Abstract
The optical and electrical properties of vanadium pentoxide films simply prepared by direct deposition from stable ethanol suspensions of V2O5 nanoparticles, are found to change after exposure to ammonia. Aimed at developing solid state ammonia sensors able to work at room temperature, spectrophotometric investigations and electrical measurements are performed on vanadium pentoxide films deposited on glass, on V2O5 films deposited on flexible substrates, having nanosilver electrodes applied on the top, and on n-Si/V2O5 heterojunctions.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Vanadium pentoxide is a versatile semiconductor, characterized by the property to change its optical and electronic behaviour reversibly, in response to a variety of stimuli including externally applied electric field [1], ultraviolet light irradiation [2], and thermal treatment [3]. In addition V2O5 is a promising candidate for gas sensing applications, because of its ability to change its electrical resistance when it is exposed to a variety of gas targets. In common with other transition metal oxides used in resistive sensors, the gas sensing performances of vanadium pentoxide in terms of sensitivity and response/recovery times, are found to improve by reducing the crystal size. V2O5 processed as nanotubes, nanowires, and nanobelts in order to maximize the surface area, has been used successfully to detect ethanol [4], ammonia [5], and ammines [6]. This paper reports on a simple synthesis to prepare nanostructured vanadium pentoxide having excellent film forming property, and low temperature sensitivity towards ammonia. The material, prepared using as starting reagent ammonium metavanadate (NH4VO3), and sintering the reaction product in air at 400°C, is found to consists of highly crystalline V2O5 nanoparticles.
2 Experimentals
The morphology and microstructural properties of the V2O5 nanoparticles are investigated by means of X-ray diffraction (XRD) and transmission electron microscopy (TEM) measurements performed using an Ital Structure APD 2000 diffractometer and a JEOL 2010 EX instrument. Optical measurements were performed using a Perkin–Elmer Lambda 9/19 spectrophotometer and a Nicolet Avatar 360 spectrometer. Electrical characterization of thin V2O5 films deposited on n-Si wafer and on flexible substrates was performed using a Keithley 2004 sourcemeter, and an Agilent 34970A multimeter. A CMOS sensor (from Sensirion) was used to check the temperature and RH into the measurement chamber. Electrical measurements were performed at constant RH, and under exposure to calibrated NH3 flux, inserting both the sensor based on V2O5 and the reference sensor into an aluminium measurement chamber. The relative humidity level inside the measurement chamber was settled and controlled by mixing steams of dry air and saturated air, using a number of digital mass flow meter controllers connected to dry air and to a bubbler held at constant temperature. NH3 fluxes were generated by a permeation tube, calibrated to flux 14 ppm ammonia when kept at 50°C. The way V2O5 films coated on quartz crystals respond to RH at constant temperature was evaluated using an automated system purchased from BioAge, able to measure the resonance frequency shifts of a coated quartz crystal.
3 Results and Discussion
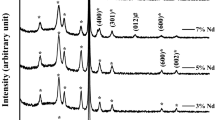
The analysis of XRD patterns (Fig. 19.1a) reveals that the material prepared starting from ammonium metavanadate consists of highly crystalline V2O5 nanoparticles, having few tens nanometers average size. High crystalline degree is confirmed by TEM measurements also (Fig. 19.1b).
In addition, TEM investigations reveal that the nanoparticles have a trend to organize aligning along the same preferential direction (Fig. 19.1c).
The film forming attitude shown by the nanoparticles allows compact films, highly adherent to the glass, plastic, and silicon substrates, to be obtained by direct deposition from ethanol nanoparticles suspensions, without the use of any binder, and without the need of any substrate surface treatment.
To investigate on the sensing properties of the nanostructured V2O5, thin films have been deposited onto AT-cut quartz crystals (10 MHz) having Al electrodes on the top. The resonance frequency of the coated quartzes changes reversibly as the RH level changes (Fig. 19.2a). The frequency shift is linearly related to the RH level in the range between 0 and 100% RH (Fig. 19.2b).
The absorption spectrum of pale yellow V2O5 films deposited on transparent substrates (Fig. 19.3a) is characterized by a band positioned between 350 and 400 nm. The films bleach when exposed in air to concentrated NH3. Exposure to NH3 has remarkable effects on the IR spectrum also (Fig. 19.3b).
Figure 19.3 shows that exposure to an elevated concentration of NH3 at high RH, has remarkable effects in the UV–VIS–IR properties of the nanocrystalline V2O5 films under investigation. Such effects are found to be persistent, and likely originate from a reaction that occurs between ammonia and the V2O5 nanocrystals, yielding ammonium metavanadate as reaction product. NH4VO3 converts back to vanadium pentoxide and the optical spectra recover their original shape, by heating the exposed films in air, at about 400°C.
Current–voltage measurements carried out in dry condition on n-Si/V2O5 heterojunctions show that exposure to calibrated amounts of ammonia in the ppm range results in a decrease of the metal oxide conductivity. This finding is evidenced by comparing the current–voltage plots of a typical n-Si/V2O5 junction before and after exposure to 7 ppm dry ammonia (Fig. 19.4a). The resistivity changes arising from the interaction between the nanocrystalline V2O5 films and NH3 in dry condition show a partial reversibility, as it is suggested by the evolution of the current measured through the junction at constant voltage, in response to a 350 s lasting pulse of 7 ppm dry ammonia (Fig. 19.4b).
Figure 19.5a shows a typical sensor based on V2O5, deposited on a transparency sheet without the need of any post-processing, by direct deposition from stable dispersions of V2O5 nanoparticles. Linearly shaped electrodes are applied on the top of the semiconducting film by using standard refillable pens, charged with water dispersions of silver nanoparticles. Figure 19.5b shows the results of electrical measurements performed on the sensor of Fig 19.5a. It can be noticed that the resistance of the device increases with exposure to ammonia.
This latter finding is in disagreement with that commonly reported in literature, according to which V2O5 behaves as n-type semiconducting oxide, having an electrical conductivity that increases when it is exposed to ammonia [5]. Such a behaviour is commonly ascribed to interaction between the metal oxide and the gas target, resulting in formation of oxygen vacancies, associated to the reduction of vanadium ions from V5+ species to V4+. This latter kind of response mechanism is that usually observed at high temperature. The response toward NH3 observed by us at low temperature, as it is described in Fig. 19.4b, shows a different trend. A possible explanation, may be that the interaction between the oxide films and ammonia is mediated by the presence of thin water layers coating the semiconducting oxide, into which NH3 dissolves, and dissociates. In such an hypothesis the metal oxide would behave as a pH sensor [7]. It is also noticed that the response of V2O5 based sensors is only partially reversible, as a certain uptake of NH3 is not released during the sensor recovery. This behaviour is ascribable to the irreversible reaction that occurs between vanadium pentoxide nanocrystals and ammonia, resulting in the formation of NH4VO3 as reaction product [8].
4 Conclusion
Nanocrystalline V2O5 films developed by direct deposition from ethanol suspensions are found to have optical and electrical properties that change after exposure to ammonia. In particular while optical changes are ascribable to NH4VO3 formation, made reversible only by heating at 400°C, sensitive electrical changes, reversible to a certain extent, are observed at low temperature, in dry conditions, by exposing the V2O5 films to few ppm ammonia. These results can be promising in view of applications of the developed material in low temperature ammonia sensing applications.
References
Xiong C, Aliev AE, Gnade B, Balkus KJ (2008) Fabrication of silver vanadium oxide and V2O5 nanowires for electrochromics. ACSNano 2:293–301
Cheremisin A, Putrolaynen V, Velichko A, Pergament A (2009) UV laser modification and selective ion-beam etching of amorphous vanadium pentoxide thin films. Phys Status Solidi A 206:1484–1487
Cui N, Teixeira V, Meng LJ, Wang R, Gao JY, Fortunato E (2008) Thermochromic properties of vanadium oxide films prepared by dc reactive magnetron sputtering. Thin Solid Films 516:1484–1488
Raj AD, Pazhanivel T, Kumar PS, Mangalaraj D, Nataraj D, Ponpandian DN (2010) Self assembled V2O5 nanorods for gas sensors. Curr Appl Phys 10:531–537
Shimizu K, Chinzei KI, Nishiyama H, Kakimoto S, Sugaya S, Matsutani W, Satsuma A (2009) Doped-vanadium oxides as sensing materials for high temperature operative selective ammonia gas sensors. Sens Actuators B 141:410–416
Raible I, Burghard M, Schlecht U, Yasuda A, Vossmeyer T (2005) V2O5 nanofibres: novel gas sensors with extremely high sensitivity and selectivity to amines. Sens Actuators B 106:730–735
Helwig A, Müller G, Sberveglieri G, Eickhoff M (2009) On the Low-Temperature Response of Semiconductor Gas Sensors. J of Sens 620720 doi:10.1155/2009/620720
Kittaka S, Hamaguchi H, Umezu T, Endoh T, Takenaka T (1997) Interaction of NH3 with H2O in the vanadium pentaoxide hydrate interlayer spaces—topotactic crystal-growth of ammonium vanadate film. Langmuir 13:1352–1358
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this paper
Cite this paper
Rizzo, G., Bonavita, A., Neri, G., Arena, A., Saitta, G. (2011). Synthesis, Characterization, and Ammonia Sensing Properties of Vanadium Pentoxide Nanocrystals. In: Neri, G., Donato, N., d'Amico, A., Di Natale, C. (eds) Sensors and Microsystems. Lecture Notes in Electrical Engineering, vol 91. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-1324-6_19
Download citation
DOI: https://doi.org/10.1007/978-94-007-1324-6_19
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-1323-9
Online ISBN: 978-94-007-1324-6
eBook Packages: EngineeringEngineering (R0)