Abstract
Networked carbon nanotubes (CNTs) films have been grown by chemical vapor deposition (CVD) technology onto miniaturized Co-coated alumina substrates for NO2 and NH3 gas sensing applications, at a sensor temperature of 150°C. The sidewalls of the CNTs films have been modified by spray-coating with two different metalloporphyrins (MPPs) consisting of a TetraPhenylPorphyrin coordinated by a central metal of zinc (Zn-TPP) and manganese (Mn-TPP) for enhanced sensitivity and tailored specificity. It was demonstrated that the gas sensitivity of the MPPs-modified CNTs-sensors significantly improved by a factor up to four-times through a catalytic effect of the MPPs. The gas sensing properties of CNTs-sensors, including MPPs-modified CNTs, are characterized by a change of the electrical conductivity in a model of charge transfer with a semiconducting p-type character. A response of the CNTs-sensor functionalized with 2 spray-layers of Mn-TPP has been measured as 0.43% to 0.5 ppm NO2, and as 0.09% to 10 ppm NH3, at 150°C. The MPPs-functionalized CNTs-sensors exhibit high sensitivity, fast response, reversibility, good repeatability, sub-ppm range detection limit.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Gas sensors based on carbon nanotubes (CNTs) have been largely studied in the form of networked films for highly-sensitive gas detection applications [1–3]. Due to very high surface-to-volume ratio, hollow nanostructure, high electron mobility, great surface reactivities and high capability of gas adsorption, CNTs have been investigated as building blocks for fabricating novel devices at nanoscale such as high-performance gas sensors and nano-platforms for biosensing.
Networked films of carbon nanotubes (CNTs) have been grown by CVD technology onto low-cost miniaturized alumina substrates. The sidewalls of the CNTs films have been modified by spray-coating with two different metalloporphyrins (MPPs) consisting of a TetraPhenylPorphyrin coordinated by a central metal of zinc (Zn-TPP) and manganese (Mn-TPP) for enhanced sensitivity and tailored specificity. Hazardous gases such as NO2 and NH3 have been detected with various responsiveness in the range of concentration from 0.1 to 1,000 ppm. The response of the chemiresistors in terms of p-type electrical conductance has been investigated as a function of the thickness of the functionalizing MPPs, at the sensor temperature of 150°C.
Carbon nanotubes (CNTs) are 1D-nanometre hollow structures rolled as single-walled or concentric multi-walled cylinders with high capability of gas molecules adsorption for enhanced gas sensitivity even at low sensor temperature. Various gas sensor nanomaterials include semiconducting metal oxides, conducting polymers, metal nanostructures and nanocomposites with nanofillers. However, it has been demonstrated that single-walled CNTs are functional nanostructures for detecting very low gas concentrations of NO2 and NH3 under ambient conditions [1]. Various principles of transduction using CNTs have been implemented for chemical sensing including field effect transistors (FET), surface acoustic waves (SAW), quartz crystal microbalance (QCM), optical fibers, electrochemical devices, chemiresistors. Here a two-pole chemiresistor has been integrated.
Surface modifications of the CNTs with different functionalizing materials have been employed to improve gas sensitivity and to tailor specificity. In fact, nanoclusters of noble metals (Au, Pt, Pd, Ag) have been used to enhance gas sensitivity of CNTs networked films, operating at a sensor temperature from room temperature to 200°C [4–7]. Moreover, metalloporphyrins consisting of TetraPhenylPorphyrins (TPP) coordinated by a central metal of zinc and manganese, are functional materials that have been prepared as highly-sensitive receptors for artificial olfaction [8] and volatile organic compounds (VOCs) detection at room temperature [9, 10].
In this study, MPP-modified CNTs networked films have been investigated for sub-ppm gas sensing of NO2, including NH3 at ppm-level, working at a temperature of 150°C.
2 Experimental Details
The scheme of the fabricated device is shown in Fig. 15.1. CNTs films were grown by CVD technology onto miniaturized alumina substrates (5 mm width × 5 mm length × 0.6 mm thickness). Co (6 nm thick) catalyst was sputtered onto alumina substrates at a working pressure of 6 × 10?2 mbar, at room temperature and a supplied RF power of 150 Watts. Then, the Co-coated substrates were placed in a quartz boat and inserted at the center of a 1-in. diameter quartz tube reactor housed in a furnace of the CVD processing chamber to grow CNTs. The chamber was evacuated up to 5 × 10?3 Torr, then the substrates were heated at 550°C upon H2 flow (100 sccm) at a working pressure of 100 Torr. Then, a carbon gaseous precursor of acetylene (C2H2) with a flow rate of 20 sccm was added to H2 gas with a flow rate of 80 sccm. The working pressure was fixed at 100 Torr with a deposition time of the CNTs layers for 30 min. After CNTs growth, the samples were equipped by vacuum thermally evaporated Cr–Au (20/300 nm) contacts to serve for the electrical measurements of the two-pole chemiresistor. The Cr/Au electrode sizes were 1 mm width × 5 mm length. The gap between two electrodes was 3 mm. The CNTs films have been modified with a metalloporphyrins layer: (5,10,15,20-tetraphenylporphyrin)zinc [ZnTPP] and (5,10,15,20-tetraphenylporphyrin)manganese chloride [MnTPPCl] have been synthesized according to literature methods [11, 12]. A layer of two distinct M-TPPs with different thickness corresponding to 1 and 2 spray-layers was deposited by spray-coating onto the surface of the CNTs networks previously grown. The M-TPPs were dispersed in solvent of chloroform for spray-coating. Metalloporphyrins were not modified to be covalently anchored onto the surface of the nanotubes, nonetheless even noncovalent interactions between metalloporphyrins and carbon nanotubes are strong enough to ensure the adhesion of the M-TPPs film.
The electrical resistance, at room temperature and upon inert atmosphere, of the un-modified, 1 spray-layer and 2 spray-layers ZnTPP, 1 spray-layer and 2 spray-layers MnTPPCl functionalized CNTs-sensors was measured as 4.51, 4.68 and 7.20, 4.65 and 6.69 k?, respectively. The presence of a given MPPs layer at increasing thickness onto the surface of the CNTs film increases the electrical resistance in the chemiresistor proportionally to the thickness of the deposited MPPs. The sheet resistance of the CNTs films was estimated in the range of 1–2 k?/square.
The fabricated CNTs sensors have been located in a test cell (500 ml volume) for gas exposure measurements. The cell case is able to host up to four chemiresistive sensors. Dry air was used as reference gas and diluting gas to air-conditioning the sensors. The gas flow rate was controlled by mass flowmeters. The total flow rate per exposure was kept constant at 1,500 ml/min. The gas sensing experiments have been performed by measuring the electrical conductance of CNTs thin films in the two-pole format upon controlled ambient of individual NH3 reducing gas and NO2 oxidizing gas in the range of 10–1,000 ppm, and 0.1–10 ppm, respectively, at sensor temperature of 150°C.
The temperature was measured with a J-type thermocouple by means of a multimeter (Agilent, 34401A). The dc electrical conductance of the CNTs-sensors has been measured by the volt-amperometric technique in the two-pole format by a multimeter (Agilent, 34401A). The sensors were scanned by a switch system (Keithley, 7001) equipped by a low-current scanner card (Keithley, 7158) with a multiplexed read-out.
3 Results and Discussion
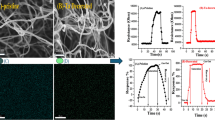
The morphology and structure of the fabricated CNTs networks has been characterized by scanning electron microscopy (SEM), as shown in Fig. 15.2. A dense network of bundles of multiple tubes consisting of multi-walled carbon nanostructures appears with a maximum length up to 5 ?m and single-tube diameter in the range of 5–40 nm. Amorphous carbon, non-nanotube material, metal impurities are present in the grown nanomaterial as well. Figure 15.3 shows the atomic force microscopy (AFM) image of the CNTs layers coated with MPPs. This demonstrates a good local coverage of the carbon nanotubes with the sprayed metalloporphyrins to enhance gas adsorption.
The measured electrical conductance of the functionalized CNTs upon exposure of a given oxidizing (NO2) or reducing (NH3) gas is modulated by a charge transfer model with p-type semiconducting characteristics. Figure 15.4 shows the typical time response in terms of electrical resistance change for four chemiresistors based on unmodified CNTs, MnTPP-modified CNTs (CNT + 1MnTPP, CNT + 2MnTPP), and ZnTPP-modified CNTs (CNT + 2ZnTPP), exposed to NH3 and NO2 gas, at 150°C. The electrical resistance of all CNTs-sensors increases (decreases) upon a single gas exposure of the NH3 reducing (NO2 oxidizing) gas due to molecules adsorption. These results demonstrate that MPPs-modified CNTs-chemiresistors are able to detect a wide range of gas concentrations of NH3 (10–1,000 ppm) and NO2 (0.5–10 ppm) with a very low limit of detection in the range of sub-ppm for NO2 and a few ppm level for NH3, at 150°C. These minimal detection limits are very interesting for environmental NO2 air-monitoring and industrial processes control NH3 detection applications.
Figure 15.5 shows the calibration curves of the resistance change of the four CNTs-chemiresistors operating at 150°C for ammonia and nitrogen dioxide. The MnTPP-modified CNTs sensor with two spray-layers exhibits the maximum response for both gases considered. This could be attributed to catalytic effects of the metalloporphyrin for enhancing gas adsorption.
4 Conclusions
CVD technology has been used to grow networked layers of multi-walled CNTs onto cost-effective miniaturized alumina substrates. CNTs-chemiresistors have been fabricated for NO2 and NH3 gas sensing applications, at a sensor temperature of 150°C. The sidewalls of the CNTs films have been modified by spray-coating with two different metalloporphyrins (MPPs) consisting of a TetraPhenylPorphyrin coordinated by a central metal of zinc (Zn-TPP) and manganese (Mn-TPP) for enhanced sensitivity compared to un-functionalized CNTs networked layers. Detection limit of 0.5 ppm NO2 and 10 ppm NH3 has been measured. Finally, MPPs-modified CNTs-sensors are very interesting for advanced nanosensors operating at moderate temperatures with low power consumption. These microsensors could be used for environmental air-monitoring applications (NO2) and industrial processes control (NH3).
References
Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, Dai H (2000) Nanotube molecular wires as chemical sensors. Science 287:622–625
Someya T, Small J, Kim P, Nuckolls C, Yardley JT (2003) Alcohol vapor sensors based on single-walled carbon nanotube field effect transistors. Nano Lett 3(7):877–881
Penza M, Cassano G, Rossi R, Alvisi M, Rizzo A, Signore MA, Dikonimos Th, Serra E, Giorgi R (2007) Enhancement of sensitivity in gas chemiresistors based on carbon nanotube surface functionalized with noble metal (Au, Pt) nanoclusters. Appl Phys Lett 90:173123
Penza M, Rossi R, Alvisi M, Cassano G, Serra E (2009) Functional characterization of carbon nanotube networked films functionalized with tuned loading of Au nanoclusters for gas sensing applications. Sens Actuators B 140:176–184
Penza M, Rossi R, Alvisi M, Cassano G, Signore MA, Serra E, Giorgi R (2008) Pt- and Pd-nanoclusters functionalized carbon nanotubes networked films for sub-ppm gas sensors. Sens Actuators B 135:289–297
Penza M, Rossi R, Alvisi M, Serra E (2010). Metal-modified and vertically aligned carbon nanotube sensors array for landfill gas monitoring applications. Nanotechnology 21:105501
Kong J, Chapline MG, Dai H (2001) Functionalized carbon nanotubes for molecular hydrogen sensors. Adv Mater 13:1384–1386
Di Natale C, Paolesse R, D’Amico A (2007) Metalloporphyrins based artificial olfactory receptors. Sens Actuators B 121:238–246
Penza M, Rossi R, Alvisi M, Valerini D, Serra E, Paolesse R, Martinelli E, D’Amico A, Di Natale C (2009) Metalloporphyrins-functionalized carbon nanotube networked films for room-temperature VOCs sensing applications. Procedia Chemistry 1:975–978
Penza M, Rossi R, Alvisi M, Signore MA, Serra E, Paolesse R, D’Amico A, Di Natale C (2010) Metalloporphyrins-modified carbon nanotubes networked films-based chemical sensors for enhanced gas sensitivity. Sens Actuators B 144:387–394
Paolesse R, Mandoj F, Marini A, Di Natale C (2004) Porphyrin based chemical sensors. Encyclopedia of nanoscience and nanotechnology, Vol. 9. American Science Publishers, Stevenson Ranch, CA, USA, pp 21–35
Monti D, Nardis S, Stefanelli M, Paolesse R, Di Natale C,D’Amico A (2009) Porphyrin-based nanostructures for sensing applications. J. Sens 856053
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this paper
Cite this paper
Penza, M. et al. (2011). Gas Microsensors with Metalloporphyrin-Functionalized Carbon Nanotube Networked Layers. In: Neri, G., Donato, N., d'Amico, A., Di Natale, C. (eds) Sensors and Microsystems. Lecture Notes in Electrical Engineering, vol 91. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-1324-6_15
Download citation
DOI: https://doi.org/10.1007/978-94-007-1324-6_15
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-1323-9
Online ISBN: 978-94-007-1324-6
eBook Packages: EngineeringEngineering (R0)