Abstract
Coronary artery disease (CAD) has two broad categories of clinical syndromes such as acute coronary syndrome (ACS) and stable angina pectoris (SAP). ACS is well recognized to be a significant contributor to both morbidity and mortality in worldwide, and it is pivotal to understand the mechanisms of ACS in order to predict the occurrence of ACS using a combination of novel imaging modalities and noninvasive biomarkers. Currently, several imaging modalities are investigated to detect vulnerable plaques. In particular, coronary angioscopy can evaluate the luminal surface by direct visualization and plays an important role to elucidate morphological interaction between the plaque and thrombus. In this review, we will focus on the differences in angioscopic findings of plaque morphology, such as plaque color and presence of thrombus in patients with ACS and those with SAP.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Acute coronary syndrome
- Stable angina pectoris
- Vulnerable plaque
- Plaque rupture
- Erosion
- Plaque color
- Thrombus
1 Introduction
The prognosis of patients with stable angina pectoris (SAP) is generally good with an incidence of death or nonfatal myocardial infarction (MI) not exceeding 2 % per year [1]. On the other hand, patients with an acute coronary syndrome (ACS) without ST elevation have much worse prognosis with a 10–15 % incidence of death or nonfatal MI within 1 year after admission [2]. Despite a similar anatomical background, there are different pathophysiologic mechanisms between ACS and SAP. ACS is a clinical syndrome characterized by acute change of ischemic symptom. Plaque disruption, platelet activation, and flow-limiting thrombus formation are recognized as key events in the pathogenesis of ACS [3]. In contrast, such changes do not occur in SAP patients. Because angiography can only provide a two-dimensional silhouette of the lumen, it is not suitable to identify plaque vulnerability. Therefore, the challenge for the future is to identify vulnerable plaques prone to disrupt before thrombotic complications occur. There are several imaging techniques that could detect local plaque vulnerability leading to an ACS [4]. Of these, only coronary angioscopy can provide detailed information of the luminal surface of a plaque, such as color, thrombus, or disruption. In the present review, we will focus on the angioscopic characteristics of ACS and SAP.
2 Characteristics of the Current Intravascular Imaging Modalities
Intravascular imaging devices can show detailed plaque composition that cannot be detected by angiography, and these findings are thought to play an important role in order to predict future cardiac events such as ACS. Each imaging device has its own advantages and disadvantages (Table 10.1) [4, 5].
Several studies have demonstrated differences in plaque components between ACS and SAP patients using different intravascular imaging devices. When comparing ACS and SAP using intravascular ultrasound imaging (IVUS), patients with ACS have more frequently positive remodeling and noncalcified attenuated plaque in the culprit lesion than in those with SAP [6, 7]. According to optical coherence tomography (OCT) findings, thrombi and plaque disruption and thin-cap fibroatheroma are frequently found within the culprit lesions of ACS patients [8]. In addition to these findings, several studies evaluated surface morphology between lesions in ACS and SAP patients using angioscopy.
The angioscopy system is classified into an occlusion balloon type (monorail on guidewire system) or a non-occlusion one (bare fiber system). Angioscopic examination using balloon occlusion type (FULLVIEW NEO, FiberTech Co., Chiba, Japan) is performed while coronary blood flow was interrupted by inflating the balloon, and blood is cleared away by the injection of 5–10 ml of lactated Ringer’s or 3 % dextran solution. On the other hand, the non-balloon occlusion type (FiberTech Co.) is designed for pullback visualization without balloon occlusion, while flushing with 3 % dextran solution through a flushing catheter.
3 Angioscopic Findings
3.1 Characteristics of Plaque Morphology
From a pathological point of view, atherosclerotic plaques can be classified into two components. These include an atheromatous and a fibrous plaque, which are generally consistent with vulnerable and stable plaques, respectively [9]. Of course, ACS patients have more plaques with characteristics of vulnerability, leading to plaque disruption and thrombosis [3]. From an angioscopic findings, vulnerable plaques show plaque disruption which is defined as having high-yellow color intensity plaque and a mural thrombus formation. Plaque disruption is further classified into two types of plaque morphology such as plaque rupture and erosion [10, 11].
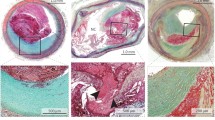
Angioscopically, plaque rupture is defined as the plaque showing dissection, fissuring, ulceration, or confirmed atheroma contents [11]. Plaque erosion is defined as eroded if it shows only reddening and erosion with no evidence of dissection, cleft, or depressed ulceration [11]. On the other hand, plaques in SAP patients usually have a smooth surface with white or light yellow color and no thrombi [12]. Representative image of plaque rupture, erosion, and stable plaque is shown in Fig. 10.1.
Representative image of plaque morphology by angioscopy. (a) Angioscopic image showed a cleft with intensive yellow plaque and a mixed thrombus. (b) Angioscopic image showed erosion on a light-yellow plaque with no evidence of dissection. (c) Angioscopic image showed a smooth surface of light-yellow plaque without thrombus
3.2 Grading of Plaque Color
Plaque color differs in patients with ACS and SAP. According to the relationship between the color of the plaque and its histopathological features, deep-yellow and yellow-red lesions represent either atheroma (53 %) or degenerated plaque (42 %), whereas pate-yellow or gray-yellow lesions were predominantly with degenerated plaque (64 %) and, to a lesser extent, with fibrous plaque (14 %) or atheroma (14 %) [13]. In SAP patients, both types of lesions, smooth gray-white and yellow lesions, were found to be equally distributed [13]. On the other hand, in ACS patients, the yellow color and plaque rupture were frequently found [13]. A previous angioscopic study reported that the number of yellow plaques in a coronary artery is an independent future risk of cardiovascular events and that patients with multiple yellow plaques per vessel have 2.2-fold higher risk of suffering ACS than patients with no or a single yellow plaque per vessel [14].
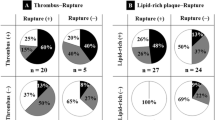
Recently, yellow plaques were further classified according to yellow color intensity: grade 0, white; grade1, light yellow; grade2, yellow; and grade3, intensive yellow (Fig. 10.2) [15]. Several studies reported that yellow color intensity was associated with plaque vulnerability. Uchida et al. reported that ACS events occurred in 3.3 % of white plaque, 7.6 % of non-glistening yellow plaque, and 68.4 % of glistening yellow plaque [16]. Ueda et al. demonstrated that yellow plaques with higher color grade have a higher incidence of thrombus on the plaque (Fig. 10.3) [15]. Furthermore, Kubo et al. revealed that there was a significant negative correlation between yellow color intensity and fibrous cap thickness evaluated by angioscopy and OCT (Fig. 10.4) [17]. Therefore, it is considered that plaque color grade assessed by angioscopy showed a good correlation with plaque stability or instability. Based on these findings, the relationship between plaque color changes and effect of statin therapy was evaluated using angioscopy. The TWINS (evaluaTion With simultaneous angIoscopy and iNtravascular ultraSound) study and TOGETHAR trial demonstrated that reduction of yellow grade detected by angioscopy occurred independently of volumetric plaque change by statin therapy [18, 19].
Relation between color grade of plaque and prevalence of thrombus. Yellow color intensity of plaque is associated with the prevalence of thrombus. (Reproduced with permission from Ref. Ueda et al. [15])
Relation between color grade of plaque and fibrous cap thickness by optical coherence of tomography. There was a significant negative correlation between yellow color intensity and fibrous cap thickness (P < 0.0001). (Reproduced with permission from Ref. Kubo et al. [17])
The major limitation of angioscopic evaluation is that color grading is often different from each observer. Therefore, in order to avoid this problem, Okada and Ishibashi, et al. have attempted a quantitative assessment color analysis using colorimetry apparatus [20, 21].
3.3 Characteristics of Thrombus
Angioscopy is superior to other imaging modalities for detecting thrombus. In angioscopic image, thrombus is usually adhering to the intima or protruding into the lumen, which persists even after flushing with lactated Ringer’s or dextran solution. Thrombi were further classified by color such as solid red, mixed red, and white mass, although the relationship between thrombus color and clinical outcome is uncertain [22–24]. A white thrombus was defined as a shaggy, irregular, and cotton-wool-like mass in which white area occupies more than two thirds. The majority of red thrombus was solid and globular, and red area occupies more than two thirds. Mixed thrombus was defined as white and red in a mosaic pattern (one third to two thirds are red) [25]. Representative images of thrombus are shown in Fig. 10.5.
A previous study reported that plaque rupture and thrombus were present in 17 % of SAP and 68 % of ACS evaluated by angioscopy, respectively [26]. However, the presence of thrombus detected by angioscopy do not necessary lead to the clinical manifestation of an ACS, although the prevalence of thrombus is higher in patients with ACS.
4 Summary
The prevalence of cardiovascular disease is growing rapidly, leading to an increasing incidence of ACS. Therefore, it is important to predict individuals at risk of plaque rupture and developing an ACS using novel modalities. Currently, several invasive and noninvasive modalities are under investigation. Coronary angioscopy gives us both plaque characteristics and the presence of thrombus as direct visualized information, which are helpful to assess the effect of preventive treatment and to make a treatment strategy of PCI such as distal protection. However, there are several limitations to perform coronary angioscopy such as the need for an invasive procedure, limited visual field, and induction of myocardial ischemia during the procedure.
Based on clinical findings using invasive imaging modalities such as angioscopy and IVUS, noninvasive screening tests for preventing ACS are developing including MRI detection of plaque inflammation, contrast-enhanced CT for assessment of noncalcified plaques, and positron emission tomography-CT for combined assessment of plaque burden and activity of the inflamed plaques. In the near future, further investigation will develop novel diagnostic procedure for plaque morphology both invasively and noninvasively.
References
Juul-Möller S, Edvardsson N, Jahnmatz B, Rosén A, Sørensen S, Omblus R. Double-blind trial of aspirin in primary prevention of myocardial infarction in patients with stable chronic angina pectoris. The Swedish Angina Pectoris Aspirin Trial (SAPAT) Group. Lancet. 1992;340:1421–5.
Yeghiazarians Y, Braunstein JB, Askari A, Stone PH. Unstable angina pectoris. N Engl J Med. 2000;342:101–14.
Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med. 1992;326(242–50):310–18.
Hamdan A, Assali A, Fuchs S, Battler A, Kornowski R. Imaging of vulnerable coronary artery plaques. Catheter Cardiovasc Interv. 2007;70:65–74.
MacNeill BD, Lowe HC, Takano M, Fuster V, Jang IK. Intravascular modalities for detection of vulnerable plaque: current status. Arterioscler Thromb Vasc Biol. 2003;23:1333–42.
Nakamura M, Nishikawa H, Mukai S, Setsuda M, Nakajima K, Tamada H, Suzuki H, Ohnishi T, Kakuta Y, Nakano T, Yeung AC. Impact of coronary artery remodeling on clinical presentation of coronary artery disease: an intravascular ultrasound study. J Am Coll Cardiol. 2001;37:63–9.
Lee SY, Mintz GS, Kim SY, Hong YJ, Kim SW, Okabe T, Pichard AD, Satler LF, Kent KM, Suddath WO, Waksman R, Weissman NJ. Attenuated plaque detected by intravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2009;2:65–72.
Jang IK, Tearney GJ, MacNeill B, Takano M, Moselewski F, Iftima N, Shishkov M, Houser S, Aretz HT, Halpern EF, Bouma BE. In vivo characterization of coronary atherosclerotic plaque by use of optical coherence tomography. Circulation. 2005;111:1551–5.
Falk E, Shah PK, Fuster V. Coronary plaque disruption. Circulation. 1995;92:657–71.
Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, Fayad Z, Stone PH, Waxman S, Raggi P, Madjid M, Zarrabi A, Burke A, Yuan C, Fitzgerald PJ, Siscovick DS, de Korte CL, Aikawa M, Juhani Airaksinen KE, Assmann G, Becker CR, Chesebro JH, Farb A, Galis ZS, Jackson C, Jang IK, Koenig W, Lodder RA, March K, Demirovic J, Navab M, Priori SG, Rekhter MD, Bahr R, Grundy SM, Mehran R, Colombo A, Boerwinkle E, Ballantyne C, Insull Jr W, Schwartz RS, Vogel R, Serruys PW, Hansson GK, Faxon DP, Kaul S, Drexler H, Greenland P, Muller JE, Virmani R, Ridker PM, Zipes DP, Shah PK, Willerson JT. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–72.
Hayashi T, Kiyoshima T, Matsuura M, Ueno M, Kobayashi N, Yabushita H, Kurooka A, Taniguchi M, Miyataka M, Kimura A, Ishikawa K. Plaque erosion in the culprit lesion is prone to develop a smaller myocardial infarction size compared with plaque rupture. Am Heart J. 2005;149:284–90.
Takano M, Mizuno K, Okamatsu K, Yokoyama S, Ohba T, Sakai S. Mechanical and structural characteristics of vulnerable plaques: analysis by coronary angioscopy and intravascular ultrasound. J Am Coll Cardiol. 2001;38:99–104.
Thieme T, Wernecke KD, Meyer R, Brandenstein E, Habedank D, Hinz A, Felix SB, Baumann G, Kleber FX. Angioscopic evaluation of atherosclerotic plaques: validation by histomorphologic analysis and association with stable and unstable coronary syndromes. J Am Coll Cardiol. 1996;28:1–6.
Ohtani T, Ueda Y, Mizote I, Oyabu J, Okada K, Hirayama A, Kodama K. Number of yellow plaques detected in a coronary artery is associated with future risk of acute coronary syndrome: detection of vulnerable patients by angioscopy. J Am Coll Cardiol. 2006;47:2194–200.
Ueda Y, Ohtani T, Shimizu M, Hirayama A, Kodama K. Assessment of plaque vulnerability by angioscopic classification of plaque color. Am Heart J. 2004;148:333–5.
Uchida Y, Nakamura F, Tomaru T, Morita T, Oshima T, Sasaki T, Morizuki S, Hirose J. Prediction of acute coronary syndromes by percutaneous coronary angioscopy in patients with stable angina. Am Heart J. 1995;130:195–203.
Kubo T, Imanishi T, Takarada S, Kuroi A, Ueno S, Yamano T, Tanimoto T, Matsuo Y, Masho T, Kitabata H, Tanaka A, Nakamura N, Mizukoshi M, Tomobuchi Y, Akasaka T. Implication of plaque color classification for assessing plaque vulnerability: a coronary angioscopy and optical coherence tomography investigation. JACC Cardiovasc Interv. 2008;1:74–80.
Okada K, Ueda Y, Takayama T, Honye J, Komatsu S, Yamaguchi O, Li Y, Yajima J, Takazawa K, Nanto S, Saito S, Hirayama A, Kodama K. Influence of achieved low-density lipoprotein cholesterol level with atorvastatin therapy on stabilization of coronary plaques: sub-analysis of the TWINS study. Circ J. 2012;76:1197–202.
Kodama K, Komatsu S, Ueda Y, Takayama T, Yajima J, Nanto S, Matsuoka H, Saito S, Hirayama A. A Stabilization and regression of coronary plaques treated with pitavastatin proven by angioscopy and intravascular ultrasound–the TOGETHAR trial. Circ J. 2010;74:1922–8.
Okada K, Ueda Y, Oyabu J, Ogasawara N, Hirayama A, Kodama K. Plaque color analysis by the conventional yellow-color grading system and quantitative measurement using LCH color space. J Interv Cardiol. 2007;20:324–34.
Ishibashi F, Mizuno K, Kawamura A, Singh PP, Nesto RW, Waxman S. High yellow color intensity by angioscopy with quantitative colorimetry to identify high-risk features in culprit lesions of patients with acute coronary syndromes. Am J Cardiol. 2007;100:1207–11.
Mizuno K, Satomura K, Miyamoto A, Arakawa K, Shibuya T, Arai T, Kurita A, Nakamura H, Ambrose JA. Angioscopic evaluation of coronary-artery thrombi in acute coronary syndromes. N Engl J Med. 1992;326:287–91.
Lehmann KG, van Suylen RJ, Stibbe J, Slager CJ, Oomen JA, Maas A, di Mario C, deFeyter P, Serruys PW. Composition of human thrombus assessed by quantitative colorimetric angioscopic analysis. Circulation. 1997;96:3030–41.
Abela GS, Eisenberg JD, Mittleman MA, Nesto RW, Leeman D, Zarich S, Waxman S, Prieto AR, Manzo KS. Detecting and differentiating white from red coronary thrombus by angiography in angina pectoris and in acute myocardial infarction. Am J Cardiol. 1999;83:94–7.
Uchida Y, Uchida Y, Sakurai T, Kanai M, Shirai S, Morita T. Characterization of coronary fibrin thrombus in patients with acute coronary syndrome using dye-staining angioscopy. Arterioscler Thromb Vasc Biol. 2011;31:1452–60.
Hong MK, Mintz GS, Lee CW, Kim YH, Lee SW, Song JM, Han KH, Kang DH, Song JK, Kim JJ, Park SW, Park SJ. Comparison of coronary plaque rupture between stable angina and acute myocardial infarction: a three-vessel intravascular ultrasound study in 235 patients. Circulation. 2004;110:928–33.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Ueno, M., Miyazaki, S. (2015). Acute Coronary Syndrome vs. Stable Angina Pectoris: Angioscopic Point of View. In: Mizuno, K., Takano, M. (eds) Coronary Angioscopy. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55546-9_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-55546-9_10
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55545-2
Online ISBN: 978-4-431-55546-9
eBook Packages: MedicineMedicine (R0)