Abstract
With the depletion of fossil fuel resources and the limited availability of petroleum-derived transport fuel, along with the contribution to global warming, the environmental benefits of renewable biofuel are seen as the best alternative source in recent years. Among the third-generation biodiesel feed stocks such as food crops (sugarcane, sugar beet, maize and rapeseed) and non-food crops (Jatropha sp., Cassava sp., lignocellulosic materials), microalgae has been hailed as the third-generation biodiesel. Microalgae are the only fuel source that can be sustainably developed in the near future, and can produce ten times more oil than oleaginous plants. Biodiesel from microalgae has received much attention world-wide in recent years due to its carbon-neutral status. The higher neutral lipid contents of microalgae also surpass terrestrial plants for biofuel production, and microalgae are the largest biomass producers. They can accumulate high concentrations of triacylglycerol as a storage lipid under photooxidative stress and other unfavorable environmental conditions within a short period of time. This chapter provides an overview of the production of biodiesel from microalgae and includes algae cultivation, biomass production, harvesting, and downstream processing, along with a list of companies aiming to develop biodiesel from microalgae.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Fossil fuels are the largest contributor of greenhouse gases. They are about to reach peak production and are predicted to be exhausted in the future due to their limited and non-renewable nature. Therefore, the current use of fossil fuels is widely recognized as unsustainable (Hemaiswarya et al. 2012). In the year 2007, the US Department of Energy reported that the burning of fossil fuels produces around 21.3 billion tonnes (≡gigatonnes [Gt]) of carbon dioxide (CO2) per year; however, it is estimated that natural processes can only absorb about half of that amount, resulting in a net increase of 10.65 billion tonnes of atmospheric CO2 per year. Carbon dioxide is one of the greenhouse gases that enhances radioactivity and contributes to global warming (a rise in the average surface temperature of the earth), which a majority of climate scientists agree is going to cause major adverse effects. Data show that, between 1989 and 2008, the proven oil reserves seem to have increased from 1,006 to 1,333 billion barrels. The annual world primary energy consumption was measured at 12,274.6 million tonnes of oil equivalent (MTOE). Fossil fuels accounted for 87.08 % of primary energy consumption, with the main fuels being oil (33.07 % share), coal (30.34 %), and natural gas (23.67 %); the minor fuels account for 17.08 % of primary energy consumption, and include nuclear energy (4.15 %), hydroelectricity (6.45 ,%), and renewables (6.48 %) (BP 2011). On the other hand, while the world oil consumption was about 86 million barrels/day in 2006, it is estimated to reach 107 million barrels/day by 2030.
Dependency on fossil fuel, primarily in the transportation sector, has encouraged research on biofuels. One-fifth of global CO2 emissions is from the transport sector. The global number of vehicles has been roughly estimated to rise to two billion by the year 2050 (Balat and Balat 2010). The transportation sector would be responsible for 80 % of this increase (US Energy Information Administration 2009) and would consume 76 % of the world oil production by 2030 (International Energy Agency 2008). A recent study has shown that microalgae biofuels have the potential to replace 17 % of oil imports used for transportation fuel in the USA by 2022 (Wigmosta et al. 2011). Moreover, following the BP oil spill in the Atlantic Ocean, the US administration is considering reducing its oil imports by one-third by 2021, using, among others, biofuels to do so (Banerjee 2011). The only possible alternative to overcome all these problems is to produce ‘algal biodiesel’.
2 Biodiesel
Biodiesel is a monoalkyl ester; the basic chemical reaction required to produce biodiesel is the transesterification of lipids, either triglycerides or oil, with alcohol (Fukuda et al. 2001). The result is a fatty acid alkyl-ester, which is the biodiesel material used in engines (e.g. fatty-acid methyl-ester [FAME]); this reaction is performed at high pH. The alcohols used include methanol and, to a lesser extent, ethanol; the main by-product is glycerol. This chemical reaction is sensitive to water. The quality and productivity of biodiesel is affected by the presence of water, because saponification reactions occur (soap formation) when water combines with the lipid. Excess unsaturated fatty acid levels are a major problem in biodiesel production because they may induce cross-linking of fatty acid chains, causing the formation of tar.
Poly-unsaturated fatty acids (PUFAs) are a potential co-product of biodiesel from microalgae. PUFAs from microalgae are a vegetable origin alternative to fish oils and other oils rich in omega-3 fatty acids. In the biodiesel process, PUFAs would be extracted prior to esterification, as these fatty acids are not the most suitable raw material for esterification. As far as biodiesel esterification is concerned, the main by-product is glycerol. Glycerol is a versatile chemical, with over 1,500 known commercial applications, though this market has become somewhat saturated due to strong growth in worldwide biodiesel production. Glycerol could be used for mixed fermentation together with sugar and protein residues from the lipid extraction step.

2.1 Advantages of Biodiesel
For several reasons, biodiesel fuel seems to be an alternative energy resource. The advantages of using biodiesel fuels are as follows.
Biodiesel has higher lubricity than petroleum diesel and is a renewable energy resource that could be sustainably supplied. It is understood that the petroleum reserves are to be depleted in less than 50 years at the present rate of consumption (Sheehan et al. 1998).
Biodiesel appears to have several favorable environmental properties: a low release of CO2 and very low sulfur content (Antolin et al. 2002; Vicente et al. 2004). The release of sulfur and carbon monoxide would be reduced by 30 and 10 %, respectively, with the use of biodiesel. Additionally, the use of biodiesel as an energy source can reduce the level of gas that is generated during combustion, and the relatively high oxygen content of biodiesel can decrease the level of carbon monoxide. Moreover, biodiesel does not contain aromatic compounds and other chemical substances that are harmful to the environment. Recent research (Sharp 1996) has indicated that 90 % of atmospheric pollution and 95 % of cancers can be decreasedwith the use of biodiesel.
Biodiesel appears to have significant economic potential; the price of fossil fuels will undoubtedly increase in the future because of their non-renewable nature (Cadenas and Cabezudo 1998). Finally, the flash point and biodegradability of biodiesel is better than diesel fuel (Ma and Hanna 1999).
2.2 Sources of Biodiesel Production
The current sources of commercial biodiesel include soybean oil, palm oil, animal fat, and waste cooking oil. These sources can be classified into three generations.

Although first-generation biofuel production capacities have increased, they have considerable economic and environmental limitations. A lack of agricultural land and deforestation (National Research Council 2007; Goldemberg and Guardabassi 2009) has led to competition with agriculture for arable land used for food production. For example, in some European countries such as France, production of first-generation biofuels using arable land available from the cultivation of oleaginous plants will not be able to support demand for biofuels by 2015 unless fallow land is saturated, which in turn would create soil impoverishment problems. According to the International Energy Agency (IEA 2008), about 1 % (14 million hectares) of the world’s available arable land is used for the production of biofuels, providing 1 % of global transport fuels. This clearly shows that an increase in the share to anywhere near 100 % is unfeasible due to the severe impact on the world’s food supply and the large areas of production land required.
Second-generation biofuels contain high amounts of free fatty acids; however, additional production steps demand increased energy and production costs (Demirbas 2008). The major limitation associated with the use of second-generation biofuels is the issue of sustainability. For biodiesel to be produced from Jatropha, half of the land area of the UK (17.5 million hectares) would be required to fulfill the diesel requirement of the UK (estimated 25 billion liters in 2008). The total energy input for the life cycle of rapeseed and soybean is estimated to be half the total energy of the fuel (Scott et al. 2010). The third largest biodiesel feedstock is palm oil. Universally, 10 % of palm oil is being used as a biodiesel source. The major disadvantage of palm oil biodiesel is poor cold flow properties, which can be overcome by blending with triglycerides to improve cold flow and cloud point properties (Sarin et al. 2009). High production of palm oil requires vast areas of natural vegetation; it can also indirectly cause damage to the ecosystem due to devastating fires. In addition, it has a negative impact on terrestrial and aquatic environments due to palm mill effluent (Ahmad et al. 2011).
Production of first- and second-generation biodiesel from crops is not affordable. Therefore, competition between biodiesel feedstock production and food production mean it is necessary to find other ways to lower the cost of biodiesel production and reduce the pressure on food and feed supplies. Biodiesel also has one major limitation: it does not fully replace other petroleum diesel and biomass-based diesels, meaning that one gallon of biodiesel does not have the same physical properties (temperature stability, energy content, etc.) as petroleum diesel and other biomass-based diesels (National Renewable Energy Laboratory 2009). Therefore, biodiesel can only be used interchangeably to a limited extent in the form of blends and in the few engines that are specifically designed to handle pure biodiesel (B100).
The reason for the differences in physical characteristics is the different types of molecules that constitute biodiesel and petroleum diesel. Biodiesel is composed of straight chain hydrocarbon esters that have one oxygen atom per molecule; petroleum diesel is composed of non-oxygenated straight and cyclic hydrocarbon chains. Biodiesel does not meet all of the stability, distribution, and engine requirements of standard diesel fuels because the compound includes oxygen, called esters. The use of pure biodiesel in the existing infrastructure can cause problems during transportation through pipelines and when used in engines; however, these difficulties can be managed without a substantial amount of monetary costs (McElroy 2007). A comparison between petro-diesel and biodiesel is shown in Table 1.
Microalgae are one of the most promising alternative and renewable feedstock sources for production of third-generation biodiesel. Microalgae have been found to have incredible oil production levels when compared with other oil seed crops such as soybeans, palm oil, etc. Biodiesel from microalgae is a sustainable development as they are carbon neutral, or reduce atmospheric CO2 as they are carbon negative (Naik et al. 2010). It is estimated that 1.8 tonnes of CO2 would be consumed (180 % reduction) by each tonne of microalgal biomass produced. This chapter mainly focused on microalgae as a potential source of biodiesel production.
3 Microalgae
Microalgae are microscopic aquatic (freshwater or marine forms) photosynthetic plants that require the aid of a microscope to be seen and can be measured in microns. Microalgae have the right oils to be converted to biodiesel. However, a microscope becomes superfluous when it comes to visualizing the potential of these organisms for biofuel production (Fig. 1a–e). With improved oil and biomass yield, algae can produce a considerably higher level of biomass and lipids per hectare than terrestrial biomass. Most microalgae are found in freshwater and marine environments; a few grow in terrestrial habitats. But the choice of microalgae species for cultivation is based on their lipid and biomass productivity as well as cultivation location. Marine and freshwater species have shown similar biomass and lipid productivities, thus making strain selection dependent on other factors (Ahmad et al. 2011). Some of the fresh and marine water microalgae and their lipid productivity (% dry weight) are shown in Table 2.
3.1 Freshwater Algae
Freshwater algae are found growing underwater on rocks, in ponds and lakes, and in mud in streams and rivers. They are usually more abundant in slower than in fast flowing streams. In inland areas, cultivation of fresh water microalgae may be more suitable and will not affect the fertile (agricultural) area in the same way as marine algae. The major drawback is contamination, and cultivation of freshwater species allows a more diverse number of species to be transmitted. There are many types of freshwater algae, identified as green algae (Chlorophyta), red algae (Rhodophyta), and diatoms (Bacillariophyta). Green algae look like strands of green hair flowing in the current. Diatoms can appear to the naked eye as brown mats of growth with soft masses or slimy layers on rocks.
3.2 Marine Water Algae
Marine forms are grown near coastal areas, salt marshes, brackish water, or even floating in the ocean. One successful way to improve the economics of biomass productivity of microalgae will be to cultivate marine microalgae (Uduman et al. 2011). Marine microalgae can be grown in saline waters, so a selective environment will serve to reduce extensive contamination. Another important factor for consideration is water availability (Mata et al. 2010). By growing marine microalgae, sea water can be used directly as an alternative to fresh water (Amaro et al. 2011). There are some disadvantages to using sea water for the growth of microalgae; sea water generally consists of marine flora that may consume microalgae. For the growth of microalgae, a large amount of water is needed to remove micro flora, or the filtering of sea water will negatively impact on the economics of production. Higher evaporation rates could result in an increase in salinity, thus the necessary adjustment of culture condition with freshwater incurs an additional cost. Osmotic shock and rupturing of cells under higher salinity may not be suitable for lipid recovery (Mata et al. 2010).
4 Why Microalgae Are Attractive
Microalgae are easy to cultivate in an aquatic medium and need less water than terrestrial crops. They can be cultivated in seawater or brackish water on non-arable land. Microalgae have a high growth rate, a short life cycle, and can be harvested continuously (Chisti 2007; Schenk et al. 2008). Microalgae double their biomass within 24 h. In fact, the biomass doubling time for microalgae during exponential growth can be as short as 3.5 h (Chisti 2007), which is significantly quicker than the doubling time of oil crops. They have the ability to reproduce themselves using simple photosynthesis to convert solar energy into chemical energy and accumulate their lipids in the form of oil, carbohydrate, and protein etc. (Schenk et al. 2008). Microalgae can grow in simple nutrients and sunlight, although the growth rate will vary with the addition of specific nutrients and aeration (Renaud et al. 1999; Pratoomyot et al. 2005; Aslan and Kapdan 2006). Many microalgae accumulate high levels of oil, which can be converted into biodiesel. Microalgae can accumulate more than 80 % of lipids on the dry weight of biomass (Chisti 2007). According to the US Department of Energy, the oil yield of algae is 10–100 times higher than conventional oilseed crops (soy, rapeseed, or tree-borne oil plantations such as Jatropha and palm). Microalgae have a high theoretical production yield of 47,000–3,08,000 L ha−1 annum−1; whereas the oil palm has the ability to produce 5,950 L of biodiesel per year (Ahmad et al. 2011).
Microalgae biomass, either terrestrial or aquatic, is considered one of the best alternative renewable energy sources (Chisti 2008; Raja et al. 2008). Microalgae biomass production may be combined with direct bio-fixation of waste CO2 (1 kg of dry algal biomass requiring about 1.8 kg of CO2). Depending on the species of microalgae, other compounds that may also be extracted include a wide range of fine chemicals and bulk products such as fats, PUFAs, oil, natural dyes, sugars, pigments, antioxidants, high-value bioactive compounds, and algal biomass, which are valuable applications in the industrial sector (Li et al. 2008a, b; Raja et al. 2008; Sathasivam et al. 2012). These high-value biological derivatives with many possible commercial applications mean that microalgae can potentially be used in a large number of biotechnology areas, including biofuels, cosmetics, pharmaceuticals, nutrition and food additives, aquaculture, and pollution prevention (Raven and Gregersen 2007; Rosenberg et al. 2008; Jacob-Lopes and Teixeira Franco 2010).
5 Classification of Microalgae Based on Metabolism
Microalgae can be divided into four major groups of algal metabolism: photoautotrophic, heterotrophic, mixotrophic, and photoheterotrophic (Chen et al. 2011). A short explanation follows.
5.1 Photoautotrophic Microalgae
Photoautotrophic microalgae build up CO2 and water into organic cell materials using energy from sunlight. In this condition, microalgae absorbs energy from light and stores it in the form of adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide phosphate (NADPH) to produce glucose in a Calvin cycle. However, inadequate light and CO2 supply are always an issue for photoautotrophic cultures. It has been reported that the addition of excess CO2 into the culture system may increase the microalgae lipid and biomass productivity, but care should be taken because an excess amount of CO2 is released in the atmosphere. Light also plays an important role because the uneven distribution of light intensity will directly affect the growth of microalgae. For example, biomass productivity is lower in photoautotrophic species than heterotrophic microalgae in both photobioreactor and open ponds—between 0.117 and 1.54 kg/m3/day, respectively (Chisti 2007).
5.2 Heterotrophic Microalgae
Heterotrophic microalgae require at least one organic nutrient from organisms or their by-products, as a carbon source for producing their own organic compounds. Heterotrophic cultures utilize organic wastes containing carbons as an energy source instead of CO2 and are independent of the light source for reproduction. Thus, the high production of lipids is enabled compared with phototrophic microalgal cultivation. Heterotrophic microalgae have the ability to accumulate a higher level of lipids than photoautotrophic microalgae (Xiong et al. 2008; Liang et al. 2009). Although it accumulates higher lipids, it is not economically feasible when the carbon sources have to be purchased, as this directly increases production costs. Carbon sources used for culturing algae include glycerol, glucose and sweet sorghum, corn powder hydrolyzate, and Jerusalem artichokes. This glucose has been widely used as a carbon source for microalgal cultivation. The most suitable glucose concentration for a high accumulation of lipids is 2 %. For example, with a glucose concentration of 0.5–8 %, the carbon source showed 44.48 % lipid content (Wu et al. 2005), whereas the glucose concentration in the normal medium does not exceed 2.4 % and accumulates lipid content as high as 57 % (Xiong et al. 2008). Sweet sorghum is another carbon source used for culturing algae. The addition of 50 % sweet sorghum juice into the culture medium instead of glucose achieved a lipid content as high as 73.4 % (Liang et al. 2010). Heterotrophic species are one of the best for the production of biodiesel (Xu et al. 2006; Martek 2008; Xiong et al. 2008). For example, Chlorella sp. grown in heterotrophic conditions showed a high level of biomass (7.4 kg/m3/day) and lipid content 50–58 % (g lipid/g dry weight) (Xiong et al. 2008).
5.3 Photoheterotrophic Microalgae
Photoheterotrophs are also referred to as photoorganitrophs, photoassimilates, and photometabolism. Photoheterotrophic microalgae use light, but obtain their carbon in organic form. For example, microalgae under stress conditions produce glycerol; these glycerols are used as a carbon source and it utilizes a light intensity of 35 μE/m−2/s−1. Yang et al. (2011) recorded an increase in the biomass concentration of Chlorella minutissima (UTEX2341) from 1.2 to 8.2 g/L.
5.4 Mixotrophic Microalgae
Mixotrophic microalgae combine both an autotrophic and a heterotrophic metabolism, simultaneously assimilating CO2. Ch. vulgaris grown with both light and glucose sources showed higher biomass productivity (254 mg/L/day). When grown in the dark under CO2 instead of glucose, the biomass productivity (151 mg/L/day) was low (Liang et al. 2009; Chen et al. 2011). Among all the mixotrophic culture conditions, algae accumulate high lipid contents even though they are rarely used in microalgal oil production. According to Cheirsilp and Torpee (2012), when freshwater Chlorella sp., marine Chlorella sp., Nannochloropsis sp., and Cheatoceros sp. were grown in mixotrophic cultures, a much higher biomass and lipid level was produced than the photoautotrophic and heterotrophic cultures.
The presence of different metabolisms can be distinguished according to pH flexibilities, which depends on the growth stoichiometry of microalgae as part of the metabolism involved. In the autotrophic metabolism, due to the consumption of CO2, the growth medium becomes alkalized, whereas, in heterotrophy, where CO2 is produced from an organic carbon source, the growth medium becomes acidulated. In a mixotrophic culture, the pH value depends on the dominating constituent metabolism, but in most cases remains constant. Some strains also have the ability to grow under photoautotrophic, heterotrophic and mixotrophic conditions (e.g. Ch. vulgaris, Haematococcus pluvialis, Arthrospira (Spirulina) platensis). Other strains, such as Selenastrum capricornutum and Scenedesmus acutus, can grow either photoautotrophically, heterotrophically, or photoheterotrophically (Chojnacka and Marquez-Rocha 2004).
6 Screening of Lipid Production Under Different Environmental Conditions
Screening of microalgae producing high levels of oil is one of the most important criteria in the optimization of biodiesel production. Oil content in microalgae can be stored as high lipid contents up to a maximum of 75 % (g lipids/g dry weight) biomass, but this is associated with low productivity. Some cultures accumulate a moderate amount of lipids (20–50 %) and, when conditions are optimized, higher productivity can be reached. The lipid content (% dry weight) of different marine and freshwater microalgae species has shown significant differences among the species (Table 2). The other factors are taken into account, such as lipid profile and the ability to grow under specific environmental conditions. The factors that affect the lipid profile are nutritional, processing, and cultivation conditions. Factors such as growth rate, quality and quantity of lipid, and strong adaptability in a changing environment to determine the preferred nutrients and nutrient uptake rates are very important in selecting better strains (Amaro et al. 2011).
The composition of fatty acids plays an important role in the characteristics of the biodiesel they produce and differs between species. Biodiesel is composed of saturated and unsaturated fatty acids with 12–22 carbon atoms, some of them of ω3 and ω6 families. The seven freshwater microalgae showed similar fatty acid composition patterns, showing that all microalgae synthesize C14:0, C16:0, C18:1, C18:2, and C18:3 fatty acids. The relative intensity of other individual fatty acid chains is species specific, e.g. Ankistrodesmus sp. consists of C16:4 and C18:4; Isochrysis sp. consists of C18:4; and Nannochloris sp. consists of C22:6, C16:2, C16:3, C20:5, C16:2, and C16:3; and Nitzschia sp. consists of C20:5 (Thomas et al. 1984; Mata et al. 2010).
Some microalgae oil yield is higher than vegetable oil; this is strain dependent. Different nutritional and environmental factors may affect the fatty acid composition. The high amount of oil production is induced under stress conditions such as nitrogen deficiency, high light intensity, phosphorous deficiency, silicon deficiency, salt stress, and other factors. For example, Botryococcus braunii under nitrogen deficiency and salt stress induced the accumulation of C20:5 whereas, all other species accumulated C18:1 (Őtleş and Pire 2001; Pratoomyot et al. 2005; Natrah et al. 2007; Hu et al. 2008; Gouveia and Oliveira 2009; Mata et al. 2010). Some microalgae have the ability to accumulate lipids under nitrogen deprivation. Nannochloropsis sp. attained 204 mg/L/day (with an average biomass productivity of 0.30 g/L/day and more than 60 % lipid content) in nitrogen-deprived media (Rodolfi et al. 2009; Veillette et al. 2012). Navicula accumulates 58 % (g lipid/g dry weight) under nitrogen deficiency, whereas the lipid content is very low (i.e.) 22–49 % (g lipid/g dry weight) under silicon (Si) deficiency. The above results clearly prove that nitrogen deprivation has a positive effect on lipid accumulation.
On the other hand, phosphate deprivation could have an effect on lipid content (Khozin-Goldberg and Cohen 2006; Xin et al. 2010). For example, when the P concentration was increased from 0.14 to 0.37 mg/L, an increase in the biomass was recorded, while the lipid content decreased from 53 to 23.5 % (g lipid/g dry weight). Silicon depletion also leads to an increase in the cellular lipid content. Under silicon starvation in the diatom, an increase of 60 % of the lipid content of Navioua pelliculosa is possible. Lipid fractions as high as 70–85 % on a dry weight basis have been reported. Such high lipid contents exceed that of most terrestrial plants. How it is that diatoms rapidly switch over from carbohydrate accumulation to lipid accumulation remains unclear. However, diatoms have great potential to accumulate microalgal lipids (Miyamoto 1997). At the initial stage of growth, more polar lipids and polyunsaturated C16 and C18 fatty acids are produced. The stationary growth phase generally consists of neutral saturated 18:1 and 16:0 long-chain fatty acids.
Light also plays an important role in lipid accumulation. Under high light intensity, Euglena gracilis and Ch. vulgaris produce polyunsaturated C16 and C18 fatty acids as well as mono- and di-galactosyl-diglycerides, sphingolipids, and phosphoglycerides. Under low temperature conditions, Monochrysis lutheri produce polyunsaturated C18 fatty acids, but in Dunaliella salina the fatty acid composition changes (Takagi et al. 2006). The above findings clearly deny that light intensity also plays a crucial role in fatty acid composition. Osmotic shock might also enhance lipid production. However, salt stress may affect the quantity of lipid within the algal cells. For example, Dunaliella cells can grow well in high NaCl concentrations. Takagi et al. (2006) observed that Dunaliella cells grown in concentrations of >1.0 M NaCl show increased biomass, but there is no significant effect on biomass in a concentration of <1.0 M NaCl. However, intracellular lipid and triglycerides were higher (67 and 56 %) in saltwater species grown in 1.0 M NaCl; the same species grown in 0.5 M NaCl accumulates a low lipid content (60 and 40 %). However, these physico-chemical treatments could also favor the synthesis of polar lipids like phospholipids or glycolipids associated with cell walls of the microalgae; such lipids are less interesting for biodiesel production (Nagle and Lemke 1990).
7 Microalgal Lipid and Biomass Productions
The medium composition is an important factors for increased microalgal growth and lipid production. Microalgae require inorganic nutrients in the form of carbon, nitrogen, and phosphorous for growth (Suh and Lee 2003; Brennan and Owende 2010; Sathasivam and Juntawong 2013). The growth medium of microalgae generally consists of macronutrients like nitrogen, phosphate, and a metal chelator. Iron is generally a micronutrient, and low concentrations of iron in a form that can be assimilated are essential for microalgae growth. In a nutrient-rich medium, Chlorella sp. are known to grow fairly. The growth of various species of Chlorella in Watanabe media containing 1.25 g/L KNO3 was demonstrated by Illman et al. (2000). Changes in culture conditions may lead to changes in metabolic pathways and result in the production of neutral lipids (Singh et al. 2011). Sometimes nutrient-rich media may cause nutrient toxicity, with lethal results (Watanabe et al. 2000). The well known microalgae and the medium used for their growth, biomass, and lipid productivity have been quoted from various literature. Stephenson et al. (2010) tested Ch. emersonii and Ch. protothecoides and achieved the highest lipid and biomass yield. Even though Ch. vulgaris and Ch. minutissima are capable of accumulating high lipid contents, lower triglycerides clearly indicated that the species is inefficient for use as a biodiesel feed stock.
Nannochloropsis has the ability to accumulate 60 % of lipids and if the culture is mixed with 2 % CO2, the biomass yield is ~0.48 g/L/d (Vunjak-Novakovic et al. 2005; Chiu et al. 2009). These results indicate that the Nannochloropsis species have a potential use as biofuel feedstock, whereas the average lipid content and biomass yield of Nannochloropsis species is low when compared with Schizochytrium limacinum, Ch. emersonii and Ch. protothecoides. The lipid accumulation of Chaetoceros calcitrans, Neochloris oleoabundans, and Scenedesmus obliquus is very low (Natrah et al. 2007; da Silva et al. 2009). Even under nitrogen-deficit conditions, N. oleoabundans is able to accumulate 37 % of lipid/dry weight, and the biomass yield is in the range of 0.05–0.09 g/L/d (Pruvost et al. 2009). These results show that the algae are unproductive as biofuel feedstocks.
8 Global Scenario of Algal Biofuels
The growth of algae is motivated around the world (Piccolo 2009), and the large-scale algal biofuel companies throughout the world are listed in Table 3. Even though algae oil production has increased worldwide, the biggest algae investment in the EU is the £26 million in the public sector funded by the UK Carbon Trust. In 2009, the Carbon Trust launched a new £8 million research program called ‘Algae Biofuels Challenge’ (ABC) (http://www.carbontrust.co.uk/News/presscentre/2008/algae-biofuel-schallenge.htm). Later, the Scottish government launched a £6 million EU project called ‘BioMara’. In 2007, a Spanish renewable energy company, Aurantia and Green Fuel Tech of Massachusetts (USA) formed a partnership through a $US92 million project to produce algae oil.
According to Piccolo (2009), countries with a coastline near the Mediterranean Sea region (roughly between 45°N and 30°N) are the best location for algae farms. In these countries south of the Mediterranean, the climate is warmer, and the temperature does not drop below 15 °C throughout the year. This kind of climate is ideal for the growth of algae in open or closed pond systems. These systems would perhaps be the most suitable, efficient, and economically feasible for the growth of algae. Many countries in the Mediterranean basin have a large potential for harvesting algae. Some countries like Israel have begun to produce several strains for fuel production and have also been harvesting algae for medicinal purposes for decades. The southern countries bordering the Mediterranean Sea, such as Morocco, Algeria, Tunisia, and Egypt, are particularly attractive because of their high temperatures and huge unused desert land. In the future, Bio3 Dakhla, an algal industry in Dakhla, Morocco, anticipates investing billions on biofuel industries and are currently producing large amounts of Spirulina for human consumption. However, countries like Libya, Cyprus, and Turkey could also harvest algae on marginal land. Although these countries do not have sufficient water resources, they grow algae in recycled brackish or saline water since the algae is from a marine source and does not require fresh water for growth. Moreover, these countries are just developing and could strongly benefit from running these algal industries. Algae farming can offer jobs for local people, and the transfer of technologies to developing countries can only be beneficial (Piccolo 2009; Singh and Gu 2010).
A complete survey has been carried out for the various aspects of algal cultivation (Edward 2009), including the profiles of various companies involved in the growth of algae for biofuel as well as other applications around the world. The classification is based on various algae farming technologies being used. The reported information has been summarized in Table 3. Some of these companies are exploring suitable regions for algae cultivation, but only a few are using open pond systems or natural settings. Initially, closed systems or photobioreactors (PBRs) were proposed to cultivate algae; these bioreactors are installed near a source of CO2, and thus serve an additional purpose of carbon sequestration. However, natural settings have the least capital cost. So regions in which enough land is available to grow algae in open ponds without interfering with the food chain are an attractive option.
9 Cultivation of Microalgae
Cultivation is the biggest part of generating biomass from microalgae. This has been done on an industrial scale for many years with the help of solar energy (photoautotrophically) and is economically feasible for large-scale production for other uses (Borowitzka 1997). There are two main cultivation systems: open pond (raceways) and closed systems (PBRs). Each system is influenced by intrinsic properties that play a major role in feasibility, including strains used, nutrients, costs of land and water, downstream process, manpower, and climatic conditions (Borowitzka 1992).
9.1 Open Pond System
The open pond production system has been used to cultivate algae since the 1950s (Borowitzka 1999). These systems can be divided into natural waters (lakes, lagoons, and ponds) and artificial systems or containers (Jiménez et al. 2003). The most commonly used cultivation system is raceway ponds. They are typically made up of closed, loop, oval-shaped recirculation channels (Fig. 1f). They are generally constructed in concrete (Fig. 2b), but plastic-covered earth-lined ponds have also been used (Brennan and Owende 2010). The plastic culture system is cheaper, easy to operate, and more durable than closed systems (Zeng et al. 2011; Rawat et al. 2011). Most paddle wheel-driven raceway ponds are 60 cm in depth, and the paddle wheels are helpful to mix and circulate the culture, thus reducing the shading effect required to stabilize algae growth and productivity (Chisti 2007; Brennan and Owende; 2010; Norsker et al. 2011). In continuous production, algae broth and nutrients are introduced near the paddle wheel to prevent sedimentation and can be circulated through the loop to the harvest point. However, ponds can have various advantages and limitations. The major limitation is that productivity is lower than closed systems, and environmental factors cannot be controlled (Chisti 2007; Borowitzka 1999; Zeng et al. 2011). Generally, ponds are more susceptible to weather conditions, evaporation, temperature, and lighting. Increases in the culture density of CO2 transfer rate and light limitations could slow down the cell growth of microalgae.
Atmospheric CO2 is used to fulfill the carbon requirement, but it contains only 0.03–0.06 %, which is not enough for the growth of microalgae. To improve the overall biomass productivity, submerged aerators may be installed to enhance CO2 absorption, these will be helpful as the proper mixing of culture can minimize the impact of both CO2 and light limitations, thus improving biomass productivity (Brennan and Owende 2010). Open pond systems occupy more land area, and contamination with other algae, bacteria, and protozoans can reduce microalgae growth (Blanco et al. 2007; Brennan and Owende 2010; Rawat et al. 2013). The selection of land and water availability for microalgae culture is another important factor. Usage of marginal and non-arable land has more advantages than the use of arable land. Maintenance and cleaning of open systems is easier and requires less energy input than PBRs (Ugwu et al. 2008; Brennan and Owende 2010; Rawat et al. 2013) and therefore have the potential for a large net energy production (Rodolfi et al. 2009). D. salina is one of the most commonly cultivated strains in open pond systems, and, during 2008, the unit price was about €2.55/kg of dry biomass, which was considered too high to justify production for biofuels (Tan 2008; Brennan and Owende 2010; Rawat et al. 2013).
9.2 Closed System
The closed system is designed to overcome all the problems associated with open ponds (contaminations and expected biomass). PBRs are continuous culture systems that can achieve expected biomasses of up to 6.7 g/L (Chisti 2007; Ranjbar et al. 2008; Bai et al. 2011). This technology is used to grow microalgae for the production of high-value compounds such as pharmaceuticals, neutraceuticals, and cosmetics that cannot be grown as a monoculture in open pond systems. Using PBRs, single species of microalgae can be grown for long durations with minimal contamination risk. PBRs have lower direct exchanges of gases and contaminations (e.g. microorganisms, dust) than open pond systems. Different PBR models (indoor and outdoor) have been developed, including tubular, flat plate, airlift, bubble column, and stirred tank (Xu et al. 2009). The principle behind the PBR is reduction of the light path, thereby increasing the amount of light received by each cell (Borowitzka 1999). The culture is generally mixed by airlift or mechanical stirring/pumping. Mixing of culture is important for gaseous exchange within the system (Brennan and Owende 2010; Rawat et al. 2013). High biomass yield, which depend on good control of culture parameters such as temperature, pH, and CO2 concentration, etc., can be achieved using closed PBRs (Suh and Lee 2003), but the capital costs are ten times higher than those of open ponds (Carvalho et al. 2006). However, the combination of both can be profitable because microalgae can be grown in open ponds while reducing contamination by undesired species (Huntley and Redalje 2008). In this culture process, the first step of microalgae production is controlled by temperature (e.g. sea water bath [16–18 °C]) PBR. Microalgae are transferred into an open pond for 5 days in a second culture step (Huntley and Redalje 2006, 2008). Closed PBRs have the advantage of high productivity, low contamination risk, efficient CO2 capture, continuous runs, and controlled growth conditions. The major drawbacks are the higher capital investment and operating costs.
9.2.1 Tubular Photobioreactor
The tubular PBR is one of the most suitable bioreactors for large-scale outdoor cultivations since they expose a large surface to sunlight. A tubular reactor consists of vertical, horizontal, inclined, or helix-shaped tubes connected with a pipe system (Molina et al. 2001; Ugwu et al. 2002; Brennan and Owende 2010). The algae-suspended fluid is able to circulate in this tubing. The tubes are generally of transparent plastics or borosilicate glass and the circulation is kept constant by a pump at the end of the system. The diameter of the tube is 0.1 m or less, and helpful for the high penetration of light into the dense culture (Chisti 2007). Either a mechanical pump or an airlift system is used to recirculate the algal cultures after allowing CO2 and O2 to be exchanged between the liquid medium and aeration gas as well as providing a mechanism for mixing (Eriksen 2008). To encourage gas exchange in the tubes, agitation and proper mixing is very important. The introduction of gas takes place at the end or beginning of the tube system. This method of introducing gas causes deficiency in CO2, a high concentration of oxygen at the end of the unit during circulation, and poor efficiency. Therefore, cultures are generally reticulated by pump, passing through a degasser at regular intervals in order to remove excess oxygen. The largest closed PBRs are tubular (e.g. the 25 m3 plants at Mera Pharmaceuticals, Hawaii [Olaizola 2000] and the 700 m3 plant in Klötze, Germany [Pulz 2001]). Tubular reactors are currently being used for the culture of high-value products such as astaxanthin (Fig 2a).
9.2.2 Bubble Column Photobioreactor
A bubble column PBR consists of a vertically arranged cylindrical column made of transparent material (Eriksen 2008). This PBR has the highest volumetric mass transfer rates, proper mixing, and controllable growth conditions (Eriksen 2008). The cost of this bioreactor is very low, and it is compact and easy to operate. The introduction of gas takes place at the bottom of the column and causes a turbulent stream to enable optimum gas exchange. At present, these types of reactors are built with a maximum diameter of 20–30 cm in order to ensure the required supply of sunlight. This type of PBR allows a reduction of harmful shear forces.
9.3 Two-Step Cultivation System
The two-step cultivation system involves a combination of raceway and PBR designs. This system has been traditionally used to develop inoculum for aquaculture operations. The main advantage of this system is the production of inoculum that is free from contamination (Schenk et al. 2008). The first step is the fast cultivation of biomass in the PBR; the second step is stress cultivation in open ponds. The first step allows for better protection of the growing biomass in the PBR during the early stages, and CO2 capture is maximized. The microalgae suspension is transferred in open ponds that have enough nutrients, are low in nitrogen, and maintain high CO2. The second step in open raceways has few problems because a high algal density is more resistant to contamination, and this phase is nutrient depleted, avoiding the growth of contaminating species. The combination of PBR and an open pond has proven efficiency for astaxanthin production (Huntley and Redalje 2006). It is currently being used by companies that are developing biofuel applications. Hybrid systems can produce as much as 20–30 tonne ha−1 of lipids annually, depending on suitable climatic conditions (Rodolfi et al. 2009).
10 Wastewater as a Source of Nutrients for the Growth of Microalgae
Wastewater is an excellent, cheap, and readily available medium for the growth of various microalgal strains (Schenk et al. 2008). Wastewater also contains macronutrients that help in the growth of microalgae (Raja et al. 2004; Hosikian et al. 2010; Rawat et al. 2011). The macronutrients present in wastewater are ammonia, nitrate, phosphate, urea, trace elements such as vitamins (biotin and thiamine), certain trace metals, and, rarely, radioisotopes. The growth of several microalgae is found to be suitable in wastewater because of the pH and dissolved CO2 concentration. Large-scale production of microalgae for biofuels using wastewater has the possibility to improve the economics of biomass production. Growth of microalgae using wastewater also has some disadvantages, such as bacterial and viral contamination that may negatively affect the biomass and production process. Use of wastewater for the growth of microalgae might lead to an adequate cleaning of the culture system (Lam and Lee 2012) and also help to reduce the eutrophication in the aquatic environment. Microalgae can also be used for the removal of rich nutrients available in wastewater (Raja et al. 2008) (e.g. C. vulgaris is used for the removal of nitrogen and phosphorous from wastewater, with an average removal efficiency of nitrogen [72 %] and phosphorus [28 %]; 3–8 mg/LNH4+ and 1.5–3.5 mg/LPO4 −3) (Aslan and Kapdan 2006). Other microalgae cultures used for the removal of nutrients are Chlorella, Scenedesmus, Spirulina spp., Nannochloris, B. brauinii, and cyanobacterium Phormidium bohneri (Lee and Lee 2001; Olguín et al. 2003; An et al. 2003; Jimenez-Pérez et al. 2004). Some companies actively involved in culturing algae using wastewater for biofuel production include Algae wheel Technologies (USA), Algal Scientific Corporation (USA), Aquaflow Binomic Corporation (New Zealand), Blue Marble Energy (USA), Community Fuels (USA), Nanoforce Incorporates (USA), and Western Michigan University (USA).
11 Harvesting Microalgal Biomass
Harvesting of microalgal biomass is one of the important steps in producing maximum biomass. It can actually contribute 20–30 % of total biomass production cost (Chisti 2007). The small size of microalgal cells (typically ranging from 2 to 200 μm) makes the harvesting process very difficult. There are a few techniques in the harvesting of microalgae, including centrifugation, flocculation, filtration and screening, gravity sedimentation, flotation, and electrophoresis techniques (Uduman et al. 2010; Brennan and Owende 2010). The selection of harvesting techniques is dependent on the physical properties of microalgae such as size, density of the slurry, intracellular biomass composition, and yield of desired products (Brennan and Owende 2010). We summarize some of the techniques that are currently being used for the recovery of microalgal biomass.
11.1 Filtration
Conventional filtration may be inadequate for biomass recovery and is most appropriate for the harvesting of large (>70 μm) microalgae such as Coelastrum proboscideum and Spirulina plantensis. The small (<30 μm) microalgae like Chlorella, Dunaliella, and Scenedesmus cannot be harvested using this technique (Mohn 1980). For recovery of smaller algal cells (<30 μm), membrane microfiltration and ultra-filtration are a technically feasible alternative to conventional filtration. Microfiltration and ultra-filtration are a form of membrane filtration using hydrostatic pressure. Microfiltration is one of the most efficient and suitable methods for harvesting fragile algal cells (Grima et al. 2003; Mata et al. 2010). Conventional filtration works under pressure or suction; filtration aids such as diatomaceous earth or cellulose can be used to increase efficiency. This method has demonstrated that the filtration process is moderately effective for large microalgae, whereby a chamber filter press can achieve a concentration factor 245 times the original concentration of C. proboscideum, to produce sludge with 27 % solids. The disadvantages of membrane filtration include the sporadic requirement for membrane replacement and elevated production costs due to energy-intensive pumping. It is more cost effective than the centrifugation process for the processing of low-culture volume (<2 m3/day). Due to the cost of membrane replacement and pumping in large-scale production (>20 m3/day), centrifugation may be one of the most economic methods for harvesting microalgae (MacKay and Salusbury 1988).
11.2 Centrifugation
Centrifugation is a method of separating algae from the medium using a centrifuge to cause the algae to settle in the bottom of a flask or tank. It is a useful device for both biolipid extraction from algae and chemical separation in biodiesel. The centrifuge works using the sedimentation principle, where centripetal acceleration is used to evenly distribute substances (presents in a solution for small-scale applications) of greater and lesser density. Centrifugation is currently considered too expensive and to increase production costs due to rising power consumption (Grima et al. 2003). This method is preferred for harvesting algal biomass especially for producing prolonged shelf-life concentrates for aquaculture usage. The main advantage of this technique is that 95 % of the cells are efficiently harvested at 13,000 × g by increasing the gravitation field (Greenwell et al. 2010) thus it increases the slurry concentration up to 150 times more and 15 % total suspended solids are technically possible to harvest (Mohn 1980). It has certain disadvantages, which include the high energy costs and potentially higher maintenance requirements (Bosma et al. 2003).
11.3 Gravity Sedimentation
Gravity sedimentation is commonly applied and is a rapid intensive method for separating microalgal biomass from huge volumes of water (or wastewater). Gravity sedimentation methods are based on Stokes’s Law (Schenk et al. 2008) (i.e. settling characteristics of suspended solids are determined by the density and radius of algae cells and induced sedimentation velocity). Higher microalgal biomass can be achieved by sedimentation using lamella separators and sedimentation tanks. This method is only suitable for harvesting of large microalgae (ca. >70 μm) such as Spirulina (Muñoz and Guieysse 2006). The success of solid removal via the gravity settling method depends on the density of the microalgal particles. Low-density microalgal particles do not settle well and are ineffectively separated by settling (Edzwald 1993). This technique has some advantages: it is cost effective, and it is possible to recycle water because no potentially toxic chemicals are used.
11.4 Flocculation
An integrated approach is needed to minimize the energy consumption of harvesting microalgae (Benemann et al. 1977). Several harvesting methods have shown that flocculation combined with flotation or sedimentation and followed further by dewatering with centrifugation or filtration is the most promising and cost-effective method (Schenk et al. 2008). This is the bulk-harvesting technique whereby the dispersed microalgal cells aggregate and form larger particles with a higher sedimentation rate. Microalgal cells are negatively charged, which prevents aggregation in aqueous suspension, so multivalent cations or cationic polymers are useful for the neutralization of microalgal cells. Flocculation can be induced in different ways. Induced chemical flocculation using multivalent metal salts like aluminum sulphate (Al2(SO4)3), ferric chloride (FeCl3), ferric sulphate (Fe2(SO4)3), or other chemical flocculants has been studied extensively (McGarry 1970; Lee et al. 1998; Papazi et al. 2010) and some of these are applied in industry. Aluminium sulphate is an effective coagulant being widely used in flocculating algal biomass (Scenedesmus and Chlorella) in the wastewater treatment process (Grima et al. 2003).
Although an easy and effective method, it is not feasible and sustainable for large-scale harvesting of microalgae because microalgae production plants have excess cationic flocculent that needs to be removed from the medium before it can be re-used, thus leading to extra operational costs (Schenk et al. 2008). Flocculation can also be induced by changing the culture conditions with the application of extreme pH, nutrient depletion, changes in temperature dissolved O2 level. The pH of the algal culture is adjusted to 10–10.6 using NaOH, followed by the addition of non-ionic polymer Magnfloc LT-25. This method of harvesting microalgal biomass leads to a concentration of 6–7 g L−1 (Knuckey et al. 2006). The process has been successfully applied to a variety of species, with flocculation efficiency of >80 %. The other method that has been proposed for flocculation of microalgae is biologically induced, where bacteria have been applied successfully in wastewater treatment (Lee et al. 2009). The disadvantage of this method is that it leads to undesirable bacterial contamination of the algal production plant. In recent years, naturally flocculating diatom Skeletonema has been used to form flocs of Nannochloropsis (Schenk et al. 2008). As diatoms have a silica-based cell wall, they require different medium composition than most microalgal strains used for biodiesel production, which leads to additional cultivation costs. Chitosan is another bio-flocculant, and this method is very sensitive to pH. For freshwater microalgae, the maximum flocculation was obtained at pH7.0, whereas, for marine species, it is low (Divakaran and Pillai 2002). The remaining water can be reused for the cultivation of algae. Heasman et al. (2000) used chitosan as a flocculant for Tetraselmis chui, Thalassiosira pseudonana, and Isochrysis sp. To complete the flocculation process of these strains, 40 mg/L of chitosan is required. On the other hand, 150 mg/L of chitosan was found to complete the flocculation process of Chaetoceros muellaris (Heasman et al. 2000). Although chemical flocculation is often reported to incur fewer operating costs to harvest algae, this method leads to significant pollution of the environment (Li et al. 2008a). The interruption of the CO2 supply in the algal system can cause algae to flocculate on its own, termed ‘autoflocculation’. In most cases, this phenomenon is associated with high pH due to photosynthetic CO2 consumption corresponding with precipitation of magnesium, calcium, phosphate, and carbonate salts with algae. Where calcium phosphate is used, excess calcium ions (positively charged) tend to react with algae (negatively charged) and they bind together to provide an autoflocculation process (Sukenik and Shelef 1984). Figure 2e shows the flocculating agents added in to the algal culture and starting to coagulate algae cells.
11.5 Flotation
Flotation is a method used in combination with flocculation to harvest algae. It is a simple method by which algae can be made to float on the surface of the medium. Due to the increase in the lipid content of the cells, some strains may float naturally (Bruton et al. 2009). The main advantage of this method is that it is cheap and it can be used to harvest large-scale microalgae. A few disadvantages of this method are that the flocculating agents may lead to contamination, and evidence of its technical viability is also very limited (Grima et al. 2003). There are two types of flotations:
11.5.1 Dissolved Air Flotation (DAF)
Dissolved air flotation (DAF) separates algae from its culture by using both froth flotation and flocculation (Fig. 2c). For the flocculation of microalgal cells, alum is used to increase the floc size before applying DAF, fine bubbles are supplied by an air compressor. Alum is a common name for several trivalent sulfates of metal such as aluminum, chromium, or iron and a univalent metal such as potassium or sodium (e.g., AlK(SO4)2).
11.5.2 Froth Flotation
This is another method of separating algae from the medium by adjusting the pH and bubbling air through a column to create a froth of algae. The algae collect in foam above the liquid level and can be separated by suction. The pH may vary depending on the algal species. The major disadvantage of the froth flotation is that it is not economically feasible.
12 Dehydration of Algal Biomass
After algal biomass is harvested, it must be processed quickly. Depending on the final product, algal biomass can be processed by dehydration or drying (Fig. 2d). Several methods area available to process algal biomass, namely, solar drying, low-pressure-shelf drying, drum drying (Prakash et al. 1997), spray drying (Desmorieux and Decaen 2005), fluidized bed drying (Leach et al. 1998), freeze drying (Grima et al. 1994), and refractance window dehydration technology (Nindo and Tang 2007). When compared with other dehydration processes, solar drying is the cheapest. However, some of the major disadvantages are that solar drying requires a long time and a large space, and there is a risk of material loss (Prakash et al. 1997).
13 Lipid Extraction
Most microalgae accumulate neutral lipids; due to the low degree of unsaturation, these lipids are essential for biodiesel production. There are several methods for extracting lipids from microalgae, namely, the Folch method, the gravimetric method, and the Bligh and Dyer method. Solvent extraction (n-hexane) and gravimetric determination are the two methods often used by researchers for the extraction of lipids from the microalgal cell with or without cell disruption (Viswanath et al. 2010). To identify and quantify microalgal lipids, other analytical methods such as thin layer chromatography (TLC), high-performance liquid chromatography (HPLC), and gas-chromatography mass-spectrometry (GC-MS) are used (Elsey et al. 2007). The choice of method depends on the efficiency, accuracy, and cost effectiveness, ease of use, high throughput capability, robustness, exactness, and reproducibility (Viswanath et al. 2010). Screening of a large number of lipid-producing microalgal samples under laboratory conditions is difficult and time consuming. Hence, to overcome this problem, attention is increasingly focused on in situ measurement of lipid content, such as Nile Red-NR staining (Fig. 1e), time-domain nuclear magnetic resonance (TD-NMR), and boron-dipyrromethene (Bodipy) (Mutanda et al. 2011).
13.1 Extraction Technique
In order to produce biodiesel from microalgae, several methods can be used for the extraction of biofuel and high-value products. The important lipid-extraction techniques are chemical solvents, supercritical CO2, physico-chemical, biochemical and direct trans-esterification.
13.2 Solvent Extraction
Microalgal oil can be extracted using chemicals such as n-hexane, chloroform, benzene, diethyl ether, and ethanol. In large-scale studies, Yaguchi et al. (1997) used choloroform-methanol blends for the extraction of lipid and obtained high lipid yields of up to 83 % (g lipid/g dry weight). n-Hexane is the most commonly used solvent for the extraction of lipids due to its lower toxicity and its affinity for non-lipid contaminants (Halim et al. 2011). For example, Miao and Wu (2006) used hexane as a solvent for the extraction of lipids from Ch. Protothecoides; the yield of lipid contents was up to 55 % (g lipid/g dry weight). Soxhlet extraction is not used at industrial scales due to a high energy requirement (Halim et al. 2011). Ethanol, octonol, or 1,8-diazabicyclo-(5.4.0)-undec-7-ene (DBU) yield lower FAME than n-hexane extraction (Samorì et al. 2010). The advantage of using solvents for lipid extraction is that they are inexpensive and very efficient for oil extraction. For the separation of other valuable products, such as beta-carotene, astaxanthin, and other essential fatty acids, from microalgae, solvent extraction is used extensively (Grima et al. 2003).
13.3 Supercritical CO2 Extraction
Supercritical fluid extraction is one of the most effective methods for the extraction of oil from microalgae (Mendes et al. 1995). CO2 is first heated and compressed until it reaches liquid-gas state or above its critical point. Harvested microalgae are added to act as a solvent. Limited methods have been studied for the recovery of lipids from microalgae and to transform them to biodiesel (Halim et al. 2011). Certain studies recovered lipid content of up to 26 % (g lipid/g dry weight) from Nannocloropsis sp. (Andrich et al. 2005). Halim et al. (2011) used supercritical CO2 extraction at 60 °C, and pressure of 30 MPa, to extract lipids from Chlorococcum sp. and obtained higher lipid contents of 5.8 % g lipid/g dry weight, whereas using hexane Soxhlet extraction obtained only 3.2 % g lipid/g dry weight. Using wet algae obtained a maximum lipid yield of 7.1 % g lipid/g dry weight, which was lower than other Botryococcus species, as the lipid is high 28.6 % g lipid/g dry weight (Lee et al. 2010). This indicates that the supercritical CO2 lipid extraction was enhanced in the presence of water with the blend of microalgae. The main advantage of this method is that it is not toxic, is easy to recover, is usable at low temperatures, and is relatively rapid because of the low viscosities and high diffusivities (Andrich et al. 2005). The disadvantage is that it requires expensive equipment (Perrut 2000) and a huge amount of energy to reach high pressure (Tan and Lee 2011).
13.4 Physico-Chemical Extraction
The physico-chemical technique can be used for the disruption of cells in order to extract lipids from microalgae (Cooney et al. 2009; Lee et al. 2010). Some of the physico-chemical techniques are microwave, autoclaving, osmotic shock, bead-beating, homogenization, freeze-drying, French press, grinding, and sonication. Microwave and bead-beating methods yield a higher lipid content from microalgal cells. In Botryococcus sp., using 5 min microwave pre-treatment increased the lipid extraction yield from 7.7 to 28.6 % g lipid/g dry weight (Lee et al. 2010).
13.5 Biochemical Extraction
Only limited studies have used biochemical extraction to extract lipids from microalgae. For example, after 72 h cellulase hydrolysis pre-treatment, 70 % of sugar was obtained, whereas the lipid content was increased slightly from 52 to 54 % g lipid/g dry weight in Chlorella sp. (Fu et al. 2010).
13.6 Direct Transesterification
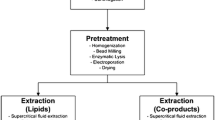
Direct transesterification is a process that mixes alcohol and a catalyst with microalgae without prior extraction. In microalgae, many catalysts have been examined, including hydrochloric (HCl) or sulphuric acid (H2SO4), but acetyl chloride (CH3COCl) produces a higher FAME yield of 56 % g FAME/g dry weight (Cooney et al. 2009). This process can be enhanced by coupling it with microwave heating. The heterogeneous catalyst (SrO) together with heating with microwaves increased the FAME yield from 7 to 37 % g FAME/g dry weight in Nannochloropsis (Koberg et al. 2011). The disadvantage of this technique is the high harvesting cost due to the necessity for dry microalgal biomass. The overall flow chart of the processing of algal biomass to biodiesel is shown in the Fig. 3.
14 Conclusion
Increases in atmospheric CO2, and depletion of petro-diesel and mineral oil reserves requires alternative environmentally friendly energy sources. To overcome this problem, production of biodiesel from microalgal biomass might be a solution to reduce CO2 emissions from industry and could also meet the global demand for transport fuels. Production of biodiesel from microalgae is an emerging technology and an economical choice because of its availability and low cost. Microalgae have several advantages over conventional crops in that less land is required and non-arable land can be cultivated; microalgae double their weight with respect to biomass within 24 h; they require low pesticide application and have no impact on food security; and microalgae biomass can also be used in other energy-generation processes after the oil has been extracted. Many companies are already achieving commercial-scale production of microalgal biofuels. Large-scale cultivation and harvesting systems are needed for the production of algal biodiesel to reduce the cost per unit area. Large-scale microalgal biomass can be achieved using open pond or PBRs, and each system has its own advantages and disadvantages. The open pond system is widely used for large-scale production due to its advantages and the potential for CO2 sequestration when compared with PBRs. Some essential factors need to be optimized for large-scale application, including strain selection, seed culture preparation, medium composition, biomass and lipid yield optimization, harvesting, and extraction of lipids from biomass. Certain other important factors include providing optimum conditions of certain parameters such as light, nutrients, temperature, and CO2 and O2 levels. However, the above parameters cannot be controlled in an open pond system. Due to the high costs involved in the harvesting and extracting of lipids from the microalgal biomass, more effort must be made to reduce process costs and to increase the quality of biodiesel.
References
Ahmad AL, Yasin NHM, Derek CJC, Lim JK (2011) Microalgae as a sustainable energy source for biodiesel production: a review. Renew Sustain Energy Rev 15:584–593
Amaro HM, Guedes AC, Malcata FX (2011) Advances and perspectives in using microalgae to produce biodiesel. Appl Energy 88:3402–3410
An J-Y, Sim S-J, Lee JS, Kim BW (2003) Hydrocarbon production from secondarily treated piggery wastewater by the green algae Botryococcus braunii. J Appl Phycol 15:185–191
Andrich G, Nesti U, Venturi F, Zinnai A, Fiorentini R (2005) Supercritical fluid extraction of bioactive lipids from the microalga Nannochloropsis sp. Eur J Lipid Sci Technol 107:381–386
Antolin G, Tinaut FV, Briceño Y, Castañno V, Pérez C, Ramirez AI (2002) Optimisation of biodiesel production by sunflower oil transesterification. Bioresour Technol 83:111–114
Aslan S, Kapdan IK (2006) Batch kinetics of nitrogen and phosphorus removal from synthetic wastewater by algae. Ecol Eng 28:64–70
Bai M-D, Cheng C-H, Wan H-M, Lin Y-H (2011) Microalgal pigments potential as byproducts in lipid production. J Taiwan Inst Chem Eng 42(5):783–786
Balat M, Balat H (2010) Progress in biodiesel processing. Appl Energy 87:1815–1835
Banerjee N (2011) U.S. to reduce oil imports by a third by 2021, Obama says. Los Angeles Times. March 31, 2011. http://articles.latimes.com/2011/mar/31/nation/la-na-obama-energy-20110331
Benemann JR, Weissman JC, Koopman BL, Oswald WJ (1977) Energy production by microbial photosynthesis. Nature 268:19–23
Blanco AM, Moreno J, Del Campo JA, Rivas J, Guerrero MG (2007) Outdoor cultivation of lutein-rich cells of Muriellopsis sp. in open ponds. Appl Microbiol Biotechnol 73:1259–1266
Bogen C, Klassen V, Wichmann J, Russa ML, Doebbe A, Grundmann M, Uronen P, Kruse O, Mussgnug JH (2013) Identification of Monoraphidium contortum as a promising species for liquid biofuel production. Bioresour Technol 133:622–626
Borowitzka MA (1992) Algal biotechnology products and processes-matching science and economics. J Appl Phycol 4:267–279
Borowitzka MA (1997) Microalgae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Borowitzka MA (1999) Commercial production of microalgae: ponds, tanks, and fermenters. Prog Ind Microbiol 35:313–321
Bosma R, van Spronsen WA, Tramper J, Wijffels RH (2003) Ultrasound, a new separation technique to harvest microalgae. J Appl Phycol 15:143–153
BP (2011) BP Statistical Review of World Energy, 07.132011. http://www.bp.com/liveassets/bp_internet/globalbp/globalbp_uk_english/reports_and_publications/statistical_energy_review_2011/STAGING/local_assets/pdf/statistical_review_of_world_energy_full_report_2011.pdf
Brennan L, Owende P (2010) Biofuels from microalgae – a review of technologies for production, processing, and extractions of biofuels and co-products. Renew Sustain Energy Rev 14:557–577
Bruton T, Lyons H, Lerat Y, Stanley M, Rasmussen MB (2009) A review of the potential of marine algae as a source of biofuel in Ireland. Dublin: Sustainable Energy Ireland 88. http://www.seai.ie/Publications/Renewables_Publications_/Bioenergy/Algaereport.pdf
Cadenas A, Cabezudo S (1998) Biofuels as sustainable technologies: perspectives for less developed countries. Technol Forecast Soc Change 58:83–103
Carvalho AP, Meireles LA, Malcata FX (2006) Microalgal reactors: a review of enclosed system designs and performances. Biotechnol Prog 22:1490–1506
Cheirsilp B, Torpee S (2012) Enhanced growth and lipid production of microalgae under mixotrophic culture condition: effect of light intensity, glucose concentration and fed-batch cultivation. Bioresour Technol 110:510–516
Chen C-Y, Yeh K-L, Aisyah R, Lee D-J, Chang J-S (2011) Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour Technol 102:71–81
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Chisti Y (2008) Biodiesel from microalgae beats bioethanol. Trends Biotechnol 26:126–131
Chiu S-Y, Kao C-Y, Tsai M-T, Ong S-C, Chen C-H, Lin C-S (2009) Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol 100:833–838
Chojnacka K, Marquez-Rocha FJ (2004) Kinetic and stoichiometric relationships of the energy and carbon metabolism in the culture of microalgae. Biotechnology 3:21–34
Cooney M, Young G, Nagle N (2009) Extraction of bio-oils from microalgae. Sep Purif Rev 38:291–325
Crimson Renewable Energy, LP. http://www.crimsonrenewable.com/biodiesel-specifications.pdf
da Silva TL, Reis A, Medeiros R, Oliveira AC, Gouveia L (2009) Oil Production towards biofuel from autotrophic microalgae semicontinuous cultivations monitorized by flow cytometry. Appl Biochem Biotechnol 159:568–578
Demirbas A (2008) Biofuels sources, biofuel policy, biofuel economy and global biofuel projections. Energy Conver Manage 49:2106–2116
Desmorieux H, Decaen N (2005) Convective drying of Spirulina in thin layer. J Food Eng 66:497–503
Divakaran R, Pillai VNS (2002) Flocculation of algae using chitosan. J Appl Phycol 14:419–422
Edward M (2009) The algal industry survey – a white paper by Dr. Mark Edward & Centre for Management Technology. http://www.ascension-publishing.com/BIZ/algal-industry-survey.pdf
Edzwald JK (1993) Algae, bubbles, coagulants, and dissolved air flotation. Water Sci Technol 27:67–81
Elsey D, Jameson D, Raleigh B, Cooney MJ (2007) Fluorescent measurement of microalgal neutral lipids. J Microbiol Meth 68:639–642
Eriksen NT (2008) The technology of microalgal culturing. Biotechnol Lett 30:1525–1536
Fu C-C, Hung T-C, Chen J-Y, Su C-H, Wu W-T (2010) Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresour Technol 101:8750–8754
Fukuda H, Kondo A, Noda H (2001) Biodiesel fuel production by transesterification of oils. J Biosci Bioeng 92:405–416
Goldemberg J, Guardabassi P (2009) Are biofuels a feasible option? Energy Policy 37:10–14
Gouveia L, Oliveira AC (2009) Microalgae as a raw material for biofuels production. J Ind Microbiol Biotechnol 36:269–274
Greenwell HC, Laurens LML, Shields RJ, Lovitt RW, Flynn KJ (2010) Placing microalgae on the biofuels priority list: a review of the technological challenges. J R Soc Interface 7:703–726
Grima EM, Medina AR, Giménez AG, Sánchez Pérez JA, Camacho FG, Sánchez JLG (1994) Comparison between extraction of lipids and fatty acids from microalgal biomass. J Am Oil Chem Soc 71(9):955–959
Grima EM, Belarbi E-H, Fernández FGA, Medina AR, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20:491–515
Halim R, Gladman B, Danquah MK, Webley PA (2011) Oil extraction from microalgae for biodiesel production. Bioresour Technol 102:178–185
Heasman M, Diemar J, O’Connor W, Sushames T, Foulkes L (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs-a summary. Aquac Res 31:637–659
Hemaiswarya S, Raja R, Carvalho IS, Ravikumar R, Zambare V, Barh D (2012) An Indian scenario on renewable and sustainable energy sources with emphasis on algae. Appl Microbiol Biotechnol 96:1125–1135
Hosikian A, Lim S, Halim R, Danquah MK (2010) Chlorophyll extraction from microalgae: a review on the process engineering aspects. Int J Chem Eng. Article ID 391632, pp 1–12
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. Plant J 54:621–639
Huntley ME, Redalje DG (2006) CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. Mitig Adapt Strateg Glob Change 12:573–608
Huntley ME, Redalje DG (2008) Continuous-batch hybrid process for production of oil and other useful products from photosynthetic microbes, volume US 2008/0118964, no 7770322, 1–9
Illman AM, Scragg AH, Shales SW (2000) Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb Technol 27:631–635
International Energy Agency (2008) World Energy Outlook 2008. OECD/IEA, Paris, France, 1–569. http://www.iea.org/textbase/nppdf/free/2008/weo2008.pdf
Jacob-Lopes E, Teixeira Franco T (2010) Microalgae-based systems for carbon dioxide sequestration and industrial biorefineries. In: Momba M, Bux F (eds) Biomass. Sciyo, Croatia, pp 135–146
Jiménez C, Cossío BR, Labella D, Xavier Niell F (2003) The feasibility of industrial production of Spirulina (Arthrospira) in southern Spain. Aquaculture 217:179–190
Jimenez-Pérez MV, Sánches-Castillo P, Romera O, Fernández-Moreno D, Pérez-Martinez C (2004) Growth and nutrient removal in free and immobilized planktonic green algae isolated from pig manure. Enzyme Microb Technol 34:392–398
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Knothe G (2010) Biodiesel and renewable diesel: a comparison. Prog Energy Combust Sci 36:364–373
Knuckey RM, Brown MR, Robert R, Frampton DMF (2006) Production of microalgal concentrates by flocculation and their assessment as aquaculture feeds. Aquac Eng 35:300–313
Koberg M, Cohen M, Ben-Amotz A, Gedanken A (2011) Bio-diesel production directly from the microalgae biomass of Nannochloropsis by microwave and ultrasound radiation. Bioresour Technol 102:4265–4269
Lam MK, Lee KT (2012) Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv 30:673–690
Leach G, Oliveira G, Morais R (1998) Spray-drying of Dunaliella salina to produce a β-carotene rich powder. J Ind Microbiol Biotechnol 20:82–85
Lee K, Lee C-G (2001) Effect of light/dark cycles on wastewater treatments by microalgae. Biotechnol Bioprocess Eng 6:194–199
Lee S, Kim S, Kim J, Kwon G, Yoon B, Oh H (1998) Effects of harvesting method and growth stage on the flocculation of the green alga Botyrococcus braunii. Lett Appl Microbiol 27:14–28
Lee AK, Lewis DM, Ashman PJ (2009) Microbial flocculation, a potentially low-cost harvesting technique for marine microalgae for the production of biodiesel. J Appl Phycol 21:559–567
Lee J-Y, Yoo C, Jun S-Y, Ahn C-Y, Oh H-M (2010) Comparison of several methods for effective lipid extraction from microalgae. Bioresour Technol 101:S75–S77
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008a) Biofuels from microalgae. Biotechnol Progr 24:815–820
Li Y, Wang B, Horsman M, Wu N, Lan CQ (2008b) Effects of nitrogen sources on cell growth and lipid accumulation of green alga Neochloris oleoabundans. Appl Microbiol Biotechnol 81:629–636
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Liang Y, Sarkany N, Cui Y, Yesuf J, Trushenski J, Blackburn JW (2010) Use of sweet sorghum juice for lipid production by Schizochytrium limacinum SR21. Bioresour Technol 101:3623–3627
Ma F, Hanna MA (1999) Biodiesel production: a review. Bioresour Technol 70:1–15
MacKay D, Salusbury T (1988) Choosing between centrifugation and crossflow microfiltration. Chem Eng J 477:45–50
Martek (2008) Martek Biosciences Corporation. In: Martek, 17.06.2010. http://seekingalpha.com/article/80126-martek-biosciences-corporation-f2q08-qtr-end-04-30-2008-earnings-call-transcript
Mata TM, Martins AA, Caetano NS (2010) Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev 14:217–232
McElroy AK (2007) Pipeline Potential. Biodiesel Magazine. January 24. http://www.biodieselmagazine.com/articles/1441/pipeline-potential
McGarry MG (1970) Algal flocculation with aluminum sulfate and polyelectrolytes. Res J Water Pollut Control Fed 42:191–201. http://www.jstor.org/stable/25036591
Mendes RL, Fernandes HL, Coelho JP, Reis EC, Cabral JMS, Novais JM, Palavra AF (1995) Supercritical CO2 extraction of carotenoids and other lipids from Chlorella vulgaris. Food Chem 53:99–103
Miao X, Wu Q (2006) Biodiesel production from heterotrophic microalgal oil. Bioresour Technol 97:841–846
Miyamoto K (1997) Renewable biological systems for alternative sustainable energy production, vol 128, FAO agricultural services bulletin. Food and Agriculture Organization of the United Nations, Osaka
Mohn FH (1980) Experiences and strategies in the recovery of biomass in mass culture of microalgae. In: Shelef G, Soeder CJ (eds) Algal biomass. Elsevier, Amsterdam, pp 547–571
Molina E, Fernández J, Acién FG, Chisti Y (2001) Tubular photobioreactor design for algal cultures. J Biotechnol 92:113–131
Muñoz R, Guieysse B (2006) Algal-bacterial processes for the treatment of hazardous contaminants: a review. Water Res 40:2799–2815
Mutanda T, Ramesh D, Karthikeyan S, Kumari S, Anandraj A, Bux F (2011) Bioprospecting for hyper-lipid producing microalgal strains for sustainable biofuel production. Bioresour Technol 102:57–70
Nagle N, Lemke P (1990) Production of methyl ester fuel from microalgae. Appl Biochem Biotechnol 24–25:355–361
Naik SN, Goud VV, Rout PK, Dalai AK (2010) Production of first and second generation biofuels: a comprehensive review. Renew Sustain Energy Rev 14:578–597
National Renewable Energy Laboratory (2009) Biodiesel handling and use guide, pp 1–56. http://www.biodiesel.org/docs/using-hotline/nrel-handling-and-use.pdf
National Research Council (2007) Water implications of biofuels production. In: National Research Council, 01.07.2010. http://nationalacademies.org/wstb
Natrah FMI, Yosoff FM, Shariff M, Abas F, Mariana NS (2007) Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J Appl Phycol 19:711–718
Nindo CI, Tang J (2007) Refractance window dehydration technology: a novel contact drying method. Dry Technol 25:37–48
Norsker N-H, Barbosa MJ, Vermuë MH, Wijffels RH (2011) Microalgal production-a close look at the economics. Biotechnol Adv 29:24–27
Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12:499–506
Olguín EJ, Galicia S, Mercado G, Pérez T (2003) Annual productivity of Spirulina (Arthrospira) and nutrient removal in a pig wastewater recycling process under tropical conditions. J Appl Phycol 15:249–257
Őtleş S, Pire R (2001) Fatty acid composition of Chlorella and Spirulina microalgae species. J AOAC Int 84:1708–1714
Papazi A, Makridis P, Divanach P (2010) Harvesting Chlorella minutissima using cell coagulants. J Appl Phycol 22:349–355
Perrut M (2000) Supercritical fluid applications: industrial developments and economic issues. Ind Eng Chem Res 39:4531–4535
Piccolo A (2009) Algae oil production and its potential in the Mediterranean region. First EMUNI Research Souk 2009 (EMUNI ReS 2009). The Euro-Mediterranean Student Research Multi-conference Unity and Diversity of Euro-Mediterranean Identities, 9th June
Prakash J, Pushparaj B, Carlozzi P, Torzillo G, Montaini E, Materassi R (1997) Microalgae drying by a simple solar device. Int J Solar Energy 18:303–311
Pratoomyot J, Srivilas P, Noiraksar T (2005) Fatty acids composition of 10 microalgal species. Songklanakarin J Sci Technol 27:1179–1187
Pruvost J, Van Vooren G, Cogne G, Legrand J (2009) Investigation of biomass and lipids production with Neochloris oleoabundans in photobioreactor. Bioresour Technol 100:5988–5995
Pulz O (2001) Photobioreactors: production systems for phototrophic microorganisms. Appl Microbiol Biotechnol 57:287–293
Raja R, Anbazhagan C, Ganesan V, Rengasamy R (2004) Efficacy of Dunaliella salina (Volvocales, Chlorophyta) in salt refinery effluent treatment. Asian J Chem 16:1081–1089
Raja R, Hemaiswarya S, Ashok Kumar N, Sridhar S, Rengasamy R (2008) A perspective on the biotechnological potential of microalgae. Crit Rev Microbiol 34:77–88
Ranjbar R, Inoue R, Shiraishi H, Katsuda T, Katoh S (2008) High efficiency production of astaxanthin by autotrophic cultivation of Haematococcus pluvialis in a bubble column photobioreactor. Biochem Eng J 39:575–580
Raven RPJM, Gregersen KH (2007) Biogas plants in Denmark: successes and setbacks. Renew Sustain Energy Rev 11:116–132
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2011) Dual role of microalgae: phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy 88:3411–3424
Rawat I, Ranjith Kumar R, Mutanda T, Bux F (2013) Biodiesel from microalgae: a critical evaluation from laboratory to large scale production. Appl Energy 103:444–467
Renaud SM, Thinh LV, Parry DL (1999) The gross chemical composition and fatty acid composition of 18 species of tropical Australian microalgae for possible use in mariculture. Aquaculture 170:147–159
Rodolfi L, Zittelli GC, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Rosenberg JN, Oyler GA, Wilkinson L, Betenbaugh MJ (2008) A green light for engineered algae: redirecting metabolism to fuel a biotechnology revolution. Curr Opin Biotechnol 19:430–436
Samorì C, Torri C, Samorì G, Fabbri D, Galletti P, Guerrini F, Pistocchi R, Tagliavini E (2010) Extraction of hydrocarbons from microalga Botryococcus braunii with switchable solvents. Bioresour Technol 101:3274–3279
Sarin R, Kumar R, Srivastav B, Puri SK, Tuli DK, Malhotra RK, Kumar A (2009) Biodiesel surrogates: achieving performance demands. Bioresour Technol 100:3022–3028
Sathasivam R, Juntawong N (2013) Modified medium for enhanced growth of Dunaliella strains. Int J Curr Sci 5:67–73
Sathasivam R, Kermanee P, Roytrakul S, Juntawong N (2012) Isolation and molecular identification of β-carotene producing strains of Dunaliella salina and Dunaliella bardawil from salt soil samples by using species-specific primers and internal transcribed spacer (ITS) primers. Afr J Biotechnol 11:16677–16687
Schenk PM, Thomas-Hall SR, Stephens E, Marx U, Mussgnug JH, Posten C, Kruse O, Hankamer B (2008) Second generation biofuels: high-efficiency microalgae for biodiesel production. Bioenergy Res 1:20–43
Scott SA, Davey MP, Dennis JS, Horst I, Howe CJ, Lea-Smith DJ, Smith AG (2010) Biodiesel from algae: challenges and prospects. Curr Opin Biotechnol 21:277–286
Sharp CA (1996) Emissions and lubricity evaluation of rapeseed derived biodiesel fuels [R]. Final Report for Montana Department of Environmental Quality. Southwest Research Institute; November 1996
Sheehan J, Dunahay T, Benemann J, Roessler P (1998) A look back at the US Department of Energy’s Aquatic Species Program- biodiesel from algae. Report NREL/TP-580–24190. National Renewable Energy Laboratory, Golden, CO, USA
Singh J, Gu S (2010) Commercialization potential of microalgae for biofuels production. Renew Sustain Energy Rev 14:2596–2610
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102:10–16
Stephenson AL, Dennis JS, Howe CJ, Scott SA, Smith AG (2010) Influence of nitrogen- limitation regime on the production by Chlorella vulgaris of lipids for biodiesel feedstocks. Biofuels 1:47–58
Suh IS, Lee C-G (2003) Photobioreactor engineering: design and performance. Biotechnol Bioprocess Eng 8:313–321
Sukenik A, Shelef G (1984) Algal autoflocculation – verification and proposed mechanism. Biotechnol Bioeng 26:142–147
Takagi M, Karseno M, Yoshida T (2006) Effect of salt concentration on intracellular accumulation of lipids and triacylglyceride in marine microalgae Dunaliella cells. J Biosci Bioeng 101:223–226
Tan HH (2008) Algae-to-biodiesel at least five to 10 years away. Energy Current: News for the Business of Energy, Singapore
Tan KT, Lee KT (2011) A review on supercritical fluids (SCF) technology in sustainable biodiesel production: potential and challenges. Renew Sustain Energy Rev 15:2452–2456
Thomas WH, Tornabene TG, Weissman J (1984) Screening for lipid yielding microalgae: activities for 1983. SERI/STR-231-2207 http://www.nrel.gov/docs/legosti/old/2207.pdf
Uduman N, Qi Y, Danquah MK, Forde GM, Hoadley A (2010) Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J Renew Sustain Energy 2:012701
Uduman N, Bourniquel V, Danquah MK, Hoadley AFA (2011) A parametric study of electrocoagulation as a recovery process of marine microalgae for biodiesel production. Chem Eng J 174:249–257
Ugwu CU, Ogbonna JC, Tanaka H (2002) Improvement of mass transfer characteristics and productivities of inclined tubular photobioreactors by installation of internal static mixers. Appl Microbiol Biotechnol 58:600–607
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Veillette M, Chamoumi M, Nikiema J, Faucheux N, Heitz M (2012) Production of biodiesel from microalgae. In: Nawaz Z (ed) Advances in chemical engineering. Intech, Croatia, pp 245–268
Vicente G, Martinez M, Aracil J (2004) Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour Technol 92:297–305
Viswanath B, Mutanda T, White S, Bux F (2010) The microalgae – a future source of biodiesel. Dyn Biochem Process Biotech Mol Biol 4:37–47
Vunjak-Novakovic G, Kim Y, Wu X, Berzin I, Merchuk JC (2005) Air-lift bioreactors for algal growth on flue gas: mathematical modeling and pilot-plant studies. Ind Eng Chem Res 44:6154–6163
Watanabe MM, Kawachi M, Hiroki M, Kasai F (2000) NIES collection list of strains. 6th ed, Microalgae and protozoa. Microbial culture collections. National Institute for Environmental Studies, Tsukuba, p 159
Wigmosta MS, Coleman AM, Skaggs RJ, Huesemann MH, Lane LJ (2011) National microalgae biofuel production potential and resource demand. Water Resour Res 47:1–13
Wu S-T, Yu S-T, Lin L-P (2005) Effect of culture conditions on docosahexaenoic acid production by Schizochytrium sp. S31. Process Biochem 40:3103–3108
Xin L, Hong-ying H, Ke G, Ying-xue S (2010) Effects of different nitrogen and phosphorus concentrations on the growth, nutrient uptake, and lipid accumulation of a freshwater microalga Scenedesmus sp. Bioresour Technol 101:5494–5500
Xiong W, Li X, Xiang J, Wu Q (2008) High-density fermentation of microalga Chlorella protothecoides in bioreactor for microbio-diesel production. Appl Microbiol Biotechnol 78:29–36
Xu H, Miao X, Wu Q (2006) High quality biodiesel production from a microalga Chlorella protothecoides by heterotrophic growth in fermenters. J Biotechnol 126:499–507
Xu L, Weathers PJ, Xiong X-R, Liu C-Z (2009) Microalgal bioreactors: challenges and opportunities. Eng Life Sci 9:178–189
Yaguchi T, Tanaka S, Yokochi T, Nakahara T, Higashihara T (1997) Production of high yields of docosahexaenoic acid by Schizochytrium sp. strain SR21. J Am Oil Chem Soc 74:1431–1434
Yang J, Rasa E, Tantayotai P, Scow KM, Yuan H, Hristova KR (2011) Mathematical model of Chlorella minutissima UTEX2341 growth and lipid production under photoheterotrophic fermentation conditions. Bioresour Technol 102:3077–3082
Zeng X, Danquah MK, Chen XD, Lu Y (2011) Microalgae bioengineering: from CO2 fixation to biofuel production. Renew Sustain Energy Rev 15:3252–3260
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer India
About this chapter
Cite this chapter
Ramaraj, S. et al. (2015). Microalgae as an Attractive Source for Biofuel Production. In: Thangavel, P., Sridevi, G. (eds) Environmental Sustainability. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2056-5_8
Download citation
DOI: https://doi.org/10.1007/978-81-322-2056-5_8
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-2055-8
Online ISBN: 978-81-322-2056-5
eBook Packages: Earth and Environmental ScienceEarth and Environmental Science (R0)