Abstract
Saponins are triterpene and steroidal glycoside compounds in which aglycone (sapogenins) are attached to one or more sugar moieties. They are polar compounds and have great diversity in their chemical structures. Different aqueous or polar solvents have been used to extract saponins. Saponins exhibit several physical and biological properties such as the formation of a stable foam, haemolysis, antimicrobial activity and defaunation of the rumen; however, there is little correlation among these properties. This paper describes the methods of saponin extraction, their chemical diversity and the effects on rumen microbial ecosystem along with metabolism of saponins in the rumen. Recent studies on methane emissions by ruminants provide evidence that saponins may have potential to be used as a antimethanogenic agent; however, the inclusion level of saponin from each source should be tested to get the optimum result. The practical utility of saponins or saponin containing plants as feed additives in sustainable and environmental friendly ruminant production warrants further investigation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
11.1 Introduction
Saponins are a structurally diverse family of plant secondary metabolites. In general, saponins consist of an aglycone attached to one or more sugar moieties. They are classified as triterpene saponins and steroid saponins based on their aglycones which can be in the form of triterpene (30 C-atoms) or steroid (27 C-atoms), respectively. Recently, Vincken et al. (2007) proposed a new classification of saponins based on the biosynthesis of carbon skeletons of the aglycone. An enormous diversity found in saponin structures was the reason for this new classification. According to this classification, 11 main classes of saponins were distinguished, i.e. dammaranes, tirucallanes, lupanes, hopanes, oleananes, taraxasteranes, ursanes, cycloartanes, lanostanes, cucurbitanes, and steroids. Out of these structures, oleanane is the most common aglycone occurring in plants with glucose, arabinose, rhamnose, xylose and glucuronic acid as the common sugars attached to it. Most saponins are defined as monodesmosides if they contain one saccharide chain attached to C3 atom of their aglycones or bidesmosides, if an additional saccharide chain is attached to C26 (furanstanol saponins) or C28 (triterpene saponins) (Güçlü-Üstündağ and Mazza 2007). Saponins can also be classified as neutral if the sugar attached to sapogenin is a common monosaccharide (glucose, xylose, arabinose etc.) or acidic if the sugar moiety contains uronic acid or one or more carboxylic groups (Lasztity et al. 1998). The aglycone component of the saponin is hydrophobic whereas the conjugated sugars are hydrophilic. Their unique structures allow saponins to give stable foams in water or aqueous solutions.
There are several recent reviews on the effect of secondary compounds including saponin or saponin containing plants on rumen function and animal production (Hart et al. 2008; Patra and Saxena 2009a). This review describes in more details saponin extraction methods, the structural diversity of saponins and their effect on rumen microbes and rumen fermentation. The information presented here could provide wider opportunities for the utilization of saponins and saponin-containing plants as feed additives in sustainable and environmental friendly ruminant production.
11.2 Biological Properties of Saponin
The most important property of saponins is their ability to form very stable foam as a consequence of their surfactant ability. The presence of polar groups in the sugar moiety together with the non polar character of the aglycone moiety enables saponins to lower surface tension in aqueous systems. Traditionally, saponin-containing plants have been used as natural washing agents.
Other biological properties of saponins include their ability to haemolyse the red blood cells, to depress protozoal populations in the rumen, and to inhibit the growth of microbes, especially fungi. The same saponin extract can exhibit several of these biological properties.
The methanol extract of pericarp of Sapindus rarak fruit could make a stable foam, haemolyse the red cells (Wina, unpublished) and also depress the protozoa population both in vitro and in vivo (Wina et al. 2005a, 2006a). Saponins extracted from guar meal in 100% methanol had the ability to haemolyse the red cells and also showed antibacterial activities against the pathogenic bacteria, Staphylococcus aureus, Salmonella typhimurium and Escherichia coli (Hassan et al. 2010a, b). Gestetner et al. (2006) found that the lucerne saponin exhibited haemolytic property and also antifungal activity. Owing to these various phenomena, several authors have tried to link the biological activities of saponins as antifungal, antiprotozoal, antimicrobial or cytotoxic activity to haemolytic activity or foaming ability (Chwalek et al. 2006; Wang et al. 2007). The foaming test and haemolysis test for saponin are simple tests. Therefore, it would be faster and easier to screen many different saponins by either foaming test or haemolysis test and to relate the result to other biological activities of saponins, then, to choose the potential saponins for further use as feed additives or medicinal application.
The result of our study with 23 saponin-containing plants, however, did not find any correlation between height of foam or haemolytic activity to the antiprotozoal activity (Wina, unpublished). Wang et al. (2007) have disclosed from their study with 63 steroidal saponins that there was no correlation between haemolytic activity and cytotoxicity.
Lin and Wang (2010) studied the haemolytic mechanism of saponins using molecular dynamic simulation technique. Saponins first penetrate easily into the lipid membrane, and then accumulate in the lipid raft micro-domain. Saponins bind cholesterol in the lipid membrane and prevent the cholesterol from the interaction with sphingomyelin. Saponin-cholesterol micelles destabilize the structure of the lipid raft micro-domain and cause disruption of the lipid bilayer, which eventually lead to the haemolysis of red cells (Lin and Wang 2010; Baumann et al. 2000). The mechanism of protozoal lysis by saponins is similar to the mechanism of haemolysis of red blood cells by saponins. However, some aglycones still showed their ability to haemolyse the red cells but lost their activity toward protozoa. The presence of intact sugar moiety is very important for its activity against protozoa. Muetzel et al. (2005) reported that the aglycone of Sapindus rarak saponins (hederagenin) did not show its ability to depress protozoa population.
From several studies, it can be concluded that antiprotozoal, antimicrobial or cytotoxicity activity of saponins cannot be predicted from the height of foam or its haemolytic activity. Direct screening of the saponin extracts or saponin containing plants based on its specific biological property is recommended.
11.3 Biodiversity of Saponins in Plant Materials
Saponins are a complex group of compounds and different species or parts of plant synthesize different types of saponins (Lasztity et al. 1998; Mahato and Garai 1998). Diversity can also occur in one location of the plants. Several saponins in one plant usually have the same aglycone but different sugar moieties. Several species of Sapindus trees are found in different parts of the world such as Sapindus rarak, Sapindus emarginatus, Sapindus mukorossi and Sapindus saponaria. Their fruits contain foaming substances: it was reported that 20 different monodesmosidic saponins have been isolated and structural elucidated in Sapindus rarak fruit’s pericarp (Hamburger et al. 1992; Asao et al. 2009).
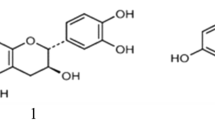
Hederagenin is the aglycone found in these saponins. When arabinose is directly attached to hederagenin and rhamnose and arabinose attached to arabinose, this saponin is called sapindoside (Fig. 11.1). When arabinose attached to hederagenin and acetyl group attached to arabinose or rhamnose, this saponin is called rarasaponin. Asao et al. (2009) have isolated and identified six different structures of rarasaponin (I to VI) from Sapindus rarak fruit’s pericarp. Other Sapindus, Sapindus emarginatus (Kanchanapoom et al. 2001), Sapindus mukorossi (Huang et al. 2008) and Sapindus saponaria (Tsuzuki et al. 2007) fruit’s pericarp contained similar saponins to Sapindus rarak with hederagenin as the aglycone. Huang et al. (2008) named the isolated saponin as sapinmusaponin, which was almost identical with rarasaponin in Sapindus rarak. They also found damarane type of saponin in the gall of Sapindus emarginatus. Kanchanapoom et al. (2001) isolated saponins with acetyl hederagenin and oleanolic acid as the aglycone in Sapindus emarginatus. Lemos et al. (1992, 1994) found other saponins in Sapindus saponaria with glucose directly attached to hederagenin and rhamnose and arabinose linked to the glucose. The complexity of these saponins has not yet received any attention in relation to their individual activity on rumen microbes.
Some saponin structures in the fruit’s pericarp of Sapindus rarak (Asao et al. 2009)
Several structures of saponin have been identified in tea (Camellia sinensis) seeds, leaves and flowers. Saponin extract from tea seed is commercially available and used for killing small fish in shrimp ponds. Recently, it has been tested for a feed additive for ruminants and as a medicinal use. Yoshikawa et al. (2005) reported that 16 different structures of saponins have been identified in tea seeds. Further, they elucidated 12 new saponins in tea seeds (Yoshikawa et al. 2007). Therefore, in tea seeds, 28 different saponins have been isolated and identified as theasaponins, camelliasaponins, floratheasaponins, etc. (Fig. 11.2). Yoshikawa et al. (2005, 2007) showed that the position of acyl group that attached to the aglycone (theasapogenol) and different groups in the sugar moieties are important from a pharmacological point of view since different positions or groups attached to the aglycone or sugar moiety cause different activities.
Some saponin structures in tea (Camellia sinesis) seed (Yoshikawa et al. 2005)
Yucca schidigera stem contained as much as 10% of steroidal saponins which consisted of 28 different structures of spirostanol and furostanol glycosides. Yucca saponins have several aglycones, i.e. sarsapogenin, markogenin, smilagenin, samogenin, gitogenin and neogitogenin. They can be monodesmosides with one sugar chain attached at 3-O (Fig. 11.3, 1–4) and bidesmosides with two sugar chains attached at 3-O and 26-O positions (Fig. 11.3, 5–7). The predominant saponins in yucca are spirostanol saponins which primarily are glycosides of sarsapogenin (66%). Thus, the major property of Yucca saponins as described by Cheeke et al. (2006) is determined by spirostanol saponins (Oleszek et al. 2001).
Quillja saponaria Molina extract contains 10% total saponins, and more than 20 different structures of triterpenoid saponin have been structurally elucidated by NMR studies (Guo et al. 2000) Further, Bankefors (2006) did structural classification of 47 Quillaja saponins including minor compounds by electrospray ionisation ion trap multiple-stage mass spectrometry in combination with multivariate analysis from the chromatographic fractions. All studied structures are bidesmosides and consist of quillaic acid (as the aglycone) which is substituted with di or tri-saccharides at C-3 and a branched oligosaccharide at C-28. Bankefors (2006) found that saponin profiles in the young plants were different compared to older specimen; hence the biological and chemical activity differed between batches from different specimen (Fig. 11.4).
The common basic saponin structure of Quillaja saponaria (Bankefors 2006)
11.4 Extraction of Saponins
Saponins from plant materials can be extracted using different techniques and solvents. The conventional techniques for saponin extraction used soxhlet, liquid-liquid or solid–liquid extraction (Berhow et al. 2002; Hassan et al. 2010a, b). These methods consume a lot of solvent, time and may lead to potentially deleterious degradation of labile compounds (Kerem et al. 2005). Therefore, in recent years, new extraction techniques include accelerated solvent extraction, supercritical fluid extraction, solid-phase microextraction, sonication, extraction with supercritical or subcritical water, and microwave-assisted extraction have been developed and are considered to be more efficient than the conventional methods (Wu et al. 2001; Kerem et al. 2005; Ligor et al. 2005; Güçlü-Üstündağ and Mazza 2007). Ultrasonication-assisted extraction of ginseng saponins was about three times faster than the liquid-liquid extraction and can be carried out at lower temperature (Wu et al. 2001). Kerem et al. (2005) reported that methanol- microwave assisted method to extract saponin of chickpea proved to be faster and more efficient than soxhlet extraction.
Since saponins are polar compounds, many saponin extraction methods used water, aqueous methanol or ethanol, absolute methanol, ethanol or n-butanol (Kaur and Arora 2009; Xu et al. 2010; Tsuzuki et al. 2007; Zhang et al. 2005). The type of aglycone, type of sugar moiety and functional group attached to aglycone or sugar moiety, the concentration of saponin influence the saponin’s ability to dissolve in different solvents, therefore different extracts exerted different activities.
Our study with Sapindus rarak fruit’s pericarps showed that different extracts had different activity on suppressing protozoa population in the in vitro rumen (Wina, unpublished). The 50% methanol extract caused the highest suppression on protozoa population in the in vitro rumen fermentation, followed by 70% methanol and ethanol extract. While Kamra et al. (2006) and Agarwal et al. (2006) showed that the methanol extract of S. mukorossi was highly detrimental to protozoa in the in vitro rumen followed by ethanol and water extracts. Goel et al. (2008a) reported that either 50% methanol or 95% methanol extract of Fenugreek or Sesbania sesban gave similar effect in depressing protozoa, while water extract of Sesbania sesban showed lower activity to depress protozoa compared to 50% or 95% methanol extract. Patra et al. (2006) found that the ethanol extract of five plants (Acacia concinna pod, Terminalia chebula, Terminalia belerica, Emblica officinalis seed pulp and Azadirachta indica seed kernel) was more active than methanol or water extract in decreasing protozoa population and in vitro methane production. Sirohi et al. (2009) reported that the acetone extracts of several plants have more effective antimicrobial property than methanol or aqueous extracts, whereas aqueous extract of Sapindus mukorossi was the best inhibitor of methane production among other extracts. Hassan et al. (2010b) showed that saponins of guar meal that dissolved in 100% methanol had antibacterial activities against Staphylococcus aureus, Salmonella typhimurium and Escherichia coli, while only those dissolved in 20% and 60% methanol stimulated Lactobacillus spp. growth. These above reports showed that different extracts of a specific plant had different activities against microorganism growth or population.
Unless it is purified, the saponins present in an extract may be in conjunction with other compounds. These compounds may or may not have an effect on the rumen fermentation. Soluble carbohydrates (simple sugars) are usually present in the methanol extract and these compounds may contribute to the increase of total production of short chain fatty acid during rumen fermentation (Wina et al. 2005b). Studies with a subfraction of methanol extract of Sapindus rarak showed that the ethyl acetate subfraction decreased protozoa numbers in the in vitro rumen fermentation much less than the aqueous subfraction (16% vs. 62%, respectively), and reduced total gas production 10% while no effect of aqueous subfraction was observed (Wina, unpublished). Beside saponins, a sesquiterpene glycoside, mukurozioside IIb has been identified in the fruit pericarp of related plant, Sapindus mukurossi and S. emarginatus (Kanchanapoom et al. 2001). This compound might dissolve in the ethyl acetate subfraction and not in the aqueous subfraction of S. rarak methanol extract
Different techniques or solvents used for saponin extraction may affect the purity of the extract. This was shown by different contents of sarsaponin in four commercial products of Yucca schidigera extract (Singer et al. 2008). Y. schidigera powder contained not only saponins but also phenolics (resveratrol, yuccaol) (Piacente et al. 2005), glycoprotein and stilbenes. These other compounds were present in non-butanol extractable fraction and exert several properties such as antiinflammatory, antioxidant (Cheeke et al. 2006), reducing air ammonia concentration and fecal odour (Piacente et al. 2005). Therefore, different results on the use of Yucca extract in the ruminant feed can be influenced by the presence of other compounds in the extract.
The use of pure saponin would allow the study of the effect of saponin on the rumen without any confounding effects from other impurities. The purification of the crude saponin extract usually requires a sequential approach from extraction, precipitation, adsorption, ultrafiltration and chromatography (Güçlü-Üstündağ and Mazza 2007)
11.5 Saponin Structure Activity Relationship
Studies on structure activity relationship mainly have been conducted to identify compounds for medical treatment or health. These findings would be useful in understanding the various results found in many animal experiments using saponins since no structure activity relationship of saponins to their activity toward ruminal microbes has been elucidated.
Saponin from Sapindus mukorossi and its monodesmosides, resulting from the partial degradation of saponin have exhibited an activity in reducing fungal growth (Saha et al. 2010). Further removal of the sugar moiety yielded a complete loss in activity. Esterification of the hydroxyl group has been found to influence the antifungal activity (Saha et al. 2010). Another study with steroidal saponins found that antifungal activity against Candida neoformans was influenced by the aglycone moiety, number and structure of sugar moieties. The sugar moiety of four or five monosaccharide units displayed remarkable antifungal activity and when the sugar moiety contained less than 4 monosaccharides units, the activity was lost (Yang et al. 2006). The spirostanol type of steroidal saponin has more antifungal activity compared to the furanstanol type (Zhang et al. 2005). Conversely, Barile et al. (2007) found that furastanol saponin has higher activity than spirostanol.
Tomatidine, a steroidal alkaloid has inhibitory activity against Saccharomyces cerevisiae (Simons et al. 2006). The removal of a single sugar from the tetrasaccharide chain of α-tomatine (the aglycone) resulted in a substantial reduction in antimicrobial activity. But, the complete loss of sugars thus forming tomatine (the aglycone) led to enhanced antifungal activity. In contrast, the triterpene aglycones i.e. oleanic acid, β-amyrin, and hederagenin, did not exhibit any inhibitory activity (Simons et al. 2006).
Saponins from several plant sources have nematicidal activity. The partial hydrolysis of saponins showed more nematicidal activity than the related saponins at the same dose. The aglycones exerted nematicidal activity with hederagenin being the most active followed by medicagenic and bayogenin (Argentieri et al. 2008).
The haemolytic activity of saponins is dependent on the nature of aglycone and the glycoside chain (sugar moiety) including the configuration of the interglycosidic linkages, the substitution pattern and the type of sugar units involved (Chwalek et al. 2006).
The different plant source and structure of saponins, structure of algycone, length and position of sugar moiety may contribute various activities toward bacteria, fungi, protozoa and further studies are warranted.
11.6 Effect of Saponins on Ruminal Microorganisms
Wina et al. (2005a) compiled the data published from 1987 to 2004 on the saponin effect on rumen fermentation both in vitro and in vivo while Hart et al. (2008) modified the same data by adding the data of total production of volatile fatty acid and methane production. Recently, Patra and Saxena (2009a) presented the in vivo data published from 1987 to 2009 on saponin effects on rumen fermentation and the performance of animals. Tables 11.1 and 11.2 present the data published from 2005 to 2010 on the effect of saponin on rumen fermentation in vitro and in vivo, respectively.
11.6.1 Protozoa
Saponins depress the protozoa population and activity in the rumen (Wallace et al. 2002). The mechanism proposed is that saponin reduces the protozoa due to the interaction of saponins with cholesterol in the outer membrane of the protozoa making a pore and, hence, lysing the protozoa membrane (Wallace et al. 2002). Another mechanism proposed suggests that saponins with its ability to reduce surface tension has a similar effect to detergent causing toxicity to protozoa (Cheeke 2000; Francis et al. 2002). The sugar moiety has an important role in depressing protozoa; once, the saponin is completely hydrolysed, the inhibitory effect on protozoa was reduced or completely disappeared (Teferedegne et al. 1999; Wang et al. 2000; Muetzel et al. 2005). Partial acid hydrolysis of lucerne saponins decreased protozoa numbers in sheep rumen (Lu and Jorgensen 1987) suggesting that partial acid hydrolysed saponins might have some sugars attached to the aglycone , thus still retained its activity to inhibit protozoa.
Most of in vitro experiments in either batch or continuous systems showed that the reduction in protozoa population caused by saponins occurred very fast and was not specific. Koenig et al. (2007) showed that the protozoal numbers in sheep rumen markedly reduced 2 h after feeding Enterolobium cyclocarpum. Entodinium protozoa were present in the rumen as the dominant protozoal species, Diplodinium, Isotricha or Dasytricha were also present in the in vitro and in vivo experiments. In these experiments, addition of saponins did not influence the proportion of protozoa species (Baah et al. 2007; Koenig et al. 2007; Suharti et al. unpublished). However, when the level of saponin increased, all protozoa species decreased (Ivan et al. 2004; Wina et al. 2005a; Suharti et al. unpublished). Saponins reduced not only the numbers but also the diversity of protozoa. This was shown by a denaturing gradient gel electrophoresis (DGGE) study which revealed a lower diversity of protozoa after 21 days feeding of 3 g of tea saponin to sheep (Wang et al. 2010). Eremoplastron dilobum band on DGGE gel disappeared on the tea saponin treatment while Entodinium furca monolobum which was indicated as a faint band on the control treatment, appeared as a strong band in the saponin treatment (Wang et al. 2010; Zhou et al. 2010). It could be concluded that saponins may depress only specific protozoa species. Predation among protozoa may also explain the higher growth of one species of protozoa than the others (Dehority 1998; Ohene-Adjei et al. 2007). More studies should be conducted to confirm whether saponins affect specific protozoa species or if there is any predation among the protozoa or both. Table 11.1 shows that the reduction of protozoa numbers by saponin in most of in vitro experiments varied from 11% to 80% while in the in vivo experiments, the reduction varied from 0% (no reduction) to 71%. The variation among the experiments could be due to the different diet, saponin source, type of saponin, level of saponin and animal species used in the different studies.
Several studies showed that the presence of saponins in the rumen caused only partially defaunation. In the in vitro fermentation, Enterolobium cyclocarpum and Sesbania sesban which both contain saponins showed inhibiting effect on protozoa population (Leng et al. 1992; Teferedegne et al. 1999). When supplementing Enterolobium cyclocarpum to sheep, however, the antiprotozoal effect of E. cyclocarpum was only transient as the protozoa numbers started to increase after 12 days (Ivan et al. 2004; Koenig et al. 2007). When sheep received Sesbania sesban supplementation, protozoa numbers also increased after several days of supplementation (Newbold et al. 1997). Protozoa numbers in sheep rumen remained lower compared to that in control sheep when fed S. rarak pericarp’s extract at the level of 0.48–0.72 g/kg body mass for 100 days (Wina et al. 2006a). However, protozoal numbers were not significantly reduced when saponin extract of S. rarak whole fruit was fed to local cattle at the level 200 mg/kg BW/day for 90 days (Suharti 2010). The S. rarak saponins do not always depress protozoa numbers in the rumen perhaps due to the difference in composition of diet, the ratio of forage to concentrate, the animal breed, the level or type of saponin extract. The inconsistent defaunation effect of saponin between in vitro and in vivo experiments occurred when using other saponin sources such as in Yucca and Quillaja saponins (Pen et al. 2006, 2007; Lovett et al. 2006; Baah et al. 2007). Probably adaptation of the ruminal microbes, the nutrient flow or dilution effect might be responsible for the different results observed in the in vitro and in vivo studies (Benchaar et al. 2008).
11.6.2 Methanogens
Recently, methanogens and their diversity have been the subject of increasing interests due to the methane production by ruminants (Firkins et al. 2008). Much research is still in progress to study methanogens in order to mitigate methane emission from ruminants.
Beside living freely in the liquid mat or living associated with particles in the rumen, some methanogens remain associated with ruminal protozoa. It was estimated that 10–20% of total methanogens exist in close association with the protozoa either on the surface (ectosymbiosis) or inside the protozoal cell (endosymbiosis). Oligotrichs have endosymbiotic methanogens and entodiniomorph protozoa have ectosymbiotic methanogens (Ohene-Adjei et al. 2007). Using fluorescent-microscopic technique, Vogels et al. (1980) showed that nine genera of protozoa from the family of Ophryoscolecidae (order Entodiniomorphida) associated with methanogens. Physical structure (pellicle, surface structure and interior structure of cell cortex) of protozoa may affect this association. Study using molecular technique revealed that about 99% of protozoa-associated methanogens belong to the family of Methanobacteriaceae (Karnati et al. 2009a), and 20 novel sequences which differed from sequences previously known for protozoa-associated methanogens were obtained from rumen samples of goat, sheep and cow (Regensbogenova et al. 2004; Morgavi et al. 2006). DGGE profile also showed that inoculation of different species of protozoa to defaunated sheep resulted in different association of methanogens (Ohene-Adjei et al. 2007). The above findings indicate that various cultured and uncultured methanogens and their association with protozoa require further study.
Protozoa hydrolysed carbohydrate to produce hydrogen which then be used directly by associated methanogens to produce methane. Ushida et al. (1997) showed the occurrence of interspecies hydrogen transfer between the rumen ciliate Polyplastron multivesiculatum and the methanogenic archebacterium, Methanosarcina barkeri. Therefore, reducing protozoa will also reduce ruminal methanogens, thus reducing methane production. Tables 11.1 and 11.2 show that the studies mostly reported the effects of saponins on ciliate protozoa, nitrogen metabolism, and methane production but only few studies reported the effect of saponins on methanogens. It was shown that saponins in the S. rarak extract at the level of 2 mg/ml in the in vitro fermentation did not affect methanogens RNA concentration (Wina et al. 2005a) The same result was observed with the feeding of S. rarak at a dose of 0.48 g/kg body to sheep (Wina et al. 2006a). In contrast, Goel et al. (2008a) found that the addition of saponin extracts from sesbania, fenugreek or knautia decreased methanogens by 78%, 22% and 21%, respectively in the in vitro rumen fermentation.
Tea saponins did not inhibit M. ruminantium as pure culture (Guo et al. 2008), but at the level of 0.4 mg/ml in the in vitro rumen fermentation, it inhibited significantly the activity of the methyl coenzyme-M reductase (mcrA) gene of Methanobrevibacter ruminantium by 76% (Guo et al. 2008). The mcrA is crucial to the final step of methanogenesis where it is involved in the reduction of the methyl group bound to coenzyme-M. Tea saponin has been reported to decrease methanogens diversity (Zhou et al. 2010), without having any effect on the relative abundance of methanogens in sheep (Mao et al. 2010). The study on the use of different saponin containing plants related to the expression or activity of mcrA gene in the rumen is very limited. Further studies are warranted to unravel the mechanism by which saponins reduce the ruminal methane production.
11.6.3 Bacteria
Beside the antiprotozoal activity, many published reports showed that saponins are antimicrobial or antifungal agents. These properties were observed when saponins were tested on pure cultures. Wang et al. (2000) showed that saponins isolated from Yucca schidigera reduced the growth of Streptococcus bovis and Ruminobacter amylophilus. Wallace et al. (1994) previously reported that yucca extract reduced the growth of S. bovis and Butyrivibrio fibrosolvens. Yucca saponins also reduced filter paper and endoglucanase activities of Ruminococcus albus and R. flavefaciens but not those of Fibrobacter succinogenes (Wang et al. 2000). Selenomonas ruminantium but not Prevotella bryantii growth, was enhanced by yucca saponins (Wang et al. 2000) while the reverse result was reported by Wallace et al. (1994). The inconsistency of these results may due to the isolation procedure and type of saponin.
The response of the mixed rumen microbes to saponins may be due to a direct effect of saponin on bacteria and an indirect effect of saponin reducing protozoal numbers. As the protozoal population decreased due to the use of saponins, the total bacteria numbers increased (Wina et al. 2005a; Pen et al. 2006; Goel et al. 2008a). However, the bacteria increase depended on the level of saponins added to the substrate. Wina et al. (2005a) found that total ruminal bacteria numbers increased significantly when S. rarak extract was included at the level of 1 mg/ml in the in vitro fermentation system, but the bacterial numbers decreased when the inclusion of saponin was increased to twofold to fourfold. It was further reported that saponins in the in vitro fermentation system caused a shift in bacterial population which was shown by different intensity of bands on DGGE gel (Suharti et al. unpublished). Further analysis of three intensified bands show that the sequences of these bands have high similarity with sequences from Prevotella ruminicola (98–100%) Pseudobutyrivibrio ruminis or Butyrivibrio fibrosolvens (99%) and Coprococcus eutactus (99%) (Suharti et al. unpublished). In dual-flow continuous culture fermenters, removing protozoa increased population of Prevotella, Eubacterium spp., Ruminococcus spp., Butyrivibrio fibrisolvens which were shown as increased intensity bands on DGGE gel (Karnati et al. 2009b).
On pure culture, the growth of Prevotella ruminicola was stimulated by the addition of yucca extract (Wallace et al. 1994). In the in vitro fermentation using cattle rumen, a significant increase of Prevotella ruminicola due to the addition of S. rarak saponin extract was also reported (Suharti et al. 2010) and this result confirmed a previous qualitative analysis by DGGE (Suharti 2010).
A preference of protozoal predation toward Butyrivibrio fibrisolvens (Williams and Coleman 1992) explains the increased intensity of band matched with B. fibrisolvens during partial defaunation. Saponins also affected three major fibrolytic bacteria, Ruminococcus albus, Ruminococcus flavefaciens and Fibrobacter succinogens in the rumen. S. rarak saponins reduced RNA concentrations of Ruminococcus albus and R. flavefaciens both in the in vitro fermentation and in vivo during short term feeding (6 days) (Wina et al. 2005a, 2006a), but this effect did not occur during a long term feeding trial on sheep (100 days). RNA concentration of Fibrobacter succinogenes was unaffected in sheep rumen during short and long term feeding of saponin (Wina et al. 2006a). Quantitative analysis by real time PCR showed that S. rarak extract did not inhibit R. flavefaciens and F. succinogenes population in the in vitro fermentation (Suharti et al. 2010). However, sesbania saponin in the in vitro fermentation increased F. succinogenes (21–45%) and Ruminococcus flavefaciens (23–40%) populations measured by real time PCR (Goel et al. 2008a). Tea saponin did not affect the number of R. flavefaciens but increased that of F. succinogenes in the in vitro system (Guo et al. 2008). The effect of tea saponin was different in the in vivo trial using Hu lambs. Mao et al. (2010) found no effect of tea saponin on relative abundance of R. flavefaciens and F. succinogenes while Zhou et al. (2010) reported a decrease of F. succinogenes but no effect on R. flavefaciens, R. albus, Butyrivibrio fibrisolvens. These inconsistent results still can not be explained and warrant more studies to be conducted.
11.6.4 Fungi
Studies on the effects of saponins on fungi were mostly performed on fungi which caused disease problems in humans or plants (Zhang et al. 2005; Barile et al. 2007; Coleman et al. 2010). Steroidal saponins from Tribulus terrestris, saponins from Allium minutiflorum, plant saponins have shown potent activity against fluconazole-resistant fungi (Zhang et al. 2005), soil-borne and air-borne pathogenic fungi (Barile et al. 2007) and Candida albicans (Coleman et al. 2010). Similar to what observed on protozoa, the mechanism of saponins inhibiting fungal growth could be the binding between saponins and sterol present in outer membrane of fungi causing its disruption (Armah et al. 1999). However, no correlation was found between antifungal activity and antiprotozoal or haemolytic activity of saponins as mentioned above. Studies on the effect of saponins on individual rumen fungi are of scarcely available. Pure culture study showed that saponins from Yucca schidigera completely inhibited the growth of ruminal fungi, Neocallimastix frontalis and Piromyces rhizinflata (Wang et al. 2000). S. rarak saponins reduced RNA concentration of ruminal fungi both in the in vitro fermentation (at the level of 2–4 mg/ml) and in vivo during short term feeding but this effect did not occur in a 100 days of feeding trial (Wina et al. 2005a, 2006a). Tea saponin showed activity to depress fungal growth in the in vitro system (Guo et al. 2008) and no such activity in the in vivo system (Mao et al. 2010; Zhou et al. 2010). Goel et al. (2008a) reported that knautia, sesbania and fenugreek extracts reduced the population of ruminal fungi in the in vitro fermentation by 30%, 38%, 65%, respectively. This fungal inhibition may or may not disappear when the extracts are given in vivo and thus further investigation is required
11.7 Adaptation and Resistance of Ruminal Microbes to Saponins
The transient effect of saponins on ruminal protozoa has been reported by several authors (Newbold et al. 1997; Ivan et al. 2004; Koenig et al. 2007). Beside transient effects, the varying effects of the same saponin on protozoa occurred. Feeding Sapindus saponaria fruits to sheep suppressed protozoal numbers as demonstrated by Diaz et al. (1993) and Navas-Camacho et al. (1993), but increased protozoal numbers in the study of Abreu et al. (2004). This variability of the anti protozoal effect exerted by saponins may be caused by an adaptation of mixed microbes to saponins although breed and exposure of the animals to different environment or diets may also contribute to the variability (Teferedegne 2000; Abreu et al. 2004). Adaptation mechanism of rumen microbes to saponins may occur. F. succinogenes was resistant to saponins. F. succinogenes is a Gram negative bacterium, with two membranes, and a thin surface carbohydrate coat of radiating fibers. The outer membrane contains of two polysaccharides, i.e. lower-molecular-mass fraction designated glycolipid and a high-molecular-mass capsular polysaccharide fraction. The presence of 2-aminoethylphosphonic acid on the surface of both polysaccharides which covalently linked to the membrane polymers, enhances membrane stability of F. succinogenes (Vinogradov et al. 2001). So, not only F. succinogenes but most of Gram negative bacteria may also exert resistance to saponins. Wang et al. (2000) reported that thickening the cell wall membrane as found in Prevotella bryantii is another adaptation mechanism of microbe to saponins.
11.8 Effect of Saponins on Rumen Fermentation
11.8.1 Methane Production
Recent reviews on the use of secondary metabolites including saponins to reduce methanogenesis (methane production) are available (Rochfort et al. 2008; Patra and Saxena 2009b).
The use of Sapindus rarak fruit’s pericarp powder, the crude extract of the fruit’s pericarp or that of the whole fruit suppressed in vitro methane production by 21%, 31% (Thalib 2004) and 10%, respectively (Suharti 2010); however there are no reports yet on the effect of S. rarak saponin on in vivo methane production. Similar effect of suppression was obtained by adding aqueous extract of Sapindus mukorrosi to the in vitro fermentation (Kamra et al. 2008; Sirohi et al. 2009). Goel et al. (2008b) found reduced methane production in the in vitro fermentation with the addition of sesbania, fenugreek or knautia leaves but not with their extracts. Saponins from Yucca schidigera or Quillaja saponaria reduced methane production in the in vitro fermentations (Pen et al. 2006; Holtshausen et al. 2009) but not in the in vivo experiments (Pen et al. 2007; Holtshausen et al. 2009). Depression on methane production occurred both in the in vitro and in vivo experiments was reported when using sarsaponin (Lila et al. 2003, 2005), and tea saponins (Hu et al. 2005; Mao et al. 2010; Zhou et al. 2010). But the depression was significant only in faunated rumen fluid, suggesting that this effect was mediated through associated effects on protozoa. In the partial defaunation by saponins, the population of methanogens that associated with protozoa would decline, hence the methane production reduced. However, saponin rich extracts of S. sesban leaves, Knautia arvensis leaves and fenugreek seeds did not decrease methane production in vitro although these extracts reduced protozoa number and methanogen population (Jayanegara et al. 2010) The lack of inhibition on methane production with decreased methanogens could be caused by changes in composition of the methanogen community and their increased efficiency of methane production (Jayanegara et al. 2010).
The formation of methane in the rumen requires the involvement of several microbes and enzymes. Reducing the activity of the methyl coenzyme-M reductase (mcrA) gene of Methanobrevibacter ruminantium by saponin (Guo et al. 2008), indicated that tea saponin inhibited the rate of methanogenesis and not the numbers of methanogens. It is likely that H2 availability rather than the number of methanogens controlled the methanogenesis. High proportion of H2 in the rumen is produced by protozoa when digesting starch, but when protozoa are inhibited, H2 production in the rumen is limited and thus methane production is reduced. Saponin may suppress the H2-producing bacteria (Wang et al. 2000) such as cellulolytic bacteria and bacteria that use pyruvate-ferredoxin oxidoreductase, hence reduce H2 availability for methanogens.
Increasing H2 utilization by organisms other than methanogens also reduce methane production. This process requires addition of an appropriate electron acceptor and an efficient type of rumen bacteria that can perfectly utilize such an acceptor in the production of acetate or propionate, hence, reduced methane production (Abdl-Rahman et al. 2010).
Thalib and Widiawati (2008) suggested a combination of feeding S. rarak saponin extract with Acetoanaerobium noterae, an acetogenic bacteria that reduced in vitro methane production by 20%. Further, Thalib et al. (2010) showed that this combination also reduced methane production in sheep by 24%.
Caution should be taken in interpreting methane production data since methane production is expressed in different units. In the in vitro system, the units of methane production could be expressed as the total production per day or concentration relative to the total gas. Hess et al. (2004) showed that methane release could be expressed relative to metabolic weight or organic matter digested or energy intake or body nitrogen retained. Different units of methane release give various values which may lead to different conclusion. From several results, it can be suggested that saponins have potential to be used as a methane reducing agent, however, the inclusion level of saponin from each source should be tested to get the optimum result.
11.8.2 Biohydrogenation of Fatty Acid
Recently, there has been a renewed interest by several researchers in the study of biohydrogenation of fatty acid in the rumen after a growing demand for producing healthy animal products for human consumption. Conjugated linoleic acid (CLA) and vaccenic acid (VA) are fatty acid intermediates produced in the rumen. CLA is known as potentially health-promoting agent since many studies showed that it contributed to cancer prevention, decreased atherosclerosis and improved immune response (Pariza 2004; Palmquist et al. 2005). The most abundant trans-18:1 isomer, vaccenic acid (18:1 trans-11) was 6.6% of total fatty acids in protozoa and 2.0% of total fatty acids in bacteria. The cis-9, trans-11 CLA was 8.6-times greater in the protozoal fraction (1.32% of total fatty acids) than in the bacterial fraction (0.15%) (Or-Rashid et al. 2007). Proportion of CLA and VA in the rumen fluid of faunated 1.6–2.5 times higher than those of defaunated cattle (Sultana et al. 2011) The supply of CLA for post rumen absorption depend on the flow of protozoa from the rumen (Yanez-Ruiz et al. 2006). Only limited data of the effects of saponins on CLA and VA concentration in the rumen is available. Addition of quillaja saponins in the dual–flow continuous culture at the level of 500–1,000 mg/L did not affect the total or individual volatile fatty acid nor change the extent of biohydrogenation (Lourenço et al. 2008). No effect of saponin from Yucca schidigera on the rate of α-linolenic acid biohydrogenation was reported when added at the level of 1.12% of dry matter feed in a Rumen simulation technique system (Khiaosa-Ard et al. 2009). Defaunation by using a certain size filter only slightly reduced biohydrogenation in the rumen (Karnati et al. 2009a) and, hence, the presence of protozoa was not necessary for biohydrogenation to occur. More studies need to be done on the effect of different saponins on biohydrogenation of fatty acid in the rumen and the production of cis-9, trans-11-CLA and VA (18:1 trans-11).
Only bacteria in the same group as the B. fibrisolvens group formed cis-9, trans-11-CLA or trans-11-18:1 as intermediates in the process of biohydrogenation of linoleic acid (Jenkins et al. 2008). The DGGE result to study rumen bacterial diversity showed that the addition of S. rarak saponin extract caused B. fibrisolvens to appear as a more intense band (Suharti et al. unpublished). Defaunation by other compounds also increased some Butyrivibrio (Karnati et al. 2009a). Further study to quantify B. fibrisolvens following saponin addition may explain about its role in the CLA production in the rumen. The effects of various phytochemicals on microbial biohydrogenation in the rumen have been discussed in details in Chap. 9.
11.8.3 Nitrogen Metabolism
Protein consumed by ruminants is partly degraded in the rumen to peptides, amino acids and finally to ammonia. Therefore, there is a relationship between ammonia concentration in the rumen and percentage of total protein in the diet (Eugene et al. 2004). Another source of ammonia in the rumen comes from the proteolysis of bacterial protein when protozoa engulf ruminal bacteria as their principal source of protein amino acids. Reduced ammonia concentrations in the rumen typically occurred when total defaunation was applied (Eugene et al. 2004). The reduction of protozoal numbers resulted in less predation of bacterial and, hence, less lysis of bacterial protein to ammonia. Therefore, the observed ammonia level in the rumen is affected by the rate of degradation of feed protein to ammonia, the rate of bacterial lysis by protozoa, and the uptake of ammonia for microbial protein synthesis. Fewer protozoa also could influence the ammonia concentration since protozoa contributed to 10–40% of the total rumen nitrogen. The excess of ammonia is diffused from the rumen, converted to urea and excreted from the animal.
Saponins which partially reduced protozoal numbers, also reduced ammonia concentration in the rumen both in the in vitro (Table 11.1) and in vivo experiments (Table 11.2). Experiments using tea saponin or S. rarak extract to partially defaunate the rumen either in vitro or in vivo showed lower ammonia production. Tables 11.1 and 11.2 showed inconsistency in the effect of saponin on ruminal ammonia production between the in vitro and in vivo experiments using the same source of saponins. Saponin extract of Yucca schidigera (Lila et al. 2005; Cardozo et al. 2004; Pen et al. 2006, 2007; Singer et al. 2008; Holtshausen et al. 2009) or Quillaja saponaria (Cheeke 2000; Pen et al. 2006, 2007; Holtshausen et al. 2009) caused different effect on ammonia production. Comparison of all the results is rather complicated because of the different source of saponin, different level of saponin, process of extraction, dietary ingredient of forage and concentrate, breed and physiology of the animal.
There is limited information on the effect of saponins on peptide and amino acids metabolism in the rumen. Bacteria are responsible for most peptide degradation within the rumen and among them, Gram-negative bacteria Prevotella ruminicola appears to be the most important peptidolytic bacteria (Broderick et al. 1991). Suharti et al. (2010) found that addition of S. rarak whole fruit extract to in vitro fermentation increased the relative abundance of Prevotella ruminicola. Further investigation is required to prove whether saponins increase the production of peptides in the rumen. However, the inhibition of rumen proteolytic activity by yucca saponins has been observed by Wallace et al. (1994). The reduction of ruminal N digestion was also observed when sheep was fed E. cyclocarpum leaves (Koenig et al. 2007). However, Muetzel et al. (2005) did not find any effect of S. rarak saponin on protein degradation in vitro. There is no effect of quillaja or yucca saponin on deaminative enzymes (Hristov et al. 1999; Baah et al. 2007).
Fenugreek extract in the continuous culture system showed no effect on large peptides, small peptides and amino acids productions in the rumen (Busquet et al. 2005) while Yucca extract increased the average peptide N concentration by 26.2% but no effect on amino acid concentration and ammonia was found (Cardozo et al. 2004).
Accumulation of peptides in the rumen fluid would be beneficial to ruminants since it would increase microbial protein synthesis and the flow of dietary amino acids to the lower gut (Griswold et al. 1996). Microbial protein synthesis (MPS) was enhanced by quillaja saponin in the in vitro fermentation (Makkar et al. 1998), S. saponaria (Abreu et al. 2004) and tea saponin in the in vivo trials (Mao et al. 2010; Zhou et al. 2010). However, MPS in vivo was not affected by applying S. rarak extract (Wina et al. 2006a, b), Quillaja or Yucca saponin (Pen et al. 2007). The application of yucca saponin at low level (15 μg/ml) increased microbial protein synthesis, in contrast, MPS reduced at high level of saponins (75 μg/ml) (Wang et al. 2000). Efficiency of MPS improved by 13% in sheep fed a diet supplemented with E. cyclocarpum (Koenig et al. 2007) and by 51% when supplemented with Biophytum petersianum Klotzsch (Santoso et al. 2007).
From the rumen, the bacteria and protozoa flow to duodenum could be observed by measuring the markers, 15Nitrogen and phosphatidylcholine (PC) in the rumen and duodenal digesta (Ivan et al. 2006). Not only the microbial protein synthesis, but also the flow of microbial protein to duodenum was enhanced by Sapindus saponaria (Abreu et al. 2004) and tea saponin (Mao et al. 2010; Zhou et al. 2010). These positive effects on the microbial protein synthesis, efficiency microbial protein synthesis, the microbial N flow and Non Ammonia Nitrogen (NAN) may help to improve dietary N utilization by ruminants (Busquet et al. 2005).
11.8.4 Carbohydrate Metabolism
Interaction of several diverse species of bacteria, fungi and protozoa facilitates the breakdown of cellulose and other carbohydrate fractions in plant materials in the rumen. With the decrease of protozoal numbers, this interaction may be disturbed as some carbohydrate degrading enzymes have less activity. Wina et al. (2005b, 2006a) showed that the xylanase activity in the rumen decreased when S. rarak saponin extract was administered to in vitro fermentation or directly to sheep. The CMCase activity was not affected in the in vitro, however, it was depressed by S. rarak extract entered the rumen of sheep. Protozoa also produced carbohydrate degrading enzyme including xylanase and CMCase (Williams and Withers 1991; Devillard et al. 1999) and a positive correlation was observed between protozoal numbers and both xylanase and CMCase activities in the sheep rumen (Wina et al. 2006a). Amylase activity in the in vitro rumen fermentation increased in the presence of saponin extract of S. rarak whole fruit (Suharti 2010). However, CMCase, xylanase, glucanase and amylase activities in heifer’s rumen were not affected by Quillaja saponin extract (Baah et al. 2007). Eventhough the fibrolytic enzyme activity might be reduced in the rumen and, hence, reducing the fibre digestibility in the rumen, the total tract digestibility was not affected by saponin addition (Wina 2005).
The products of carbohydrate degradation in the rumen would be volatile fatty acids. The higher total VFA production was observed by S. rarak extract addition in the in vitro (Wina et al. 2005b; Suharti et al. unpublished) and in cattle rumen (Suharti 2010) indicating that saponin improved the fermentation while many experiments showed no effect or depressed effect of saponin on total VFA production. Consistent results were seen in the in vitro system and both sheep and cattle feeding trials in that the molar proportion of propionate increased in the presence of S. rarak saponin extract (Tables 11.1 and 11.2). Y. schidigera and Q. saponaria extracts increased propionate production in vitro (Pen et al. 2006) but not in sheep rumen (Pen et al. 2007). There are other reports in agreement and showing an increase in the molar proportion of propionate in the presence of saponin although some did not agree with this result (see Tables 11.1 and 11.2). The higher molar proportion of propionate could be due to higher activity or numbers of Prevotella ruminicola to form propionate. Prevotella ruminicola in pure culture increased in the presence of Y. schidigera saponin fraction (Wallace et al. 1994) and its relative abundance as a percentage to the total bacteria increased significantly in the presence of S. rarak extract in the in vitro fermentation (Suharti et al. 2010). Lower acetate and butyrate production which were the major fermentation end products in isolated protozoa caused a shift in molar proportion of individual VFAs to a higher proportion of propionate and a reduced ratio of acetate to propionate. A higher molar proportion of propionate means a higher glucogenesis and would be beneficial for animals fed high roughage diets that are commonly found in tropical countries where available sources for feed are mainly roughages of low quality. The potential usage of saponin extract as a natural substance to improve propionate production requires more study.
11.9 Metabolism of Saponins in the Rumen
Rumen microbes produce a variety of intracellular or extracellular enzymes. These enzymes hydrolyse all substances at different rates. Saponins are glycosylated compounds and are highly soluble in water or aqueous medium. Saponins can dissolve easily in the rumen and therefore be readily degraded by the various glycosidases produced by the different microbes. In the in vitro study, Makkar and Becker (1997) reported that 81% of quillaja saponins still undegraded in buffered rumen liquor up to 9 h of incubation and were degraded rapidly after this time. Wina (2005) studied the degradation of S. rarak saponin in the in vitro rumen and showed that after 6 h of incubation only one saponin compound could be detected by thin layer chromatography. With further incubation for 12 h, this compound was detected with lower intensity. Using Rusitec system, Wang et al. (1998) found that after 2 h of incubation, most of yucca saponins (93.5%) remained intact in the rumen but with longer incubation, the degradation process occurred quite rapid and more than 50% of saponins disappeared at 8 h of incubation (Wang et al. 2000). Different rates of saponin degradation are shown in the in vitro systems (Makkar and Becker 1997; Wang et al. 1998, 2000; Wina 2005) and the degradation rate seems faster in the in vivo system especially if the saponin was directly introduced to the rumen. Saponins from an extract of Costus speciosus rhizomes was rapidly hydrolysed at 1 h after dosing to the rumen (Meagher et al. 2001). However, Wina (2005) did not find any degradation product of S. rarak saponins 1 h after feeding. The degradation products (aglycone structures) appeared after 2 h of feeding (Fig. 11.5). Hederagenin (He) as the aglycone of Sapindus rarak saponins was detected up to 4 h after feeding and then, it disappeared and perhaps structurally changed to several other degradation products after 24 h of the saponin extract feeding.
Sapogenin fraction extracted from rumen of control and SE (saponin extract) fed goat taken at 1, 2, 4 and 24 h after feeding and separated on thin layer of Silica gel plate. C1–C5 represented the metabolites of saponins (in the form of sapogenin), He hederagenin, the main aglycone of Sapindus rarak saponins
Flåøyen et al. (2002) reported that the saponin from Yucca schidigera was hydrolysed in the rumen to its main algycone, sarsapogenin, but then they assumed that this sarsapogenin was oxidized and reduced at C-3 position to become the corresponding epi-sarsapogenins. This result was in agreement with their previous study with saponins from Narthecium ossifragum which caused photosensitization to sheep (Flåøyen et al. 2001). Several free aglycones are formed in the rumen and the ring structure was not being degraded further in the rumen (Flåøyen et al. 2001, 2002). Fibrobacter succinogenes was reported to deglycosylate yucca saponin (Wang et al. 2000). There might be many other rumen microbes that have glycosidases activity and can hydrolyse saponin but have never been reported yet.
11.10 Summary of the Effects of Saponin on Rumen Fermentation
A scheme of effects of saponins on rumen microorganisms, activities and its products is presented in Fig. 11.6. Evidence obtained from numerous experiments suggests that the effects of saponins or saponin containing plants initially affected the microorganism in the rumen. Effect of saponin or its degradative products on microbial intestinal or caecum of ruminants, however, are limited known.
When saponins enter the rumen, the first effect is to reduce protozoal population (Fig. 11.6) Total bacterial numbers increase as less predation of bacteria by protozoa after saponins reduce protozoal population. The increase in total bacteria numbers means that the microbial protein synthesis enhances, hence, increases the microbial nitrogen flow to post rumen or intestine. Individual bacteria responses differently to saponins. Several studies show that the activity of methanogens but not methanogens population is reduced by saponins, resulting in a decrease in the methane produced in the rumen. Three major fibrolytic bacteria (F. succinogenes, R. flavefaciens and R. albus) are unaffected by saponin in the in vivo system Similar to fibrolytic bacteria, ruminal fungi are not affected by saponin. Protozoa and fungi possess carbohydrases (CMCase, xylanase), therefore partial defaunation by saponin causes a reduction in carbohydrate degrading enzymes. The reduced activity of these enzymes by saponin causes NDF digestibility in buffered rumen to decrease (Wina et al. 2005a). Saponin does not inhibit the activities of carbohydrate degrading enzymes in sheep or cattle rumen and, hence, does not impair total tract digestibility. Saponin increases VFA and propionate production in the rumen, therefore, it is expected that glucogenesis in post rumen is enhanced.
With reduced protozoal population, increase of total bacteria population, propionate production, microbial biomass production, microbial nitrogen flow and glucogenesis and without any effect of saponin on total tract digestibility, it is expected that the performance of ruminant is improved by saponin addition.
11.11 Conclusion
Various methods of extracting saponins from plant materials and the diversity of saponins influenced the activity of rumen microorganisms. Saponins could manipulate the rumen fermentation by depressing the protozoal population in the rumen. The consequences of reduced protozoal population leads to some changes in the rumen microbial composition and population, a shift in individual volatile fatty acids toward higher propionate and increased microbial nitrogen flow to duodenum. Saponins could also decrease methane production with or without decreasing the numbers of methanogenic archaea. Evaluation on the dose of specific saponins to be added into the diet and the interaction of saponins with different composition of diets require further studies so that saponins could be potentially used as a defaunating agent, a propionate enhancer and an inhibitor for methane production in the rumen.
Abbreviations
- NMR:
-
nuclear magnetic resonance
- VFA:
-
volatile fatty acid
- DGGE:
-
denaturing gradient gel electrophoresis
- PCR:
-
polymerase chain reaction
- CLA:
-
conjugated linoleic acid
- VA:
-
vaccenic acid
- RNA:
-
ribonucleic acid
- MPS:
-
microbial protein synthesis
References
Abdl-Rahman MA, Sawiress FAR, Abd El-Aty AM (2010) Effect of sodium lauryl sulfate-fumaric acid coupled addition on the in vitro rumen fermentation with special regard to methanogenesis. SAGE-Hindawi Access to Research. Vet Med Int 2010:7 pp, Article ID 858474. doi:10.4061/2010/858474
Abreu A, Carulla JE, Lascano CE et al (2004) Effects of Sapindus saponaria fruits on ruminal fermentation and duodenal nitrogen flow of sheep fed a tropical grass diet with and without legume. J Anim Sci 82:1392–1400
Agarwal N, Kamra DN, Chaudhary LC et al (2006) Effect of Sapindus mukorossi extracts on in vitro methanogenesis and fermentation characteristics in buffalo rumen liquor. J Appl Anim Res 30:1–4
Argentieri MP, D’Addabbo T, Tava A et al (2008) Evaluation of nematicidal properties of saponins from Medicago spp. Eur J Plant Pathol 120:189–197. doi:10.1007/s10658-007-9207-8
Armah CN, Mackie AR, Roy C et al (1999) The membrane-permeabilizing effect of avenacin A-1 involves the reorganization of bilayer cholesterol. Biophys J 76:281–290
Asao Y, Morikawa T, Xie YY et al (2009) Structures of acetylated oleanane-type triterpene saponins, rarasaponins IV, V, and VI, and anti-hyperlipidemic constituents from the pericarps of Sapindus rarak. Chem Pharm Bull 57:198–203
Baah J, Ivan M, Hristov AN et al (2007) Effects of potential dietary antiprotozoal supplements on rumen fermentation and digestibility in heifers. Anim Feed Sci Technol 137:126–137
Bankefors J (2006) Structural classification of Quillaja saponins by electrospray ionization ion trap multiple-stage mass spectrometry in combination with multivariate analysis. Licentiate thesis, Swedish University of Agricultural Sciences, Uppsala, 44 pp
Barile E, Bonanomi G, Antiganani V et al (2007) Saponins from Allium minutiflorum with antifungal activity. Phytochemistry 68:596–603
Baumann E, Stoya G, Volkner A et al (2000) Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem 102:21–35
Benchaar C, McAllister TA, Chouinard PY (2008) Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, Quebracho condensed tannin, or Yucca schidigera saponin extracts. J Dairy Sci 91:4765–4777. doi:10.3168/jds.2008-1338
Berhow MA, Cantrell CL, Duval SM et al (2002) Analysis and quantitative determination of group B saponins in processed soybean products. Phytochem Anal 13:343–348
Broderick GA, Wallace RD, Orskov ER (1991) Control of rate and extent of protein degradation. In: Tsuda T, Sesako Y, Kawashima R (eds) Physiological aspects and metabolism in ruminant. Academic, San Diego, pp 542–592
Busquet M, Calsamiglia S, Ferret A et al (2005) Screening for effects of plant extracts and active compounds of plants on dairy cattle rumen microbial fermentation in a continuous culture system. Anim Feed Sci Technol 123–124:597–613
Cardozo PW, Calsamiglia S, Ferret A et al (2004) Effects of natural plant extracts on ruminal protein degradation and fermentation profiles in continuous culture. J Anim Sci 82:3230–3236
Cheeke PR (2000) Actual and potential applications of Yucca schidigera and Quillaja saponaria saponins in human and animal nutrition. In: Proceedings of the American Society of Animal Sciences, Indianapolis, 10 p, 1999. http://www.asas.org/JAS/symposia/proceeding/0909.pdf
Cheeke PR, Piacente S, Oleszek W (2006) Anti-inflammatory and anti-arthritic effects of Yucca schidigera: a review. J Inflamm 3:6–13. doi:10.1186/1476-9255-3-6
Chwalek M, Lalun N, Bobichon H et al (2006) Structure–activity relationships of some hederagenin diglycosides: haemolysis, cytotoxicity and apoptosis induction. Biochim Biophys Acta 1760:1418–1427
Coleman JJ, Okoli I, Tegos GP et al (2010) Characterization of plant-derived saponin natural products against Candida albicans. Am Chem Soc Chem Biol 5:321–332. doi:10.1021/cb900243b
Dehority AB (1998) Microbial interactions in the rumen. Rev Fac Agron (LUZ) 15:69–86
Devillard E, Newbold CJ, Scott KP et al (1999) A xylanase produced by the rumen anaerobic protozoan Polyplastron multivesiculatum shows close sequence similarity to family 11 xylanases from gram-positive bacteria. FEMS Microbiol Lett 181:145–152
Diaz A, Avendano M, Escobar A (1993) Evaluation of Sapindus saponaria as a defaunating agent and its effects on different ruminal digestion parameters. Livest Res Rural Dev 5:1–6
Eugene M, Archimede H, Sauvant D (2004) Quantitative meta-analysis on the effects of defaunation of the rumen on growth, intake and digestion in ruminants. Livest Prod Sci 85:81–97
Firkins JL, Karnati SKR, Yu Z (2008) Linking rumen function to animal response by application of metagenomics techniques. Aust J Exp Agric 48:711–721
Flåøyen A, Wilkins AL, Debg D et al (2001) Ovine metabolism of saponins: evaluation of a method for estimating the ovine uptake of steroidal saponins from Narthecium ossifragum. Vet Res Commun 25:225–238
Flåøyen A, Wilins AL, Sandvik M (2002) Ruminal metabolism in sheep of saponins from Yucca schidigera. Vet Res Commun 26:159–169
Francis G, Keem Z, Makkar HPS et al (2002) The biological action of saponins in animal systems: a review. Br J Nutr 88:587–605
Gestetner B, Assa Y, Henis Y et al (2006) Lucerne saponin IV. Relationship between their chemical constitution and haemolytic and antifungal activities. J Sci Food Agric 22:10–26
Goel G, Makkar HPS, Becker K (2008a) Effects of Sesbania sesban and Carduus pycnocephalus leaves and Fenugreek (Trigonella foenum-graecum L.) seeds and their extracts on partitioning of nutrients from roughage- and concentrate-based feeds to methane. Anim Feed Sci Technol 147:72–78
Goel G, Makkar HPS, Becker K (2008b) Changes in microbial community structure, methanogenesis and rumen fermentation in response to saponin-rich fractions from different plant materials. J Appl Microbiol 105:770–777. doi:10.1111/j.1365-2672.2008.03818.x
Griswold KE, Hoover WH, Miller TK et al (1996) Effect of form of nitrogen on growth of ruminal microbes in continuous culture. J Anim Sci 74:483–491
Güçlü-Üstündağ Ö, Mazza G (2007) Saponins: properties, applications and processing. Crit Rev Food Sci Nutr 47:231–258. doi:10.1080/10408390600698197
Guo S, Falk E, Kenne L et al (2000) Triterpenoid saponin containing an acetylated branched D-fucosyl residue from Quillaja saponaria Molina. Phytochemistry 53:861–868
Guo YQ, Liu JX, Lu Y et al (2008) Effect of tea saponin on methanogenesis, microbial community structure and expression of mcrA gene, in cultures of rumen microorganisms. Lett Appl Microbiol 47:421–426
Hamburger M, Slacanin I, Hostettmann K et al (1992) Acetylated saponins in molluscicidal activity from Sapindus rarak: unambiguous structure determination by proton nuclear magnetic resonance and quantitative analysis. Phytochem Anal 3:231–237
Hart KJ, Yanez-Ruiz DR, Duval SM et al (2008) Plant extracts to manipulate rumen fermentation. Anim Feed Sci Technol 147:8–35
Hassan SM, Byrd JA, Carwright AL et al (2010a) Hemolytic and antimicrobial activities differ among saponin-rich extracts from guar, quillaja, yucca, and soybean. Appl Biochem Biotechnol 162:1008–1017. doi:10.1007/s12010-009-8838-y
Hassan SM, Hag AU, Byrd JA et al (2010b) Haemolytic and antimicrobial activities of saponin-rich extracts from guar meal. Food Chem 119:600–605
Hess HD, Beuret RA, Lötscher M et al (2004) Ruminal fermentation, methanogenesis and nitrogen utilization of sheep receiving tropical grass hay-concentrate diets offered with Sapindus saponaria fruits and Cratylia argentea foliage. Anim Sci 79:177–189
Holtshausen L, Chaves AV, Beauchemin KA et al (2009) Feeding saponin-containing Yucca schidigera and Quillaja saponaria to decrease enteric methane production in dairy cows. J Dairy Sci 92:2809–2821. doi:10.3168/jds.2008-1843
Hristov AN, McAllister A, Van Herk FH et al (1999) Effect of Yucca schidigera on ruminal fermentation and nutrient digestion in heifers. J Anim Sci 77:2554–2563
Hu WL, Wu YM, Liu JX et al (2005) Tea saponins affect in vitro fermentation and methanogenesis in faunated and defaunated rumen fluid. J Zhejiang Univ Sci B 6:787–792
Huang HC, Wu MD, Tsai WJ et al (2008) Triterpenoid saponins from the fruits and galls of Sapindus mukorossi. Phytochemistry 69:1609–1616
Ivan M, Koenig KM, Teferedegne B et al (2004) Effect of the dietary Enterolobium cyclocarpum foliage on the population dynamics of rumen ciliate protozoa in sheep. Small Rumin Res 52:81–91
Ivan M, Koenig KM, Morgavi DP et al (2006) Duodenal flow and digestibility in fauna-free sheep and in sheep monofaunated with the Entodinium caudatum or Polyplastron multivesiculatum species of rumen ciliate protozoa. Br J Nutr 95:469–476
Jayanegara A, Goel G, Makkar HPS et al (2010) Reduction in methane emissions from ruminants by plant secondary metabolites: effects of polyphenols and saponins. In: Odongo NE, Garcia M, Viljoen GJ (eds) Sustainable improvement of animal production and health. FAO, Rome, pp 151–157
Jenkins TC, Wallace RJ, Moate PJ et al (2008) Recent advances in biohydrogenation of unsaturated fatty acids within the rumen microbial ecosystem. J Anim Sci 86:397–412. doi:10.2527/jas.2007-0588
Kamra DN, Agarwal N, Chaudhary LC (2006) Inhibition of ruminal methanogenesis by tropical plants containing secondary compounds. Int Congr Ser 1293:156–163
Kamra DN, Patra AK, Chatterjee PN et al (2008) Effect of plant extracts on methanogenesis and microbial profile of the rumen of buffalo: a brief overview. Aust J Exp Agric 48:175–178
Kanchanapoom T, Kasai R, Yamasaki K (2001) Acetylated triterpene saponins from the Thai medicinal plant, Sapindus emarginatus. Chem Pharm Bull 49:1195–1197
Karnati SKR, Sylvester JT, Ribeiro CVM et al (2009a) Investigating unsaturated fat, monensin, or bromoethanesulfonate in continuous cultures retaining ruminal protozoa. I. Fermentation, biohydrogenation, and microbial protein synthesis. J Dairy Sci 92:3849–3860. doi:10.3168/jds.2008-1436
Karnati SKR, Yu Z, Firkins JL (2009b) Investigating unsaturated fat, monensin, or bromo ethanesulfonate in continuous cultures retaining ruminal protozoa II. Interaction of treatment and presence of protozoa on prokaryotic communities. J Dairy Sci 92:3861–3873. doi:10.3168/jds.2008-1437
Kaur GJ, Arora DS (2009) Antibacterial and phytochemical screening of Anethum graveolens, Foeniculum vulgare and Trachyspermum ammi. BMC Complement Altern Med 9:30. doi:10.1186/1472-6882-9-30
Kerem Z, German-Shashoua H, Yarden O (2005) Microwave-assisted extraction of bioactive saponins from chickpea (Cicer arietinum L). J Sci Food Agric 85:406–412
Khiaosa-Ard R, Bryner SF, Scheeder MRL et al (2009) Evidence for the inhibition of the terminal step of ruminal α-linolenic acid, biohydrogenation by condensed tannins. J Dairy Sci 92:177–188. doi:10.3168/jds.2008-1117
Koenig KM, Ivan M, Teferedegne BT et al (2007) Effect of dietary Enterolobium cyclocarpum on microbial protein flow and nutrient digestibility in sheep maintained fauna-free, with total mixed fauna or with Entodinium caudatum monofauna. Br J Nutr 98:504–516. doi:10.1017/S0007114507723930
Lasztity R, Hidvegi M, Bata A (1998) Saponins in food. Food Rev Int 14:371–390
Lemos TLG, Mendes AL, Sousa MP et al (1992) New saponin from Sapindus saponaria. Fitoterapia 93:515–517
Lemos TLG, Sousa MP, Mendes AL et al (1994) Saponin from Sapindus saponaria. Fitoterapia 95:557
Leng RA, Bird SH, Klieve A et al (1992) The potential for tree forage supplements to manipulate rumen protozoa to enhance protein to energy ratios in ruminants fed on poor quality forages. In: Speedy A, Pugliese PL (eds) Legume trees and other fodder trees a protein sources for livestock, vol 102, Food and Agriculture Organization, Rome, pp 177–191
Ligor T, Ludwiczuk A, Wolski T et al (2005) Isolation and determination of ginsenosides in American ginseng leaves and root extracts by LC-MS. Anal Bioanal Chem 383:1098–1105. doi:10.1007/s00216-005-0120-8
Lila ZA, Mohammed N, Kanda S et al (2003) Effect of sarsaponin on ruminal fermentation with particular reference to methane production in vitro. J Dairy Sci 86:3330–3336
Lila ZA, Mohammed N, Kanda S et al (2005) Sarsaponin effects on ruminal fermentation and microbes, methane production, digestibility and blood metabolites in steers. Asian-Aust J Anim Sci 18:1746–1751
Lin F, Wang RX (2010) Hemolytic mechanism of dioscin proposed by molecular dynamics simulations. J Mol Model 16:107–118. doi:10.1007/s00894-009-0523-0
Lourenço M, Cardozo PW, Calsamiglia S et al (2008) Effects of saponins, quercetin, eugenol, and cinnamaldehyde on fatty acid biohydrogenation of forage polyunsaturated fatty acids in dual-flow continuous culture fermenters. J Anim Sci 86:3045–3053. doi:10.2527/jas.2007-0708
Lovett DK, Stack L, Lovell S et al (2006) Effect of feeding Yucca schidigera extract on performance of lactating dairy cows and ruminal fermentation parameters in steers. Livest Sci 102:23–32
Lu CD, Jorgensen NA (1987) Alfalfa saponins affect site and extent of nutrient digestion in ruminants. J Nutr 117:919–927
Mahato SB, Garai S (1998) Triterpenoid saponins. In: Herz W, Kirby GW, Moore RE, Steglich W, Tamm CH (eds) Progress in the chemistry of organic natural products. 74. Springer, Wien/New York, pp 1–196
Makkar HPS, Becker K (1997) Degradation of quillaja saponins by mixed culture of rumen microbes. Lett Appl Microbiol 25:243–245
Makkar HPS, Sen S, Blummel M et al (1998) Effect of fractions containing saponins from Yucca schidigera, Quillaja saponaria and Acacia auriculoformis on rumen fermentation. J Agric Food Chem 46:4324–4328
Mao HL, Wang JK, Zhou YY et al (2010) Effects of addition of tea saponins and soybean oil on methane production, fermentation and microbial population in the rumen of growing lambs. Livest Sci 129:56–62
Meagher LP, Smith BL, Wilkins AL (2001) Metabolism of diosgenin-derived saponins: implication for hepatogenous photosensitization diseases in ruminants. Anim Feed Sci Technol 91:157–170
Morgavi DP, Jouany JP, Martin C et al (2006) Archaeal community structure diversity in the rumen of faunated and defaunated sheep. Int Congr Ser 1293:127–130
Muetzel S, Akpagloh R, Becker K (2005) Sapindus rarak saponins do not affect rumen protein degradation in vitro. Proc Soc Nutr Physiol (German) 14:73
Navas-Camacho A, Laredo MA, Cuesta H et al (1993) Effect of supplementation with a tree legume forage on rumen function. Livest Res Rural Dev 5:58–71
Newbold CJ, El Hassan SM, Wang JM et al (1997) Influence of foliage from African multipurpose trees on activity of rumen protozoa and bacteria. Br J Nutr 78:237–249
Ohene-Adjei S, Teather RM, Ivan M et al (2007) Post inoculation protozoan establishment and association patterns of methanogenic archaea in the ovine rumen. Appl Environ Microbiol 73:4609–4618. doi:10.1128/AEM.02687-06
Oleszek W, Sitek M, Stochmal A et al (2001) Steroidal saponins of Yucca schidigera Roeszl. J Agric Food Chem 49:3292–3296
Or-Rashid MM, Odongo NE, McBride BW (2007) Fatty acid composition of ruminal bacteria and protozoa, with emphasis on conjugated linoleic acid, vaccenic acid, and odd-chain and branched-chain fatty acids. J Anim Sci 85:1228–1234. doi:10.2527/jas.2006-385
Palmquist DL, Lock AL, Shingfield KJ et al (2005) Biosynthesis of conjugated linoleic acid in ruminants and humans. Adv Food Nutr Res 50:179–217
Pariza MW (2004) Perspective on the safety and effectiveness of conjugated linoleic acid. Am J Clin Nutr 79:1132S–1136S
Patra AK, Saxena J (2009a) The effect and mode of action of saponins on the microbial populations and fermentation in the rumen and ruminant production. Nutr Res Rev 22:204–219
Patra AK, Saxena J (2009b) Dietary phytochemicals as rumen modifiers: a review of the effects on microbial populations. Antonie Van Leeuwenhoek 96:363–375. doi:10.1007/s10482-009-9364-1
Patra AK, Kamra DN, Agarwal N (2006) Effect of plant extract on in vitro methanogenesis, enzyme activities and fermentation of feed in rumen liquor of buffalo. Anim Feed Sci Technol 128:276–291
Pen B, Sar C, Mwenya B et al (2006) Effects of Yucca schidigera and Quillaja saponaria extracts on in vitro ruminal fermentation and methane emission. Anim Feed Sci Technol 129:175–186
Pen B, Takaura K, Yamaguchi S et al (2007) Effects of Yucca schidigera and Quillaja saponaria with or without 1–4 galacto-oligosaccharides on ruminal fermentation, methane production and nitrogen utilization in sheep. Anim Feed Sci Technol 138:75–88
Piacente S, Pizza C, Oleszek W (2005) Saponins and phenolics of Yucca schidigera Roezl: chemistry and bioactivity. Phytochem Rev 4:177–190
Poungchompu O, Wanapat M, Wachirapakorn C et al (2009) Manipulation of ruminal fermentation and methane production by dietary saponins and tannins from mangosteen peel and soapberry fruit. Arch Anim Nutr 63:389–400. doi:10.1080/17450390903020406
Regensbogenova M, McEwan NR, Javorsky P et al (2004) A re-appraisal of the diversity of the methanogens associated with the rumen ciliates. FEMS Microbiol Lett 238:307–313
Rochfort S, Parker AJ, Dunshea FR (2008) Plant bioactives for ruminant health and productivity: review. Phytochemistry 69:299–322
Saha S, Walia S, Kumar J et al (2010) Structure–biological activity relationships in triterpenic saponins: the relative activity of protobassic acid and its derivatives against plant pathogenic fungi. Pest Manag Sci 66:825–831
Santoso B, Kilmaskoss A, Sambodo P (2007) Effects of saponin from Biophytum petersianum Klotzsch on ruminal fermentation, microbial protein synthesis and nitrogen utilization in goats. Anim Feed Sci Technol 137:58–68
Simons V, Morrissey JP, Latijnhouwers M et al (2006) Dual effects of plant steroidal alkaloids on Saccharomyces cerevisiae. Antimicrob Agents Chemother 50:2732–2740. doi:10.1128/AAC.00289-06
Singer MD, Robinson PH, Salem AZM et al (2008) Impacts of rumen fluid modified by feeding Yucca schidigera dairy cows on in vitro gas production of 11 common dairy feedstuffs as well as animal performance. Anim Feed Sci Technol 146:242–258
Sirohi SK, Pandey N, Goel N et al (2009) Microbial activity and ruminal methanogenesis as affected by plant secondary metabolites in different plant extracts. Int J Environ Sci Eng 1:52–58
Soliva CR, Zeleke AB, Cl’ement C et al (2008) In vitro screening of various tropical foliages, seeds, fruits and medicinal plants for low methane and high ammonia generating potentials in the rumen. Anim Feed Sci Technol 147:53–71
Suharti S (2010) Modification of rumen microbe diversity and fermentation of cattle using lerak (Sapindus rarak) saponin. PhD dissertation, Bogor Agricultural University, Indonesia (Indonesian)
Suharti S, Astuti DA, Wina E et al Effects of whole fruit lerak (Sapindus rarak DC) extract on in vitro ruminal fermentation, methane production and microbial diversity. Lett Appl Microbiol (unpublished)
Suharti S, Wina E, Astuti DA et al (2010) The dynamic of rumen microbes in the in vitro fermentation of different ratios of forage and concentrate in the presence of whole lerak (Sapindus rarak) fruit extract. In: Presented in 2nd symposium on gastrointestinal ecology, Nanjing Agricultural University, Nanjing, 23–25 May 2010
Sultana H, Miyazawa K, Kanda S et al (2011) Fatty acid composition of ruminal bacteria and protozoa, and effect of defaunation on fatty acid profile in the rumen with special reference to conjugated linoleic acid in cattle. Anim Sci J 82:434–440
Teferedegne B (2000) New perspective on the use of tropical plants to improve ruminant nutrition. Proc Nutr Soc 59:209–214
Teferedegne B, McIntosh F, Osuji PO et al (1999) Influence of foliage from different accessions of the subtropical leguminous tree, Sesbania sesban on ruminal protozoa in Ethiopian and Scottish sheep. Anim Feed Sci Technol 78:11–20
Thalib A (2004) In vitro study of effectiveness of saponin from Sapindus rarak fruit as methanogenesis inhibitor on ruminal digestion system. J Ilmu Ternak &Veteriner 9:164–171 (in Indonesian)
Thalib A, Widiawati Y (2008) Effect of Acetoanaerobium noterae bacteria addition in the diet on methane production and performance of sheep. J Ilmu Ternak &Veteriner 13:273–278
Thalib A, Widiawati Y, Haryanto B (2010) Utilization of complete rumen modifier on sheep fed high fibrous forages. J Ilmu Ternak & Veteriner 15(2):97–104 (in Indonesian)
Tsuzuki JK, Svidzinki TIE, Shinobu CS et al (2007) Antifungal activity of the extracts and saponins from Sapindus saponaria L. An Acad Bras Cienc 79:577–583
Ushida K, Newbold CJ, Jouany JP (1997) Interspecies hydrogen transfer between the rumen ciliate Polyplastron multivesiculatum and Methanosarcina barkeri. J Gen Appl Microbiol 43:129–131
Vincken JP, Heng L, de Groot A et al (2007) Saponins, classification and occurrence in the plant kingdom. Phytochemistry 68:275–297
Vinogradov E, Egbosimba EE, Perry MB et al (2001) Structural analysis of the carbohydrate components of the outer membrane of the lipopolysaccharide-lacking cellulolytic ruminal bacterium Fibrobacter succinogenes S85. Eur J Biochem 268:3566–3576
Vogels G, Hoppe WF, Stumm CK (1980) Association of methanogenic bacteria with rumen ciliates. Appl Environ Microbiol 40:608–612
Wallace RJ, Arthaud L, Newbold CJ (1994) Influence of Yucca schidigera extract on ruminal ammonia concentrations and ruminal microorganism. Appl Environ Microbiol 60:1762–1767
Wallace RJ, McEwan NR, McIntosh FM et al (2002) Natural products as manipulators of rumen fermentation. Asian-Aust J Anim Sci 15:1458–1468
Wang Y, McAllister TA, Newbold CJ et al (1998) Effect of Yuca schidigera extract on fermentation and degradation of steroidal saponins in the rumen simulation technique (RUSITEC). Anim Feed Sci Technol 74:143–153
Wang Y, McAllister TA, Yanke LJ et al (2000) In vitro effects of steroidal saponins from Yucca schidigera extract on rumen microbes. J Appl Microbiol 88:887–896
Wang Y, Zhang Y, Zhu Z et al (2007) Exploration of the correlation between the structure, hemolytic activity, and cytotoxicity of steroid saponins. Bioorg Med Chem 15(7):2528–2532
Wang JK, Ye JA, Liu JX (2010) Effects of tea saponins on rumen microbiota, rumen fermentation, methane production and growth performance – a review. Trop Anim Health and Prod DOI: 10.1007/s11250-011-9960-8 (on line 26 august 2011)
Williams AG, Coleman GS (1992) The rumen protozoa. Springer, New York
Williams AG, Withers SE (1991) Effect of ciliate protozoa on the activity of polysaccharide-degrading enzymes and fibre breakdown in the rumen ecosystem. J Appl Bacteriol 70:144–155
Wina E (2005) The utilization of Sapindus rarak DC. Saponins to improve ruminant production through rumen manipulation. PhD dissertation, University of Hohenheim Verlag Grauer, Beuren, Stuttgart
Wina E, Muetzel S, Hoffmann E et al (2005a) Saponins containing methanol extract of Sapindus rarak affect microbial fermentation, microbial activity and microbial community structure in vitro. Anim Feed Sci Technol 121:159–174
Wina E, Muetzel S, Becker K (2005b) The impact of saponins or saponin-containing plant materials on ruminant production – a review. J Agric Food Chem 53:8093–8105
Wina E, Muetzel S, Becker K (2006a) The dynamics of major fibrolytic microbes and enzyme activity in the rumen in response to short- and long-term feeding of Sapindus rarak saponins. J Appl Microbiol 100:114–122
Wina E, Muetzel S, Becker K (2006b) Effects of daily and interval feeding of Sapindus rarak saponins on protozoa, rumen fermentation and digestibility in sheep. Asian-Aust J Anim Sci 19:1580–1587
Wu J, Liu L, Chau FT (2001) Ultrasound-assisted extraction of ginseng saponins from ginseng roots and cultured ginseng cells. Ultrason Sonochem 8:347–352
Xu M, Rinker M, McLeod KR et al (2010) Yucca schidigera extract decreases in vitro methane production in a variety of forages and diets. Anim Feed Sci Technol 159:18–26
Yanez-Ruiz DR, Scollan ND, Merry RJ et al (2006) Contribution of rumen protozoa to duodenal flow of nitrogen, conjugated linoleic acid and vaccenic acid in steers fed silages differing in their water-soluble carbohydrate content. Br J Nutr 96:861–869. doi:10.1017/BJN20061927
Yang CR, Zhang Y, Jacob MR et al (2006) Antifungal activity of C-27 steroidal saponins. Antimicrob Agents Chemother 50:1710–1714. doi:10.1128/AAC.50.5.1710-1714.2006
Yoshikawa M, Morikawa T, Li N, Nagatomo A et al (2005) Bioactive saponins and glycosides. XXIII.1) Triterpene saponins with gastroprotective effect from the seeds of Camellia sinensis –Theasaponins E3, E4, E5, E6, and E7. Chem Pharm Bull 53:1559–1564
Yoshikawa M, Morikawa T, Nakamura S et al (2007) Bioactive saponins and glycosides. XXV.1) Acylated oleanane-type triterpene saponins from the seeds of tea plant (Camellia sinensis). Chem Pharm Bull 55:57–63
Zhang JD, Cao YB, Xu Z et al (2005) In vitro and in vivo antifungal activities of the eight steroid saponins from Tribulus terrestris L. with potent activity against fluconazole-resistant fungal. Biol Pharm Bull 28:2211–2215
Zhou YY, Mao HL, Jiang F et al (2010) Tea saponins reduce ruminal methane emission through the inhibitory effect on protozoa in Hu sheep. In: Presented in greenhouse gas animal agriculture conference IV, Alberta-Canada, 4–8 Oct 2010
Acknowledgements
The author would like to give a sincere thank to the anonymous reviewer for his valuable suggestions to improve the quality of this paper. Data presented in Fig. 11.5 were obtained from the research supported by the Indonesian Agency for Agricultural Research and Development during the author’s PhD program at University of Hohenheim, Germany.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Wina, E. (2012). Saponins: Effects on Rumen Microbial Ecosystem and Metabolism in the Rumen. In: Patra, A. (eds) Dietary Phytochemicals and Microbes. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3926-0_11
Download citation
DOI: https://doi.org/10.1007/978-94-007-3926-0_11
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-3925-3
Online ISBN: 978-94-007-3926-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)