Abstract
Recent progress in multidisciplinary therapies for osteosarcoma promises the ability to perform successful limb salvage surgery. Endoprosthetic reconstruction following resection of a bone tumor has a history of more than 70 years, and modern endoprosthesis provides intraoperative flexibility to fill the bony defect and structural stability to allow for immediate weight bearing. Although prosthetic reconstruction has become a standard surgical technique, there remain many unresolved problems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
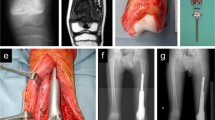
Osteosarcoma is an aggressive malignant neoplasm that exhibits osteoblastic differentiation and produces malignant osteoid [1]. It is most prevalent in children and young adults. Because of the advances in imaging, surgical techniques, and adjuvant chemotherapy protocols, osteosarcoma patients have had a considerable improvement in prognosis during the past 25 years [2–5]. Recent progress in these multidisciplinary therapies had made it possible to perform successful limb salvage surgery, which is considered to be the standard procedure for the majority of patients with osteosarcoma involving the extremities (Fig. 9.1) [2, 6–9].
Overview of the endoprosthetic reconstruction for extremity osteosarcoma. A radiography (a) and a MR image (b) indicated the osteosarcoma of the distal femur (yellow triangle). Osteosarcoma of the distal femur was resected with wide margin including surrounding muscles (c) and reconstructed using distal femoral endoprosthesis (d). Postoperative radiographic finding showed the good alignment of the reconstructed lower extremity without aseptic loosening (e)
In this chapter, I review the advantages of endoprosthetic reconstruction and the history of endoprosthesis in Europe, the USA, and Japan and point out issues and perspectives associated with endoprosthetic reconstruction following the wide excision of osteosarcoma located in the extremities.
2 Advantages of Endoprosthetic Reconstruction
There are three types of reconstruction strategies used for the massive bone defect that is left following the resection of osteosarcoma. The first strategy is biological reconstruction, including allogeneic bone grafting, autologous recycled bone grafting, and vascularized bone grafting [10]. The second reconstructive strategy is prosthetic reconstruction, and the third is reconstruction using recycled bone/allograft-endoprosthesis composite [11]. Biological reconstruction has a major advantage with respect to durability, when the graft bone is implanted into the intercalary defect of the long bone after resection of a diaphyseal bone tumor [10]. However, because most osteosarcoma arises in the meta-diaphysis of the long bones, the surgical indications for biological reconstruction are limited to special cases of osteosarcoma patients [1]. Thus, prosthetic reconstruction has become the standard surgical technique in developed countries.
Various types of prostheses have been developed and applied for the reconstruction of a bone defect after tumor resection. The current models of prostheses include modular segments, a wrought stem, a kinetic rotating hinge around the knee, circumferential porous coating around the endoprosthesis at the bone–prosthesis junction, and loopholes for soft-tissue attachment [12]. The advantages of the current modular prostheses include durability, intraoperative flexibility to fill the bony defect, and structural stability to allow for immediate weight bearing [12–15] (Fig. 9.1).
3 History of Endoprostheses in Europe and the USA
In 1943, the first metallic endoprosthesis was implanted into the proximal femur after resection of a giant cell tumor of bone by Austin Moore and Harold Bohlman in the USA [16]. This first endoprosthesis was made of a cobalt–chromium (Co–Cr) alloy. The Stanmore endoprosthesis also has a long clinical history of development and was first implanted in 1949 [17, 18]. It was a custom-made endoprosthesis with cemented fixation. The GUEPAR endoprosthesis (Benoit-Gerrard Company, Caen, France) was a custom-made Co–Cr cemented tumor endoprosthesis with a single-axis hinged knee which was used in France beginning in 1972 [19].
During the subsequent decades, many exploratory treatments were performed using endoprostheses made from cobalt–chromium alloy [20–23] and other materials, including stainless steel [24], polythene [25], and acrylic [26, 27] for treating bone defects after tumor resection. Although orthopedic oncologists had been interested in these tumor prostheses, these prostheses were only considered for the treatment of patients who refused amputation, because of their low reliability [28].
It was not until the late 1970s that endoprosthetic implants began to emerge as a valuable treatment option in orthopedic oncology. The late 1970s was an epoch-making turning point in the treatment of osteosarcoma. First, because of the development of antineoplastic drugs, including high-dose methotrexate and adriamycin, orthopedic oncologists began to use chemotherapy for osteosarcoma [29]. Chemotherapy was initially performed as adjuvant therapy following the removal of the primary tumor in order to kill the microscopic residual disease. Currently, the standard chemotherapeutic treatment for osteosarcoma is a combination of neoadjuvant and adjuvant chemotherapy [29].
Second, new diagnostic imaging modalities had become available in the field of musculoskeletal tumors. Although plain radiography is an essential modality in the early diagnosis of bone tumors, it was not sufficient to visualize the intraosseous/soft-tissue extent of bone tumors. Owing to the developments and popularization of computed tomography (CT) [30] in the late 1970s and magnetic resonance imaging (MRI) in the mid-1980s [31], orthopedic tumor surgeons could correctly evaluate the bony and soft-tissue extension of tumors [13, 30, 31]. Progress in limb salvage surgery and endoprosthetic reconstruction of bone defect was based on the advances in imaging modalities and chemotherapy protocols.
Because the advances in imaging modalities and chemotherapy protocols enabled the safe tumor excision in the latter 1970s, limb-sparing surgery using endoprosthetic reconstruction was expected to become a realistic surgical procedure. To provide patient-specific endoprostheses that could be modified during surgery without the cost and delay of a custom endoprosthesis, modular systems were developed. Prior to the development of modular systems, physicians were unable to provide an optimal-fitting endoprosthesis during surgery. Modular systems allowed surgeons to use components with the best size and length for each patient (Fig. 9.2).
Modular prosthesis for the reconstruction of the bone defect following the resection of extremity bone tumor. Owing to the development of modular prosthesis, physicians can provide an optimal-fitting prosthesis during surgery. The indicated system is the Kyocera Modular Limb Salvage system (KMLS system)
The first clinical application of a modular ceramic endoprosthesis was indicated for a humeral bone tumor by Salzer et al. [31]. The first modular knee prosthetic systems were developed by Kotz in 1982 and were published as the Kotz modular femur tibia reconstruction system (KMFTR) in 1986 [32]. Although the KMFTR showed good clinical outcomes, the KMFTR was developed into the Howmedica Modular Resection System (HMRS) in 1988. The HMRS had several new features, including a porous coating on the metal surface to promote extracortical bone bridging and one side plate on the femoral stem, and in 1996, a rotating hinge knee was added to the system [13]. Based on the clinical success of the rotating hinge mechanism, a new Global Modular Replacement System (GMRS), which is the next generation of both HMRS and MRS (modular replacement system), was developed in 2002. The GMRS has a unique cementless titanium stem made of titanium alloy with titanium plasma spray, and its proximal 3 cm is coated with hydroxyapatite.
Modular endoprosthetic systems thereby simplified reconstructive surgical procedures. Stanmore Implants produced the METS proximal femur replacement system and METS distal femoral replacement system (designed as a modular system) in 2001 and 2002, respectively. Since 1992, the MUTARS (Modular Universal Tumour and Revision System, Implantcast, Buxtehude, Germany) has been used for the treatment of major bone defects of the lower and upper extremities. The fixation is achieved with an intramedullary stem, which can be inserted either cementless or cemented. In the cementless version, the stem is inserted press-fit in order to achieve sufficient primary stability. The stem has a rough surface with a pore size of 100 μm to allow the bone to grow onto the surface. The stem has a hexagonal cross section and is made of titanium alloy (TiAl6V4) in the cementless version and of Co–Cr–Mo alloy in the cemented version [33].
4 History of Endoprosthesis in Japan
In Japan, the first prosthetic reconstruction was performed by Dr. Katayama at Jikei University School of Medicine in January 1962, for a chondrosarcoma patient using an acrylic hinge-type knee endoprosthesis, which was manufactured at Nemoto Shokai (Tokyo, Japan) [27, 34]. This custom-made endoprosthesis was made from stainless steel, with the surface covered with an acrylate resin, including the metallic hinge axis, in order to reduce the intermetallic friction (Fig. 9.3a). The judgment whether or not bone cement should be used for the stem fixation was left to the surgeon’s discretion. These acrylic hinge-type knee prostheses were applied in 35 patients between 1961 and 1969 and, thereafter, were used for more than 600 patients until 1998 after modifying the implant design and the material from stainless steel to titanium alloy (Fig. 9.3b). Although there have been no systemic long-term follow-up studies of this endoprosthesis, Yamawaki et al. reported a patient who retained the endoprosthesis 40 years after the initial surgery [34].
The hinge-type knee prosthesis manufactured at Nemoto Shokai (Tokyo, Japan). The first endoprosthetic reconstruction in Japan was performed in January 1962 at Jikei University School of Medicine. The prosthesis was acrylic hinge-type knee prosthesis (a). These Jikei-type hinge knee prostheses were used until 1998 modifying the implant design and the material from stainless steel to titanium alloy (b)
In 1984, the KMFTR was approved by the Japanese Ministry of Health, Labour and Welfare and was widely used for the reconstruction of bone defects after resection of a bone tumor. However, the KMFTR was designed for Caucasian physical constitution and was sometimes too large in size [35–40] and too heavy in weight for Asia-Pacific patients. Therefore, Kyocera Corp. (Kyoto, Japan) made two types of prostheses. One type of Kyocera endoprosthesis is a full ceramic custom-made endoprosthesis, the C-TKP (Fig. 9.4a), and the other is a metallic endoprosthesis, the PHK series (Fig. 9.4b–d). The C-TKP was a unique endoprosthesis in that the extraosseous component was made of aluminum, and the stem component was made of sapphire. Although the C-TKP was used from 1988 to 1994, it showed a risk of stem breakage.
Tumor endoprostheses manufactured by KYOCERA Medical Corporation. The first prosthesis was a full ceramic custom-made prosthesis, the C-TKP (a), which was used from 1988 to 1994. After the development of Physio Hinge Total Knee System Type I (PHK I) (b) and PHK II (c), PHK III (d) was introduced in 1997. In April 2002, the Kyocera Medical Limb Salvage system (KMLS system) was developed by a collaboration between the JMOG and KYOCERA Medical Corp. (e). The PHK III remains a distal femoral prosthesis with a cement fixation stem in the product line of the KMLS system
In 1987, the Japanese Musculoskeletal Oncology Group (JMOG) developed an initial prosthetic system with a unique loose hinge for distal femoral bone replacement, the KYOCERA Physio Hinge Total Knee System Type I (PHK I) (KYOCERA Corp. Kyoto, Japan) (Fig. 9.4b). However, several mechanical defects in the hinge joint of the PHK I led to the development of the PHK II (Fig. 9.4c) with a conventional hinge joint in May 1990. Although the PHK II had good clinical performance, new modular endoprosthesis with a rotating hinge joint, the PHK III, was introduced in April 1997 (Fig. 9.4d) [12]. The PHK III was designed for Asian patients, including Japanese, who have a smaller anatomical architecture of the knee joint. PHK III was expected to have various advantages in contrast to widely used endoprosthesis.
The PHK III has a unique semi-rotator hinge joint which allows maximal flexion of 142° and internal/external rotation of 5° [12]. The metallic parts of the PHK III are made of lightweight and high-strength titanium alloy with good biocompatibility and bio-stability and allow scanning by magnetic resonance imaging (MRI). As a result, the PHK III is extremely lightweight. For example, when the PHK III was used for an 11 cm bony defect of the distal femur, the total weight was about 660 g, whereas reconstruction of a 12 cm bony defect using the HMRS reaches a weight of about 1200 g. The metallic surface of the hinge shaft and the rotator, which creates friction between high-density polyethylenes, was fabricated using surface-hardening treatment by azote-ionic inpouring to obtain greater durability of the hinge joint. The rotation sleeve, plate, and shaft sleeve were made of ultrahigh molecular weight polyethylene. A multi-institutional cooperative study by the JMOG showed that the 5-year overall prosthetic survival rates, prosthetic survival rate without aseptic loosening, and limb preservation rate were 85 %, 90 %, and 86 %, respectively. The mean functional score according to the classification system of the Musculoskeletal Tumor Society was 20.5 points (68 %) [12].
In April 2002, the Kyocera Modular Limb Salvage system (KMLS system) was developed by a collaboration between the JMOG and KYOCERA Medical Corp. (Figs. 9.4e and 9.5) [15]. The KMLS system is a modular endoprosthesis system which was developed for the reconstruction of a defect after resection of a tumor located at the proximal humerus, proximal femur, distal femur, proximal tibia, or distal humerus. The distal femoral endoprosthesis of the KMLS system has the same mechanical construction of the prosthetic component as the PHK III (Fig. 9.4d, e), but is characterized by the addition of a cementless stem component. The PHK III remains a distal femoral endoprosthesis with a cement fixation stem in the product line of the KMLS system. A multi-institutional cooperative study by the JMOG showed that the 5-year overall endoprosthetic survival rate was 80.0 %, and the 5-year limb salvage rate was 94.5 %. The mean function score according to the scoring system of the Musculoskeletal Tumour Society was 21.8 points (72.5 %) [15]. This modular system provided patient-specific endoprostheses that could be modified during surgery without the cost and delay of a custom endoprosthesis (Fig. 9.2).
Kobe Steel, Material Ltd. (Kobe, Japan) also developed a tumor endoprosthesis made from titanium alloy, mainly in collaboration with Kyoto University. The K-MAX Hip System H-1 (Fig. 9.6a) was applied for the reconstruction of a proximal femoral bone defect in 1990, and the K-MAX Knee System K-2 (Fig. 9.6b) was used for the reconstruction of a distal femoral bone defect in 1994. The K-MAX Hip System H-1 was the world’s first tumor prostheses made of titanium alloy. Although these simple prostheses promised stable clinical outcomes, the medical material businesses of Kobe Steel, Ltd. Material and Kyocera Corporation was integrated, and the processing technologies of for ceramics and metals were inherited by KYOCERA Medical Corporation.
5 Problems with the Modern Endoprostheses and Future Perspectives
Although endoprosthetic reconstruction of a massive bone defect following tumor resection has become a standard surgical technique, there remain many unresolved problems which may require revision surgery. The prosthetic 5- and 10-year survival rate for distal femoral prostheses in previous studies ranged from 57 % to 93 % and 0 %–88 %, respectively. The 5-year limb preservation rates were 86–96 % [14, 17, 18, 41–49]. These results mean that about 10 % of patients required limb amputation due to an endoprosthesis-related complication. Thus, orthopedic tumor surgeons require endoprostheses with better durability.
Breakage of the components, including the stem components and hinge mechanism, sometimes lead to revision surgery (Fig. 9.7a). The thin stem is especially associated with a high risk of stem breakage. In the PHK III series, breakage of the femoral stem could be attributed to the fact that all four broken stems reported were small-sized stems with a 10 mm diameter [12]. Therefore, small stems less than 10 mm in diameter should be avoided. However, the patients with osteosarcoma are mainly children with a small physique. Moreover, the average size of Japanese knees was found to be 5–10 % smaller than that of Caucasians [50, 51]. Thus, further metallurgical improvement of the metal strength is warranted.
Infection is currently the most common serious complication of endoprostheses, because patients who undergo surgery for a neoplastic condition are often subjected to extensive soft-tissue dissection and long operations and are immunosuppressed [47, 52] (Fig. 9.7b). Several reports have indicated that the endoprosthesis-associated infection rates were 2–15 % [14, 17, 18, 44–49, 52–59]. Infections may result in the amputation of the patients’ limbs. In addition, the cost of treating an infected endoprosthesis is considerable, and infections often recur.
An analysis of clinical factors which caused endoprosthesis loss or amputation suggested that the soft-tissue condition significantly influenced the duration of the infection control period and likelihood of limb salvage [60]. The use of muscle rotation flaps or a vascularized musculocutaneous flap should be considered when only the rectus femoris muscle has been preserved [14].
Several promising technologies are aimed at decreasing the risk of infection following limb preservation. Recently, it was reported that the use of silver-coated prostheses reduced the infection rate [61]. In 2012, a European CE mark was awarded to the ionic silver-treated massive endoprosthesis tumor system (“METS”) as a modular implant system from Stanmore Implants. Silver-coated prostheses are also produced by MUTARS (Implantcast Ltd, Buxtehude, Germany). Hardes et al. reported that the use of silver-coated megaprostheses resulted in a reduced infection rate by analyzing a total of 51 patients who were treated with a silver-coated proximal femur replacement or proximal tibia replacement [61]. Another option to prevent endoprosthetic infection is the use of iodine-coated prostheses. Shirai et al. performed a clinical trial of iodine-coated endoprostheses in 47 patients with malignant bone tumors or pyogenic arthritis [62]. The iodine-coated endoprostheses were used to prevent infection in 21 patients and to treat active infections in 26 patients. Infections were prevented in 20 out of 21 patients. In 26 treatment cases involving one- or two-stage revision surgery, the infection subsided without any additional surgery in all cases. An iodine-coated endoprosthesis may play an important role not only in prophylaxis for infections but also in the treatment of an infected endoprosthesis.
Aseptic loosening of the endoprosthesis is one of common reasons for endoprosthesis revision [12, 14, 15, 17, 54, 58, 63] (Fig. 9.7c). Most endoprostheses have a stem component with a round cross section and circumferential porous coating around the endoprosthesis at the bone–prosthesis junction. To promote the initial bone formation at the bone–prosthesis junction, several technical improvements have been introduced in the tumor prosthesis. Since 1991, a cementless version of the Stanmore endoprosthesis has been available with a hydroxyapatite-coated titanium stem. Additionally, parts of the implant body are HA-coated to enhance the extracortical bone bridging [33]. Some types of cementless endoprostheses, including the KMFTR/HMRS and KMLS, had a side plate to obtain the initial stem stability [13, 15, 32, 57]. However, it appeared that these side plates caused the stress shielding of the cortical bone at the end of the residual bone and led to stem loosening [13, 15, 32, 57]. Therefore, the GMRS, which is the next generation of both HMRS and MRS, has a proximally arranged fin at the cementless press-fit stem to obtain rotational stability. The stems are made of titanium alloy with titanium plasma spray, and the proximal 3 cm of the endoprosthesis is coated with hydroxyapatite. Although the shape of the cross section of the stem component is important to prevent intramedullary stem loosening, most of the prostheses had a stem component with a round cross section. The cementless stem of the MUTARS has a hexagonal cross section and is inserted press-fit in order to achieve sufficient primary stability [33].
The rotating hinge mechanism was associated with better function and was expected to achieve lower rates of loosening in the previous reports [14, 59, 64–66]. In a follow-up study, Kawai et al. [14] compared a fixed hinge knee endoprosthesis, the HSS (Hospital for Special Surgery: Biomet, Warsaw, IN), with a rotating hinge knee, the FINN endoprosthesis (Biomet). The HSS showed aseptic loosening in 31.4 % of cases compared to 6.5 % in the FINN group. The 5-year survivorship was 64 % for the HSS endoprosthesis and 82 % for the FINN endoprosthesis. Thus, a rotating hinge mechanism can reduce the mechanical stress, thus reducing the intramedullary stress.
Most limb salvage procedures performed today are reconstructed using modular tumor endoprostheses with long cemented stems and uncemented stems. Compressive osseointegration fixation (Compress: Biomet) is a new technology for the fixation of tumor endoprostheses [67]. The principal potential advantages of this technique include preservation of the bone stock, prevention of stress shielding, and a short working length of bone necessary for fixation of the implant. Using compressive osseointegration technology, a compressive force is continuously loaded across the bone–implant interface and the bone at the bone–implant interface hypertrophies with time. Although there have been no long-term follow-up studies of this technology yet, Monument et al. indicated that the overall survival rate was 67 % and the endoprosthesis-related implant survival was 89 % after a minimum of 5 years of clinical follow-up. To more accurately assess this fixation strategy, longer clinical follow-up studies are needed.
In conclusion, because of the advances in imaging, surgical techniques, and adjuvant chemotherapy protocols, osteosarcoma patients have had a considerable improvement in prognosis during the past 25 years [2–5]. Recent progress made in these multidisciplinary therapies has made it possible to perform successful limb salvage surgery, and this is now considered to be the standard procedure for the majority of the patients with osteosarcoma involving the extremities. Endoprosthetic reconstruction promises intraoperative flexibility to fill the bony defect and immediate structural stability to allow for weight bearing. Although prosthetic reconstruction has become a standard surgical technique, there remain a lot of unresolved problems which may lead to the need for revision surgery. Thus, orthopedic tumor surgeons are urgently seeking endoprostheses with better durability.
References
Fletcher CDM, DM, Bridge JA, Hogendoorn P, Mertens F. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: International Agency for Research on Cancer; 2013.
Veth R, van Hoesel R, Pruszczynski M, Hoogenhout J, Schreuder B, Wobbes T. Limb salvage in musculoskeletal oncology. Lancet Oncol. 2003;4(6):343–50.
Ferrari S, Palmerini E. Adjuvant and neoadjuvant combination chemotherapy for osteogenic sarcoma. Curr Opin Oncol. 2007;19(4):341–6.
Whelan J, Seddon B, Perisoglou M. Management of osteosarcoma. Curr Treat Options Oncol. 2006;7(6):444–55.
Onikul E, Fletcher BD, Parham DM, Chen G. Accuracy of MR imaging for estimating intraosseous extent of osteosarcoma. Am J Roentgenol. 1996;167(5):1211–5.
Kneisl JS, Finn HA, Simon MA. Mobile knee reconstructions after resection of malignant tumors of the distal femur. Orthop Clin North Am. 1991;22(1):105–19.
Skaliczki G, Antal I, Kiss J, Szalay K, Skaliczki J, Szendri M. Functional outcome and life quality after endoprosthetic reconstruction following malignant tumours around the knee. Int Orthop. 2005;29(3):174–8. Epub 2005 Apr 14.
Marulanda GA, Henderson ER, Johnson DA, Letson GD, Cheong D. Orthopedic surgery options for the treatment of primary osteosarcoma. Cancer Control. 2008;15(1):13–20.
Swallow CJ, Catton CN. Local management of adult soft tissue sarcomas. Semin Oncol. 2007;34(3):256–69.
Kunz P, Bernd L. Methods of biological reconstruction for bone sarcoma: indications and limits. Recent Results Cancer Res. 2009;179:113–40.
Benedetti MG, Bonatti E, Malfitano C, Donati D. Comparison of allograft-prosthetic composite reconstruction and modular prosthetic replacement in proximal femur bone tumors: functional assessment by gait analysis in 20 patients. Acta Orthop. 2013;84(2):218–23.
Matsumine A, Ueda T, Sugita T, Yazawa Y, Isu K, Kawai A, Japanese Musculoskeletal Oncology Group, et al. Clinical outcomes of the KYOCERA physio hinge total knee system type III after the resection of a bone and soft tissue tumor of the distal part of the femur. J Surg Oncol. 2011;103(3):257–63.
Kotz RI. Progress in musculoskeletal oncology from 1922–2012. Int Orthop. 2014;38(5):1113–22.
Kawai A, Lin PP, Boland PJ, et al. Relationship between magnitude of resection, complication, and prosthetic survival after prosthetic knee reconstructions for distal femoral tumors. J Surg Oncol. 1999;70(2):109–15.
Nakamura T, Matsumine A, Uchida A, Kawai A, Nishida Y, Kunisada T, et al. Clinical outcomes of Kyocera modular limb salvage system after resection of bone sarcoma of the distal part of the femur: the Japanese Musculoskeletal Oncology Group study. Int Orthop. 2014;38(4):825–30.
Moore AT, Bohlman HR. Metal hip joint: a case report. J Bone Joint Surg Am. 1943;25(3):688–92.
Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. 1996;78(1):5–13.
Unwin PS, Cobb JP, Walker PS. Distal femoral arthroplasty using custom-made prostheses. The first 218 cases. J Arthroplasty. 1993;8(3):259–68.
Mascard E, Anract P, Touchene A, Pouillart P, Tomeno B, Mascard E, et al. Complications from the hinged GUEPAR prosthesis after resection of knee tumor. 102 cases. Rev Chir Orthop Reparatrice Appar Mot. 1998;84:628–37.
Brav EA, Mc FJ, Miller JA. The replacement of shaft defects of long bones by metallic prostheses. Am J Surg. 1958;95(5):752–60.
Loomis LK. Internal prosthesis for upper portion of femur; a case report. J Bone Joint Surg Am. 1950;32(A:4):944–6.
Venable CS. An elbow and an elbow prosthesis; case of complete loss of the lower third of the humerus. Am J Surg. 1952;83(3):271–5.
Moore AT. The self-locking metal hip prosthesis. J Bone Joint Surg Am. 1957;39-A(4):811–27.
Horwitz T. Use of a shaft prosthesis in the treatment of surgically resistant nonunion of the humerus. Bull Hosp Joint Dis. 1955;16(1):37–44.
Seddon HJ, Scales JT. A polythene substitute for the upper two-thirds of the shaft of the femur. Lancet. 1949;2(6583):795.
Macausland WR. Replacement of the lower end of the humerus with a prosthesis; a report of four cases. West J Surg Obstet Gynecol. 1954;62(11):557–66.
Katayama R, Maezawa T. Total replacement of the knee joint by prosthesis: the first report. J Jpn Orthop Assoc. 1968;42:187–92.
Hwang JS, Mehta AD, Yoon RS, Beebe KS. From amputation to limb salvage reconstruction: evolution and role of the endoprosthesis in musculoskeletal oncology. J Orthop Traumatol. 2014;15(2):81–6.
Rosen G, Murphy ML, Huvos AG, Gutierrez M, Marcove RC. Chemotherapy, en bloc resection, and prosthetic bone replacement in the treatment of osteogenic sarcoma. Cancer. 1976;37(1):1–11.
Destouet JM, Gilula LA, Murphy WA. Computed tomography of long-bone osteosarcoma. Radiology. 1979;131(2):439–45.
Zimmer WD, Berquist TH, McLeod RA, Sim FH, Pritchard DJ, Shives TC, Wold LE, May GR. Magnetic resonance imaging of osteosarcomas. Comparison with computed tomography. Clin Orthop Relat Res. 1986;208:289–99.
Kotz R, Ritschl P, Trachtenbrodt J. A modular femur-tibia reconstruction-system. Orthopedics. 1986;9:1639–52.
Heisel C, Kinkel S, Bernd L, Ewerbeck V. Megaprostheses for the treatment of malignant bone tumours of the lower limbs. Int Orthop. 2006;30(6):452–7.
Yamawaki K, Aoki H, Shimizu K, Mori T, Kidokoro K. Revision of acrylic hinge-type knee prosthesis after a follow-up of almost 40 years. J Orthop Sci. 2006;11(1):97–102.
Siu D, Rudan J, Wevers HW, Griffiths P. Femoral articular shape and geometry. A three-dimensional computerized analysis of the knee. J Arthroplasty. 1996;11(2):166–73.
Hitt K, Shurman 2nd JR, Greene K, McCarthy J, Moskal J, Hoeman T, et al. Anthropometric measurements of the human knee: correlation to the sizing of current knee arthroplasty systems. J Bone Joint Surg Am. 2003;85-A Suppl 4:115–22.
Wang SW, Feng CH, Lu HS. A study of Chinese knee joint geometry for prosthesis design. Chin Med J (Engl). 1992;105(3):227–33.
Uehara K, Kadoya Y, Kobayashi A, Ohashi H, Yamano Y. Anthropometry of the proximal tibia to design a total knee prosthesis for the Japanese population. J Arthroplasty. 2002;17(8):1028–32.
Bhudhikanok GS, Wang MC, Eckert K, Matkin C, Marcus R, Bachrach LK. Differences in bone mineral in young Asian and Caucasian Americans may reflect differences in bone size. J Bone Miner Res. 1996;11(10):1545–56.
Low FH, Khoo LP, Chua CK, Lo NN. Kinematic analysis of total knee prosthesis designed for Asian population. Crit Rev Biomed Eng. 2000;28(1–2):33–40.
Torbert JT, Fox EJ, Hosalkar HS, Ogilvie CM, Lackman RD. Endoprosthetic reconstructions: results of long-term followup of 139 patients. Clin Orthop Relat Res. 2005;438:51–9.
Kawai A, Healey JH, Boland PJ, Athanasian EA, Jeon DG. A rotating-hinge knee replacement for malignant tumors of the femur and tibia. J Arthroplasty. 1999;14(2):187–96.
Mittermayer F, Krepler P, Dominkus M, Schwameis E, Sluga M, Heinzl H, et al. Long-term followup of uncemented tumor endoprostheses for the lower extremity. Clin Orthop Relat Res. 2001;388:167–77.
Roberts P, Chan D, Grimer RJ, Sneath RS, Scales JT. Prosthetic replacement of the distal femur for primary bone tumours. J Bone Joint Surg Br. 1991;73(5):762–9.
Muschler GF, Ihara K, Lane JM, Healey JH, Levine MJ, Otis JC, et al. A custom distal femoral prosthesis for reconstruction of large defects following wide excision for sarcoma: results and prognostic factors. Orthopedics. 1995;18(6):527–38.
Ham SJ, Schraffordt Koops H, Veth RP, van Horn JR, Molenaar WM, Hoekstra HJ. Limb salvage surgery for primary bone sarcoma of the lower extremities: long-term consequences of endoprosthetic reconstructions. Ann Surg Oncol. 1998;5(5):423–36.
Griffin AM, Parsons JA, Davis AM, Bell RS, Wunder JS. Uncemented tumor endoprostheses at the knee: root causes of failure. Clin Orthop Relat Res. 2005;438:71–9.
Biau D, Faure F, Katsahian S, Jeanrot C, Tomeno B, Anract P. Survival of total knee replacement with a megaprosthesis after bone tumor resection. J Bone Joint Surg Am. 2006;88(6):1285–93.
Sharma S, Turcotte RE, Isler MH, Wong C. Cemented rotating hinge endoprosthesis for limb salvage of distal femur tumors. Clin Orthop Relat Res. 2006;450:28–32.
Mensch JS, Amstutz HC. Knee morphology as a guide to knee replacement. Clin Orthop Relat Res. 1975;112:231–41.
Miyake T. Studies on the sizes and shapes of Japanese knee and their applications to the design of knee prosthesis. J Jpn Orthop Assoc. 1978;52:865–79.
Jeys LM, Grimer RJ, Carter SR, Tillman RM. Periprosthetic infection in patients treated for an orthopaedic oncological condition. J Bone Joint Surg Am. 2005;87(4):842–9.
Jeys LM, Grimer RJ, Carter SR, Tillman RM. Risk of amputation following limb salvage surgery with endoprosthetic replacement, in a consecutive series of 1261 patients. Int Orthop. 2003;27(3):160–3. Epub 2003 Feb 8.
Heisel C, Breusch SJ, Schmid G, Bernd L. Lower limb salvage surgery with MUTARS endoprostheses: 2 to 7 year results. Acta Orthop Belg. 2004;70(2):142–7.
Hardes J, Gebert C, Schwappach A, et al. Characteristics and outcome of infections associated with tumor endoprostheses. Arch Orthop Trauma Surg. 2006;26:289–96.
Shin DS, Weber KL, Chao EY, et al. Reoperation for failed prosthetic replacement used for limb salvage. Clin Orthop Relat Res. 1999;358:53–63.
Capanna R, Morris HG, Campanacci D, Del Ben M, Campanacci M. Modular uncemented prosthetic reconstruction after resection of tumours of the distal femur. J Bone Joint Surg Br. 1994;76(2):178–86.
Kawai A, Muschler GF, Lane JM, Otis JC, Healey JH. Prosthetic knee replacement after resection of a malignant tumor of the distal part of the femur. Medium to long-term results. J Bone Joint Surg Am. 1998;80(5):636–47.
Bickels J, Wittig JC, Kollender Y, Henshaw RM, Kellar-Graney KL, Meller I, et al. Distal femur resection with endoprosthetic reconstruction: a long-term followup study. Clin Orthop Relat Res. 2002;400:225–35.
Morii T, Morioka H, Ueda T, Araki N, Hashimoto N, Kawai A, et al. Deep infection in tumor endoprosthesis around the knee: a multi-institutional study by the Japanese musculoskeletal oncology group. BMC Musculoskelet Disord. 2013;14:51.
Hardes J, von Eiff C, Streitbuerger A, Balke M, Budny T, Henrichs MP, et al. Reduction of periprosthetic infection with silver-coated megaprostheses in patients with bone sarcoma. J Surg Oncol. 2010;101(5):389–95.
Shirai T, Tsuchiya H, Nishida H, Yamamoto N, Watanabe K, Nakase J, et al. Antimicrobial megaprostheses supported with iodine. J Biomater Appl. 2014;29(4):617–23.
Zeegen EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivorship analysis of 141 modular metallic endoprostheses at early followup. Clin Orthop Relat Res. 2004;420:239–50.
Choong PF, Sim FH, Pritchard DJ, Rock MG, Chao EY. Megaprostheses after resection of distal femoral tumors. A rotating hinge design in 30 patients followed for 2–7 years. Acta Orthop Scand. 1996;67(4):345–51.
Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Endoprosthetic replacement of the distal femur for bone tumours: long-term results. J Bone Joint Surg Br. 2007;89(4):521–6.
Myers GJ, Abudu AT, Carter SR, Tillman RM, Grimer RJ. Erratum. J Bone Joint Surg Br. 2007;89(5):706.
Monument MJ, Bernthal NM, Bowles AJ, Jones KB, Randall RL. What are the 5-year survivorship outcomes of compressive endoprosthetic osseointegration fixation of the femur? Clin Orthop Relat Res. 19 June 2014 [Epub ahead of print].
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Japan
About this chapter
Cite this chapter
Matsumine, A. (2016). Endoprosthetic Reconstruction for Extremity Osteosarcoma. In: Ueda, T., Kawai, A. (eds) Osteosarcoma. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55696-1_9
Download citation
DOI: https://doi.org/10.1007/978-4-431-55696-1_9
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55695-4
Online ISBN: 978-4-431-55696-1
eBook Packages: MedicineMedicine (R0)