Abstract
Representations constitute an important part of chemistry knowledge. This paper revisits the notion of the term, symbolic, in the chemistry triangle proposed by Johnstone using the theoretical lens of social semiotics. In doing so, this paper proposes a framework of chemistry learning that highlights representational re-description and coordination as key mechanisms for facilitating connections among the three domains of knowledge: chemical phenomenon (perceptual-experiential level), macroscopic (theoretical-descriptive level) and submicroscopic (theoretical-explanatory level). This paper illustrates how this framework can be used to explore student meaning making of changes of state by examining students’ interactions with the phenomena of melting and boiling and with the multiple representations of the phases of matter introduced in the classroom. The findings revealed the opportunities and challenges which emerged from student meaning making with multiple representations in the process of developing an understanding of the submicroscopic view of phase change. It also highlighted the support needed to facilitate such meaning making through representational re-description and coordination in order for students to develop a deep understanding of the logical connections between the particular model and the macroscopic patterns of the observed phenomena.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The particulate nature of matter (PNM) is one of the most important ideas in science (Harrison & Treagust, 2002), serving as a core building block for learning within a discipline of science (Tsaparlis & Sevian, 2013), and a core idea in the science curricula in many countries (e.g. National Research Council, 2012; Australian National Science Curriculum, ACARA). Since the 1970s, there has been much attention paid to identifying students’ ideas of particles such as atoms and molecules (e.g. Harrison & Treagust, 2002; Johnson, 1998; Novick & Nussbaum, 1978). Research in this area has repeatedly and consistently shown that students ranging from a young age to university level experience great difficulties in understanding the ideas behind the particulate nature of matter and its associated concepts (Driver & Project, 1994; Ayas et al., 2010). Students were found to have developed intuitive ideas during the early years of schooling and only change to scientifically acceptable understandings to a limited extent after formal instruction (Stavy, 1988; Talanquer, 2009). A major challenge identified in the literature is a view of matter as continuous and static instead of a scientific view of matter as consisted of discontinuous particles in constant motion separated by empty space (Novick & Nussbaum, 1978; Johnson, 1998).
Previous approaches to overcome students’ difficulties with the PNM tended to adopt a conceptual change approach framed within a fundamentally cognitivist tradition in which students’ alternative conceptions or mental models are challenged (Gabel, 1998; Tsaparlis & Sevian, 2013). Recent accounts of conceptual learning emphasize the situated nature of learning and highlight a view of conceptual knowledge as implicit, perceptual and context dependent rather than being propositional and abstract. This shift in perspectives foregrounds the roles of representational practice in supporting learning (Tytler & Prain, 2010).
The importance of representations in chemistry learning has been highlighted in an emerging body of research that focuses on the development of students’ representational competence in a range of social and disciplinary contexts (e.g. Kozma et al., 2000; diSessa, 2004; Adadan et al., 2010; Lehrer & Schauble, 2013). This body of research demonstrates that students’ ability to connect and coordinate multiple modes of representations is critical for building scientific understandings of natural phenomena in general (Ainsworth, 2006) and for successful problem solving in chemistry in particular (Kozma, 2003; Adadan et al., 2010). This recognition of the fundamental roles of multiple representations as meaning making resources in developing a high-level understanding in chemistry offers a promising direction to develop theories of chemistry learning in social contexts. It also points to a need to investigate social and material practices to identify how multiple representations are generated and made available in classrooms, and how the meanings of these representations are interactively constructed (Wu, 2003).
This paper revisits the chemistry triangle proposed by Johnstone (1982) using the theoretical lens of social semiotics (Peirce, 1998; Lemke, 1998). Based on this reconsideration, a framework of chemistry learning is proposed to highlight the representational practices involved in the construction of chemistry knowledge in classroom settings. This paper illustrates how this framework can be used to explore student meaning making of chemistry representations and the particulate nature of matter in a secondary science classroom. Drawing upon data from video recordings of a sequence of three lessons focusing on phase change, this paper explores these two research questions:
-
1)
How did the students make sense of the different levels of representation (macroscopic, submicroscopic or symbolic) introduced and discussed in a Grade 7 science classroom?
-
2)
What are the affordances and challenges which emerged from student meaning making with multiple representations for the purpose of developing an understanding of their submicroscopic views of phase change?
Multiple Representations and Johnstone’s Triangle of Chemistry Knowledge
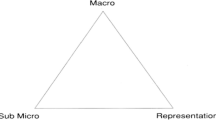
Representations constitute an important part of chemistry knowledge. Chemistry knowledge is often conceptualized in terms of three levels: (1) descriptive and functional; (2) representational and (3) explanatory (Johnstone, 1982). Johnstone’s chemistry triangle (Johnstone, 1982) described these three levels of chemical knowledge as a triangle, labelled as macroscopic, symbolic and submicroscopic (see Fig. 1). This model of chemistry knowledge has been influential in shaping chemistry education for the past 30 years (Gilbert & Treagust, 2009; Reid, 2021). Researchers have been drawing on the Johnstone’s triangle in multiple ways, as a framework for guiding curriculum writing (Gilbert & Treagust, 2009), a pedagogical framework for chemistry teaching (Gabel, 1993) and as an analytical framework for identifying student understanding (Devetak & Glažar, 2014). A significant number of research studies in chemistry education have focused on characterizing the challenges faced by students to integrate the different levels of chemistry knowledge (Gilbert & Treagust, 2009) or chemistry representations (Gabel, 1998; Treagust et al., 2003). These studies have demonstrated that many learners struggle to develop meaningful connections between the three levels of chemistry knowledge or representations (Gilbert & Treagust, 2009) needed for them to move from an instrumental to a relational understanding of chemistry concepts (Treagust et al., 2003).
Johnstone’s triangle (Johnstone, 1982)
Researchers have interpreted the components of the chemistry triangle in different ways (Gilbert & Treagust, 2009; Talanquer, 2011; Taber, 2013). Among the three levels of knowledge proposed by Johnstone (1982), the symbolic (representational) domain has been most ambiguous and confusing. While Johnstone’s initial idea of ‘representational’ as one of the ‘three levels of thought’ (Johnstone, 2000, p. 9), expressed as ‘symbolic’ in the triangle in Fig. 1, others have taken symbolic to be one of the three levels of representations (Treagust et al., 2003; Gilbert & Treagust, 2009). In rethinking the symbolic domain in the chemistry triangle, Talanquer (2011) argued that the distinction between the actual theoretical models used to explain chemistry phenomena and the manipulating of iconic representations to capture the features of the phenomena has been blurred in educational discussions. He further pointed out that the visual language of chemistry is often comprised of symbols and icons to represent the properties and behaviours of chemical substances and processes, and therefore gives them a hybrid status between signs and models, which can sit within any of the three domains. Similarly, Taber (2013) questioned the ontological status of the symbolic domain and argued that the original symbolic domain in Johnstone’s triangle is ambiguous because symbolic representations are essential for representing and communicating effectively in chemistry learning. Instead of regarding the symbolic level as a discrete level of chemistry knowledge, Taber (2013) suggested a revised model that considers chemical phenomena as the experiential domain together with the two domains of conceptual knowledge: macroscopic (theoretical-descriptive level) and submicroscopic (theoretical-explanatory level). Taber’s revised model allows for the consideration of various forms of representations (including symbolic representations in Johnston’s triangle) as bridges to connect the macroscopic and the submicroscopic views in chemistry, providing a useful approach to understanding teaching and learning of chemistry in classroom settings. This emphasis on the act of representing and communicating using representations in chemistry learning is consistent with Mahaffy’s (2006) proposal of adding a fourth dimension: namely human element, into Johnstone’s triangle to highlight the need for connecting chemistry with the real world and with a student’s experience.
Revisiting Johnstone’s Chemistry Triangle from the Perspective of Social Semiotics
For the purpose of investigating the social and material practices in classrooms, this paper revisits Johnstone’s chemistry triangle from the perspective of social semiotics as influenced by the work of Peirce (1998), Lemke (1998) and several other researchers (Bezemer & Kress, 2008; Airey & Linder, 2009; Tang, 2016). Instead of seeing representations as signifying some hidden reality, social semiotics adopts a functional approach to meaning making by investigating how the various semiotic resource systems such as words, images, symbols and actions have evolved to enable us to communicate with each other by making particular kinds of meanings. For Peirce (1998), each representation is a sign which can be interpreted by other signs in the process of meaning making. This chain of signs is grounded in bodily experiences of perception and action in a particular context. Therefore, it is less about the nature of signs but more about how people use signs of different kinds to make meaning in social contexts. Lemke (1998) considers meaning making as always being a material process as well as a social semiotic practice. Every material sign, such as a line graph, can be the product of an action or interaction, or an ‘actant’ in the process of action and interaction (Lemke, 2003). Following Kozma and others (Kozma et al., 2000), the material process refers to features of physical phenomena and symbolic representations that can be perceived and manipulated. Semiotic practice such as seeing a representation as standing for unseen entities and processes is something we learn to do as members of a community (Lemke, 1998). As such, the act of representing can be viewed as a process of making meanings through which representations are used as signs to make particular kinds of meanings in a social context such as in a classroom community (Tang, 2016). The social and material aspects involved in meaning making in science were explored further by Airey and Linder (2009) who coined the term ‘disciplinary discourse’ to encompass a complex set of representations, tools and activities of a discipline. They argued that developing conceptual understanding in science involves the flexibility to use a variety of multi-modal representational resources to develop a disciplinary way of knowing.
Interpreting the chemistry triangle from this social semiotic perspective highlights the role of representational practice in the construction of chemistry knowledge. This includes the use of linguistic, visual-spatial and symbolic representations to support the communication and connection building among the three domains of knowledge: chemical phenomenon (perceptual-experiential level), macroscopic (theoretical-descriptive level) and submicroscopic (theoretical-explanatory level). This paper proposes a framework of chemistry learning (see Fig. 2) that builds on Taber’s (2013) revised model of the Johnstone’s triangle but offers further elaboration of the arrows in the revised model.
A social semiotic interpretation of the chemistry triangle (Adapted from Taber, 2013)
The solid double-headed arrows in this model of chemistry learning involve two processes: (1) coordination of multiple representations (Kozma, 2003; Prain & Tytler, 2012); and (2) representational ‘re-description’ (Lehrer & Schauble, 2013) of one modal representation to another. In a classroom situation, the process of ‘coordination’ and ‘re-description’ of representations is socially and discursively accomplished through classroom interactions. In the case of phase change, it involves the following: (1) a representational re-description of everyday experience and observation of phenomena, e.g. melting of ice, in macroscopic terms such as temperature changes as measured formally by a thermometer and presented in mathematical or graphical representations; and (2) coordination of mathematical, graphic and submicroscopic representations to account for the physical entities and processes underlying the experienced phenomena. The development of meaningful connections between different domains can also involve transduction between modes (Bezemer & Kress, 2008; Airey & Linder, 2009), for example, the ability to create or identify iconic representations of a system in the form of particulate drawings, and translate them into written representations. Rather than seeing the correspondence of different levels of representations, this paper argues that this representational re-description and coordination involves a chain of argumentation and modelling processes that align different perspectives and representations together for the purpose of understanding the same phenomenon. In other words, it requires developing and stabilizing alignment between the phenomena and a system of models, e.g. empirical models, data models and explanatory models (Manz et al., 2020), to support students to move from everyday descriptions to formal representations.
In the following sections, the framework of chemistry learning outlined above will be used to investigate classroom interactions in an Australian secondary science classroom. Specifically, this study examines the interplay between students’ experience with the chemical phenomena and representations during meaning making events focusing on the chemistry concept of phase change. The analysis identifies how the meanings of different levels of chemical representations were socially organized and interactively constructed in the classroom. By doing so, this paper intends to illustrate how this framework of chemistry learning could potentially help us to generate insight into student learning difficulties with chemistry representations and draw implications to inform classroom instruction.
Data Sources
The data reported in this paper were drawn from a project entitled Causal Connections in Science Classrooms funded by the Australian Research Council. This project was conducted in three science classrooms across two demographically distinct school settings in Melbourne, employing a complementary accounts methodology developed by Clarke (1997). This involved capturing of classroom interactions through a multi-camera approach, complemented by participants’ reconstructive account of classroom events through video-stimulated post-lesson interviews. For each classroom, a lesson sequence between 6 and 10 lessons was video recorded over a period of approximately 3 weeks each, using a four-camera approach: one whole class camera, one teacher camera and two focus student group cameras. Video-stimulated post-lesson individual interviews were conducted with the teacher and two focus group students after every lesson, focusing on the events that were perceived as important or interesting for the participants. Other materials obtained include copies of lesson materials, students’ written work, students’ results on the International Benchmark Test for Science, students’ class tests and the teachers’ questionnaires.
This paper focuses on a Grade 7 classroom in a local government school. The science teacher, Mr. Gardner, has nearly 16 years of teaching experience. At the time of data generation, he had been teaching Grade 7 science for 8 years in the same school. The science class was a mixed ability class with 27 students including 11 females and 16 males. The science lessons, designed by the science teacher, were conducted in the science lab in the school. For the purpose of focusing on student meaning making with representations, this paper will focus on a group of four students: Keith, Sydney, Brant and Lionel, who have been working together in science lessons for some time. This mixed ability group was chosen based on the teacher’s recommendation that this group usually worked well together and could articulate their thinking well in science classes.
Several steps were undertaken to analyze the data. The video recordings of lessons and post-lesson interviews were fully transcribed. Three transcripts of classroom interactions were generated, including one for the teacher-student interactions and two transcripts of focus students’ group interactions. In the first step, the video recordings of the lessons and transcripts were viewed several times to identify the conceptual foci of the talk and actions undertaken by the participants in each lesson. Segments of each lesson that focus on the discussion of key representations, either created by the students or introduced by the teacher, were selected for further analysis, guided by the research questions. In the second step, the transcripts of these lesson segments were analyzed through discourse analysis (Wells & Arauz, 2006), focusing on the shift of conceptual foci demonstrated in the classroom talk, including a shift in attention, an articulation of relationships between concepts or a shift in levels of representations e.g. macro to submicro. Each turn of talk was also examined in terms of its function in the exchange of information and the social status accorded by the participants to a particular piece of information exchanged, see Table 2 for an example of this analysis. This analysis of classroom video and transcripts was conducted in parallel with two other PhD students who were working on the same data set from the project (see Martin et al., 2021). The validation of the analysis reported in this paper was carried out through multiple discussions with the other two PhD students in research meetings with the PhD supervisor, to reach agreement on the coding categories and the interpretations of the coded events.
The analysis of classroom videos was complemented by the analysis of the post-lesson interviews with the teacher and students, and artefacts collected. Adopting a thematic analysis approach by Braun and Clarke (2013), the teacher’s interviews and planning materials were viewed several times to identify objectives of each lesson and key conceptual foci. Students’ interviews and their written work were analyzed to identify meaning making of chemical phenomena under investigation and of representations introduced and discussed in the classroom.
An Overview of the Lesson Sequence on Phase Change
The unit of work was designed and conducted by the teacher with little intervention from the research team. Given that the focus of this paper is on students’ exploration of the particulate nature of matter, three consecutive lessons (Lessons 4–6) focusing on changes of state and an introduction to the Particle Theory of Matter were chosen from the sequence (Fig. 3). Table 1 provides an overview of the three lessons, including the learning objectives of each lesson identified from the post-lesson teacher interviews and unit planner for the topic. Key activities and main representations were identified through the video analysis of the recorded lessons.
As mentioned earlier, understanding the submicroscopic view of phase change involves interpreting observable macroscopic properties of matter and its changes by means of a submicroscopic model of particles that is beyond what we can perceive with our senses. As demonstrated in the brief overview of the three lessons, there seems to be a progressive move from observations of the phenomena of melting and boiling during the practical work (Fig. 4), to a macroscopic description of temperature changes occurred during the phase changes (Fig. 5) and to a submicroscopic explanation in terms of changes in arrangement of particles (Fig. 6). The graphs of temperature changes produced by the students as part of their practical reports can help to anchor their perceptual experiences of the phenomena and support their interpretation of the written statements of the Particle Theory of Matter in order to develop a submicroscopic explanation of melting and boiling.
Results
This section reports a detailed analysis of the three-lesson sequence to identify how the students made sense of the representations introduced and discussed in the classroom. Students’ understanding of phase change will be discussed through the interactions of the four students: Sydney, Lionel, Keith and Brant, and the student interviews conducted immediately after each lesson.
Developing a Macroscopic Description of Phase Changes
In this lesson sequence, the chemical phenomena involve two phase changes: melting and boiling of water. The practical work in Lesson 4 (see Fig. 4) provided students with material experience and gave students access to observable changes in temperature as measured by a thermometer and changes in the state of water from ice, to water and to steam. It should be noted that the students would most likely have experienced these phenomena in their everyday life, but what is different in this practical work is the systematic measuring of temperature changes during the phase changes. The graphic representations of the temperature data can allow students to examine the everyday phenomena from a different perspective and support students to develop a submicroscopic explanation of phase change.
Discussion of the Line Graph of Temperature Data.
Table 2 shows the whole class discussion of the results generated from the practical work undertaken by the students in Lesson 4. Sydney’s line graph (Fig. 5) was chosen to be the focus of the following whole class discussion.
In this episode, the teacher attempted to help students to establish links between their observations during the practical work and the temperature change as demonstrated in the line graph drawn on the board. The classroom discussion drew students’ attention to the three sections of the line graph: the bottom horizontal segment, the top horizontal segment and the slope in the middle section of the graph. In an attempt to help students to see the underlying principles of melting and boiling, Mr. Gardner drew students’ attention to the features of each section of the line graph by pointing and tracing the line on the graph, and linked each section of the graph to the physical event occurred during the experiment without simply telling the class how to interpret the graph. Nonetheless, it seems that there are ambiguities in terms of what the horizontal segments of the graph represent as the teacher implied that the bottom horizontal section represents water in a solid state [Turns 3–12]. He also pointed at the end point of that section when stating ‘melting’ [Turn 42]. This could be interpreted as referring to the entire segment or that specific point that the teacher was pointing at as ‘melting’. For the top horizontal section of the graph, Mr. Gardner did not give specific answers, but he pointed at the end of the segment when he uttered ‘we said that’s boiling’ [Turn 42]. Similarly, this gesture could be interpreted as if the teacher was referring to the whole horizontal section or to a specific point. Only the slope was clearly stated as representing trend of temperature change when water is in its liquid state.
Interpreting the Line Graph of Melting and Boiling.
The line graph was created based on the temperature measurements that the students recorded during their experiment. It should be noted that as a symbolic representation, the interpretation of the line graph involves coordination of a range of semiotic resources, including the connections between the physical phenomena which occurred during the experiments, the numerical and graphical representations of the results and the scientific concepts of states of matter and changes of state. The graphical representation was intended by the teacher to highlight the relationship between the change of temperature and the change of state; that is, when a substance is undergoing a phase change, the temperature remains the same. As such, it serves as a bridge to support students to make connections between the observed phenomena and the macroscopic conceptualizations (see Fig. 2). However, the analysis of student practical reports, Table 3, revealed that the students had difficulties in connecting the line graph with their observations of the phenomena, and with the conceptual ideas introduced in the lessons because they struggled to understand the underlying logic behind phase changes.
Student difficulty in interpreting the graphical representations of phase change can be attributed to many factors. Firstly, such recognition requires the students to have drawn a graph that is at least similar to the one on the board (Fig. 5), with two horizontal lines and a slope. However, many students’ graphs did not show the three sections clearly as intended by the teacher, see graphs in Table 3. Secondly, as revealed in the post-lesson interviews with the students in the focus group, it appears that they did not pay much attention to the phase change during their practical work, but attended primarily to the changes in temperature as measured and recorded in their books. Since the students were only asked to think about what they had seen in terms of states of matter after the practical work, they had no perceptual cues to help them to identify these connections nor any written observational record, and they had to rely on their memory of the event to make sense of the data and the graphs.
Furthermore, while the concepts of melting and boiling have scientific meanings that are separate from a state of matter e.g. solid, the distinction between changes of state and states of matter is not that clear in everyday language. In everyday use, the words melting or boiling could refer to the process of applying heat until ice melts or water boils, which combines both states of matter and changes of state in each term. Such differences in everyday use and scientific use of the same word can cause students’ difficulties in identifying and distinguishing the two concepts as displayed on their line graphs. This confusion was evident in student practical work which shows that almost all groups turned off their Bunsen burners after the third or fourth reading, despite the instruction on the worksheet clearly indicated that they should ‘continue the measurement until the water has been boiling for 3 or 4 minutes’. While this mistake may be interpreted as an indication that the students did not read the instruction carefully, it is also reasonable to suspect that some students might not understand the word boiling in this context. For example, the instruction ‘boil water’ may be interpreted as either ‘heat water’ or ‘heat water until it boils’. The fact that almost all the groups stopped the experiment even before the water commenced boiling suggests that they employed the everyday interpretation of the word boiling which is often associated with the appearance of bubbles in the water (Erickson & Tiberghien, 1985).
The Notions of Melting and Boiling Points.
The recognition of the temperature plateau during a phase change also involves student understanding of the notions of melting and boiling points. In the whole class discussion before the practical activity, Mr. Gardner elicited student prior knowledge about water, among which the boiling point of water was mentioned by one of the students to be 100°C. The class also discussed some factors that might affect the boiling of water e.g. air pressure and purity. However, the examination of student work during the class and their practical reports demonstrates that the students did not have an adequate understanding of the concept of a boiling point, particularly that the temperature remains constant while boiling, and its specificity to a substance.
Analysis of the private interactions among the focus group students revealed that some students seemed to think that there was no limit to how hot water can be, and the temperature of the boiling water was directly associated with the temperature of the heating source or due to the limit of the thermometer. The following excerpt illustrates part of the discussion among the four boys when they were working on question 6 of the worksheet, Fig. 4, which required them to make predictions about the temperature of the water 10 min after it started boiling (Table 4).
In this episode, Keith’s question and Sydney’s responses suggest that both of them were associating the temperature of water with the strength of flame of the Bunsen burner. While this is a reasonable connection for the students to make, it indicates that they have not yet understood the concept of a boiling point and its specificity to a particular substance. The post-lesson interview with Sydney confirmed the interpretation above in that he regarded the temperature of the water as directly related to the temperature of the heating source. He suggested that the temperature of water ‘will rise, and then it will stop and be steady again because that would be the maximum heat from the Bunsen burner’ (L06-INT-Sydney). Since it has already been acknowledged in the whole class discussion that the boiling point varies with air pressure, it is not unreasonable for the students to infer that other factors, e.g. heat source, would also affect the boiling point. From the perspective of social semiotics, while the whole class discussion, Table 2, helped the students to establish the referent of the line graph: the relationship between temperature changes and phase changes, identifying this relationship requires coordinating of a range of semiotic and representational resources including an understanding of the specificity of a boiling point to a particular substance. In this case, developing an explanation for this temperature plateau during the phase change requires an understanding of the notion of latent heat and what is happening to the liquid at the molecular level (Erickson & Tiberghien, 1985). The introduction of the Particle Theory became a crucial step to enable the students to conceptualize the process of melting and boiling, and other changes undergone by matter. But as we will see in the following section, the introduction of Particle Theory of Matter imposed further challenges as it required the students to be able to interpret the macroscopic properties and changes of matter by means of a submicroscopic model, which was inaccessible simply using their senses.
Making Sense of Submicroscopic Views of Phase Change
Introduction to the Particle Model of Matter.
Following the whole class discussion of the line graph in Lesson 5, the students spent the next twenty minutes writing their report, and answering the questions listed on the practical worksheet (see Fig. 4). The teacher then asked the class to copy some notes about the Particle Theory of Matter from the board, see Table 1, followed by a classroom discussion focusing on the phrase ‘too small to see’, and whether a synchrotron can be used to ‘see’ particles. The next three statements of the Particle Theory of Matter were introduced in the following lesson (Lesson 6). To assist the students in answering question 7 on the worksheet (see below), Mr. Gardner also provided diagrams of the line graph on the overhead projector (Fig. 5). From the teacher’s perspective, the particle diagrams of melting and boiling provide background information that could assist the students to answer question 7 on the practical worksheet (L06-INT-Teacher).
Students’ Explanation of Melting and Boiling.
Following the introduction to the Particle Model of Matter and the particle diagrams of melting and boiling, the students were asked to respond to question 7 on their practical worksheet:
The temperature did not increase while the ice was melting and while the water was boiling — even though there was a constant supply of energy from the burner. Use the particle model to explain where that energy was going.
The examination of student practical reports (Table 3) shows that three out of four focus students provided a similar answer by stating that the particles are trying to break away from each other and trying to get away from the heat. It appears that this response was generated from a discussion with the teacher. The following excerpt illustrates the conversation between Mr. Gardner, Brant, Lionel and Keith (Table 5).
In this episode, the particles were regarded as ‘wanting’ to break bonds and to escape from heat. Such personification of particles was evident in Keith’s summary that ‘particles are afraid of heat’ [Turn 14]. In this way, the explanation refers to heat as a cause for changes of state. In addition, heat and energy were discussed as two separate concepts. While ‘heat’ was considered as the cause for particle movement, ‘energy’ was represented as a property of water, which can be given out or gained back.
The analysis of students’ understanding as identified from their private interactions as well as post-lesson interviews revealed difficulties that the students confronted in formulating the underlying mechanisms of how changes occurred to matter in terms of the particle model, and how this might be connected to observable macroscopic phenomena. One explanation provided by the boys’ group in consultation with the teacher referred to heat as the cause for particles escaping, ‘break bonds to escape’. This explanation makes sense at the macroscopic level, at which the water was seen to be evaporating and disappearing into the air, but such a causal mechanism is insufficient to explain the emergent process at the submicroscopic level.
Discussion
Understanding the particulate nature of matter involves considering observable phenomena in the light of a submicroscopic model, in which matter is considered as being constituted by a system of particles in constant interaction with each other. It requires students to accept a discontinuous view of matter, and to be able to understand the differences and connections between the macroscopic and submicroscopic levels of the same phenomenon. Drawing upon a social semiotics interpretation of the chemistry triangle, this paper provides an analysis of a three-lesson sequence on the topic of phase change, with the intention to identify student meaning making with the multiple representations introduced in the classroom. Learning with representations involves not only identifying the referent but also interpreting it by other signs in order to establish the meaning of a representation. The findings can be summarized in the following points.
In this lesson sequence, multiple representations were introduced and discussed in the classroom, each providing a different entry point to the phenomenon of phase change. The practical work of melting and boiling provided students with perceptual experience of changes of state. This experience was re-described in the form of a line graph, highlighting the relationships between temperature change and phase change. The introduction of the written statements of the Particle Theory of Matter and diagrammatic representations were intended for the students to visualize the submicroscopic processes in terms of particle movement and distribution, in order to develop a submicroscopic explanation of phase change. This sequential introduction of the multiple representations of the same phenomena could potentially help the students to gain a deeper understanding of the phenomena and the associated concepts. However, while the students were presented with multiple representations, they only interacted with the macroscopic and symbolic levels as they carried out the experiment and wrote the practical report. There was a limited attempt to link the observations with what was happening at the submicroscopic level. In response to the first research question, this study highlights that the use of multiple representations of the same phenomena alone is not sufficient without being supported by a chain of argumentation and modelling processes for students to connect and coordinate these representations and associated concepts to make sense of the observed phenomena. As discussed in the results section, the students seemed to struggle to make connections between the line graph and the chemical phenomena which occurred during the practical activity. Even though the diagrammatic representations of melting and boiling provided a visual description of the process of melting and boiling at the particle level, the students struggled to provide explanations to account for the constant temperature during a phase change. This is not surprising given the complexity of such explanations involving the coordination of multiple representational resources and conceptual understandings to identify the possible mechanisms for the constant temperature during a phase change. In this case, understanding the logical connections between the particulate model and the macroscopic patterns is critical, which was not well supported and guided by the teacher in the classroom discussions. As a result, students’ capabilities of interpreting the phenomena in particle terms were largely dependent on the perceptual appearance of the phenomena, or what they could see at the macroscopic level, for example, attributing the rise of temperature to the source of energy. The students tended to use the macroscopic properties and processes to explain the invisible submicroscopic ones, rather than the opposite, for example, ‘particles are afraid of heat’. This is consistent with previous findings from the literature (Pozo & Crespo, 2005; Nakhleh et al., 2005). Students’ capability to use the submicroscopic level to explain the macroscopic properties of matter was further hampered by their lack of clarity about the relationship between the submicroscopic model and the macroscopic phenomena, which was not explored in the classroom. The modelling of the macroscopic phenomena could have been achieved by including a discussion of the submicroscopic level of representation during the practical activity and by asking students to draw what they perceived was occurring to the water at this level. However, the teacher did not provide such opportunities because for the teacher, the focus of the practical work has been on student discovery learning: ‘I always think practical work is at its best when kids are making discoveries’ (0:01:06, Lesson 4 teacher interview).
In response to the second research question, a further challenge identified in the analysis is the discrepancy between everyday language and scientific language. Language is the most pervasive system of semiotic resources, and the ways in which scientists use specialized languages and common language in specialized ways index the discourses of the communities of scientific disciplines (Lemke, 1998). The difference between every day and scientific meanings of language has been widely acknowledged by many researchers (e.g. Yore & Treagust, 2006). As a formal language, scientific language has its own defined meanings, which in many cases differ from everyday use. In the case presented in this paper, the word boiling in science refers to a change of state when the entire liquid is heated to the boiling point and continues until the liquid turns completely into gas. In contrast, in everyday use, boiling is used to mean heating a liquid, bubbling observed as the liquid is heated or actual evaporation. Confusion was also identified about the meanings and distinctions made for scientific terms such as energy, heat and temperature. The analysis reported in this paper clearly demonstrates that the ambiguity in the use of scientific terms contributed to student difficulties in understanding the concept of changes of state and its particulate explanation. From the perspective of social semiotics, simply stating the definition of a term is not sufficient for developing student understanding of the underlying concept. Similar to the line graph and the visual diagram of particles, terms such as heat or energy need to be explored in conjunction with other relevant signs and representations to enable student meaning making.
Conclusion and Implications for Classroom Practice
Representations are essential for representing and communicating ideas effectively in chemistry. Drawing upon a social semiotic perspective, this paper clarifies the notion of the term, symbolic, in Johnstone’s chemistry triangle. Rather than seeing symbolic (representations) as a discrete domain of knowledge, this paper proposes a framework of chemistry learning that considers representations and the act of representing as supporting the communication and connection among the three domains of knowledge: chemical phenomenon (perceptual-experiential level), macroscopic (theoretical-descriptive level) and submicroscopic (theoretical-explanatory level). The reconsideration of the chemistry triangle from the perspective of social semiotics, represented in the proposed framework of chemistry learning, provides strong explanatory accounts for the learning difficulties identified in the empirical study reported in this paper. Moreover, this study reveals that the concepts related to phase changes are very complex and a teacher should consider very carefully the order in which the various aspects are introduced. For example, a teacher should consider whether the students are familiar with the concepts of energy and heat before introducing them to the topic of phase changes. Having students draw the submicroscopic representations at various points in the graph could provide the teacher with an indication of the level of students’ understanding of the phase changes.
This study also points to the need to rethink the processes of meaning making with signs in chemistry learning as an implication of recognizing the representational nature of concepts and conceptual learning in chemistry education. In relation to the revised triangle shown in Fig. 2, this shift in focus to representations and representational practice as a bridge between the three domains of knowledge highlights the importance of representational re-description and coordination as key mechanisms for facilitating student conceptual understanding in chemistry, represented as solid double-headed arrows in the triangle. As a implication of this shift, more attention is needed to both theorize and empirically investigate the types of representational practice in classrooms that support the connection building between the three domains of knowledge. The teacher’s role in opening up discussion of representations is critical (Tytler & Prain, 2010). This discussion should allow students to engage in a chain of argumentation and modelling processes that align different perspectives and representations together for the purpose of gaining a deep understanding the same phenomenon (Manz et al., 2020), which is largely missing from the classroom studied in this paper. In some regards, the findings in this paper are not surprising and the ideas proposed are not entirely new. Nevertheless, it is hoped that this renewed focus on representational re-description and coordination, and associated argumentation and modelling processes could potentially provide some future directions for researchers to design and develop new approaches based on more sophisticated research informed pedagogy to support student understanding of the particulate nature of matter.
References
Adadan, E., Trundle, K. C., & Irving, K. E. (2010). Exploring grade 11 students' conceptual pathways of the particulate nature of matter in the context of multirepresentational instruction. Journal of Research in Science Teaching, 47(8), 1004–1035.
Ainsworth, S. (2006). DeFT: A conceptual framework for considering learning with multiple representations. Learning and Instruction, 16(3), 183–198.
Airey, J., & Linder, C. (2009). A disciplinary discourse perspective on university science learning: Achieving fluency in a critical constellation of modes. Journal of Research in Science Teaching, 46(1), 27–49.
Ayas, A., Özmen, H., & Çalik, M. (2010). Students’ conceptions of the particulate nature of matter at secondary and tertiary level. International Journal of Science and Mathematics Education, 8(1), 165–184.
Bezemer, J., & Kress, G. (2008). Writing in multimodal texts: A social semiotic account of designs for learning. Written Communication, 25(2), 166–195.
Braun, V., & Clarke, V. (2013). Successful qualitative research: A practical guide for beginners. Sage.
Clarke, D. J. (1997). Chapter 7: Studying the classroom negotiation of meaning: Complementary accounts methodology. Journal for Research in Mathematics Education. Monograph, 9, 98–111.
Devetak, I., & Glažar, S. A. (2014). Learning with understanding in the chemistry classroom. Springer.
diSessa, A. (2004). Metarepresentation: Naive competence and targets for instruction. Cognition and Instruction, 22(3), 293–331.
Driver, R., & Project. (1994). Making sense of secondary science: research into children's ideas. Routledge.
Erickson, G., & Tiberghien, A. (1985). Heat and temperature. In R. Driver, E. Guesne, & A. Tiberghien (Eds.), Children’s ideas in science (pp. 52–66). Open University Press.
Gabel, D. L. (1993). Use of the particle nature of matter in developing conceptual understanding. Journal of Chemical Education, 70(3),193–194.
Gabel, D. (1998). The complexity of chemistry and implications for teaching. In B. J. Frasers & K. G. Tobin (Eds.), International handbook of science education (pp. 233–248). Kluwer Academic.
Gilbert, J. K., & Treagust, D. (2009). Multiple representations in chemical education (Vol. 4). Springer.
Harrison, A. G., & Treagust, D. F. (2002). The particulate nature of matter: Challenges in understanding the submicroscopic world. In J. K. Gilbert, O. D. Jong, R. Justi, D. F. Treagust, & J. H. V. Driel (Eds.), Chemical education: Towards research-based practice (Vol. 17, pp. 189–212). Kluwer Academic Publishers.
Johnson, P. (1998). Progression in children’s understanding of ‘basic’ particle theory: A longitudinal study. International Journal of Science Education, 20(4), 393–412.
Johnstone, A. H. (1982). Macro- and microchemistry. School Science Review, 64, 377–379.
Johnstone, A. H. (2000). Teaching of chemistry – Logical or psychological? Chemistry Education Research and Practice, 1, 9–15.
Kozma, R. (2003). The material features of multiple representations and their cognitive and social affordances for science understanding. Learning and Instruction, 13(2), 205–226.
Kozma, R., Chin, E., Russell, J., & Marx, N. (2000). The roles of representations and tools in the chemistry laboratory and their implications for chemistry learning. The Journal of the Learning Sciences, 9(2), 105–143.
Lehrer, R., & Schauble, L. (2013). Representational re-description as a catalyst of conceptual change. In B. M. Brizuela & B. E. Gravel (Eds.), “Show me what you know”: Exploring student representations across STEM disciplines (pp. 244–249). Teachers College Press.
Lemke, J. L. (1998). Teaching all the languages of science: Words, symbols, images, and actions. Paper presented at the Conference on science education in Barcelona. Retrieved September 17, 2020, from http://academic.brooklyn.cuny.edu/education/jlemke/papers/barcelon.htm.
Lemke, J. L. (2003). Mathematics in the middle: Measure, picture, gesture, sign, and word. In M. Anderson (Ed.), Educational perspectives on mathematics as semiosis: From thinking to interpreting to knowing (pp. 215–234). Legas.
Mahaffy, P. (2006). Moving chemistry education into 3D: A tetrahedral metaphor for understanding chemistry. Union Carbide Award for Chemical Education. Journal of Chemical Education, 83(1), 49–55.
Manz, E., Lehrer, R., & Schauble, L. (2020). Rethinking the classroom science investigation. Journal of Research in Science Teaching, 57(7), 1148–1174.
Martin, J., Xu, L., & Seah, L. H. (2021). Discourse analysis and multimodal meaning making in a science Classroom: Meta-methodological insights from three theoretical perspectives. Research in Science Education, 51(1), 187–207.
Nakhleh, M. B., Samarapungavan, A., & Saglam, Y. (2005). Middle school students’ beliefs about matter. Journal of Research in Science Teaching, 42(5), 581–612.
National Research Council. (2012). A framework for K-12 science education: Practices, crosscutting concepts, and core ideas. Board on Science Education, Division of Behavioral and Social Sciences and Education. The National Academies Press.
Novick, S., & Nussbaum, J. (1978). Junior high school pupils’ understanding of the particulate nature of matter: An interview study. Science Education, 62(3), 273–281.
Peirce, C. S. (1998). The essential Peirce: Selected philosophical writings (Vol. 2). Indiana University Press.
Pozo, J. I., & Crespo, M. A. G. (2005). The embodied nature of implicit theories: The consistency of ideas about the nature of matter. Cognition and Instruction, 23(3), 351–387.
Prain, V., & Tytler, R. (2012). Learning through constructing representations in science: A framework of representational construction affordances. International Journal of Science Education, 34(17), 2751–2773.
Reid, N. (2021). The Johnstone triangle: The Key to understanding chemistry. Royal Society of Chemistry.
Stavy, R. (1988). Children’s conception of gas. International Journal of Science Education, 10(5), 553–560.
Taber, K. S. (2013). Revisiting the chemistry triplet: Drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education. Chemistry Education Research and Practice, 14(2), 156–168.
Talanquer, V. (2009). On cognitive constraints and learning progressions: The case of “structure of matter”. International Journal of Science Education, 31(15), 2123–2136.
Talanquer, V. (2011). Macro, submicro, and symbolic: The many faces of the chemistry “triplet”. International Journal of Science Education, 33(2),179–195.
Tang, K. S. (2016). The interplay of representations and patterns of classroom discourse in science teaching sequences. International Journal of Science Education, 38(13), 2069–2095.
Treagust, D., Chittleborough, G., & Mamiala, T. (2003). The role of submicroscopic and symbolic representations in chemical explanations. International Journal of Science Education, 25(11), 1353–1368.
Tsaparlis, G., & Sevian, H. (2013). Concepts of matter in science education (Vol. 19). Springer.
Tytler, R., & Prain, V. (2010). A framework for re-thinking learning in science from recent cognitive science perspectives. International Journal of Science Education, 32(15), 2055–2078.
Wells, G., & Arauz, R. M. (2006). Dialogue in the classroom. The Journal of the Learning Sciences, 15(3), 379–428.
Wu, H. K. (2003). Linking the microscopic view of chemistry to real-life experiences: Intertextuality in a high-school science classroom. Science Education, 87(6), 868–891.
Yore, L. D., & Treagust, D. F. (2006). Current realities and future possibilities: Language and science literacy - empowering research and informing instruction. International Journal of Science Education, 28(2-3), 291–314.
Funding
The research was funded by the Australian Research Council and directed by the late Professor David Clarke. The author would like to dedicate this paper in honour of Professor Clarke who supervized her doctoral study as part of this ARC-funded project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xu, L. Towards a Social Semiotic Interpretation of the Chemistry Triangle: Student Exploration of Changes of State in an Australian Secondary Science Classroom. Int J of Sci and Math Educ 20, 705–726 (2022). https://doi.org/10.1007/s10763-021-10190-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10763-021-10190-1