Abstract
The sun is the primary driver of terrestrial atmospheric phenomena and energy source for the earth. It emits radiation over a large energy band and ejects highly energetic plasma fluxes of charged particles into space. The sun is an active star that (i) goes through a 12-year maximum–minimum emission cycle, (ii) has huge, non-periodic eruptions in solar flares and coronal mass ejections, and (iii) has nearly equipartition of energy between particle and radiation fluxes. Variations in these emissions interact with all atmospheric layers down to the earth surface. The precise nature of these interactions can be examined through microscopic physics at atomic and molecular levels for illustration of physical and chemical processes, and their impact on macroscopic problems such as global climate change, and more localized manifestations such as the atmospheric brown cloud (ABC) prominent in Asia. I will describe some of these calculations for atomic and molecular species such as carbon, nitrogen, oxygen, sulfur and their compounds. While the visible and near-infrared solar radiation penetrates through, more energetic components in the ultraviolet (UV) and the x-ray are absorbed by the upper layers of the atmosphere and thus provide protecting shields for life on earth. The atmosphere has been maintaining a fine energy balance of solar radiation entering the earth by radiating the same amount into space. Certain atmospheric gases trap radiation energy and reflect back near earth’ surface to heat it up in an energy cycle. This phenomenon is known as the Greenhouse effect and has maintained the average earth surface temperature at 14°C. However, for over a 100 years the balance is being perturbed due to changes in atmospheric compositions of greenhouse gases. More energy is being trapped than released, leading to global warming and climate change. Depletion of atmospheric ozone molecules has created holes for harmful radiation to reach earth’s surface. The basic scientific data in current atmospheric models lack accurate parameters for fundamental atomic and molecular processes, and hence provide predictions which have large uncertainties. We aim to explore the sensitivity of numerical simulations to accurately predict the response of the earth’s atmosphere to changes in elemental and molecular composition. To wit, what is the effect of including new high-accuracy photoionization and radiative transition rates in C, N, O, H2O, etc. in climate models? How do temporal-spatial and temperature-density dependencies of fundamental physical and chemical parameters and rates affect the absorption of solar radiation by the ABC? Such studies should lead to an improved understanding of global warming and climate change processes, and help in the future steps.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Chemical Compositions and Global Warming
Global warming is directly related to solar irradiance. The earth is our home planet and the sun is an integral part of our lives. The sun is responsible for virtually all energy that reaches the earth’s surface. The interaction of solar radiation with atmospheric gases establishes the atmospheric temperature. The atmosphere contains number of component gases: 78.08% nitrogen (N2), 20.95% oxygen (O2), 0.93% argon, 0.038% carbon dioxide (CO2) and traces of some others. About 1–4% could be water vapor depending on the climate. The atmospheric gases which absorb and trap energy causing the earth to heat up in a cyclic process are the greenhouse gases. The natural constituent greenhouse gases are water vapor, which causes about 36–70% of the heating, carbon dioxide (CO2) about 9–26%, methane (CH4) about 4–9%, and ozone (O3) about 3–7%. These gases have maintained a constant average earth temperature by a natural balance as they radiate about the same amount of energy that they absorb. Even a slight change in the concentration of particular gases in the atmosphere can prevent heat from being radiated out into space and upset this fine balance. The current global warming is a result of such imbalance. Over the last 100 years, the earth is seen going through a climate change, that is, changes in its weather systems, rainfall and temperatures. The average temperature of the earth’s near-surface air and oceans has increased by 0.74 ± 0.18 C (1.33 ± 0.32°F) during the last 150 years ending in 2005. These changes can be caused naturally either, as a result of changes in the way oceans and the atmosphere interact, or from changes in the amount of energy received from the sun. Recent measurements indicate that the earth is presently absorbing solar energy of 0.85 ± 0.15 W/m2 more than it is emitting into space.
Atmospheric concentrations of various greenhouse gases have been increasing slowly over the years and are believed to causing global warming. These concentrations are considerably higher than at any time during the last 650,000 years, the period for which reliable data has been extracted from ice cores. Concentration of greenhouse gas CO2 has seen a steady rise. Currently about 6.5 billion tonnes of carbon dioxide are emitted globally each year, mostly through burning fossil fuel such as, coal, oil and gas by industries and domestic transportations, and through land use by deforestation and agricultural processes. The Intergovernmental Panel on Climate Change (IPCC) Special Report on Emissions Scenarios predicts that this increment in atmosphere may increase to from 541 to 970 ppm by the year 2100. Since the industrial revolution the concentrations of greenhouse gases have increased by human activities. The increases are +35% for carbon dioxide since 1,700, +150% for methane and +20% for nitrous oxide. Other greenhouse gases are also on the rise, such as, aerosol gases like hydrofluorocarbons (HFC) and chlorofluorocarbon (CFC). The conclusion of IPCC research indicated that most of the temperature increase since the mid-twentieth century is due to the increase in these anthropogenic gases. However, increase of CO2 is believed to be the main gas accountable for global warming. Present technology cannot prevent the release of carbon dioxide, although there is some that can reduce the amount of gas being released.
The effect of global warming through heating up the earth’s surface, oceans and atmosphere is causing devastating damage for the inhabitants of our planet. A rise in temperature of just one or two degrees will result in changing weather patterns and lead to increased flooding, desertification, crop failures, freshwater shortages, increase of salinity in inland water, and storms. The polar snow caps are retreating and sea levels are rising. United Nations Environment Programme (UNEP) has shown that glaciers melting has more than doubled in places. Some of the biggest losses have occurred in the Alps and Pyrenees mountain ranges in Europe. Estimates for 2006 indicate shrinkage of 1.4 m of ‘water equivalent’, compared to half a meter in 2005. A huge Antarctic ice sheet, 160 square mile piece which is more than seven times the size of Manhattan, of the Wilkins ice shelf collapsed into the ocean near the end of February 2008. The piece had been attached to Antarctica for hundreds, or may be even 1,500 years. Retreat of glaciers have created a number of glacier lakes in the Himalayas mountains. Bhutan has about 24 such lakes. The swollen lakes pose the risk of outbursts and flooding to the populated areas. Sea level is rising by 0.8 mm/year. IPCC estimates from its research on around 100 glaciers that the rise may be over 20 cm by 2100, where the increase of 1–23 cm will be due to melting of glaciers alone. Its data shows significant shrinkage taking place in Asia, European countries including Austria, Norway, Sweden, Italy, Spain and Switzerland. The overall headline figures from the IPCC expect global sea levels to rise by between 11 and 88 cm this century, and to rise further after that. However, these figures are based on computer modeling. With more accurate data on how solar radiation is being trapped, the models are expected to provide more precise predictions.
Another cause for concern in the climate change crisis is the depletion of ozone, O3, in the atmosphere. O3 is a molecule with three oxygen atoms which interacts efficiently with solar UV radiation. In amount ozone is rare in our atmosphere, averaging about three molecules for every 10 million air molecules. Despite of this small amount, O3 plays a vital role in the upper atmosphere (stratosphere) and the lower atmosphere (troposphere). Stratospheric ozone (often referred to as “good ozone”) plays a beneficial role by absorbing most of the biologically damaging ultraviolet, called UV-B, sunlight. Without the filtering action of the ozone layer, more UV-B would penetrate the atmosphere and would reach the earth’s surface.
The absorption of ultraviolet radiation by ozone creates a source of heat, and is the reason for temperature rise as one goes to higher altitudes. Ozone thus plays a key role in the temperature structure of the earth’s atmosphere. On the other hand, surface-level tropospheric ozone (often called “bad ozone”) is destructive to living systems when comes in direct contact. Studies show harmful effects of ozone on crop production, forest growth, and human health. Concern has been raised as ground-based and satellite-borne instruments measurement showed decreasing amount of stratospheric ozone, while increase in tropospheric ozone. Over some parts of Antarctica, up to 60% of the total overhead of ozone depleted during Antarctic spring (September–November). This phenomenon is known as the Antarctic ozone hole. In the Arctic polar regions, similar processes occur that have also led to significant chemical depletion of the column ozone during late winter and spring in seven out of the last 11 years. Smaller, but still significant, stratospheric decreases have been seen over other more populated regions of the earth. Increases in surface UV-B radiation have been observed in association with local decreases in stratospheric ozone. In addition, near-surface ozone is causing problem as a key component of photochemical “smog”, especially in many cities. The scientific evidence from studies by the international research community has shown that human-produced chemicals are responsible for the observed depletions of the ozone layer. The ozone-depleting compounds, such as carbon tetrachloride, methyl chloroform which are commonly known as halocarbons, contain various combinations of the chemical elements chlorine, fluorine, bromine, carbon, and hydrogen. One example is the halons which contain carbon, bromine, fluorine, and (in some cases) chlorine, used as fire extinguishants. The other compound, known as chlorofluorocarbon contain only chlorine, fluorine, and carbon. These human-produced gases are used in many applications, such as, refrigeration, air conditioning, foam blowing, cleaning of electronics components, and as solvents. Upon usage, these float in the atmosphere up to the stratosphere and break down ozone into oxygen which then ionizes and escapes into space.
2 Atmospheric Brown Cloud (ABC)
Recently, another problem which is worsening the climate has been of much concern. It is the atmospheric brown cloud (ABC) which blocks solar radiation from reaching the earth surface by partly reflecting out and partly absorbing the sunlight. ABC is more pronounced in Asia – especially China, Arabian Peninsula, India, Bangladesh, Korea, Japan etc. where it is enhancing the global warming. ABC is the thick haze formed in humid condition, usually in winter when the monsoon is with no rainfall to wash the pollution. The ingredients are airborne particles and pollution due to coal powered industrial soot, biomass burning, vehicle emissions, burning of woods, dung, and crops. This pollution layer was detected during intensive field observation under the Indian Ocean Experiment (INODEX) in 1999. Later the United Nations Environment Program (UNEP) supported a project called ABC to study the pollution. The brown cloud that hangs over southern Asia could precipitate an environmental disaster that could affect billions of people. A recent CSIRO study found that the Asian brown cloud is also affecting rainfall in Australia. A new study shows that getting rid of it could actually help increase the rice harvests in the subcontinent. ABC is believed to have reduced sunlight, by about 20% since 1970s, by reflecting part of it back into space. This cools the surface and thus reduces the evaporation and causes less monsoon rainfall. It also absorbs the sunlight and thus raises solar atmospheric heating. Model suggests 50% temperature raised in the area is due to ABC (Ramanathan et al. 2007) and is believed to cause melting the Himalayan glaciers. However, the model included the data for solar heating with uncertainty of about a factor of four. The study of solar heating of the earth’s atmosphere is crucial in precise modeling of its impact on the environment, and future plans. The following sections describe and discuss briefly our present day understanding, and how further study can lead to our projected aim.
3 Solar Irradiation of the Earth
Study of interaction of sunlight with atmospheric gases is an integral part of understanding global warming and future predictions. The sun brightens up and warm up the day as the earth spins every day, and causes seasons as the earth moves along its orbit. The earth is much smaller than the sun, its radius being 110 times smaller than that of the sun and only a small fraction of solar emission irradiates the earth. The sun, is however, an “unQuiet” star. During active period of its cycle of 11 years of maximum and minimum activity, it erupts with explosions that eject large amount of particles and radiation into space as solar flares which can affect the earth considerably. Features in solar activity are being elucidated through multi-wavelength spectroscopy using observations by space based satellites, such as, SOHO, Chanda x-ray Observatory, aided by detailed theoretical atomic and molecular calculations. The “Halloween” solar storm that occurred on October 28, 2003, was well documented by the space observatories Chandra and SOHO. Sun’s active spots were detected especially by SOHO. Its mass spectrometer, LASCO, detected large coronal mass ejections around the sun, which is covered at the center by the detector. Eight hours after ejection from the sun LASCO was swarmed by the ejected particles in the form of a proton shower. Emission of x-rays in KeV energy range peaked in the radiation spectrum of solar flares. The emission bumps were found to be produced by He-like Ca, Fe, and Ni. Solar x-ray emission spectra may be analyzed by observing dielectronic satellite (DES) lines that form when an electron colliding with a two-electron He-like ion forms an excited 3-electron system. An atomic state with two electrons in excited levels is known as a doubly excited autoionizing state. An autoionizing state decays quickly by emitting a photon, which forms a DES line and a stable bound state. Study of DES lines (e.g. Nahar and Pradhan 2006) provides various diagnostics, such as temperature, density, and chemical abundances of the plasma surrounding the sun.
Materials, such as electrons, protons, and heavy ions ejected out into space by the solar explosions can be dangerous as these can damage tissue, break strands of DNA, and lead to diseases like cancer. The powerful electromagnetic pulses during a solar storm can also affect satellites and communications, and can even disrupt electrical service over long distances. However, earth holds protective shields around it. Its magnetic field channels these particles around the earth, funneling some of it to the poles to produce the most commonly noticed effect, the glowing aurorae. The upper layers of earth’s atmosphere deflect and block part of radiation. For example ozone in stratosphere blocks most of ultraviolet, x-rays, and Gamma rays, and the lower atmosphere scatters and burns most of the incoming particles.
While the earth’s atmosphere blocks most of the harmful radiation, it lets visible light, most radio waves, and some infrared light through, making it possible to study the universe with ground-based telescopes at these wavelengths. Atmospheric opacity gives the measure of radiation transport such that less opacity means more radiation can pass through. Most of the infrared light coming to earth is absorbed by water vapor and carbon dioxide in the earth’s atmosphere. Observations of astronomical objects with radiation heavily attenuated by the atmosphere are carried out by space-based telescopes.
4 Solar Energy Distribution and Greenhouse Effect
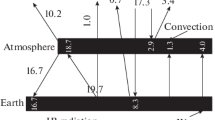
The most important scientific input for climate change models is related to the greenhouse effect. Of the total solar radiation reaching the earth, 30% is reflected back to space (6% by air, 20% by clouds, and 4% by the surface of the earth),19% is absorbed by atmosphere (16% by atmospheric gases, 3% by clouds) and 51% by the earth surface. Fifty-one percent of energy absorbed by the earth’s surface is reradiated into the atmosphere (21% as infrared emission, 7% in sensible heat flux, 23% in latent heat flux). The atmosphere absorbs this 51% of long wavelength infrared radiation, plus the original 19% of short wavelength radiation absorbed directly from the sun, and then reradiates the total 70% back to space (6% radiated directly and 64% radiated from clouds and atmosphere). Hence, a stable relation is maintained between the atmospheric thermal structure and solar radiation.
In the energy cycle of the greenhouse effect, part of the energy is trapped to heat the earth surface. Direct overhead sunlight at the top of the atmosphere provides 1,366 W/m2. Due to geometric effects and reflective surfaces only 235 W/m2 is absorbed, 67 W/m2 by air and 168 W/m2 by land and water. Earth’s surface temperature is raised only to −18 C by 168 W/m2. However, the greenhouse gases absorb the outgoing and reflected radiation, and thus trapping more radiation in the atmosphere and subsequently reflecting it back to the lower layers of the atmosphere closer to the surface which raises the earth’s temperature. The gases absorb 452 W/m2 thermal infrared radiation emitted by the earth’s surface. Of the total energy 519 W/m2 (= 67 + 452) it delivers 324 W/m2 (62%) back to earth and transmits the remainder 195 W/m2 (38%) to space. The total energy 492 W/m2 (168 from sunlight +324 from atmosphere) raises earth’s surface temperature to +14°C. This energy recycling process, that is the greenhouse effect, is an essential piece of the earth’s climate. Greenhouse effect has maintained abundant liquid water for the earth’s habitants. A more detailed study of the effect requires one to know how atmospheric atoms and molecules interact with radiation, discussed next.
5 Atomic and Molecular Processes of Photo-Absorption, Photon-Emission, and Atmospheric Opacity
The macroscopic phenomena due to solar irradiance of the earth are related to a number of atomic and molecular processes involving light and atmospheric gases which absorb and emit radiation at different wavelengths, that is, photons of different energy. Followings are the processes of absorption and emission of a photon. A photon is described by hν where ν is the photon frequency and h is the Planck’s constant. Its interaction with an atomic or molecular species, X+Z of charge Z, can increase the charge with loss of an electron.
-
1.
Photoexcitation – an electron in an atomic or molecular system, X+Z , absorbs the photon and jumps to a higher excited state while remaining in the atomic or molecular system:
$$ {\text{X}}^{\text{ + Z}}+\text{h}\nu \to {\text{X}}^{\text{ + Z}}{}^{*}$$The asterisk (*) denotes an excited state.
-
2.
De-excitation (inverse of excitation) – an electron in an excited atomic or molecular state emits energy in the form of a photon and drops down to the ground level:
$$ {\text{X}}^{\text{ + Z*}}\to {\text{X}}^{\text{ + Z}}\text{ + h}\nu $$ -
3.
Photoionization/photo-dissociation/photo-electric effect – an electron absorbs a photon and exits the atom/molecule:
$$ {\text{X}}^{\text{ + Z}}\text{ + h}\nu \to {\text{X}}^{\text{ + Z + 1}}\text{ + e}$$ -
4.
Electron-ion recombination (inverse of photoionization) – a free electron loses its energy as a photon and combines to an ion:
$$ {\text{X}}^{\text{ + Z}}+\text{e}\to {\text{X}}^{\text{ + Z + 1}}+\text{h}\nu $$ -
5.
Collisional excitation – an atom or a molecules goes to an excited state by the impact of a free electron, and decays by emitting a photon.
$$ {\text{X}}^{\text{ + Z}}+\text{e}\to {\text{X}}^{\text{ + Z*}}+\text{e ′}$$
The first two processes are confined to the atomic system and hence emit or absorb photons of particular energy related to quantized atomic levels. The parameters of interest relevant to the processes are called oscillator strengths (f-values) and radiative decay rates (A-values) respectively. They produce only lines in the spectrum. However, the other three processes show characteristic features over a range of energies in their cross sections. Study of these features in cross sections are crucial for any precise modeling. For example, sun’s ultraviolet radiation breaks down O2 and N2 molecules into atoms, and then photo-ionizes them in the ionosphere. Nahar (1998) and Nahar and Pradhan (1997) carried out calculations for photoionization cross section (σ PI) with photon energy which showed that the ionization does not vary smoothly with energy, but goes through resonances at different energies. The resonant peaks indicate huge enhancement of ionization at particular energies (Fig. 3.1).
Nahar (1995, 1996) studied the characteristic patterns for electron combining with a doubly ionized (two electrons stripped off) sulfur forming a singly ionized sulfur. Her work showed that at low temperature the recombination rate is very high, and goes down with higher temperatures since high velocity electrons have less time to combine. However, when the temperature is high enough to form resonant states, the recombination increases, and forms a peak which is followed by a smooth decay (Fig. 3.2).
Total recombination rate for singly ionized sulfur, S II, from doubly ionized sulfur S III (Nahar and Pradhan 2006). The recombination rates shows that starting high at very low temperature it decreases until at a higher temperature where it peaks due to resonances in the process
Due to various processes between the gases and sunlight, earth’s atmospheric opacity spectrum shows various windows of radiation transmission reaching the earth. For example, (i) oxygen molecules, O3 and O2, and atomic oxygen (O) absorb ultraviolet radiation, (ii) water and oxygen absorb some infrared, microwave and radio frequencies, and (iii) carbon dioxide absorbs infrared frequency photons, Determination of atmospheric opacity, which provides the measures of radiation transfer, depends on the above processes. For example, monochromatic opacity (for a particular photon frequency), k ν, depends on the photo-excitation parameter, f i j, oscillator strength for transition from level i to level j,
where Ni = ion density in state i, ϕ is a profile factor, and the rest are constants, and on photoionization cross sections σ PI
The total opacity depends on the interaction of radiation with all atoms and molecules in the atmosphere. Complete atmospheric modeling requires opacities and parameters of all other processes. Two international projects have undertaken large-scale computations for precise understanding of astronomical objects, the Opacity Project (1995, 1996), and the Iron Project (Hummer et al. 1993), involving collaborators from the US (Ohio State U, NASA-Goddard, Rollins), U.K., France, Germany, Belgium, Venezuela, and Canada. These two projects entail all radiative and collisional processes described above using an ab initio R-matrix method. A huge amount of accurate atomic data for many atoms and ions from hydrogen to nickel are available through databases: TOPbase, TIPbase at CDS (France) at http://vizier.u-strasbg.fr/topbase/topbase.html, Ohio Supercomouter Center at http://opacities.osc.edu, and NORAD-Atomic-Data at: www.astronomy.ohio-state.edu/∼nahar/nahar_radiativeatomicdata/index.html. These resources can also be utilized for atmospheric models.
A detailed solar spectrum at the earth’s surface after attenuation by the chemical constituents of the atmosphere and without consideration of change of change in greenhouse gases (e.g. R. Kurucz, CfA, Harvard University, kukurucz.harvard.edu/sun/fluxatlas) shows that the lines corresponding to various photon absorptions are closely lying for ultra-violet photons indicating high absorption of the radiation and less dense for optical radiation indicating less absorption. Yellow is absorbed minimum (reason for the Sun to look yellow). Absorption of lines increases in the infrared range due to absorption mostly by water. The best calculations for H2O opacity in atmosphere were done using over 800M transitions.
With changes in the atmospheric composition the spectrum will obviously change. It may be noted that some bands that are saturated (i.e. 100% of radiation in that band is absorbed) can be affected by further increases in greenhouse gas concentrations. The greenhouse gases will cause the radiation to be captured closer to the earth’s surface and increase the temperature. Detailed and accurate study of atmospheric opacities will therefore provide more accurate radiation transfer with varying components, solar heating of the earth, and more precise predictions of global warming and climate change. Such calculations will require high-performance large-scale atomic and molecular calculations, as, for example, carried out at the Ohio Supercomputer Center in Columbus, Ohio.
Finally, we mention a few space observatories engaged in atmospheric research. NASA, along with space agencies from other countries, has a fleet of satellites known as the A-Train to study the atmosphere affecting the global temperature. Satellite Aqua collects information on earth’s water cycle, Cloudsat studies clouds for their role in regulating the climate, Calipso studies the way aerosols interact with clouds, Parasol can distinguish natural from human-produced aerosols and measures polarized light, Glory measures the energy budget of the earth and determines the global temperature, and Aura maps global pollution. NASA’s Orbiting Carbon Observatory (OCO) was launched to pinpoint key locations on the earth’s surface where CO2 is being emitted and absorbed, but was lost in space while setting in its orbit. However, Japan’s Gosat is carrying out similar observations on global greenhouse effects through spectroscopy of the sunlight reflected off the earth’s surface, in which the constituent colors determine how much carbon dioxide and molecular oxygen are present. Two carbon observatories, A-SCOPE (Advanced Space Carbon and Climate Observation of Planet Earth) and BIOMASS, are planned to be launched from Europe in 2016.
6 Conclusion
-
The sun is the main source of energy for living conditions that depend on the thermal structure in the atmosphere.
-
The relation between solar irradiation and earth’s atmosphere needs to be understood; atomic and molecular processes are inherent to atmospheric models. Numerical simulation requires complex quantum-mechanical calculations for atomic and molecular processes using high-performance computing platforms.
-
Large number of atomic parameters for radiative processes in the atmosphere are needed. A concerted MULTI-DISCIPLINARY effort is crucial in solving the problem of global warming and protect our home planet.
-
PLAN: Calculation of accurate atmospheric opacities and models.
Abbreviations
- ABC:
-
Atmospheric brown cloud
- CFC:
-
Chlorofluorocarbon
- CSIRO:
-
Council of Scientific & Ind. Research
- DES:
-
Dielectric satellite
- HFC:
-
Hydrofluorocarbon
- INODEX:
-
Indian Ocean Experiment
- IPCC:
-
International Government Panel on Climate Change
- OCO:
-
Orbiting Carbon Observatory
- UNEP:
-
UN Environment Program
- UV:
-
Ultraviolet
References
Hummer DG, Berrington KA, Eissner W, Pradhan Anil K, Saraph HE, Tully JA (1993) Atomic data from the IRON project. 1: goals and methods. Astron Astrophys 279:298–309
Nahar SN (1995, 1996) Electron-ion recombination rate coefficients for Si I, Si II, S II, S III, C II and C-like ions C I, N II, O III, F IV, Ne V, Na VI, Mg VII, Al VIII, Si IX, and S XI. Astrophys Suppl J 101:423–434, 106:213–214
Nahar SN (1998) Photoionization cross sections and oscillator strengths for oxygen ions: O i-O vii. Phys Rev A 58:3766–3782
Nahar SN, Pradhan AK (1997) Electron-ion recombination rate coefficients, photoionization cross sections, and ionization fractions for astrophysically abundant elements I. carbon and nitrogen. Astrophys J Suppl 111:339–355
Nahar SN, Pradhan AK (2006) Dielectronic satellite spectra of helium like iron and nickel from the unified recombination method. Phys Rev A. 73:062718-1-8
Ramanathan V, Ramana MV et al (2007) Warming trends in Asia amplified by brown cloud solar absorption. Nature 448:575–578
The Opacity Project Team (1995, 1996) The Opacity Project, vol 1 & 2. Institute of Physics, London
Acknowledgements
Partially supported by a NASA grant.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Nahar, S.N. (2010). Solar Irradiance of the Earth’s Atmosphere. In: Lal, R., Sivakumar, M., Faiz, S., Mustafizur Rahman, A., Islam, K. (eds) Climate Change and Food Security in South Asia. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-9516-9_3
Download citation
DOI: https://doi.org/10.1007/978-90-481-9516-9_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-9515-2
Online ISBN: 978-90-481-9516-9
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)