Abstract
In cricket brains a neuropil area in the anterior ventral protocerebrum next to the pedunculus and the α-lobe is involved in the control of singing behaviour. Command interneurons for singing have dendrites in this neuropil, whereas their axon descends towards the ventral nerve cord. A bilateral calling song command neuron has been identified which drives the singing central pattern generator with tonic spike activity. The control of courtship and rivalry song via the brain is not yet resolved at a cellular level. Electrical and pharmacological brain stimulation reliably elicit normal and fictive singing in crickets. The central pattern generating network for singing seems to extend from the metathoracic ganglion complex to the first unfused abdominal ganglion A3. Crickets immediately stop singing and do not recover, once the connectives anterior to A3 are cut. Opener interneurons have been identified in A3, which modify and reset the singing motor pattern. The response properties of opener and closer interneurons upon hyperpolarising current injection indicate that post-inhibitory rebound mechanisms may be central to motor pattern generation underlying singing. Recordings of flight interneurons and singing interneurons prove that both motor patterns are controlled by separate neuronal networks.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Brain stimulation
- Command interneurons
- Calling song
- Fictive singing
- Central pattern generator
- Abdominal ganglia
1 Introduction
Crickets show a conspicuous acoustic behaviour for intraspecific communication. Males sing with rhythmic opening and closing movements of their raised front wings and thereby produce a vigorous rivalry song on encounter with another male, a loud calling song to attract females or the more gentle courtship song to initiate mating (Alexander 1962). For more than 50 years, two principle questions about the neural basis of their singing behaviour have been central to cricket neurobiology (Huber 1960). How are the different song patterns controlled by the brain and what is the organisation of the neural networks for singing motor pattern generation? Since the review by Kutsch and Huber (1989), considerable progress has been made at the cellular level to answer both questions. Intracellular recordings of brain neurons allowed identifying command neurons that control the calling song. Furthermore, electrical and pharmacological brain stimulations have been established as a reliable method to elicit fictive singing in the dissected nervous system. This allowed localising the singing network in the ventral nerve cord and characterising component neurons of the central pattern generator (CPG) for singing.
2 Control of Singing Behaviour by the Brain
Brain stimulation experiments by Huber (1955, 1960) and electrical stimulation of fibres in the neck connectives (Otto 1971; Bentley 1977) demonstrated that descending brain neurons control the singing behaviour in G. campestris and T. oceanicus. Tonic activation of these neurons was sufficient to elicit rivalry, calling and/or courtship song, respectively. The brain does not generate the rhythmic motor pattern for singing, but rather drives the singing motor network in the ventral nerve cord by tonic descending activity. Based on the experimental evidence, these brain neurons seemed to be specific labelled lines for the activation of motor activity, i.e. command neurons for singing. Electrical and mechanical brain stimulation experiments pointed towards the anterior protocerebrum as a principle region involved in the control of singing (Huber 1960). Intracellular recordings in the brain of tethered male G. bimaculatus finally identified a bilateral pair of descending brain interneurons that can be characterised as command neurons for the control of singing (Hedwig 2000).
2.1 Descending Command Neurons for Calling Song
The command neuron’s cell body is located at the dorsal side of the brain, the neurite projects anteriorly and dendrites arborise in the anterior protocerebrum ventral to the pedunculus (Fig. 10.1a). The axon crosses the midline ventral to the central body complex and gives off some collaterals that end in the left and right tritocerebrum before the axon finally descends in the contralateral connective in a medial position. The arborisation pattern of the neuron in the ventral nerve cord has not yet been described.
Command neuron for calling song in the cricket Gryllus bimaculatus. (a) Arborisation pattern of the neuron in the brain, dendrites are located in the anterior protocerebrum, and the axon descends in the contralateral connective. (b) Depolarising current injection increases the discharge rate of the neuron and elicits calling song as indicated by opening-closing forewing movements and recording of the sound pattern. CB central body, MB mushroom body, Pd pedunculus, ON optical nerve, AN antennal nerve, α-L alpha-lobe, Ax axon of the neuron, Con connective (Modified from Hedwig 2000)
When the dendrite of this neuron is penetrated by a microelectrode, transient spike activity of this cell elicits rudimentary singing movements of the front wings. Systematic manipulation of the neuron’s activity by intracellular current injection demonstrates that its spike activity is sufficient for calling song motor activity to occur. When the activity in one of the two mirror-image neurons is enhanced to 60–80 AP/s, the cricket will gradually raise its front wings and start sonorous singing of the calling song (Fig. 10.1b). Singing behaviour is maintained for the duration of enhanced interneuron activity. With the end of current injection, the interneuron spike activity as well as the chirp rate of singing decreases until singing stops. During singing the neuron’s spike activity exhibits a weak modulation in the chirp rhythm, the significance of which is not yet evident. Spiking activity of the neuron is also necessary for singing to occur. In crickets which started to sing spontaneously, or after stable singing had been evoked by sequences of current injection into the command neuron, singing can be stopped by hyperpolarising current injection that blocks spiking of the neuron. Thus, in G. bimaculatus, this descending brain neuron is sufficient and necessary to control the calling song behaviour (Hedwig 2000) and therefore represents an example of a classical command neuron (Kupfermann and Weiss 1978).
Transitions from calling song to either courtship song or rivalry song were reported during electrical connective stimulation in G. campestris (Otto 1971) and single fibre stimulation in T. oceanicus (Bentley 1977). Therefore, the activity level of a single descending interneuron may be sufficient to control different song patterns in T. oceanicus, whereas in G. campestris different fibres might be involved. In G. bimaculatus, preliminary data indicate separate command neurons for the control of calling, rivalry and courtship song (Hedwig unpublished); however, so far the evidence for the descending control of the different song patterns is not yet conclusive.
2.2 Brain Structures for Singing and Targeted Pharmacological Brain Stimulation
Labelling the calling song command neuron with fluorescent tracers and subsequent histological analysis of the brain revealed the region between the pedunculus and the α-lobe as housing the main dendritic branches of the command neuron. Comparing the stimulation sites that elicited calling song (Huber 1960) with the arborisation site of the calling song command neuron (Hedwig 2000) reveals a close match between the command neuron’s dendritic arborisations and effective stimulation sites. In parasagittal sections the corresponding neuropil area stands out as a circular region within the ventral protocerebrum (Fig. 10.2a–d).
(a) Brain neuropil area involved in the control of calling song as indicated by the dendritic arborisation pattern of the command neuron. (b) Histological parasagittal section of the brain with the projection area of the calling song command neuron identified as circular neuropil in front of the pedunculus, indicated by white square. (c) Electrical stimulation sites in the anterior brain and (d) microinjection sites of cholinergic agonists that elicited calling song. (e) Sequence of calling song after injection of ACh into the brain and subsequent termination of the behaviour by GABA injection. β-L beta-lobe, DC deutocerebrum, CN command neuron; other abbreviations as in Fig. 10.1 (Modified from Hedwig 2006)
Otto (1978) demonstrated that each of the three song patterns can be released by local injection of acetylcholine (ACh) into the brain. The identification of a specific brain neuropil involved in the control of singing allowed to systematically target this area in pharmacological brain stimulation experiments (Fig. 10.2e). Testing of neuroactive substances revealed that also cholinergic agonists such as nicotine or muscarine are effective in releasing singing (Wenzel and Hedwig 1999). Microinjections of nicotine elicited short bouts of singing behaviour with a short latency, whereas muscarine gradually activated singing activity within 60 s that then lasted for several minutes. A very effective way to release enduring singing activity is the injection of eserine, an ACh-esterase inhibitor that causes a gradual accumulation of ACh in the neuropil around the injection site. Correspondingly, eserine injection will activate singing only after several minutes, but then it can last for several hours. Once singing has been elicited by cholinergic drugs, it can be stopped by local brain injection of the inhibitory transmitter GABA. Thus, the pathway for singing is not only under a cholinergic but also under GABAergic control, which may be required for fast and effective termination of singing. The pharmacological experiments do not allow to conclude weather the command neurons are directly activated by cholinergic substances or driven by presynaptic neurons with cholinergic receptors. Under natural conditions singing behaviour seems to depend on a circadian input as the males preferentially produce the calling song at species-specific times of the day (Wiedenmann and Loher 1984; Nakatani et al. 1994; Fergus and Shaw 2013). In contrast rivalry and courtship song may require more specific sensory inputs as these song types are only generated after physical encounter with conspecific males or females, respectively (Adamo and Hoy 1994, 1995).
3 Motor Pattern Generation Underlying Singing
Sound production in crickets is based on rhythmic opening and closing movements of the front wings. As the antagonistic wing-opener and wing-closer muscles and their motoneuron machinery are located in the anterior thoracic segments (Kutsch and Huber 1989; Hennig 1989), it was initially assumed that the CPG for singing might be located in the corresponding ganglia of the thoracic nerve cord. Recent studies, however, revealed a spatial separation between the mesothoracic ganglion that produces the motor output and the neural network that generates the singing motor pattern, which apparently spans from the metathoracic to the first unfused abdominal ganglion (Hennig and Otto 1996; Schöneich and Hedwig 2011). The localisation of the singing CPG eventually allowed to analyse this network at the cellular level (Schöneich and Hedwig 2012).
3.1 Locating the Central Pattern Generator for Singing
Early behavioural observations in G. campestris and A. domesticus with lesions in the CNS lead to the assumption that the neural network generating the singing motor pattern may be housed in the mesothoracic ganglion, where it receives descending commands from the brain and sensory information from the terminal genital apparatus (Huber 1955, 1960; Kutsch and Otto 1972). More systematic micro-lesioning of the thoracic nervous system in combination with recordings of wing movements and muscle activity indicated that the metathoracic ganglion complex (T3-A2, comprising the third thoracic and the two subsequent abdominal neuromers) houses the singing CPG (Hennig and Otto 1996). However, the observation that local cooling of the thoracic ganglia has only a minor effect on song pattern generation (Pires and Hoy 1992) and the structure of descending thoracic interneurons which are activated during singing (Hennig 1990) pointed towards a contribution of the abdominal ganglia to singing pattern generation. To clarify the situation, further connective lesioning experiments in the abdominal nerve cord were required. In a G. bimaculatus preparation where fictive singing was elicited by pharmacological brain stimulation , the connectives between the abdominal ganglia were systematically sectioned, while singing motor activity was monitored by extracellular recording the activity of singing motoneurons in the mesothoracic wing nerve N3A (Fig. 10.3). In the nerve recording, rhythmically alternating spike activity of wing-opener and wing-closer motoneurons reliably reflects the syllable pattern of the calling song chirps. When connectives were transected anywhere between A4 and the terminal ganglion, TAG singing continued although the motor pattern became more variable as more ganglia were disconnected. After the A3-A4 connective was disrupted, singing motor activity continued for another minute or so and then gradually waned. All animals stopped singing immediately when the A2-A3 connectives were cut, and they never recovered over the next hour of observation. These experiments in fictive singing crickets demonstrate that the abdominal ganglion A3 is essential for the motor pattern generation of the calling song and indicate that other abdominal ganglia may contribute as well.
(a) Cricket CNS with the location of pharmacological brain stimulation and the recording site for the fictive singing motor activity indicated. (b) Sectioning of abdominal connectives and their effect on fictive singing; numbered scissor symbols indicate different cuts. Singing motor activity fails immediately if connectives anterior to A3 are sectioned (Modified from Schöneich and Hedwig 2011)
3.2 Identification and Functional Characterisation of Singing CPG Interneurons
The interneurons of the central singing network can be classified as opener and closer interneurons depending on the wing movement and corresponding muscle/motoneuron activity they precede with their rhythmic spike activity. Accordingly, opener interneurons depolarise and spike in the wing-opening phase of each syllable and are inhibited in the wing-closing phase, whereas closer interneuron activity always starts with an inhibition in the wing-opening phase. With evidence that the singing network extends into the abdominal nerve cord, experiments to analyse the singing CPG and to identify its components do now focus on the metathoracic and anterior abdominal ganglia. Interneurons with rhythmic membrane potential oscillations in phase with the syllable pattern of the calling song and an axon descending from the metathoracic into the abdominal ganglia chain were first identified by Hennig (1990) in T. commodus. Recently, interneurons have been identified in G. bimaculatus that clearly qualify as elements of the calling song CPG (Schöneich and Hedwig 2012).
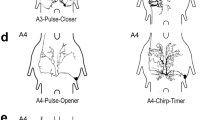
The singing interneurons exist as bilateral mirror-image pairs and exhibit characteristic arborisations in the dorsal midline neuropils of the fused metathoracic and/or the first unfused abdominal ganglion. Their spike activity precedes the corresponding motoneuron spike bursts in the wing nerve by 5–15 ms. Component neurons of the central pattern generator, however, should also alter or reset the ongoing singing rhythm whenever their activity is disturbed by intracellular current injection (Selverston 2010). Neurons that comply with these criteria were found in both the metathoracic T3 and the first unfused abdominal ganglion A3 (Schöneich and Hedwig 2012). One of the identified CPG neurons is an ascending opener interneuron with its cell body and extensive dendritic arborisations in the abdominal ganglion A3 (A3–AO; Fig. 10.4a). It’s ascending axon projects in all three thoracic ganglia, giving off distinct collaterals in the metathoracic ganglion complex as well as the meso- and prothoracic ganglion.
(a) Structure of the bilateral neuron pair A3–AO with soma and dendrites in A3 and axonal projection in the fused metathoracic ganglion complex. (b) Activity pattern of A3–AO during fictive singing ; depolarising current injection elicits additional singing motor activity that resets the ongoing chirp pattern. (c) Phase-response diagram; the shift in the chirp rhythm depends linearly on stimulus phase (Modified from Schöneich and Hedwig 2012)
The spike activity of A3–AO has a strong impact on singing motor activity. Stimulation of the neuron by constant depolarising current injection in its dendrite leads to continuous depolarisation-hyperpolarisation cycles of its membrane potential for the duration of the current pulse. Each depolarisation phase produces a short burst of action potentials, which reliably elicits rhythmically alternating bursts of wing-opener and wing-closer motoneurons reflecting the syllable pattern of the calling song motor activity in the ipsi- and contralateral wing nerves. Depolarising current pulses of just 100 ms duration elicited strictly three depolarisation-hyperpolarisation cycles and the motor pattern of a three-syllable chirp (Fig. 10.4b), whereas 500 ms current pulses caused unnaturally long chirps with 14–15 syllables. Evoking activity in just one of the two A3–AO interneurons is sufficient to continuously drive the coordinated motor pattern of the calling song. Dye-coupling between the bilateral mirror-image pair of A3–AO neurons indicates that they may be electrically coupled via gap junctions.
Eliciting additional chirps by current injection in the A3–AO dendrite during chirp intervals reliably resets the chirp pattern, as the interval between the last syllable of the elicited chirp and the first syllable of the subsequently generated chirp remains constant (Fig. 10.4c). Depolarising current injection within a chirp, however, does not reset the singing rhythm. Interestingly, membrane potential oscillations that elicit additional syllables can also be evoked by the release from hyperpolarising current injection. The rhythmic activity may arise from post-inhibitory rebound depolarisation properties of the membrane in combination with feedback inhibition by yet unidentified closer interneurons. A post-inhibitory rebound in the A3–AO opener interneuron is also supported by the observation that for the last syllable of each chirp, the inhibition that terminates the large amplitude depolarisation of its dendrite is always followed by a small subthreshold depolarisation.
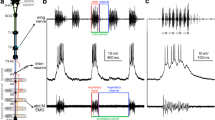
The importance of inhibition and post-inhibitory rebound depolarisation is more obvious in closer interneurons, where each syllable of fictive singing starts with an inhibition in phase with the opener motor activity followed by a depolarising membrane potential and spike activity in phase with the closer motor activity (Fig. 10.5). In some closer interneurons, volleys of inhibitory postsynaptic potentials (IPSPs) can be recorded several seconds before singing activity starts, and over a sequence of singing motor activity, the membrane potential gradually drops by few millivolt. Hyperpolarising current injection into closer interneurons reliably evokes a subsequent rebound depolarisation that triggers spiking activity. This clearly demonstrates that post-inhibitory rebound depolarisation occurs in these neurons and may be a basic neural mechanism underlying singing motor pattern generation.
(a) Intracellular recording of a non-identified closer interneuron in A2 during fictive singing . An average of 30 individual IPSPs (asterisks) shows subsequent post-inhibitory rebound depolarisation (arrow head). Dashed lines indicate the resting membrane potential. (b) Rebound depolarisation triggers spiking after the dendrite is released from 4 nA hyperpolarising current injection (Modified from Schöneich and Hedwig 2012)
3.3 Functional Organisation of the Singing Pattern Generating Network
The remarkable consistency of the singing pattern in intact animals (Verburgt et al. 2011) compared to the motor activity of pharmacologically induced fictive singing (Schöneich and Hedwig 2012) demonstrates that the underlying CPG operates virtually independent of sensory feedback. During sonorous singing, mechanosensory feedback merely adjusts the angular position of the moving front wings to ensure the required engaging force (Möss 1971; Elliott and Koch 1983).
Whereas in the G. bimaculatus calling song, the interval between subsequent opener activation may gradually increase within the chirps, there is a strict latency coupling between opener and closer motoneuron activity (Kutsch 1969; Kutsch and Huber 1989; Schöneich and Hedwig 2011). Based on motoneuron recordings, Bentley (1969) speculated that the wing-closer activity might be triggered by post-inhibitory rebound depolarisation in order to explain the tight temporal coupling between opener and closer motor activity. Thorough statistical analysis, however, did not support a direct motoneuron coupling (Elepfandt 1980). As antagonistic singing motoneurons may be recruited as agonists during flight motor activity (Hennig 1989), a direct synaptic motoneuron coupling would not be functional. However, as the closer interneurons are inhibited in phase with the depolarisation of the opener interneurons, post-inhibitory rebound in closer interneurons could secure the latency-fixed coupling to the opener activity.
Intracellular recordings of singing interneurons in G. bimaculatus (Schöneich and Hedwig 2012) indicate that inhibitory connections between opener and closer interneurons with post-inhibitory rebound properties may form a rather simple neural network to generate the motor pattern underlying rhythmic sound production. Computer modelling already demonstrated that small circuits of reciprocally inhibitory neurons exhibiting weak post-inhibitory rebound produce stable rhythmic output whenever the network is tonically activated by a command neuron (Perkel and Mulloney 1974; Satterlie 1985). Further studies are, however, needed to identify the remaining elements of the singing pattern generator in G. bimaculatus and to investigate their detailed circuitry and membrane properties. This should also reveal the neural mechanisms that control the chirp rhythm, which may include activity-dependent changes of membrane conductance, presynaptic inhibition of the command neuron input and/or periodical recovery of strongly depressing synapses.
4 Singing and Flight: Two Behaviours of Rhythmic Wing Movements
Crickets use their wings for different rhythmic behaviours. Whereas singing consists of rhythmical opening-closing movements of the front wings, flight comprises continuous up-down movements of front wings and hindwings. The motor patterns for singing and flight share the alternating activation of some antagonistic wing muscles and motoneurons, and in some crickets both behaviours are based on a similar cycle period. Early studies on the flight and stridulatory motor patterns in G. campestris (Huber 1962; Kutsch 1969) therefore suggested that both behaviours may be driven by a common central oscillator, while the chirps of the calling song were described as short flight sequences.
A comparison of the synaptic and spike activation patterns of singing and flight interneurons in T. commodus (Hennig 1990), however, revealed that flight interneurons are not activated during singing motor activity and that singing interneurons were inhibited during flight (Fig. 10.6). The study also demonstrated that there is no strict coupling between the singing and flight motor pattern when these two rhythmic behaviours, which are normally mutually exclusive, are elicited at the same time. Although the most conclusive experiments would require intracellular recordings of the corresponding CPG interneurons, these experiments convincingly demonstrate that the flight and singing patterns are generated by functionally separate networks that do not share a common pool of interneurons.
Activity patterns of singing interneurons in T. commodus during generation of the singing and flight motor pattern. Although rhythmically active during singing, closer (a) and opener (b) interneurons are not activated or even inhibited during the generation of the flight motor pattern. Calibration: vertical: 20 mV (top) and 25 mV (bottom); horizontal 100 ms (Modified from Hennig 1990)
5 Future Perspectives
Acoustic communication in crickets is based on three different song patterns. The command neurons for calling song have been identified, but the control of courtship song and rivalry song and the generation of the underlying motor patterns are yet to be understood. Further systematic recordings of central neurons will be required.
The dendritic arborisation pattern of the calling song command neurons identifies a neuropil area in front of the pedunculus to be involved in the control of singing behaviour. As calling song generation is under circadian control, we may expect direct functional links between the circadian clock in the medulla and this area of the ventral protocerebrum (Yukizane et al. 2002).
At the level of calling song pattern generation, closer interneurons have been recorded in the metathoracic ganglion complex and the first unfused abdominal ganglion A3 but have not been anatomically identified yet. Furthermore, the singing CPG might have homologous interneurons in the more posterior abdominal ganglia which may contribute to motor pattern generation. Such an organisation would shed new light on the evolution of the singing CPG in crickets.
The enormous variety of song patterns makes crickets an ideal model system to study evolution and species-specific alterations in pattern generating systems. In Hawaiian Laupala crickets, sexual selection of song patterns caused a rapid evolution of sister species, identical in morphological traits but clearly different in respect to their songs (Mendelson and Shaw 2005). With the basic elements of the neural networks for singing revealed, comparative studies should now allow us to understand which of the network properties have been conserved and at which level changes occurred that allowed for the species-specific motor patterns.
Moreover crickets are on the verge of becoming a genetic model system (Hamada et al. 2009; Watanabe et al. 2012). Having genetic tools available to generate knockouts or to introduce suited reporter genes for cell lines and neuronal activity in the central nervous system will provide an enormous step forward. As compared to other species, in crickets these tools could be easily combined with intracellular recording techniques and a wealth of existing data on identified neurons.
References
Adamo SA, Hoy RR (1994) Mating behaviour of the field cricket Gryllus bimaculatus and its dependence on social and environmental cues. Anim Behav 47:857–868
Adamo SA, Hoy RR (1995) Agonistic behaviour in male and female field crickets, Gryllus bimaculatus, and how behavioural context influences its expression. Anim Behav 49:1491–1501
Alexander RD (1962) Evolutionary change in cricket acoustical communication. Evolution 16(4):443–467
Bentley DR (1969) Intracellular activity in cricket neurons during generation of song patterns. J Comp Physiol A 62(3):267–283
Bentley D (1977) Control of cricket song patterns by descending interneurons. J Comp Physiol A 16(1):19–38
Elepfandt A (1980) Morphology and output coupling of wing muscle motoneurons in the field cricket (Gryllidae, Orthoptera). Zool Jahrb Physiol 84:26–45
Elliott CJH, Koch UT (1983) Sensory feedback stabilizing reliable stridulation in the field cricket Gryllus campestris L. Anim Behav 31:887–901
Fergus DJ, Shaw KL (2013) Circadian rhythms and period expression in the Hawaiian cricket genus Laupala. Behav Genet 43(3):241–253
Hamada A, Miyawaki K, Honda-Sumi E, Tomioka K, Mito T, Ohuchi H, Noji S (2009) Loss-of-function analyses of the fragile X-related and dopamine receptor genes by RNA interference in the cricket Gryllus bimaculatus. Dev Dyn 238(8):2025–2033
Hedwig B (2000) Control of cricket stridulation by a command neuron: efficacy depends on the behavioral state. J Neurophysiol 83(2):712–722
Hedwig B (2006) Pulses, patterns and paths: neurobiology of acoustic behaviour in crickets. J Comp Physiol A 192:677–689
Hennig RM (1989) Neuromuscular activity during stridulation in the cricket Teleogryllus commodus. J Comp Physiol A 165:837–846
Hennig RM (1990) Neuronal control of the forewings in two different behaviours: stridulation and flight in the cricket, Teleogryllus commodus. J Comp Physiol A 167(5):617–627
Hennig RM, Otto D (1996) Distributed control of song pattern generation in crickets revealed by lesions to the thoracic ganglia. Zoology 99:268–276
Huber F (1955) Sitz und Bedeutung nervӧser Zentren für Instinkthandlungen beim Männchen von Gryllus campestris L. Z Tierpsychol 12(1):12–48
Huber F (1960) Untersuchungen über die Funktion des Zentralnervensystems und insbesondere des Gehirnes bei der Fortbewegung und der Lauterzeugung der Grillen. J Comp Physiol A 44(1):60–132
Huber F (1962) Central nervous control of sound production in crickets and some speculations on its evolution. Evolution 16(4):429–442
Kupfermann I, Weiss KR (1978) The command neuron concept. Behav Brain Sci 1:3–10
Kutsch W (1969) Neuromuscular activity in three cricket species during various behavioural patterns. J Comp Physiol A 63(4):335–378
Kutsch W, Huber F (1989) Neural basis of song production. In: Huber F, Moore TE, Loher W (eds) Cricket behaviour and neurobiology. Cornell University Press, Ithaca, pp 262–309
Kutsch W, Otto D (1972) Evidence for spontaneous song production independent of head ganglia in Gryllus campestris L. J Comp Physiol A 81(1):115–119
Mendelson TC, Shaw KL (2005) Rapid speciation in an arthropod. Nature 433:375–376
Möss D (1971) Sense organs in the wing region of the field cricket (Gryllus campestris L.) and their role in the control of stridulation and wing position. J Comp Physiol A 73(1):53–83
Nakatani I, Adachi T, Murayama O (1994) Selection of light or darkness, locomotor, and stridulatory activities in the cricket, Gryllus bimaculatus DeGeer (Orthoptera: Gryllidae). J Insect Physiol 40:1007–1015
Otto D (1971) Central nervous control of sound production in crickets. J Comp Physiol A 74(3):227–271
Otto D (1978) Changes of parameters in cricket song (Gryllus campestris L.) after injection of drugs into the brain. Verh Dtsch Zool Ges 245
Perkel DH, Mulloney B (1974) Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science 185(4146):181–183
Pires A, Hoy RR (1992) Temperature coupling in cricket acoustic communication II. Localization of temperature effects on song production and recognition networks in Gryllus firmus. J Comp Physiol A 171:79–92
Satterlie RA (1985) Reciprocal inhibition and post-inhibitory rebound produce reverberation in a locomotor pattern generator. Science 229:402–404
Schöneich S, Hedwig B (2011) Neural basis of singing in crickets: central pattern generation in abdominal ganglia. Naturwissenschaften 98:1069–1073
Schöneich S, Hedwig B (2012) Cellular basis for singing motor pattern generation in the field cricket (Gryllus bimaculatus DeGeer). Brain Behav 2(6):707–725
Selverston AI (2010) Invertebrate central pattern generator circuits. Philos Trans R Soc B 365(1551):2329–2345
Verburgt L, Ferreira M, Ferguson JWH (2011) Male field cricket song reflects age, allowing females to prefer young males. Anim Behav 81:19–29
Watanabe T, Ochiai H, Sakuma T, Horch HW, Hamaguchi N, Nakamura T, Bando T, Ohuchi H, Yamamoto T, Noji S, Mito T (2012) Non-transgenic genome modifications in a hemimetabolous insect using zinc-finger and TAL effector nucleases. Nat Commun 3:1–8
Wenzel B, Hedwig B (1999) Neurochemical control of cricket stridulation revealed by pharmacological microinjections into the brain. J Exp Biol 202:2203–2216
Wiedenmann G, Loher W (1984) Circadian control of singing in crickets: two different pacemakers for early-evening and before-dawn activity. J Insect Physiol 30:145–151
Yukizane M, Kaneko A, Tomioka K (2002) Electrophysiological and morphological characterization of the medulla bilateral neurons that connect bilateral optic lobes in the cricket, Gryllus bimaculatus. J Insect Physiol 48:631–641
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer Japan KK
About this chapter
Cite this chapter
Schöneich, S., Hedwig, B. (2017). Neurons and Networks Underlying Singing Behaviour. In: Horch, H., Mito, T., Popadić, A., Ohuchi, H., Noji, S. (eds) The Cricket as a Model Organism. Springer, Tokyo. https://doi.org/10.1007/978-4-431-56478-2_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-56478-2_10
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-56476-8
Online ISBN: 978-4-431-56478-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)