Abstract
Carbon (C) sequestration in terrestrial ecosystems and here specifically in soils is currently discussed as a potential strategy to contribute to reducing atmospheric carbon dioxide (CO2) concentrations. However, increases in soil C stocks may also have adverse effects on the exchange of greenhouse gases (GHGs) between terrestrial ecosystems and the atmosphere. In view of the unprecedented perturbation of the global nitrogen (N) cycle, increases in soil C stocks and the ongoing saturation of terrestrial ecosystems with reactive forms of N (Nr) may result in a stimulation of soil nitrous oxide (N2O) emissions. These largely unexplored ecosystem C-N interactions and their importance for biosphere-atmosphere GHG exchange need to better understood to finally assess the climate benefits of C sequestration in soils.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
19.1 Introduction
Decomposition of soil carbon (C) stocks following conversion of natural soils to agricultural soils and intensification of agricultural management has significantly contributed to a depletion of soil organic carbon pools (SOC) worldwide. Lal (2004) pointed out that following cultivation of natural soils 60% or up to 75% of the SOC pools may be lost in temperate or tropical regions, respectively. It has been estimated that since 1850 changes in land use may have resulted in a loss of carbon (C) from terrestrial ecosystems of about 156 Pg C (Houghton 2007), with losses of SOC due to mineralization amounting to about one-third or 52 ± 8 Pg C and losses due to erosion to 26 ± 9 Pg C (Lal 2004). In the last years the reversion of historical SOC losses via the intentional implementation of improved land management practices – e.g., higher inputs of residue C, adoption of less intensive cropping systems or temporary vegetative cover between agricultural crops – is discussed as a potential strategy to contribute to reducing atmospheric carbon dioxide (CO2) concentrations (Smith et al. 2008; Conant et al. 2011). However, increasing SOC stocks will not only affect ecosystem C cycling, but also ecosystem nitrogen (N) cycling and soil microbial processes involved in the production and oxidation of other non-CO2 greenhouse gases (GHGs), namely nitrous oxide (N2O) and methane (CH4). In addition, changes in global N cycling as driven by human activities need also to be considered while implementing strategies to increasing soil C sequestration, since additional N inputs and increased availability of soil C may alter the net balance of the biosphere atmosphere exchange of GHGs at the ecosystem as well as at the landscape scale. This chapter will therefore provide examples of how C-N interactions and changes in N trace gas exchange between terrestrial ecosystems and the atmosphere may potentially affect the climate benefits of C sequestration in soils.
19.1.1 Pertubation of the Global Nitrogen Cycling and Soil Carbon-to-Nitrogen Ratios
Human activities have not only resulted in a perturbation of the global C cycle, but even more significantly in an unprecedented change of global N cycling. Following the introduction of industrial ammonia (NH3) production via the Haber-Bosch process N fertilizers have increasingly been used to meet the demand of the growing world population for food and feed (Erisman et al. 2009). Furthermore, N fixing crops are increasingly cultivated also adding N to the biosphere. Finally, reactive N (Nr) compounds are also created incidentally during combustion processes. The human perturbation of the natural N cycle has led to an unprecedented accumulation of Nr in the biosphere (Erisman et al. 2009). For 2005 it is estimated that due to human activities, 187 Tg of N have been added to the global biosphere, which approximately relates to a doubling of global N cycling (Galloway et al. 2008). Not all of this N is ultimately denitrified, but a substantial, though hardly to quantify amount of Nr may remain in terrestrial ecosystems and here mainly in the soil. Galloway et al. (2004) estimated that from the 0.268 Pg N which were added annually during the 1990s to terrestrial ecosystems approximately 0.060 Pg N year−1 may be stored in terrestrial ecosystems, i.e., 20–25% of the total input of Nr to terrestrial ecosystems. In contrast, it is assumed that in pre-industrial times Nr inputs and outputs were balanced. In conclusion, it is very much likely to assume that Nr is accumulating in the global biosphere and that Nr stocks of all terrestrial ecosystems are increasing.
What is the consequence of this? Batjes (1996) estimated that global soil C stocks for 0–30 cm are in the range of 906–969 Pg C and that to 100 cm soil depth 2,150–2,300 Pg C may be stored. For soil N stocks the respective numbers are: = 63–67 Pg N for the 0–30 cm soil layer and 133–140 Pg N for 0–100 cm soil layer, respectively. Following Galloway et al. (2004) that from the 0.268 Pg N year−1 added to terrestrial ecosystems 0.060 Pg N year−1 are remaining in ecosystems, and assuming that the major fate is its storage in soils this will lead to a narrowing of soil C:N ratios (Fig. 19.1). If soil C stocks are not increasing and 10% of the additional Nr is stored in the topsoil (0–30 cm) the global average soil C:N ratio may narrow for the first 30 cm of soil from 14.4 to 13.8 in the coming 100 years (Fig. 19.1). However, soil C:N ratios may further widen if soils get re-carbonized, whereas if soil C loss due to cultivation of soils continues the narrowing of soil C:N ratio may accelerate (Fig. 19.1).
Changes in global soil N stocks from 2000 to 2100 assuming that soil C stocks are remaining unchanged, anthropogenic N inputs are remaining at the same level as in the 1990s and that total N additions to terrestrial ecosystems are equaling 0.268 Pg N year−1 (Galloway et al. 2004) and that about 10% or 0.030 Pg year−1 of the N input to terrestrial ecosystems are either stored in the topsoil (0–30 cm) or in soils to 100 cm depth. (a) Shows changes in total soil N stocks, whereas (b) displays resulting changes in the soil C:N ratio either for 0–30 cm or 0–100 cm soil depth, respectively. Data for global soil N and soil C stocks were taken from Batjes (1996). Dashed lines in the (b) are indicating changes in the soil C:N ratio if C loss due to cultivation continues or if soils are re-carbonized. For both scenarios changes in soil C stocks (either 0–30 cm or 0–100 cm) at a rate of 78 Pg C 100 year−1 were assumed
Significant changes in forest floor and mineral topsoil soil C:N ratios following simulated increased atmospheric N deposition has been observed e.g., in a study by Andersson et al. (2002) for a series of Picea abies (L.) stands in Sweden or by Lovett and Goodale (2011) for a mixed oak wood stand in Northeastern US. In a report for the German Environmental Agency, Kiese et al. (2009) provided data for a pine forest in the Northeastern German Lowlands where due to increased atmospheric N deposition forest floor C:N ratios decreased within 20 years from 31 to 27 and in the top 10 cm of the mineral soil from 26 to 23, respectively (Fig. 19.2). These observed decreases could be simulated well by a biogeochemical model based on reported data of atmospheric N deposition at the forest site (Kiese et al. 2009).
Observed changes in forest floor and soil (0–10 cm) C:N ratios (points lower graph) of a pine stand in the Northeastern German Lowlands as a response to increased atmospheric N deposition (upper graph). The lower graph also displays simulated changes in soil C:N ratios using the biogeochemical model MOBILE-DNDC, and historic and predicted climate and N deposition data as drivers. For further details see Kiese et al. (2009)
19.1.2 Coupling of Carbon and Nitrogen Turnover Processes
As pointed out earlier the re-carbonization of the biosphere has been highlighted recently as an important approach to mitigate the increase and to consolidate atmospheric CO2 concentrations by converting the atmospheric CO2 into biotic or abiotic C sequestered in vegetation or soil pools. Due to the tight-coupling of C and N cycles in soils and ecosystems (Fig. 19.3) – e.g., mineralization of organic matter does not only lead to break down of C substrates and the release of CO2 but also to the liberation of inorganic N – anthropogenic induced changes in rates of biotic C-sequestration in terrestrial ecosystems will directly affect N turnover processes in soils, and thus, also the biosphere–atmosphere exchange of gaseous N and C compounds (Li et al. 2005).
Coupling of ecosystem C and N cycling and possible consequences of an acceleration of both cycles with regard to soil-atmosphere C and N exchange. +: enhancement −: attenuation (Figure modified following Blagodatsky et al. 2011)
Consequently, increased rates of C sequestration in soils are likely to accelerate not only C but also N turnover, in particular when agricultural N fertilizer use and atmospheric N deposition in many regions globally remain at the present high levels. Increased C and N cycling may result in increased soil anaerobiosis, due to increased microbial oxygen consumption during mineralization of organic matter. This may lead to increases in soil anaerobiosis, which may result in elevated soil N2O emissions and a weakening of the sink strengths of upland soils for atmospheric CH4 (Butterbach-Bahl et al. 2011), due to a stimulation of denitrification and methanogenesis versus plant and microbial N immobilization or CH4 oxidation in soils.
19.2 Soil Organic Carbon and Magnitude of Nitrous Oxide Emissions
In their literature review on C:N interactions, Li et al. (2005) revealed a very significant positive relationship between SOC content of soils and the magnitude of N2O emissions (Fig. 19.4). There are several reasons why SOC contents are a major control of soil N2O emissions. First, organic C is the basis for any biotic mineralization process and with increasing SOC contents – except for conditions of strict anaerobiosis like in peatlands or if soil moisture/temperature is hampering microbial activity like in arid and semi-arid regions – mineralization activities are increasing too. The oxygen consumption by microbial mineralization may result in an increased frequency and increased spatial extend of anaerobic microsites, where mineralized and oxidized inorganic Nr may be transformed by nitrification and denitrification processes into N2O. Second, the availability of labile C substrates is a prerequisite for denitrification besides the availability of oxidized Nr substrates. Simultaneous availability of sufficient concentrations of readily available C and Nr substrates for denitrification is more likely to occur in soils rich in organic matter, while in soils with low organic C content denitrification activity and, thus, also N2O formation, may even be hampered due to missing C substrates. Such a situation is e.g. occurring in many arable soils in the North China Plain, one of the key regions of food production in China. For this region, several reports indicate, that soil N2O emissions are rather low at mineral fertilizer application of up to 600 kg N ha−1 year−1 with annual cumulative emission rates of <4 kg N2O-N ha−1 year−1 (Ju et al. 2011; Liu et al. 2010). Also the N fertilizer N2O emission factor of 0.5–0.7% of added fertilizer (Ding et al. 2007) for wheat-maize (Triticum spp.-Zea mays L.) rotations in the North China Plain is significantly below the global average of 1% as suggested by IPCC (2006). The main reason for low N2O emissions seems to be the low C content of the soils and not the availability of nitrate (NO −3 ), which accumulates in the soil. Most likely C substrate supply limits denitrification and, thus, also N2O production by denitrification. If these soils are re-carbonized, the large stocks of inorganic Nr in surface and subsurface soils may largely get denitrified, thereby potentially releasing significant amounts of N2O as by-products of denitrification. Comparable situations, i.e., that the availability of readily available C is limiting denitrification and N2O formation has been described for agro-ecosystems as well as for natural ecosystems (Morley and Baggs 2010; Kammann et al. 2008; Butterbach-Bahl and Dannenmann 2011).
Comparison of observed and simulated annual N2O emissions from agricultural soils. Symbols indicate soil organic carbon (SOC) content in soil in kg SOC kg−1 soil. A general trend to higher N2O emissions for higher SOC is apparent in both field and simulated results (Figure adapted from Li et al. 2005)
19.3 Soil C:N Ratio and Microbial N Turnover Processes
The major long-term sink of atmospheric Nr input into ecosystems is soil organic matter (SOM), as was shown in many 15N-tracing experiments (Morier Jaquet et al. 2008; Nadelhoffer et al. 1999; Tietema et al. 1998). Since Nr deposition via atmospheric pathways is often exceeding plant N demand in natural and semi-natural ecosystems, the atmospheric N input has led to N saturation of natural and semi-natural ecosystems, resulting in a decrease in the soil C:N ratio both in the organic layer and in the mineral soil of natural and semi-natural ecosystems within large regions of Europe, Asia and North America (Galloway et al. 2004; Corré et al. 2007; Velthoff et al. 2011; Butterbach-Bahl et al. 2011). Even if atmospheric Nr input in terrestrial ecosystems may not further increase in future, the C:N ratio in mineral topsoils will further decrease (Fig. 19.1). The N saturation and associated decline in soil C:N ratios involves detrimental effects such as soil eutrophication, -acidification, reduced plant biodiversity and reduction in tree population, while it has a large potential to increase C sequestration in plant biomass and eventually also in soil (Snyder et al. 2009; Velthoff et al. 2011).
Besides these effects, the C:N ratio is – as well as total C and N content and -availability – a major driver of soil microbial N turnover processes. This is not only the case for processes such as ammonification, nitrification, denitrification and microbial immobilization of inorganic N, but for the relative importance or even dominance of single processes such as nitrification over competing processes such as microbial ammonium (NH +4 ) immobilization. In particular, the relative importance of nitrification and denitrification over the processes of microbial immobilization of inorganic Nr (and mycorrhizal/plant uptake) determine whether there are large or small losses of N2O from soil (Tietema and Wessel 1992; Stockdale et al. 2002; Butterbach-Bahl et al. 2011). By regulating this delicate balance, the C:N ratio plays a crucial role in the characterization of the N cycle, i.e., if it is open (high N loss along gaseous and hydrological pathways) or closed (internal N cycling facilitates ecosystem N retention). In the following section, the effect of the C:N ratio on ecosystem Nr retention and loss is explained at the level of the single N cycle processes (Fig. 19.5).
Schematic representation of the relationships between soil C:N ratio and gross rates of inorganic N production (ammonification, nitrification) and consumption (microbial immobilization). When C:N ratios narrow below a value of approximately 25, e.g. as a consequence of atmospheric Nr deposition, gross ammonification as well as nitrification will increase, while heterotrophic microbial immobilization of inorganic Nr will decrease. Thus, the index of microbial Nr retention in the soil (i.e., the ratio between gross microbial immobilization and total gross inorganic N production) is decreasing, indicating an opening of the N cycle and increased N2O emissions. Mechanisms of impacts of the C:N ratio on single N cycle processes are provided in the text
The lower the soil C:N ratio, the larger the N yield during depolymerization and mineralization of a given amount of SOM. Thus, ammonification will increase with decreasing C:N ratio (Frankenberger and Abdelmagid 1985; Hart et al. 1994). Subsequently, the balance of partitioning of NH +4 to the potentially competing microbial processes of heterotrophic immobilization into biomass of free living microorganisms and autotrophic nitrification (i.e. conversion to NO3), as well as plant or mycorrhizal uptake of NH +4 , determines whether N is retained in the ecosystem or subject to increased risk of N loss via gaseous or hydrological pathways.
Due to the long residence time of Nr in plant biomass, plant Nr uptake results in persistent ecosystem N retention until disturbances such as harvest or fire. However, plants are in general poor competitors for Nr against microbes at the process level (Rennenberg et al. 2009). Even in fast growing spruce (Picea spp) forests, plant N uptake may at least be one order of magnitude lower than gross N mineralization, thus consuming only for a minor part of inorganic N produced in soil (Kreutzer et al. 2009).
Heterotrophic NH +4 assimilating microorganisms generally tend to outcompete autotrophic nitrifiers (Verhagen and Laanbroek 1991; Hart et al. 1994; Verhagen et al. 1995; Booth et al. 2005). However, this may not necessarily be the case in agricultural soils, when there is no NH +4 limitation (Burger and Jackson 2003). The availability of labile C compounds is the major controller of heterotrophic microbial immobilization of NH +4 -N (Woodmansee and Duncan 1980; Compton and Boone 2002; Booth et al. 2005; Accoe et al. 2004, 2005). At decreasing C:N ratios, microbial N uptake may be C-limited, leaving more NH +4 available to the process of autotrophic nitrification, thus opening pathways to potential N loss such as N2O emission to the atmosphere.
In contrast to nitrification, the incorporation of NH +4 -N into microbial cell walls, characterized by short residence times, leads to retention of N in the ecosystem. This may be of short-term, when there is dieback and remineralization of microbial residues leading to a rapid and repeated re-allocation of mineral N to the soil NH +4 pool. However, despite the rapid turnover of microbial biomass in soil, microbial immobilization can also lead to long-term stabilization of N. Among the processes are microbially mediated long-term N stabilization by the accumulation of bacterial-derived residues such as proteins, amino acids and amino sugars on the surface of clay minerals (Sollins et al. 2006). Decreasing soil C:N ratios could also reduce the long-term N stabilization in soil via reducing microbial immobilization, which could contribute to increased risk of N loss. Hence, the C:N ratio is of crucial importance for the balance of NH +4 partitioning to processes promoting either N retention or N loss. Beside its effect on nitrification at the substrate level, the C:N ratio is also affecting the balance of consumption of the end product of nitrification. Specifically, heterotrophic microbial NO −3 immobilization will also be dependent on C availability, i.e., decreasing with decreasing C:N ratio, promoting accumulation of soil NO −3 , which would be available for denitrification and associated N2O production and –loss.
Via its effects on ammonification, nitrification as well as on microbial immobilization, the soil C:N ratio is a crucial determinant of the microbial Nr retention capacity (Booth et al. 2005). The effect of the soil C:N ratio on microbial N retention was shown in some field studies, e.g., for a Rendzic Leptosol in a mountainous beech (Fagus sylvatica L.) forest in Southern Germany. In this study, variations in soil C:N ratio triggered by different microclimate and management were found to be correlated with microbial N retention, i.e., decreased C:N ratios lead to decreased relative importance of microbial N retention (Dannenmann et al. 2006, 2007).
By promoting nitrification, the decreasing C:N ratio may also promote the formation of N2O as a by-product. Besides its effect on nitrification, also the formation of N2O as a facultative end-product of denitrification may be increased, when a narrow C:N ratio is leading to NO −3 accumulation in soil, promoting denitrification in general, while impairing the last step of denitrification, i.e. the conversion of N2O to the end product dinitrogen (N2) catalyzed by the enzyme N2O reductase (e.g., Groffman et al. 2006; Wang et al. 2011). Thus, narrowing C:N ratios favour both N2O production by nitrification and denitrification. However, denitrification requires also labile C substrates. In this context, increased root exudation of monomeric C compounds, promoted by generally improved plant growth as a consequence of atmospheric Nr deposition and, in particular, due to increased atmospheric CO2 levels, may further promote denitrification (Kammann et al. 2008; Butterbach-Bahl and Dannenmann 2011). Denitrification may be further promoted by increased soil respirations and plant water use efficiency, both increasing the soil anaerobic volume in a changing climate (Butterbach-Bahl and Dannenmann 2011). Hence, in a changing climate, NO −3 accumulation in soil as a consequence of Nr deposition and narrowed C:N ratios may increasingly meet environmental conditions favourable for denitrification, bearing the potential for high N2O emissions from soil.
19.4 Soil Carbon-to-Nitrogen Ratios and Soil Nitrogen Trace Gas Emissions
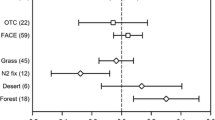
The most important soil microbial processes involved in the formation of N2O and nitric oxide (NO) are nitrification and denitrification. The magnitude of both processes will strongly depend on the availability of Nr substrates. For autotrophic nitrification NH3 is the relevant Nr substrate, while for denitrification inorganic Nr oxides as well as labile C substrates are needed. Heterotrophic nitrifiers may use also organic N compounds instead of NH3 to produce NO −3 and produce as side products as well NO and N2O. Therefore, a close link between Nr and C availability and N2O emissions can be expected. Specifically, close relationship between soil C:N ratios and magnitude of N trace gas emissions have been shown for forest soils in various climate zones (Fig. 19.6). Based on soil emission measurements at various tropical rain forest sites in Queensland, Australia, and a detailed analysis of soil properties, Breuer et al. (2000) showed that topsoil C:N ratios are a significant predictor for understanding the spatial variability of soil N2O emissions. Comparable results were also reported by Pilegaard et al. (2006) while analyzing environmental controlling variables of soil N2O and NO emissions for 15 different forest ecosystem sites across Europe (Fig. 19.6). At least for N2O a significant relationship was found with exponential increasing soil N2O emission rates with decreasing soil C:N ratios in the topsoil. The study of Klemedtsson et al. (2005) on N2O emissions from drained forested histosols in Sweden shows that a strong negative relationship between N2O emissions and soil C:N ratios exist. This relationship has been successfully used to estimate emissions at other sites in Finland and Germany.
The exponential increase in forest soil N2O emissions with decreasing C:N ratios is in good agreement with the observation that the risk for elevated NO −3 leaching from forest soils is increasing exponentially if soil C:N ratios are smaller than 25 (Gundersen et al. 2006; Butterbach-Bahl et al. 2011). In both cases the ecosystem N retention capacities are likely to be exceeded and losses of N to the hydrosphere and atmosphere are increasing. With regard to the re-carbonization of soils this clearly indicates, that the N status of the ecosystem needs to be explored, e.g., by recording soil C:N ratios as indicators – and it will be necessary to consider total ecosystem N inputs either by atmospheric N-input and/or by atmospheric-N-input plus organic or inorganic fertilizers or even weathering of bedrock N (Morford et al. 2011).
19.5 Nitrogen Availability and Ecosystem Carbon Sequestration
Input of Nr to terrestrial ecosystems is also a measure to increase ecosystem C sequestration. For agricultural systems Nr has been reported to play an important role in soil C storage either by promoting crop dry matter production and/ or by chemically stabilizing C in the soil (Snyder et al. 2009). However, though several studies have shown that nitrogen fertilization results in higher levels of soil C over time (Paustian et al. 1992; Wilts et al. 2004) the stimulating effect may be rather small (about 2%) as shown in the meta-data analysis by Liu and Greaver (2009). Besides agricultural ecosystems, several recent studies have shown that Nr deposition to European forest ecosystems has resulted in increased forest growth and C sequestration (De Vries et al. 2006, 2011; Schulze et al. 2010). With regard to the net climate balance of atmospheric Nr deposition, C sequestration was thereby largely outweighing reductions in soil CH4 uptake or stimulations in soil N2O emissions (De Vries et al. 2011). However, long-term assessments are still missing and the stimulation of forest growth may cease following a few decades while stimulative effects on soil N2O emissions may persist over longer periods of time.
19.6 Conclusions
Re-carbonization of terrestrial ecosystems is likely to be a sustainable strategy to stabilize or even lowering of atmospheric CO2 concentrations. Nevertheless, it still needs to be assessed if specifically an increase in soil C stocks will indeed lead to a net reduction of GHG emissions from terrestrial ecosystems, i.e., if benefits due to C sequestration are not specifically outweighed by the stimulation of N2O emissions from soils. The reason for this is the unprecedented perturbation of the global N cycle. If current rates of direct (fertilization) and indirect reactive N inputs (mainly atmospheric N deposition) continues, C:N ratios in soils may further narrow even if additional C is sequestered in soils. Both, C accumulation as well as the narrowing of soil C:N ratios may result in a stimulation of microbial N turnover and associated emissions of N2O, which has as a global warming potential of about 300 over a 100-year period as compared to CO2. Therefore, a thorough quantification of the biogeochemical interaction of C and N needs to be incorporated into assessment frameworks to accurately evaluate the climate benefits of a re-carbonization of the terrestrial environment.
Abbreviations
- NH3 :
-
ammonia
- NH +4 :
-
ammonium
- C:
-
Carbon
- CO2 :
-
carbon dioxide
- N2 :
-
dinitrogen
- GHGs:
-
greenhouse gases
- CH4 :
-
methane
- NO −3 :
-
nitrate
- NO:
-
nitric oxide
- N:
-
nitrogen
- N2O:
-
nitrous oxide
- Nr:
-
reactive forms of N
- SOC:
-
soil organic carbon pools
- SOM:
-
soil organic matter
References
Accoe F, Boeckx P, Busschaert J et al (2004) Gross N transformation rates and net N mineralization rates related to the C and N contents of soil organic matter fractions in grassland soils of different age. Soil Biol Biochem 36:2075–2087
Accoe F, Boeckx P, Videla X et al (2005) Estimation of gross nitrogen transformations and nitrogen retention in grassland soils using FLUAZ. Soil Sci Soc Am J 69:1967–1976
Andersson P, Berggren D, Nilsson I (2002) Indices for nitrogen status and nitrate leaching from Norway spruce (Picea abies (L.) Karst.) stands in Sweden. For Ecol Manage 157:39–53
Batjes NH (1996) Total carbon and nitrogen in the soils of the world. Eur J Soil Sci 47:151–163
Blagodatsky S, Grote R, Kiese R et al (2011) Modelling of microbial carbon and nitrogen turnover in soil with special emphasis on N-trace gases emission. Plant Soil 346:297–330
Booth MS, Stark JM, Rastetter E (2005) Controls on nitrogen cycling in terrestrial ecosystems: a synthetic analysis of literature data. Ecol Monogr 75:139–157
Breuer L, Papen H, Butterbach-Bahl K (2000) N2O-emission from tropical forest soils of Australia. J Geophys Res 105:26353–26378
Burger M, Jackson LE (2003) Microbial immobilization of ammonium and nitrate in relation to ammonification and nitrification rates in organic and conventional cropping systems. Soil Biol Biochem 35:29–36
Butterbach-Bahl K, Dannenmann M (2011) Denitrification and associated soil N2O emissions due to agricultural activities in a changing climate. Curr Opin Environ Sci 3:389–395
Butterbach-Bahl K, Gundersen P, Ambus P et al (2011) Nitrogen processes in terrestrial ecosystems. In: Sutton MA, Howard CM, Erisman JW et al (eds) The European nitrogen assessment: sources effects, and policy perspectives. Cambridge University Press, Cambridge
Compton JE, Boone RD (2002) Soil nitrogen transformations and the role of light fraction organic matter in forest soils. Soil Biol Biochem 34:933–943
Conant RT, Ogle SM, Paul EA et al (2011) Measuring and monitoring soil organic carbon stocks in agricultural lands for climate mitigation. Front Ecol Environ 9:169–173
Corré M, Brumme R, Veldkamp E et al (2007) Changes in nitrogen cycling and retention processes in soils under spruce forests along a nitrogen enrichment gradient in Germany. Glob Change Biol 13:1509–1527
Cui F, Yan G, Zhou Z et al (2011) Annual nitrous oxide and nitric oxide emissions from a wheat-maize cropping system on a calcareous soil in the North China Plain. Plant Soil (submitted)
Dannenmann M, Gasche R, Ledebuhr A et al (2006) Effects of forest management on soil N cycling in beech forests stocking on calcareous soils. Plant Soil 287:279–300
Dannenmann M, Gasche R, Papen H (2007) Nitrogen turnover and N2O production in the forest floor of beech stands as influenced by forest management. J Plant Nutr Soil Sci 170:134–144
De Vries W, Reinds GJ, Gundersen P et al (2006) The impact of nitrogen deposition on carbon sequestration in European forests and forest soils. Glob Change Biol 12:1151–1173
De Vries W, Kros J, Reinds GJ et al (2011) Quantifying impacts of nitrogen use in European agriculture on global warming potential. Curr Opin Environ Sci 3:291–302
Ding WX, Meng L, Cai ZC, Han FX (2007) Effects of long-term amendment of organic manure and nitrogen fertilizer on nitrous oxide emission in a sandy loam soil. J Environ Sci China 19:185–193
Erisman JW, Sutton MA, Galloway J et al (2009) How a century of ammonia synthesis changed the world. Nat Geosci 1:636–639
Frankenberger WT, Abdelmagid HM (1985) Kinetic parameters of nitrogen mineralization rate of leguminous crops incorporated into soil. Plant Soil 87:257–271
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present, future. Biogeochemistry 70:153–226
Galloway JN, Townsend AR, Erisman JW et al (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Groffman PM, Altabet MA, Böhlke JK et al (2006) Methods for measuring denitrification: diverse approaches to a difficult problem. Ecol Appl 16:2091–2122
Gundersen P, Schmidt IK, Raulund-Rasmussen K (2006) Leaching of nitrate from temperate forests – effects of air pollution and forest management. Environ Rev 14:1–57
Hart S, Nason GE, Myrold DD, Perry DA (1994) Dynamics of gross nitrogen transformations in an old-growth forest: the carbon connection. Ecology 75:880–891
Houghton RA (2007) Balancing the global carbon budget. Annu Rev Earth Planet Sci 35:313–347
IPCC (2006) IPCC Guidelines for National Greenhouse Gas Inventories, prepared by the National Greenhouse Gas Inventories Programme, IGES, Hayama, Japan
Ju X, Lu X, Gao Z et al (2011) Processes and factors controlling N2O production in an intensively managed low carbon calcareous soil under sub-humid monsoon conditions. Environ Pollut 159:1007–1016
Kammann C, Müller C, Grünhage L et al (2008) Elevated CO2 stimulates N2O emissions in permanent grassland. Soil Biol Biochem 40:2194–2205
Kiese R, Wochele S, Grote R et al (2009) Modellierung und kartierung räumlich differenzierter wirkungen von stickstoffeinträgen in Ökosysteme im rahmen der UNECE-Luftreinhaltekonvention. Final report for the “FuE-Vorhaben FKZ 205 85 239”, German Environmental Protection Agency (Umweltbundesamt), Dessau, Germany
Klemedtsson L, Von Arnold K, Weslien P et al (2005) Soil C:N ratio as a scalar parameter to predict nitrous oxide emissions. Glob Change Biol 11:1142–1147
Kreutzer K, Butterbach-Bahl K, Rennenberg H et al (2009) The complete nitrogen cycle of a N-saturated spruce forest ecosystem. Plant Biol 11:694–700
Lal R (2004) Soil carbon sequestration impacts on global climate change and food security. Science 304:1623–1627
Li C, Frolking S, Butterbach-Bahl K (2005) Carbon sequestration can increase nitrous oxide emissions. Clim Change 72:321–338
Liu L, Greaver TL (2009) Nitrogen addition stimulates emissions of biogenic greenhouse gases in terrestrial and wetland ecosystems. Ecol Lett 12:1103–1117
Liu C, Zheng X, Zhou Z et al (2010) Nitrous oxide and nitric oxide emissions from an irrigated cotton field in Northern China. Plant Soil 1–2:123–134
Lovett GM, Goodale CL (2011) A new conceptual model of nitrogen saturation based on experimental nitrogen addition to an oak forest. Ecosystems 14:615–631
Morford SL, Houlton BZ, Dahlgren RA (2011) Increased forest ecosystem carbon and nitrogen storage from nitrogen rich bedrock. Nature 477:78–81
Morier Jaquet I, Guenat C, Siegwolf R et al (2008) Dynamics of atmospheric nitrogen deposition in a temperate calcareous forest soil. J Environ Qual 37:2012–2021
Morley N, Baggs EM (2010) Carbon and oxygen controls on N2O and N2 production during nitrate reduction. Soil Biol Biochem 42:1864–1871
Nadelhoffer KJ, Downs MR, Fry B (1999) Sinks for 15N-enriched additions to an oak forest and a red pine plantation. Ecol Appl 9:72–86
Paustian K, Parton WJ, Persson J (1992) Modeling soil organic matter in organic amended and N-fertilized long-term plots. Soil Sci Soc Am J 56:476–488
Pilegaard K, Skiba U, Ambus P et al (2006) Factors controlling regional differences in forest soil emission of nitrogen oxides (NO and N2O). Biogeosciences 3:615–661
Rennenberg H, Dannenmann M, Gessler A et al (2009) Nitrogen balance in forest soils: nutritional limitation of plants under climate change stresses. Plant Biol 11:4–23
Schulze ED, Ciais P, Luyssaert S et al (2010) The European carbon balance, part 4: integration of carbon and other trace-gas fluxes. Glob Change Biol 16:1451–1469
Smith J, Jones M Jr, Houghton L et al (1999) Future of health insurance. N Engl J Med 965:325–329
Smith P, Martino D, Cai Z et al (2008) Greenhouse gas mitigation in agriculture. Philos Trans R Soc Lond B Biol Sci 363:789–813
Snyder CS, Bruulsema TW, Jensen TL et al (2009) Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric Ecosyst Environ 133:247–266
Sollins P, Swanston C, Kleber M et al (2006) Organic C and N stabilization in a forest soil: evidence from sequential density fractionation. Soil Biol Biochem 38:3313–3324
Stockdale E, Hatch DJ, Murphy DV et al (2002) Verifying the nitrification to immobilization ratio (N/I) as a key determinant of potential nitrate loss in grassland and arable soils. Agronomie 22:831–838
Tietema A, Wessel WW (1992) Gross nitrogen transformations in the organic layer of acid forest ecosystems subjected to increased atmospheric nitrogen input. Soil Biol Biochem 24:943–950
Tietema A, Emmett B, Gundersen P et al (1998) The fate of 15N labelled nitrogen deposition in coniferous forest ecosystems. For Ecol Manage 101:19–27
Velthoff G, Barot S, Bloem J et al (2011) Nitrogen as a threat to European soil quality. In: Sutton MA, Howard CM, Erisman JW et al (eds) The European nitrogen assessment: sources effects, and policy perspectives. Cambridge University Press, Cambridge
Verhagen FJM, Laanbroek HJ (1991) Competition for ammonium between nitrifying and heterotrophic bacteria in dual energy-limited chemostats. Appl Environ Microbiol 57:3255–3263
Verhagen FJM, Laanbroek HJ, Woldendorp JW (1995) Competition for ammonium between plant roots and nitrifying and heterotrophic bacteria and the effects of protozoan grazing. Plant Soil 170:241–250
Wang R, Willibald G, Feng Q et al (2011) Measurement of N2, N2O, NO, and CO2 emissions from soil with the gas-flow-soil-core technique. Environ Sci Technol 45:6066–6072
Wilts AR, Reicosky DC, Allmaras RR et al (2004) Long-term corn residue effects: harvest alternatives, soil carbon turnover, and root-derived carbon. Soil Sci Soc Am J 68:1342–1351
Woodmansee RG, Duncan DA (1980) Nitrogen and phosphorus dynamics and budgets in annual grasslands. Ecology 61:893–904
Acknowledgments
We thank the German Science Foundation (Deutsche Forschungsgemeinschaft) for generously funding the work of Michael Dannenmann through contract number DA 1217/2-1. This work is a contribution to the EU funded integrated project NitroEurope.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Butterbach-Bahl, K., Dannenmann, M. (2012). Soil Carbon and Nitrogen Interactions and Biosphere-Atmosphere Exchange of Nitrous Oxide and Methane. In: Lal, R., Lorenz, K., Hüttl, R., Schneider, B., von Braun, J. (eds) Recarbonization of the Biosphere. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4159-1_19
Download citation
DOI: https://doi.org/10.1007/978-94-007-4159-1_19
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4158-4
Online ISBN: 978-94-007-4159-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)