Abstract
Carbonate karst is characterized by subterranean drainage and contains the most biodiverse groundwater faunas globally. These faunas, which include a suite of higher taxa largely restricted to karst subterranean waters, comprise species that characteristically are narrow range endemics. They typically possess a suite of adaptations to subterranean life that render them especially vulnerable to anthropogenic disturbance. Most such faunas depend on imported energy, largely in the form of dissolved organic carbon, or on chemoautotrophic energy in particular circumstances. Karst is especially vulnerable to surface inputs at both local and broad scales owing to the absence of, or thin soil cover, and by the presence of open conduits that can transport materials such as sediments, pollutants, or nutrients to the deep subterranean waters without amelioration. Management actions – such as sustaining water supply, control of pollution and nutrification, regulating resource extraction, catchment surface management to sustain recharge and prevent siltation, and control of human access – may need to be applied at very different scales, ranging from a small cave, or extending to an entire catchment which may comprise extensive areas outside the karst itself.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Karst is characterized by the predominance of subterranean drainage, the fauna of which this chapter is concerned. Karst is renowned for hosting obligate subterranean species (stygobionts) and the first such species described was the Olm, Proteus anguinus Laurenti, 1768, a blind, depigmented, salamander inhabiting cave streams in the classical Kras (karst) area of Slovenia, believed in mediaeval times to be the dragon larvae. Stygobionts often comprise ancient relictual lineages isolated underground millions of years ago, but this is not always the case. So, the evolution of subterranean life is a continuing process and the movement of fauna or the flow of genes between surface and subterranean populations needs to be maintained. Consequently, the thresholds to the subterranean world, including springs, caves, and sinkholes, are important elements in the management of groundwater fauna in karst. For the purpose of this chapter, I group all underground waters inhabited by fauna within the term groundwater although drips, pools, underground rivers, and streams within caves are not typically referred to as groundwater.
The last two decades have seen the recognition of groundwater as a significant source of biodiversity (Danielopol et al. 2000) that warrants protection both in its own right (Danielopol 1998) and also for the provision of environmental services of direct bearing on human well being (Boulton et al. 2008). The karst areas of the world support the most diverse assemblages of subterranean aquatic species (var. stygofauna, stygiofauna) and this chapter raises issues pertinent to their management and conservation.
Groundwater ecosystems have been recognized as dynamic systems comparable in complexity to surface ecosystems (Rouch 1977; Gibert et al. 1994), although they lack primary production. Although within cave species richness (α-diversity) is not especially great (Culver and Sket 2000), the great change in species composition between systems/karst areas (β-diversity) results in a high species richness in larger regions (γ-diversity). In some areas, the species richness of groundwater fauna exceeds that in surface waters, an increased knowledge that has arisen through both greater research effort and as a result of molecular analyses showing that there are many cryptic species in groundwater (Bradford et al. 2010). Consequently, previously widespread species are recognized to comprise an array of species each more circumscribed geographically, that is endemic to quite small areas (Proudlove and Wood 2003; Page et al. 2008; Trontelj et al. 2009; Zakšek et al. 2009). Elsewhere, it has arisen as a result of research in areas previously considered lacking in groundwater fauna, such as in Australia where more than 750 species of groundwater fauna were reported within about a decade (Humphreys 2008). The Balkan Peninsula is the longest most thoroughly researched karst region with more than 650 stygobiont species (plus 975 species of troglofauna; Sket et al. 2004) and Slovenia has the highest density of stygobionts with 114 species (Culver et al. 2004). Whereas six European countries (Belgium, France, Italy, Portugal, Slovenia, Spain) combined harbor 1059 stygobiont species (Michel et al. 2009), only 269 species of stygobiont are known from the 48 contiguous states of USA (Culver et al. 2003), with no more than 80 species in any karst region. Strategies for conservation of cave fauna on a regional basis, beyond the scope of this chapter, are addressed elsewhere (Culver et al. 2001; Ferreira et al. 2007; Gibert and Culver 2009 ; Malard et al. 2009; Michel et al. 2009).
The management of groundwater species in karst environments typically invokes images of cave streams and pools, and sometimes springs, but it requires consideration of a far wider range of subterranean habitats to encompass the entire karst ecosystem (Rouch 1977), and at a much larger scale, the extent of which may extend far beyond the karst system itself. The nature of karst, its past and present climatic, altitudinal, geomorphic, cultural, and developmental contexts vary widely both within and between biogeographic regions and nations. Consequently, it is not appropriate here to prescribe management practices but rather to raise some general principles and issues pertinent to the karst manager when contemplating the management of groundwater fauna in their particular circumstance and what needs to be considered and what questions need positing of researchers. Thus, it is not intended to provide a comprehensive coverage of the management issues, rather, to provide a guide to the issues of which managers needs to be aware and to provide pointers to where the information may be found or what direction research may need to take to address the management issues.
The management of each karst basin faces a unique set of circumstances for which it is not possible to be generally prescriptive. Humphreys (2002) identified three needs for groundwater fauna – a place to live, food, and oxygen – in order to focus attention on essential general elements of groundwater ecology without prescription. He further developed a string of issues that were known to impact groundwater fauna generally, or that may be expected so to do by extrapolation from surface waters or general ecological principles (Humphreys 2009).
The European PASCALIS project, comprising the karst rich nations of Belgium, France, Italy, Portugal, Slovenia, and Spain, is the first international attempt to assess groundwater fauna with a view to optimizing conservation planning (Michel et al. 2009). The general issue of conservation of subterranean fauna at a regional, national, and global scale is not covered here but is addressed in Culver and Pipan (2009) with leads to the pertinent literature.
To provide background to the conservation of karst aquatic fauna, there are many books and manuals dealing with the details of karst and water management at various levels of detail. Exemplar texts are, by topic: caves (biology – Culver and Pipan 2009; entity – Gillieson 1996); encyclopaedias (caves – Culver and White 2005; caves and karst – Gunn 2004; biospeleology, Juberthie and Decu 1994, 2000, 2001); fauna (Botosaneanu 1986); groundwater biology (Gibert et al. 1994; Griebler et al. 2001); hydrogeology (Ford and Williams 2007); mapping (Culver et al. 2001); protection and management (Watson 1997; Tercafs 2001; Jones et al. 2003); subterranean ecosystems (Wilkens et al. 2000; Culver and Pipan 2009); dedicated journal issues (Humphreys 1993b; Humphreys and Harvey 2001; Austin et al. 2008; Gibert and Culver 2009); dedicated journals (Subterranean Biology, formerly Mémoires de Biospéologie parts of International Journal of Speleology); and publications of the Karst Waters Institute, USA.
2 The Nature of Groundwater Fauna
Subterranean aquatic fauna comprise elements dependent to differing degrees on groundwater. They include stygobionts (var. stygobites) that are obligatorily dependent on groundwater throughout their life cycle, stygophiles that spend only part of their life cycle in groundwater, and stygoxens that opportunistically inhabit subterranean waters (Gibert et al. 1994). These degrees of dependence are reflected in the extent of their adaptation to subterranean life, being most marked in stygobites that characteristically lack visual and body pigments and so appear white and eyeless, are commonly translucent, and often vermiform permitting passage through small voids. They also exhibit behavioral adaptations that compensate, amongst others, for lack of vision (review: Langecker 2000; Parzefall 2000), and physiological adaptations such as low metabolic rate to compensate for the scarcity of food (review: Coineau 2000; Langecker 2000). Subterranean species typically have no resting life stage, exhibit a K-selected or A(adversity)-selected life history strategies and respond to environmental scarcity with delayed maturation, delayed reproduction, and lowered fertility (sensu Greenslade 1983; Southwood 1988). For example, tiny Bathynellacea (Antrobathynella stammeri and Bathynella sp.) may produce, but a single egg and take 9 months to mature (Coineau 2001; Giere 2009: 99).
Stygobionts typically occupy a very small geographic extent and are numerically rare, both factors that increase their vulnerability to extinction through stochastic environmental and population events, and genetic inbreeding. In the USA, 44% of stygobionts were limited to a single county (Culver et al. 2000), while in Western Australia, groundwater calcrete formations each harbor an endemic fauna, rich in diving beetles and crustaceans (Humphreys 2008). Aspects of rarity are discussed by Rabinowitz et al. (1986) and developed in the context of subterranean animals, together with population estimations, by Culver and Pipan (2009).
These attributes of groundwater animals have various management implications. The differing degree of dependence on groundwater means that some part of the fauna may require connection with the water surface even the air above, to complete the life cycle and that this connectivity is a prerequisite to maintain the faunal assemblage. For example, stygobiont diving beetles (Dytiscidae) breath at the water surface while stygobiont crustaceans in the same system respire within the water; so the crustaceans, but not beetles, could potentially disperse through subterranean water lacking contact with the air. Conversely, stygobiont species, typically long-lived and lacking resting stages, are dependent on the permanent presence of groundwater. Attributes such as low metabolic rate that adapt stygiobionts to the low food environment make them susceptible to competitive displacement by surface species if subterranean food energy is increased through pollution.
Stygobionts overwhelmingly comprise crustaceans belonging to many higher taxa, including those with broad ecological and geographical distributions (such as Copepoda, Ostracoda, Amphipoda, Isopoda, and Decapoda) (Fig. 13.1), and those with narrow ecological and geographical affinities, entirely or largely restricted in their distribution to subterranean waters (such as Bathynellacea, Remipedia, Thermosbaenacea, and Spelaeogriphacea) (Botosaneanu 1987). Many other higher taxa are represented amongst stygiofauna, including vertebrates (fishes, amphibians), meiofauna, hydroids, sponges, flatworms, nematodes, segmented worms, snails, mites, and beetles. Although this chapter focuses largely on visible fauna, there is a plethora of smaller meiofauna (Giere 2009) barely studied in groundwater sediments, and microbiota (Bacteria, Archaea) (Chapelle 2001; Griebler and Lueders 2009).
Examples of stygobiontic animals. (a) Eleotrid cave fish Milyeringa veritas and synbranchid eel, Ophisternon candidum, from Cape Range, Western Australia; (b) thermosbaenacean Halosbaena tulki from anchialine system, Cape Range, Western Australia; (c) Allocrangonyx pellucidus from Oklahoma, USA; (d) isopod Monolistra (Monolistra) monstruosa Sket from western Bosnia and Herzegovina. (a) and (b) are associated with an anchialine ecosystem. Photo credits: (a and b) Douglas Elford, Western Australian Museum; (c) courtesy of John Holsinger; (d) Boris Sket
Subterranean habitats can be very old and groundwater fauna may survive under extreme conditions and in isolated subterranean habitats through geological eras (Longley 1986; Wilson 2008) and consequently through major changes in climate and geological context (Humphreys 2000b, 2008). It used to be thought that stygobites were largely a phenomenon in karst terrains in temperate regions; however, over the last two decades, it has been recognized that stygobites occur widely, both in terms of geology and climate. Speciose stygal communities occur in the tropics (Deharveng and Bedos 2000; Humphreys 2008) but are largely absent in areas closer to the poles. However, stygobites occur below the Pleistocene ice sheet in Iceland inhabiting water-kept liquid by geothermal heat (Bjarni et al. 2007). Groundwater fauna occurs in aquifers formed within a wide variety of substrates, such as fractured rock, alluvial gravels, sandstone, and lava (Humphreys 2008), but it is most widespread and prolific in karst systems. Groundwater fauna is known from fresh, marine, and inland hypersaline waters (Humphreys et al. 2009), from thermal springs (Monod 1924), and from beneath the ice sheets (see above). It is known from both unconfined and confined aquifers – artesian systems to depths of up to 1 km in Morocco (Essafi et al. 1998) and widely in systems dependent on non-tradition energy sources (Engel 2005), such as chemotrophic systems in Edwards Aquifer, USA (Longley 1992), Movile Cave, Romania (Sarbu 2000), Frasassi Cave, Italy (Sarbu et al. 2000), Ayyalon Cave, Israel (Por 2007), and the anchialine system in Mayan Blue Cenote, Mexico (Pohlman et al. 2000). Accordingly, most undisturbed saturated karst systems outside the polar regions are expected to support groundwater fauna.
Stygal species often represent ancient phylogenetic and geographical relictual lineages (review: Humphreys 2000) that have been isolated underground for many millions of years (e.g., Leys et al. 2003; Wilson 2008). However, under certain conditions, a lineage may independently invade the subterranean realm on numerous occasions, such as with the development of regional aridity (Leys et al. 2003; Cooper et al. 2008). Other stygal species are recent invaders with close surface relatives, or species that still have both surface and subterranean populations, both amongst vertebrates (Strecker et al. 2003, 2004) and invertebrates (Carlini et al. 2009). Thus, colonization of the subterranean realm is a continuing process and so it is not just those species markedly adapted to subterranean life, which often attract most attention, but also putative colonizers that deserve protection, together with the gateway to and from the subterranean realm, be it a resurgence or sink. There are hints, also, of subterranean lineages recolonizing the surface (Prendini et al. 2010; Kornicker et al. 2010).
‘Connectivity is a primary process influencing ecosystem function and the distribution, abundance and persistence of all biota’ (Lindenmayer et al. 2008). Surface and underground streams may both provide avenues of connectivity within a karst basin (Verovnik et al. 2004; Carlini et al. 2009) and so need to be considered as a whole in karst management, as in other systems. Conversely, cave populations inhabiting subsurface basins may be quite disconnected from spring resurgences because individuals of a species may be too large to traverse interstitial channels in phreatic sediments and porous rock where conduits confining the cave stream pass below the water table (Ford and Williams 2007), as was noted for Gammarus minus (Carlini et al. 2009) and Niphargus virei (Malard et al. 1997). Even meiofauna, such as Parabathynellidae, may be unable to traverse fine phreatic sediments between calcrete aquifer “islands” in a desert landscape (Guzik et al. 2008). In contrast, at karst outcrop areas, the spring ecotone may not modify the population structure of the drifting population and consequently sampling at springs proved to be an effective way to study the population dynamics of the amphipod Niphargus virei within the aquifer (Malard et al. 1997). It is widely recognized that vegetation provides the organic carbon that forms the basis of subterranean food webs but its role in connectivity is often overlooked. Roots may penetrate karst to a depth of at least 50 m (Gillieson 1996). Roots transport organic matter directly to the groundwater, both by growth and by the movement of sap and so provide a direct connection between the photosynthetic tissue and groundwater. Secondly, roots provide connection between groundwater and plant transpiration and this affects groundwater levels. One consequence is that tree plantations may lower groundwater levels and threaten cave invertebrate faunas (Jasinska and Knott 2000).
The degree of local endemism in karst subterranean fauna is unparalleled. Most stygal species are considered to occur over short distances, few more than 200 km and many very much less (e.g., Leys et al. 2003; Finston et al. 2007, 2009; Cooper et al. 2008; Page et al. 2008; Eberhard et al. 2009; Trontelj et al. 2009), commonly restricted to single caves (Iliffe and Bishop 2007). This constraint may be further strengthened as many cryptic taxa are being found further restricting the range of a given putative species (Page et al. 2008; Trontelj et al. 2009). Short range endemism makes the persistence of a species vulnerable to local scale impacts, such as quarrying, draining, or impoundments. Similarly, they may be vulnerable to loss through hybridization or by competitive exclusion by mixing of faunas through flooding by impoundments, or tunnelling to manage water supply or as a bypass for engineering and mining projects (Humphreys 2008).
The total species richness in subterranean water is difficult to determine because, as is generally the case (May 1976), most species are rare. The task of evaluating the diversity is compounded by limited access to an environment that, unlike the deep ocean, does not yield readily to technological solutions. Despite intense sampling, subterranean systems typically yield additional species for prolonged periods and species accumulation curves analyzed using various algorithms are used to estimate total species richness of a system, examples of which are found for the PASCALIS project (Dole-Olivier et al. 2009). Additional species are still being found in Vjetrenica (Bosnia & Hetzegovina) and Mammoth (USA) caves after 150 years of sampling (Lučić and Sket 2003). An exception to this is sampling at a very small spatial scale, by which means Pipan and Culver (2007a) were able readily to sample a full complement of species of copepods from epikarst drip water.
3 Scale
Scale affects most aspects of karst management, such as practicality of management, susceptibility to disturbance, temporal lags in hydrology, dispersal and vicariance of fauna, and intrinsic stability. Karst groundwater fauna are dependent on subterranean water-filled voids that may vary in scale through several orders of magnitude (Boulton 2001), from the spaces between particles in alluvia to massive caves and conduits in some karsts such as the longest known underground river, the 180 km long Ox Bel Ha cave system that links 130 cenotes (QRSS 2009) on the Yucatán Peninsula, Mexico. In addition, groundwater fauna occurs extensively in the smaller voids of carbonate deposits, even in the absence of overt karstification at the surface (Humphreys 2001a). Within these systems, the physicochemical attributes of karst groundwater also vary, from the redox gradients around sediment particles through finely structured vertical gradients in anchialine waters (Seymour et al. 2007), to the wide range of temporal and spatial scales enshrined in aquifer flowpaths (Morgan 1993). Groundwater residence time is a significant factor in the hydrogeochemical evolution of groundwater, the properties of which are important to aquifer ecology. For example, changed flow affects the flux of organic carbon in groundwater that has a significant influence on the physicochemical variables such as pH and redox (Eh) (Pérez del Villar et al. 2004).
Although most karst fauna is collected in caves, most fauna probably occurs in the much more extensive void space in the leads and fissures far too small for people to enter. One approach to examine this has been to filter the entire drainage from a karst system (Rouch 1977, 1986) but it could be tested by having a cavernous area with many boreholes to enable sampling of both means of access within the same karst. However, multiple bores are rarely present in areas with accessible caves. Culver et al. (2009) surmounted this by sampling from individual cave drips (1–1,000 m) effectively obtaining point source diversity of the epikarst fauna. They demonstrated that frequency of occurrence of stygobiont copepods at these very small scales could predict occurrence of stygobiont species at one to two orders of magnitude of larger scales and argued that this supported dispersal at these scales. Conversely, Shabarova and Pernthaler (2010) sampled three cave pools, between 722 and 913 m below the entrance of Bärenschacht cave, Switzerland, and showed very high microbial diversity but minimal overlap, the pools sharing only one of 150 taxa (OTUs). They suggested that this may partly be the result of habitat filtering by different hydrochemical properties but largely due to hydrological properties with vadose pools serving as static collector of microbial diversity, while epiphreatic pools served as ephemeral habitat during the passage of bacteria between terrestrial habitats and rivers or estuaries.
The source of recharge water, whether from within (autogenic) or from outside (allogenic) the karst basin, has a great influence on the natural properties of the water (Table 13.1) and on the potential for influx of contaminants and nutrients. Allogenic karst may require that landscape-scale management has, of necessity, to extend upstream into non-karst landscape to provide adequately for the protection of the karst aquatic fauna.
The genetic variation in populations of stygobionts may vary widely in spatial scale. Guzik et al. (2009) demonstrated significant genetic structuring within populations of three sympatric species of subterranean diving beetles (Dytiscidae) within 3.5 km2 of a superficial karst in Western Australia. This study indicated that the three sister species had different population histories with episodes of population expansion, various degrees of spatial heterogeneity in the distribution of genetic variation, isolation by distance and coalescence. Conversely, in anchialine karst and pseudokarst such as lava, genetic structuring may be strong between adjacent areas in the same region (Cape Range Australia, Page et al. 2008; Hawaii, Santos 2006), or negligible within and between islands throughout the Hawaiian archipelago (Russ et al. 2010). Spatial scale has temporal scale consequences in karst. For example, groundwater “ages” with distance along its flowpath and so has different properties, typically being more nearly carbonate saturated with time (distance) and depleted of oxygen by microbial and eukaryote respiration, while excretion changes the chemical and redox conditions. The degree of change will depend in part on the duration of the biological and geochemical impacts (Humphreys 2008), and by the buffering effect reducing the temporal variation in flow rate.
4 Nature of Karst and Karst Hydrogeology
A succinct authoritative account of karst hydrogeology is provided by White (2005), and a fuller account by Ford and Williams (2007).
The development, structure, and hydraulic properties of karst affect the suitability of karst for groundwater fauna and the range of habitats available. Karst is characterized by the presence of a water-filled network of fractures in carbonate rocks (limestones, dolostones, and metacarbonates) but that part of the karst pertinent to groundwater ecology is not necessarily obvious. As water infiltrates to groundwater (saturated zone), it traverses many different pathways with varying velocities and so there are varying degrees of storage in the vadose (unsaturated) zone (Fig. 13.2). This epikarst is the site of significant biodiversity containing short range endemics, especially of micro-crustaceans (Culver et al. 2009; Pipan and Culver 2007a, b; Pipan et al. 2006). Conversely, conduits are known from a depth of more than 1,000 m and deep aquifers may contain rich (Edwards Aquifer, Texas; Longley 1992) or sparse stygobiont faunas (Plaine de Fès, Morocco; Essafi et al. 1998).
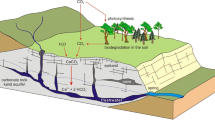
Schematic section of karst showing how the subterranean voids are variously interconnected both internally and externally facilitating or impeding the movement of energy, materials, and organisms. Other subterranean habitats have similar attributes working at different temporal and spatial scales but largely lacking open conduit flow (From Eberhard and Humphreys 2003, reproduced with permission from University of New South Wales Press and S.M. Eberhard)
Although most karst hydrology has been developed from studies in caves and resurgences, there are many areas (e.g., buried karst) where only bores (wells) can provide access for both hydrogeological (Smart and Worthington 2004a) and biological sampling (Humphreys 2001a; Allford et al. 2008). In karst, boreholes typically intercept a few small conduits (<1 cm) that represent fractures that have been greatly enlarged by solution and which can be further enlarged if a bore is used for water extraction (Smart and Worthington 2004b). Poorly maintained or poorly constructed bores can become routes for contamination of the groundwater due to downward leakage of surface water, or upward leakage of groundwater from deeper aquifers, along the wall of the bore. While this can clearly have implications for subterranean fauna, both by mixing separate faunas and changing water quality, bore construction protocols are typically a regulatory issue that the karst manager needs to ensure that they are compliant throughout the catchment of the karst.
It is the dissolution of bedrock (karstification) that results in carbonate rocks becoming productive aquifers (Smart and Worthington 2004a), as well as suitable habitat for a range of stygiofauna with different lifestyles. However, matrix porosity is commonly two orders of magnitude greater than fracture and channel porosity (Smart and Worthington 2004a) and, although the small voids of the matrix make it unimportant as living space for groundwater fauna, the matrix porosity may support stable autochthonous microbial endokarst communities (Farnleitner et al. 2005) thereby affecting water quality. Conversely, seasonality/variation in water level due to temporal effects is important as it allows the deposition of sediments/clays, which are prime sites for biogeochemical activity, and organic carbon which is the basis of food on which stygiofauna ultimately rely (general account: Humphreys 2009). Hydraulic conductivity increases by 101–102 times from matrix to fracture, and from fracture to conduit domains (Worthington 1999). In consequence, the draining of karst following periodic recharge may occur in distinct phases as the different karst domains and pondings unload their water (Fig. 13.3) and this has consequences for the fauna. For example, sampling the fauna flushed from a spring draining an entire karst enabled understanding of the different domains within the karst. The rapid transit of water through open conduits (Mangin 1975) carried with it a groundwater fauna distinct from that found in the long residence time water of the karst matrix (Rouch 1977, 1986), an example whereby stygobionts can also be a tool to understand and monitor attributes of karstic groundwater (Malard et al. 1994; Humphreys 2009; Bonacci et al. 2009). However, recent work has suggested that these distinct phases of drainage may be due to the draining of ponded water behind constrictions in conduits (Eisenlohr et al. 1997; Covington et al. 2009).
Hydrographs showing asynchronous changes in physico-chemical parameters in Stemler Cave, St Clair County, Illinois, over a period of 10 days through a major flood event (17–19 January) (From Taylor and Webb 2000a)
The lack of light in subterranean waters means that primary production is lacking (the “truncated ecosystem” of Gibert and Deharveng 2002) and so energy input is generally imported (allochthonous) from the surface. Consequently, depth below the surface and lateral distance from inflow are issues of importance because they are related to the coupling of the groundwater physicochemical and biotic variables with the surface environment, both in terms of flux and temporal lags. For example, organic carbon flux decreases with depth below the soil surface and with distance along the groundwater flow path (review: Humphreys 2009). The duration of flow within an aquifer affects the chemical composition of the water (e.g., carbonate saturation), and is inversely related to the dissolved oxygen and organic carbon concentration that are utilized by the microbiota and fauna (cf Humphreys 2009). Thus, pertinent information on fauna management may be found in the hydrogeochemistry of karst aquifers, informing on the degree of connectivity of its different parts, the heterogeneity of the aquifer matrix, anisotropy – the time course of recharge and discharge events, and the chemical evolution of the groundwater.
5 Where Groundwater Fauna Occur in Karst
Understanding of the integration of biological and hydrological processes in karst, sometimes termed ecohydrology, is at an early stage, but Bonacci et al. (2009) provide background discussion and a framework for progress, a subject that overlaps with hydrogeoecology, an emerging discipline that encompasses all groundwater (Hancock and Boulton 2009). Karst aquatic biodiversity is partitioned between a number of different major habitats that may need to be managed separately. The cave streams and lakes, that are the dominant feature in many caves and the focus of most work on karst stygiofauna, may contain only a fraction of the fauna, both in terms of numbers of species and numbers of individuals. Most of the void space in karst is of dimensions far too small to access by people and in these areas, probably most of the fauna live – variously termed crevicular or mesocavernous voids (Fišer and Zagmajster 2009) –, but there are also rarer elements such as gour (rimstone) pools, permanent sheet flow down vertical walls (cave hygropetric, Sket 2004), meiofauna within sediments (Giere 2009) within the karst, and drips from epikarst, and others (Culver and Pipan 2009). They have been variously sampled by hand collecting, netting resurgences, boreholes or epikarst, or by baiting to attract such fauna to larger voids (sampling methods, Camacho 1992).
Although diverse stygal communities do occur in some deep confined aquifers, stygobites are most abundant and speciose in shallow unconfined aquifers. Gibert (1986) studied the entire 10.5 km2 Dorvan-Cleyzieu karst drainage basin in the Jura Mountains, eastern France from where water drains primarily through the Grotte du Pissoir. She determined the evapotranspiration, runoff, and infiltration to derive the hydrological budget for the entire karst. Most carbon infiltrating the epikarst as dissolved organic carbon (DOC) rather than particulate carbon (POC), a result that is in accord with the later work of Simon et al. (2007). Although large numbers of animals enter karst from the surface (Rouch 1991), or via the epikarst (Pipan 2005), and carbon also enters the karst water as drift in cave streams, their combined total is small compared with DOC. Despite the great insights such a study provides on the functioning of the karst system, this study is still unique. This demonstrates the importance of connectivity (Lindenmayer et al. 2008) with the surface, and elsewhere, as a prime issue in karst management.
6 Threats to Karst Groundwater Fauna
Small scale endemism places stygobionts at risk through stochastic processes (Sket 1999) and from single land use changes. It is difficult to assess the degree of threat to groundwater fauna generally because in most places there is, or has been until recently, a lack of information on subterranean systems. Lack of information is highlighted in Australia where at least 750 species of groundwater fauna were found mostly in the 10 years to 2008, and largely in areas where no stygiofauna had previously been recorded (Humphreys 2008). There are, nonetheless, records of species loss and it is certain that local populations of invertebrates, fishes, and salamanders have been extirpated and some species probably lost (Veni 1987; Elliott 1993, 1994). Since some species are endemic to a single cave or a small cluster of caves, and many caves have been disturbed, filled, quarried, mined, submerged, drained, or polluted (Table 13.2), it is probable that some species have disappeared recently without our knowledge (Lewis 1996). Culver and Pipan (2009:10.4) provide many examples of threats to subterranean faunas in general (Fig. 13.4).
Schematic diagram of a karst system (1), with an enlargement (2) of a section of a subterranean stream (18), depicting some routes of connectivity that may be managed to protect and or restore invertebrate communities. A stream flows onto the karst (3) from non-limestone region (allogenic) and descends underground at a sinkhole (4) forming a subterranean stream of river (5) which leaves the karst system via a vauclusian spring (6). The characteristics of the allogenic water is determined by the geology and land use in the catchment (7), and this is modified by passage through the karst and allogenic recharge (9) that together determine suitability for subsequent users, including human (8). Nutrients and pollutants in rain (9) and from arable and pastoral cropping (10) percolate to the groundwater through the thin soil cover typical of karst, or more directly from septic systems and sewage injection (11). Plantations increase evapotranspiration by intercepting rainfall and draw water from shallow groundwater (12). Deep roots may penetrate up to 50 m depth drawing on water, and providing carbon to the subterranean fauna by root growth and sap flow (13), sometime forming mats at the water surface providing habitat (19). The underground river conduit (5) passes below and above the regional water table (14) which is freshwater but overlies a saline layer (green). The saline layer is up-coning through the Ghyben-Herzberg effect in the downstream regions as a result of overdrawing of water from the aquifer (15), or as a result of seawater intrusion from the coast (not depicted). Upward and downward meanders in the river conduit serve as traps for high density (16) and low density non-aqueous phase liquids (17) that can consequently accumulate within the karst system from both allogenic and autogenic sources and smother sediments and conduit walls, and the organisms, including biofilm. Within a small part of the karst (18) enlarged (2) the underground river (5) flows through a conduit cut through the matrix of the limestone (24) which forms the majority of the water storage capacity of the karst and which connects with the conduit through fractures (23) which also provide access for roots (13). The sediments and gravel banks (22), both below the water and periodically flooded, provide habitat for fauna and substrate for biofilms, and may be destabilised through trampling by people. Some fauna is specialised to inhabit sheet water flows on walls, (20) or amongst the floor speleothems (cave formations). Dripping stalactites (21) provide effective sampling sites for epikarst fauna, and stalagmites growth using palaeoclimate methods provides information on the history of the cave and local climate, sometimes into deep history. For geochemical aspects refer to Fig. 3 in Humphreys (2009)
Poulson (1968) attributed the present rarity of the cave fish Amblyopsis spelaea in the Mammoth Cave System to silting and flooding associated with deforestation, forest fires, and water engineering projects. Groundwater fauna are largely threatened by changes in water level (Longley 1992; Rouch et al. 1993) and water quality, the removal of matrix (Humphreys 2009), and sedimentation smothering surfaces (Eberhard 1999; Hamilton-Smith and Eberhard 2000), and clogging voids although the activity of stygobionts may counteract this (Nogaro et al. 2006).
6.1 Pollution
There are many texts dealing with the issue of karst contamination (e.g., introductory – Schindel et al. 2004; major text – Fetter 2001; dispersed – Boyer 2004; point source – Schindel and Hoyt 2004; remediation – Schindel et al. 2004; protection – Drew and Dunne 2004; karst contamination in a USA – Elliott 2000; nitrates – Katz 2005; chlorinated solvents – Wolfe and Haugh 2001).
Pollution is the contamination of groundwater with substances not naturally present or changing the concentration of substances naturally present outside the natural range which may be harmful to life. Subterranean waters are typically poor in organic energy and subterranean fauna generally have low metabolic rates and other physiological adaptation to this low energy environment (Coineau 2000). An increase in energy input into the groundwater as a result of pollution (Fig. 13.5) may permit the successful invasion of surface forms into a previously oligotrophic environment (Notenboom et al. 1994; Malard et al. 1994).
Artificial exposure of groundwater ecosystems in groundwater calcrete bodies in Australia. (a), Road quarry with sinkhole development, an unusual feature in calcrete (Mt Padbury Station, WA); (b), Road quarry used for cattle watering, Lake Way Station, WA; c, specially constructed stock (cattle) watering point (Napperby Station, NT) (Photo: W.F. Humphreys). Such opening eliminate stygal habitat by making lakes of groundwater, increase nitrification of groundwater, and increases salinity by enhanced evaporation
While surface waters are more readily ameliorated by photodegradation and by the high oxic conditions, once pollutants enter groundwater, remediation is rarely practicable, typically very difficult and often impossible. Thus, the focus needs to be on prevention of contamination, and this is especially the case in karst environments that are highly vulnerable to pollution from both solid and liquid sources. This arises because karst typically lacks deep soil and from the rapid water flow through a conduit network, produced by karstification, that enables rapid transport of contaminants to the water table with little natural remediation (Ray 2005). In many cases, a diffuse network of conduits, such as those of the Dinaric Karst, makes tracking and prediction of pollution difficult (Sket 2005).
Acid mine drainage can become a pervasive issue for karst management. For example, the Rand goldfields on South Africa are overlain by compartmentalized dolomitic karst that was partly, but widely, drained to enable mining. For over a century, mine workings have penetrated the karst, including the continental drainage divide. When mines are depleted and pumping stops, sulfide rich water rises to the surface, is oxidized, and the resulting acid water, rich in uranium, decant to both sides of the continental drainage, divides and adversely affects both groundwater and surface water quality (Winde 2006).
As outlined by Webb et al. (1994), contaminants in solution, such as salts, acids, and endocrine mimics, will largely follow the dynamics of the water movement and the impact on the fauna will be determined by their physical and chemical properties. Hydrophobic pollutants, especially non-aqueous phase liquids (NAPLs), are a particular concern as they are transported relatively rapidly within the aquifer, depending on groundwater velocity and conduit morphology, where they may smother matrix surfaces, including biofilm. Dense NAPLs sink to the bottom and smother surfaces, mix with the benthos and are incorporated in sediments, whereas low density NAPLs rise to the water surface preventing gaseous exchange and may accumulate in elevated parts of conduits. Consequently, both phases may lead to persistent pollution from a single point source pollution event (Schindel and Hoyt 2004).
Webb et al. (1994) provide a clear exposition of contaminant issues in Illinois in respect of groundwater fauna. Lewis et al. (1983) and Lewis (1996) reconstruct the processes that led to the elimination, as a result of sewage and heavy metal pollution, and the restoration of the subterranean fauna of Hidden River (Horse) Cave, Kentucky, which included Typhlichthys cavefish, Orconectes crayfish, and Caecidotea isopods. In this case, following rehabilitation, the fauna repopulated the cave from relatively unpolluted, upstream tributaries. Such dramatic examples should not mask awareness that even sublethal concentrations of insecticides in water may provide significant environmental stress, as indicated by fluctuating asymmetry on larval development (Chang et al. 2009) and behavioral abnormalities (Sandahl et al. 2004).
Toxicological studies of groundwater fauna are problematic as many stygobionts are naturally rare and are difficult to maintain in the laboratory. This has prompted the use of proxies in an attempt to address this problem (Hose 2005), but not without dissent (Humphreys 2007; Hose 2007). Meiofauna population densities are substantially greater than the mesofauna that is typically used in studies and offer potential to allow statistically robust toxicological experiments on groundwater species. However, as with other faunal categories, meiofaunal elements are differentially affected by pollutants, for example, atrazine was lethal to about 70% of several species of harpacticoid copepods but barely affected nematodes (Bejarano et al. 2005).
Special mention should be made of anchialine systems – near coastal groundwater affected by marine tides but lacking surface connection with the sea, typically markedly stratified with freshwater overlying seawater – not least because they are home to a remarkable diversity of higher taxa, especially crustaceans (Fig. 13.1), globally confined to this ecosystem and considered to be tethyan in distribution (Sket 1996). The most spectacular systems are in the karst platforms of the Bahamas Banks (Daenekas et al. 2009), and on the Yucatán peninsula, Mexico (Iliffe 1993; Iliffe and Bishop 2007) where a ring of cenotes surrounds the Chicxulub meteorite impact site – that arguably caused the end Cretaceous mass extinction – interconnected by caves including the Ox Bel Ha system (180 km). Although they may be extreme environments (Sket 1986; Humphreys 2001b), anchialine systems are considered especially vulnerable to pollution (Iliffe et al. 1984), those in Quintana Roo being polluted by sewage disposal by injection into the seawater underlying the freshwater layer (Beddows 2004).
7 Anthropogenic Changes to Water Regime
Although karst aquifers are a major source of water for human use, little is known about the impacts of water abstraction on ecosystems within aquifers (Rouch et al. 1993), especially deep aquifers (Longley 1992). Rouch et al. (1993) conducted a high discharge pumping test in a sinkhole to investigate its effect on the movement of stygiofauna out of the saturated zone of the Baget karst (Ariège, France). The water in the sinkhole was lowered by 21 m on three occasions over 4 days, and as a result, the micro-crustacean drift (mainly harpacticoid copepods) from the karst increased and the site had not recovered after 1 year. The diverse groundwater fauna of the Edwards Aquifer, Texas, which includes both vertebrate and invertebrate stygobites with both marine and freshwater affinities, is under threat from over-extraction of water which remove almost all of the natural recharge. The adverse effects are caused by loss of spring flow, dewatering of parts of the karst, and saltwater intrusion (Longley 1992). The Texas blind salamander, Typhlomolge rathbuni, inhabits the artesian part of the aquifer and is threatened by over-pumping (Elliott 2000). The use of Valdina Farms Sinkhole, Texas, as a recharge well for the Edwards Underground Water District appears to have extirpated the only known population of the salamander, Eurycea troglodytes, which is now probably extinct (Elliott 2000).
Owing to growing human demand on water resources, to enhance water storage, natural or treated waters are increasingly being used to artificially recharge aquifers but the effects on subterranean fauna are seldom studied. While this may seem a slight insult compared to industrial waste disposal, the impacts may be substantial. Injection in a sinkhole of 12,000 m3 day−1 of treated wastewater water had marked effects on the occurrence of stygobiont species in fractured limestone in Nardò (southern Italy). In the Castro subregion alone, seven stygobionts, eight stygophile, and eight stygoxene species were not collected after the injection of reclaimed water started in 1991. However, the response differed between species and some omnivores, such as the stygobiont mysid Spelaeomysis bottazzii, were favored (Masciopinto et al. 2006).
Floods, and other large scale disturbances, can be important drivers of ecosystem and landscape processes (Lindenmayer et al. 2008). Increases in water level may also affect karst systems, the most overt being impoundments which may change the hydrodynamics and sediment transport within the karst as well as changing the water levels. Impoundments are commonly on the surface, but there is an increasing promotion and use of subterranean impoundments (Mengxiong 1987; Milanović 2004a) to utilize the storage capacity of karst areas while preserving surface utility and, important in hotter and arid climates, where the water is not lot by evaporation. Impoundments can have negative effect on both surface and subsurface water regimes. The plugging of river beds and ponors with cement to allow impoundments endangered the Olm, Proteus anguinus, by blocking connections between karst channels and surface, and it was also at rick by flushing during reservoir operations (Milanović 2004b).
Mammoth Cave, Kentucky, at 591 km, the world’s longest cave system, has been well documented ecologically. The Green River, which naturally back-flooded the cave, was dammed in 1906 resulting in higher than natural flooding of the cave. Further impoundments in the 1970s reduced flood height but extended the period of flooding. These changes have had wide ranging ecological consequences with documented changes to Palaemonias ganteri (Kentucky blind shrimp), the cavefish Amblysoma spelaea, the crayfish Orconectes pellucidus, and two species of Caecidotea isopods, probably as a result of siltation, rather than the increase in toxins or organic enrichment (Poulson 1996). This detailed study may have more general implications because global climate change models on some aquifer systems predict changes in the amount of rainfall and in the seasonality of floods and low water (Scibek et al. 2008).
Changes, such as urbanization, that increase the magnitude or intensity of flood peaks in cave streams, may increase scouring of sediments. Wicks et al. (2010) found that the critically endangered pink planarian, Macrocotyla glandulosa, in Devils Icebox cave system, Missouri, occurred mainly in areas where numerical simulation of flow indicated stable sediment not prone to scouring.
8 Cave Visitors
Globally, caves and hot springs within caves, cenotes, and anchialine passages are visited by people for research, recreation, medical, spiritual needs, and mass tourism. Although they impact on a minor part of karst systems, they can have detrimental impacts on stygiofauna and each would require impact specific management. For example, the mass tourism in Waitomo Caves is renowned for the spectacular light display of the New Zealand Glowworm (Pugsley 1984; Broadley and Stringer 2002), and metal pollution from coins in the tourist section of the La Corona lava tube, Lanzarote, Canary Islands (Iliffe and Bishop 2007). The Yucatán is also a major centre for cave diving tourism and marine fish follow divers’ lights into the caves and eat the anchialine fauna. This can be prevented by the simple expedient of divers turning off their lights while entering the cave from the sea.
Sediment banks are important locations of fauna in cave waters as they are areas of deposition and therefore of concentrations of organic matter which nourishes the biofilm (Dickson 1979) on which the fauna depend. Cavers following stream passages through caves may trample sediment banks, destabilize them, mix the contents, and disrupt the fauna. Caving protocols may reduce such impacts generally, and route marking and/or closures to entry of significant passages may protect them.
9 Inventory
A major impediment to protection and management of subterranean fauna is lack of knowledge of their current status, even of their presence – see the above example of Western Australia, a highly mineraliferous region, where a rich subterranean fauna occurred unknown until recently – exacerbated in some regions by a culture, now largely past, of secrecy amongst speleologists. However, in some places, this may be replaced by consultants who may retain information to protect some perceived advantage. A prime requirement for the management and conservation of cave fauna and the ecosystems in which they occur is an inventory to permit mapping of the distribution of the fauna in space and time to determine the species richness and assessment of their ecological status and vulnerability (Schneider and Culver 2004). This argument, generally applicable, is especially pertinent to karst subterranean aquatic systems which have exceptionally demanding requirements owing to difficulties of access, the hydrogeological complexities of the karst milieu, the perceived high level of vulnerability of the fauna (Box 13.1), and the exceptional degree of species endemism.
Inventory can occur at many levels of detail largely reflecting the financial and human resources available; a detailed study is shown in Fig. 13.6 and Box 13.2 in relation to the habitat of an endangered species. While major resources for long-term inventory of fauna may be available to inform the management of karst aquatic fauna in affluent and karst aware jurisdictions, this is a minority position. Even in industrial countries, the sampling for inventory work is often largely the province of capable amateur speleologists, with professionals involved only for specialist identification, and taxonomic and systematic development. In many, perhaps most, parts of the world, inventory, if at all, is elementary and the province of intermittent amateur and perhaps professional speleological expeditions is often associated with institutes devoted to taxonomy and systematics.
Average number of animals per m2 (N = 7 samples/month/cave) for all taxa in cave stream substrate of Fogelpole Cave, Monroe County, Illinois. The four identified Amphipoda are Gammarus troglophilus, G. acherondytes, Crangonyx forbesi and Bactrurus brachycaudus (From Taylor and Webb 2000b, with permission)
Nonetheless, there are useful guidelines to follow whatever level of inventory is possible for a given karst. This may range from a one-off collection establishing a minimum species occurrence baseline, through to the routine monitoring of the distribution and abundance of the cave fauna that will permit the detection of subtle changes in conditions and allow for the possibility of remedial action in the face of adversity. Hence, a basic requirement of management is to have an inventory of spatial information on the fauna and, as a minimum, to record what is where, when, in what, how and by whom were the data gathered. Each category can be expanded as human and financial resources and knowledge improvement (Table 13.3). A specimen progressively becomes a formally described species located in a phylogeny and placed in an historical biogeographic context. The aquatic habitat can later be expanded to include the hydrological and physicochemical properties of the water and its variation. An individual’s field notes may progressively develop to become a compilation of multivariate data through time in a GIS system. But it is the minimum level of data that provides an essential baseline against which to start to make management assessment. This is often lacking for specific areas and even across major regions before profound changes to the landscape were made, for example in Australia (Hamilton-Smith and Eberhard 2000) and North America (Elliott 2000: 685). There are few areas of the world where subterranean fauna are a specific requirement for environmental impact assessments; Western Australia is one among them (EPA 2003). Although US research infers for management that groundwater quality is best protected by protecting the ecosystem (Job and Simons 1994), only in Switzerland has the maintenance of functioning aquifer ecosystems enshrined in ordinance (GSchV 1998).
Generally, most species cannot be identified from field photographs and so specimen collection, and arrangement for their long term preservation, storage, and data base, is an essential corollary of inventory work. The long term storage, particularly of the type material from which new species have been described, is best handled by institutions maintained to manage and research fauna collections, typically state museums – voucher material to aid local identification can be maintained at the laboratories involved with research in a particular karst region. Although sparsely utilized, taxonomic expertise is essential in all ecological studies (Bortolus 2008), but is especially important where high β-diversity results from the very short range endemism characteristic of subterranean fauna. Once the fauna of a particular cave or karst is well characterized, then conservation strategies can be supported by non-lethal life history sampling (Venarsky et al. 2007).
Data derived from karst fauna management are essentially field data ultimately derived from all scales of biological organization, spanning orders of magnitude of temporal and spatial scale, and which is very heterogeneous in format and content. The protocols established for the Resource Discovery Initiative for Field Station (RDIFS) in the USA (Brunt and Michener 2009) provide a valuable framework on which to establish both a karst specific data collection and management, and the means of intergenerational and between karst region sharing of research and training and management information to better monitor their own responsibility and to establish a broad research base on which to undertake the management of karst aquatic fauna. RDIFS is focused on enabling cross jurisdictional exchange of data by promoting consistent terminology, standard field methods, and quality assurance and control of data.
10 Management in Faunistic Ignorance
Although the subterranean biodiversity warrants protection in its own right and this is best achieved by the application of ecology to cave and karst management (Whitten 2009), the protection of the quality and quantity of human water supply provides the strongest avenue indirectly to aid the protection of stygiofauna. Real protection of karst waters depends on protection of the entire catchment and so, as mentioned above, landscape management may need to extend beyond the jurisdiction of the karst manager (Jones et al. 2003: 36). At its most basic, but rarely possible, the area and the catchment can be maintained in its original, unaltered state. However, global fallout of anthropogenic products negates pristine areas especially as even sub-lethal concentrations involved may induce developmental (Chang et al. 2009) and behavioral abnormalities (Sandahl et al. 2004). Boulton (2009) considered that the “biggest challenge as aquatic conservationists is to increase (and sustain) public and political awareness of the importance of groundwaters and GDEs, how they are threatened, and the need for applied research on groundwater processes and response functions to help managers assess groundwater resource use.” At the local level, effective engagement by the karst manager with those responsible for water quality for human consumption is advocated, as they often have authority over the water and are better resourced for effective action if they are persuaded of the synergy between water quality and aquatic ecosystems. Such linkage could be explored between the studies of Masciopinto et al. (2006) and Masciopinto et al. (2007) of the Salento peninsula, southern Italy.
11 Education and Protection
Danielopol and Pospisil (2004) emphasized reasons for protection of groundwater fauna that are useful in educational programs for the protection of karst, including scientific, moral, and practical economic arguments to protect groundwater organisms and prevent deterioration of the environment. Management of karst groundwater can be implemented only if the occupiers of the land, both within the karst and the upstream catchment, and visitors, are made aware of the properties of karst systems that make their groundwater fauna particularly vulnerable to anthropogenic disturbance. In consequence, any management of stygiofauna will, of necessity, be part of an integrated and sustained educational effort of comprehensive karst management, a topic that is extensively developed elsewhere (Watson et al. 1997; Tercafs 2001). “A major emphasis on education is crucial if we are to effectively implement changes in land-use practices associated with urbanization and agricultural activities in..... [southwestern Illinois], and changes that help sustain the aquatic cave community will also improve the quality of life for..... residents in this karst area” (Taylor and Webb 2000a). The value of cave species to the public, often, is considered low, but cave species have potential scientific, practical, and educational value. For example, they may serve as “indicator species” in karst areas as a natural alarm for regulators and public health agencies to groundwater contamination, and they may provide ecosystem services by contributing to maintaining groundwater quality or aquifer porosity. Interestingly, a survey showed that visitors were willing to pay an increased entry fee (ca. 30%) to Yanchep National Park, Western Australia, to support a recovery plan for a root mat ecosystem containing a cryptic fauna threatened by groundwater drawdown (Perriam et al. 2008).
There is a substantial literature on the legislative approach to species and community protection, that includes subterranean fauna, in many countries (e.g., Elliott 2000; Hamilton-Smith and Eberhard 2000; Juberthie 2000) and this is reflective of the increased consideration for groundwater protection to maintain biodiversity (Danielopol et al. 2004). Although groundwater fauna are included in various international treaties and conventions, aquifer ecosystems, generally, are specifically protected at a national scale only in Switzerland by the Water Protection Ordinance that defines both water quality standards and ecological goals, “groundwater biocenosis [ecosystem] should be in a natural state adapted to the habitat and characteristics of water that is not or only slightly polluted” (GSchV 1998).
Awareness of the vulnerability of species and communities may be raised by inclusion of sites in listing of threatened fauna at local, national, and international levels, such as a Ramsar site (Beltram 2004) or Redbook listing (IUCN 2008). The listing of species under national endangered species legislation typically requires a level of scientific knowledge that is unavailable for rare subterranean species (but see Box 13.2). Where possible, despite the rather haphazard and arbitrary listing processes, listed species may protect associated unlisted species by proxy (Elliott 1990), particularly pertinent to karst groundwaters that often contain short range endemic species.
12 Some Management Actions
Alteration of land use practice is probably one of the principle factors affecting subterranean waters in karst because changes to or removal of vegetation changes water quality and flow regimes. The increased water yield exacerbates flooding in caves, while the more rapid run-off from cleared land increases peak discharge to cave streams and so previously permanent streams may become periodic. Four short examples of management actions on stygiofauna are given here, all from Australia.
-
(1)
In south-western Australia, root mats develop in water table caves and they and the associated fungi are grazed by a rich stygiofauna (Jasinska and Knott 2000), and these root mat communities are listed under both Western Australian and Australian Commonwealth fauna protection legislation. Groundwater levels are declining, at Yanchep, north of Perth, as a result of excessive water extraction from the Gnangara Mound aquifer (Perriam et al. 2008), and, at Jewel Cave, Augusta, from unknown causes, hypothesized to be due to reduced rainfall infiltration as a result of increased understorey growth in the karri forest resulting from a change in fire frequency (Eberhard 2004). Stranded root mats and associated invertebrate communities are dying and, considerable efforts have been made to maintain artificially from bores the water supply at Yanchep, but with poor success, owing to iron rich water (Venn 2008).
-
(2)
The operations of a limestone quarry at Ida Bay, Tasmania, caused adverse impacts on aquatic cave fauna as a result of sedimentation, eutrophication, and toxins that resulted in the extinction of fauna in Chesterman Cave (Eberhard 1995). In Exit Cave, the sedimentation restricted the distribution of hydrobiid snails, and while the quarry was operational, snail abundance was significantly lower in sediment affected streams than in control streams. A rehabilitation program was initiated after closure of the quarry to prevent further environmental degradation by restoring natural in-flow regimes and limiting further influx of sediment. Subsequently, snail populations were similar in control and sediment affected sites (Eberhard 1999).
-
(3)
Barrow Island comprises a low limestone anticline on the shallow North West Shelf, Australia, and was declared an A-class reserve in 1910, the highest level of conservation protection in Western Australia. Since 1961, it has been a production oilfield requiring disposal of ever increasing volumes of oil contaminated “produced water”. From 1968–1979 and 1987–1994, produced water was discharged nto superficial karst to “B Block caves” and “F Block caves” respectively. In 1996 alone, this amounted to 2.3 × 106 m3 of hypersaline water (40–45 g L−1 TDS) disposed with an residual oil content of 100–2,000 ppm (Wapet 1996), that is between 230 and 4,600 m3 of oil. Neither the anchialine nature of the ecosystem (Humphreys 2001b), nor the existence of a high conservation value groundwater fauna were recognized until the 1990s (Humphreys 1993b) and now known to include endemic species of crustaceans and fish. Although disposal of produced water in the superficial karst was subsequently stopped, in favor of disposal to deeper geological formations, the lack of pre-impact surveys of subterranean fauna precludes complete assessment of any impact of oil field operations on the original stygiofauna of this A-class reserve.
-
(4)
Cryptic species generally are increasingly being exposed with the aid of molecular methods (Bickford et al. 2007; Bradford et al. 2009). It is commonly found that isolated populations of stygiofauna (Fig. 13.7), especially amongst the various amphipod families, show deep phylogenetic divergences based on DNA although they cannot easily be separated using morphological criteria (Finston et al. 2004, 2007; Cooper et al. 2007). The discovery of stygobiont fauna in a groundwater calcrete deposit near a new iron ore mine, Ore Body 23, at Newman, Western Australia, resulted in delay in commissioning of the mine. The initial diversity assessment was made on stygiobiont paramelitid amphipods – a group that has proven to be intractable to morphological study (Cooper et al. 2007) – which indicated 14 species of amphipod were present (Bradbury 2000), making it a global hotspot for amphipods. Molecular analysis was eventually commissioned which suggested a much lower diversity of amphipods (Finston and Johnson 2004; Finston et al. 2004), although the local endemism of other stygobiontic taxa was ultimately found (Karanovic 2006; Finston et al. 2007; Reeves et al. 2007). Subterranean fauna was previously unknown from this region, but it was later recognized to contain a globally significant subterranean biodiversity (Humphreys 2008); procedures introduced to ensure subterranean fauna were included in the environmental assessment process (EPA 2003), and a broad scale regional assessment of the stygiofauna was undertaken by the fauna authority (Eberhard et al. 2009; Karanovic 2006, 2007).
13 Conclusions
There are about 126,000 described freshwater animal species representing 9.5% of the total number of animal species recognized globally. As surface freshwaters cover only about 0.01% of the total surface of the globe, freshwater ecosystems support a disproportionately large fraction of the world’s total biodiversity (Balian et al. 2008b) that is disproportionately threatened (37% of freshwater fish species are threatened; IUCN 2009). Despite increased awareness of inland waters in many regions and innumerable restoration projects, the biodiversity and biological resources of inland waters face a major crisis related to water resource integrity that is linked with the essential ecosystem services provided by aquatic ecosystems (Balian et al. 2008a). Freshwater systems are being destroyed at an increasing rate as a result of extraction of water for domestic use, for industry and irrigation, by draining and infilling, and by gross contamination by industrial and agrochemical pollutants and salination. Constrained in distribution by its milieu, freshwater fauna globally is consequently under immense pressure. While these influences are apparent in surface waters, they are well hidden in groundwater which comprises 97% of all liquid freshwater resources on earth (L’Volich 1974) and about 25% of the world’s population is supplied largely or entirely by karst waters (Ford and Williams 2007).
Knowledge of the biodiversity within all types of groundwater is rapidly increasing and untangling the ecosystem services it provides has commenced (Boulton et al. 2008; Hancock and Boulton 2009); but the task is challenging and new challenges emerge, such as climate change. Karst systems provide the more dynamic and accessible groundwater systems but also those most readily contaminated and through which contaminants can most rapidly spread. Maintenance of the biodiversity within karst waters is the default option in the absence of empirical evidence of what constitutes key components of these simplified ecosystems.
References
Adams M, Humphreys WF (1993) Patterns of genetic diversity within selected subterranean fauna of the Cape Range peninsula, Western Australia: systematic and biogeographic implications. In: Humphreys WF (ed.), The biogeography of Cape Range, Western Australia. Rec West Aust Mus, Suppl 45: 145–164
Allford A, Cooper SJB, Humphreys WF et al (2008) The ecology and distribution of groundwater fauna in a limestone aquifer: does sampling alter the story? Invert Syst 22:127–138
Austin AD, Cooper SJB, Humphreys WF (eds.) (2008) Subterranean connections: biology and evolution in troglobiont and groundwater ecosystems. Invert Syst 22:85–310
Balian EV, Lévêque C, Segers H et al (eds.) (2008a) Freshwater animal diversity assessment. Hydrobiologia 595:1–637
Balian EV, Lévêque C, Segers H et al (2008b) The freshwater animal diversity assessment: an overview of the results. Hydrobiologia 595:627–637
Beddows PA (2004) Yucatán phreas, Mexico. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 786–788
Bejarano AC, Pennington PL, DeLorenzo ME et al (2005) Atrazine effects on meiobenthic assemblages of a modular estuarine mesocosm. Mar Pollut Bull 50:1398–1404
Beltram G (2004) Ramsar sites – wetlands of international importance. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 619–621
Bickford D, Lohman DJ, Sodhi NS et al (2007) Cryptic species as a window on diversity and conservation. Trends Ecol Evol 22:148–155
Bjarni K, Kristjánsson BK, Svavarsson J (2007) Subglacial refugia in Iceland enabled groundwater amphipods to survive glaciations. Am Nat 170:292–296
Bonacci O, Pipan T, Culver D (2009) A framework for karst ecohydrology. Environ Geol 56:891–900
Bortolus A (2008) Error cascades in the biological sciences: the unwanted consequences of using bad taxonomy in ecology. Ambio 37:114–118
Botosaneanu L (ed.) (1986) Stygofauna mundi: a faunistic, distributional, and ecological synthesis of the world fauna inhabiting subterranean waters (including the marine interstitial). EJ Brill, Leiden
Botosaneanu L (1987) A new thalassostygobiont species of Cyathura (Isopoda: Anthuridea) from the South-East Pacific. Stygologia 3:296–304
Boulton AJ (2001) ‘Twixt two worlds: taxonomic and function biodiversity at the surface water/groundwater interface. Rec West Aust Mus Supp 64:1–13
Boulton AJ (2009) Recent progress in the conservation of groundwaters and their dependent ecosystems. Aquat Conserv Mar Freshw Ecosys 19:731–735
Boulton AJ, Fenwick GD, Hancock PJ et al (2008) Biodiversity, functional roles and ecosystem services of groundwater invertebrates. Invert Syst 22:103–116
Boyer DG (2004) Groundwater pollution: dispersed. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 403–404
Bradbury JH (2000) Western Australian stygobiont amphipods (Crustacea: Paramelitidae) from the Mt Newman and Millstream regions. Rec West Aust Mus, Suppl No. 60:1–102
Bradford T, Adams M, Humphreys WF et al (2009) DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone. Mol Ecol Resour. doi:10.1111/j.1755-0998.2009.02706.x
Bradford T, Adams M, Humphreys WF, Austin AD, Cooper SJB (2010) DNA barcoding of stygofauna uncovers cryptic amphipod diversity in a calcrete aquifer in Western Australia’s arid zone. Molecular Ecology Resources 10:41–50
Broadley RA, Stringer IAN (2002) Prey attraction by larvae of the New Zealand glowworm Arachnocampa luminosa (Diptera: Mycetophilidae). Invert Biol 120:170–177
Brunt JW, Michener WK (2009) The resource discovery initiative for field stations: enhancing data management at North American biological field stations. BioSci 59:482–487
Camacho AI (ed.) (1992) The natural history of biospeleology. Mus Nac Cienc Natur, Madrid
Carlini DB, Manning J, Sullivan PG et al (2009) Molecular genetic variation and population structure in morphologically differentiated cave and surface populations of the freshwater amphipod Gammarus minus. Mol Ecol 18:1932–1945
Chang X, Zhai B, Wang B et al (2009) Effects of the mixture of avermectin and imidacloprid on mortality and developmental stability of Copera annulata (Odonata: Zygoptera) larvae. Biol J Linn Soc 96:44–50
Chapelle FH (2001) Ground-water microbiology and geochemistry, 2nd edn. Wiley, New York
Coineau N (2000) Adaptations to interstitial groundwater life. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 189–210
Coineau N (2001) Syncarida. In: Juberthie C, Decu V (eds.) Encyclopaedia biospeologica. Societé de Biospéologia and Académie Roumanie. Moulis (CNRS)/Bucharest, Romania, pp 863–876
Cooper SJB, Bradbury JH, Saint KM et al (2007) Subterranean archipelago in the Australian arid zone: mitochondrial DNA phylogeography of amphipods from central Western Australia. Mol Ecol 16:1533–1544
Cooper SJB, Saint KM, Taiti S et al (2008) Subterranean archipelago II: mitochondrial DNA phylogeography of stygobitic isopods (Oniscidea: Haloniscus) from the Yilgarn region of Western Australia. Invert Syst 22:195–206
Covington MD, Wicks CM, Saar MO (2009) A dimensionless number describing the effects of recharge and geometry on discharge from simple karstic aquifers. Water Resour Res 45:W11410. doi:10.1029/2009WR008004
Culver DC, Pipan T (2009) The biology of caves and other subterranean habitats. Oxford University Press, Oxford
Culver D, Pipan T, Schneider K (2009) Vicariance, dispersal and scale in the aquatic subterranean fauna of karst regions. Freshw Biol 54:918–929
Culver DC, Sket B (2000) Hotspots of subterranean biodiversity in caves and wells. J Cave Karst Stud 62(1):11–17
Culver D, White W (eds.) (2005) Encyclopedia of caves. Academic, San Diego
Culver DC, Master LL, Christman MC et al (2000) Obligate cave fauna of the 48 contiguous United States. Conserv Biol 14:386–401
Culver DC, Deharveng L, Gibert J et al (eds.) (2001) Mapping subterranean biodiversity. Cartographie de la biodiversité souterraine. In: Proceedings of the international workshop held 18–20 Mar 2001, Laboratoire Souterrain du CNRS, Moulis, Ariège, France. Karst Waters Institute, Petersburg
Culver DC, Christman MC, Elliott WR et al (2003) The North American obligate cave fauna: regional patterns. Biodivers Conserv 12:441–468
Culver DC, Christman MC, Sket B et al (2004) Sampling adequacy in an extreme environment: species richness patterns in Slovenian caves. Biodivers Conserv 13:1209–1229
Culver DC, Pipan T, Schneider K (2007) Vicariance, dispersal and scale in the aquatic subterranean fauna of karst regions. Freshw Biol 54:918–929
Daenekas J, Iliffe TM, Yager J et al (2009) Speleonectes kakuki, a new species of Remipedia (Crustacea) from anchialine and sub-seafloor caves on Andros and Cat Island, Bahamas. Zootaxa 2016:51–66
Danielopol DL (1998) Conservation and protection of the biota of karst: assimilation of scientific ideas through artistic perception. J Cave Karst Res 60:67
Danielopol DL, Pospisil P (2004) Why and how to take care of subterranean aquatic microcrustaceans? In: Gibert J (ed.) World subterranean biodiversity. Proceedings of an international symposium, 8–10 Dec 2004. Laboratoire des Hydrosystèmes Fluviaux, Villeurbanne, pp 29–35
Danielopol DL, Pospisil P, Rouch R (2000) Biodiversity in groundwater: a large scale view. Trends Ecol Evol 15:223–224
Danielopol DL, Gibert J, Griebler C et al (2004) Incorporating ecological perspectives in European groundwater management policy. Environ Conserv 31:185–189
Deharveng L, Bedos A (2000) The cave fauna of Southeast Asia: origin, evolution and ecology. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 603–632
Dickson GW (1979) The importance of cave mud sediments in food preference, growth and mortality of the troglobitic amphipod Crustacean Crangonyx antennatus packard (Crangonyctidae). Crustaceana 36:129–140
Dole-Olivier M-J, Castellarini F, Coineau N et al (2009) Towards an optimal sampling strategy to assess groundwater biodiversity: comparison across six European regions. Freshw Biol 54:777–796
Drew DP, Dunne S (2004)Vulnerability mapping for the protection of karst aquifers. Environment Agency England and Wales, Bristol, R & D Technical Report W6-032/TR, 99 pp
Eberhard SM (1995) Impact of a limestone quarry on aquatic cave fauna at Ida Bay in Tasmania In: ‘Proceeding of the 11th Australian Cave and Karst Management Association conference, Tasmania, May 1995, pp 125–137
Eberhard S (1999) Cave fauna management and monitoring at Ida Bay, Tasmania. Nature Conservation Report 99/1. Parks & Wildlife Service, Tasmania, pp 1–37
Eberhard SM (2004) Ecology and hydrology of a threatened groundwater-dependent ecosystem: the Jewel Cave karst system in Western Australia. PhD thesis, Murdoch University, Perth
Eberhard SM, Halse SA, Williams MR et al (2009) Exploring the relationship between sampling efficiency and short-range endemism for groundwater fauna in the Pilbara region, Western Australia. Freshw Biol 54:885–901
Eberhard SM, Humphreys WF (2003) The crawling, creeping and swimming life of caves. In Finlayson B, Hamilton-Smith E (ed.) Australia Underground: A Tribute to Joe Jennings. Sydney: University of New South Wales Press
Eisenlohr L, Király L, Bouzelboudjen M, Rossier Y (1997) Numerical simulation as a tool for checking the interpretation of karst spring hydrographs. J Hydrol 193:306–315
Elliott WR (1990) Endangered species, endangered caves. Nat Speleological Soc News 48:225–231
Elliott WR (1993) Cave fauna conservation in Texas. In: Foster, DL (ed.) Proceeding of the National cave Management symposium, Bowling Green, 1991. American Cave Consv Assoc, Horse Cave, pp. 323–337
Elliott WR (1994) Conservation of Texas caves and karst. In: Elliott WR, Veni G (eds.) The caves and karst of Texas, Convention guidebook. National Speleological Society, Huntsville, pp 85–97
Elliott WR (2000) Conservation of the North American cave and karst biota. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 665–689
Engel AS (2005) Chemoautotrophy. In: Culver D, White W (eds.) Encyclopedia of caves. Academic, San Diego, pp 90–102
EPA (2003) Consideration of subterranean fauna in groundwater and caves during environmental impact assessment in Western Australia. Guidance Statement No. 54. Environmental Protection Authority, Perth
Essafi K, Mathieu J, Berrady I et al (1998) Qualité de l’eau et de la faune au niveau de forages artésiens dans la Plaine de Fès et la Plaine des Beni-Sadden. Premiers résultats. Mém Biospéol 25:157–166
Farnleitner AH, Wilhartitz I, Ryzinska G et al (2005) Bacterial dynamics in spring water of alpine karst aquifers indicates the presence of stable autochthonous microbial endokarst communities. Env Microbiol 7:1248–1259
Ferreira D, Malard F, Dole-Olivier MJ et al (2007) Obligate groundwater fauna of France: diversity patterns and conservation implications. Biodiv Conserv 16:567–596
Fetter CW (2001) Contaminant hydrogeology, 4th edn. Prentice Hall, Upper Saddle River
Finston TL, Johnson MS (2004) Geographic patterns of genetic diversity in subterranean amphipods of the Pilbara, Western Australia. Mar Freshw Res 55:619–628
Finston TL, Bradbury JH, Johnson MS et al (2004) When morphology and molecular markers conflict: a case history of subterranean amphipods from the Pilbara, Western Australia. Anim Biodivers Conserv 27:83–94
Finston TL, Johnson MS, Humphreys WF et al (2007) Cryptic speciation in two widespread subterranean amphipod genera reflects historical drainage patterns in an ancient landscape. Mol Ecol 16:355–365
Finston TL, Francis CJ, Johnson MS (2009) Biogeography of the stygobitic isopod Pygolabis (Malacostraca: Tainisopidae) in the Pilbara, Western Australia: evidence for multiple colonisations of the groundwater. Mol Phylogenet Evol 52:448–460
Fišer C, Zagmajster M (2009) Cryptic species from cryptic space: the case of Niphargus fongi sp. n. (Amphipoda, Niphargidae). Crustaceana 82:593–614
Ford DC, Williams P (2007) Karst hydrogeology and geomorphology. Wiley, Chichester
Gibert J (1986) Ecologie d’un système karstique jurassien. Hydrogéologie, dérive animale, transits de matières, dynamique de la population de Niphargus (Crustacé Amphipode). Mém Biospéol 13:1–379
Gibert J, Culver DC (2009) Assessing and conserving groundwater biodiversity: an introduction. Freshw Biol 54:639–648
Gibert J, Deharveng L (2002) Subterranean ecosystems: a truncated functional biodiversity. Bioscience 52:473–481
Gibert J, Danielopol DL, Stanford JA (eds.) (1994) Groundwater ecology. Academic, San Diego
Giere O (2009) Meiobenthology: the microscopic mobile fauna of aquatic sediments, 2nd edn. Springer, Berlin/Hamburg
Gillieson DS (1996) Caves: processes, development, and management. Blackwell, Oxford
Greenslade PJM (1983) Adversity selection and the habitat templet. Am Nat 122(3):352–365
Griebler C, Lueders T (2009) Microbial biodiversity in groundwater ecosystems. Freshw Biol 54:649–677
Griebler C, Danielopol DL, Gibert J et al (eds.) (2001) Groundwater ecology: a tool for management of water resources. Office for Official Publications of the European Commission, Luxembourg
GSchV (1998) Gewässerschutzverordnung (Swiss Water Ordinance) 814.210, Der Schweizer Bundesrat, Bern, Switzerland
Gunn J (ed.) (2004) Encyclopedia of caves and karst science. Fitzroy Dearborn, London
Guzik MT, Abrams KM, Cooper SJB et al (2008) Phylogeography of the ancient Parabathynellidae (Crustacea: Bathynellacea) from the Yilgarn region of Western Australia. Invert Syst 22:205–216
Guzik MT, Cooper SJB, Humphreys WF et al (2009) Fine-scale comparative phylogeography of a sympatric sister species triplet of subterranean diving beetles from a single calcrete aquifer in Western Australia. Mol Ecol 18:3683–3698
Hamilton-Smith E, Eberhard S (2000) Conservation of cave communities in Australia. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 647–664
Hancock P, Boulton AJ (2009) Preface: hydrogeoecology, the interdisciplinary study of groundwater dependent ecosystems. Hydrogeol J 17:1–3
Hose GC (2005) Assessing the need for groundwater quality guidelines for pesticides using the species sensitivity distribution approach. Hum Ecol Risk Assess 11:951–966
Hose GC (2007) Response to Humphreys’ (2007) comments on Hose GC (2005) ‘Assessing the need for groundwater quality guidelines for pesticides using the species sensitivity distribution approach. Hum Ecol Risk Assess 13:241–246
Humphreys WF, ed (1993b) The biogeography of Cape Range, Western Australia. Rec West Aust Mus Suppl 45:1–248
Humphreys WF (2000) The hypogean fauna of the Cape Range peninsula and Barrow Island, north western Australia. In Wilkens H, Culver DC, Humphreys WF (eds) Ecosystems of the World 30, Subterranean Ecosystems, pp. 581–601. Elsevier, Amsterdam
Humphreys WF (2000b) Karst wetlands biodiversity and continuity through major climatic change – an example from arid tropical Western Australia. In: Gopal B, Junk WJ, Davis JA (eds.) Biodiversity in wetlands: assessment, function and conservation, vol 1. Backhuys, Leiden, pp 227–258
Humphreys WF (2001a) Groundwater calcrete aquifers in the Australian arid zone: the context to an unfolding plethora of stygal biodiversity. In: Humphreys WF, Harvey MS (eds.), Subterranean biology in Australia 2000, Rec West Aust Mus, Suppl 64, pp 63–83
Humphreys WF (2001b) The subterranean fauna of Barrow Island, northwestern Australia, and its environment. Mém Biospéol Int J Subterr Biol 28:107–127
Humphreys WF (2002) Keynote address: Groundwater ecosystems in Australia: an emerging understanding. In: Proceedings of the international association of hydrogeologists conference, Darwin, 12–17 May 2002, CD-ROM
Humphreys WF (2007) Comment on assessing the need for groundwater quality guidelines for pesticides using the species sensitivity distribution approach by Hose (2005). Hum Ecol Risk Assess 13:236–240
Humphreys WF (2008) Rising from down under: developments in subterranean biodiversity in Australia from a groundwater fauna perspective. Invert Syst 22:85–101
Humphreys WF (2009) Hydrogeology and groundwater ecology: does each inform the other? In (P.J. Hancock, J.H. Randall. A.J. Boulton, eds) Special issue, Hydrogeoecology, the interdisciplinary study of groundwater dependent ecosystems. Hydrogeol J 17:5–21
Humphreys WF, Adams M (1991) The subterranean aquatic fauna of the North West Cape peninsula, Western Australia. Rec West Aust Mus 15:383–411
Humphreys WF, Harvey MS (eds.) (2001) Subterranean biology in Australia 2000. Rec West Aust Mus Suppl 64: 1–242
Humphreys WF, Watts CHS, Cooper SJB et al (2009) Groundwater estuaries of salt lakes: buried pools of endemic biodiversity on the western plateau, Australia. Hydrobiologia 626:79–95
Iliffe TM (1993) Fauna troglobia acuática de la Península de Yucatán. In: Salazar-Vallejo SI, González NE (eds.) Biodiversidad marina y costera de México. Conabio and Cicro, México, pp 673–686
Iliffe TM, Bishop RE (2007) Adaptations to life in marine caves. In: Safran P (ed.) Fisheries and aquacultur, In: Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers, Oxford. http://www.eolss.net. Retrieved 25 Sep 2007
Iliffe TM, Jickells TD, Brewer MS (1984) Organic pollution of an inland marine cave from Bermuda. Mar Environ Res 12:173–189
IUCN (2008) http://www.iucn.org/about/work/programmes/species/red_list/review/
Jasinska EJ, Knott B (2000) Root-driven faunas in cave waters. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 287–307
Job CA, Simons JJ (1994) Ecological basis for management of groundwater in the United States: statutes, regulations, and a strategic plan. In: Gibert J, Danielopol DL, Stanford JA (eds.) Groundwater ecology. Academic, San Diego, pp 523–540
Jones WK, III Hobbs HH, Wicks CM et al (2003) Recommendations and guidelines for managing caves on protected lands. Special publication 8. Karst Waters Institute, Charles Town
Juberthie C (2000) Conservation of subterranean habitats and species. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, Subterranean ecosystems. Elsevier, Amsterdam, pp 691–700
Juberthie C, Decu V (eds.) (1994) Encyclopaedia biospeologica, vol 1. Société internationale de Biospéologie, Moulis
Juberthie C, Decu V (eds.) (2000) Encyclopaedia biospeologica, vol 2. Société internationale de Biospéologie, Moulis
Juberthie C, Decu V (eds.) (2001) Encyclopaedia biospeologica, vol 3. Société internationale de Biospéologie, Moulis
Karanovic T (2006) Subterranean copepods (Crustacea, Copepoda) from the Pilbara region in Western Australia. Rec West Aust Mus Suppl 70:1–239
Karanovic I (2007) Candoninae Ostracodes from the Pilbara region in Western Australia. Crustaceana Monogr 7:1–432
Katz BG (2005) Nitrate contamination in karst groundwaters. In: Culver D, White W (eds.) Encyclopedia of caves. Academic, San Diego, pp 415–418
Kornicker LS, Humphreys WF, Danielopol DL et al (2010) Ontogeny of an anchialine ostracod from Western Australia and comments on the origin and distribution of Halocyprididae. Crustaceana 83(6):715–752
L’Volich MI (1974)World water resources and their future. (English translation AGU), Mysl’ PH, Moscow
Langecker TG (2000) The effect of continuous darkness on cave ecology and cavernicolous evolution. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 135–157
Lewis JJ (1996) Cave bioinventory as a management tool. In: Rea GT (ed.), Proceeding of the 1995 national cave management symposium, Spring Mill State Park, Mitchell, pp 228–236. Indiana Karst Conservancy, Indianapolis
Lewis JJ, Lewis TM, Eckstein J (1983) A biological reconnaissance of a polluted cave stream: the hidden river groundwater basin. Cave Research Foundation Annual Report 1982: 9–10
Leys R, Watts CHS, Cooper SJB, Humphreys WF (2003) Evolution of subterranean diving beetles (Coleoptera: Dytiscidae: Hydroporini, Bidessini) in the arid zone of Australia. Evolution 57:2819–2834
Lindenmayer D, Hobbs RJ, Montague-Drake R et al (2008–01) A checklist for ecological management of landscapes for conservation. Ecol Lett 11(1):78–91
Longley G (1986) The biota of the Edwards Aquifer and the implications for paleozoogeography. In: Abbott PL, Woodruff CM (eds.) The Balcones Escarpment, central Texas. Geological Society of America, San Antonio, pp 51–54
Longley G (1992) The subterranean aquatic ecosystem of the Balcones Fault Zone Edwards Aquifer in Texas – threats from overpumping. In: Stanford JA, Simons JJ (eds.) Proceedings of the 1st international conference on groundwater ecology, American Water Resources Association, Bethesda, pp 291–300
Lučić I, Sket B (2003) Vjetrenica. Pogled u dušu zemlje. Savez speleologa Bosne i Hercegovine and Hrvatsko biospeleoško društvo, Zagreb, Croatia
Malard F, Crague G, Turquin M-J et al (1994) Monitoring karstic groundwater: the practical aspects of subterranean biology. Theor Appl Karst 7:115–126
Malard F, Turquin M–J, Magniez G (1997) Filter effect of karstic spring ecotones on the population structure of the hypogean amphipod Niphargus virei. In: Gibert J, Mathieu J, Fournier F (eds.) Groundwater/surface water ecotones: biological and hydrological interactions and management option. Cambridge University Press, Cambridge, pp 42–56
Malard F, Boutin C, Camacho AI et al (2009) Diversity patterns of stygobiotic crustaceans across multiple spatial scales in Europe. Freshw Biol 54:756–776
Mangin A (1975) Contribution à l’étude hydrodynamique des aquifères karstiques [Contribution to the hydrodynamic study of karstic aquifers]. Annls Spéléol 30:21–124
Masciopinto C, Semeraro F, La Mantia R et al (2006) Stygofauna abundance and distribution in the fissures and caves of the Nardò (southern Italy) fractured aquifer subject to reclaimed water injections. Geomicrobiol J 23:267–278
Masciopinto C, La Mantia R, Carducci A et al (2007) Unsafe tap water in households supplied from groundwater in the Salerno region of southern Italy. J Water Health 5:129–148
May RM (1976) Patterns in multi-species communities. In: May RM (ed.) Theoretical ecology: principles and applications. Blackwell, Oxford, pp 142–162
Mengxiong C (1987) Groundwater resources and development in China. Env Geol 10:141–147
Michel G, Malard F, Deharveng L et al (2009) Reserve selection for conserving groundwater biodiversity. Freshw Biol 54:861–876
Milanović PT (2004a) Water resources engineering in karst. CRC Press, Boca Raton
Milanović P (2004b) Dams and reservoirs on karst. In: Gunn J (ed.) Encyclopedia of caves and karst science, pp 277–279. Fitzroy Dearborn, London Monod T (1924) Sur un type nouveau de malacostrace: Thermosbaena mirabilis, nov gen. nov. sp. Bull Soc Zool France 49:58–68
Morgan KH (1993) Development, sedimentation and economic potential of palaeoriver systems of the Yilgarn Craton of Western Australia. Sediment Geol 85:637–656
Nogaro G, Mermillod-Blondin F, Francois-Carcaillet F et al (2006) Invertebrate bioturbation can reduce the clogging of sediment: an experimental study using infiltration sediment columns. Freshw Biol 51:1458–1473
Notenboom J (2001) Managing ecological risks of groundwater pollution. In: Griebler C, Danielopol DL, Gibert J et al (eds.) Groundwater ecology: a tool for management of water resources, Director-general for research. European Communities, Luxembourg, pp 247–262
Notenboom J, Plénet S, Turquin M-J (1994) Human interference and management implications. In: Gibert J, Danielopol DL, Stanford JA (eds.) Groundwater ecology. Academic, San Diego, pp 477–504
Page TJ, Humphreys WF, Hughes JM (2008) Shrimps down under: evolutionary relationships of subterranean crustaceans from Western Australia (Decapoda: Atyidae: Stygiocaris). PLoS ONE 3(2):1–12, e1618
Parzefall J (2000) Ecological role of aggressiveness in the dark. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 221–228
Pérez del Villar L, Garralón A, Delgado A et al (2004) Hydrogeochemical evolution and C isotope study of groundwaters from “Mina Fe” U deposit (Salamanca, Spain): implications for processes in radwaste disposal. App Geochem 20:465–485
Perriam J, Tapsuwan S, Burton M et al (2008) Value of the Yanchep caves: assessing Yanchep National Park visitor’s willingness to pay for environmental improvement to the caves. Technical report, CSIRO: Water for a Healthy Country National Research Flagship, Canberra
Pipan T (2005) Epikarst – a promising habitat. Založba ZRC, Ljubljana
Pipan T, Culver D (2007a) Copepod distribution as an indicator of epikarst system connectivity. Hydrogeol J 15:817–822
Pipan T, Culver DC (2007b) Regional species richness in an obligate subterranean dwelling fauna – epikarst copepods. J Biogeog 34:854–861
Pipan T, Christman MC, Culver DC (2006) Dynamics of epikarst communities: microgeographic pattern and environmental determinants of epikarst copepods in Organ Cave, West Virginia. Am Midl Nat 156:75–87
Pohlman JW, Cifuentes LA, Iliffe TM (2000) Food web dynamics and biogeochemistry of anchialine caves: a stable isotope approach. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 345–357
Por D (2007) Ophel: a groundwater biome based on chemoautotrophic resources. The global significance of the Ayyalon cave finds, Israel. Hydrobiologia 592:1–10
Poulson TL (1968) Aquatic cave communities. Cave Research Foundation Annual Report, 1968:16–18
Poulson TL (1996) Research aimed at management problems should be hypothesis-driven: case studies in the Mammoth Cave Region. In: Rea GT (ed.) Proceedings of the 1995 national cave management symposium, Spring Mill State Park, Mitchell, Indiana. Indiana Karst Conservancy, Indianapolis, pp 267–273
Prendini L, Francke OF, Vignoli V (2010) Troglomorphism, trichobothriotaxy and typhlochactid phylogeny (Scorpiones, Chactoidea): more evidence that troglobitism is not an evolutionary dead-end. Cladistics 26:117–142
Proudlove G, Wood PJ (2003) The blind leading the blind: cryptic subterranean species and DNA taxonomy. Trends Ecol Evol 18:272–273
Pugsley C (1984) Ecology of the New Zealand glowworm, Arachnocampa luminosa (Diptera: Keroplatidae), in the Glowworm Cave, Waitamo. J Roy Soc N Z 14:387–407
QRSS (2009) Quintana Roo speleological survey, The National Speleological Society. www.caves.org/project/qrss Accessed 28 July 2009
Rabinowitz D, Cairns S, Dillon T (1986) Several forms of rarity and their frequency in the flora of the British Isles. In Soule ME (ed.) Conservation Biology: the science of scarcity and diversity. Sinauer Associates Inc. Publishers, Sunderland Massachusetts
Ray JA (2005) Sinking streams and losing streams. In: Culver D, White W (eds.) Encyclopedia of caves. Academic, San Diego, pp 509–514
Reeves J, Deckker P, Halse S (2007) Groundwater ostracods from the arid Pilbara region of northwestern Australia: distribution and water chemistry. Hydrobiologia 585:99–118
Rouch R (1977) Considérations sur l’écosystème karstique [considerations on the karstic ecosystem]. C R Acad Sci Paris Sér D 284:1101–1103
Rouch R (1986) Sur l’écologie des eaux souterraines dans le karst [on the ecology of subterranean waters in karst]. Stygologia 2:352–398
Rouch R (1991) Structure de peuplement des harpacticides dans le milieu hyporhéique d’un ruisseau des Pyrénées [population structure of harpacticoids in the hyporheic zone of a Pyreneean brook]. Ann Limnol 27:227–241
Rouch R, Pitzalis A, Descouens A (1993) Effets d’un pompage à gros dèbit sur le peuplement des Crustacès d’un aquifére karstique [the effect of large flow pumping on the population of crustaceans in a karstic aquifer]. Annal Limnol 29:15–29
Russ A, Santos SR, Muir C (2010) Genetic population structure of an anchialine shrimp, Metabetaeus lohena (Crustacea: Alpheidae), in the Hawaiian Islands. Revist Biol Trop 58:159–170
Sandahl JF, Baldwin DH, Jenkins JJ et al (2004) Odor-evoked field potentials as indicators of sublethal neurotoxicity in juvenile coho exposed to copper, chlorpyrifos, and esfenvalerate. Can J Fish Aquat Sci 61:404–413
Santos SR (2006) Patterns of genetic connectivity among anchialine habitats: a case study of the endemic Hawaiian shrimp Halocaridina rubra on the Island of Hawai‘i. Mol Ecol 15:2699–2718
Sarbu SM (2000) Movile cave: a chemoautotrophically based groundwater ecosystem. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 319–343
Sarbu SM, Galdenzi S, Menichetti M et al (2000) Geology and biology of Frasassi caves in Central Italy: an ecological multidisciplinary study of a hypogenic underground karst system. In: Wilkens H, Culver DC, Humphreys WF (eds.) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam, pp 359–378
Schindel G, Hoyt J (2004) Groundwater pollution: point source. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 404–406
Schindel G, Johnson S, Smart C (2004) Groundwater pollution: remediation. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 406–407
Schneider K, Culver DC (2004) Estimating subterranean species richness using intensive sampling and rarefraction curves in a high density cave region in West Virginia. J Cave Karst Stud 66:39–45
Scibek J, Allen DM, Whitfield PH (2008) Quantifying the impacts of climate change on groundwater in an unconfined aquifer that is strongly influenced by surface water. In: Dragoni W, Sukhija BS (eds.) Climate change and groundwater. Special publication 288. Geological Society, London, pp 79–98
Seymour JR, Humphreys WF, Mitchell JG (2007) Stratification of the microbial community inhabiting an anchialine sinkhole. Aquat Microb Ecol 50:11–24
Shabarova T, Pernthaler J (2010) Karst pools in subsurface environments: collectors of microbial diversity or temporary residence between habitat types. Environ Microbiol 12:1061–1074
Simon KS, Pipan T, Culver DC (2007) A conceptual model of the flow and distribution of organic carbon in caves. J Cave Karst Stud 69:279–284
Sket B (1986) Ecology of the mixohaline hypogean fauna along the Yugoslav coast. Stygologia 2:317–338
Sket B (1996) The ecology of anchihaline caves. Trends Ecol Evol 11:221–255
Sket B (1999) The nature of biodiversity in hypogean waters and how it is endangered. Biodivers Conserv 8:1319–1338
Sket B (2004) The cave hydgopetric – a little known habitat and its inhabitants. Arch Hydrobiol 160:413–424
Sket B (2005) Dinaric karst. In: Culver DC, White WB (eds.) Encyclopedia of caves. Elsevier, Burlington, pp 158–165
Sket B, Paragamian K, Trontelj P (2004) Census of the obligate subterranean fauna of the Balkan Peninsula. In: Griffith HI (ed.) Balkan biodiversity. Kluwer, Dordrecht, pp 309–322
Smart C, Worthington SRH (2004a) Groundwater in karst. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 394–397
Smart C, Worthington SRH (2004b) Groundwater in karst: borehole hydrology. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 397–398
Southwood TRE (1988) Habitat, the templet for ecological strategies? J Anim Ecol 46:337–365
Strecker U, Bernatchez L, Wilkens H (2003) Genetic divergence between cave and surface populations of Astyanax in Mexico (Characidae, Teleostei). Mol Ecol 12:699–710
Strecker U, Víctor H, Faúndez VH et al (2004) Phylogeography of surface and cave Astyanax (Teleostei) from Central and North America based on cytochrome b sequence data. Mol Phylogenet Evol 33:469–481
Taylor SJ, Webb DW (2000a) Human impacts on groundwater quality and subterranean aquatic biota in southwestern Illinois. Illinois Natural History Survey Reports No 361:2–3
Taylor SJ, Webb DW (2000b) Subterranean amphipoda (Crustacea) of Illinois’ Salem Plateau: spatial and temporal components of microdistribution. Illinois Natural History Survey, Center for Biodiversity, Technical Report 2000(27):1–62
Tercafs R (2001) The protection of the subterranean environment. Conservation principles and management tools. Production Services Publishers, Luxembourg
Trontelj P, Douady CJ, Fišer C et al (2009) A molecular test for cryptic diversity in groundwater: how large are the ranges of macro-stygobionts? Freshw Biol 54:727–744
Venarsky MP, Wilhelm FM, Anderson FE (2007) Conservation strategies supported by non-lethal life history sampling of the U.S. Federally listed Illinois Cave Amphipod, Gammarus acherondytes. J Crust Biol 27:202–211
Veni G (1987) Valdina farms sinkhole: hydrogeologic and biologic evaluation. Report for Edwards Underground Water District, San Antonio
Venn D P (2008) A changing cultural landscape: Yanchep National Park, Western Australia. MA thesis, Faculty of Education and Arts, Edith Cowan University, Mt Lawley, Western Australia
Verovnik R, Sket B, Trontelj P (2004) Phylogeography of subterranean and surface populations of water lice Asellus aquaticus (Crustacea: Isopoda). Mol Ecol 13:1519–1532
Wapet (1996) Protection of Barrow Island groundwater. Report to the National Parks and Nature Conservation Authority 1996. Western Australian Petroleum Pty Ltd, Perth
Watson J (1997) Guidelines for cave and karst protection. WCPA Working Group on Cave and Karst Protection/IUCN, Gland
Webb DW, Taylor SJ, Krejca JK (1994) The biological resources of Illinois’ caves and other subterranean environments. Illinois Natural History Survey, Center for Biodiversity, Technical Report 1993(8):1–168
White WB (2005) Hydrogeology of karst aquifers. In: Culver DC, White WB (eds.) Encyclopedia of caves. Elsevier, Burlington, pp 293–300
Whitten T (2009) Applying ecology for cave management in China and neighbouring countries. J Appl Ecol 46:520–523
Wicks C, Noltie DB, Peterson EW et al (2010) Disturbances in the habitat of Macrocotyla glandulosa (Kenk). Ecohydrology 3:116–125
Wilkens H, Culver DC, Humphreys WF (eds.) (2000) Ecosystems of the world, vol 30, Subterranean ecosystems. Elsevier, Amsterdam
Wilson GDF (2008) Gondwanan groundwater: subterranean connections of Australian phreatoicidean isopods (Crustacea) to India and New Zealand. Invert Syst 22:301–310
Winde F (2006) Long-term impacts of gold and uranium mining on water quality in dolomitic regions – examples from the Wonderfonteinspruit catchment in South Africa. In: Broder JM, Hasche-Berger A (eds.) Uranium in the environment: mining impact and consequences. Springer Berlin, Heidelberg, pp 807–816
Wolfe WJ, Haugh CJ (2001) Preliminary conceptual models of chlorinated-solvent accumulation in karst aquifers. In: Kuniansky EL (ed.), U.S. geological survey karst interest group proceedings, Water-Resources Investigations Report 01–4011:157–162
Worthington SRH (1999) A comprehensive strategy for understanding flow in carbonate aquifers. In: Palmer AN, Palmer MV, Sasowsky ID (eds.) Karst modeling. Special publication 5. Karst Waters Institute, Charles Town, pp 30–37
Worthington SRH, Smart C (2004) Groundwater in karst: conceptual models. In: Gunn J (ed.) Encyclopedia of caves and karst science. Fitzroy Dearborn, London, pp 399–401
Zakšek V, Sket B, Gottstein S et al (2009) The limits of cryptic diversity in groundwater: phylogeography of the cave shrimp Troglocaris anophthlamus (Crustacea: Decapoda: Atyidae). Mol Ecol 18:931–946
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2011 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Humphreys, W.F. (2011). Management of Groundwater Species in Karst Environments. In: van Beynen, P. (eds) Karst Management. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-1207-2_13
Download citation
DOI: https://doi.org/10.1007/978-94-007-1207-2_13
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-1206-5
Online ISBN: 978-94-007-1207-2
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)