Abstract
One of the most important ideas about meaningful learning in chemistry—the triple nature of chemical concepts is further developed in this chapter by Tsaparlis. His text entitled “Linking the Macro with the Submicro Levels of Chemistry: Demonstrations and Experiments that Can Contribute to Active/Meaningful/Conceptual Learning” discusses the chemistry as a multirepresentational structure. Studies have shown that students have great difficulties when trying to grasp concepts at the submicro level. In this chapter, a set of demonstrations and experiments is proposed that, if properly used in teaching by means of active-learning methodology, can contribute to meaningful learning and conceptual understanding of the particulate concepts of matter by properly linking the macro with the submicro levels. Different laboratory work is presented, and the importance of linking different levels of chemical concepts presentations is proposed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Conceptual Understanding
- Potassium Permanganate
- Molar Heat Capacity
- Meaningful Learning
- Potassium Permanganate Solution
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
According to Johnstone (1991, 2000, 2007, 2010), Johnstone and Wham (1982), modern chemistry has three main components: the macro and tangible (dealing with experiments and observations of concrete substances), the symbolic and mathematical or representational (dealing with symbols, equations, and calculations), and the molecular and invisible or submicro (dealing with molecules, atoms, structure and bonding) (see Fig. 3.1). This multi-representational structure (the ‘triplet relationship’) is very important for understanding chemistry (Gilbert and Treagust 2009).
Once we have embedded this structure in long-term memory, we can use it as a powerful tool for looking at the world. However this is not easy. Dealing with levels other than the macro at the early treatments of school chemistry leads to working-memory overload, hence makes learning difficult or impossible. Johnstone (2007, 2010) maintains that almost all the areas of conceptual difficulties and misconceptions that have been studied by researchers over the past 30 years are attributable to the early introduction of the levels other than the macro.
To avoid this overload, we must keep things tangible, staying with the macro level “until pupils have formed new concepts before we attempt to introduce ‘explanations’ based on micro considerations” (Jonhstone 2007, p. 9). Laboratory experiences provide direct contact with substances and phenomena, and so “are essential throughout science education.” Physical science taught without experiments (this must be the case in many countries) is highly unsatisfactory. In addition, experiments and demonstrations are a powerful tool for linking the three levels of chemistry (Tsaparlis 2009). The new kinds of concept at the submicro level take a long time to develop, making chemistry a complex, difficult, and for many students an unpopular subject. A question then arises: does school chemistry follow an orthodox way to lead students to constructing its multi-representational structure shown in Fig. 3.1?
The actual school chemistry of today, as it is taught and tested all over the world by many if not most teachers, places the emphasis on learning rules and algorithms, which enable conscientious students to respond with success to examination questions, including relatively complicated computational questions. Examples of such ‘dexterity’ are the placing of electrons in electron shells and subshells or in orbitals, the rote learning of oxidation numbers of the elements, the writing of chemical formulas, the balancing of chemical equations, the calculation of heats of reactions, etc. If we turn however to matters of conceptual understanding, we realize that our students are as a rule ignorant and cannot answer questions such as: why chlorine appears with so many oxidation numbers, why spontaneous endothermic reactions exist, and why reactions lead in general to equilibrium?
Concentrating on the structural concepts, we present to students as absolute truth the foundation of the whole edifice of chemistry. Students have to accept the teacher’s word for questions such as: (1) How do we know that molecules and atoms exist? (2) What data forced us to accept that the molecules of several elements are diatomic? (3) How the chemical formulae of compounds are determined? (4) How did we discover the structure of the atom and nucleus? (5) How electric charge and the mass of the electron were measured? (6) How the atomic numbers of the elements were determined? (7) On what experimental evidence the placing of electrons in shells and in orbitals was based? (8) What is an atomic or a molecular orbital? (9) How do we know that atoms in molecules vibrate, and that molecules in gases and in liquids rotate?
The Lack of Deep Understanding is a Real Problem of School Chemistry
In a research project that aimed to examine what students assume that contributes to success in school chemistry, Rop (1999) found that success in chemistry can be defined as “doing the work” and “getting good grades on tests” (p. 221). There were, however, some other students for whom a different definition of success seemed to be in operation. For such students, school chemistry does not require them “to understand molecules, atoms, and the ways things work in the real world”: (1) “When I do not understand it, I don’t like it [chemistry]”; (2) “[To really understand chemistry,] we have to know that it’s there [conceptualization of real atoms and molecules] but I can’t grasp it. It doesn’t make sense to me that all this stuff [the student points to tables and chairs] would be made of little things”; (3) “… but there must be something awesome out there: [an unseen but wonderful world of moving, acting electrons and atoms—somewhere beyond the constant plodding on of daily life in chemistry class.”] (p. 229).
Corpuscular/particulate and structural concepts constitute the corner stone of chemistry. These concepts are highly abstract, lacking both in perceptible examples and perceptible attributes, and should be considered formal in the Piagetian sense: hence “it is quite likely that they cannot be totally understood without some formal reasoning” (Herron 1978). Tsaparlis (1997a) employed the following perspectives, and arrived at the same conclusion about pupils’ difficulties in learning the atomic and molecular concepts: (1) the Piagetian developmental perspective, (2) the Ausbelian theory of meaningful learning, (3) the information processing theory, and (4) the alternative conceptions movement.
The adoption in teaching of a three-cycle method which separately covers the macro, the representational, and the submicro levels of chemistry should be considered seriously as a good method for introductory chemistry (Georgiadou and Tsaparlis 2000). In the macro cycle, which occupied half of the teaching time, the students became familiar with chemical substances and their properties. Central here was the use of experiments, while chemical notation as well as atoms and molecules were not included. Applying the spiral curriculum, the representational cycle covered the same course material, but added chemical formulas and equations. Finally, the submicro cycle brought atoms and molecules into play. Evaluation of the method, by means of end-of-school-year tests as well as by beginning-of-next year repeat of the same tests, showed that the three-cycle method made the largest single positive effect, compared with a traditional control class and a class in which teaching methods proposed by psychologist R. Case were applied. [According to Case (1978a, b), successful instruction must somehow accomplish the following two objectives: (a) to demonstrate to students that their current strategy must and can be improved upon and (b) to minimize the load on students’ working memory.]
Mention should also be made of a freshman chemistry curriculum, in which the topic of atomic structure is delayed until the second semester (Toomey et al. 2001). In this curriculum, concept development is linked to the observable behavior of matter, while the submicroscopic and symbolic realms are introduced by engaging students in some of the detective work that established the relative atomic masses of the elements and formulas of simple compounds. In this way, students have an opportunity to become familiar with the relationships among facts, definitions, hypotheses, deductions, and predictions, which are central to the enterprise of science. Similarly, Nelson (2002) proposed a way for teaching chemistry progressively, starting with observations at a macroscopic level, interpreting these at an atomic and molecular level, and then at an electronic and nuclear level. Finally, Tsaparlis with colleagues wrote a textbook for eighth-grade chemistry with emphasis given to the macroscopic phenomena and concepts that are treated qualitatively, using constructivist and meaningful-learning teaching methods, while the particulate concepts are delayed (Tsaparlis et al. 2010).
A time then comes, be that early or delayed, that we have to introduce in our chemistry courses the particulate concepts of molecule, atom, electron, etc. It is not then surprising that most chemistry and general science courses introduce these concepts in an almost axiomatic, quasi dogmatic way (see above). Niaz and Rodriguez (2000) defined criteria based on history and philosophy of science, and used them to evaluate presentation of atomic structure in general chemistry textbooks. They found that most of the newer (1970–1992) and older (1929–1967) textbooks not only ignore history and philosophy of science, but also present experimental findings as a ‘rhetoric of conclusions.’ It was concluded that such presentations are not conducive toward a better understanding of scientific progress.
In this chapter, the aim is to propose a set of demonstrations and experiments that, if properly used in teaching by means of active-learning methodology, can contribute to meaningful learning and conceptual understanding of the particulate concepts of matter. Although essentially all meaningful learning is ‘active’ in the sense that the learner actively links new learning with his/her pre-existing knowledge and understanding (see Chap. 1), in practice, the application of such teaching methodologies is primarily the job and responsibility of the teacher, and less so of the textbooks. The demonstrations and experiments can be used of course in teacher-centered receptive-learning approaches, but the outcomes in terms of quality of learning might not be the desired ones. Taking into account that secondary school teachers have often a limited knowledge about the findings of educational research (Costa et al. 2000), it is necessary that teachers are aware of active-learning methodology.
Teaching for Active Learning and Conceptual Understanding
Ausubel’s Theory of Meaningful Learning
There are various theories of teaching that can contribute to conceptual understanding. One could start with Ausubel’s theory of meaningful learning (Ausubel 2000), which concentrates on the influence of prior knowledge on how learning occurs and is based on the golden rule of educational practice, which states that teaching should be done according to what students already know. Ausubel postulates that meaningful learning occurs when the learner’s appropriate existing knowledge interacts with the new learning. On the contrary, if such interaction does not occur, the result is rote learning. The interaction is realized by means of the so-called subsumers, that is, any concept, principle or generalizing idea that the student already knows, and which provides association or anchorage for the components of the new knowledge.
All structural concepts must be built on new ground, that is, the proper subsumers/anchorages for this knowledge should pre-exist in students’ minds. In this spirit, we have to admit that chemistry needs basic concepts from physics, such as mass, density, weight, atmospheric pressure, temperature, heat, energy. At higher levels, chemistry is further dependent on physics. According to research findings (Harris 1983), a group of 40 students, who had completed high school chemistry and physics, achieved considerably higher in first-year college general chemistry (79.0 % with standard deviation 9.2) than an equal number (40) who lacked prior physics preparation (63.2 % with s.d. 13.0); that is, physics is deemed an important factor for success in college chemistry. This gives a rationale for physics before chemistry or for chemists to establish physics ideas before chemical ones are attached.
Needless to add that mathematics is also important to physics and chemistry learning, contributing to the complexity of these subjects. Mathematics is essential for the meaningful learning of physics and chemistry, but for this to happen it must be coupled with understanding of the underlying physical concepts. Several studies (Griffith 1985; Hudson and Liberman 1982; Hudson and McIntire 1977; Liberman and Hudson 1979) have attempted to correlate mathematical skill and student reasoning ability with success in physics. It appears that mathematical skills seem to be necessary but not sufficient for success in physics. There are students with marginal mathematical skills, but with well-developed logical and conceptual skills who can be successful in physics. Related to this is the fact that different instructors may place different demands on the students with regard to mathematical ability. Some may be content with the capacity of students to connect physics and chemistry (especially physical chemistry) with mathematics, while others may pay more attention to mathematical operations and calculations.
Constructivism and Active Learning
According to the Alternative Conceptions Movement, students form their own models for atoms, molecules, and bonding which are at variance with the scientific views taught in schools. Griffiths (1994) has critically reviewed students’ chemistry misconceptions and has enumerated 67 misconceptions about matter and 14 misconceptions about chemical bonding (see also Griffiths and Preston 1992). A similar review has also considered the same concepts (Garnett et al. 1995). These students’ concepts are explained by means of the theory of constructivism. It is the duty of teachers, firstly to recognize their students’ ideas, and secondly to take them into account in planning and performing their teaching, so that the aim of conceptual change is fulfilled. Constructivist teaching and learning (von Glasersfeld 1989) are the banner of modern science education.
Active learning refers to several models of instruction that give the learner the initiative in the learning process (Bonwell and Eison 1991). One form of active learning is discovery learning (guided-discovery learning), the idea of which goes back to John Dewey, but it was fully developed in the 1960s by Bruner (1961). It takes place in problem solving situations. Inquiry-based learning is also a form of active instructional method that developed during the discovery learning movement. In inquiry-based learning, priority is given not to the mere acquisition of knowledge by the student but to the student developing experimental and analytical skills.
To sum up, according to Johnstone (2007, p. 10), “there are a number of messages from research which, if applied, would make our students’ experience of science more meaningful, enjoyable and yet intellectually demanding and satisfying. These messages are: (1) What we learn is controlled by what we already know; (2) Learners can process only a limited amount of information at one time; (3) Science concepts exist on more than one intellectual level; (4) Many scientific concepts are of a different kind from everyday concepts; (5) Learners need to start with concepts built from tangible experience and developed later to include inferred concepts.”
It is pertinent to emphasize at this point that although Ausubel’s meaningful learning and constructivism have been presented here as separate, Ausubel’s main ideas are compatible with constructivism. This follows from the fact that, as commented earlier, essentially all meaningful learning is ‘active,’ and by coupling this with the above argument that constructivist learning is compatible with active learning.
Constructivist and Active Approaches to Teaching Particulate Concepts
A number of years ago, an international seminar (Linse et al. 1990) was dedicated to the relation of macroscopic phenomena to (sub)microscopic particles. Ben-Zvi et al. (1990) confirmed that the root of many difficulties that beginning chemistry students have are due to the deficient understanding of the atomic model and how it is used to explain phenomenology and the laws of chemistry. Appropriate models are essential also to explain the link between energy transfer and temperature change in chemical changes, as well as the link between the molecular model and the energy transfer. Having studied students’ relevant views and the problems concerning macro–micro relationships in the area of structure and reaction, the authors proceeded to propose a teaching unit to help overcome students’ difficulties. The unit employed a well-known statistical-thermodynamics model, coupled with mechanical models, to explain the energy changes accompanying reactions.
Meheut and Chomat (1990) attempted to make 13–14 year old children build up a particulate model of matter by working out a sequence of experimental facts, starting from properties of gases (compression, diffusion), then moving on to solids, leaving the liquids to last. On the other hand, Millar (1990) placed the emphasis on employing everyday contexts (on the basis of the Salters’ approach: Hills et al. 1989), using, e.g., a piece of cloth (which is made of fabrics, made of threads, and made of fibers) to move from the macroscopic to the submicroscopic level (see also Tsaparlis 1989). For Millar, many children need time and experience to appreciate that gases are really matter, so he suggested that it may be wise to start with solids, and postpone consideration of gases until later.
Finally, in a collective volume, Nussbaum (1998), after critically reviewing the various relevant propositions in the 1990 international seminar, dealt with the constructivist teaching of particulate theories, using the history-and-philosophy-of-science approach. Vacuum physics is, according to Nussbaum, the right starting point for particulate physics. Only the existence of a vacuum can justify the noncontinuous nature of matter, hence its particulate nature. In addition, vacuum allows for motion of the particles. Nussbaum bases his introduction of the particulate model on the study of air and other gases, and maintains that the study of the particulate model is a long process of conceptual change, in which students’ wrong ideas can play a positive role.
As stated already, in this chapter, the aim is to propose ways that can contribute to conceptual understanding and to meaningful/active-learning methodologies for the teaching and learning of the particulate concepts. Though I subscribe to Nussbaum’s position that the concept of vacuum is central for a conceptual understanding of particulate concepts, for younger students, I am in favor of starting with liquids, then taking up solids, and leaving (in agreement with Millar) gases to last. Of necessity, some discussion of the properties of gases is essential too. Emphasis will be placed on discussing prerequisite physics concepts and techniques that are deemed essential for realizing the above aim.
Physical science taught without experiments is highly unsatisfactory. Experiments and demonstrations are a powerful tool for providing direct contact with substances and phenomena, as well for linking the three levels of chemistry (Tsaparlis 2009). To carry out and to interpret the experiments, students could work in groups of 2–4. In this way, cooperative learning is encouraged and promoted. If it is not feasible for students themselves to carry out the experiments, the teacher should use demonstrations, where the experiment is carried out in front of the class by a pair of students under the guidance of the teacher.
Needless to add that the psychology of learning requires that, before dipping into the submicro world of particles, students must be fairly familiar with the relevant phenomena at the macro-level. In Ausubel’s terminology, it is important to introduce macro anchorages before the submicro concepts are introduced. Again, this makes it imperative that basic physics ideas are established before chemical ones.
Introduction of the Concept of the Molecule
To introduce the concept of the molecule, one needs certain macroscopic concepts and phenomena, such as the phenomenon of diffusion, states of matter, kinetic theory, changes of states of matter, and the concept of temperature. Note that many ideas described below are from an introductory chemistry text aimed at 12 year olds (Johnstone and Morrison 1964).
Diffusion
It is common experience and knowledge that if we open a bottle containing a volatile liquid, e.g., ether, the ether vapor escapes and diffuses into the atmosphere. Similarly, a crystal of potassium permanganate placed on the surface of water is seen soon to dissolve and diffuse into the water. By adding water to the potassium permanganate solution and stirring, we observe that the initially purple color becomes pink and eventually almost disappears. The crystal may have spread itself through water more than a million times its own volume! What has been happening to the crystal as it dissolved? Does the crystal stretches like rubber or has it broken down into minute pieces (‘particles’), which disperse themselves through the water?
Is there a limit to the spreading? If one blows some light dust such as fine, dry chalk powder on to the surface of some water contained in a large dish, and then adds one drop of an oily material to the centre of the water surface, the oily material spreads out on the surface. Is there a limit to how far the oil spreads? Again, the oil may have spread out like a rubber sheet, and there is a limit to how far a sheet will stretch. The particle picture is different.
An analogy will help here. Let us allow small wooden balls to float on water, representing the dust on the surface. Then a beaker of wooden balls of another color (perhaps and size), representing particles of oil is poured into the center of the surface. The balls are not on top of each other, but form a single layer. The limit to the spreading comes when there are no more balls piled on top of each other. Again two pictures seem to fit: (1) the oil has spread out like a rubber sheet; (2) the oil has spread out to give a layer one particle thick, and has pushed the dust to the edge of the bowl. [An experiment based on this principle, using a stearic acid film, can be used for determining the value of the Avogadro constant (Ift and Roberts 1975).]
Collapsing Balloons
Gases can help us resolve the above dilemma with respect to a rubber-like stretching macro material or a particulate structure. Two identical balloons are filled to the same size one with air, the other with helium, and the necks of both are airtight (this can be checked by dipping the necks into water and observing if leaking of gas occurs). The balloons are then left until the next chemistry lesson, when we observe that the balloons have become smaller, with the helium-filled balloon being much smaller. Checking and ruling out the possibility of gas leaking from the necks of the balloons, we are left with the explanation that very small holes must exist in the balloon rubber, which allows the gases to escape. Which of our two models fits now? The particle idea is more appropriate for explaining this experiment: the particles of helium are smaller than those of air, and they must escape through the holes at a higher rate.
Ever-Moving Particles
In the experiment of the crystal of potassium permanganate placed on the surface of water, without any stirring, it is seen that the crystal soon dissolves and diffuses into the water. What is the cause of the observed movement? An analogy can be of help here.
Consider an overcrowded bus. A passenger wanting to alight at a certain station finds it hard to move to the door of the bus, and has to ask and even to push other passengers to make his or her way to the door. The situation in the case of a bus with few passengers is quite different where movement of a passenger is unobtrusive.
Since movement is observed in the case of diffusion, one needs a micro-picture of a gas or a liquid, which allows for empty spaces to be there. Because most gases are colorless and thus invisible, the case of gas is more complicated. A liquid however makes things straightforward: in a liquid, these empty spaces are not directly observable, but instead we sense the continuous presence of the macro-material. The empty spaces make it necessary for the material to be present in distinct lumps. These lumps can be defined as the molecules.
Dissolving salt in water or mixing ethanol and water leads to the fact that the eventual volume of the solution is smaller than the combined volume of salt and water or ethanol and water. A particle model can help explain the observations: fill a beaker with small balls and pour sand to fill the spaces—you do not raise the level of the balls above the top of the beaker.
Half fill a small test tube with a solution of gelatin in water. After the gelatin sets (like the familiar table jellies (Jell-O)), pour some yellow potassium chromate solution on top of it. Take another test tube with gelatin in it, and place a crystal of blue copper sulfate on top.
In the case of solid materials, diffusion is more difficult (or even impossible), and this can be accounted for by assuming that molecules are closely packed in making the crystals, but still leaving empty spaces. Note that the properties of crystals make it imperative for the molecules of a substance to be of the same kind and size (Jones and Childers 1984).
Brownian Motion
One can see movement by watching under a microscope slide on which very fine pieces of blue poster paint are suspended in water. Similarly, observations are possible using some very fine specs of smoke in air, placed in a small box which has two glass windows, with light shining through the side, and observed through the other window by means of a microscope. A model like that helps explain Brownian motion. This kind of movement can be brought about by the bombardment of a large particle with many smaller ones. Similarly, the movement of the smoke and the paint may be due to the bombardment by unseen water or air particles in motion.
From our experiments and our thinking, we have the following picture of the composition of materials: (1) Matter is made up of very small particles, too small to be seen by the human eye; (2) The particles of different substances may have different sizes; (3) The particles are extremely light; (4) The particles are in motion, but different kinds of particles may move at different speeds.
Difference of Properties of a Substance and its Molecule
We can reformulate our definition of molecules as ‘the building blocks of substances,’ in the same way as bricks are the building blocks of walls, or the rings of a chain, or the threads of a textile. However, many students believe that molecules maintain all (physical and chemical) properties of the macroscopic material, e.g., temperature, physical state, hardness, etc. Thus, a single water molecule is assumed to be like a very tiny droplet of water. This is caused mainly by a faulted definition of a molecule as “the smallest particle of a substance that still retains all [physical and chemical] properties of a mass of the substance.” Such a definition is given by some authors (e.g., Fine 1978; Merill 1973) or implied by others (e.g., Sherman and Sherman 1983), and is misleading in suggesting that bulk properties can be attributed to the individual particles (IUPAC 1993). Analogies can be useful in this respect: a wall and the bricks, a chain and its rings, a textile and its fibers (Tsaparlis 1989).
Temperature
Let us consider two visually identical cups of water, one containing water at 5 °C, the other at 30 °C. There is nothing we can see that causes the water in the two cups having a different temperature. We know of course the origin of the temperature difference (for instance cooling or heating the water), but what is there inside water that is responsible for the different temperature? The different rates of diffusion of potassium permanganate in cold and warm water can provide the links to the different rates of motion of particles with temperature change.
Change of Physical State
‘Change of physical state’ is a topic usually studied within physics. However its relation to chemistry in connection with the concepts of molecules and their varying movement and interaction in the three states is very strong (Meheut and Chomat 1990). An analogy could help here: let us consider a plastic cylinder sitting on a vibrator filled with small polystyrene balls with a card disc sitting on them. This is the solid state. As the vibrator is switched on, on low power, the balls begin to move slightly as a liquid. As the energy input is increased the balls begin to fly about and lift the card. As the energy input increases further, the card rises further showing expansion. This analogy takes in physical state and change of state as well as some idea of energy and temperature change.
The Concept of Energy
In principle, energy is an interdisciplinary scientific concept (Tsaparlis and Kampourakis 2000). However, it is studied more systematically in physics courses. The concept of energy (especially chemical energy) as well as the concept of interaction are very difficult for young students (Duit 1986; Duit and Häußler 1994). Yet they are essential to many aspects of chemistry and physics. For this reason, the integrated physics and chemistry program proposed by Tsaparlis and Kampourakis (2000) introduces energy from the introductory lesson. Energy is necessary to study changes of state, the concept of temperature, as well as chemical reactions. In addition, it is required as a discriminating factor in distributing electrons to electron shells and to orbitals.
Very helpful for the understanding of the concept of energy is the concept of gravitational energy and especially of gravitational potential energy, that is, is the energy that arises from gravitational force (from the gravitational interaction). In addition to potential energy, we need the principle of energy minimization, as predictor of the most stable (ground-state) electron configuration of atoms and molecules.
In atoms and molecules, potential energy is electrostatic, arising through Coulomb forces. Basic concepts from electricity are essential here. Attach two inflated balloons by a string. Rub both on your hair to give them the same charge and hold the middle of the string. The balloons stand apart as like charges repel. The attraction can be seen by rubbing a balloon on your hair and then taking it away from your head slowly. The hair has a charge opposite to that on the balloon and the hair stands on end, attracted to the balloon.
In contrast to the case of gravitational energy (where we usually set the zero at ground level, so the potential energy at points above the ground assumes positive values), we define the electrostatic potential energy at infinite distance from the nucleus of an atom as zero; hence all energy values at finite distances from the nucleus have a negative sign.
Finally, considering the topics of relative sizes of ions and patterns in ionization energies, Taber (1998) concluded that chemistry teachers base their relevant presentations on the principles of Coulomb electrostatics. However, many students do not have the same background in physics as their teacher, with the result that they apply alternative assumptions in the context of interactions in atoms and molecules.
Vibrational and Rotational Spectroscopies
With more mature students (at upper secondary level and in university general chemistry), vibrational spectra can be used for justifying the concept of vibrating molecules. Rotational (microwave spectra) or the rotational structure of vibrational spectra of gases can be used to rationalize rotation of molecules. It is true that spectra are a theme usually studied in physics, but its strong connections with chemistry should not be overlooked.
The Concept of the Atom
To introduce the concept of atom by means of active/constructivist and meaningful-learning methodology, one certainly needs to dwell on the historical aspects of this concept. Historical experimental evidence for the existence of atoms has been invoked by Jones et al. (1984): the law of definite proportions of Proust, Dalton’s atomic theory, Gay Lussac’s law, Avogadro’s hypothesis, and Faraday’s law of electrolysis. Niaz and Rodriguez (2001) have used examples from the topics of atomic structure, kinetic theory, covalent bonding, and the law of multiple proportions, to illustrate how a History-and-Philosophy-of-Science perspective can facilitate students’ conceptual understanding.
Toomey et al. (2001) have shaped a program which follows a historical approach. Observations about gases, liquids, and solids are used to support the atomic theory. Further, the laws of definite and multiple proportions are used to suggest that atoms may be bonding to one another to form molecules when a compound is formed. The gas laws are introduced next, and further connected to the kinetic theory. Students deduce that an oxygen atom should be eight times as massive as a hydrogen atom, and the concept of relative atomic mass unit is introduced. Following that, students are introduced to experimental observations about the volumes of gases that react with each other when the temperature and pressure have the same initial and final values. The law of combining volumes is introduced next, and the fact that two volumes of the product gases are produced in various reactions. The Avogadro’s hypothesis follows, and students are asked to use the hypothesis and the experimental facts about combining volumes to make various deductions. Returning to the kinetic theory, relative velocities of different gases at the same temperature are compared, and their relative particle masses predicted using Graham’s law.
Nelson (2002) suggested that students should be introduced to the following phenomena, which can be demonstrated with suitable experiments (e.g., Fowles 1957; Nelson 1996a; Sienko et al. 1984): law of conservation of mass; phenomenon of constant composition; phenomenon of multiple proportions; phenomenon of proportionate gaseous volumes.
There are several indications that matter may be made up of atoms: Many solids are crystalline; this can be explained in terms of the regular packing of small particles. Gases are much more compressible than liquids or solids, and when they condense there is a large reduction of volume. These observations can be explained if gases comprise separate particles, which come together in the liquid or solid state. These may be atoms or clusters of atoms (molecules). When a small quantity of olive oil is poured on to a large pool of water, the oil only spreads over a limited area of the surface. These considerations, along with the phenomena of the previous paragraph, lead to the following theory of matter, after Dalton and Avogadro (Nelson 2002): (1) Matter is made up of atoms; (2) The atoms of an element are all the same, and differ from those of other elements (provisional statement); (3) Chemical reactions involve changes in which atoms are combined, but not in their number; (4) Atoms of different elements often combine in different ratios; (5) These ratios are often small whole numbers; (6) Avogadro’s hypothesis.
This theory explains the law of conservation of mass, constant composition, multiple proportions, and proportionate gaseous volumes, as well as the fact that in the reaction between hydrogen and chlorine to form hydrogen chloride, the volumes are in the ratio 1:1:2. This leads to hydrogen comprising hydrogen molecules H2m, chlorine comprising chlorine molecules Cl2n, and hydrogen chloride comprising hydrogen chloride molecules HmCln (with m not necessarily equal to n). Also, postulate six enables the masses of molecules to be compared. For example, the density of hydrogen at STP (standard temperature and pressure) is 0.08988 g/L and of oxygen 1.4290 g/L, thus 1.4290/0.08988 = 15.899. The result is approximate because of the pressure; the limiting value at low pressures is 15.875. If the mass of a hydrogen molecule (μ) is provisionally made the unit of mass for atoms and molecules, the mass of an oxygen molecule is therefore about 16 μ.
To establish the atomic composition of a molecule, a further principle needs to be added to (after Cannizzaro). This is: The mass of an atom of an element is the smallest mass of the element found in any molecule containing it.
The conclusion that matter is made up of atoms and molecules is supported by the results of the kinetic theory of gases. Electron tunneling microscopy can be useful at this point for providing images of atom arrangements on metallic surfaces, while mass spectra are useful for the modern way of establishing the relative atomic masses, as well as relative molecular masses. In addition, X-ray diffraction patterns can be used with students at upper secondary level (Tsaparlis 2004).
Electrons and Electron Configurations
In Toomey et al. (2001) approach, electrons are not introduced until week 12, and atomic number is not introduced until week 1 of semester two. On the other hand, topics like quantum numbers and orbitals have been eliminated, and replaced in semester two by presentations that use comparison of ionization energies to suggest the existence of different energy levels in atoms.
The classic experiments that proved the existence of electrons, and determined its charge and mass (Thomson, Millikan) are a must for upper secondary students; similarly Goldsteins’s experiment that proved the existence of protons. Models and computer simulations can be very useful here. In addition, atomic spectra, fluorescent tubes with inert gases, as well as the coloring of a flame by metals and salts are useful resources for teaching.
Chemical Bonding
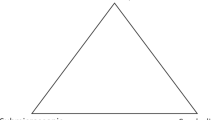
Nelson (1994) distinguished among three “limiting types” of binary compounds: metallic, salt-like (ionic), and nonmetallic. These represent extremes, and most binary compounds fall somewhere in between these extremes. In practice, determining the degree of salt-like character is difficult, since it requires accurate electrochemical measurements at high temperatures on melts. The diagram in Fig. 3.2 places the limiting types at the corners of a triangle. From the triangle, we deduce that there are four intermediate types, A, B, C, and D (see also Table 3.1). The properties of the intermediate types can be inferred from the properties of the limiting types. Thus:
- Type A :
-
High transparency, no luster, weak electrolytic conductivity in fused state. Examples: beryllium chloride, BeCl2, and zinc chloride, ZnCl2. These are colorless, and in the fused state they conduct electricity electrolytically. Their conductivities are only a fraction, however, of those of fused MgCl2 and CaCl2 (about 0.5 Ω−1 m−1, as compared with about 100 Ω−1 m−1)
- Type B :
-
High opacity, possible some luster, semiconducting in solid and liquid. Example: iron monoxide FeO, which is black and has a conductivity at room temperature of 2 × 103 Ω−1 m−1. Electrolytic conduction in the melt is negligible
- Type C :
-
High opacity, possibly some luster, both electrolytically conducting and semiconducting (i.e., with a direct current, chemical decomposition takes place, but less than the amount required by Faraday’s laws)
- Type D :
-
Like C, but lower conductance. Example of type C/D: Dicopper sulfide, Cu2S, which is also black. At room temperature, it is a semiconductor, with a conductivity of 3 Ω1 m−1. At higher temperatures, the conductivity rises, and electrolytic conduction makes a contribution, reaching about 85 % at 400 °C
The distinction between molecular and nonmolecular substances is an important one in chemistry, and can be done without having to appeal to X-ray crystallography, by classifying substances on the basis of volatility and solubility (Nelson 1996b). Finally, the analogy of the covalent chemical bond as an “atomic tug-of-war” (Tsaparlis 1984) is useful for teaching the concept of covalent bond, as well as the distinction between polar and nonpolar covalent bonds.
The Amount of Substance Concept
Central in school chemistry are numerical/stoichiometric calculations that determine the masses (and of volumes in the case of gases) of substances that are consumed and/or produced in chemical reactions (Schmidt 1994). Fundamental here is the amount of substance (Mole) concept. There has been a large literature of the 1970s, the 1980s and the 1990s that focused on the complexity of this concept and the difficulties students and teachers encounter in dealing with and using it (e.g., Bent 1985; Cervellati et al. 1982; Dierks 1981; Duncan and Johnstone 1978; Furió et al. 2000; Ingle and Shayer 1971; Lazonby et al. 1984; Nelson 1991; Novick and Menis 1976; Schmidt 1994; Staver and Lumpe 1993, 1995; Stromdahl et al. 1994; Tullberg et al. 1994). A series of demonstrations will be described below that aim to build the amount of substance concept as a unifying concept in chemistry. They have been used by Johnstone:
-
Compare the volumes of equal moles of an organic homologous series (e.g., methanol, ethanol, propanol, butanol, pentanol) to see patterns.
-
Compare moles of sugars to “see” mono and disaccharides.
-
Compare the volumes of equal moles of finely ground halides with the same cation (e.g., NaCl, NaBr, NaI) to “see” relative halide ion sizes.
-
Look at molar heat capacities for partners. Take, for instance, the metals Li, Mg, and Al. Their specific heat capacities are respectively: 3,390, 1,030, and 900 J kg−1 K−1. By multiplying each of these values by the corresponding relative atomic mass, we find the following values for the molar heat capacities: 23,730, 24,720, 24,300 J kmol−1 K−1, which are very close to each other. Similar values are found for other elements.
-
Similarly, let us look at molar gas volumes for patterns. For the gaseous elements H2, He, N2, and Ne, the corresponding densities under STP are: 0.09, 0.18, 1.25, and 0.90 gL−1. If we divide the corresponding relative molecular or atomic mass by each of these values, we find the following values for the molar volumes at STP: 22.2, 22.2, 22.4, 22.2 Lmol−1. Similar values are found for other species.
-
Compare a 20 l drum (approximate volume of one mole water vapour) with one mole (18 mL of liquid water).
Quantum Chemical Concepts
Atomic and molecular orbitals and related concepts are highly abstract, and their introduction in high school course may be problematic (Papaphotis and Tsaparlis 2008a, b; Tsaparlis 1997a, b; Tsaparlis and Papaphotis 2002). Alternative ways that avoid the orbitals at both the high school and the general chemistry level might be preferable. Gillespie maintained that Lewis structures, and the VSEPR model are sufficient for high school, while the electron-domain model is sufficient for general chemistry, with emphasis placed on electron density rather than orbitals (Gillespie 1991, 1992a, b, c; Gillespie et al. 1994, 1996; Gillespie and Mata 2001). For instance, using the physical repulsion of balloons (e.g., two, three, four blown balloons tied together) can lead us to VSEPR, and then we could go a very long way in organic and inorganic chemistry (Johnstone et al. 1981), without the need to discuss orbitals and hybridization.
Concluding Remarks
To have a good conceptual understanding of particulate and structural concepts, students need a firm grasp of the underlying physics concepts, for which the use of the history and philosophy of science is very useful. According to Niaz and Rodriguez (2000), this can be introduced in the classroom not necessarily through formal courses in the history of chemistry or comments and anecdotes, but rather by incorporating the ‘heuristic principles’ that guided the scientists to elaborate their theories. On the other hand, modern techniques (mass, electronic, vibrational, and rotational spectra, electron tunneling microscopy, X-ray diffraction) can be quite conducive to the active/constructivist, and meaningful learning approach, but these should be mostly reserved for the more advanced students at the upper secondary level and for students in university general chemistry.
It is important to realize that we need more knowledgeable teachers, both with respect to the content of science, and the active/constructivist, and meaningful learning methodologies. In point of fact, according to the chart of the American Association for the Advancement of Science (NCRTL 1994), future teachers must know science and their subject of specialty (physics, chemistry, biology) more deeply than it usually is the case. On the other hand, Gillespie (1997) has suggested that new chemistry textbooks should be written that should aim on the one hand to be interesting for the vast majority of students, and on the other hand to be providing them with an understanding of chemistry. Finally, when is understanding (e.g., of atomic structure) sufficiently good and complete? This question sets up a paradox: “the more one learns about some aspect of the world, the more aware one is likely to become of the depth of one’s ignorance of it. That does not necessarily mean that as a consequence of learning, one’s understanding actually decreases, but simply that one’s appreciation of the complexity of that aspect of the world is likely to increase—which may be, after all, a better understanding of a fundamental sort” (Rop 1999, p. 233).
References
Ausubel, D. P. (2000). The acquisition and retention of knowledge: A cognitive view. Dordrecht: Kluwer Academic.
Bent, H. A. (1985). Should the mole concept be X-rated? Journal of Chemical Education, 62(1), 59.
Ben-Zvi, R., Silberstein, J., & Mamlok, R. (1990). Macro-micro relationships: A key to the world of chemistry. In P. L. Lijnse, P. Licht, W. De Vos, & A. J. Waarlo (Eds.), Relating macroscopic phenomena to microscopic particles (pp. 183–197). Utrecht: University of Utrecht, Centre for Science and Mathematics Education.
Bonwell, C. C., & Eison, J. A. (1991). Active learning: Creating excitement in the classroom. AEHE-ERIC Higher Education Report No. 1. Washington, D.C.: Jossey-Bass.
Bruner, J. S. (1961). The act of discovery. Harvard Educational Review, 31(1), 21–32.
Case, R. (1978a). Implications of developmental psychology for the design of instruction. In R. Glaser, A. Lesgold, J. Pellegrino, & J. Fokkema (Eds.), Cognitive psychology and instruction (pp. 441–463). New York: Plenum.
Case, R. (1978b). Intellectual development from birth to adulthood: A new-Piagetian interpretation. In R. S. Siegler (Ed.), Children’s thinking: What develop. New Jersey: Hillsdale, Erlbaum.
Cervellati, R., Montuschi, A., Perugini, D., Grimellini-Tomasini, N., & Pecori Balandi, B. (1982). Investigation of secondary school students’ understanding of the mole concept in Italy. Journal of Chemical Education, 59(10), 852–856.
Costa, N., Marques, L., & Kempa, R. (2000). Science teachers’ awareness of findings from educational research. Chemistry Education Research and Practice, 1(1), 31–36.
Dierks, W. (1981). Teaching the mole. European Journal of Science Education, 3(2), 145–158.
Duit, R. (1986). In search of an energy concept. In R. Driver & R. Millar (Eds.), Energy matters (pp. 67–101). Leeds: University of Leeds.
Duit, R., & Häußler, P. (1994). Learning and teaching energy. In P. J. Fensham, R. F. Gunstone, & R. T. White (Eds.), The content of science: A constructivist approach to its teaching and learning (pp. 185–200). London: The Falmer Press.
Duncan, I. M., & Johnstone, A. H. (1978). The mole concept in chemistry. Education in Chemistry, 10(6), 213–214.
Fine, L. W. (1978). Chemistry (2nd ed.). Baltimore: Williams & Wilkins.
Fowles, G. (1957). Lecture experiments in chemistry (4th ed.). London: Bell.
Furió, C., Azcona, R., Guisasola, J., & Ratcliffe, M. (2000). Difficulties in teaching the concepts ‘amount of substance’ and ‘mole’. International Journal of Science Education, 22(12), 1285–1304.
Garnett, P. J., Garnett, P. J., & Hackling, M. W. (1995). Refocusing the chemistry lab: A case for laboratory-based investigations. Australian Science Teachers Journal, 41(2), 26–32.
Georgiadou, A., & Tsaparlis, G. (2000). Chemistry teaching in lower secondary school with methods based on: a) Psychological theories; b) the macro, representational, and submicro levels of chemistry. Chemistry Education Research and Practice, 1(2), 217–226.
Gilbert, J. K., & Treagust, D. F. (Eds.) (2009). Multiple representations in chemical education. Dordrecht: Springer.
Gillespie, R. J. (1991). What is wrong with the general chemistry course? Journal of Chemical Education, 68(3), 192–194.
Gillespie, R. J. (1992a). The VSEPR model revisited. Chemical Society Reviews, 21(1), 59–68.
Gillespie, R. J. (1992b). Multiple bonds and the VSEPR model. Journal of Chemical Education, 69(2), 116–121.
Gillespie, R. J. (1992c). Electron densities and the VSEPR model of molecular structure. Canadian Journal of Chemistry, 70(3), 742–750.
Gillespie, R. J. (1997). Reforming the general chemistry textbook. Journal of Chemical Education, 74(5), 484–485.
Gillespie, R. J., Eaton, D. R., Humphreys, D. A., & Robinson, E. A. (1994). Atoms, molecules and reactions: An introduction to chemistry. Englewood Cliffs: Prentice-Hall.
Gillespie, R. J., & Matta, C. F. (2001). Teaching the VSEPR model and electron densities. Chemistry Education Research and Practice, 2(2), 73–90.
Gillespie, R. J., Spencer, J. N., & Moog, R. S. (1996). Demystifying introductory chemistry, Parts 1 & 2. Journal of Chemical Education, 73(7), 617–626.
Griffith, W. T. (1985). Factors affecting performance in introductory physics courses. American Journal of Physics, 53(9), 839–842.
Griffiths, A. K. (1994). A critical analysis and synthesis of research on students’ chemistry misconceptions. In H. J. Schmidt (Ed.), Problem solving and misconceptions in chemistry and physics (pp. 70–79). Hong Kong: ICASE.
Griffiths, A. K., & Preston, K. R. (1992). Grade-12 students’ misconceptions relating to fundamental characteristics of atoms and molecules. Journal of Research in Science Teaching, 29(6), 611–628.
Harris, S. P. (1983). Physics, an important factor in the success of general college chemistry students. Journal of Chemical Education, 60(9), 739–740.
Herron, J. D. (1978). Piaget in the classroom. Journal of Chemical Education, 55(3), 165–170.
Hills, G., Holman, J., Lazonby, J., Raffan, J., & Waddington, D. (1989). Introducing chemistry: The Salters’ approach. London: Heinemann Educational Books.
Hudson, H. T., & Liberman, D. (1982). The combined effect of mathematics skills and formal operational reasoning on student performance in the general physics course. American Journal of Physics, 50(12), 1117–1119.
Hudson, H. T., & McIntire, W. R. (1977). Correlation between mathematical skills and success in physics. American Journal of Physics, 45(5), 470–471.
Ift, J. B., & Roberts, J. L, Jr. (1975). Frantz/Malm’s essentials of chemistry in the laboratory. San Francisco: Freeman.
Ingle, R., & Shayer, M. (1971). Conceptual demand in Nuffield ‘O’ level chemistry. Education in Chemistry, 8(5), 182–183.
IUPAC (1993). Recommendations for language, symbols and representation in chemistry: Atom. International Newsletter on Chemical Education, (39), 7–10.
Johnstone, A. H. (1991). Thinking about thinking. International Newsletter on Chemical Education, (6), 7–11.
Johnstone, A. H. (2000). The presentation of chemistry—Logical or psychological? Chemistry Education Research and Practice, 1(1), 9–15.
Johnstone, A. H. (2007). Science education: We know the answers, let’s look at the problems. In Proceedings of the 5 th Greek Conference Science Education and New Technologies in Education (Vol. 1, pp. 1–13). Retrieved from http://www.kodipheet.chem.uoi.gr/fifth_conf/pdf_synedriou/teyxos_A/1_kentrikes_omilies/1_KO-4-Johnstone.pdf
Johnstone, A. H. (2010). You can’t get there from here. Journal of Chemical Education, 87(1), 22–27.
Johnstone, A. H., & Morrison, T. I. (1964). Chemistry takes shape (Vol. 1). London: Heinemann.
Johnstone, A. H., Morrison, T. I., & Reid, N. (1981). Chemistry about us. London: Heinemann.
Johnstone, A. H., & Wham, A. J. B. (1982). The demands of practical work. Education in Chemistry, 19(3), 71–73.
Jones, E. R, Jr, & Childers, R. L. (1984). Experimental evidence for the existence of atoms. The Physics Teacher, 22(6), 354–360.
Lazonby, J. N., Morris, J. E., & Waddington, D. J. (1984). The muddlesome mole. Education in Chemistry, 19(4), 109–111.
Liberman, D., & Hudson, H. T. (1979). Correlation between logical abilities and success in physics. American Journal of Physics, 47(9), 784–786.
Lijnse, P. L., Licht, P., DeVos, W., & Warlo, A. J. (Eds.). (1990). Relating macroscopic phenomena to microscopic particles. Utrecht: CD-β Press.
Meheut, M., & Chomat, A. (1990). The bounds of children’s atomimism: An attempt to make children build up a particulate model of matter. In P. L. Lijnse, P. Licht, W. De Vos, & A. J. Waarlo (Eds.), Relating macroscopic phenomena to microscopic particles (pp. 266–282). Utrecht: CD-β Press.
Merrill, M. A. (1973). Chemistry: Process and prospect. Columbus: Bell & Howell.
Millar, R. (1990). Making sense: What use are particle ideas to children. In P. L. Lijnse, P. Licht, W. De Vos, & A. J. Waarlo (Eds.), Relating macroscopic phenomena to microscopic particles (pp. 283–293). Utrecht: CD-β Press.
NCRTL (National Center for Research on Teaching and Learning) (1994). A blueprint for the education of project 2061 science teachers. East Lansing: Michigan State University.
Nelson, P. G. (1991). The elusive mole. Education in Chemistry, 28(4), 103–104.
Nelson, P. G. (1994). Classifying substances by electrical character. Journal of Chemical Education, 71(1), 24–26.
Nelson, P. G. (1996a). Demonstrating constant composition. Education in Chemistry, 33(1), 22.
Nelson, P. G. (1996b). To be a molecule, or not to be? Education in Chemistry, 33(5), 129–130.
Nelson, P. G. (2002). Teaching chemistry progressively: From substances, to atoms and molecules, to electrons and nuclei. Chemistry Education Research and Practice, 3(2), 215–228.
Niaz, M., & Rodriguez, M. A. (2000). Teaching chemistry as rhetoric of conclusions or heuristic principles—A history and philosophy of science perspective. Chemistry Education Research and Practice, 1(3), 315–322.
Niaz, M., & Rodriguez, M. A. (2001). Do we have to introduce history and philosophy of science or is it already ‘inside’ chemistry? Chemistry Education Research and Practice, 2(2), 159–164.
Novick, S., & Menis, J. (1976). A study of student perceptions of the mole concept. Journal of Chemical Education, 53(11), 720–722.
Nussbaum, J. (1998). History and philosophy of science and the preparation for constructivist teaching: The case of particle theory. In J. J. Mintzes, J. H. Wandersee, & J. D. Novak (Eds.), Teaching science for understanding—A human constructivist view (pp. 165–194). New York: Academic Press.
Papaphotis, G., & Tsaparlis, G. (2008a). Conceptual versus algorithmic learning in high school chemistry: The case of quantum chemical concepts. Part 1, Statisitcal analysis of a quantitative study. Chemistry Education Research and Practice, 9(4), 323–331.
Papaphotis, G., & Tsaparlis, G. (2008b). Conceptual versus algorithmic learning in high school chemistry: The case of quantum chemical concepts. Part 2, Students’ common errors, misconceptions, and difficulties in understanding. Chemistry Education Research and Practice, 9(4), 332–340.
Rop, J. (1999). Student perspectives on success in high school chemistry. Journal of Research in Science Teaching, 36(2), 221–237.
Schmidt, H. J. (1994). Stoichiometric problem solving in high school chemistry. International Journal of Science Education, 16(2), 191–200.
Sherman, A., & Sherman, S. J. (1983). Chemistry and our changing world. Englewood Cliffs: Prentice Hall.
Sienko, M. J., Plane, R. A., & Marcus, S. T. (1984). Experimental chemistry (6th ed.). Tokyo: McGraw-Hill.
Staver, J. R., & Lumpe, A. T. (1993). A content analysis of the presentation of the mole concept in chemistry textbooks. Journal of Research in Science Teaching, 30(4), 321–337.
Staver, J. R., & Lumpe, A. T. (1995). Two investigations of student understanding of the mole concept and its use in problem solving. Journal of Research in Science Teaching, 32(2), 177–193.
Stromdahl, H., Tulberg, A., & Lybeck, L. (1994). The quantitatively different conceptions of 1 mole. International Journal of Science Education, 16(1), 17–26.
Taber, K. S. (1998). The sharing-out of nuclear attraction: Or I can’t think about physics in chemistry. International Journal of Science Education, 20(8), 1001–1014.
Toomey, R., DePierro, R., & Garafalo, F. (2001). Helping students to make inferences about the atomic realm by delaying the presentation of atomic structure. Chemistry Education Research and Practice, 2(3), 183–202.
Tsaparlis, G. (1984). The chemical bond as an atomic tug-of-war. Journal of Chemical Education, 61(8), 677.
Tsaparlis, G. (1989). What a single molecule does not look like–Two analogies and their effect on learning. Abstracts of papers of the American Chemical Society, 198, 176-CHED.
Tsaparlis, G. (1997a). Atomic and molecular structure in chemical education. Journal of Chemical Education, 74(8), 922–926.
Tsaparlis, G. (1997b). Atomic orbitals, molecular orbitals, and related concepts: Conceptual difficulties among chemistry students. Research in Science Education, 27(2), 271–287.
Tsaparlis, G. (2004). Atomic structure. In J. J. Lagowski (Ed.), Chemistry: Foundations and applications (Vol. 1, pp. 78–87). New York: MacMillan Reference-Thomson Gale.
Tsaparlis, G. (2009). Learning at the macro level: The role of practical work. In J. K. Gilbert & D. F. Treagust (Eds.), Multiple representations in chemical education (pp. 109–136). Dordrecht: Springer.
Tsaparlis, G., & Kampourakis, K. (2000). An integrated physical-science (physics and chemistry) introduction for lower-secondary level (grade 7). Chemistry Education Research and Practice, 1(2), 277–290.
Tsaparlis, G., Kolioulis, D., & Pappa, E. (2010). Lower-secondary introductory chemistry course: A novel approach based on science-education theories, with emphasis on the macroscopic approach, and the delayed meaningful teaching of the concepts of molecule and atom. Chemistry Education Research and Practice, 11(2), 107–117.
Tsaparlis, G., & Papaphotis, G. (2002). Quantum-chemical concepts: Are they suitable for secondary students? Chemistry Education Research and Practice, 3(2), 129–144.
Tulberg, A., Stromdahl, H., & Lybeck, L. (1994). Students’ conceptions of 1 mole and educators’ conceptions of how they teach the “mole”. International Journal of Science Education, 16(2), 145–156.
von Glasersfeld, E. (1989). Cognition, construction of knowledge, and teaching. Synthese, 80(1), 121–140.
Acknowledgments
I would like to express my gratitude to Professor Alex H. Johnstone for his work and ideas, and the discussions I have had with him, all of which largely influenced this work. I also thank the two anonymous reviewers who made numerous constructive suggestions that contributed greatly to the improvement of this chapter.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Tsaparlis, G. (2014). Linking the Macro with the Submicro Levels of Chemistry: Demonstrations and Experiments that can Contribute to Active/Meaningful/Conceptual Learning. In: Devetak, I., Glažar, S. (eds) Learning with Understanding in the Chemistry Classroom. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4366-3_3
Download citation
DOI: https://doi.org/10.1007/978-94-007-4366-3_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4365-6
Online ISBN: 978-94-007-4366-3
eBook Packages: Humanities, Social Sciences and LawEducation (R0)