Abstract
Тhe microbiological transformation of oil hydrocarbons to humic-like substances (HLS) is considered in this chapter. The transformation process was carried out in aqueous medium with oil being the only carbon source. The urea and metal oxide additives influence the substance formation dynamics and increase their concentration. The humic-like substances were analyzed by IR spectroscopy, spectrophotometry, polyacrylamide gel electrophoresis, and elemental composition. Soil humic substances were used as comparison samples. The results of the analysis prove kindred nature of soil humic matter and compounds produced by microbial oil transformation.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
As usual research of humic substances is a study of formed humic compounds.
In research works (Fukushima et al. 2009; Plaza et al. 2005; Droussi et al. 2009), humic acids (HA) are studied during the composting process. The changes of their physical and chemical properties are determined during the humification of organic material. In research work (Yavmetdinov et al. 2003) humic-like substances were obtained by wood-destroying fungi, which were cultivated on oat straw. The carried out physical and chemical analysis confirmed the humic nature of the obtained compounds. The results of these studies indicate the possibility of obtaining humic-like substances (HLS) at a laboratory. But in the mentioned studies used, substrate material contained or could contain HLS originally.
The purpose of this work is demonstration of the possibility to transform raw materials not containing humic substances – oil, to HLS by microorganisms.
Materials and Methods
The aqueous mineral medium was used for the cultivation of microorganisms. The tank oil obtained from Yarino-Kamennolozhskoe field (Perm Krai, Russia) was used as carbon source for microorganisms and was added by a ratio 1 mL per 100 mL of medium. A cumulative culture of microorganisms was obtained from activated sludge.
During the incubation, solution was sampled, then filtered, acidified by adding 10% HCl to pH 2, and centrifuged. The extinction coefficient (λ = 400 nm) of supernatant [fulvic acid (FA) solution] was determined by spectrophotometry (UNIC 1201, Russia), and the precipitate was dissolved in 5 mL of 0.1 M NaOH, and the extinction coefficient for getting solution (solution of humic acids) was also measured. Sampling of aliquots was repeated during 2.5 months. The obtained data were used for graphing. In the control sample a sterile oil sample and sterilized mineral medium without addition of cultures were used. The humic substances formation did not occur in the control sample. The urea (concentration 0.7%) or a mixture of metals Al3+ + Si4+ (0.02% concentration) was added to solution to determine the effect of changing medium composition on process development.
HA and FA obtained from sod-podzolic soils of Perm Krai, Russia, were used as comparison samples.
HA and FA identification was done by FTIR- spectrometry (FSM – 1201, Russia). Elemental analysis was carried out by the analyzer CNHS-932 (Leco Corporation USA). Electrophoresis of 10% polyacrylamide gel of HA and FA and the obtained HLS was conducted according to the method proposed by Trubetskoy et al. (1993).
Results and Discussion
It is known that the oil biodegradation process is accompanied by humic substance formation (Bazhenova et al. 2000). Our earlier work (Vialih and Ilarionov 2010) considered the dynamics of quantitative and qualitative changes in humic matter in soil. It was shown that the changes in HA and FA content during the oil remediation process are not alike. At the same time it is impossible to say definitely about the source of their formation, because HA could initially be traced in the soil.
In this work at the beginning it was stated that used raw material (oil) did not contain humic substance, because the alkaline extract of oil was not colored.
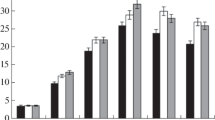
The control sample was not colored either. This sample was sterilized and did not contain microorganisms. Thus, we can conclude that HLS formation is closely connected with microbial activity. Based on the results the dependence of HLS alkaline solution extinction coefficient to the process time was made (Fig. 1).
The dynamics in the ratio of extinction coefficient of humic (a) and fulvic (b) acid solutions to time during the oil microbiological transformation: 1 without any additional substances in the solution, 2 the urea (concentration 0.7%) was added, 3 metal ions Al3+ + Si4+ (0.02% concentration) were added
After 20 days of the experiment, formation of HLS could be observed. From the data it is evident that formation of humic-like compounds was uneven. However, at this moment it is not possible to identify the reason for this dynamics.
From the data presented in the graphs, it is obvious the addition of urea and metal oxides affected the HLS concentration in solution. However, if the effect of urea can be observed after the first 20 days of the process, the effect of metals on the humic substances content was noticeable only after 40 days. In solutions containing both urea and metal oxides, a significant concentration of HLS was revealed. This phenomenon can be explained by the fact that humic substances have a complex structure and polydisperse composition and contain a large amount of hydroxyl and carboxyl groups and aromatic structures.
The urea influence on humic-like matter concentration in the solution could be explained by its properties, to be more exact, by the presence of a highly polar bond (C═O). Dissolved urea increases the ionic strength, thereby increasing the number of dissociated weakly polar-OH and-COOH groups of organic compounds, leading to an increase in the number of compound charged particles, which can simultaneously be in solution and does not aggregate to form larger micelles, which would precipitate.
The influence of metals on the increase in the extinction coefficient of the HLS solution can be accounted for by the metals’ property to form complex compounds with organic compounds containing hydroxyl and carboxyl groups, as well as aromatic fragments. The formation of complex compounds that are more associated structures usually leads to the increase in the extinction of coefficient of solution.
Dried and purified specimens of humic substances isolated from soil and obtained by microbial oil transformation were analyzed by infrared spectrometry. The produced spectra indicate that specimens of different origin are similar as far as the basic spectral band sets were the same in both cases. However, the spectra of humic acids could be characterized by more intensive peaks typical for intermolecular and intramolecular hydrogen bonds that, in particular, may be related to the HA ability to absorb large amounts of moisture from the atmosphere.
Contents of the main elements (C, O, H, N) lie within the range typical for HA and FA and given in the literature (Table 1).
During the process of electrophoresis HA and FA of different genesis were divided into three major fractions. Electrophoretic mobility of identical fractions of different preparations was the same, which can be explained by the close value for the following variables: the molecular weight fraction and its common charge.
Conclusions
The analysis confirms a kindred nature of humic substances isolated from soil and produced by microbial petroleum transformation.
These results can be the basis for development of technologies for processing oil-contaminated objects into a valuable product – humic compounds. A more detailed study of the proposed model of the oil products’ transformation by microorganisms to humic acids could provide answers to some unresolved questions: What is the molecular structure of these substances? What are consequently all their properties? Up to now scientists treat HA as “a black box.” They vary external parameters and observe changes in the final products, but they are unable to explain the mechanism of interactions.
References
Bazhenova, O.K., U.K. Burlin, B.A. Sokolov, and V.E. Khain. 2000. Geology and geochemistry of oil and gas. Moscow: Moscow State University.
Droussi, Z., V. D’Orazio, M. Hafidi, and A. Ouatmane. 2009. Elemental and spectroscopic characterization of humic-acid-like compounds during composting of olive mill by-products. Journal of Hazardous Materials 163: 1289–1297.
Fukushima, M., M. Yamamoto, T. Komai, and K. Yamamoto. 2009. Studies of structural alterations of humic acids from conifer bark residue during composting by pyrolysis-gas chromatography/mass spectrometry using tetramethylammonium hydroxide (TMAH-py-GC/MS). Journal of Analytical and Applied Pyrolysis 86: 200–206.
Plaza, C., A. Polo, N. Senesi, and G. Brunetti. 2005. Acid–base properties of humic and fulvic acids formed during composting. Environmental Science and Technology 39: 7141–7146.
Trubetskoy, O.A., L.Y. Kudryavtseva, and L.T. Shirshova. 1993. Soil humic substances fractionation by polyacrylamide gel electrophoresis in the presence of denaturing agents. Soil Science 8: 122–125.
Vialih, E.A., and S.A. Ilarionov. 2010. Humic substance formation during oil compounds destruction process. Environment Protection in Oil and Gas Area 11: 50–52.
Yavmetdinov, I.S., E.V. Stepanova, V.P. Gavrilova, B.V. Lokshin, I.V. Perminova, and O.V. Koroleva. 2003. Preparation and characterization of humic substances synthesized by wood-destroying fungi, agents of the ‘White Rot’. Applied Biochemistry and Microbiology 39: 293–301.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Zhejiang University Press and Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Vialih, E.A., Ilarionov, S.A. (2013). Microbiological Oil Transformation to Humic-Like Substances. In: Xu, J., Wu, J., He, Y. (eds) Functions of Natural Organic Matter in Changing Environment. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5634-2_17
Download citation
DOI: https://doi.org/10.1007/978-94-007-5634-2_17
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5633-5
Online ISBN: 978-94-007-5634-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)