Abstract
Nonocclusion type of angioscopy was developed in Japan. The design of the device is relatively simple, consisting of a probe catheter and fiber catheter. Low molecular weight dextran is injected through the outer probe, removing red blood cells from the vessel wall in order to create a clear field of view for observing the lesion. This paper will describe the procedural requirements to undertake nonocclusion type of angioscopy.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Angioscopy visualizes atherosclerotic lesions in vivo, with yellow plaque indicating lipid-rich plaque and high vulnerability [1–10]. Nonocclusion type of angioscopy has been used to assess plaque morphology in patients with acute coronary syndrome and plaque stabilization or regression by statin treatment in a number of clinical trials [11–13]. Recently, some reports have evaluated neointimal coverage and thrombus formation after drug-eluting stent implantation by angioscopy [14–17].
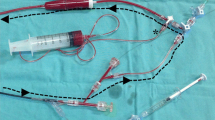
The type of angioscopy system (Fig. 5.1a) comprises of a light source and a CCD camera. There are two types of angioscopy systems, which are distinguished according to the use of an occlusion balloon. Both balloon occlusion and nonocclusion types of catheters are commercially available in Japan. In particular, the unique feature of the nonocclusion type of angioscopic procedure is that the lumen of the vascular can be observed without requiring a blood occlusion, minimizing myocardial ischemia compared to a balloon occlusion type.
Fiber catheter of nonocclusion type of angioscopy and imaging system. (a) This device has a built-in a light source and a CCD camera. (b) This catheter is 6,000 pixels. This probing catheter consists of inner catheter and outer catheter. A tube and a T-shaped stopcock for the connection with dextran are required. 5 ml syringes for flashbulbs are also necessary
Although angioscopy is a commercially available and FDA-approved optical imaging technology that has been available for many years, its use in theUnited States has been largely restricted to research applications rather than clinical practice. Limitations of the technology include the requirement to clear the coronary lumen of blood by the infusion of saline, the large catheter diameter that limits the evaluation of distal segments, the inability to pass significant lesions, and the difficulties associated with accurate interpretation of the images, which therefore mandate expertise [18, 19]. Balloon occlusion-induced myocardial ischemia and the complicated procedure arising from the use of an occlusion type angioscopy may be a concern in the United States.
Nonocclusion type of angioscopy was developed in Japan in order to eliminate the risk associated with the balloon occlusion device, with approval obtained from the Ministry of Health, Labor and Welfare. The design of the device is relatively simple, consisting of a probe catheter and fiber catheter. Low molecular weight dextran is injected through the outer probe, removing red blood cells from the vessel wall in order to create a clear field of view for observing the lesion. This paper will describe the procedural requirements to undertake nonocclusion type of angioscopy.
2 Nonocclusion Type of Angioscopy System
A description of the angioscopy device and catheter is outlined in Fig. 5.1a, b. The physician operates the fiber and probe catheters, while a 2nd operator flushes low molecular weight dextran through the manifold.
3 Preparation of Nonocclusion Type of Angioscopy
-
1.
Preparation of nonocclusion type of angioscopy (VISIBLE Fiber (FT-203F; Fiber Tech Co. Ltd., Tokyo, Japan), a fiber imaging system, and a console (Inter-tec Medicals Co. Ltd., Osaka, Japan) is as follows: both the inner and outer probing catheter are flushed with heparin and assembled.
-
2.
The 2nd operator assembles the manifold, ensuring that no air is introduced with the low molecular weight dextran. A 5 ml syringe is also attached (Fig. 5.2).
-
3.
A 0.014-in. guidewire is inserted through the probing catheter, introduced via a guiding catheter (>6Fr) for coronary angioplasty and advanced to lesion location, while viewing fluoroscopic images on the monitor (Fig. 5.3).
-
4.
Then, the inner catheter and guidewire are removed, and the 5 ml syringe is connected while confirming presence of reverse blood flow and that no air has been introduced. The fiber catheter is then inserted.
4 Preparation for the Probing Catheter
The probing catheter requires rinsing with saline solution. The outer and inner catheters are then separated and rinsed using a 10 ml syringe which is also effective in removing residual air. The probe catheter is rinsed in a tray (it is important to ensure that the catheter does not dry out). The catheter should be carefully rinsed to ensure that no residual air remains (residual air bubbles in the tip of the probing catheter will result in interference of the field of imaging). The inner catheter is placed within the outer catheter while immersed in saline solution. As the catheter is relatively thin, the use of a small tray may affect the ability to insert the catheter effectively, and excessive force may result in damage to the catheter. The catheter is easily inserted in a straight position, and should resistance be encountered, it may be effective to pre-insert the guidewire in the inner catheter prior to insertion into the outer catheter (Fig. 5.4). If there is residual air inside the catheter, air bubbles or ST elevation on the electrocardiograph is observed during the procedure; a 20–30 ml syringe of saline solution should be used to repetitively flush the coronary arteries and wash out any air post procedure. When the probing catheter in the angioscopy catheter set cannot cross over severe stenosis, an aspiration catheter (>7Fr) for thrombectomy can be used instead of the original probing catheter.
5 Focus Alignment of Angioscopy
In order to align the focus of the angioscopy catheter, the tip should be placed close to a dry gauze in order to confirm the imaging. The image should be focused to align either the 2 × 2 or 3 × 3 markers (Fig. 5.5). The focusing dial should be adjusted carefully and the focus confirmed. Following repetitive use, the tip should be cleaned with damp gauze and the focus readjusted. When protein becomes attached to the tip, the image may become unfocused. When large particles are observed on the image, it may be resultant from particles contained within the connection rather than the catheter tip. The connection should be wiped with either gauze or cotton swab or the “white balance” button reset.
6 Insertion of the Fiber Catheter into the Probing Catheter
Similar to other catheters, the fiber catheter is easily damaged under excessive bending. When the fiber is broken, sufficient light is unable to reach the catheter tip. When product is not in use, it is recommended to place a cover over the fiber when stored. The probing catheter is inserted beyond the target location. Care should be given that the guiding catheter does not become disengaged when the probing catheter is inserted into tortuous vasculature (the guiding catheter should ideally be coaxially positioned). It might be impossible to advance the fiber catheter when the guiding catheter is bent. When the catheter is placed in position, the inner catheter and guidewire are removed (quickly removing product may result in the introduction of air). Use a 2.5 ml syringe to remove any residual air, and confirm the backflow of blood. When reverse blood flow is not observed, the catheter may have become wedged in a smaller vessel diameter, and under minimal negative pressure, the catheter should be pulled slightly proximal. Not correcting this issue may result in an ST elevation.
7 Care During Insertion
The 2nd operator should introduce low molecular dextran removing residual air with the Y-connector connected. The fiber catheter is then inserted though probing catheter. Further flushing of dextran should be performed again with the catheter inserted at a 40–50 cm depth to ensure removal of all residual air. Finally, the fiber catheter is further inserted just prior to the tip of the guide catheter. If the guiding catheter tip is positioned at a bend, some resistance may be incurred during insertion. If the fiber catheter is within the guiding catheter, it can be advanced further even under some resistance. If the guiding catheter is excessively bent, the guiding catheter positioning should be corrected. Insertion resistance will be increased if the probing catheter is also placed in a bent position. A fluoroscopic marker is located at the tip, and the catheter should be advanced 1 cm at a time. The catheter should be inserted as straight as possible to avoid damage to the catheter. Excessive force may result in the fiber catheter breaking.
When image recording is commenced, it can be difficult to determine the vessel location and whether target location is pre- or posttreatment. All significant lesion locations should be recorded on cine angiography while imaging, noting details such as the position of the catheter and the grade of the plaque.
8 Careful Consideration During Imaging
It is important that the lead physician be aware of the positioning of both probing catheter tip and fiber catheter when pulling the probing catheter back. The image in Fig. 5.6a is the correct positioning.
The positioning of fiber catheter in probing catheter. (a) The fiber catheter placed at correct position from the tip of probing catheter. Yellow plaque clearly evident. (b) When the fiber catheter is far from the tip of the probing catheter, the observational range is small, and the whole image is dark. The image is improved by reducing the distance to the tip of the catheter. In addition, if blood removal was not adequately performed by appropriate dextran flushing because of strong blood flow, the image might be improved by increasing the light quantity without bringing the catheter closer to the tip
When the fiber catheter is positioned far from the tip of probing catheter, the observational range is small, and the whole image is dark as in Fig. 5.6b. The image is improved by reducing the distance between catheters. In addition, if blood removal was inadequate by appropriate dextran flushing due to strong blood flow, the image might be improved by increasing light quantity without reducing the distance between catheters. As flushing may cause a slight offset, it is important to correct the positioning. There is a 1–2 s delay between pullback and the image, making it difficult to determine accurate positioning if retracted too quickly. Further, it is important that the fiber catheter is not retracted before the probing catheter as this may result in injury to the vessel.
9 Complication with Procedure of Angioscopy
The general complications of this nonocclusion type of angioscopy are similar to the imaging modality in other blood vessels, for example, catheter-induced spasm, intimal injury, deformation of stent, etc., and there are no specific complications. However, it is important that we do not push it forcibly when the probing catheter cannot reach for the target lesion because of resistance with a severe stenosis, calcification, bending vessel, and the stent edge when we advance probing catheter in the vessel.
10 Future Prospects
The inability to see through blood flow because of its opaque nature and the resulting need to remove blood from the visual field using dextran-flushing technique remain the primary obstacles to the widespread and routine use of nonocclusion type of angioscopy to evaluate the luminal wall.
At present, certain angioscopic techniques may possibly recognize the presence of coronary artery calcification, but assessment/quantification of coronary artery calcification is not a current application of this evolving technique.
However, angioscopy is able to observe vulnerable plaque and arteriosclerotic change, as well as identify the presence of thrombus, currently not feasible with an IVUS or OCT system. It is considered that the use of this device will become more prevalent, although the advancement of hardware of the nonocclusion type of angioscopy system is a requirement moving forward.
References
Spears JR, Marais HJ, Serur J, Pomerantzeff O, Geyer RP, Sipzener RS, Weintraub R, Thurer R, Paulin S, Gerstin R, Grossman W. In vivo coronary angioscopy. J Am Coll Cardiol. 1983;1:1311–14.
Sherman CT, Litvack F, Grundfest W, Lee M, Hickey A, Chaux A, Kass R, Blanche C, Matloff J, Morgenstern L, et al. Coronary angioscopy in patients with unstable angina pectoris. N Engl J Med. 1986;315:913–19.
Uchida Y, Tomaru T, Nakamura F, Furuse A, Fujimori Y, Hasegawa K. Percutaneous coronary angioscopy in patients with ischemic heart disease. Am Heart J. 1987;114:1216–22.
Mizuno K, Arai T, Satomura K, Shibuya T, Arakawa K, Okamoto Y, Miyamoto A, Kurita A, Kikuchi M, Nakamura H, et al. New percutaneous transluminal coronary angioscope. J Am Coll Cardiol. 1989;13:363–8.
Siegel RJ, Ariani M, Fishbein MC, Chae JS, Park JC, Maurer G, Forrester JS. Histopathologic validation of angioscopy and intravascular ultrasound. Circulation. 1991;84:109–17.
White CJ, Ramee SR, Collins TJ, Mesa JE, Jain A. Percutaneous angioscopy of saphenous vein coronary bypass grafts. J Am Coll Cardiol. 1993;21:1181–5.
Mizuno K, Miyamoto A, Satomura K, Kurita A, Arai T, Sakurada M, Yanagida S, Nakamura H. Angioscopic coronary macromorphology in patients with acute coronary disorders. Lancet. 1991;337:809–12.
Ueda Y, Asakura M, Hirayama A, Komamura K, Hori M, Kodama K. Intracoronary morphology of culprit lesions after reperfusion in acute myocardial infarction: serial angioscopic observations. J Am Coll Cardiol. 1996;27:606–10.
Mizote I, Ueda Y, Ohtani T, Shimizu M, Takeda Y, Oka T, Tsujimoto M, Hirayama A, Hori M, Kodama K. Distal protection improved reperfusion and reduced left ventricular dysfunction in patients with acute myocardial infarction who had angioscopically defined ruptured plaque. Circulation. 2005;112:1001–7.
Ueda Y, Asakura M, Yamaguchi O, Hirayama A, Hori M, Kodama K. The healing process of infarct-related plaques: insights from 18 months of serial angioscopic follow-up. J Am Coll Cardiol. 2001;38:1916–22.
Hirayama A, Saito S, Ueda Y, Takayama T, Honye J, Komatsu S, Yamaguchi O, Li Y, Yajima J, Nanto S, Takazawa K, Kodama K. Qualitative and quantitative changes in coronary plaque associated with atorvastatin therapy. Circ J. 2009;73(4):718–25.
Hirayama A, Saito S, Ueda Y, Takayama T, Honye J, Komatsu S, Yamaguchi O, Li Y, Yajima J, Nanto S, Takazawa K, Kodama K. Plaque-stabilizing effect of atorvastatin is stronger for plaques evaluated as more unstable by angioscopy and intravenous ultrasound. Circ J. 2011;75(6):1448–54.
Kodama K, Komatsu S, Ueda Y, Takayama T, Yajima J, Nanto S, Matsuoka H, Saito S. Hirayama A Stabilization and regression of coronary plaques treated with pitavastatin proven by angioscopy and intravascular ultrasound–the TOGETHAR trial. Circ J. 2010;74(9):1922–8.
Kotani J, Awata M, Nanto S, Uematsu M, Oshima F, Minamiguchi H, Mintz GS, Nagata S. Incomplete neointimal coverage of sirolimus-eluting stents: angioscopic findings. J Am Coll Cardiol. 2006;47(10):2108–11.
Oyabu J, Ueda Y, Ogasawara N, Okada K, Hirayama A, Kodama K. Angioscopic evaluation of neointima coverage: sirolimus drug-eluting stent versus bare metal stent. Am Heart J. 2006;152(6):1168–74.
Awata M, Kotani J, Uematsu M, Morozumi T, Watanabe T, Onishi T, Iida O, Sera F, Nanto S, Hori M, Nagata S. Serial angioscopic evidence of incomplete neointimal coverage after sirolimus-eluting stent implantation: comparison with bare-metal stents. Circulation. 2007;116(8):910–16.
Takayama T, Hiro T, Akabane M, Kawano T, Ichikawa M, Kanai T, Fukamachi D, Haruta H, Saito S, Hirayama A. Degree of neointimal coverage is not related to prevalence of in-stent thrombosis in drug-eluting stents: a coronary angioscopic study. Int J Cardiol. 2012;156(2):224–6.
Suter MJ, Nadkarni SK, Weisz G, Tanaka A, Jaffer FA, Bouma BE, Tearney GJ. Intravascular optical imaging technology for investigating the coronary artery. JACC Cardiovasc Imaging. 2011;4(9):1022–39.
Fujii K, Hao H, Ohyanagi M, Masuyama T. Intracoronary imaging for detecting vulnerable plaque. Circ J. 2013;77(3):588–95.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Takayama, T. (2015). System and Procedure of Nonocclusion Type of Angioscopy. In: Mizuno, K., Takano, M. (eds) Coronary Angioscopy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55546-9_5
Download citation
DOI: https://doi.org/10.1007/978-4-431-55546-9_5
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55545-2
Online ISBN: 978-4-431-55546-9
eBook Packages: MedicineMedicine (R0)