Abstract
The influence of the concentration and composition of humic acids (HAs) on the biodegradation of petroleum hydrocarbons was investigated. The HAs were obtained from oxidized brown coal by alkaline extraction upon the mechanical treatment of coal in the presence of 3 and 8 wt % NaOH (HA1 and HA2, respectively). It was found that solid-phase alkaline hydrolysis in the presence of 8 wt % NaOH with the subsequent separation of HA2 with water led to an increase in the degree of aromaticity and the number of phenolic groups. The soil microflora stimulated by HA1 and HA2 had an increased destructive oil-oxidizing activity. A decrease in the concentration of phenanthrene in solution due to the formation of a complex upon interaction with humic acids was established by spectrophotometry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
The detoxification, purification, and reclamation of soils contaminated with oil and petroleum products is an important problem of considerable current interest in the activities of oil producing, transporting, and refining enterprises. Light fractions and aromatic hydrocarbons are the most toxic components of oil. The group of polycyclic aromatic hydrocarbons (PAHs), which are characterized by pronounced mutagenicity and carcinogenicity, is a great danger to living organisms [1].
Methods for eliminating the consequences of oil pollution of soil and water basins are based on the biodegradation of oil hydrocarbons and the binding and detoxification of organic pollutants in addition to sorption processes [2]. The intensity and nature of the biodegradation of petroleum hydrocarbons in soil depends on the nutrient medium and the functional activity of hydrocarbon-oxidizing microorganisms present in the soil [3, 4]. In this case, not only the introduction of biological preparations containing hydrocarbon-oxidizing microorganisms but also the activation of the aboriginal microflora of contaminated objects is implemented [2]. The raw materials for the production of biological preparations are peat and brown coal, the surfactants of which are humic acids (HAs), which stimulate the activity of microorganisms in the biodegradation of hydrocarbons [5–7].
The sorption and detoxifying properties of HAs in relation to organic pollutants depend on their functional composition. The ability to enter into ionic and donor–acceptor interactions and form hydrogen bonds is determined by the presence of a wide range of functional groups in HA molecules in combination with aromatic fragments [8, 9]. Because of this, HAs bind ecotoxicants (pesticides, polycyclic aromatic hydrocarbons, and heavy metals) into nontoxic complexes [10–13].
The structural-group parameters of HAs are determined by the method of their isolation [14–16]. It was found that the yield of HAs was reduced at minimum alkali amounts, but at the same time their degree of aromaticity and biological activity were higher [17]. The conditions of mechanical treatment (MT) of solid caustobioliths make it possible to decrease the alkalinity of the medium and, at the same time, to increase the yield and the detoxifying ability of HAs [18, 19].
The aim of this work was to study the effect of the method of isolation of HAs on their detoxifying properties in relation to petroleum hydrocarbons.
EXPERIMENTAL
The test materials were humic acids (HAs) isolated from oxidized brown coal (Chui-Kenul deposit, China). The technical characteristics of the oxidized coal were the following: ash content, 16.8 wt %; moisture content, 16.7 wt %; and HA content, 35.7 wt %.
The mechanical treatment of coal was carried out in an AGO-2 planetary mill (Novosibirsk) in the presence of 3 and 8 wt % NaOH (chemically pure grade, KhPK-Grupp, Penza) [20, 21].
Humic substances were extracted with 0.1 N NaOH at a temperature of 90°C for 1 h (pH 11.5) from the original coal and coal after MT with 3 wt % (HA1) and with distilled water at 20°C (pH 8.5) from coal after MT with 8 wt % NaOH (HK2). The solutions were filtered, and HAs were precipitated with 0.1 N HCl (pH 2). The HA precipitate was washed on the filter with distilled water.
The concentrations of acidic ionogenic groups in HAs were determined by potentiometric titration. A weighed HA portion was dissolved in a 0.1 N solution of NaOH, and a saturated solution of NaCl (pure grade, KhPK-Grupp, Penza) was added to create a constant ionic strength of the solution; the mixture was titrated with a 0.1 N solution of HCl (Khimprom, Kemerovo).
The elemental composition of humic acids was determined on a Vario El Cube elemental analyzer (Germany). The fragment composition was obtained by 13C NMR spectrometry on a Bruker 300 radiospectrometer (Germany) at an operating frequency of 100 MHz using Fourier transform with accumulation. The sweep width of the spectrum was about 26 000 Hz, the recording time of the free induction decay signal was 0.6 s, the interval between pulses was 8 s at a pulse width of 90°, and the spectrum accumulation time was 24 h. The sample (50–70 mg) of a preparation was dissolved in 0.7 cm3 of 0.3 M NaOD.
The antioxidant activity (AOA) K was determined by a voltammetric method of cathodic oxygen reduction using a mercury film electrode. The supporting electrolyte was a phosphate buffer solution (IMS, Moscow, pH 6.8). An analyte sample of 5 × 10–3 g was dissolved in 5 mL of 0.1 N NaOH. The antioxidant activity K reflects the amount of oxygen and active oxygen radicals reacted with an antioxidant per minute [22].
where I is the current of electroreduction (O2 ER) in the presence of HA in solution, μA; I0 is the current of О2 ER in the absence of HA in solution, μA; \(C_{{{{{\text{O}}}_{2}}}}^{0}\) is the initial concentration of oxygen in the solution, μmol/l; and t is the process time, min.
To create oil pollution, highly paraffinic oil in an amount of 15 g/kg was introduced into sod-podzolic soil with vigorous stirring. The detoxifying agents of oil pollution were HA solutions at pH 7 and a concentration of 0.005–0.05 wt %. The abundance of soil microflora was determined for eight weeks by inoculation on selective media using an example of heterotrophs of different physiological groups. The abundance of microorganisms in clean and oil-contaminated soils served as a reference control.
The concentrations of bitumoids in oil-contaminated soil were determined using extraction with chloroform. Paraffin-naphthenic hydrocarbons (PNHs), aromatic hydrocarbons (AHs), resins, and asphaltenes were separated by column chromatography from crude oil and bitumoids in the samples of oil/soil/HA (OSHA), oil/soil/HA1 (OSHA1), and oil/soil/HA2 (OSHA2).
The individual composition of n-alkanes was analyzed on an Agilent-6890 gas chromatograph (the United States). Calibration was carried out using n-C20.
To quantify the interaction of phenanthrene with HAs in an aqueous medium, we used spectrophotometry on an Agilent Cary Win spectrophotometer (the United States). A HA solution with a concentration of 0.005–0.01 g/L was added to the test solution with a known concentration of phenanthrene (5 × 10–5–4 × 10–4 g/L), and the optical density was determined at the same wavelength. The experimental spectra were mathematically processed using the Assayer software.
RESULTS AND DISCUSSION
It is well known that the yield and composition of HAs are significantly affected by the conditions of alkaline extraction. According to published data [12, 21], extraction with an increased alkali amount should be performed in order to maximize the extraction of HAs from coal. It was found experimentally that 35.7 wt % HAs was extracted from the oxidized coal upon alkaline extraction, and 55.4 wt % was extracted in the MT of coal with 3 wt % NaOH; at 8 wt % NaOH, mechanocomposites were formed, from which 79.0 wt % water-soluble humic substances containing 61.4 wt % HAs were extracted with water.
According to the results of the elemental analysis, HA1 and HA2 were enriched in oxygen to a greater extent in comparison with HAs isolated by alkaline extraction (Table 1). The 13C NMR-spectroscopic data indicated equally high degrees of aromaticity (ΣCar + CarO) of the molecules. In HA1 and HA2, the fraction of oxidized aromatic fragments represented as СarO/Car + CarO increased, and the concentration of oxidized alkyl fragments СalkO/Сalk decreased (Table 1). According to the results of potentiometric titration of oxygen-containing groups in HA2, the concentration of phenolic hydroxyls increased to a greater extent and the amount of carboxyl groups decreased (Table 2). Milder conditions of the alkaline extraction of HA2 (pH 8.5) determined the fragment and functional composition of the molecules.
In the oxidation of organic substances, hydroperoxides are formed as primary reaction products; they decompose into radicals, which initiate oxidation chains with the formation of reactive oxygen species (ROS) to cause oxidative stress. ROS are constantly formed in living cells as products of normal oxygen metabolism, and they are responsible for their destruction [23]. The neutralization of radicals and the termination of chain reactions in biological objects are carried out by antioxidants (AOs). The mechanism of action of HA AOs is the disproportionation reaction of ROS [22].

Antioxidant activity (K) significantly increases in the HA2 sample, the fraction of aromatic fragments and phenolic hydroxyls in the molecules of which is higher. This ensures the control of ROS and is an important protective system in biochemical processes (Table 2).
According to Zherebtsov et al. [17], a decrease in alkalinity on extraction led to an increase in the degree of aromaticity and in the number of hydroxyl groups in the composition of HA1 and HA2, which are capable of participating in redox reactions.
Oil oxidation begins immediately after it enters the soil. The common features of this process are the destruction of methane–naphthene fractions, a relative increase in the fraction of resinous substances in oil, and the conversion of some oil components into forms insoluble in organic solvents. The rate of changes in the concentrations of individual hydrocarbons and fractions depends on the natural and climatic zones, the composition of the original oil, and the stimulation of oil biodegradation.
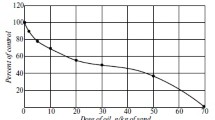
The amount of bitumoids in soil without HA additives after the eight-week experiment was 96.0 wt % (Fig. 1). When the soil was treated with aqueous solutions of humic acids, a maximum degree of oil degradation was observed at a concentration of 0.03 wt %. At higher concentrations, a decrease in the stimulating effect of HAs was noted, which was reflected in the biodegradation of petroleum hydrocarbons. It follows from this experiment that the process of oil biodegradation at low concentrations prevailed over adsorption, which manifested itself at higher HA concentrations.
The abundance of heterotrophic microorganisms was determined in clean and oil-contaminated soils after cultivation for eight weeks simultaneously with oil degradation (Fig. 2). In clean soil, heterotrophic microorganisms were present in an amount of 3.5 × 105 cell/g. In oil-contaminated soil, their abundance sharply decreased and increases to a maximum upon the addition of a solution of HA2 with a concentration of 0.03 wt %.
A correlation between the degradation and abundance of microflora was reflected in the composition of bitumoids extracted from oil-contaminated soil. Table 3 shows that the amount of paraffin–naphthene hydrocarbons in the bitumoids decreased in the test time interval, and the concentration of aromatic hydrocarbons decreased to a lesser extent. At the same time, the concentration of resins and asphaltenes increased. Changes in the composition of bitumoids were related to an increase in the activity of the hydrocarbon-oxidizing soil microflora. An increase in bioavailability was associated with the formation of a HA complex with hydrocarbons and its targeted transfer to bacterial cells. Yudina et al. [24] found that HAs isolated after the MT of coals were characterized by an increase in surface activity, which was associated with an increase in the hydrophobicity of molecules and the number of oxygen-containing groups.
It is well known that, among the petroleum components, normal-structure HCs are the first to undergo microbiological degradation. Changes in the composition of n-alkanes in the bitumoid samples of OSHA and OSHA2 most clearly demonstrated the destruction of hydrocarbons (Fig. 3). The concentration of С12–С19 hydrocarbons in the n-alkanes from bitumoids decreased. The molecular-weight-distributions of n-alkanes in oil and the bitumoids of OSHA and OSHA2 exhibited maximums at С17–С18, С19–С21, and С22–С24, respectively.
Table 4 summarizes characteristics used to evaluate oil degradation. The concentration of С12–С18 n-alkanes in oil was higher than that in bitumoids from oil-contaminated soil by a factor of 1.5–1.6. At the same time, the concentration of C19–C27 n-alkanes, which were less susceptible to microbiological degradation over the specified period of time, increased in the bitumoids of OSHA and OSHA2. The decrease in the fraction of medium molecular weight hydrocarbons was evidenced by a change in the n-C17/n-C27 ratio. The biochemical oxidation of hydrocarbons is associated with changes in the branching of hydrocarbon chains. The value of iC19 + iC20/n-C17 + n-C18 makes it possible to assess the depth of oil biodegradation processes. Table 4 indicates that the concentration of iC19 + iC20 decreased to a greater extent in the bitumoid of OSHA2. Thus, the presence of humic acids in soil stimulates the process of biochemical oxidation.
It is well known that tri- and tetracyclic hydrocarbons in oil are prone to biodegradation [25]. The role of HAs in reducing the toxicity of petroleum AHs is to bind them into complexes due to the presence of a hydrophobic aromatic framework in the structure. This fact was confirmed by the spectrophotometric determination of the concentration of phenanthrene absorbed by HA molecules. The formation of an HA–AH complex depends on the concentrations of HA and phenanthrene (Fig. 4). The maximum absorption of phenanthrene by the HA sample was observed at a concentration of 5 × 10–4 g/L and amounted to 43 rel %. With an increase in the concentration of phenanthrene, the residual fraction of the toxic substance in the solution increased to indicate the limited absorption capacity of HAs. The greatest effect of phenanthrene binding was observed in the presence of HA2 molecules, which are characterized by a higher degree of aromaticity. Large micelle-like aggregates, which impede the access of phenanthrene molecules to aromatic fragments, were formed as the HA concentration was increased; therefore, their interaction in this case was not so significant.
CONCLUSIONS
The soil microflora stimulated by mobile aqueous solutions of humic acids has an increased destructive oxidative activity toward oil. The maximum destruction of oil and an increase in the abundance of heterotrophic microorganisms were observed in the presence of 0.03 wt % HAs, the molecules of which were characterized by greater hydrophobicity and amount of oxygen-containing groups. An increase in the HA concentration caused a decrease in the level of degradation of petroleum hydrocarbons, which can be associated with inhibition of the activity of microorganisms.
The stimulation of the oxidative destruction of petroleum hydrocarbons by HA solutions was confirmed by a decrease in the amount of light hydrocarbons and an increase in the fraction of resinous components. A model experiment with the detoxification of phenanthrene showed that a significant decrease in its concentration was achieved in the presence of humic acids with a higher fraction of aromatic fragments in their composition.
REFERENCES
Grechishcheva, N.Yu., Perminova, I.V., and Me-shcheryakov, S.V., Ekol. Prom–st' Rossii, 2016, vol. 20, no. 1, p. 30. https://doi.org/10.18412/1816-0395-2016-1-30-36
Filatov, D.A., Krivtsov, E.B., Sviridenko, N.N., Golovko, A.K., and Altunina, L.K., Neftekhimiya, 2017, vol. 57, no. 8, p. 386. https://doi.org/10.7868/S0028242117040050
Prince, R.C., Hedgpeth, B.M., Redman, A.D., and Butler, J.D., Open J. Marine Sci., 2019, vol. 9, no. 3, p. 113.
Ivanov, A.A., Yudina, N.V., Mal’tseva, E.V., Matis, E.Ya., and Svarovskaya, L.I., Eurasian Soil Sci., 2010, vol. 43, no. 2, p. 210. https://doi.org/10.1134/S1064229310020110
Prince, R.C., Hedgpeth, B.M., Redman, A.D., and Butler, J.D., Open J. Marine Sci., 2019, vol. 9, no. 3, p. 113.
Pineda-Flores, G., Boll-Arguello, G., Lira-Galeana, C., and Mesta-Howard, A.M., Biodegradation, 2004, vol. 15, no. 3, p. 145. https://doi.org/10.4236/ojms.2019.93009
Chikere, C.B., Okpokwasili, G.C., and Chikere, B.O., Afr. J. Biotechnol., 2009, vol. 8, no. 11, p. 2535.
Sutton, R., Environ. Sci. Technol., 2005, vol. 39, no. 23, p. 9009. https://doi.org/10.1021/es050778q
Liang, Y-N., Britt, D.W., and McLean, J.E., Appl. Microbiol. Biotechnol., 2007, vol. 74, no. 6, p. 1368.
Smith, K.E., Thullner, M., Wick, L.Y., and Harms, L.Y., Environ. Sci. Technol., 2009, vol. 43, no. 19, p. 7205. doi org/https://doi.org/10.1021/es803661s
Yudina, N.V., Mal’tseva, E.V., Chaikovskaya, O.N., and Nechaev, L.V., Russ. J. Phys. Chem. A, 2011, vol. 85, no. 9, p. 1558. https://doi.org/10.1134/S0036024411090147
Aniefiok, E., Hanney, N.F., and Semple, K.T., Int. J. Environ. Bioremed. Biodegrad., 2015, vol. 3, no. 2, p. 40. https://doi.org/10.12691/ijebb-3-2-1
Shirshin, E.A., Budylin, N.Yu., Fadeev, V.V., and Perminova, I.V., Photochem. Photobiol. Sci., 2016, vol. 15, p. 842. https://doi.org/10.1039/c6pp00052e
Tchaikovskaya, O.N., Yudina, N.V., Mal’tseva, E.V., and Sokolova, I.V., Luminescence, 2005, no. 20, p. 187. https://doi.org/10.1002/bio.818
Skripkina, T.S., Bychkov, A.L., Tikhova, V.D., and Lomovskii, O.I., Solid Fuel Chem., 2018, vol. 52, no. 6, p. 356. https://doi.org/10.3103/S0361521918060101
Skripkina, T.S., Bychkov, A.L., Tikhova, V.D., Smolyakov, B.S., and Lomovsky, O.I., Environ. Technol. Innovation, 2018, vol. 11, p. 74. https://doi.org/10.1016/j.eti.2018.04.010
Zherebtsov, S.I., Votolin, K.S., Malyshenko, N.V., Smotrina, O.V., Dugarzhan, Zh., and Ismagilov, Z.R., Solid Fuel Chem., 2019, vol. 53, no. 5, p. 253. https://doi.org/10.3103/S0361521919050124
Tchaikovskaya, O.N., Yudina, N.V., Nechaev, L.V., and Mal’tseva, E.V, Luminescence, 2016, vol. 31, no. 5, p. 1098. https://doi.org/10.1002/bio.3077
Savel'eva, A.V., Mal’tseva, E.V., Yudina, N.V., and Lomovskii, O.I., Khim. Interesakh Ustoich. Razvit., 2016, vol. 24, no. 2, p. 263. https://doi.org/10.15372/KhUR20160221
Urazova, T.S., Bychkov, A.L., and Lomovskii, O.I., Russ. J. Appl. Chem., 2014, vol. 87, no. 5, p. 651. https://doi.org/10.1007/s11172-015-0997-0
Yudina, N.V., Savel’eva, A.V., and Linkevich, E.V., Solid Fuel Chem., 2019, vol. 53, no. 1, p. 29. https://doi.org/10.3103/S0361521919010099]
Yudina, N.V., Savel’eva, A.V., Ivanov, A.A., Korotkova, E.I., and Lomovskii, O.I., Russ. J. Appl. Chem., 2004, vol. 77, no. 1, p. 46. https://doi.org/10.1023/B:RJAC.0000024574.01023.fe
Klein, O.I., Kulikova, N.A., Filimonov, I.S., Koro-leva, O.V., and Konstantinov, A.I., Soils Sediments, 2018, no. 4, p. 1355. https://doi.org/10.1007/s11368-016-1507-1
Yudina N.V., Savel’eva, A.V., and Lomovskii, O.I., Khim. Interesakh Ustoich. Razvit., 2019, no. 4, p. 637. https://doi.org/10.15372/KhUR2019156
Antipenko, V.R., Bakanova, O.S., and Filatov, D.A., Neftekhimiya, 2019, vol. 50, no. 5, p. 508. .https://doi.org/10.1134/S0028242119050022
Funding
This work was carried out within the framework of a state contract at the Institute of Petroleum Chemistry, Siberian Branch, Russian Academy of Sciences (project no. 1210315000498).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by V. Makhlyarchuk
About this article
Cite this article
Linkevich, E.V., Yudina, N.V. & Savel’eva, A.V. Role of Humic Acids in the Detoxification of Petroleum Hydrocarbons in Soil. Solid Fuel Chem. 55, 332–337 (2021). https://doi.org/10.3103/S0361521921050049
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.3103/S0361521921050049