Abstract
The reactions of ozone with alkenes are of importance within atmospheric chemistry as a non-photolytic source of the oxidant radicals OH, HO2 and RO2. While OH yields are relatively well constrained, few data exist for production of HO2 or RO2. We report direct measurements of total radical yields from a range of small (C2–C5) alkenes, using LIF and PERCA techniques within large simulation chamber experiments. OH yields are found to be consistent with established understanding, while HO2 yields are substantially smaller than previous measurements suggest, but in good agreement with those assumed within current atmospheric chemical mechanisms.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
12.1 Introduction
Alkenes, unsaturated hydrocarbons, are emitted to the atmosphere from a range of natural and anthropogenic sources, notably biogenic emissions of isoprene, C5H8, and the isoprenoid terpenes (C10) and sesquiterpenes (C15). Alkenes can contribute up to 30% of the total OH sink in urban regions, and a higher proportion forested environments; atmospheric degradation of alkenes contributes to the production of ozone in the presence of nitrogen oxides, and leads to the production of substituted and/or oxygenated degradation products, which may act as precursors to, or contribute to the formation of, secondary organic aerosol (SOA). In addition to degradation driven by reaction with OH and NO3, alkene oxidation may be initiated by reaction with ozone, a process which leads to the dark, non-photolytic production of radical intermediates; detailed analyses of measurements from atmospheric field campaigns have shown ozonolysis to account for up to 30% of the total OH radical production. Understanding the yields of OH, HO2 and RO2 radicals, and their dependence upon atmospheric conditions, is essential to quantify this important contribution to atmospheric oxidising capacity.
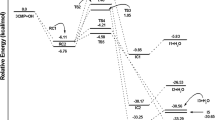
Gas-phase alkene ozonolysis is believed to proceed via the Criegee mechanism [7], illustrated in Fig. 12.1. Ozonolysis is highly exothermic, initiated by the electrophilic cycloaddition of ozone across the C═C double bond to form an unstable 1,2,3-trioxolane (hereafter referred to as a primary ozonide, POZ) (R1). This intermediate is high in energy and rapidly decomposes at the central C–C bond and one of the O–O bonds. Given that the O–O bond can break at two different sites, a pair of carbonyl oxides (hereafter referred to as Criegee Intermediates, CIs) and stable carbonyl molecules can be formed (R2a and R2b).
The CI and carbonyl co-product produced from the exothermic decomposition of the POZ possess a significant amount of vibrational excitation. This energy enables further unimolecular reactions of the excited CI to occur but is not sufficient for the decomposition of the carbonyl molecule [6] – Fig. 12.2. The distribution of decomposition products of the POZ is dependent upon the substitution of the alkene. Different CIs behave as distinct chemical entities as demonstrated by the range of detected experimental products, dependent upon the extent of the substitution of the CI and distribution of energy following decomposition of the POZ. Substituted CIs can be formed in a syn (i.e. with the alkyl substituent on the same side of the CI as the terminal O atom) configuration or anti configuration, with a substantial barrier to interconversion between them. Briefly, syn- and di-substituted CIs are thought to predominantly decompose through isomerisation via a five-membered transition state to give an excited hydroperoxide species which subsequently decomposes to give OH and a vinoxy radical (the “hydroperoxide mechanism” – e.g. [17] and references therein). The proportion of the vibrationally excited CI that does not isomerise/decompose is suggested to be collisionally stabilised and can therefore undergo bimolecular reactions [25]. In general, at low pressures, energy rich CIs undergo prompt decomposition to yield OH and a vinoxy radical, which subsequently reacts near-instantaneously with O2 to form a peroxy radical [9]. At higher pressures (i.e. under boundary layer conditions) the CI may be collisionally stabilised (Fig. 12.2, R4) and can thermally decompose to generate OH and a vinoxy radical (R3b) or undergo rearrangement through a dioxirane structure. The dioxirane structure can decompose to various products including HO2 (R6), via a ‘hot’ acid intermediate.
The vinoxy radical formed alongside OH (Fig. 12.2) will react with oxygen in the atmosphere to form an excited β-oxo peroxy radical, which may be stabilised or undergo decomposition forming CO, a (secondary) stable carbonyl species and OH [19]. However, this pathway to OH formation is only thought to be significant if an aldehydic hydrogen is present. The stabilised β-oxo peroxy radical may then undergo self- or cross-reaction with other peroxy radicals to form stable species such as glyoxal, glycolaldehyde, peroxides and secondary carbonyls.
The fate of the anti-CI and of the CH2OO CI formed from terminal alkenes is discussed in detail elsewhere [1]. Briefly, the anti-CI (and CH2OO) can undergo rearrangement through a dioxirane structure, which can decompose to various products including OH, HO2, CO, CO2, H2O and alkyl molecules via a ‘hot’ acid/ester intermediate (e.g. [6]). The syn and anti-CIs can also undergo stabilisation followed by bimolecular reaction, but studies suggest that stabilisation is a minor process for di-substituted and syn mono-substituted CIs, as their lifetime with respect to the vinyl hydroperoxide mechanism is thought to be substantially shorter than the time required for bimolecular processes to occur [10, 26]. Collisional stabilisation is therefore more likely to occur for the anti-CI (for which the hydroperoxide route is unavailable), potentially enabling bimolecular reactions to proceed with many atmospherically relevant species such as H2O, NO2, SO2 and CO [6, 13, 18]. In this article we review the results of a detailed study of the ozonolysis of a series of small-chain alkenes (ethene – isoprene), with a focus upon the production of the radical species OH an HO2, and their dependence upon experimental conditions (e.g. humidity).
12.2 Experimental Approach
The experimental work was performed in the European Photoreactor (EUPHORE) in Valencia, Spain, coupled with detailed chemical box modelling analysis for data interpretation. The EUPHORE facility comprises two large scale atmospheric simulation chambers, used for studying the mechanisms of atmospheric processes. Briefly, each chamber consists of a 197 m3 hemispherical reactor, formed from fluorine-ethene-propene (FEP) Teflon foil (127 μm thickness), and fitted with housings which exclude ambient sunlight. Detailed descriptions of the chambers and their instrumentation are given elsewhere [3]. In this study a range of analytical instrumentation was used, including traditional monitors (O3, CO, HCHO, H2O), Fourier transform infrared spectroscopy (FTIR) and chemical ionisation reaction time-of-flight mass-spectrometry (CIR-TOF-MS – [34]) for the detection of precursor and product species, including oxygenated derivatives. Radical species were monitored using laser induced fluorescence (LIF – [4, 32]) and peroxy radical chemical amplifier (PERCA – [12]) for the detection of OH/HO2 and HO2 + ΣRO2 respectively.
All ozonolysis experiments were performed with the chamber housings closed to exclude ambient light/photochemical effects (j(NO2) < 2 × 10−6 s−1), at near atmospheric pressure and at ambient temperature (294–298 K). All experiments were conducted under NOx-free conditions. In the absence of NOx and sunlight, chamber wall radical production has been shown to be negligible [35]. For “dry” experiments, the relative humidity was low (in simulation chamber terms: <1% RH). The experimental procedure, starting with a clean flushed chamber (NMHC < 0.2 ppbV, CH4 = ambient i.e. 1,800 ppbV, NOy < 1 ppbV), was to add SF6 (as a dilution tracer), followed by ozone (50–500 ppbV) and in certain cases an OH scavenger (CO or cyclohexane, in concentrations such that ≥95% of any OH produced was scavenged rather than reacting with the precursor alkene) was introduced prior to ozone injection. To initiate the reaction, a known aliquot of alkene (20–500 ppbV) was injected into the chamber and the evolution of reactants and products monitored over timescales of 1–3 h, at a time resolution ranging from seconds (e.g. LIF) to 10 min (FTIR scan time). For “wet” experiments, where the relative humidity was increased to ca. 30%, water was added to the chamber through a nebuliser prior to the addition of the reactants.
The EUPHORE data was analysed using a detailed chemical box model, based upon the Master Chemical Mechanism (MCM: http://mcm.leed.ac.uk/MCM) version 3.1 [5, 15, 31], incorporating an extended and updated version of the ozonolysis mechanism of interest (c.f. [1]). Within the model, the POZ and CI were assumed to decompose rapidly (compared with the timescale of the subsequent chemistry) to form radical products and stable species, or stabilised CIs, and were therefore not assigned individual rate constants. Rate constants for the bimolecular reactions of the SCI were taken directly from the MCM. The cyclohexane photo-oxidation mechanism, extracted from MCMv3.1, was also updated and extended as outlined in Alam et al. [1]. Simulations were initialised at the time point at which the maximum measured alkene mixing ratio was observed. Temperature, relative humidity and dilution rates were averaged over the duration of each experiment, as the variation in these parameters on the experimental timescale was minimal. Four analytical stages were performed, in each case to determine the overall yields of specific products from the overall fast ozonolysis reaction (i.e. the CI formation/decomposition chemistry). Briefly, alkene/O3 reaction rate coefficients were optimised for experiments performed in the presence of a radical scavenger, followed by the optimisation of the branching ratios of the POZ decomposition, forming the respective pairs of carbonyl products (and CIs). Overall carbonyl yields were derived using model optimisation, by minimising the sum of squares of residuals between the simulated and observed concentrations, and classically, by (dilution corrected) mass balance calculations; both methods were in excellent agreement. Finally, HOx radical yields were determined by optimising the branching ratio for the isomerisation/decomposition of the syn-CI to minimise the sum of squares of residuals between simulated and observed OH/HO2 concentrations. The OH yield from the ozonolysis of ethene (0.17; [1]) was applied to the decomposition of the CH2OO CI formed in all terminal alkene systems. The model optimization process accounted for further reactions of OH and HO2, and for secondary formation processes. It is important to note that the overall HOx yields obtained through this approach, relative to flux through each alkene-ozone reaction, are reasonably absolute and independent of the HOx production route implemented in the model, but their attribution to specific reaction pathways of the ozonolysis system (e.g. Criegee decomposition branching ratios) is dependent upon the assumed mechanism. In the discussion below we draw inferences regarding the likely mechanistic origin of the observed OH and HO2, from the variation in yields with co-products and experimental conditions.
12.3 OH Production
Figure 12.3 shows a typical temporal profile of the OH steady state concentration as measured by the LIF system, and model simulation comparisons for cis-2-butene ozonolysis. The data illustrate that MCMv3.1 overestimates (in the case of cis-2-butene) the OH yield, compared to the present work. (In this instance it is likely that this arises from the assumed 50:50 split of the syn/anti CIs in the MCM 3.1 mechanism – the lower yield obtained here (and in other studies – [14]) suggests that the anti-conformer of the CI is preferentially formed (alongside acetaldehyde) from the primary ozonide decomposition in this system – [29]).
Figure 12.4 shows the OH yields obtained for ethene, propene, 1-butene, 2-methylpropene, cis-2-butene, trans-2-butene and 2,3-dimethyl-2-butene, compared to those from other studies [2, 23, 24, 27, 28, 30] plotted as a function of the equivalent IUPAC recommendations for OH yields [14]. The uncertainties in the results from this work represent the combined (2σ) statistical uncertainty from repeated determinations propagated with the corresponding OH measurement calibration uncertainty (27%; [4]). The results are well correlated with the IUPAC values; as the literature studies mainly exploit indirect methods to detect OH, by the use of OH scavenger (e. g. [2]) and tracer (e.g. [30]) techniques, or indirect observation by matrix-isolation electron spin resonance [24] and PERCA [28] the agreement with the direct OH observations in this work is encouraging. The results are consistent with the isomerisation/decomposition of a given CI to a vinyl hydroperoxide and OH; the basis of the OH yield structure activity relationship (SAR) of Rickard et al. [30].
Comparison of the OH yields from this work and literature studies, with the current [14] OH yield recommendations (abscissa). Filled circles: this work; filled squares: Rickard et al. [30]; open squares: Atkinson et al. [2]; filled triangle: Mihelcic et al. [24]. The dashed line shows the 1:1 correlation
These OH production yields (YOH) correspond to the formation of OH via the (fast) direct decomposition/isomerisation of the CI, after taking secondary sources into account within the model; for example OH formation via HO2 + O3, from decomposition of the excited β-oxo peroxy radical [19] and the reactions of acyl peroxy radicals + HO2 [8, 16].
12.4 HO2 Production
The yields of HO2 (YHO2) for the alkenes studied were found to be significantly larger in the absence of OH radical scavengers (specifically, carbon monoxide) – particularly for the 2-methylpropene and 2,3-dimethyl-2-butene ozonolysis systems, where “YHO2” values were found to be significantly greater than unity (1.51 and 1.74 respectively). It is likely that in the absence of radical scavengers the retrieved HO2 concentrations are in fact biased high due to interference from the decomposition of β-hydroxyalkyl peroxy radicals, formed from the OH + alkene reactions, within the LIF instrument [11]. We therefore disregard the HO2 observations/yields obtained in the absence of added CO, and focus upon the data recorded with excess CO present in the following. The OH yields previously obtained were employed within the model to distinguish direct HO2 formation from indirect production via the OH + CO reaction. Figure 12.5 shows the temporal profile of HO2 measured by LIF in a propene ozonolysis experiment, along with the HO2 model results. The first stage of the experiment illustrates the β-hydroxyalkyl peroxy radical HO2 interference effect noted above – the HO2 levels are overestimated. Following addition of CO, the observed HO2 increases slightly (increased through the conversion of OH to HO2, offset by removal of the interferant RO2 species) – this part of the experiment represents the base case scenario for retrieval of the HO2 yield. In the third part of the experiment, water is added to increase the relative humidity to ca. 30%, and the observed HO2 levels (shown corrected for the LIF system calibration humidity dependence) decrease. The dashed line in Fig. 12.5 represents the modelled HO2 using an overall yield of 0.09, while the solid lines in sections 1, 2 and 3 show the individually optimized yields of 0.61, 0.09 and 0.02, respectively. The inference from Fig. 12.5 then is that interference effects increase the retrieved “HO2” in the absence of CO (section 1); the HO2 yield in the absence of H2O is 9% (section 2), and upon addition of H2O, the HO2 levels decrease, to a greater extent than can be accounted for by the humidity dependence of the HO2 recombination reaction (which is included in the model), corresponding to a reduction in the HO2 yield from propene ozonolysis with increasing humidity (section 3).
HO2 production in the ozonolysis of propene (section 1) and its response to the addition of CO (section 2; 62 min +) and H2O (section 3; 144 min +). Data show the observed concentrations of HO2 (open circles) plus model simulations with direct HO2 yields of 0.61, 0.09 and 0.02 for sections 1–3 of the experiment respectively (solid lines), and for a single fixed yield of 0.09 throughout (dashed lines) for a propene ozonolysis
The dry yields of HO2 obtained here are compared with those from other studies in Fig. 12.6. The overestimate of the “HO2 yield” obtained in the absence of CO, from the RO2 interference, is clearly apparent (filled squares) – these data are not considered further. Our measured yields are in good agreement with measurements for ethene and propene obtained by PERCA, and for isoprene by direct observation by LIF, in the absence of an OH scavenger [22]. The yields obtained in this work are substantially smaller than those reported by Wegener et al. [33] – these values were obtained indirectly from analysis of alkene and ozone turnover in the course of long-duration experiments in the SAPHIR chamber. It is difficult to directly account for the difference between these studies – while the turnover approach is in principle independently sensitive to the HOx (OH and HO2) levels present, the sensitivity within the alkene and ozone decays is small (compared with reaction and dilution), reflected in the uncertainty of these values. The HO2 yields obtained here, and by Qi et al. [28] and Malkin et al. [22], are in good agreement with those implemented within the MCMv3.1, at least under dry conditions.
Comparison of HO2 yields for small chain alkenes investigated during this study with literature values. The abbreviations T2B, C2B and TME are trans-2-butene, cis-2-butene and 2,3-dimethyl-2-butene respectively. Filled squares – this work, no CO present (values overestimated due to RO2 interference). Open squares – this work, excess CO experiments (valid HO2 data); filled triangles – Wegener et al. [33]; light filled diamonds (ethene, propene) – Qi et al. [27, 28]; dark filled diamond (isoprene) – Malkin et al. [22]; star (ethene) – Mihelcic et al. [24] and open circles – model yields, MCMv3.1
The humidity dependence of OH production was not studied in this work; the majority of previous studies have found no evidence for any variation with water vapour (e.g. [17] and references therein), although some indications of variations with H2O have been reported (e.g. [33]). As noted above, HO2 yields decreased upon addition of water vapour for the propene – ozone system (0.09 ± 0.02 to 0.02 ± 0.01). Reductions in HO2 yields upon addition of water were also observed for ethene (0.10 ± 0.03 to 0.05 ± 0.01 – [1]) and cis-2-butene (albeit from a low starting point: 0.03 ± 0.01 to 0.00 ± 0.01), but interestingly not to any significant extent for 2-methyl propene (0.36 ± 0.10 to 0.38 ± 0.10). These data suggest that water vapour is able to intercept (at least part of) the HO2 formation chemistry in (at least some) alkene ozonolysis reactions. The trend is consistent with the reduction in HO2 yields observed with increasing humidity by Wegener et al. [33] – where yield reductions of ca. 20% were observed for ethene, propene and isobutene (but not for 1-butene) when going from dry conditions to ca. 10 mbar H2O, albeit with considerable (≈50%) uncertainty. Recently, Leather et al. [20] have reported an increase in the yield of formic acid formation from ethene ozonolysis with increasing humidity, attributed to H2O reacting with the (stabilized) Criegee intermediate in this system. Together, these studies indicate that the yield of HO2 from alkene ozonolysis may vary with humidity, implying that competition may occur within the mechanism between radical production through decomposition, and bimolecular reaction, of the stabilized Criegee intermediate (most likely, through decomposition of the “hot” acid intermediate which results from isomerisation of the anti-CI and/or CH2OO).
12.5 Atmospheric Implications
A constrained zero-dimensional box model was used to quantify the role of alkene ozonolysis to radical production, under ambient conditions observed during the TORCH (Tropospheric Organic Chemistry) field experiment performed in a suburban location to the North-East of London during July and August 2003. This period coincided with an air pollution event and heatwave, leading to elevated ozone and VOC levels compared with the mean for the location and season [21]. The model was constrained to observed levels of long-lived species (NOx, O3, H2O, VOCs, HCHO), meteorological parameters and photolysis rates, and used to calculate the relative contribution of the different (primary) OH and HO2 production channels, employing the HOx yields derived from the experiments described above. For OH, ozonolysis was found to account for 29% of primary production (dominated by O3 + hν/O(1D) + H2O), while for HO2 ozonolysis accounted for 8% of primary production, which was dominated by aldehyde, particularly HCHO, photolysis (this simple calculation neglects the nested contribution of ozonolysis to the aldehyde loading). Three caveats apply to these values – total OH production was dominated by radical cycling, with the reactions of HO2 with NO and O3 accounting for 88% of the total flux into OH; HONO photolysis was not included, as HONO observations were unavailable, and is likely to make a substantial contribution, and the ozonolysis radical yields used from this work were those obtained under dry conditions. If, as hypothesised above, radical yields are reduced in the presence of water vapour, and hence potentially other reaction partners (e.g. NO), these figures for the importance of ozonolysis in the atmosphere may be regarded as upper limits.
12.6 Conclusions
The production of OH and HO2 radicals from the ozonolysis of a range of small alkenes has been studied through a simulation chamber approach using the EUPHORE facility, including direct observations of OH and HO2 via laser-induced fluorescence, with the data obtained analysed in conjunction with a detailed chemical box model to obtain radical yields. OH yields are in agreement with previous measurements, and are consistent with the dominant mechanistic source being the rapid isomerisation and decomposition of syn-CIs (Criegee Intermediates) via the hydroperoxide mechanism. Yields of HO2 were lower than those inferred in some other recent studies, but were in good agreement with those implemented in the Master Chemical Mechanism (version 3.1) under dry conditions. Analysis of atmospheric field data confirmed ozonolysis as a significant source of OH and HO2 radicals in the summertime semi-polluted continental boundary layer. Evidence for a reduction in HO2 yield with increasing humidity was observed for ethene, propene and cis-2-butene, implying that these and other similar calculations may overestimate HOx production from alkene ozonolysis under ambient humidity conditions.
References
Alam MS, Camredon M, Rickard AR, Carr T, Wyche KP, Hornsby KE, Monks PS, Bloss WJ (2011) Total radical yields from tropospheric ethene ozonolysis. Phys Chem Chem Phys 13:11002–11015
Atkinson R (1997) Gas-phase tropospheric chemistry of volatile organic compounds: 1. alkanes and alkenes. J Phys Chem Ref Data 26:215–290
Becker KH (1996) EUPHORE: final report to the European Commission. contract EV5V-CT92-0059. Bergische Universität Wuppertal, Germany
Bloss WJ, Lee JD, Bloss C, Heard DE, Pilling MJ, Wirtz K, Martin-Reviejo M, Siese M (2004) Evaluation of the Calibration of a laser-induced fluorescence instrument for the measurement of OH radials in the atmosphere. Atmos Chem Phys 4:571–583
Bloss C, Wagner V, Jenkin ME, Volkamer R, Bloss WJ, Lee JD, Heard DE, Wirtz K, Martin-Reviejo M, Rea G, Wenger JC, Pilling MJ (2005) Development of a detailed chemical mechanism (MCMv3. 1) for the atmospheric oxidation of aromatic hydrocarbons. Atmos Chem Phys 5:641–664
Calvert JG, Atkinson R, Kerr JA, Madronich S, Moortgat GK, Wallington TJ, Yarwood G (2000) The mechanism of atmospheric oxidation of the alkenes. Oxford University Press, New York
Criegee R (1975) Mechanism of ozonolysis. Angew Chem 14:745–752
Dillon TJ, Crowley JN (2008) Direct detection of OH formation in the reactions of HO2 with CH3C(O)O2 and other substituted peroxy radicals. Atmos Chem Phys 8:4877–4889
Donahue NM, Drozd GT, Epstein SA, Presto AA, Kroll JH (2011) Adventures in ozoneland: down the rabbit-hole. Phys Chem Chem Phys 13:10848–10857
Fenske JD, Hasson AS, Ho AW, Paulson SE (2000) Measurement of absolute unimolecular and bimolecular rate constants for CH3CHOO generated by the trans-2-butene reaction with ozone in the gas phase. J Phys Chem A 104:9921–9932
Fuchs H, Bohn B, Hofzumahaus A, Holland F, Lu KD, Nehr S, Rohrer F, Wahner A (2011) Detection of HO2 by laser-induced fluorescence: calibration and interferences from RO2 radicals. Atmos Meas Tech 4:1209–1225
Green TJ, Reeves CE, Fleming ZL, Brough N, Rickard AR, Bandy BJ, Monks PS, Penkett SA (2006) An improved dual channel PERCA instrument for atmospheric measurements of peroxy radicals. J Environ Monit 8:530–536
Hatakeyama S, Akimoto H (1994) Reactions of Criegee intermediates in the gas phase. Res Chem Intermed 20:503–524
IUPAC (2009) International union of pure and applied chemistry subcommittee on gas kinetic data evaluation. http://www.iupac-kinetic.ch.cam.ac.uk/
Jenkin ME, Saunders SM, Pilling MJ (1997) The tropospheric degradation of volatile organic compounds: a protocol for mechanism development. Atmos Environ 31:81–104
Jenkin ME, Hurley MD, Wallington TJ (2007) Investigation of the radical product channel of the CH3C(O)O2 + HO2 reaction in the gas phase. Phys Chem Chem Phys 9:3149–3162
Johnson D, Marston G (2008) The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem Soc Rev 37:699–716
Johnson D, Lewin AG, Marston G (2001) The effect of Criegee-intermediate scavengers on the OH yield from the reaction of ozone with 2-methylbut-2-ene. J Phys Chem A 105:2933–2935
Kuwata KT, Hasson AS, Dickinson RV, Petersen EB, Valin LC (2005) Quantum chemical and master equation simulations of the oxidation and isomerization of vinoxy radicals. J Phys Chem A 109:2514–2524
Leather KE, McGillen MR, Cooke MC, Utembe SR, Archibald AT, Jenkin ME, Derwent RG, Shallcross DE, Percival CJ (2011) Acid-yield measurements of the gas-phase ozonolysis of ethene as a function of humidity using Chemical Ionisation Mass Spectrometry (CIMS). Atmos Chem Phys Discuss 11:25173–25204
Lee JD, Lewis AC, Monks PS, Jacob M, Hamilton JF, Hopkins JR, Watson NM, Saxton JE, Ennis C, Carpenter LJ, Carslaw N, Fleming Z, Bandy BJ, Oram DE, Penkett SA, Slemr J, Norton E, Rickard AR, Whalley LK, Heard DE, Bloss WJ, Gravestock T, Smith SC, Stanton J, Pillin MJ, Jenkin ME (2006) Ozone photochemistry and elevated isoprene during the UK heatwave of August 2003. Atmos Environ 40:7598–7613
Malkin TL, Goddard A, Heard DE, Seakins PW (2010) Measurements of OH and HO2 yields from the gas phase ozonolysis of isoprene. Atmos Chem Phys 10:1441–1459
McGill CD, Rickard AR, Johnson D, Marston G (1999) Product yields in the reactions of ozone with Z-but-2-ene, E-but-2-ene and 2-methylbut-2-ene. Chemosphere 38:1205–1212
Mihelcic D, Heitlinger M, Kley D, Musgen P, Volz-Thomas A (1999) Formation of hydroxyl and hydroperoxy radicals in the gas-phase ozonolysis of ethene. Chem Phys Lett 301:559–564
Niki H, Maker PD, Savage CM, Breitenbach LP, Hurley MD (1987) FTIR spectroscopic study of the mechanism for the gas-phase reaction between ozone and tetramethylethylene. J Phys Chem 91:941–946
Olzmann M, Kraka E, Cremer D, Gutbrod R, Andersson S (1997) Energetics, kinetics, and product distributions of the reactions of ozone with ethene and 2, 3-dimethyl-2-butene. J Phys Chem A 101:9421–9429
Qi B, Sato K, Imarnura T, Takami A, Hatakeyama S, Ma Y (2006) Production of the radicals in the ozonolysis of ethene: a chamber study by FT-IR and PERCA. Chem Phys Lett 427:461–465
Qi B, Yang B, Wang ZQ, Yang HY, Liu L (2009) Production of radicals in the ozonolysis of propene in air. Sci China B Chem 52:356–361
Rathman WCD, Claxton TA, Rickard AR, Marston G (1999) A theoretical investigation of OH formation in the gas-phase ozonolysis of E-but-2-ene and Z-but-2-ene. Phys Chem Chem Phys 1:3981–3985
Rickard AR, Johnson D, McGill CD, Marston G (1999) OH yields in the gas-phase reactions of ozone with alkenes. J Phys Chem A 103:7656–7664
Saunders SM, Jenkin ME, Derwent RG, Pilling MJ (2003) Protocol for the development of the Master Chemical Mechanism, MCM v3 (Part A): tropospheric degradation of non-aromatic volatile organic compounds. Atmos Chem Phys 3:161–180
Siese M, Becker KH, Brockmann KJ, Geiger H, Hofzumahaus A, Holland F, Mihelcic D, Wirtz K (2001) Direct measurement of OH radicals from ozonolysis of selected alkenes: a EUPHORE simulation chamber study. Environ Sci Technol 35:4660–4667
Wegener R, Brauers T, Koppmann R, Bares SR, Rohrer F, Tillmann R, Wahner A, Hansel A, Wisthaler A (2007) Simulation chamber investigation of the reactions of ozone with short-chained alkenes. J Geophys Atmos 112. doi: 10.1029/2006JD007531
Wyche KP, Blake RS, Ellis AM, Monks PS, Brauers T, Koppmann R, Apel EC (2007) Technical note: performance of chemical ionization reaction time-of-flight mass spectrometry (CIR-TOF-MS) for the measurement of atmospherically significant oxygenated volatile organic compounds. Atmos Chem Phys 7:609–620
Zador J, Turanyi T, Wirtz K, Pilling MJ (2006) Measurement and investigation of chamber radical sources in the European Photoreactor (EUPHORE). J Atmos Chem 55:147–166
Acknowledgements
The contributions of the staff at EUPHORE is gratefully acknowledged, in particular Paco Alacreu, Mónica Vázquez, Mila Rodenas, Amalia Muñoz, and Teresa Vera Espallardo. This work was funded by the UK Natural Environment Research Council (NERC) as part of the TRAPOZ – Total Radical Production from Alkene Ozonolysis project, Grant Ref. NE/E016081/1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Bloss, W.J. et al. (2013). Production of the Atmospheric Oxidant Radicals OH and HO2 from the Ozonolysis of Alkenes. In: Barnes, I., Rudziński, K. (eds) Disposal of Dangerous Chemicals in Urban Areas and Mega Cities. NATO Science for Peace and Security Series C: Environmental Security. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-5034-0_12
Download citation
DOI: https://doi.org/10.1007/978-94-007-5034-0_12
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-5033-3
Online ISBN: 978-94-007-5034-0
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)