Abstract
Scientific evidence of the efficacy of magnetoencephalography (MEG) is currently lacking except in diagnosing epilepsy. In the present study, we performed a review of clinical MEG studies on neurodegenerative disorders using a website bibliographic survey. We searched MEDLINE for MEG papers on neurodegenerative disorders published before December 2014 using the following keywords: a representative diagnosis such as amyotrophic lateral sclerosis (ALS) and magnetoencephalography or MEG. We further narrowed the search to 30 papers based on the levels of evidence and abstract contents; 3 papers on ALS, 18 papers on Parkinson disease, and 9 papers on multiple sclerosis were included in the final review. Levels of evidence were classified as follows: grade I, no paper; grade II, 19 papers; grade III, 2 papers; and grade IV, 9 papers. The majority of studies were conducted with a small number of patients. However, MEG has the advantage of being able to detect spontaneous activity in small brain regions and to measure functional network activity between multiple brain areas or coherent activity between deep brain nuclei and distinct cortical areas. Accordingly, MEG allows the assessment of functional changes in certain diseases and provides novel insights into disease-specific pathophysiology, such as in Parkinson disease.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
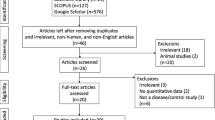
Although magnetoencephalography (MEG) provides noninvasive information regarding the localization of epileptic foci in patients with epilepsy, scientific evidence of the utility of MEG in diagnosing various other neurologic diseases, defining disease status, and predicting disease progression or prognosis remains lacking. To evaluate clinical utility of MEG in patients diagnosed as having neurodegenerative diseases, we reviewed clinical MEG studies on neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS) or motor neuron disease, multiple sclerosis (MS), Parkinson disease (PD), and spinocerebellar degeneration, based on a website bibliographic survey. We identified MEG studies on neurodegenerative disorders published before December 2014 by searching MEDLINE using the following keywords: a representative diagnosis such as ALS and magnetoencephalography or MEG. We further narrowed the search to 30 papers based on the levels of evidence and abstract contents; 3 papers on ALS, 18 papers on PD, and 9 papers on MS were included in the final review. The evidence level and recommendations were judged in each paper based on published criteria, as shown in Table 12.1 [1]. In this chapter, we provide a brief overview of the clinical and pathophysiological features of each abovementioned neurodegenerative disease and the current status of MEG research related to each disease. We further discuss the present capabilities and future possibilities of MEG in relation to neurodegenerative diseases.
2 Magnetoencephalography (MEG) in Amyotrophic Lateral Sclerosis (ALS)
2.1 Clinical and Pathophysiological Features of ALS
Amyotrophic lateral sclerosis (ALS), often referred to as Lou Gehrig’s disease, is a progressive neurodegenerative disease that affects both upper motor neurons in the brain and lower motor neurons in the spinal cord. Amyotrophy means neurogenic atrophy of muscle; lateral sclerosis refers to the firmness of affected spinal cords reported by pathologists at autopsy [2]. Lateral sclerosis results from the proliferation of astrocytes and the scarring of the lateral columns of the spinal cord in response to degeneration of the corticospinal tracts. Early symptoms of ALS include increasing muscle weakness that predominantly involves the arms and legs, speech, swallowing, or breathing. The disease begins focally and then spreads relentlessly. However, some motor neurons innervating the ocular muscles are spared, and sensation and bladder functions also are spared during the course of the disease. Whereas approximately 10 % of ALS cases are inherited as dominant traits and approximately 25 % of inherited ALS patients result from mutations in the gene encoding superoxide dismutase 1 (SOD1), the cause of sporadic ALS remains unknown [2]. Cognitive impairment in ALS was considered uncommon until recently. Overt frontotemporal dementia (FTD) occurs in approximately 15 % of ALS patients, but up to 50 % of ALS patients are classified as impaired if measured by neuropsychological tests [3].
2.2 MEG Study in Patients with ALS
A summary of three MEG studies of ALS is presented in Table 12.2. A number of divergent approaches to the study of MEG in ALS are observed. Pekkonen et al. [4] reported that the P50m and N100m responses or MMNm of auditory-evoked fields, the magnetic counter part of mismatch negativity potentials, are augmented in ALS patients with severe bulbar signs, indicating that auditory processing underlying stimulus detection, and subsequent memory-based comparison processes are abnormal in ALS. Boyajian et al. [5] performed single-dipole analysis of focal delta–theta activity in ALS patients and demonstrated the presence of slow-wave bursts generated from the frontal, temporal, and parietal cortices but not occipital areas, indicating widespread cortical dysfunction in patients with ALS. By performing MEG before and after swallowing in normal controls and ALS patients, Teismann et al. [6] demonstrated event-related desynchronization (ERD) in beta and low gamma bands in bilateral sensorimotor areas in control subjects; however, the ERD response was predominantly on the right side in ALS patients with difficulty in swallowing. This right hemispheric predominance in the activation of the primary motor cortex during volitional swallowing may be the only sign of cortical plasticity in dysphagic ALS patients.
3 Magnetoencephalography (MEG) in Parkinson Disease
3.1 Clinical and Pathophysiological Features of Parkinson Disease
Parkinson disease (PD) is a progressive neurodegenerative disorder involving varying combinations of bradykinesia, rest tremor, rigidity, and loss of postural adjustment. The pathological hallmark of idiopathic PD is the degeneration of the dopaminergic cells in the substantia nigra pars compacta that project to the striatum and a number of other basal ganglia nuclei. Dopaminergic hypofunction in cortico-basal ganglia circuits is thought to underlie the majority of motor disturbances observed in PD as dopamine replacement or dopamine receptor agonist administration has demonstrated efficacy in reducing motor disturbances. Several hypotheses have been proposed to account for the mechanisms underlying the pathogenesis of motor disturbances in PD. Of these hypotheses, the “rate model” [7] or standard “antagonist balance model” [8] posits that the pathophysiology of bradykinesia or hypokinesia is as follows. Loss of dopaminergic input to the striatum gives rise to increased activity of the indirect pathway; the striatum to the external segment of the globus pallidus (GPe) to the subthalamic nucleus (STN) to the internal segment of the globus pallidus (GPi), and decreased activity in the direct pathway; and the striatum to the GPi [7]. Both of these changes would lead to a net increase in the activity of neurons in the GPi and substantia nigra pars reticulata. This increase in basal ganglia output would then result in increased inhibition of thalamocortical and midbrain tegmental neurons and account for the hypokinetic features of PD [7]. A modification of the standard model, known as the “center–surround model,” states that the two pathways interact in a center–surround organization similar to that described in the visual system [9]. In this model, desired movement is normally achieved via activation of the direct pathway, and undesired movement due to competing motor programs is suppressed via activation of the indirect pathway that causes surrounding inhibition [9]. In PD, STN hyperactivity leads to excessive inhibition of all movements, both desired and undesired, leading to akinesia and bradykinesia, whereas STN hypoactivity results in decreased suppression of undesired movements and its florid expression in the form of hemiballism [8, 9]. Another emerging hypothesis regarding the pathogenesis of PD is the “abnormal firing pattern model [7, 8].” Studies employing microelectrode recordings from MPTP parkinsonian primates and PD patients have demonstrated abnormal firing pattern in the indirect pathway, i.e., increased oscillatory and synchronized activity in GPi, GPe, and STN neurons [7, 8]. Abnormal neural oscillations in the low frequency range of 5–8 Hz may contribute to rest tremor [7, 8]. In addition, abnormal oscillation and increased synchronization at the 15–30 Hz frequency range (beta band) may either block the normal flow of information through the basal ganglia or be associated with a loss of neuronal selectivity, resulting in the slowing or prevention of movements [7, 8]. High-frequency deep brain stimulation (DBS) has been shown to improve motor deficits in PD patients by suppressing oscillatory beta activity of the basal ganglia.
Recently, an increasing number of studies have examined PD-related non-motor symptoms, such as hyposmia, autonomic dysfunctions, mood disorders, sleep disorders, sensory disorders, and cognitive deficits [10]. In accordance with these clinical symptoms, accumulated pathological studies of sporadic or idiopathic PD patients have provided evidence that PD involves a multisystem degenerative process, possibly initiated by the migration of pathogens to the brain from the stomach or nose [11], involving not only the dopaminergic but also the noradrenergic, serotoninergic, cholinergic, and other systems [10]. During the progression of PD, Lewy bodies have been shown to extend from brainstem areas to multiple cortical areas, leading to the onset of dementia [10]. Therefore, it is now accepted that the majority of PD patients develop cognitive deficits with prolonged disease duration in contradiction to the original description of PD by James Parkinson.
3.2 MEG Studies in Patients with Parkinson Disease
A summary of 18 MEG studies of PD is presented in Table 12.3. MEG studies of PD include a number of different approaches dependent on the subject of interest. Eight articles were published by the VU University Medical Center in Amsterdam [12–19]. In earlier studies, the frequency spectrum of spontaneous MEG, or odor stimulus-conditioned MEG, was analyzed in sensor space of ten cortical regions covering the bilateral frontal, central, temporal, parietal, and occipital areas [12, 13]. As a result of sensor-based synchronization likelihood (SL) analysis of odor stimulus-conditioned MEG, Boesveldt et al. [13] found a decrease in beta (β) local SL and an increase in delta (δ) interhemispheric SL in controls but not patients with PD (for further details, see Table 12.3). Gómez et al. [14] also investigated sensor-space functional connectivity in PD patients by obtaining Lempel–Ziv complexity (LZC) values calculated by channel-by-channel analysis and demonstrated lower LZC values in PD patients compared to controls. Recently, Olde Dubbelink et al. [15–18] published four papers focusing on changes in functional connectivity and the possible contribution of such changes to cognitive decline in PD patients. First, the relationship between sensor-space frequency spectral power and cognitive function was assessed with decreased cognitive performance shown to be associated with increased delta (0.5–4 Hz) and theta (4–8 Hz) power, as well as decreased in alpha1 (8–10 Hz), alpha2 (10–13 Hz), and gamma (30–48 Hz) power. Increased motor impairment was found to be associated with increased theta power only [15]. Next, using a beamforming approach to measure brain activity in 78 cortical regions, a source-space analysis was performed to assess frequency spectral power and the phase lag index (PLI), as a measure of functional connectivity, with PD patients and controls examined twice with an interval of 4 years [16]. In patients with PD, longitudinal analyses over a 4-year period revealed decreased alpha1 and alpha2 band connectivity in multiple seed regions associated with motor or cognitive deterioration (see Table 12.3) [16]. In a further study with the same PD patients and controls, Olde Dubbelink et al. [17] applied minimum spanning tree (MST) network analyses as another measure of functional connectivity and found lower leaf number and lower tree hierarchy in the alpha2 (10–13 Hz) frequency band in PD patients compared to controls [17]. A 4-year longitudinal analysis in PD patients demonstrated progressive decreases in local clustering in multiple frequency bands concurrently with decreases in path length in the alpha2 (10–13 Hz) frequency band [17]. More recently, Olde Dubbelink et al. [18] analyzed the results of MEG testing and cognitive functions in PD patients over a 7-year period and evaluated predictive factors for the development of PD-related dementia (PDD). The authors concluded that the combination of impaired fronto-executive task performance and low beta power was associated with the highest dementia risk [18]. Vardy et al. [19] assessed primary motor cortex activity during rest and rhythmic movement in PD patients. This study reported that PD patients displayed more power in the alpha (7–11 Hz) band and less power in the beta (13–30 Hz) band during both rest and motor activity compared to controls, indicating that the slowing of neural activity is a structural, systemic phenomenon in PD that progresses over time [19].
Several MEG studies from other institutes have used different methods to investigate the pathophysiology of movement disorders in PD. One approach for understanding the mechanisms of tremor or hypokinesia is the simultaneous recording of spontaneous MEG and electromyograms (EMG) from the hand muscle of the more severely affected side in PD. Pollok et al. measured coherent activity between the primary motor hand area (M1) and the hand muscle EMG or between multiple motor-related brain areas during muscle contraction or rest [20] with or without the administration of the study drug [21]. In control subjects, the beta frequency power of M1 contralateral to movement was decreased during muscle contraction compared to the ipsilateral side [20]. This pattern appeared to be attenuated in de novo PD patients and fully reversed in medicated PD patients [20]. During the period without drug administration in PD patients, EMG power spectrum analysis revealed discernible peaks at the tremor frequency (4.8 Hz) and double the tremor frequency (9.4 Hz) [21]. Coherent activity at double the tremor frequency was found in the contralateral primary sensorimotor cortex (M1/S1) and several other areas: the premotor cortex (PMC), supplementary motor area (SMA), secondary somatosensory cortex (S2), posterior parietal cortex (PPC), and thalamus contralateral to the EMG recorded site, and ipsilateral cerebellum [21]. The coherence strength of each cerebro–cerebral coupling was seen to decrease with improvements in tremor during drug administration (Table 12.3) [21].
Deep brain stimulation (DBS) neurosurgery allows the assessment of the interactions between populations of neurons in the human cerebral cortex and basal ganglia in PD patients. Another approach for understanding the pathogenesis of motor disturbances in PD is the scrutinization of cortico-basal ganglia network activity. There have been five papers that have used simultaneous recording of MEG and local field potentials (LFP) of the STN to map cortico-STN coherence. Litvak et al. [22] identified two spatially and spectrally separated cortico-STN networks: a temporoparietal-STN network in the alpha (7–13 Hz) band and a predominantly frontal-STN network in the beta (15–35 Hz) band. Dopaminergic medications were shown to modulate the resting beta network by increasing beta coherence between the STN region and ipsilateral prefrontal cortex. Litvak et al. [23] further investigated cortico-STN coherence in PD patients during the performance of synchronous and sequential finger movements or during the administration of the dopamine prodrug, levodopa. Discrete peaks in M1 and STN power were observed at 60–90 Hz and at 300–400 Hz. All power peaks increased with either synchronous or sequential finger movement and levodopa treatment. Only STN activity at 60–90 Hz was coherent with activity in M1. Based on directionality analysis, STN gamma activity at 60–90 Hz was found to contribute to gamma activity in M1 [23]. Hirschmann et al. also studied cortico-STN coherence using simultaneous MEG and STN-LFP recording [24–26]. During rest, coherent activity in the low (12–20 Hz) and high (20–35 Hz) beta range was observed in the sensorimotor and premotor cortex on the ipsilateral side to STN-LFP recording [24]. Coherence in the alpha range (7–12 Hz) was observed at various locations in the ipsilateral temporal lobe [24]. During the motor task, beta coherence between STN-LFP and M1 was identified and seen to decrease following the administration of levodopa [25]. M1-muscular coherence in alpha and beta frequency bands was decreased by movement but was unchanged by medication [25]. This study also observed strong alpha band coherence between the superior temporal gyrus (STG) and STN in all experimental conditions and that motor tasks and the administration of levodopa had no effect on STG-STN coherence [25]. Hirschmann et al. further investigated cortical activity coherent to EMG spectral power produced by tremor [26]. Increases in cerebral synchronization at tremor frequencies were observed in a rest tremor network that included the STN, M1, premotor, and posterior parietal cortex contralateral to the tremulous limb [26]. Analysis of the imaginary part of coherency revealed tremor-dependent coupling between these cortical areas at tremor frequency and double the tremor frequency [26].
An alternative approach to the investigation of PD pathophysiology was undertaken by Airaksinen et al. [27–29] in a study comparing brain responses with or without DBS. In this study, a spatiotemporal signal space separation (tSSS) method was used to reduce DBS artifacts during MEG recording. When assessing auditory-evoked fields, the ipsilateral auditory N100m responses in the right hemisphere were found to be augmented by 10 % during DBS [27]. A trend toward increased contralateral N100m responses and somatosensory P60m responses was observed in response to bilateral DBS [27]. Spontaneous MEG during DBS demonstrated a nonsignificant decrease in mean source strength at the 6–10 Hz range (mu rhythm) and lower and higher beta ranges over pericentral cortical regions [28]. During DBS, the severity of rigidity correlated with 6–10 Hz and 12–20 Hz somatomotor source strengths when patients had their eyes open [28] (Table 12.3). When DBS was not being applied, action tremor severity correlated with pericentral 6–10 Hz and 12–20 Hz and occipital alpha source strength when patients had their eyes open [28]. By recording MEG during rest and movement, Airaksinen et al. [29] demonstrated sensor-space corticomotor coherence peaks at 13–25 Hz in 15 out of 19 PD patients. In addition, corticomotor coherence peaks at 6–13 Hz and 4–6 Hz were observed in 15 and 5 patients, respectively. The effect of DBS on corticomotor coherence peaks was variable among individual patients [29].
4 Magnetoencephalography (MEG) in Multiple Sclerosis (MS)
4.1 Clinical and Pathophysiological Features of Multiple Sclerosis (MS)
Multiple sclerosis (MS) is a common chronic demyelinating disease of the central nervous system (CNS) with a highly variable clinical course. MS is characterized by the spatial and temporal progression of lesions affecting the brain, spinal cord, and/or optic nerves [30]. Exacerbations and remissions occur frequently. The symptoms and signs of MS usually indicate the presence of more than one lesion, and some of them may be transient. Multiple sclerosis is a clinical diagnosis that requires appropriate expertise to confirm the spatial and temporal progression of CNS lesions and exclude other possible diseases [30]. Magnetic resonance imaging (MRI) is essential for the diagnosing of MS as it allows the visualization of MS plaques representing white matter inflammation, demyelination, and glial scarring (sclerosis). The identification of oligoclonal immunoglobulin G bands in cerebrospinal fluid may support the diagnosis of MS. Although autoimmune processes are thought to underlie the pathogenesis of MS, there are currently no serological tests with utility in diagnosing MS [30]. For the 10–20 years before MRI was introduced as a diagnostic tool for MS, evoked potentials were used as an important diagnostic tool for the detection of clinically silent CNS lesions. Currently, evoked potentials are considered less sensitive than MRI. However, visual-evoked potentials are still considered to have utility in providing evidence of optic nerve demyelination through the demonstration of markedly delayed P100 wave of normal amplitude [30].
4.2 MEG Studies in Patients with Multiple Sclerosis
A summary of nine MEG studies of MS is presented in Table 12.4. Two different approaches to the use of MEG to study MS were identified. Three of the nine papers focus on cortical somatosensory network activity following electrical finger stimulation or median nerve stimulation. Tecchio et al. [31] identified source activity representing the thumb and little finger at around 24 ms poststimulus and estimated sensory cortical connectivity as the phase locking between these source activities in the gamma frequency range. In this study, an altered pattern of the intracortical connectivity index was observed in MS patients (see Table 12.4) compared to controls. Dell’Acqua et al. [32] examined the profiles of M20 and M30 responses following median nerve stimulation in MS patients. Although the latency and signal strength for M20 were not affected, the analysis of M30 responses demonstrated prolonged latency, decreased signal strength, and asymmetry of right and left M30 dipole locations [32]. Hagiwara et al. [33] analyzed contralateral SI and bilateral SII responses following median nerve stimulation. In MS patients, the mean latencies of all contralateral SI responses were prolonged, the signal strength of the N20m response was decreased, and induced SI gamma activity was relatively reduced [33]. Although the latencies of bilateral SII responses were within the normal range, phase locking in the induced gamma-band activity between SI and SII during the time interval of 30–100 ms poststimulus was diminished in MS patients, indicating impaired cortical somatosensory network activity.
The remaining six of the nine MEG studies in MS were published by the VU University Medical Center in Amsterdam [34–39]. Hardmeier et al. [34] performed sensor-space analyses of frequency power spectrum and functional connectivity in spontaneous MEG. Functional connectivity between MEG sensors was assessed by calculating the synchronization likelihood (SL), and the resulting weight matrix was used to compute eigenvector centrality (EC) [34]. Eigenvector centrality values in the theta (θ) band were higher over both parietal areas in MS patients compared to controls. Further, EC values in the upper alpha (α) (8–10 Hz) and beta (β) (13–30 Hz) bands over left temporal regions, and the gamma (γ) (30–48 Hz) band over right parietal regions, were lower in MS patients compared to controls [34]. In a further study of the same MS patients and controls, Schoonheim et al. [35] performed graph theoretical analysis to assess functional connectivity. Sensor-space analyses of the frequency power spectrum demonstrated increased synchronization in the theta (θ) (4–8 Hz),low alpha (α) (8–10 Hz), and beta (β) (10–13 Hz) bands and decreased synchronization in the upper alpha (α) (10–13 Hz) band in MS patients compared to controls [35]. In the graph theoretical analysis, the lower alpha (α) (8–10 Hz) band demonstrated increased clustering coefficient and path length values, indicating a change toward a more regular network topology in MS patients. Tewarie et al. published four studies of spontaneous MEG in MS patients focusing on network functional connectivity [36–39]. Using a beamforming approach (synthetic aperture magnetometry, SAM), source-space analyses were performed in 78 cortical regions. First, the phase lag index (PLI) was determined to calculate the asymmetry of the distribution of phase differences between the two time series [36]. Lower functional connectivity (lower PLI) was observed in the α2 band in the default mode network (DMN), and the visual processing network and higher functional connectivity (higher PLI) were observed in the beta (β) (13–30 Hz) band in the DMN and the temporoparietal network in MS patients [36]. The authors posited that altered functional connectivity may underlie the clinical and cognitive dysfunction in MS. In the second paper, minimum spanning tree (MST) network analyses were performed [37]. MSTs were found to differ between MS patients and controls in the alpha2 (α2) (10–13 Hz), beta (β) (13–30 Hz), and theta (θ) (4–8 Hz) bands [37]. The MSTs in the alpha2 (α2) (10–13 Hz) band of MS patients were characterized by a larger eccentricity and lower leaf fraction and “tree hierarchy” [37]. These changes indicated a loss of hierarchical structure and were associated with poor cognitive performance. Similar findings were reported by a further study with a large number of MS patients and controls [38, 39] (Table 12.4). In MS patients, higher PLI values were present in the delta (δ) (0.5–4 Hz) band in many cortical areas, except for the right temporal and occipital areas, and in the theta (θ) (4–8 Hz) band in many cortical areas [38]. Lower PLI values were observed in the alpha2 (α2) (10–13 Hz) band in occipital, temporal, and parietal areas. MST analyses demonstrated different MST topology only in the alpha2 (α2) band in MS patients, reflecting a lower leaf fraction, lower degree of divergence, and lower tree hierarchy in the alpha2 (α2) frequency band of MS patients [38]. A lower degree of divergence also was observed in all frequency bands, except the gamma (γ) (30–48 Hz) band, in MS patients [39].
5 General Remarks
The present review of studies examining the clinical application of MEG in neurodegenerative diseases such as ALS, PD, and MS reveals the future potential and limitations of MEG as a diagnostic tool or neurophysiological marker. The simultaneous recording of MEG and STN-LFP in PD patients who underwent neurosurgery for STN-DBS provided an opportunity to explore functional network activity between the STN and distinct cortical areas. These studies in PD patients, in conjunction with experimental studies of MPTP parkinsonian primates, have provided invaluable data allowing the testing of emerging hypotheses regarding the pathogenesis of hypokinesia or bradykinesia in PD [22–25], the “abnormal firing pattern model,” and novel insights into the pathogenesis of rest tremor in PD [20, 21, 26]. In addition, regardless of diagnosis such as PD and MS, patients with cognitive decline or impairment demonstrated altered or disruptive network functional connectivity during spontaneous MEG [15–18, 36–39]. Thus, functional connectivity analyses using spontaneous MEG may provide data with utility in informing the diagnosis of PD-related dementia or the presence of frontotemporal dementia during the early stages of ALS. Further, spontaneous MEG may also have clinically utility in diagnosing and predicting cognitive decline in patients suffering from other neurodegenerative diseases including multisystem atrophy, spinocerebellar degeneration, and progressive supranuclear palsy. However, network functional connectivity analyses of spontaneous MEG in PD patients or MS patients were repeatedly performed in the same institute with MEG examinations performed on a limited number of patients and control subjects. Therefore, studies from other institutes or collaborations between many institutions with a large number of participants are required to evaluate the validity of functional connectivity analyses and confirm the relationship between abnormal functional connectivity analysis results of spontaneous MEG and cognitive decline or deficits, thereby enhancing the clinical utility of MEG examinations.
References
McCormick KA, Fleming B. Clinical practice guidelines. The Agency for Health Care Policy and Research fosters the development of evidence-based guidelines. Health Prog. 1992;73:30–4.
Brown RH, Cannon SC, Rowland LP. Chapter 14: Diseases of the nerve and motor unit. In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of neural sciences. 5th ed. New York: McGraw-Hill Professional; 2012. p. 307–30.
Gordon PH. Amyotrophic lateral sclerosis: an update for 2013 clinical features, pathophysiology. Manag Ther Trials Aging Dis. 2013;4(5):295–310.
Pekkonen E, Osipova D, Laaksovirta H. Magnetoencephalographic evidence of abnormal auditory processing in amyotrophic lateral sclerosis with bulbar signs. Clin Neurophysiol. 2004;115(2):309–15.
Boyajian RA, Amo C, Otis SM, Romine JS, Smith RA. Magnetic source imaging of cortical dysfunction in amyotrophic lateral sclerosis. Am J Phys Med Rehabil. 2008;87(6):427–37.
Teismann IK, Warnecke T, Suntrup S, Steinsträter O, Kronenberg L, Ringelstein EB, Dengler R, Petri S, Pantev C, Dziewas R. Cortical processing of swallowing in ALS patients with progressive dysphagia—a magnetoencephalographic study. PLoS One. 2011;6(5):e19987. doi:10.1371/journal.pone.0019987.
Wichmann T, DeLong MR. Chapter 43: The basal ganglia. In: Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, editors. Principles of neural sciences. 5th ed. New York: McGraw-Hill Professional; 2012. p. 982–98.
Gale JT, Amirnovin R, Williams ZM, Flaherty AW, Eskandar EN. From symphony to cacophony: pathophysiology of the human basal ganglia in Parkinson disease. Neurosci Biobehav Rev. 2008;32(3):378–87.
Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425.
Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004;318(1):121–34.
Hawkes CH, Del Tredici K, Braak H. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol. 2007;33(6):599–614.
Stoffers D, Bosboom JL, Deijen JB, Wolters EC, Berendse HW, Stam CJ. Slowing of oscillatory brain activity is a stable characteristic of Parkinson’s disease without dementia. Brain. 2007;130(Pt 7):1847–60.
Boesveldt S, Stam CJ, Knol DL, Verbunt JP, Berendse HW. Advanced time-series analysis of MEG data as a method to explore olfactory function in healthy controls and Parkinson’s disease patients. Hum Brain Mapp. 2009;30(9):3020–30.
Gómez C, Olde Dubbelink KT, Stam CJ, Abásolo D, Berendse HW, Hornero R. Complexity analysis of resting-state MEG activity in early-stage Parkinson’s disease patients. Ann Biomed Eng. 2011;39(12):2935–44.
Olde Dubbelink KT, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW. Cognitive decline in Parkinson’s disease is associated with slowing of resting-state brain activity: a longitudinal study. Neurobiol Aging. 2013;34(2):408–18.
Olde Dubbelink KT, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Hillebrand A, Berendse HW. Resting-state functional connectivity as a marker of disease progression in Parkinson’s disease: a longitudinal MEG study. Neuroimage Clin. 2013;2:612–9.
Olde Dubbelink KT, Hillebrand A, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Berendse HW. Disrupted brain network topology in Parkinson’s disease: a longitudinal magnetoencephalography study. Brain. 2014;137(Pt 1):197–207.
Olde Dubbelink KT, Hillebrand A, Twisk JW, Deijen JB, Stoffers D, Schmand BA, Stam CJ, Berendse HW. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology. 2014;82(3):263–70.
Vardy AN, van Wegen EE, Kwakkel G, Berendse HW, Beek PJ, Daffertshofer A. Slowing of M1 activity in Parkinson’s disease during rest and movement – an MEG study. Clin Neurophysiol. 2011;122(4):789–95.
Pollok B, Makhloufi H, Butz M, Gross J, Timmermann L, Wojtecki L, Schnitzler A. Levodopa affects functional brain networks in Parkinsonian resting tremor. Mov Disord. 2009;24(1):91–8.
Pollok B, Krause V, Martsch W, Wach C, Schnitzler A, Südmeyer M. Motor-cortical oscillations in early stages of Parkinson’s disease. J Physiol. 2012;590(Pt 13):3203–12.
Litvak V, Jha A, Eusebio A, Oostenveld R, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Resting oscillatory cortico-subthalamic connectivity in patients with Parkinson’s disease. Brain. 2011;134(Pt2):359–74.
Litvak V, Eusebio A, Jha A, Oostenveld R, Barnes G, Foltynie T, Limousin P, Zrinzo L, Hariz MI, Friston K, Brown P. Movement-related changes in local and long-range synchronization in Parkinson’s disease revealed by simultaneous magnetoencephalography and intracranial recordings. J Neurosci. 2012;32(31):10541–53.
Hirschmann J, Özkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Distinct oscillatory STN-cortical loops revealed by simultaneous MEG and local field potential recordings in patients with Parkinson’s disease. Neuroimage. 2011;55(3):1159–68.
Hirschmann J, Özkurt TE, Butz M, Homburger M, Elben S, Hartmann CJ, Vesper J, Wojtecki L, Schnitzler A. Differential modulation of STN-cortical and cortico-muscular coherence by movement and levodopa in Parkinson’s disease. Neuroimage. 2013;68:203–13.
Hirschmann J, Hartmann CJ, Butz M, Hoogenboom N, Ozkurt TE, Elben S, Vesper J, Wojtecki L, Schnitzler A. A direct relationship between oscillatory subthalamic nucleus-cortex coupling and rest tremor in Parkinson’s disease. Brain. 2013;136(Pt 12):3659–70.
Airaksinen K, Mäkelä JP, Taulu S, Ahonen A, Nurminen J, Schnitzler A, Pekkonen E. Effects of DBS on auditory and somatosensory processing in Parkinson’s disease. Hum Brain Mapp. 2011;32(7):1091–9.
Airaksinen K, Butorina A, Pekkonen E, Nurminen J, Taulu S, Ahonen A, Schnitzler A, Mäkelä JP. Somatomotor mu rhythm amplitude correlates with rigidity during deep brain stimulation in Parkinsonian patients. Clin Neurophysiol. 2012;123(10):2010–7.
Airaksinen K, Mäkelä JP, Nurminen J, Luoma J, Taulu S, Ahonen A, Pekkonen E. Cortico-muscular coherence in advanced Parkinson’s disease with deep brain stimulation. Clin Neurophysiol. 2014 (in press). doi:http://dx.doi.org/10.1016/j.clinph.2014.07.025.
Leary S, Giovannoni G, Howard R, Miller D, Thompson A. Chapter 10: Multiple sclerosis and demyelinating diseases. In: Clarke C, Howard R, Rossor M, Shorvon SD, editors. Neurology: a queen square textbook. Singapore: Willy-Blackwell; 2009. p. 411–47.
Tecchio F, Zito G, Zappasodi F, Dell’ Acqua ML, Landi D, Nardo D, Lupoi D, Rossini PM, Filippi MM. Intra-cortical connectivity in multiple sclerosis: a neurophysiological approach. Brain. 2008;131(Pt 7):1783–92.
Dell’Acqua ML, Landi D, Zito G, Zappasodi F, Lupoi D, Rossini PM, Filippi MM, Tecchio F. Thalamocortical sensorimotor circuit in multiple sclerosis: an integrated structural and electrophysiological assessment. Hum Brain Mapp. 2010;31(10):1588–600.
Hagiwara K, Okamoto T, Shigeto H, Ogata K, Somehara Y, Matsushita T, Kira J, Tobimatsu S. Oscillatory gamma synchronization binds the primary and secondary somatosensory areas in humans. Neuroimage. 2010;51(1):412–20.
Schoonheim MM, Geurts JJ, Landi D, Douw L, van der Meer ML, Vrenken H, Polman CH, Barkhof F, Stam CJ. Functional connectivity changes in multiple sclerosis patients: a graph analytical study of MEG resting state data. Hum Brain Mapp. 2013;34(1):52–61.
Hardmeier M, Schoonheim MM, Geurts JJ, Hillebrand A, Polman CH, Barkhof F, Stam CJ. Cognitive dysfunction in early multiple sclerosis: altered centrality derived from resting-state functional connectivity using magnetoencephalography. PLoS One. 2012;7(7):e42087. doi:10.1371/journal. pone.0042087.
Tewarie P, Schoonheim MM, Stam CJ, van der Meer ML, van Dijk BW, Barkhof F, Polman CH, Hillebrand A. Cognitive and clinical dysfunction, altered MEG resting-state networks and thalamic atrophy in multiple sclerosis. PLoS One. 2013;8(7):e69318. doi:10.1371/journal.pone.0069318.
Tewarie P, Hillebrand A, Schoonheim MM, van Dijk BW, Geurts JJ, Barkhof F, Polman CH, Stam CJ. Functional brain network analysis using minimum spanning trees in Multiple Sclerosis: an MEG source-space study. Neuroimage. 2014;88:308–18.
Tewarie P, Steenwijk MD, Tijms BM, Daams M, Balk LJ, Stam CJ, Uitdehaag BM, Polman CH, Geurts JJ, Barkhof F, Pouwels PJ, Vrenken H, Hillebrand A. Disruption of structural and functional networks in long-standing multiple sclerosis. Hum Brain Mapp. 2014;35(12):5946–61. doi:10.1002/hbm.22596.
Tewarie P, Schoonheim MM, Schouten DI, Polman CH, Balk LJ, Uitdehaag BM, Geurts JJ, Hillebrand A, Barkhof F, Stam CJ. Functional brain networks: linking thalamic atrophy to clinical disability in multiple sclerosis, a multimodal fMRI and MEG study. Hum Brain Mapp. 2015;36(2):603–18.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Ozaki, I., Hashimoto, I. (2016). Neurodegenerative Disorders. In: Tobimatsu, S., Kakigi, R. (eds) Clinical Applications of Magnetoencephalography. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55729-6_12
Download citation
DOI: https://doi.org/10.1007/978-4-431-55729-6_12
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55728-9
Online ISBN: 978-4-431-55729-6
eBook Packages: MedicineMedicine (R0)