rev Clin Gerontol 4:5–20
Thomas H, Ougham H, Hörtensteiner S (2001) Recent advances in the cell biology of chlorophyll catabolism. Adv Bot Res 35:1–52
Thomas H, Ougham HJ, Wagstaff C, Stead AD (2003) Defining senescence and death. J Exp Bot 54:1127–1132
Thomson WW, Whatley JM (1980) Development of non-green plastids. Annu Rev Plant Physiol 31:375–394
Ulanowicz RE, Hannon BM (1987) Life and the production of entropy. Proc R Soc Lond B 232:181–192
Van Doorn WG, Yoshimoto K (2010) Role of chloroplasts and other plastids in ageing and death of plants and animals: a tale of Vishnu and Shiva. Ageing Res Rev 9:117–130
Vothknecht UC, Soll J (2005) The protein import pathway in chloroplasts: a single tune or variations on a common theme? In: Møller SG (ed) Plastids, annual plant reviews, vol 13. Blackwell, Oxford, pp 157–179
Waters M, Pyke K (2005) Plastid development and differentiation. In: Møller SG (ed) Plastids, annual plant reviews, vol 13. Blackwell, Oxford, pp 30–59
Wilson KE, Ivanov AG, Oquist G, Grodzinski B, Sahan F, Huner NPA (2006) Energy balance, organellar redox status and acclimation to environmental stress. Can J Bot 84:1355–1370
Yon-Kahn J, Hervé G (2010) Molecular and cellular enzymology. Springer, Berlin, pp 63–84
Zavaleta-Mancera HA, Franklin KA, Ougham HJ, Thomas H, Scott IM (1999a) Regreening of senescent Nicotiana leaves 1. Reappearance of NADPH-protochlorophyllide oxidoreductase and light harvesting chlorophyll a/b binding protein. J Exp Bot 50:1677–1682
Zavaleta-Mancera HA, Thomas BJ, Thomas H, Scott IM (1999b) Regreening of senescent Nicotiana leaves II. Redifferentiation of plastids. J Exp Bot 50:1683–1689
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Raval, M.K., Mishra, B.K., Biswal, B., Biswal, U.C. (2013). Chloroplast Development: Time, Dissipative Structures and Fluctuations. In: Biswal, B., Krupinska, K., Biswal, U. (eds) Plastid Development in Leaves during Growth and Senescence. Advances in Photosynthesis and Respiration, vol 36. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5724-0_2
Download citation
DOI: https://doi.org/10.1007/978-94-007-5724-0_2
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5723-3
Online ISBN: 978-94-007-5724-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)