Abstract
The present study used an optical imaging paradigm to investigate plastic changes in the auditory cortex induced by fear conditioning, in which a sound (Conditioned Stimulus, CS) was paired with an electric foot-shock (Unconditioned Stimulus, US). We report that, after conditioning, auditory information could be retrieved on the basis of an electric foot-shock alone. Before conditioning, the auditory cortex showed no response to a foot-shock presented in the absence of sound. In contrast, after conditioning, the mere presentation of a foot-shock without any sound succeeded in eliciting activity in the auditory cortex. Additionally, the magnitude of the optical response in the auditory cortex correlated with variation in the electrocardiogram. The area activated in the auditory cortex, in response to the electric foot-shock, also showed a considerable correspondence to that elicited by the CS sound. These results suggest that integration of different sensory modalities in the auditory cortex was established by fear conditioning.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Sensory cortices are defined by responses to physical stimuli in specific modalities. Recently, however, human neuroimaging studies have shown auditory cortex activation without sound. Little is known about how the brain produces such activity. Here we study this topic with an entirely different paradigm, based on fear conditioning, using optical imaging with a voltage-sensitive dye [1] in the guinea pig auditory cortex. Our data show that foot shocks, through conditioning, can activate the auditory cortex in the absence of sound.
The traditional view that auditory cortex neurons are only activated by sound stimuli required revision when functional magnetic resonance imaging in humans showed blood flow changes in auditory cortex during silence [2–4]. It was confirmed that mental imagery [2] and stimulus expectancy [3] create virtual acoustic representations, which may be responsible for the perception of sounds that are physically absent, as when subjects report hearing a familiar song continue even if the music has stopped. The latter finding was obtained when subjects were given no specific instructions, suggesting that the phenomenon had an obligatory aspect [4]. We reasoned that if auditory cortex activation can occur autonomously as a consequence of over learning (e.g., a familiar song), then similar results might be obtained with nonhuman animals in a conditioning paradigm. To investigate effects of conditioning on auditory cortex activity, we used a fear-conditioning paradigm in guinea pigs, pairing pure tones with foot shocks. Fear conditioning and other behavioral and pharmacological paradigms have induced plasticity in various aspects of mammalian auditory cortical representations, but the possibility of signals in the absence of sound, elicited through auditory fear conditioning, remained unexplored.
In this work, to investigate whether auditory information could be retrieved by electric foot-shock after the conditioning or not, the auditory response to a foot-shock was investigated in three groups: normal conditioning, sham conditioning, and naïve.
2 Methods
The experiments were performed in accordance with the guidelines of the Animal Experiments Committee of Tamagawa University. Nineteen guinea pigs of 250–450 g, 3–6 weeks old females were used as experimental subjects. Three groups (normal conditioning, sham conditioning and naïve) were prepared. Six guinea pigs in each normal/sham conditioning group were fear-conditioned while they were awake in the test cage, through a grid shock floor. Fear conditioning was carried out by using a pure tone (CS) and an electric foot-shock (US). The normal/sham conditioning protocols is shown in Fig.1. After the conditioning, the animals were anesthetized and underwent surgery for optical recording. The auditory cortex was stained for 40–60 min with a voltage-sensitive dye, RH795 (0.125 mg/ml, dissolved in saline; molecular Probes). While a pure-tone (duration: 5 s, frequency: 4–16 kHz, acoustic pressure: 65 dB SPL) was presented to the right ear or an electric shock (duration: 0.5 s, current intensity: 0.5–1.5 mA) was applied to hind legs, response from the left auditory cortex was recorded. 100 × 100 ch MOS imaging device (MiCAM ULTIMA-L, Brainvision Inc., Tokyo) was used for recording the fluorescent signals from the cortex. The wavelengths of excitation and emission were 540 ± 30 nm and > 600 nm, respectively.
3 Results
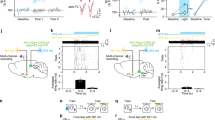
Figure 2 shows the comparison of the ΔRR value measured before and after normal/sham conditioning under anesthesia, where ΔRR is the variation of the R-R interval in the electrocardiogram (ECG). There was a significant difference between pre and post in the normal conditioning group (P < 0.01), suggesting that the guinea pigs in the normal conditioning group were fear conditioned. On the other hand, there was no significant difference between pre and post in the sham conditioning group. Moreover, there was also a significant difference between normal and sham conditioning groups after the conditioning (P < 0.05).
Comparison of the ΔRR value measured before and after the normal/sham conditioning. White and gray color bars represent the ΔRR measured before and after the conditioning, respectively. The four bars represent the mean value of the ΔRR measured for 10 s after CS onset. Error bar is SEM. Normal: n = 6, Sham: n = 6, *P < 0.05, **P < 0.01
Next, the neural responses in the auditory cortex to electric foot-shocks alone were investigated. Figure 3a shows the optical responses to foot-shock alone in the three groups. The neural responses in the auditory cortex could be clearly observed in both the normal and the sham conditioning groups, but no response could be observed in the naïve group. Figure 3b shows the time course of the optical response to foot-shock alone in the three groups. No response was observed in the naïve group, whereas responses were observed in both the normal and sham conditioning groups. ECG data show that the guinea pigs of the sham conditioning group were not statistically significantly conditioned. However there was a tendency of a difference between pre and post conditioning, implying that some level of conditioning might have occurred. The time course of the normal tone response (gray line) shows a peak onset latency of approximately 40 ms and an offset latency of approximately 100 ms. However, the time course of the neural responses in the auditory cortex to electric foot-shock alone (black lines) shows an onset latency of 120 ms and a very long duration of over 500 ms, suggesting that the time course of the optical response to electric foot-shock was very different from that of the responses to pure tones.
In order to confirm the correlation between the ΔRR and the optical response, we calculated correlation coefficient. The correlation coefficient R amounted to −0.68, P < 0.01, implying that there was a significant negative correlation between the ΔRR and the optical responses. Next, the areas of the cortical responses to the CS sounds and the electric foot-shocks were compared. As shown in Fig. 4a, there seemed to be a correlation between the shape and location of the activated area in response to the CS sound and electric foot-shock, although there was a large difference in time scale. In order to compare quantitatively, the normalized cross-correlation between the activated area in response to the CS/non-CS sounds and that to the electric foot-shock was calculated. The normalized cross-correlation R was defined as:
where fi,j and gi,j are the (i, j) th element of the matrix F and G. The matrix F and G represent 100 × 100 ch optical imaging data in response to electric foot-shock and CS/non-CS tone stimuli, respectively. Thus, N, M = 100 in the above formula. In the matrix F and G, the numerical values of fi,j and gi,j were converted into 0 or 1 from the real optical imaging data ΔF/F (%) according to the following rule: fi,j and gi,j = 0 when ΔF/F ≤ 3SD, and fi,j and gi,j = 1 when ΔF/F > 3SD (standard deviation) of the baseline. \( \overline f \) and \( \overline g \) are mean value of fi,j and gi,j, respectively. Figure 4b shows that the activated area in response to electric foot-shock statistically significantly had a larger cross-correlation value for tone response to the CS sound compared to the non-CS sounds.
4 Discussions
It has been reported that neural circuits involving the medial geniculate body, the amygdala, the basal forebrain (BF) and the auditory cortex are very important for fear conditioning using sound and foot-shock, and that the plastic changes in the auditory cortex are induced by acetylcholine (ACh) released from the basal forebrain to the cortex during conditioning [5]. ACh affects pyramidal neurons and promotes plastic changes in the auditory cortex [6]. We propose the following hypothesis about neural mechanisms of auditory fear conditioning. Figure 5 shows a schematic diagram of the hypothesized neural circuit including the auditory cortex, the thalamus, the amygdala and the basal forebrain based on Weinberger’s model [7]. First, CS (tone) information is directly transferred to the auditory cortex by way of MGv (the ventral subdivision of the medial geniculate body) in the thalamus, such that pyramidal neurons in layers II and III produce EPSP (normal tone response). Next, US (foot-shock) information is transferred to the amygdala by way of MGm in thalamus, and ACh is released from BF to the auditory cortex. As a result, LTP and/or LTP-like plastic changes occur in MGm (the magnocellular subdivision of the medial geniculate body) and in the pyramidal neurons in layers II and III of the auditory cortex, where EPSP is induced in response to CS. Then, after conditioning, when US alone is presented, learning-dependent increase in ACh release from BF occurs and US information is transferred to the auditory cortex by way of MGm, eliciting EPSP in the pyramidal neurons where LTP and/or LTP-like plastic changes were induced by conditioning. This, in turn, would result in the emergence of the neural responses in the auditory cortex to a foot-shock alone, and might explain the correlation between the auditory activated area in response to CS sound and US foot-shock. We conclude that integration of different sensory modalities in the auditory cortex was established by fear conditioning.
References
Hosokawa, Y., Sugimoto, S., Kubota, M., Taniguchi, I., Horikawa, J. (2004) Optical imaging of binaural interaction in multiple fields of the guinea pig auditory cortex. Neuroreport, 15, 1093–1097.
Kraemer, D.J., Macrae, C.N., Green, A.E., Kelley, W.M. (2005) Musical imagery: sound of silence activates auditory cortex. Nature, 434, 158.
Voisin, J., Bidet-Caulet, A., Bertrand, O., Fonlupt, P. (2006) Listening in silence activates auditory areas: a functional magnetic resonance imaging study. J. Neurosci., 26, 273–278.
Zatorre, R.J. & Halpern, A.R. (2005) Mental concerts: Musical imagery and auditory cortex. Neuron, 47, 9–12.
Kilgard, M.P. & Merzenich, M.M. (1998) Cortical map reorganization enabled by nucleus basalis activity. Science, 279, 1714–1718.
Bandrowski, A.E., Moore, S.L., Ashe, J.H. (2001) Cholinergic synaptic potentials in the supragranular layers of auditory cortex. Synapse, 41, 118–130.
Weinberger NM, Bakin JS (1998) Learning-induced physiological memory in adult primary auditory cortex: Receptive field plasticity, model, and mechanisms. Audiology & Neuro-Otology 3: 145–167.
Acknowledgments
This work was supported by the twenty-first Century Center of Excellence (COE) Program and the Global COE Program at Tamagawa University and Grants-in-Aid for Scientific Research (A) 19200014, Young Scientists (B) 21700435 and Scientific Research on Innovative Areas 21120006 from MEXT in Japan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer Science+Business Media Dordrecht
About this paper
Cite this paper
Ide, Y., Takahashi, M., Lauwereyns, J., Tsukada, M., Aihara, T. (2013). Integration of Hetero Inputs to Guinea Pig Auditory Cortex Established by Fear Conditioning. In: Yamaguchi, Y. (eds) Advances in Cognitive Neurodynamics (III). Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4792-0_100
Download citation
DOI: https://doi.org/10.1007/978-94-007-4792-0_100
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4791-3
Online ISBN: 978-94-007-4792-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)