Abstract
Owing to the significant biological activities, quinazoline derivatives have drawn more and more attention in the synthesis and bioactivities research. This chapter summarizes the recent advances in the investigations of synthesis and biological activities of quinazoline derivatives. According to the main method the authors adopted in their research design, those synthetic methods include microwave-assisted reaction, ultrasound-promoted reaction, metal-mediated reaction, water reaction, and phase-transfer catalysis reaction. The biological activities of the synthesized quinazoline derivatives are also discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
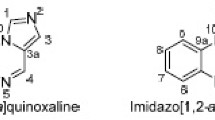
Quinazoline is a compound made up of a fused benzene ring and a pyrimidine ring (Figs. 13.1 and 13.2). Its chemical formula is C8H6N2. Quinazoline appears as a yellow crystalline substance. Any derivative of quinazoline may be described as a quinazoline compound.
Quinazoline derivatives, which belong to the N-containing heterocyclic compounds, have caused universal concerns due to their wide and distinct biopharmaceutical activities. Researchers have already determined many therapeutic activities of quinazoline derivatives, including anticancer [1–4], anti-inflammatory [5, 6], antibacterial [7–10], analgesic [5, 9], antiviral [11], anti-cytotoxic [12], antispasmodic [9, 13], antituberculosis [14], antioxidant [15], antimalarial [16], antihypertensive [17], antiobesity [18], antipsychotic [19], antidiabetic [20], etc. Medicinal chemists synthesized a variety of quinazoline compounds with different biological activities by installing various active groups to the quinazoline moiety using developing synthetic methods. Potential applications of the quinazoline derivatives in fields of biology, pesticides, and medicine have also been explored. This chapter summarizes the representative synthetic methods, either traditional or novel, and categorized them as microwave-assisted reaction, metal-catalyzed reaction, ultrasound-promoted reaction, and phase-transfer catalysis. In addition, the bioactivity researches of quinazoline derivatives are also discussed in order to provide valuable reference for future synthesis and biological investigation of these compounds.
2 Naturally Occurring Quinazoline-Based Compounds
The quinazoline alkaloids form a small but important group of naturally occurring bases, which were isolated from a number of different families in the plant kingdom. Witt and Bergman [21] review the chemistry of quinazoline alkaloids, viz. chrysogine, luotonine A, tryptanthrin, febrifugine, and rutaecarpine. The only non-alkaloid naturally occurring quinazoline is the potent neurotoxin called tetrodotoxin, which was isolated from the Japanese puffer fish and from the California newt. Arborine was isolated by two Indian groups from Glycosmis arborea [22]. Vasicine was discovered in Adhatoda vasica [23] and was found to show bronchodilator activity. The bronchodilator activity of vasicine, vasicinone, and 3,4-dihydro-4-oxoquinazoline was studied in detail but was in no way comparable with known bronchodilator drugs [24].
Experiments in the USA and China during World War II led to the isolation of two compounds called febrifugine (Fig. 13.3) and isofebrifugine from Dichroa febrifuga with known antimalarial activity (a Chinese herb). Further studies suggested that it must be the diastereoisomers of febrifugine that posses the antimalarial properties. Baker and co-workers [25] studied the antimalarial activities of synthesized samples of dl-febrifugine and found only one-half activity than the naturally occurring compound. Further, they discovered that it was actually the isofebrifugine that possessed the antimalarial properties, but was highly toxic in isolation to the other compounds found in the naturally occurring alkaloid [26]. Finally, the antimalarial activity of febrifugine and many of its synthetic derivatives were confirmed by Hewitt and collaborators [27].
A hypotensive red alkaloid isolated from the Brazilian plant Hortia arborea England was called hortiamine. One of the most potent nonprotein neurotoxin tetrodotoxin was isolated from certain varieties of the Japanese puffer fish. Several quinazoline derivates show antimalarial properties against Plasmodium gallinaceum. The most effective was 6-chloro-2-ethyl-3,4-dihydro-4-oxo-3-p-pyrimidin-2′-ylsulfa-moylphenylquinazoline [28].
Cytotoxic alkaloids of the fumiquinazoline (Fig. 13.4) family have been isolated from different fungi including a strain of the fungus Aspergillus fumigatus, Acremonium sp., Ecteinascidia turbinata, and Neosartorya fischeri. The first total synthesis of fumiquinazolines has been described by Snider and Zheng [29].
3 Microwave Methodology of Quinazolines Synthesis
3.1 Microwave-Assisted Synthesis of Quinazoline Compounds
Microwave-assisted organic synthesis is becoming popular with organic chemists, and comprehensive chapters have become available in recent years. Microwave heating is very convenient to use in organic synthesis. The heating is instantaneous, very specific, and there is no contact required between the energy source and the reaction vessel. Recent interest has been focused on “dry media” synthesis and particularly on solvent-free procedures using various mineral oxides and solvent-less reactions with neat reactants in the absence of a catalyst or solid support. Furthermore, the diversity-generating potential of multicomponent reactions (MCRs) has been recognized and their utility in preparing libraries to screen for functional molecules is well appreciated. Consequently, the design of novel MCRs is an important field of research. In this section, some selected literature examples of quinazoline synthesis by these methodologies are discussed.
Compared to traditional heating methods, microwave heating could expand the reaction range as well as shorten the reaction time from a few days or hours to a few minutes. Thus, when applied in fields of organic synthesis, pharmaceutical chemistry, and high-throughput chemistry, microwave heating shows greater advantage than traditional heating methods [30–33].
Luo et al. reported the first microwave-assisted synthesis of new quinazoline derivates containing α-aminophosphonate [34]. In their method, N′-(substituted-2-cyanophenyl)-N, N-dimethyl-formamidine derivatives, and dialkyl amino(phenyl) were adopted as the raw materials to react in 4:1 volume ratio of isopropanol to acetic acid solvent for 20 min under microwave irradiation (100 psi) and obtained 24 quinazoline compounds, two of which had similar activity as commercial reagent ningnanmycin (Scheme 13.1).

Scheme 13.1
Tu et al. reported a fast one-pot, microwave-assisted synthesis of polysubstituent imidazo[1,2-a]quinoline, pyrimido [1,2-a]quinoline, and quinolino[1,2-a]quinazoline derivatives [35]. They explored the optimal reagent, volume, and heating temperature by testing different reagents under different reaction times and temperatures. Then, under optimal conditions (2.0 ml glycol), several aldehydes were separately made to react with various enaminones and malononitrile to obtain different products (Scheme 13.2).

Scheme 13.2
In the synthetic research conducted by Kidwai et al. [36], the target compounds quinazoline derivatives were obtained by heating an equimolar amount of aldehyde, 5,5-dimethyl-1,3-cyclohexanedione (dimedone), and urea/thiourea under microwave irradiation in the absence of solvent and catalyst (Scheme 13.3).

Scheme 13.3
3.2 Niementowski Quinazoline Synthesis
The striking improvement in the Niementowski quinazoline synthesis has been fulfilled using microwave irradiation (Scheme 13.4). Using microwave irradiation and/or Appel’s salt, new efficient routes to various substituted and fused quinazolines have been developed by Besson et al. [37, 38].

Scheme 13.4
3.3 MultiComponent One-Pot Synthesis of Quinazolines
One-pot synthesis of 4(3H)-quinazolinones from amines and formic acid (or orthoesters) was developed, and recently, more detailed procedures using inorganic solid support and neat one-pot procedures under microwave irradiation have been developed by Dandia et al. [39] (Scheme 13.5) and Liu et al. [40]. Also facile one-pot synthesis of 2,4(1H,3H)-quinazolinediones has been developed recently as a green chemical procedure.

Scheme 13.5
4 Ultrasound-Promoted Synthesis of Quinazoline
In critical synthesis, ultrasonic assistance is needed to meet the high requirements for temperature and pressure. For instance, in Bischler cyclization, the most traditional synthetic methods for quinazoline derivatives, high temperature (above 120 °C) and high pressure are needed for at least 5 h in saturated ammonia alcohol solution. Various syntheses applying this method contain the passage of ammonia through a mixed melt of the amino compound and sodium acetate at a temperature higher than 160 °C in which ultrasonic promotion is demanded.
Zhang et al. [41] reported an ultrasound-assisted synthesis of novel quinazoline derivatives (Scheme 13.6), including a four-step synthesis of quinazoline core and the optimization of the Bischler cyclization [42].

Scheme 13.6
We had an interest in utilizing 2-phosphoranylideneamino-benzoyl derivatives as building blocks, particularly in view of anthranilic acids as important biological precursors of various alkaloids such as glomerine, vascine, and microbial products like tryptanthrin and anthramycine.
Thus, acylation of N-methylamides with 2-azidobenzoyl chloride (readily available from 2-azidobenzoic acid [43]) forms imides which upon treatment with triphenylphosphine (TPP) in the course of consecutive Staudinger reaction/intramolecular aza-Wittig reaction yields exclusively 3-methylquinazolin-4(3H)-ones quantitatively (Scheme 13.7) [44, 45]. This procedure provides simple and efficient quinazolinone annelation of amides and lactams.

Scheme 13.7
Safari et al. [45] successfully demonstrated for the first time that Cu powder and ultrasound 300 w/H2O could be used as an excellent and efficient catalyst for convenient synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives under solvent-free conditions and microwave irradiation (Scheme 13.8). The protocol proves to be efficient and environmentally benign in terms of easy workup, high yields, and ease of recovery of catalyst. In addition, the present method is superior in terms of green media, the amount of catalyst, and reaction time.

Scheme 13.8
Bharathi et al. [46] successfully synthesized the TiO2 nanoparticles using aqueous Annona squamosa peel extract (Scheme 13.9). These synthesized TiO2 nanoparticles were characterized using UV, XRD, and TEM and used as a catalyst for 2,3-dihydro-3-methyl-2-phenylquinazolin-4(1H)-one analogue synthesis.

Scheme 13.9
Muthukrishnan et al. [47] discovered that the EtOAc fraction of Glycosmis pentaphylla leaf extract inhibits the juvenile hormone III biosynthesis in vitro of corpora allata from 3-day-old females of the field cricket Gryllus bimaculatus. The bioactive compound responsible for this activity was identified as the quinazolone alkaloid arborine. This alkaloid also exhibited larvicidal activity against the mosquito.
Derivatives of 2-methyl-3-(o-tolyl)-4(3H)-quinazolone (Scheme 13.10) bearing new substituents on the 2-methyl group have been synthesized. It was established that most substitutions at this position reduce or remove the CNS-depressant activity of methaqualone [48].

Scheme 13.10 Aza-Diels–Alder reaction
Imino-Diels–Alder reaction [49] containing the coupling of imine and electron-rich alkene gradually became a powerful tool for the synthesis of quinazoline derivatives [50]. In Povarov imino-Diels–Alder reaction, aniline and ethyl glyoxalate were chosen as substrates. And two molecules of α-iminoesters, which were obtained from the condensation of aniline and ethyl glyoxalate, were hypothesized to form the direct additive product. Cascade imino-Diels–Alder reaction conducted by Chen et al. [51] (Scheme 13.11) was extended from the Povarov imino-Diels–Alder reaction. In this research, researchers chose the same substrates as in the Povarov imino-Diels–Alder reaction, adopted various kinds of Lewis acids as catalysts, and finally produced quinazoline derivatives. Iron powder was determined as the optimized catalyst with highest yields.

Scheme 13.11 Aza-Wittig reaction
Aza-Wittig reaction, which generally precedes in cascade with easy operation under mild reaction conditions, is widely used in the synthesis of N-heterocycles [52]. He et al. reported a kind of tandem Staudinger–Aza-Wittig–nucleophilic addition reaction to synthesize indolo[1,2-c]quinazolines recently [53]. Results showed that the nitrogen evolution through the Staudinger reaction halted during the initial 2 h and surprisingly produced the final product indolo[1,2- c]quinazolines directly from the reaction mixture (Scheme 13.12).

Scheme 13.12
A synthetic method for 2-alkoxy-3H-quinazolin-4-ones was reported by Ding et al. in 2004 [54]. In this study, 12 novel 2-alkoxy-3H-quinazolin-4-ones were synthesized from carbodiimide, which was obtained from aza-Wittig reaction of iminophosphorane with aromatic isocynate (Scheme 13.13).

Scheme 13.13
5 Water-Mediated Quinazoline Synthesis
Organic reactions in water, without the use of any harmful organic solvents, are of great interest because water is nontoxic, nonflammable, abundantly available, and inexpensive. Thus, water as the reaction medium is generally considered a cheap, safe, and environmentally benign alternative to synthetic solvents. Furthermore, because of the low solubility of common organic compounds in water, the use of water as a solvent often makes the purification of products very easy by simple filtration or extraction.
A convenient and clean water-mediated synthesis of a series of indolo[1,2-c]quinazoline derivatives was reported using alternative nonconventional energy sources. The products are obtained in shorter times with excellent yields (78–89 %) from the MCR of 2-aminobenzimidazole, malononitrile, and carbonyl compounds [55]. In their research, 2-(2-halophenyl)-1H-indoles and (aryl)methanamines were adopted as raw materials to generate corresponding Schiff base via the Ullmann reaction. Then, gas as oxidant, 3 equiv K2CO3 as base, and 10 mol% Cu(OAc)2 as catalyst were revealed as the optimum conditions to conduct aerobic oxidative C–H amination under solvent-free conditions or water (Scheme 13.14).

Scheme 13.14
Jiang et al. also reported a one-pot synthesis of 5,12-dihydroindolo[2,1-b]quinazolines [56]. N-(2-bromobenzyl)-2-iodoani-line and malononitrile were adopted as the raw materials to afford the desired compound through copper-catalyzed intramolecular C–N coupling reaction (Scheme 13.15).

Scheme 13.15
Dabiri and Mostafa reported [57] a rapid, efficient, and one-pot procedure for the synthesis of mono- and di-substituted (3H)-quinazolin-4-ones in the presence of an AlCl3/ZnCl2 mixture supported on silica gel, under solvent-free conditions or water.

Scheme 13.16
An efficient and eco-friendly method is reported for the synthesis of 2-substituted-2,3-dihydroquinazolin- 4(1H)-ones from direct cyclocondensation of anthranilamide with aldehydes and ketones using N-propylsulfamic acid supported on magnetic Fe3O4 as a recoverable and recyclable nanocatalyst in good to excellent yields in water (Scheme 13.17). The characteristic advantages of this catalyst are rapid, simple, and efficient separation using an appropriate external magnet, which minimizes the loss of catalyst during separation and is reusable without significant loss of activity up to ten cycles.

Scheme 13.17
The procedure does not involve the use of any additional reagent/catalyst, produces no waste, and represents a green synthetic protocol with high atom economy. The combination of microwave irradiation, ultrasonic irradiation, and aqueous-mediated conditions using MCRs lead to enhanced reaction rates, higher yields of pure products, easier workup, and sometimes selective conversions. Consequently, this protocol should be welcome in these environmentally aware days.
6 Metal-Catalyzed Synthesis of Quinazoline Derivatives
In 1993, several catalytic methods have been developed for the synthesis of 4(3H)-quinazolinones via transition-metal catalyzed reductive N-heterocyclization. For example, palladium-catalyzed cyclocarbonylations of halides with appropriate reactants provided regioselective synthesis of 4(3H)-quinazolinone derivatives and indoloquinazolines [58]. Also, selenium-catalyzed reductive N-heterocyclization to quinazolinones has been developed. Copper-catalyzed heteroannulation with alkynes has been developed as highly region- and stereoselective route to 2-(2-arylvinyl)-1,2,3,4-tetrahydroquinazolin-4-ones by Kundu et al. [59] (Scheme 13.18). Recently, condensation of anthranylamide with various aldehydes to 4-quinazolinones has been found to give excellent yields in the presence of cupric chloride. For synthesis of quinazoline derivatives, various coupling reactions have been utilized after synthesis of quinazoline-2,4(1H,3H)-diones via palladium-catalyzed oxidative coupling. For example, synthesis of diarylquinazolines by iron-catalyzed cross-coupling reaction and diaminoquinazolinones by palladium-catalyzed amination have been developed.

Scheme 13.18
6.1 Titanium-Catalyzed Reaction
A convenient method for the synthesis of 3-substituted quinazolin-4(3H)- ones using the convergent reactions of formic acid, a primary amine, and isatoic anhydride under solvent-free conditions and with brief microwave irradiation is described.
Natural and synthetic molecules with the core of quinazoline ring system show a wide range of biological activities [60–64]. The chemotherapeutic use of quinazoline alkaloids may date back to the ancient Chinese treatment of malaria with the herbal preparations from Dichroa febrifuja [65]. At present, some synthetic quinazoline-based drugs such as metolazone, quinethazone, and prazosin have acquired medicinal approval for their unique pharmacological indices and many others are under clinical evaluation [66–71]. In this synthesis, a series of quinazoline derivatives were afforded by adopting anhydrous THF as solvent and the TiCl4–Zn system as reducing agent. Several representative synthetic routes were selected [72, 73]. In many such cases, the role of solvents as heat dispersants are no longer needed. The so-called solvent-free reactions are eco-friendly and, in view of green chemistry’s desire for avoiding solvent hazards, are in demand (Scheme 13.19).

Scheme 13.19
6.2 Palladium-Catalyzed Reaction
Palladium-catalyzed coupling reaction, which plays a vital role in the pharmaceutical industry, is widely applied in the chemical synthesis industry and laboratories as an efficient method for the formation of C–C and C–heteroatom bond. Qiu et al. [74] determined the optimum conditions for the palladium-catalyzed three-component synthesis of quinazolino[3,2-a]quinazolines as follows: amine (3.0 equiv), isocyanide (3.0 equiv), carbodiimide (0.2 mmol), Pd(OAc)2 (5 mol%), and Cs2CO3 (3.0 equiv) in 3.0 ml toluene (Scheme 13.20).

Scheme 13.20
6.3 Organometallic Reagents
Various quinazolines from 2-aminobenzonitrile using organometallic reagents have been developed by Bergman et al. [75–76]. The topical synthetic methodologies such as iminophosphorane-mediated synthesis (aza-Wittig methodology), microwave-assisted synthesis, solid-phase synthesis, and application of organometallic reagents, etc. will be discussed retrospectively, focusing on the pathways to quinazoline, quinazoline-4-one (Scheme 13.21), and their derivatives.

Scheme 13.21
6.4 PTSA-catalyzed Reaction
Rossi et al. have utilized the tandem aza-Wittig/electrocyclization principle for synthesis of quinazoline ring starting from N-imidoyl iminophosphorane [77]. Other unique synthetic strategies with N-vinyliminophosphoranes and benzotriazolyl derivatives (Scheme 13.22) have also been developed demonstrating the maturity and excellent prospects of iminophosphorane-mediated syntheses.

Scheme 13.22
Quinazolines and their spiro derivatives are also available via MCRs in water. The three-component condensation of isatoic anhydride, primary amines, and aromatic aldehydes or isatin to give 2,3-dihydroquinazolin-4(1H)-ones or spirooxindole derivatives (Scheme 13.23) was performed in water using ethylenediamine diacetate (EDDA) as catalyst [78].

Scheme 13.23
7 Antimalarial Activity
Several bio-active natural products such as febrifugine (Fig. 13.5) and isofebrifugine (Fig. 13.6) contain quinazolinone moieties with potential antimalarial [79] activity.
Quinazolinone derivatives attract widespread attention due to the diverse biological activities associated with them. Rutaecarpine (Fig. 13.7) and luotonine A [80] (Fig. 13.8) are the two natural quinazoline-fused compounds exhibiting very potent pharmacological values.
8 Conclusions
Conventional synthetic methods for quinazoline derivatives, still in general use, including microwave-assisted reaction, ultrasound-promoted reaction, metal-mediated reaction, water reaction, and phase-transfer catalysis reaction for the synthesis of this important heterocyclic compounds are discussed. It could be seen from the examples compiled above that some novel synthetic methods are in constant development and different methods are adopted in the synthesis of different quinazoline analogues. On the other hand, it is known that substituents at different positions affect the activity differently. By careful observation of the recent researches, substituted quinazoline analogues remain a majority among the products. However, with the deepening and development of researches, substituent groups at other positions are also achieved and studied increasingly, such as the construction of N-heterocyclic quinazolines by introduction of active groups into the 3-position of the quinazoline core. It is worth mentioning that N-heterocyclic quinazolines with more rigid and complicated structures were synthesized successively, some of which showed excellent biodynamic derivatives. In addition, it could be drawn from the research progress discussed above that enhancement of activity by the splicing method of installing various active groups is and will still be the main method for drug design and reconstruction of quinazoline derivatives. We hope that the information contained here encourages the readers to make use of these green protocols for the efficient and eco-friendly construction of novel heterocyclic frameworks.
References
Chandregowda V, Kush AK, Chandrasekara RG (2009) Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur J Med Chem 44:3046–3055
Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AA, Abdel-Hamide SG, Al-Obaid AM, El-Subbagh HI (2006) Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone analogs. Bioorg Med Chem 14:8608–8621
Vasdev N, Dorff PN, Gibbs AR, Nandanan E, Reid LM, Neil JPO’, VanBrocklin HF (2005) Synthesis of 6-acrylamido-4-(2-[18F] fluoroanilino) quinazoline. A prospective irreversible EGFR binding probe. J Lablelled Compd Rad 48:109–115
Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, Gibson KH (2002) An orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res 62:5749–5754
Alagarsamy V, Solomon VR, Dhanabal K (2007) Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H -quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg Med Chem 15:235–241
Baba A, Kawamura N, Makino H, Ohta Y, Taketomi S, Sohda T (1996) Studies on disease-modifying antirheumatic drugs: synthesis of novel quinoline and quinazoline derivatives and their anti-inflammatory effect. J Med Chem 39:5176–5182
Rohini R, Muralidhar Reddy P, Shanker K, Hu A, Ravinder V (2010) Antimicrobial study of newly synthesized 6-substituted indolo[1,2-c]quinazolines. Eur J Med Chem 45:1200–1205
Antipenko L, Karpenko A, Kovalenko S, Katsev A, Komarovska-Porokhnyavets E, Novikov V, Chekotilo A (2009) Synthesis of new 2-thio-[1,2,4]triazolo[1,5-c] quinazoline derivatives and its antimicrobial activity. Chem Pharm Bull 57:580–585
Jatav V, Kashaw S, Mishra P, Gupta V (2008) Synthesis and antimicrobial activity of some new 3–[5-(4-substituted)phenyl-1,3,4-oxadiazole-2yl]-2-styrylquinazoline-4(3H)-ones. Med Chem Res 17:205–211
Aly AA (2003) Synthesis of novel quinazoline derivatives as antimicrobial agents. Chin J Chem 21:339–346
Li H, Huang R, Qiu D, Yang Z, Liu X, Ma J, Ma Z (1998) Synthesis and bioactivity of 4-quinazoline oxime ethers. Prog Nat Sci 8:359–365
Chandrika PM, Yakaiah T, Narsaiah B, Sridhar V, Venugopal G, Rao JV, Kumar KP, Murthy USN, Rao ARR (2009) Synthesis leading to novel 2,4,6-trisubstituted quinazoline derivatives, their antibacterial and cytotoxic activity against THP-1, HL-60 and A375 cell lines. Indian J Chem 48B:840–847
Paneersalvam P, Raj T, Ishar PSM, Singh B, Sharma V, Rather BA (2010) Anticonvulsant activity of Schiff bases of 3-amino-6,8-dibromo-2-phenyl-quinazolin-4(3H)-ones. Indian J Pharm Sci 72:375–378
Nandy P, Vishalakshi MT, Bhat AR (2006) Synthesis and antitubercular activity of Mannich bases of 2-methyl-3H-quinazolin-4-ones. Indian J Heterocycl Chem 15:293–294
Saravanan G, Alagarsamy V, Prakash CR (2010) Synthesis and evaluation of antioxidant activities of novel quinazoline derivatives. Int J Pharm Pharm Sci 2:83–86
Lakhan R, Singh OP, Singh-J RL (1987) Studies on 4 (3H)-quinazolinone derivatives as anti-malarials. J Indian Chem Soc 64:316–318
Hess HJ, Cronin TH, Scriabine A (1968) Antihypertensive 2-amino-4(3H)-quinazolinones. J Med Chem 11:130–136
Sasmal S, Balaji G, Kanna Reddy HR, Balasubrahmanyam D, Srinivas G, Kyasa S, Sasmal PK, Khanna I, Talwar R, Suresh J, Jadhav VP, Muzeeb S, Shashikumar D, Harinder Reddy K, Sebastian VJ, Frimurer TM, Rist Ø, Elster L, Högberg T (2012) Design and optimization of quinazoline derivatives as melanin concentrating hormone receptor 1 (MCHR1) antagonists. Bioorg Med Chem Lett 22:3157–3162
Alvarado M, Barceló M, Carro L, Masaguer CF, Raviña E (2006) Synthesis and biological evaluation of new quinazoline and cinnoline derivatives as potential atypical antipsychotics. Chem Biodivers 3:106–117
Malamas MS, Millen J (1991) Quinazolineacetic acids and related analogs as aldose reductase inhibitors. J Med Chem 34:1492–1503
Wagner H, Wolff P (1977) New natural products and plant drugs with pharmacological, biological or therapeutical activity, vol 3. Springer, Berlin, p 642
Malon MH (1977) New natural products and plant drugs with pharmacological, biological or therapeutical activity, vol 3. Springer, Berlin, pp 23–25
Inderjit K, Chauhan PK (2012) Antioxidant and antimicrobial activity of leaf extract of adhatoda vasica against the bacteria isolated from the sputum samples of asthmatic patients. Int J Drug Res Tech 2:273–278
Rachana, Basu S, Kumar M (2011) Review & future perspectives of using vasicine, and related compounds. Indo-Global J Pharm Sci 1:85–98
Baker BR, Schaub RE, McEvoy FJ, Williams JH (1952) An antimalarial alkaloid from hydrangea. xii. synthesis of 3-[β-keto-γ-(3-hydroxy-2-piperidyl) propyl]-4-quinazolone, the alkaloid. J Org Chem 17:132–140
Yasuo T, Miyo O (2005) Concise Synthesis of dl-Febrifugine. Chem Pharm Bull 53:868–869
Koepfli JB, Mead JF et al (1947) An alkaloid with high antimalarial activity from Dichroa febrifuga. J Am Chem Soc 69:1837
Gujral ML, Saxena PN, Tiwari RS (1955) Comparative evaluation of quinazolones: a new class of hypnotics. Indian J Med Res 43:637–641
Snider BB, Zeng H (2003) First total synthesis of fumiquinazolines. J Org Chem 68:545
Mavandadi F, Lidstrom P (2004) Microwave- assisted chemistry in drug discovery. Curr Top Med Chem 4:773–792
Gedye R, Smith F, Westaway K, Ali H, Baldisera L et al (1986) The use of microwave ovens for rapid organic synthesis. Tetrahedron Lett 27:279–282
Liu YP, Yin DC, Chen HT, Sun BG (2010) Rapid synthesis of flavor compound 4-ethyloctanoic acid under microwave irradiation. Int J Mol Sci 11:4165–4174
Cleophax J, Liagre M, Loupy A, Petit A (2000) Application of focused microwaves to the scale-up of solvent-free organic reactions. Org Process Res Dev 4:498–504
Luo H, Hu D, Wu J, He M, Jin L, Yang S, Song B (2012) Rapid synthesis and antiviral activity of (quinazolin-4-ylamino)methyl-phosphonates through microwave irradiation. Int J Mol Sci 13:6730–6746
Tu S, Li C, Li G, Cao L, Shao Q, Zhou D, Jiang B, Zhou J, Xia M (2007) Microwave-assisted combinatorial synthesis of polysubstituent imidazo[1,2-a] quinoline, pyrimido[1,2-a]quinoline and quinolino[1,2-a]quinazoline derivatives. J Comb Chem 9:1144–1148
Kidwai M, Saxena S, Khan MKR, Thukral SS (2005) Synthesis of 4-aryl-7, 7-dimethyl-1,2,3,4,5,6,7,8-octahydroquinazoline-2-one/thione-5-one derivatives and evaluation as antibacterials. Eur J Med Chem 40:816–819
Besson T, Alexandre F-R, Berecibar A (2003) Synthesis of Niementowski Quinazolinone. Tetrahedron 59:141
Besson T, Alexandre F-R, Berecibar A (2005) microwave irradiation and/or Appel’s salt, new efficient routes to various substituted and fused quinazolines. Tetrahedron 59:141
Dandia A, Singh R, Sarawgi P (2005) Green chemical multi-component one-pot synthesis of fluorinated 2,3-disubstituted quinazolin-4(3H)-ones under solvent-free conditions and their anti-fungal activity. J Fluor Chem 126:307
Liu J-F, Lee J, Dalton AM, Bi G, Baldino CM, McElory E, Brown M (2005) Facile one-pot synthesis of 2,4(1H,3H)-quinazolinediones has been developed recently as a green chemical procedure. Tetrahedron Lett 46:1241
Zhang L, Gao Z, Peng C, Bin ZY, Zhao D, Wu J, Xu Q, Li JX (2012) Ultrasound-promoted synthesis and immunosuppressive activity of novel quinazoline derivatives. Mol Divers 16:579–590
Khalil AK (2005) Phase-transfer catalyzed alkylation and cycloalkylation of 2-mercaptoquinazolin-4(3H)-one. Phosphorus Sulfur Silicon Relat elem 180:2533–2541
Ardakan MN, Smalley RK, Smith RH (1983) Acylation of N-methylamides with 2-azidobenzoyl chloride. J Chem Soc Perkin Trans 1:2501
Takeuchi H, Eguchi S (1989) Aza–Wittig reaction yield exclusively 3-methylquinazolin-4(3H)-ones. Tetrahedron Lett 30:3313
Safari J, Gandomi-Ravandi S (2013) Microwave-accelerated three components cyclocondensation in the synthesis of 2,3-dihydroquinazolin-4(1H)-ones promoted by Cu-CNTs. J Mol Catal A Chem 371:135–140
Bharathi A, Roopan SM, Kajbafvala A, Padmaja RD, Darsana MS, Kumari GN (2013) Catalytic activity of TiO2 nanoparticles in the synthesis of some 2,3-disubstituted dihydroquinazolin-4(1H)-ones. Chin Chem Lett 25:324–326. http://dx.doi.org/10.1016/j.cclet.2013.11.040
Muthukrishnan J, Seifert K, Hoffmann KH, Lorenz MW (1999) Inhibition of juvenile hormone biosynthesis in Gryllus bimaculatus by Glycosmis pentaphylla leaf compounds. Phytochemistry 50:249–254
Ager I R, Harrison D R, Kennewell P D, Taylor J B (1977) Synthesis of 2-methyl-3-(o-tolyl)-4(3H)-quinazolone. J Med Chem 20:380
Povarov LS (1967) α, β-Unsaturated ethers and their analogues in reactions of diene synthesis. Rus Chem Rev 36:656–669
Reymond S, Cossy J (2008) Copper-catalyzed Diels-alder reactions. Chem Rev 108:5359–5406
Chen X, Wei H, Yin L, Li X (2010) A convenient synthesis of quinazoline derivatives via cascade imimo-Diels-Alder and oxidation reaction. Chin Chem Lett 21:782–786
Molina P, Vilaplana MJ (1994) Iminophosphoranes useful building blocks for the preparation of nitrogen-containing heterocycles. Synthesis 19:1197–1218
He P, Nie YB, Wu J, Ding MW (2011) Unexpected synthesis of indolo[1,2-c] quinazolines by a sequential ugi 4CC-Staudinger-aza-Wittig-nucleophilic addition reaction. Org Biomol Chem 9:1429–1436
Ding MW, Yang SJ, Chen YF (2004) Synthesis and fungicidal activities of 2-alkoxy-3H-quinazolin-4-ones. Chin J Org Chem 24:923–926
Sang P, Xie YJ, Zou JW, Zhang YH (2012) Copper-catalyzed sequential Ullmann N-arylation and aerobic oxidative C-H amination: a convenient route to indolo[1,2-c]quinazoline derivatives. Org Lett 14:3894–3897
Jiang M, Li J, Wang F, Zhao YC, Zhao F, Dong XC, Zhao WL (2012) A facile copper-catalyzed one-pot domino synthesis of 5,12-dihydroindolo[2,1-b] quinazolines. Org Lett 14:1420–1423
Dabiri M, Salehi P, Mohammadi AA, Baghbanzadeh M (2005) One-pot procedure for the synthesis of mono-and disubstituted (3H)-quinazolin-4-ones. Synth Commun 35:279
Battistuzzi G, Cacchi S, Fabrizi G, Marinelli F, Parisi LM (2002) Regioselective synthesis of 4(3H)-quinazolinone derivatives and indoloquinazolines. Org Lett 4:1355
Kundu NG, Chaudhuri G (2001) Region- and stereoselective route to 2-(2-arylvinyl)-1,2,3,4- tetrahydroquinazolin-4-ones. Tetrahedron 57:6833
Saxena S, Verma M, Saxena AK, Shanker K (1991) Antiinflammatory quinazolinones. Indian J Pharm Sci 53:48–52
Srivastava B, Shukla JS, Prabhakar YS, Saxena AK (1991) Synthesis and QSAR of 2,3,6,8- substituted 1,3-quinazolin-4(3H)-ones as potential anthelmintics. Indian J Chem 30B:332–339
Fisnerova L, Brunova B, Maturova E, Grimova J, Tikalova J, Kocfeldova Z (1991) Preparation of keto derivatives of 4(3H)-quinazolinone as analgesics. Chem Pharm Bull 115:293
Abdel-Rahman MM, Mangoura SA, El-Bitar HI (1990) Possible CNS depressant effects of some newly synthesized quinazolinone derivatives. Bull Pharm Sci Assiut Univ 13:137–144
Hori M, Iemura R, Hara H, Ozaki A, Sukamoto T, Ohtaka H (1990) Novel 4-phenoxy-2-(1-piperazinyl)quinazolines as potent anticonvulsive and antihypoxic agents. Chem Pharm Bull 38:681–687
Brown DJ (1984) In Comprehensive Heterocyclic Chemistry 2:148
Jackman AL, Kimbell R, Aherne GW, Brunton L, Jansen G, Stephens TC, Smith MN, Wardleworth JM, Boyle FT (1997) Cellular pharmacology and in vivo activity of a new anticancer agent, ZD9331: a water-soluble, nonpolyglutamatable, quinazoline-based inhibitor of thymidylate synthase. Clin Cancer Res 3:911–921
Rafi I, Taylor GA, Calvete JA, Boddy AV, Balmanno K, Bailey N, Lind M, Calvert AH, Webber S, Jackson RC, Johnston A, Clendeninn N, Newell DR (1995) Clinical pharmacokinetic and pharmacodynamic studies with the nonclassical antifolate thymidylate synthase inhibitor 3,4- dihydro-2-amino-6-methyl-4-oxo-5-(4-pyridylthio)-quinazolone dihydrochloride(AG337) given by 24-hour continuous intravenous infusion. Clin Cancer Res 1:1275–1284
Brown DJ (1996) Quinazolines. In: Taylor EC (ed) The chemistry of heterocyclic compounds supplement I, vol 55. Wiley, New York, p 1123
Seijas JA, Va´zquez-Tato, MP, Martı´nez MM (2000) Microwave-enhanced synthesis of 4-aminoquinazolines. Tetrahedron Lett 41:2215–2217
Alexandre FR, Berecibar A, Besson T (2002) Microwave-assisted Niementowski reaction: back to the roots. Tetrahedron Lett 43:3911–3913
Dandia A, Singh R, Sarawgi P (2005) Green chemical multi-component one-pot synthesis of fluorinated 2,3-disubstituted quinazolin-4(3H)-ones under solventfree conditions and their anti-fungal activity. J Fluor Chem 126:307
Perreux L, Loupy A (2001) A tentative rationalization of microwave effects in organic synthesis according to the reaction medium, and mechanistic considerations. Tetrahedron 57:9199–9223
Lidstrom P, Tierney J, Wathey B, Westman J (2001) Microwave-assisted organic synthesis—a review. Tetrahedron 57:9225–9283
Qiu G, He Y, Wu J (2012) Preparation of quinazolino[3,2-a]quinazolines via a palladium-catalyzed three-component reaction of carbodiimide isocyanide and amine. Chem Commun 48:3836–3838
Bergman J, Brynolf A, Elman B, Vuolinen A (1986) Various quinazolines from 2-aminobenzonitrile using organometallic reagents. Tetrahedron 42:3697
Wiklund P, Bergman J (2003) Synthesis, and application of organometallic reagents. Org Biomol Chem 1:36
Matheson SL, Brahimi F, Jean-Claude BJ (2004) The combi-targeting concept: intracellular fragmentation of the binary epidermal growth factor (EGFR)/DNA targeting “combi-triazene” SMA41. Biochem Pharmacol 67:1131–1138
Wang GW, Miao CB (2006) Environmentally benign one-pot multi-component approaches to the synthesis of novel unsymmetrical 4-arylacridinediones. Green Chem 8:1080–1085
Bauer L, Suresh KS (1963) Synthesis of anti-malarial compound. J Org Chem 28:1604
Baek DJ, Park YK, Heo HI, Lee MH, Yang ZY, Chio MH (1998) Synthesis of 5-substituted quinazolinone derivatives and their inhibitory activity in vitro. Bio Org Med Chem Lett 83:287
Acknowledgments
The authors are thankful to Prof. G. L. Talesara, Retd. Professor, Department of Chemistry, Mohan Lal Sukhadia University Udaipur, and Dr. Sunil Jhakoria, Dean, Faculty of Arts, Science and Technology (FASC), Mody University of Science and Technology, for their constant encouragement during this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer India
About this chapter
Cite this chapter
Dangi, R., Chundawat, N., Ameta, K. (2014). Synthesis and Biological Evaluation of Some Quinazoline Heterocyclic Derivatives. In: Ameta, K., Dandia, A. (eds) Green Chemistry: Synthesis of Bioactive Heterocycles. Springer, New Delhi. https://doi.org/10.1007/978-81-322-1850-0_13
Download citation
DOI: https://doi.org/10.1007/978-81-322-1850-0_13
Published:
Publisher Name: Springer, New Delhi
Print ISBN: 978-81-322-1849-4
Online ISBN: 978-81-322-1850-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)