Abstract
The cholesterol-dependent cytolysins (CDCs) are a family of β-barrel pore-forming toxins secreted by Gram-positive bacteria. These toxins are produced as water-soluble monomeric proteins that after binding to the target cell oligomerize on the membrane surface forming a ring-like pre-pore complex, and finally insert a large β-barrel into the membrane (about 250 Å in diameter). Formation of such a large transmembrane structure requires multiple and coordinated conformational changes. The presence of cholesterol in the target membrane is absolutely required for pore-formation, and therefore it was long thought that cholesterol was the cellular receptor for these toxins. However, not all the CDCs require cholesterol for binding. Intermedilysin, secreted by Streptoccocus intermedius only binds to membranes containing a protein receptor, but forms pores only if the membrane contains sufficient cholesterol. In contrast, perfringolysin O, secreted by Clostridium perfringens, only binds to membranes containing substantial amounts of cholesterol. The mechanisms by which cholesterol regulates the cytolytic activity of the CDCs are not understood at the molecular level. The C-terminus of perfringolysin O is involved in cholesterol recognition, and changes in the conformation of the loops located at the distal tip of this domain affect the toxin-membrane interactions. At the same time, the distribution of cholesterol in the membrane can modulate toxin binding. Recent studies support the concept that there is a dynamic interplay between the cholesterol-binding domain of the CDCs and the excess of cholesterol molecules in the target membrane.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Cholesterol
- Membranes
- Pore-forming toxins
- Cholesterol-dependent cytolysins
- Membrane structure
- Cholesterol activity
- Transmembrane beta-barrel
- Transmembrane pore
- Fluorescence spectroscopy
- Perfringolysin
- Lipid cluster
20.1 Introduction
The cholesterol-dependent cytolysins (CDCs) are a growing group of β-barrel pore-forming toxins secreted by Gram-positive bacteria (Farrand et al., 2008; Gelber et al., 2008; Heuck et al., 2001; Jefferies et al., 2007; Mosser and Rest, 2006), and the first members were discovered more than a century ago (see Alouf et al., 2006 for a historical background on the CDCs). To date, there are complete amino acid sequences for 28 species distributed among the phyla of Firmicutes (genera of Bacillus, Paenibacillus, Lysinibacillus, Listeria, Streptococcus, and Clostridium), and of Actinobacteria (genera of Arcanobacterium and Gardenella) (Table 20.1). Most of the CDCs have a cleavable signal sequence and are therefore secreted to the extracellular medium via the general secretion system (see Harwood and Cranenburgh, 2008). A few exceptions are species of the genus Streptoccocus (S. pneumoniae, S. mitis, and S. pseudoneumoniae) that produce CDC without a signal sequence. The secretion mechanism for these CDCs is unclear (Jefferies et al., 2007; Marriott et al., 2008). After secretion to the extracellular medium, the CDCs fold into water-soluble monomeric proteins, travel and bind to the target membrane, and oligomerize on the membrane surface forming characteristic arcs and ring-like structures which are responsible for cytolysis. Several reviews have been published describing the recent advances in the structural and mechanistic studies of the CDCs (Alouf et al., 2006; Giddings et al., 2006; Gilbert, 2005; Rossjohn et al., 2007; Tweten, 2005). Here, we will focus on the role played by cholesterol during the transformation of the CDC from a water-soluble monomer to a membrane-inserted oligomeric complex. Although the cholesterol-dependent inhibition of the activity for these toxins was one of the first biochemical properties attributed to the family (Arrhenius, 1907), the molecular mechanism of the cholesterol-toxin interaction remains as one of the least understood aspects in the study of the CDC family.
20.2 Mechanism of Pore Formation
The 28 CDC family members listed in Table 20.1 show a significant degree of amino acid identity (from 28.1 to 99.6%) and similarity (greater than 45.7%), with amino acid sequences ranging from 471 to 665 amino acids in length. A comparison of
the primary structure of these proteins shows that they share a very low degree of similarity at their N-terminus, in part because different species employ distinct signal sequences for secretion, but also because some of the CDC members possess additional domains located in this region (e.g., Farrand et al., 2008). If we consider just the conserved core shared by all CDCs and required for pore-formation activity [amino acids 38–500 in perfringolysin O (PFO)], the amino acid identity and similarity among different members becomes higher than 36.7 and 58%, respectively (sequence length of analyzed sequences range from 462 to 469, Fig. 20.1). Therefore, from the analysis of the primary structure of these toxins we can anticipate that all the CDCs will exhibit similar activities and three-dimensional structures.
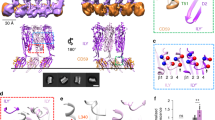
The first crystal structure for a CDC was solved for PFO by Rossjohn and colleagues (1997). The crystal structure for two other CDCs, intermedylisin (ILY) and anthrolysin (ALO), have been solved so far, and all of them share similar secondary and tertiary structure (Bourdeau et al., 2009; Polekhina et al., 2005). They have a high β-strand content and their structures have been divided into four domains, with the C-terminal domain (domain 4 or D4) being the only independent and continuous domain (Fig. 20.2A) (Polekhina et al., 2006).
PFO secreted by the pathogen Clostridium perfringens is a prototypical CDC (Tweten et al., 2001). To describe the general mechanism of pore-formation for the CDC we will depict the current knowledge of the PFO cytolytic mechanism which starts with the binding of the toxin to the target membrane and concludes with the insertion of a large transmembrane β-barrel (Fig. 20.2A).
Analysis of the primary structure for the CDCs reveals a high degree of identity and similarity among them. Only the sequence for the conserved core of the CDCs was used for the analysis (corresponding to PFO amino acids 38–500). If more than one sequence was available for individual species, only one was used in the analysis. The databank access numbers are provided in Table 20.1. Sequence relationships were calculated using the MatGat 2.02 alignment program using the BLOSUM 62 matrix and open and extension gap penalties of 12 and 1, respectively (Campanella et al., 2003). The identity scores occupy the upper triangle (in bold) with scores higher than 70% shaded in dark gray, and those at 50–70% in light gray. Similarity scores in the lower triangle where shaded in dark gray if higher than 80% and in light gray if between 70 and 80%
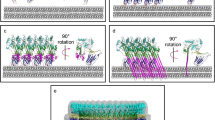
Pore formation mechanism for the CDCs. Secreted as water-soluble monomeric proteins, the toxins bind to the target membrane and oligomerize into a ring-like structure called the pre-pore complex. A poorly understood conformational change then leads to the insertion of the TMHs into the bilayer to form the aqueous pore. (A) Stages of PFO pore formation. The defined PFO structural domains are numbered. The membrane bilayer is depicted with cholesterol molecules (ovals) intercalated between the phospholipid constituents. Membrane binding is accomplished as D4 interacts with membrane regions having free cholesterol molecules available. Subsequent allosteric rearrangements within the monomer promote oligomerization and pore-formation. (B) Conformational changes in domain 3 of PFO are required for monomer–monomer association and β-barrel pore formation. Each stage corresponds to the stage shown above in (A). The TMH1 is shown as bicolor and the TMH2 in black. The small β5 strand is shown as a black loop. The aromatic residues involved in the alignment of the β-strands are shown as open rectangles. Adapted from Ramachandran et al. (2004), with permission
Upon encountering a cholesterol-containing membrane, PFO oligomerizes and spontaneously inserts into the bilayer to form a large transmembrane pore (∼35–50 monomers per oligomer; approximately 250 Å in diameter, Fig. 20.2), (Czajkowsky et al., 2004; Dang et al., 2005; Mitsui et al., 1979; Olofsson et al., 1993). The C-terminus of PFO (D4) encounters the membrane first (Fig. 20.2A, I, Heuck et al., 2000; Nakamura et al., 1995; Ramachandran et al., 2002). The binding of D4 triggers the structural rearrangements required to initiate the oligomerization of PFO monomers (Ramachandran et al., 2004; Soltani et al., 2007a) and formation of a pre-pore complex on the membrane surface (Fig. 20.2A, II, Heuck et al., 2003; Shepard et al., 2000; Tilley et al., 2005). Pore formation commences when two amphipathic β-hairpins from each PFO molecule insert and span the membrane (Fig. 20.2A, III, Hotze et al., 2002; Shatursky et al., 1999; Shepard et al., 1998). The concerted insertion of two transmembrane β-hairpins (TMHs) from ∼35 PFO monomers then creates the large transmembrane β-barrel that penetrates the membrane (Dang et al., 2005; Tilley et al., 2005). This general mechanism of pore-formation is followed by most CDCs, however, some variations have been observed for specific members and they will be described in the following sections.
20.2.1 Localizing the Target Membrane
The first step in the CDC cytolytic cascade is the recognition of the target cell (Fig. 20.2A, I). The CDC binds to the target membrane by recognizing a specific membrane lipid, cholesterol, or by recognizing a membrane-anchored protein in the case of ILY (Giddings et al., 2004). Cholesterol-recognition provides specificity towards eukaryotic cells in general, and the glycosylphosphatidylinositol-anchored protein CD59 provides specificity for human cells. While it has been shown that ILY interacts with the CD59 receptor forming a 1:1 complex (Lachapelle et al., 2009), the interaction of other CDCs with cholesterol is less well understood. Independently of the recognition mechanism, it appears that all CDCs bind to the target membranes via D4 (Nagamune et al., 2004; Soltani et al., 2007a).
20.2.2 Grouping Forces on the Membrane Surface: Pre-pore Formation
After successful recognition of the target membrane, the CDC oligomerize in the membrane surface to form a membrane-bound pre-pore complex (Fig. 20.2, II). Formation of a pre-pore complex seems to be a common feature of the β-barrel pore-forming toxins (Heuck et al., 2001; Miller et al., 1999; Shepard et al., 2000; Walker et al., 1992). In general, the secreted monomeric proteins do not oligomerize spontaneously in solution, and it has been shown that the binding of the toxins to the target membrane is required to trigger the monomer-monomer association (Abdel Ghani et al., 1999; Lachapelle et al., 2009; Ramachandran et al., 2004). Although oligomerization has been observed in the absence of membranes for certain CDCs (e.g., pneumolysin, (PLY) Gilbert et al., 1998; Solovyova et al., 2004), it only occurs when the toxin concentration is relatively high (in the micromolar range or higher), compared to the low concentration needed for efficient oligomerization when incubated with natural membranes. The difference in efficiency between oligomerization in solution and at the surface of a cell membrane suggests that the cells in some way promote the association of toxin monomers. In general, oligomerization of β-barrel pore-forming toxins requires the exposure of hidden polypeptide regions involved in the monomer-monomer interaction (Heuck and Johnson, 2005; Heuck et al., 2001). In the CDC, this process is triggered by conformational changes induced by protein-lipid interactions (e.g., PFO, Ramachandran et al., 2004) or by conformational changes induced by protein-protein interactions (e.g., ILY, Lachapelle et al., 2009).
Ramachandran et al. (2004) have shown that in the water-soluble form of the toxin, oligomerization is prevented by blocking access to one edge of a core β-sheet in the monomer (Fig. 20.2B). This blockage prevents its association with the edge of the core β-sheet in the neighboring monomer, thus impeding formation of an extended β-sheet. Specifically and importantly, premature association of PFO molecules (before they bind to the appropriate membrane surface) is prevented by the presence of β5, a short polypeptide loop that hydrogen bonds to β4 in the monomer, and thereby prevents its interaction with the β1 strand in the adjacent monomer. This feature is conserved in all crystal structures so far reported for the CDCs (i.e., PFO, ILY, and ALO).
The structural changes associated with converting a CDC from a water-soluble monomer to a membrane-inserted oligomer extend through much of the molecule. The binding of D4 to the membrane surface immediately elicits a conformational change in domain 3, more than 70 Å above the membrane (Abdel Ghani et al., 1999; Heuck et al., 2000; Ramachandran et al., 2002, 2004, 2005). This conformational change rotates β5 away from β4 and thereby exposes β4 to the aqueous medium where it can associate with the always-exposed β1 strand of another PFO molecule, to initiate and promote oligomerization (Fig. 20.2B).
Such an extensive network of structural linkages within a CDC can be advantageous because it reduces the chance of prematurely entering a structural transition that exposes a TMH. By allosterically linking different domains or regions of the protein, the system can couple separate interactions (e.g., binding to the membrane and binding to another subunit) and thereby ensure that pore formation proceeds only when the necessary criteria are met. Given the important allosteric communication between the membrane binding domain and the pore-forming domain, it is not surprising that the most conserved regions on these proteins are located among inter-domain segments, forming an almost continuous path with its origin at the tip of D4 and terminus at the segments that form the amphipathic TMHs (Fig. 20.3). Interestingly, while most of the surface exposed residues of the CDCs are not very conserved, the residues at the surface of the D4 tip, involve in membrane interaction, are highly conserved.
Comparison of PFO homologs reveals a conserved core backbone. Alignment and comparison of the composite members of the CDC family reveals conserved regions that extend from the tip of the membrane recognition domain, D4, through the regions involved in oligomerization and membrane insertion. (A) Cartoon representation of PFO with the conserved residues shown in black. (B) Surface representation of PFO the conserved core highlighted in black. It is postulated that this conserved backbone is especially adapted to allosterically communicate successful, cholesterol-dependent membrane binding, and thus permit subsequent conformational adaptations that favor oligomerization and pore formation. Alignment of the 28 CDC sequences was effected using the PRALINE multiple sequence alignment tool using a BLOSUM62 matrix with open and extension gap penalties set at 12 and 1, respectively, a PSI-BLAST pre-profile processing with iterations set at 3, e-value cut off set at 0.01, non-redundant data bases, and a DSSP-defined secondary structure search using PSIPRED (Simossis et al., 2005). PFO structure representation was rendered using PyMol (DeLano Scientific LLC)
Establishment of an oligomeric complex in the membrane surface facilitates the formation of a transmembrane pore because the insertion of a single amphipathic β-hairpin into a membrane is not energetically favored. In a hydrophobic environment that lacks hydrogen bond donors or acceptors, isolated β-hairpins cannot achieve the hydrogen-bond formation necessary to lower the thermodynamic cost of transferring the polar atoms of the polypeptide backbone into the hydrocarbon interior (White and Wimley, 1999). However, this energy barrier is circumvented if the β-strands are inserted as β-sheets and form closed structures such as a β-barrel. For monomeric β-barrel membrane proteins such as OmpA, a concerted folding mechanism has been observed in vitro, in which the hydrogen bonds formed between adjacent β-chains presumably favor the insertion of the β-barrel into the membrane (Kleinschmidt, 2006; Tamm et al., 2004). Similarly, the formation of a pre-pore complex may be required to allow the concerted, and perhaps simultaneous, insertion of the β-hairpins from individual monomers, thereby overcoming the energetic barrier of inserting non-hydrogen-bonded β-strands into the membrane bilayer. Whereas it is clear that the formation of a complete ring (or pre-pore complex) on the membrane surface will minimize the energetic requirements for inserting a β-barrel into the membrane, it is likely that the insertion of incomplete rings can also occur if monomer recruitment into the oligomer slows down. In the absence of additional monomers, the incomplete pre-pore complexes observed in vitro (or metastable arc structures) will be trapped, and they may have enough time to insert into the membrane and form a pore (Gilbert, 2005). Insertion of an arc may well form a transmembrane pore by itself, or in association with other arcs (double arc structures, see Palmer et al., 1998). A minimal number of monomers must be required to overcome the energetic barrier of inserting an arc-like β-sheet into the membrane. It has been shown that independently of the toxin/lipid ratio, the pores formed by PFO and streptolysin O (SLO) are at least large enough to allow the passage of proteins with an approximate diameter of 100 Å (Heuck et al., 2003).
In summary, a coordinated train of events regulates the proper assembly of the CDC oligomeric complex at the surface of the target membrane. Formation of these oligomeric structures facilitates the insertion of numerous TMHs, which are required to form the large transmembrane β-barrel.
20.2.3 Perforating the Membrane: Insertion of a Large β-Barrel
A characteristic of the CDC that distinguishes them from most other β-barrel pore-forming toxins is the use of two amphipathic β-hairpins per monomer to form the large transmembrane barrel (Heuck and Johnson, 2005; Heuck et al., 2001; Shatursky et al., 1999). In the water-soluble monomeric configuration of the CDC these TMHs are folded as short α-helices, presumably to minimize the exposure of the hydrophobic surfaces (Heuck and Johnson, 2005). These helices, located at either side of the central β-sheet in domain 3, extend and insert into the membrane bilayer (Shatursky et al., 1999; Shepard et al., 1998). The conversion of short α-helices to amphipathic β-hairpins constituted a new paradigm for how pore-forming toxins transform from a water-soluble to membrane-inserted conformation. This structural transformation has been recently found in eukaryotic pore-forming proteins, as revealed by the structure of the membrane attack complex/perforin superfamily members (Hadders et al., 2007; Rosado et al., 2007). After insertion, the hydrophobic surfaces of the TMHs are exposed to the non-polar lipid core of the membrane and the hydrophilic surfaces face the aqueous pore. A concerted mechanism of insertion ensures that the hydrophilic surfaces of the hairpins remain exposed to the aqueous medium, and not to the hydrophobic core of the membrane. Such a coordinated insertion requires the displacement of membrane bilayer lipids as the aqueous pore is formed in the membrane.
The creation of a circular hole, having a radius of nearly 150 Å, in a liposomal membrane requires the displacement of about 1000 phospholipid molecules in each monolayer (or about 800 phospholipids plus 800 cholesterol molecules, because the average surface area occupied by one phospholipid molecule plus one cholesterol molecule is ∼90 Å2 in a 1:1 phospholipid/cholesterol mixture) (Heuck et al., 2001; Lecuyer and Dervichian, 1969). Analysis of the release of markers encapsulated in liposomes when using limiting concentrations of PFO or SLO have shown that both the small markers and the large markers are released at the same rate. Therefore, it appears that all of these lipid molecules leave or are displaced from the pore formed by these CDCs at the same time (Heuck et al., 2003), though not all agree (Palmer et al., 1998).
A direct comparison of the cytolytic mechanism of PFO and ILY showed that whereas ILY does not require cholesterol for binding, pore-formation is subsequently entirely dependent on the presence of cholesterol in the target membrane (Giddings et al., 2003). Employing a series of ILY mutants that block pore formation at different stages, Hotze and colleagues have shown that ILY remains engaged with its receptor (human CD59) throughout the assembly of the pre-pore complex, but it is released from CD59 upon the transition to the membrane-inserted oligomer (Lachapelle et al., 2009). Upon release from the receptor, ILY is anchored to the membrane via D4 suggesting that this domain still conserves the cholesterol-binding properties of other CDC members (note that insertion of the ILY β-barrel does not occur if cholesterol is depleted from the membrane).
After pre-pore formation, the insertion of the PFO TMHs requires the appropriate intermonomer β-strand alignment. Ramachandran et al. (2004) suggested that the π-stacking interaction between Y181 and F318 guides the alignment of the TMHs of adjacent monomers (Fig. 20.2B). Interestingly, while Y181 is completely conserved in the 28 members of the CDC family, F318 is not. Instead of phenylalanine, this position is occupied by valine in lectinolysin, vaginolysin, and PLY, by isoleucine in ILY, and alanine in pyolysin. It will be interesting to determine if a mutation of the conserved PFO-Y181-equivalent in ILY results in a pre-pore blocked derivative, as observed in PFO.
20.3 The Role of Cholesterol in Membrane Binding
Among all the different lipids that shape the vast diversity of cell membranes, the presence of cholesterol is a distinguishing feature of mammalian cells. The CDCs have evolved to take advantage of this feature of mammalian membranes, and their ability to perforate the target membrane is totally dependent on the presence of cholesterol (Giddings et al., 2003; Palmer, 2004).
In liposomal membranes containing only phosphatidylcholine and cholesterol, more than 30 mole % cholesterol is required for CDCs such as tetanolysin (Alving et al., 1979), SLO (Rosenqvist et al., 1980), and PFO (Heuck et al., 2000; Ohno-Iwashita et al., 1992), to bind and create a pore in the bilayer. For PFO, no binding at all is detected when the cholesterol concentration in the liposomal membrane is less than ∼30 mole% of the total lipids (Flanagan et al., 2009; Heuck et al., 2000; Nelson et al., 2008). Thus, if cholesterol acts solely as a receptor, and hence as a PFO binding ligand, reducing the cholesterol concentration in the bilayer should only affect the kinetics of the cytolytic process. In other words, lowering the amount of cholesterol in the membrane should result in a longer time required for PFO to form a transmembrane pore. However, the sharp transition observed in the binding isotherm of PFO suggests that the basis of this recognition is more complex than a simple encounter frequency between PFO and individual cholesterol molecules (Heuck et al., 2000).
20.3.1 Domain 4 and Membrane Recognition
The initial members of the CDC family were characterized by their sensitivity to oxygen and cholesterol (Alouf et al., 2006). Toxins isolated from culture supernatants were inactivated by exposure to oxygen present in the air or when pre-incubated with cholesterol. While the oxygen-dependent inactivation of the toxins could be reversed by incubation with thiol-based reducing agents, inactivation by pre-incubation with cholesterol was not reversible. A direct consequence of these findings was that the discovery of new CDC members was strongly influenced by the search for these two distinguishing features in the newly encountered hemolytic toxins: i.e. inhibition by oxygen and cholesterol. Therefore, it is not surprising that the first sequences obtained for CDCs revealed that all of them contained a conserved undecapeptide which was critical for cholesterol recognition, and a unique cysteine in this segment that was sensitive to aerobic oxidation. This correlation led researchers to postulate that the conserved undecapeptide, and attendant cysteine constituted the cholesterol-binding site for the CDC. However, advancements in recombinant DNA technology soon allowed researchers to show that this unique cysteine was not essential for cholesterol recognition. First, the replacement of this cysteine with alanine rendered a protein that remained hemolytic (Michel et al., 1990; Pinkney et al., 1989; Saunders et al., 1989; Shepard et al., 1998). Second, the sequence of newly discovered CDC members showed that this cysteine was indeed replaced by alanine during the evolution of different Gram-positive species (Billington et al., 2001; Nagamune et al., 2000).
New protein homologues of the CDCs are being revealed as new genomes are sequenced, and these new family members show greater variability in the amino acid sequence of this segment. The multi-sequence alignment for the 28 CDC sequences shows that 20% of the CDCs contain amino acid substitutions in the conserved undecapeptide. Based on this newly accumulated evidence, the original view of the conserved undecapeptide as the cholesterol binding site is being replaced by alternative models for membrane-binding. It has been shown that one of the CDCs, intermedilysin (ILY) recognize the target membrane by the specific binding to a human protein receptor CD59, and it is therefore possible that other members may also bind to the target membrane by as yet unidentified protein receptors (Bourdeau et al., 2009). In addition to the undecapeptide, other well conserved peptide loops located at the tip of D4 may contribute to the cholesterol recognition motif (Ramachandran et al., 2002; Soltani et al., 2007a; Soltani et al., 2007b).
20.3.1.1 The Conserved Loops
PFO D4 has a 4 stranded β-sandwich structure that interacts with the membrane surface only at one end, via the distal loops that interconnect the eight β-strands that form the domain (Fig. 20.4A, Ramachandran et al., 2002; Rossjohn et al., 1997; Soltani et al., 2007a). Superimposition of the D4 α-carbons for PFO, ALO, and ILY reveals that the global structure of D4 is well conserved among these members. The main differences arise in the conformation of the undecapeptide, involved in toxin-membrane interaction, and in the loops that are close to the domain 2-D4 interface (Fig. 20.4A).
The three dimensional structure of D4 is highly conserved in the CDC family. (A) Comparison of D4 from three CDC homologs highlights the conserved architecture of this C-terminal domain. A cartoon, upper left, clarifies the threading of 2 β-sheets and loops in the β-sandwich and indicates the spatial organization of the undecapeptide, L1, and L2. The α-backbone for the D4 domains of PFO, ILY, and ALO were superimposed using PyMol (DeLano Scientific LLC; available at http://www.pymol.org). (B) Alignment of the sequence for the 28 CDC family members reveals substantial conservation in loops L1, L2 and the undecapeptide. While integrity of the undecapeptide was long recognized for being critical to the cholesterol-dependent activity of these toxins, other loops are also important. Residues conserved in all sequences are shaded in black, and highly conserved residues are shaded in gray. Protein names are as in Fig. 20.2. Residue numbers correspond to the PFO sequence. Multiple sequence alignment was effected as indicated in Fig. 20.3
Three of the four loops located at the distal tip of D4 are highly conserved among the CDC members: the conserved undecapeptide (also known as the Trp-rich loop), L1, and L2 (Fig. 20.4B). The L3 loop is less conserved and is located farther away from the unique cysteine residue. Recent data obtained by Tweten and colleagues suggest that in addition to the undecapeptide, the other D4 loops (L1–L3) may also play a role in the cholesterol-dependent recognition of the CDC (Soltani et al., 2007b). Single amino acid modifications in these loops prevented the binding of PFO to cholesterol-rich liposomes, and abolished the pre-pore to pore transition for ILY in a cholesterol-dependent manner. Both of these events involve the association of the D4 with the cholesterol-containing membrane. It has become clear that the three-dimensional arrangement of the undecapeptide and the L1–L3 loops is important for the association of the CDC with the cholesterol-containing membrane (Giddings et al., 2003; Polekhina et al., 2005; Soltani et al., 2007a, b).
Interestingly, changes in the pH of the medium which affect the conformation of D4 also influence the cholesterol-toxin interaction. A reduction of the pH from 7.5 to 6.0 induces a conformational change in PFO causing the tryptophan residues to be more exposed to the aqueous solvent, and also alters the threshold for the minimal cholesterol concentration required to trigger binding of PFO to liposomal membranes (Nelson et al., 2008). Since no major changes are expected to occur in the structure of the membrane in between pH 7.5 and 6.0, one can assume that protonation of certain amino acids in PFO may alter the D4 conformation, and as a consequence, its ability to recognize cholesterol in the target membrane. A related effect has been observed for listeriolysin O (LLO), a CDC recognized for having an optimum acidic pH for activity (Bavdek et al., 2007). However, the loss of activity of LLO at neutral pH can be rescued by increasing the concentration of cholesterol in the membrane.
Given that conformational changes in D4 can alter the cholesterol-dependent properties of the CDC, one can speculate that the conformational change triggered by the binding of ILY to the CD59 receptor (Soltani et al., 2007a), may modulate the cholesterol-dependent association with the membrane required for pore-formation.
Unfortunately, despite the various high-resolution structures available for the CDCs, and the multiple functional data obtained by modification of amino acids located at the D4 loops, it is still unclear how cholesterol modulates the conformational changes required to anchor the toxin to the membrane and to insert a large transmembrane β-barrel. Furthermore, is not clear if the binding of PFO (and related CDCs) is triggered by the binding of a single cholesterol molecule (Geoffroy and Alouf, 1983; Nollmann et al., 2004; Polekhina et al., 2005), or by the recognition of a more complex cholesterol-arrangement in the bilayer structure (Bavdek et al., 2007; Flanagan et al., 2009; Heuck and Johnson, 2005; Heuck et al., 2007; Nelson et al., 2008).
20.3.2 Searching for Cholesterol in the Membrane
The binding of a protein domain to a membrane surface is in general, a two-step process that involves the initial formation of non-specific collisional complex, followed by the formation of a tightly bound complex. The first step is diffusional and may involve electrostatic interactions, and the second step stabilizes the initial interaction by membrane penetration of non-polar amino acids and/or specific interactions between the protein and the membrane lipids (Cho and Stahelin, 2005). The initial membrane association locates non-polar amino acids close to the interfacial region of the bilayer, facilitating their exposure to the hydrophobic core. Non-polar amino acids are not usually exposed to the protein surface, and therefore conformational changes are required to expose them to the membrane.
Exposure of the aromatic residues located in the undecapeptide occurs upon membrane binding, though they do not penetrate deeply into the bilayer core (Heuck et al., 2003; Nakamura et al., 1998; Sekino-Suzuki et al., 1996). The sensitivity of the undecapeptide to amino acid changes suggests that the exposure of aromatic amino acids and membrane binding requires precise conformational changes and/or a particular three-dimensional conformation. A conformational change in the undecapeptide that modulates cholesterol binding and membrane anchoring has been suggested for PFO (Rossjohn et al., 1997), however the binding site for cholesterol, if any, remains elusive.
It has become apparent that in addition to the three dimensional structure of the binding-domain, the arrangement of the cholesterol molecules in the bilayer is also critical for successful binding. In a membrane, the cholesterol molecules are mobile in the non-polar core of the bilayer with an orientation nearly parallel to the acyl chains of the phospholipids. The non-polar hydrocarbon tail of the molecule orients towards the center of the bilayer, and the 3-β-OH group locates close to the ester bonds formed by the fatty acid chains and the glycerol backbone of the phospholipids near the membrane-water interface. Compared to the phospholipid head groups, the polar group of the cholesterol molecule is not highly exposed at the membrane surface. Therefore, it is not strange that at such relatively low concentrations, few cholesterol molecules should be available to interact with water-soluble molecules (e.g., cholesterol oxidase, cyclodextrins or CDCs) (Lange et al., 1980).
20.3.2.1 Cholesterol Availability in Membrane Bilayers
In multi-component membranes, the availability of cholesterol at the membrane surface is regulated by the interactions between cholesterol and other the components of the membrane (phospholipids, glycolipids and proteins). The more the cholesterol interacts with the othere membrane components, the less available it will be to interact with extra-membranous molecules. Factors that affect the interaction of cholesterol with phospholipids are the length of the acyl chains, the presence of double bonds in these chains, the size of the polar head-groups, and the ability of the phospholipid to form hydrogen bonds with the hydroxyl group of cholesterol (Ohvo-Rekilä et al., 2002).
When cholesterol is added to a membrane containing a single phospholipid species, two phases appear in a concentration-dependent manner (Mouritsen and Zuckermann, 2004; Sankaram and Thompson, 1991). This suggests that instead of randomly distributing among the membrane phospholipids, cholesterol associates with the phospholipids, presumably forming stoichiometric complexes (Radhakrishnan and Mcconnell, 1999). When the phospholipids are in excess, most of the cholesterol molecules form complexes with phospholipids. These complexes are immiscible in the pure phospholipid phase and therefore a two-phase mixture appears in the membrane. Increasing the cholesterol concentration will increase the population of the complexes until they form a single phase containing the complexes with a minor presence of uncomplexed phospholipids and cholesterol molecules. Beyond this point, the added cholesterol molecules (free cholesterol) will mix with the complexes until they reach the solubility limit and precipitate out of the membrane (Mason et al., 2003). Cholesterol molecules do not form stable single bilayers in aqueous solution, so when present in excess they cannot form a new stable and extended phase. The free cholesterol molecules in excess are likely to have a tendency to “fly” away from the membrane, and outside the membrane due to their low solubility they will be prone to associate to form multi-bilayer crystals in aqueous solution (Harris, 1988).
The formation of phospholipid-cholesterol complexes can explain the low interaction detected between cyclodextrins and cholesterol when the membrane sterol is present in low amounts (Mcconnell and Radhakrishnan, 2003). An alternative model to account for this behavior was proposed by Huang and Feigenson (1999). These authors propose that the hydrophobic effect positions the phospholipid head groups toward the membrane surface to protect the hydrophobic molecule of cholesterol from the unfavorable contact with water. When the concentration of cholesterol in the membrane achieves and exceeds the protective capacity of the head-groups, the tendency for the sterol molecules to exit the membrane will increase.
Both models provide a reasonable explanation for the increased accessibility of cholesterol at high sterol/phospholipid ratios, and the consensus is that they are not mutually exclusive (Lange and Steck, 2008; Mesmin and Maxfield, 2009). Binding (and/or pore-formation) of the CDCs occurs at high cholesterol concentration where free cholesterol becomes available, and therefore any of these models can be used to explain the experimental observations.
In more complex lipids mixtures, when more than one phospholipid is present in the membrane, the total cholesterol content will distribute unevenly between any formed phases (Goñi et al., 2008; Veatch and Keller, 2002). How much cholesterol is present in each phase will be governed by the interaction between cholesterol and the components (lipids and proteins) present in the phases (Epand, 2006).
20.3.2.2 The Role of Other Lipids
The pioneering work of Ohno-Iwashita and colleagues on the binding of PFO to membranes showed that the phospholipid composition affects the arrangement of cholesterol in the membrane (see also Chapter 22). Using a protease-nicked derivate of PFO they showed that the binding of the toxin was not only influenced by the total amount of cholesterol present in the membrane, but also by the phospholipid composition. They found that this PFO derivative preferentially binds to cholesterol-rich membranes composed of phospholipids with 18-carbon acyl chains (Ohno-Iwashita et al., 1992, 1991). An effect on cholesterol state in the membrane by ceramides and glycerolipids was also suggested by Zitzer et al. (2003), based on their studies of SLO pore-formation in liposomal membranes prepared with different phospholipids. Lipids having a conical molecular shape appear to effect a change in the energetic state of membrane cholesterol that in turn augments the interaction of the sterol with the cholesterol-specific cytolysin. Interestingly, these authors also showed that SLO was active when membranes were prepared solely with the enantiomeric cholesterol, suggesting that the effect associated with the presence of cholesterol may be other than a site specific binding event (Zitzer et al., 2003).
A more systematic analysis of the interaction of PFO D4 with membranes prepared with different phospholipds and sterols revealed that PFO binding to the bilayer and the initiation of the sequence of events that culminate in the formation of a transmembrane pore depend on the availability of free cholesterol at the membrane surface (Flanagan et al., 2002; Flanagan et al., 2009; Nelson et al., 2008). These studies also showed that changes in the acyl chain packing of the phospholipids and cholesterol in the membrane core do not correlate with PFO binding. Taken together, all these studies suggest than the binding of PFO (and SLO) to the membrane is triggered when the concentration of cholesterol exceeds the association capacity of the phospholipids, and this cholesterol excess is then free to associate with the toxin (Fig. 20.5).
PFO only binds to membranes containing free cholesterol molecules. Examples of mechanisms for cholesterol-dependent anchoring of PFO to the membrane surface: (A) PFO cannot stably bind to the bilayer if there are no free cholesterol molecules available in the membrane surface. (B) At high cholesterol concentrations free cholesterol molecules become available (black ovals), and D4 can anchor to the bilayer. In this example, a single cholesterol molecule binds to D4 and induces the conformational changes required to expose the D4 loops to the bilayer core. (C) Alternatively, the interplay between D4 and the membrane result in the redistribution of the lipids at the surface, clustering the free cholesterol molecules underneath the tip of D4. Anchoring may be accomplished by the interaction of multiple hydroxyl groups located in the cholesterol-rich cluster and the conserved amino acids of the loops
The requirement of such high cholesterol content in membranes was initially associated with the binding of PFO to cholesterol-rich domains (or membrane rafts) (Ohno-Iwashita et al., 2004; Waheed et al., 2001). However, recent results indicate that this assertion may require further analysis and consideration. It was found that the incorporation of sphingomyelin, a necessary component for the formation of membrane rafts, inhibited rather than promoted the binding of PFO to membranes (Flanagan et al., 2009). No correlation was found between PFO binding, and the amount of the detergent-resistant fraction in membranes, a fraction usually associated with membrane rafts (Flanagan et al., 2009). Incorporation of sterols that promote the formation of ordered membrane domains was not critical to promoting the PFO-membrane interaction (Nelson et al., 2008). Therefore, one needs to be cautious when employing PFO as a probe to reveal the presence of membrane rafts in cellular membranes. Rather than recognizing a particular membrane “raft”, PFO seems to bind to membranes containing free cholesterol (or where cholesterol has a high chemical activity).
20.3.2.3 Cholesterol Alone Is Enough
It was long known that incubation of SLO (Duncan and Schlegel, 1975; Johnson et al., 1980), PFO (Mitsui et al., 1979), cereolysin (Cowell and Bernheimer, 1978), alveolysin (Johnson et al., 1980), PLY (Johnson et al., 1980), and LLO (Vazquez-Boland et al., 1989) with cholesterol dispersed in aqueous solution produced the typical aggregated sterol-toxin complexes. For PFO and SLO, typical ring- and arc-like structures were observed after incubation with cholesterol at concentrations above its solubility limit (i.e., higher than 5 μM Duncan and Schlegel, 1975, Haberland and Reynolds, 1973, Harris et al., 1998, Mitsui et al., 1979).
To clarify the role of cholesterol in PFO cytolysis, the extent to which the different steps of the cytolytic mechanism could be elicited solely by the presence of cholesterol was analyzed (Heuck et al., 2007). Using site-directed fluorescence labelling of PFO in combination with multiple independent fluorescence techniques (Heuck and Johnson, 2002; Johnson, 2005), it was revealed that a selective interaction between the undecapeptide and the D4 loops with cholesterol dispersed in aqueous solution is indistinguishable from the interaction of PFO with cholesterol-containing membranes. Binding solely to cholesterol aggregates in aqueous solution is sufficient to initiate the coupled conformational changes that extend throughout the toxin molecule from the tip of D4 to the TMHs. Moreover, it was found that the topology of D4 bound to cholesterol aggregates was identical to the one observed in liposomal membranes, and that the binding of PFO to cholesterol aggregates was sufficient to trigger the conformational change in domain 3 that has been associated with oligomerization (Heuck et al., 2007; Ramachandran et al., 2004). As previously observed for SLO in cholesterol micro-crystals (Harris et al., 1998), oligomerization and formation of typical arc and ring structures were observed in the presence of cholesterol microcrystals. Surprisingly, none of these changes were produced by epicholesterol, a sterol that differs from cholesterol only in that the hydroxyl group is directed axially instead of equatorial (Heuck et al., 2007).
Taking advantage of the inability of PFO to recognize epicholesterol, competition experiments were done to examine how cholesterol packing in the bilayer affects the interactions with the membrane. More than 48 mole % cholesterol is required for PFO to bind to POPC-cholesterol liposomes (Flanagan et al., 2009). However, when the epicholesterol was mixed with cholesterol to maintain the concentration of total sterols constant at 48 mole %, and to reduce the net amount of cholesterol in the membrane, it was shown that in this case considerable binding of PFO was found with as little as 19 mole % cholesterol. Epicholesterol apparently intercalates in the bilayer and competes with cholesterol for association with phospholipids, as reported for other membrane intercalating agents (Lange et al., 2005). These data therefore confirmed that there are at least two distinctive states of cholesterol in a typical membrane bilayer: one in which cholesterol is readily accessible for binding to proteins such as PFO (free cholesterol), and one in which the sterol is associated with surrounding membrane components that reduce its exposure to the surface (e.g., phospholipid headgroups may obscure access to sterols associated with phospholipid acyl chains).
The selective binding of PFO to cholesterol aggregates and not to epicholesterol aggregates, suggests that the failure to bind epicholesterol when incorporated in membrane bilayers is not related to the packing or association of this sterol with the phospholipids. This failure is rather caused by the inappropriate orientation of the hydroxyl group (Murari et al., 1986), which it may be required for the specific docking of the sterol molecule to a binding pocket located in D4 (Fig. 20.5B, Rossjohn et al., 2007). Alternatively, the hydroxyl group may need to be properly exposed at the surface of a lipid cluster, that may then act as a platform for the anchoring of the D4 loops (Fig. 20.5C). Such a cluster may be preformed on the membrane before binding, or formed as a result of the interaction of D4 with the bilayer surface. Redistribution of lipids after protein-binding has been observed for LLO (Gekara et al., 2005), and other proteins (e.g., Heimburg et al., 1999).
The PFO and SLO specific binding to cholesterol aggregates and microcrystals (Harris et al., 1998; Heuck et al., 2007), together with the need for more than 30 mole% cholesterol in membranes to trigger binding (Flanagan et al., 2009; Heuck et al., 2000; Nelson et al., 2008), suggest that the role of cholesterol in the cytolytic mechanism of the CDC may be more complex than solely binding to a specific binding site. An alternative explanation would be the need of a cluster of cholesterol molecules at the membrane surface to provide a docking platform for the D4 loops (Gekara et al., 2005, Heimburg et al., 1999, Heuck and Johnson, 2005). Interestingly, the binding of pore-forming toxins to lipid clusters have been reported for Staphylococcus aureus α-hemolysin (Valeva et al., 2006), and the need for small cholesterol clusters have been recently suggested for the binding of LLO to membranes (Bavdek et al., 2007). Further work is needed to unambiguously determine the mechanism by which cholesterol specifically anchors the CDC to the target membrane.
20.4 Conclusions and Future Perspectives
Recent studies support the concept that there is a complex interplay between the structural arrangement of the CDC D4 loops and the distribution of cholesterol in the target membrane (Bavdek et al., 2007; Flanagan et al., 2009; Giddings et al., 2003; Heuck and Johnson, 2005; Nelson et al., 2008; Polekhina et al., 2005; Ramachandran et al., 2002; Soltani et al., 2007a; Soltani et al., 2007b). Modifications in the lipid composition alter the cholesterol arrangement in the membrane, and as a consequence, the binding of the CDC (Flanagan et al., 2009; Nelson et al., 2008). At the same time, modifications to the structure of the CDC due to mutations, changes in the pH of the medium or other factors, apparently modifies the threshold for the amount of cholesterol required to trigger binding (Bavdek et al., 2007; Nelson et al., 2008; Moe & Heuck, unpublished).
The presence of free cholesterol molecules at the membrane surface seems to be critical to trigger the binding of most CDCs. A direct inference from these findings is that the exposure of cholesterol at the membrane surface may be facilitated by the action of other membrane-damaging toxins or enzymes secreted by these pathogens like, for example phospholipase C. Such toxins cleave the head-groups of phospholipids, and consequently increase the exposure of cholesterol molecules (or availability of free cholesterol) to the membrane surface. Cooperation between the CDC and different phospholipase C molecules contribute to the pathogenesis of at least two organisms. A synergic effect has been reported for the action of PFO and α-toxin in clostridial myonecrosis (Awad et al., 2001), and both phospholipase C and LLO have been identified as key factors for the vacuolar dissolution and cell-to-cell spreading mechanism of Listeria monocytogenes (Alberti-Segui et al., 2007).
Complete understanding of the mechanism of pore formation for the CDCs at the molecular level will require high-resolution structures of the initial (water-soluble monomer), the final (membrane-inserted pore/oligomer), and any intermediate pre-pore state involved in the cytolytic process (including complexes with receptors or lipids). Great progress has been achieved to this end, but there is much more to be accomplished. A few crystal structures for monomeric CDCs are currently available (PFO, ILY, ALO, Bourdeau et al., 2009 ; Polekhina et al., 2005; Rossjohn et al., 1997), and the low resolution structure for the pre-pore complex and the membrane-inserted oligomer of PLY have been obtained by cryo-electron microscopy (Tilley et al., 2005).
It has become clear that the analysis of complex biological systems, in particular those involving membranes, benefits from the combination of high-resolution structural techniques (e.g., X-ray crystallography, nuclear magnetic resonance and electron microscopy) and spectroscopic analysis of probes incorporated at specific positions in the proteins (e.g., electron paramagnetic resonance, fluorescence spectroscopy) (Cowieson et al., 2008; Heuck and Johnson, 2002; Hubbell et al., 2000). In addition to providing structural information, by monitoring the spectral signal of these probes as a function of time, one can determine the kinetics of the discrete steps of the pore-formation mechanism (Heuck et al., 2000, , 2003) and the dynamics of the structural transformations (Columbus and Hubbell, 2002).
Understanding the CDC function in the establishment of the diseases caused by various Gram-positive pathogens is far from complete (Marriott et al., 2008; Schnupf and Portnoy, 2007). The actual role of CDCs in bacterial pathogenesis may be more complex than merely forming a transmembrane pore. For example, it has been proposed that SLO is involved in protein translocation during Streptococcus pyogenes infection (Madden et al., 2001; Meehl and Caparon, 2004).
The involvement of protein receptors in the mechanism of certain CDC is another area that requires further investigation. The discovery of the ILY receptor illuminated two distinct roles for cholesterol in the cytolytic mechanism of this CDC (Giddings et al., 2003). ALO’s strong preference for targeting the apical side of gut epithelial cells suggests that a receptor (other than cholesterol) may be present in these cells (Bourdeau et al., 2009). Clearly, there is much to be discovered concerning the complex and fascinating roles played by the CDC in bacterial pathogenesis.
Abbreviations
- CDCs:
-
cholesterol-dependent cytolysins
- PFO:
-
perfringolysin O
- ILY:
-
intermedilysin
- PLY:
-
pneumolysin
- SLO:
-
streptolysin O
- ALO:
-
anthrolysin
- TMH/s:
-
transmembrane β-hairpin/s
- D4:
-
domain 4
- L1, L2, and L3:
-
loop 1, loop 2 and loop 3
References
Abdel Ghani, E. M., Weis, S., Walev, I., Kehoe, M., Bhakdi, S. and Palmer, M., 1999, Streptolysin O: inhibition of the conformational change during membrane binding of the monomer prevents oligomerization and pore formation. Biochemistry 38: 15204–15211.
Alberti-Segui, C., Goeden, K. R. and Higgins, D. E., 2007, Differential function of Listeria monocytogenes listeriolysin O and phospholipases C in vacuolar dissolution following cell-to-cell spread. Cell. Microbiol. 9: 179–195.
Alouf, J. E., Billington, S. J. and Jost, B. H., 2006, Repertoire and general features of the family of cholesterol-dependent cytolysins. In Alouf, J. E. and Popoff, M. R. (Eds.) The Comprehensive Sourcebook of Bacterial Protein Toxins. 3rd ed., pp. 643–658, Oxford, England, Academic Press.
Alving, C. R., Habig, W. H., Urban, K. A. and Hardegree, M. C., 1979, Cholesterol-dependent tetanolysin damage to liposomes. Biochim. Biophys. Acta 551: 224–228.
Arrhenius, S., 1907. Immunochemistry. The application of the principles of physical chemistry to the study of the biological antibodies. New York, The Macmillian Company.
Awad, M. M., Ellemor, D. M., Boyd, R. L., Emmins, J. J. and Rood, J. I., 2001, Synergistic effects of alpha-toxin and perfringolysin O in Clostridium perfringens-mediated gas gangrene. Infect. Immun. 69: 7904–7910.
Bavdek, A., Gekara, N. O., Priselac, D., Gutierrez Aguirre, I., Darji, A., Chakraborty, T., Macìœek, P., Lakey, J. H., Weiss, S. and Anderluh, G., 2007, Sterol and pH interdependence in the binding, oligomerization, and pore formation of listeriolysin O. Biochemistry 46: 4425–4437.
Billington, S. J., Songer, J. G. and Jost, B. H., 2001, Molecular characterization of the pore-forming toxin, pyolysin, a major virulence determinant of Arcanobacterium pyogenes. Vet. Microbiol. 82: 261–274.
Bourdeau, R. W., Malito, E., Chenal, A., Bishop, B. L., Musch, M. W., Villereal, M. L., Chang, E. B., Mosser, E. M., Rest, R. F. and Tang, W.-J., 2009, Cellular functions and x-ray structure of anthrolysin O, a cholesterol-dependent cytolysin secreted by Bacillus anthracis. J. Biol. Chem. 284: 14645–14656.
Campanella, J., Bitincka, L. and Smalley, J., 2003, MatGAT: An application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinf. 4: 29.
Cho, W. and Stahelin, R. V., 2005, Membrane-protein interactions in cell signaling and membrane trafficking. Annu. Rev. Biophys. Biomol. Struct. 34: 119–151.
Columbus, L. and Hubbell, W. L., 2002, A new spin on protein dynamics. Trends Biochem. Sci. 27: 288–295.
Cowell, J. L. and Bernheimer, A. W., 1978, Role of cholesterol in the action of cereolysin on membranes. Arch. Biochem. Biophys. 190: 603–610.
Cowieson, N. P., Kobe, B. and Martin, J. L., 2008, United we stand: combining structural methods. Curr. Opin. Struct. Biol. 18: 617–622.
Czajkowsky, D. M., Hotze, E. M., Shao, Z. and Tweten, R. K., 2004, Vertical collapse of a cytolysin prepore moves its transmembrane beta-hairpins to the membrane. EMBO J. 23: 3206–3215.
Dang, T. X., Hotze, E. M., Rouiller, I., Tweten, R. K. and Wilson-Kubalek, E. M., 2005, Prepore to pore transition of a cholesterol-dependent cytolysin visualized by electron microscopy. J. Struct. Biol. 150: 100–108.
Duncan, J. L. and Schlegel, R., 1975, Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein-cholesterol interaction. J. Cell Biol. 67: 160–174.
Epand, R. M., 2006, Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 45: 279–294.
Farrand, S., Hotze, E., Friese, P., Hollingshead, S. K., Smith, D. F., Cummings, R. D., Dale, G. L. and Tweten, R. K., 2008, Characterization of a streptococcal cholesterol-dependent cytolysin with a Lewis y and b Specific Lectin Domain. Biochemistry 47: 7097–7107.
Flanagan, J. J., Heuck, A. P. and Johnson, A. E. (2002) Cholesterol-phospholipid interactions play an important role in perfringolysin O binding to membrane. FASEB J., 16, A929.
Flanagan, J. J., Tweten, R. K., Johnson, A. E. and Heuck, A. P., 2009, Cholesterol exposure at the membrane surface is necessary and sufficient to trigger perfringolysin O binding. Biochemistry 48: 3977–3987.
Gekara, N. O., Jacobs, T., Chakraborty, T. and Weiss, S., 2005, The cholesterol-dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cell Microbiol. 7: 1345–1356.
Gelber, S. E., Aguilar, J. L., Lewis, K. L. T. and Ratner, A. J., 2008, Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 190: 3896–3903.
Geoffroy, C. and Alouf, J. E., 1983, Selective purification by thiol-disulfide interchange chromatography of alveolysin, a sulfhydryl-activated toxin of Bacillus alvei. Toxin properties and interaction with cholesterol and liposomes. J. Biol. Chem. 258: 9968–9972.
Giddings, K. S., Johnson, A. E. and Tweten, R. K., 2003, Redefining cholesterol’s role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. USA 100: 11315–11320.
Giddings, K. S., Johnson, A. E. and Tweten, R. K., 2006, Perfringolysin O and Intermedilysin: Mechanisms of Pore Formation by the Cholesterol-Dependent Cytolysins. In Alouf, J. E. and Popoff, M. R. (Eds.) The Comprehensive Sourcebook of Bacterial Protein Toxins. 3rd ed., pp. 671–679, Oxford, England, Academic Press.
Giddings, K. S., Zhao, J., Sims, P. J. and Tweten, R. K., 2004, Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 11: 1173–1178.
Gilbert, R. J., 2005, Inactivation and activity of cholesterol-dependent cytolysins: what structural studies tell us. Structure (Camb.) 13: 1097–1106.
Gilbert, R. J. C., Rossjohn, J., Parker, M. W., Tweten, R. K., Morgan, P. J., Mitchell, T. J., Errington, N., Rowe, A. J., Andrew, P. W. and Byron, O., 1998, Self-interaction of pneumolysin, the pore-forming protein toxin of Streptococcus pneumoniae. J. Mol. Biol. 284: 1223–1237.
Goñi, F. M., Alonso, A., Bagatolli, L. A., Brown, R. E., Marsh, D., Prieto, M. and Thewalt, J. L., 2008, Phase diagrams of lipid mixtures relevant to the study of membrane rafts. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids 1781: 665–684.
Haberland, M. E. and Reynolds, J. A., 1973, Self-association of cholesterol in aqueous solution. Proc. Natl. Acad. Sci. USA 70: 2313–2316.
Hadders, M. A., Beringer, D. X. and Gros, P., 2007, Structure of C8 α-MACPF reveals mechanism of membrane attack in complement immune defense. Science 317: 1552–1554.
Harris, J. R., 1988, Electron microscopy of cholesterol. Micron Microsc. Acta 19, 19–31.
Harris, J. R., Adrian, M., Bhakdi, S. and Palmer, M., 1998, Cholesterol-streptolysin O interaction: An EM study of wild-type and mutant streptolysin O. J. Struct. Biol. 121: 343–355.
Harwood, C. R. and Cranenburgh, R., 2008, Bacillus protein secretion: an unfolding story. Trends Microbiol., 16, 73–79.
Heimburg, T., Angerstein, B. and Marsh, D., 1999, Binding of peripheral proteins to mixed lipid membranes: Effect of lipid demixing upon binding. Biophys. J. 76: 2575–2586.
Heuck, A. P., Hotze, E. M., Tweten, R. K. and Johnson, A. E., 2000, Mechanism of membrane insertion of a multimeric β-barrel protein: perfringolysin O creates a pore using ordered and coupled conformational changes. Mol. Cell 6: 1233–1242.
Heuck, A. P. and Johnson, A. E., 2002, Pore-forming protein structure analysis in membranes using multiple independent fluorescence techniques. Cell Biochem. Biophys. 36: 89–101.
Heuck, A. P. and Johnson, A. E., 2005, Membrane recognition and pore formation by bacterial pore-forming toxins. In Tamm, L. K. (Ed.) Protein-Lipid Interactions. From Membrane Domains to Cellular Networks, pp. 165–188, Weinheim, Wiley-VCH.
Heuck, A. P., Savva, C. G., Holzenburg, A. and Johnson, A. E., 2007, Conformational changes that effect oligomerization and initiate pore formation are triggered throughout perfringolysin O upon binding to cholesterol. J. Biol. Chem. 282: 22629–22637.
Heuck, A. P., Tweten, R. K. and Johnson, A. E., 2001, beta-Barrel pore-forming toxins: intriguing dimorphic proteins. Biochemistry 40: 9065–9073.
Heuck, A. P., Tweten, R. K. and Johnson, A. E., 2003, Assembly and topography of the prepore complex in cholesterol-dependent cytolysins. J. Biol. Chem. 278: 31218–31225.
Hotze, E. M., Heuck, A. P., Czajkowsky, D. M., Shao, Z., Johnson, A. E. and Tweten, R. K., 2002, Monomer-monomer interactions drive the prepore to pore conversion of a beta -barrel-forming cholesterol-dependent cytolysin. J. Biol. Chem. 277: 11597–11605.
Huang, J. and Feigenson, G. W., 1999, A microscopic interaction model of maximum solubility of cholesterol in lipid bilayers. Biophys. J. 76: 2142–2157.
Hubbell, W. L., Cafiso, D. S. and Altenbach, C., 2000, Identifying conformational changes with site-directed spin labeling. Nat. Struct. Mol. Biol. 7: 735–739.
Jefferies, J., Nieminen, L., Kirkham, L.-A., Johnston, C., Smith, A. and Mitchell, T. J., 2007, Identification of a secreted cholesterol-dependent cytolysin (Mitilysin) from Streptococcus mitis. J. Bacteriol. 189: 627–632.
Johnson, A. E., 2005, Fluorescence approaches for determining protein conformations, interactions and mechanisms at membranes. Traffic 6: 1078–1092.
Johnson, M. K., Geoffroy, C. & Alouf, J. E. (1980) Binding of cholesterol by sulfhydryl-activated cytolysins. Infect. Immun., 27, 97–101.
Kleinschmidt, J. H. (2006) Folding kinetics of the outer membrane proteins OmpA and FomA into phospholipid bilayers. Chem. Phys. Lipids, 141, 30–47.
Lachapelle, S., Tweten, R. K. and Hotze, E. M., 2009, Intermedilysin-receptor interactions during assembly of the pore complex: assembly intermediates increase host cell susceptibility to complement-mediated lysis. J. Biol. Chem. 284: 12719–12726.
Lange, Y., Cutler, H. B. and Steck, T. L., 1980, The effect of cholesterol and other intercalated amphipaths on the contour and stability of the isolated red cell membrane. J. Biol. Chem. 255: 9331–9337.
Lange, Y. and Steck, T. L., 2008, Cholesterol homeostasis and the escape tendency (activity) of plasma membrane cholesterol. Prog. Lipid Res. 47: 319–332.
Lange, Y., Ye, J. and Steck, T. L., 2005, Activation of membrane cholesterol by displacement from phospholipids. J. Biol. Chem. 280: 36126–36131.
Lecuyer, H. and Dervichian, D. G., 1969, Structure of aqueous mixtures of lecithin and cholesterol. J. Mol. Biol. 45: 39–57.
Madden, J. C., Ruiz, N. and Caparon, M., 2001, Cytolysin-mediated translocation (CMT): a functional equivalent of type III secretion in gram-positive bacteria. Cell 104: 143–152.
Marriott, H. M., Mitchell, T. J. and Dockrell, D. H., 2008, Pneumolysin: a double-edged sword during the host-pathogen interaction. Curr. Mol. Med. 8: 497–509.
Mason, P. R., Tulenko, T. N. and Jacob, R. F., 2003, Direct evidence for cholesterol crystalline domains in biological membranes: role in human pathobiology. Biochim. Biophys. Acta 1610: 198–207.
Mcconnell, H. M. and Radhakrishnan, A., 2003, Condensed complexes of cholesterol and phospholipids. Biochim. Biophys. Acta 1610: 159–73.
Meehl, M. A. and Caparon, M. G., 2004, Specificity of streptolysin O in cytolysin-mediated translocation. Mol. Microbiol. 52: 1665–1676.
Mesmin, B. and Maxfield, F. R., 2009, Intracellular sterol dynamics. Biochim. Biophys. Acta, Mol. Cell. Biol. Lipids 1791: 636–645.
Michel, E., Reich, K. A., Favier, R., Berche, P. and Cossart, P., 1990, Attenuated mutants of the intracellular bacterium Listeria monocytogenes obtained by single amino acid substitutions in listeriolysin O. Mol. Microbiol. 4: 2167–2178.
Miller, C. J., Elliott, J. L. and Collier, R. J., 1999, Anthrax protective antigen: prepore-to-pore conversion. Biochemistry 38: 10432–10441.
Mitsui, K., Sekiya, T., Okamura, S., Nozawa, Y. and Hase, J., 1979, Ring formation of perfringolysin O as revealed by negative stain electron microscopy. Biochim. Biophys. Acta 558: 307–313.
Mosser, E. and Rest, R., 2006, The Bacillus anthracis cholesterol-dependent cytolysin, Anthrolysin O, kills human neutrophils, monocytes and macrophages. BMC Microbiol. 6: 56.
Mouritsen, O. G. and Zuckermann, M. J., 2004, What’s so special about cholesterol? Lipids 39: 1101–1113.
Murari, R., Murari, M. P. and Baumann, W. J., 1986, Sterol orientations in phosphatidylcholine liposomes as determined by deuterium NMR. Biochemistry 25: 1062–1067.
Nagamune, H., Ohkura, K., Sukeno, A., Cowan, G., Mitchell, T. J., Ito, W., Ohnishi, O., Hattori, K., Yamato, M., Hirota, K., Miyake, Y., Maeda, T. and Kourai, H., 2004, The human-specific action of intermedilysin, a homolog of streptolysin O, is dictated by domain 4 of the protein. Mol. Microbiol. 48: 677–692.
Nagamune, H., Whiley, R. A., Goto, T., Inai, Y., Maeda, T., Hardie, J. M. and Kourai, H., 2000, Distribution of the intermedilysin gene among the anginosus group streptococci and correlation between intermedilysin production and deep-seated infection with Streptococcus intermedius. J. Clin. Microbiol. 38: 220–226.
Nakamura, M., Sekino, N., Iwamoto, M. and Ohno-Iwashita, Y., 1995, Interaction of .theta.-toxin (perfringolysin O), a cholesterol-binding cytolysin, with liposomal membranes: change in the aromatic side chains upon binding and insertion. Biochemistry 34: 6513–6520.
Nakamura, M., Sekino-Suzuki, N., Mitsui, K.-I. and Ohno-Iwashita, Y., 1998, Contribution of tryptophan residues to the structural changes in perfringolysin O during interaction with liposomal membranes. J. Biochem. 123: 1145–1155.
Nelson, L. D., Johnson, A. E. and London, E., 2008, How interaction of perfringolysin O with membranes is controlled by sterol structure, lipid structure, and physiological low pH: insights into the origin of perfringolysin O-lipid raft interaction J. Biol. Chem. 283: 4632–4642.
Nollmann, M., Gilbert, R., Mitchell, T., Sferrazza, M. and Byron, O., 2004, The role of cholesterol in the activity of pneumolysin, a bacterial protein toxin. Biophys. J. 86: 3141–3151.
Ohno-Iwashita, Y., Iwamoto, M., Ando, S. and Iwashita, S., 1992, Effect of lipidic factors on membrane cholesterol topology - mode of binding of θ-toxin to cholesterol in liposomes. Biochimica et Biophysica Acta 1109: 81–90.
Ohno-Iwashita, Y., Iwamoto, M., Mitsui, K.-I., Ando, S. and Iwashita, S., 1991, A cytolysin, θ-toxin, preferentially binds to membrane cholesterol surrounded by phospholipids with 18-carbon hydrocarbon chains in cholesterol-rich region. J. Biochem. 110: 369–375.
Ohno-Iwashita, Y., Shimada, Y., Waheed, A., Hayashi, M., Inomata, M., Nakamura, M., Maruya, M. and Iwashita, M., 2004, Perfringolysin O, a cholesterol-binding cytolysin, as a probe for lipid rafts. Anaerobe 10: 125–134.
Ohvo-Rekilä, H., Ramstedt, B., Leppimäki, P. and Peter Slotte, J., 2002, Cholesterol interactions with phospholipids in membranes. Prog. Lipid Res. 41: 66–97.
Olofsson, A., Hebert, H. and Thelestam, M., 1993, The projection structure of Perfringolysin O (Clostridium perfringens θ-toxin). FEBS Lett. 319: 125–127.
Palmer, M., 2004, Cholesterol and the activity of bacterial toxins. FEMS Microbiol. Lett. 238: 281–289.
Palmer, M., Harris, R., Freytag, C., Kehoe, M., Tranum-Jensen, J. and Bhakdi, S., 1998, Assembly mechanism of the oligomeric streptolysin O pore: the early membrane lesion is lined by a free edge of the lipid membrane and is extended gradually during oligomerization. EMBO J. 17: 1598–1605.
Pinkney, M., Beachey, E. and Kehoe, M., 1989, The thiol-activated toxin streptolysin O does not require a thiol group for cytolytic activity. Infect. Immun. 57: 2553–2558.
Polekhina, G., Feil, S. C., Tang, J., Rossjohn, J., Giddings, K. S., Tweten, R. K. and Parker, M. W., 2006, Comparative three-dimensional structure of cholesterol-dependent cytolysins. In Alouf, J. E. and Popoff, M. R. (Eds.) The Comprehensive Sourcebook of Bacterial Protein Toxins. Third ed., pp. 659–670, Oxford, England, Academic Press.
Polekhina, G., Giddings, K. S., Tweten, R. K. and Parker, M. W., 2005, Insights into the action of the superfamily of cholesterol-dependent cytolysins from studies of intermedilysin. Proc. Natl. Acad. Sci. USA 102: 600–605.
Radhakrishnan, A. and Mcconnell, H. M., 1999, Condensed complexes of cholesterol and phospholipids. Biophys. J. 77: 1507–1517.
Ramachandran, R., Heuck, A. P., Tweten, R. K. and Johnson, A. E., 2002, Structural insights into the membrane-anchoring mechanism of a cholesterol-dependent cytolysin. Nat. Struct. Mol. Biol. 9: 823–827.
Ramachandran, R., Tweten, R. K. and Johnson, A. E., 2004, Membrane-dependent conformational changes initiate cholesterol-dependent cytolysin oligomerization and intersubunit beta-strand alignment. Nat. Struct. Mol. Biol. 11: 697–705.
Ramachandran, R., Tweten, R. K. and Johnson, A. E., 2005, The domains of a cholesterol-dependent cytolysin undergo a major FRET-detected rearrangement during pore formation. Proc. Natl. Acad. Sci. USA 102: 7139–7144.
Rosado, C. J., Buckle, A. M., Law, R. H. P., Butcher, R. E., Kan, W.-T., Bird, C. H., Ung, K., Browne, K. A., Baran, K., Bashtannyk-Puhalovich, T. A., Faux, N. G., Wong, W., Porter, C. J., Pike, R. N., Ellisdon, A. M., Pearce, M. C., Bottomley, S. P., Emsley, J., Smith, A. I., Rossjohn, J., Hartland, E. L., Voskoboinik, I., Trapani, J. A., Bird, P. I., Dunstone, M. A. and Whisstock, J. C., 2007, A common fold mediates vertebrate defense and bacterial attack. Science 317: 1548–1551.
Rosenqvist, E., Michaelsen, T. E. and Vistnes, A. I., 1980, Effect of streptolysin O and digitonin on egg lecithin/cholesterol vesicles. Biochim. Biophys. Acta 600: 91–102.
Rossjohn, J., Feil, S. C., Mckinstry, W. J., Tweten, R. K. and Parker, M. W., 1997, Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell 89: 685–692.
Rossjohn, J., Polekhina, G., Feil, S. C., Morton, C. J., Tweten, R. K. and Parker, M. W., 2007, Structures of perfringolysin O suggest a pathway for activation of cholesterol-dependent cytolysins. J. Mol. Biol. 367: 1227–1236.
Sankaram, M. B. and Thompson, T. E., 1991, Cholesterol-induced fluid-phase immiscibility in membranes. Proc. Natl. Acad. Sci. USA 88: 8686–8690.
Saunders, F. K., Mitchell, T. J., Walker, J. A., Andrew, P. W. and Boulnois, G. J., 1989, Pneumolysin, the thiol-activated toxin of Streptococcus pneumoniae, does not require a thiol group for in vitro activity. Infect. Immun. 57: 2547–2552.
Schnupf, P. and Portnoy, D. A., 2007, Listeriolysin O: a phagosome-specific lysin. Microbes Infect. 9: 1176–1187.
Sekino-Suzuki, N., Nakamura, M., Mitsui, K.-I. and Ohno-Iwashita, Y., 1996, Contribution of individual tryptophan residues to the structure and activity of θ-toxin (perfringolysin O), a cholesterol-binding cytolysin. Eur. J. Biochem. 241: 941–947.
Shatursky, O., Heuck, A. P., Shepard, L. A., Rossjohn, J., Parker, M. W., Johnson, A. E. and Tweten, R. K., 1999, The mechanism of membrane insertion for a cholesterol-dependent cytolysin: A novel paradigm for pore-forming toxins. Cell 99: 293–299.
Shepard, L. A., Heuck, A. P., Hamman, B. D., Rossjohn, J., Parker, M. W., Ryan, K. R., Johnson, A. E. and Tweten, R. K., 1998, Identification of a membrane-spanning domain of the thiol-activated pore-forming toxin Clostridium perfringens perfringolysin O: an alpha-helical to beta-sheet transition identified by fluorescence spectroscopy. Biochemistry 37: 14563–14574.
Shepard, L. A., Shatursky, O., Johnson, A. E. and Tweten, R. K., 2000, The mechanism of pore assembly for a cholesterol-dependent cytolysin: formation of a large prepore complex precedes the insertion of the transmembrane beta-hairpins. Biochemistry 39: 10284–10293.
Simossis, V. A., Kleinjung, J. and Heringa, J., 2005, Homology-extended sequence alignment. Nucl. Acids Res. 33: 816–824.
Solovyova, A. S., Nollmann, M., Mitchell, T. J. and Byron, O., 2004, The solution structure and oligomerization behavior of two bacterial toxins: pneumolysin and perfringolysin O. Biophys. J. 87: 540–552.
Soltani, C. E., Hotze, E. M., Johnson, A. E. and Tweten, R. K., 2007a, Specific protein-membrane contacts are required for prepore and pore assembly by a cholesterol-dependent cytolysin. J. Biol. Chem. 282: 15709–15716.
Soltani, C. E., Hotze, E. M., Johnson, A. E. and Tweten, R. K., 2007b, Structural elements of the cholesterol-dependent cytolysins that are responsible for their cholesterol-sensitive membrane interactions. Proc. Natl. Acad. Sci. USA 104: 20226–20231.
Tamm, L. K., Hong, H. and Liang, B., 2004, Folding and assembly of beta-barrel membrane proteins. Biochim. Biophys. Acta 1666: 250–263.
Tilley, S. J., Orlova, E. V., Gilbert, R. J., Andrew, P. W. and Saibil, H. R. (2005) Structural basis of pore formation by the bacterial toxin pneumolysin. Cell 121: 247–256.
Tweten, R. K., 2005, Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73: 6199–6209.
Tweten, R. K., Parker, M. W. and Johnson, A. E., 2001, The cholesterol-dependent cytolysins. Curr. Top. Microbiol. Immunol. 257: 15–33.
Valeva, A., Hellmann, N., Walev, I., Strand, D., Plate, M., Boukhallouk, F., Brack, A., Hanada, K., Decker, H. and Bhakdi, S., 2006, Evidence that clustered phosphocholine head groups serve as sites for binding and assembly of an oligomeric protein pore. J. Biol. Chem. 281: 26014–26021.
Vazquez-Boland, J. A., Dominguez, L., Rodriguez-Ferri, E. F., Fernandez-Garayzabal, J. F. and Suarez, G., 1989, Preliminary evidence that different domains are involved in cytolytic activity and receptor (cholesterol) binding in listeriolysin O, the Listeria monocytogenes thiol-activated toxin. FEMS Microbiol. Lett. 53: 95–99.
Veatch, S. L. and Keller, S. L., 2002, Organization in lipid membranes containing cholesterol. Phys. Rev. Lett. 89: 268101.
Waheed, A., Shimada, Y., Heijnen, H. F. G., Nakamura, M., Inomata, M., Hayashi, M., Iwashita, S., Slot, J. W. and Ohno-Iwashita, Y., 2001, Selective binding of perfringolysin O derivative to cholesterol-rich membrane microdomains (rafts). Proc. Natl. Acad. Sci. USA 98: 4926–4931.
Walker, B., Krishnasastry, M., Zorn, L. and Bayley, H., 1992, Assembly of the oligomeric membrane pore formed by Staphylococcal alpha-hemolysin examined by truncation mutagenesis. J. Biol. Chem. 267: 21782–21786.
White, S. H. and Wimley, W. C., 1999, Membrane protein folding and stability: physical principles. Annu. Rev. Biophys. Biomol. Struct. 28: 319–365.
Zitzer, A., Westover, E. J., Covey, D. F. and Palmer, M., 2003, Differential interaction of the two cholesterol-dependent, membrane-damaging toxins, streptolysin O and Vibrio cholerae cytolysin, with enantiomeric cholesterol. FEBS Lett. 553: 229–231.
Acknowledgments
Work in the authors’ laboratory was supported by a Scientist Development Grant from the American Heart Association to A.P.H
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2010 Springer Science+Business Media B.V.
About this chapter
Cite this chapter
Heuck, A.P., Moe, P.C., Johnson, B.B. (2010). The Cholesterol-Dependent Cytolysin Family of Gram-Positive Bacterial Toxins. In: Harris, J. (eds) Cholesterol Binding and Cholesterol Transport Proteins:. Subcellular Biochemistry, vol 51. Springer, Dordrecht. https://doi.org/10.1007/978-90-481-8622-8_20
Download citation
DOI: https://doi.org/10.1007/978-90-481-8622-8_20
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-90-481-8621-1
Online ISBN: 978-90-481-8622-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)