Abstract
Calcium (Ca) is an essential element for plant growth, as calcium deficiency causes various disorders in some types of horticultural crops. The most significant calcium deficiency disorder is blossom-end rot (BER) of fruit vegetables. In tomato (Solanum lycopersicum), one of the most important vegetables in the world, the incidence of BER often becomes a serious problem in agricultural production and results in financial losses. The typical external symptoms of BER in tomato are water-soaked tissues, necrosis, and discoloring of tissues in the distal portion of the fruit. BER develops in the necrotic region of the parenchymal tissue surrounding young seeds and the distal placenta in the internal tissue of the fruit. The symptoms and causes of BER have been extensively studied, and BER is assumed to be related to Ca deficiency of the fruit. Here, we reviewed symptoms and physiological mechanisms of BER that are related to Ca concentration in fruit tissue and focus on recent molecular genetic research on tomato BER.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
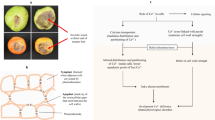
Calcium (Ca) is an essential element for plants: it maintains the integrity of the plasma membrane, the structure of the cell wall, and is involved in intracellular signaling (White and Broadley 2003; Hepler 2005). Ca deficiency induces various physiological disorders such as bitter pit in apple (Malus domestica), black heart in celery (Apium graveolens var. dulce), tip burn in leafy vegetables, and cracking in tomato (Solanum lycopersicum), apple, and cherry (Prunus spp.) fruit (Kirkby and Pilbeam 1984; White and Broadley 2003). These physiological disorders induce severe economic and productivity losses. In fruit vegetables, such as tomato, blossom-end rot (BER) at the distal portion of the fruit is caused by Ca deficiency. The typical symptoms of BER in tomato are necrosis and discoloring of tissues in the distal portion of the fruit (Fig. 9.1).
Blossom-end rot (BER) in tomato fruit. a Induction of BER occurs within 2 weeks after fruit set, and the distal portion of BER fruit exhibits necrosis and discoloring during the early stages of fruit development. b BER enhances softening, causes premature ripening, results in small fruit, and exhibits water-soaked symptoms at the distal portion of the fruit. c A necrotic region also develops in the internal parenchymal tissue surrounding the young seeds and in the distal placenta
The incidence of BER is also observed in other fruit vegetables such as bell pepper (Capsicum annuum), watermelon (Citrullus lanatus), and eggplant (Solanum melongena) (Taylor and Locascio 2004; Aktas et al. 2005; Silber et al. 2005). BER symptoms in bell pepper are enhanced by reactive oxygen species (ROS) production in the apoplast at the most sensitive stage to BER. BER symptoms in bell pepper are suppressed by manganese (Mn), which inhibits ROS production (Aktas et al. 2005). A negative correlation between the incidence of BER and fruit Mn concentrations has been reported in bell pepper (Silber et al. 2005). Therefore, Mn concentration may be related to the incidence of BER in bell pepper fruit, but further studies are needed to demonstrate this hypothesis (Silber et al. 2005).
Several previous studies about BER have focused on tomato fruit. Tomato is one of the most important vegetables in the world, and its global production is increasing. The incidence of BER results in a 50 % loss in the worldwide tomato production (Taylor and Locascio 2004), and thus induces serious problems in agricultural productions and results in financial losses. The incidence of BER is related to environmental factors such as high salinity and high temperature (Taylor and Locascio 2004). Salinity treatment or water deficiency to increase the soluble solids content of tomato often induces BER in fruit (Saito et al. 2006). BER is believed to be a Ca deficiency disorder in tomato fruit because of the high incidence of BER during plant growth under low-Ca conditions, the low tissue Ca content in fruit with BER, and the reduced incidence of BER after spraying plants with Ca (Ho et al. 1993; White and Broadley 2003; Ho and White 2005). However, fruits with BER occasionally contain equal or higher Ca content than that of healthy fruits (Saure 2001). Therefore, high Ca content in the fruit tissue is not always critical in the prevention of the incidence of BER. In this chapter, we describe the physiological mechanisms of BER incidence that relate to Ca concentration in the fruit tissue and the molecular genetics of tomato BER.
2 BER Symptom Development
Initial BER symptoms are membrane leakage of cell solutes, cell plasmolysis, and membrane breakdown (Saure 2001; Ho and White 2005; De Freitas et al. 2011). Subsequently, the fruit surface exhibits water-soaked symptoms, and the tissue at the distal portion of the fruit becomes discolored and necrotic. BER enhances fruit softening in tomato and bell pepper, causes premature ripening, and results in small fruit (Aktas et al. 2005). In the internal tissue of the fruit, BER develops in the necrotic region of the parenchymal tissue surrounding young seeds and in the distal placenta (Adams and Ho 1992; Ho and White 2005).
BER of tomato fruit is considered to be the result of Ca deficiency, and especially the concentration of Ca is reportedly low in the distal portion of the fruit (Bradfield and Guttridge 1984; Adams and Ho 1992). BER is induced within 2 weeks after fruit set when fruit cell expansion is most rapid (Saure 2001; De Freitas et al. 2011). The results suggest that, when the fruit grows rapidly and demands Ca, an insufficient Ca supply to cells in rapidly developing tissue causes BER despite a sufficient Ca concentration in the whole fruit (Ho and White 2005). The incidence of BER is related to the daily irradiance and temperature, which controls fruit cell expansion (Ho et al. 1993). A previous study showed that plum tomatoes are more susceptible to BER than round tomatoes, and BER is never observed in cherry tomatoes or wild relatives of tomato (Ho and White 2005). Therefore, it is important to consider the genetics of BER incidence as well as the environmental effects on the induction of BER.
3 Calcium Movement and the Incidence of BER in Tomato Fruit
As already stated, BER in tomato fruit is induced by Ca deficiency in cells of the distal portion of the fruit within 2 weeks after pollination. Ca is transported through the xylem (Michael and Kirkby 1979; Kirkby and Pilbeam 1984; Jeschke and Pate 1991). Tomato cultivars susceptible to BER have a lower capacity for Ca transport through the fruit xylem network than nonsusceptible cultivars (Belda et al. 1996). As fruit cell expansion advances, the density of xylem vessels decreases in the distal portion of fruit where there are fewer and narrower xylem vessels compared to the proximal end (Belda and Ho 1993; Ho et al. 1993; Belda et al. 1996). The xylem/phloem ratio also decreases toward the distal end of the fruit (Ho and White 2005). These findings strongly suggest that low Ca transport capacity to the distal end of fruit may cause BER.
Environmental factors that reduce Ca fluxes into the developing fruit may induce BER, such as high canopy transpiration rates diverting the xylem stream preferentially to leaves and high electrical conductivity (EC) impairing xylem development within the fruit (Ho and White 2005). BER is also induced by other growing conditions such as low Ca or low phosphorus (P) supply, high magnesium (Mg), high nitrogen (N), high potassium (K), or high salinity, drought or waterlogging in the root zone, and low humidity or high light and temperature in the shoot environment (Ho and White 2005). These conditions that induce fruit BER limit absorption and transition of Ca (or Ca2+) from the root zone.
Some Ca2+ transport proteins occur in plant cells (Fig. 9.2), and studies on these proteins have progressed in Arabidopsis thaliana (Mäser et al. 2001). Cation exchangers (CAXs) are the integral membrane proteins that transport Ca2+ using the proton (H+) or sodium (Na+) gradient generated by primary transporters. They are mainly localized in the vacuole membrane and transport Ca2+ into the vacuole (Hirschi et al. 1996; Cheng et al. 2005; Shigaki et al. 2006; Manohar et al. 2011). Ca2+-ATPases are integral membrane proteins mainly localized in the plasma membrane and actively transport Ca2+ to the apoplast against substantial concentration gradients in plant cells using ATP (Axelsen and Palmgren 2001; White and Broadley 2003). Calcium-permeable channels (Ca2+ channels) are localized in the plasma membrane (White 2000; White and Broadley 2003). Knowledge about these Ca2+ transport proteins in fruit crops is poor, and further genetic and physiological studies of these transport proteins in fruits may reveal novel findings regarding the incidence of BER that relates to Ca2+ movement.
Summary of Ca2+ transport system in plant cells based on White and Broadley (2003) and Manohar et al. (2011). Cation exchangers (CAXs) are integral membrane proteins that transport Ca2+ using the H+ or Na+ gradient. Plant CAXs are mainly localized in the vacuole membrane and transport Ca2+ into the vacuole. Ca2+-ATPases are mainly localized in the plasma membrane and actively transport Ca to the apoplast against substantial concentration gradients using ATP. Calcium-permeable channels (Ca2+ channel) are localized in the vacuole and plasma membrane
4 Stabilization of the Plasma Membrane with Ca2+
A cellular mechanism for the induction of BER by Ca2+ deficiency is described in this section. The largest Ca2+ pool in plant tissue is in the cell wall, where at least 60 % of the total Ca2+ content is found (Demarty et al. 1984). Higher membrane leakage is observed during an early stage of BER incidence and is responsible for the deficiency of apoplastic free Ca2+ (Clarkson and Hanson 1980; Kirkby and Pilbeam 1984; Hirschi 2004). Apoplastic free Ca2+ stabilizes the plasma membrane by bridging phosphate and carboxylate groups of phospholipids and proteins at the membrane surface (Clarkson and Hanson 1980; Kirkby and Pilbeam 1984; Hirschi 2004). The apoplastic level of Ca2+ is maintained at certain thresholds to prevent excessive membrane leakiness and damage (Kirkby and Pilbeam 1984; Picchioni et al. 1998).
Transgenic tomato that overexpresses CAX (sCAX1) from A. thaliana shows an enhanced BER development compared to that of nontransgenic plants (Park et al. 2005a; De Freitas et al. 2011). This study revealed that the sCAX1-expressing fruit reduces cytosolic and apoplastic Ca2+ concentrations, affecting the plasma membrane structure, and leads to the development of BER symptoms in the fruit tissue, although water-soluble Ca concentrations in sCAX1-expressing fruit tissue are higher than those in a nontransgenic control (De Freitas et al. 2011). Pectin methylesterases (PMEs), which increases the Ca2+ bound to the cell wall, also affect apoplastic Ca2+ concentrations. Apoplastic water-soluble Ca2+ was increased, and membrane leakage and BER incidence were decreased by suppressing PME gene expression in transgenic tomato fruit (De Freitas et al. 2012). These reports indicate that BER could be triggered by low concentration of apoplastic Ca2+, which stabilizes the plasma membrane.
5 Genes Affecting the Incidence of BER
The incidence of BER can be regulated by changing apoplastic Ca2+ concentrations in transgenic tomato plants. However, few studies have been performed on the isolation of endogenous genes affecting the incidence of BER. As already stated, the incidence of BER could be related to rapid cell expansion, resulting in a low concentration of apoplastic Ca2+ in the distal portion of tomato fruit within 2 weeks after anthesis. Because this early stage of fruit development is regulated by auxins and gibberellins (Vriezen et al. 2008), the analysis of auxin- and gibberellin-related genes such as cell wall-modifying proteins would be useful for indentifying the relationship between early fruit development and BER incidence (Catalá et al. 2000).
Ca2+ transport proteins such as CAXs and Ca2+-ATPase (White and Broadley 2003) could be involved in the incidence of BER. Overexpression of the Arabidopsis CAX gene in tomato, carrot, and potato increases calcium content in edible parts (Park et al. 2004, 2005a, b; De Freitas et al. 2011), and that in petunia enhances cadmium tolerance and accumulation (Wu et al. 2011). Overexpression of the Arabidopsis CAX gene in tomato also increases the incidence of BER, as described. These studies used the Arabidopsis CAX gene; however, the information on endogenous CAX gene in fruit crops is inadequate. Because whole-genome sequencing has been previously reported in tomato (The Tomato Genome Consortium 2012), the relationship between tomato CAX genes and BER incidence can be investigated efficiently based on the Arabidopsis CAX family functional classification information (Mäser et al. 2001).
Because wild species of tomato are generally stress tolerant and the occurrence of BER has not been reported (Ho and White 2005), wild species may have genes that inhibit the incidence of BER. Genetic variation in wild species is valuable for breeding modern cultivars, in which natural biodiversity has been lost from domestication. However, it is difficult to evaluate the agriculturally important traits of wild species because most of the useful traits are quantitative, and genetic variation in wild species has a negative effect on agricultural productivity.
To resolve these problems, introgression lines (ILs), which contain genomic segments of Solanum pennellii LA716 replaced by homologous regions in the background of the cultivated tomato S. lycopersicum “M82”, have been developed (Eshed et al. 1992; Eshed and Zamir 1994). Solanum pennellii, a wild species with small green fruit, has some useful traits (Eshed and Zamir 1995; Eshed et al. 1996). Seventy-six tomato ILs, which cover the entire genome, lack most negative traits of wild species and are useful for evaluating individual quantitative trait loci (QTLs) (Lippman et al. 2007). Some useful genes that relate to fruit sugar concentration and fruit weight have been isolated using these lines (Frary et al. 2000; Fridman et al. 2004; Gur et al. 2010). ILs and DNA markers are also available for pepper QTL analyses (Thabuis et al. 2004; Zygier et al. 2005; Eggink et al. 2014). Thus, ILs are potentially useful for genetic studies on the incidence of BER, which is reportedly controlled by a QTL.
The incidence of BER in IL8-3, one of the ILs that carries a S. pennellii chromosome segment on chromosome 8 of M82, is reportedly lower than that in independently cultivated M82 (Fig. 9.3) (Uozumi et al. 2012). The gene locus affecting the incidence of BER has been mapped to a narrow region on chromosome 8, which is covered by two BAC clones. The isolation of BER tolerance genes using ILs is expected in the near future. It is also important to explore further genetic material other than ILs using the S. pennellii chromosome.
Incidence of blossom-end rot (BER) in Solanum lycopersicum “M82” and the tomato introgression line “IL8-3” (Uozumi et al. 2012). Percentage of fruit with BER per total number of fruits is shown for the M82 and IL8-3 lines. About 65 % of the total fruits exhibited BER symptoms in M82; IL8-3 exhibited <30 %. Each value represents the mean and standard error (SE; n = 11) in the first, second, third, and total inflorescences. Values with ** and * of IL8-3 are significantly different compared with M82 at P < 0.01 and 0.05, respectively, by the t test
6 Conclusion
BER is observed in various fruit vegetables such as tomato, bell pepper, watermelon, and eggplant. Although BER is a well-known physiological disorder of tomato fruit induced by calcium deficiency, it may occur despite a sufficient Ca content in the fruit because BER is related to Ca movement determined by xylem vessels in the fruit. BER is induced by insufficient Ca in the distal portion of the fruit tissue and occurs within 2 weeks after anthesis when fruit cell expansion is most rapid. Therefore, the incidence of BER is affected by daily irradiance and temperature, which affect fruit cell expansion as well as Ca supply. Studies using transgenic tomato indicated that BER could be triggered by a low concentration of apoplastic Ca2+, which stabilizes the plasma membrane. A QTL for BER tolerance has been identified using ILs that contains a S. pennellii chromosome segment. The tomato genome project has recently been completed, and the entire genome sequence is now available (The Tomato Genome Consortium 2012). A huge quantity of information on tomato and its wild relatives has been revealed with next-generation sequencing technology (Koenig et al. 2013). Therefore, progress on genetic analysis and isolation of genes related to BER is expected in Solanaceae fruit crops.
References
Adams P, Ho LC (1992) The susceptibility of modern tomato cultivars to blossom-end rot in relation to salinity. J Hortic Sci 67:827–839
Aktas H, Karni L, Chang D-C, Turhan E, Bar-Tal A, Aloni B (2005) The suppression of salinity-associated oxygen radicals production, in pepper (Capsicum annuum) fruit, by manganese, zinc and calcium in relation to its sensitivity to blossom-end rot. Physiol Plant 123:67–74
Axelsen KB, Palmgren MG (2001) Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol 126:696–706
Belda R, Ho LC (1993) Salinity effects on the network of vascular bundles during tomato fruit development. J Hortic Sci 68:557–564
Belda RM, Fenlon JS, Ho LC (1996) Salinity effects on the xylem vessels in tomato fruit among cultivars with different susceptibilities to blossom end rot. J Hortic Sci 71:173–179
Bradfield EG, Guttridge CG (1984) Effects of night-time humidity and nutrient solution concentration on the calcium content of tomato fruit. Sci Hortic 22:207–217
Catalá C, Rose JKC, Bennett AB (2000) Auxin-regulated genes encoding cell wall-modifying proteins are expressed during early tomato fruit growth. Plant Physiol 122:527–534
Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138:2048–2060
Clarkson DT, Hanson JB (1980) The mineral nutrition of higher plants. Annu Rev Plant Physiol 31:239–298
De Freitas ST, Padda M, Wu Q, Park S, Mitcham EJ (2011) Dynamic alternations in cellular and molecular components during blossom-end rot development in tomatoes expressing sCAX1, a constitutively active Ca2+/H+ antiporter from Arabidopsis. Plant Physiol 156:844–855
De Freitas ST, Handa AK, Wu Q, Park S, Mitcham EJ (2012) Role of pectin methylesterases in cellular calcium distribution and blossom-end rot development in tomato fruit. Plant J 71:824–835
Demarty M, Morvan C, Thellier M (1984) Calcium and the cell wall. Plant Cell Environ 7:441–448
Eggink PM, Tikunov Y, Maliepaard C, Haanstra JP, de Rooij H, Vogelaar A, Gutteling EW, Freymark G, Bovy AG, Visser RG (2014) Capturing flavors from Capsicum baccatum by introgression in sweet pepper. Theor Appl Genet 127:373–390
Eshed Y, Zamir D (1994) A genomic library of Lycopersicon pennellii in L. esculentum: a tool for fine mapping of genes. Euphytica 79:175–179
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield associated QTL. Genetics 141:1147–1162
Eshed Y, Abu-Abied M, Zamir D (1992) Lycopersicon esculentum lines containing small overlapping introgressions from L. pennellii. Theor Appl Genet 83:1027–1034
Eshed Y, Gera G, Zamir D (1996) A genome-wide search for wild species alleles that increase horticultural yield of processing tomatoes. Theor Appl Genet 93:877–886
Frary A, Grandillo S, Knaap E, Cong B, Liu J, Meller J, Elber R, Alpert KB, Tanksley SD (2000) fw2.2: a quantitative trait locus key to the evolution of tomato fruit size. Science 289:85–88
Fridman E, Carrari F, Liu YS, Fernie AR, Zamir D (2004) Zooming in on a quantitative trait for tomato yield using interspecific introgressions. Science 305:1786–1789
Gur A, Osorio S, Fridman E, Fernie AR, Zamir D (2010) hi2-1, a QTL which improves harvest index, earliness and alters metabolite accumulation of processing tomatoes. Theor Appl Genet 121:1587–1599
Hepler PK (2005) Calcium: a central regulator of plant growth and development. Plant Cell 17:2142–2155
Hirschi KD (2004) The calcium conundrum. Both versatile nutrient and specific signal. Plant Physiol 136:2438–2442
Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci USA 93:8782–8786
Ho LC, White PJ (2005) A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann Bot 95:571–581
Ho LC, Belda R, Brown M, Andrews J, Adams P (1993) Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. J Exp Bot 44:509–518
Jeschke WD, Pate JS (1991) Cation and chloride partitioning through xylem and phloem within the whole plant of Ricinus communis L. under conditions of salt stress. J Exp Bot 42:1105–1116
Kirkby EA, Pilbeam DJ (1984) Calcium as a plant nutrient. Plant Cell Environ 7:397–405
Koenig D, Jimenez-Gomez JM, Kimura S, Fulop D, Chitwood DH, Headland LR, Kumar R, Covington MF, Devisetty UK, Tat AV, Tohge T, Bolger A, Schneeberger K, Ossowski S, Lanz C, Xiong G, Taylor-Teeples M, Brady SM, Pauly M, Weigel D, Usadel B, Fernie AR, Peng J, Sinha NR, Maloof JN (2013) Comparative transcriptomics reveals patterns of selection in domesticated and wild tomato. Proc Natl Acad Sci USA 110:E2655–E2662
Lippman ZB, Semel Y, Zamir D (2007) An integrated view of quantitative trait variation using tomato interspecific introgression lines. Curr Opin Genet Dev 17:545–552
Manohar M, Shigaki T, Hirschi KD (2011) Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol 13:561–569
Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJM, Sanders D, Harper JF, Tchieu J, Gribskov M, Persans MW, Salt DE, Kim SA, Guerinot ML (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126:1646–1667
Michael JA, Kirkby EA (1979) Estimation of potassium recirculation in tomato plants by comparison of the rates of potassium and calcium accumulation in the tops with their fluxes in the xylem stream. Plant Physiol 63:1143–1148
Park S, Kim CK, Pike LM, Smith RH, Hirschi KD (2004) Increased calcium in carrots by expression of an Arabidopsis H+/Ca2+ transporter. Mol Breed 14:275–282
Park S, Cheng NH, Pittman JK, Yoo KS, Park J, Smith RH, Hirschi KD (2005a) Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiol 139:1194–1206
Park S, Kang TS, Kim CK, Han JS, Kim S, Smith RH, Pike LM, Hirschi KD (2005b) Genetic manipulation for enhancing calcium content in potato tuber. J Agric Food Chem 53:5598–5603
Picchioni GA, Watada AE, Conway WS, Whitaker BD, Sams CE (1998) Postharvest calcium infiltration delays membrane lipid catabolism in apple fruit. J Agric Food Chem 46:2452–2457
Saito T, Fukuda N, Nishimura S (2006) Effects of salinity, planting density and lateral shoot leaves under truss on yield and total soluble solids of tomato fruits grown in hydroponics. Hortic Res 5:415–419
Saure MC (2001) Blossom-end rot of tomato (Lycopersicon esculentum Mill.) a calcium or a stress related disorder? Sci Hortic 90:193–208
Shigaki T, Rees I, Nakhleh L, Hirschi KD (2006) Identification of three distinct phylogenetic groups of CAX cation/proton antiporters. J Mol Evol 63:815–825
Silber A, Bruner M, Kenig E, Reshef G, Zohar H, Posalski I, Yehezkel H, Shmuel D, Cohen S, Dinar M, Matan E, Dinkin I, Cohen Y, Karni L, Aloni B, Assouline S (2005) High fertigation frequency and phosphorus level: effects on summer-grown bell pepper growth and blossom-end rot incidence. Plant Soil 270:135–146
Taylor MD, Locascio SJ (2004) Blossom-end rot: a calcium deficiency. J Plant Nutr 27:123–139
Thabuis A, Palloix A, Servin B, Daubèze AM, Signoret P, Hospital F, Lefebvre V (2004) Marker-assisted introgression of 4 Phytophthora capsici resistance QTL alleles into a bell pepper line: validation of additive and epistatic effects. Mol Breed 14:9–20
The Tomato Genome Consortium (2012) The tomato genome sequence provides insights into fleshy fruit evolution. Nature (Lond) 485:635–641
Uozumi A, Ikeda H, Hiraga M, Kanno H, Nanzyo M, Nishiyama M, Kanahama K, Kanayama Y (2012) Tolerance to salt stress and blossom-end rot in an introgression line, IL8-3, of tomato. Sci Hortic 138:1–6
Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C (2008) Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol 177:60–76
White PJ (2000) Calcium channels in higher plants. Biochim Biophys Acta 1465:171–189
White PJ, Broadley MR (2003) Calcium in plants. Ann Bot 92:487–511
Wu Q, Shigaki T, Williams KA, Han JS, Kim CK, Hirschi KD, Park S (2011) Expression of an Arabidopsis Ca2+/H+ antiporter CAX1 variant in petunia enhances cadmium tolerance and accumulation. J Plant Physiol 168:167–173
Zygier S, Chaim AB, Efrati A, Kaluzky G, Borovsky Y, Paran I (2005) QTLs mapping for fruit size and shape in chromosomes 2 and 4 in pepper and a comparison of the pepper QTL map with that of tomato. Theor Appl Genet 111:437–445
Acknowledgment
The authors thank Ms. Ai Uozumi for providing the picture in Fig. 9.1.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Japan
About this chapter
Cite this chapter
Ikeda, H., Kanayama, Y. (2015). Blossom-End Rot in Fruit Vegetables. In: Kanayama, Y., Kochetov, A. (eds) Abiotic Stress Biology in Horticultural Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55251-2_9
Download citation
DOI: https://doi.org/10.1007/978-4-431-55251-2_9
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55250-5
Online ISBN: 978-4-431-55251-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)