Abstract
Blossom end rot (BER) is mainly a calcium (Ca2+) deficiency-related physiological disorder of fruits that affects various crop production worldwide including tomato (Solanum lycopersicum). The visible symptoms of BER include cell wall disintegration, cell plasmolysis, and water-soaked signs at the distal end of the fruits. During fruit development increase in cell expansion and a decrease in the transport of Ca2+ to the distal part of the fruits, can lead to the development of BER. It is hypothesized that insufficient Ca2+ is available for essential apoplastic and cytoplasmic functions during the cell expansion phase of fruits when the cellular Ca2+ demand exceeds the Ca2+ supply. Therefore, abnormal intracellular Ca2+ content or signals, cause weakening of cell walls, and a loss of cellular integrity, potentially leading to cell death and the outward manifestations of BER. The occurrence of BER in tomatoes is also influenced by environmental factors that affect the cellular growth of the fruits. These factors such as drought, high salinity, high temperature, insufficient xylem tissue development, phytohormones, and oxidative stress can influence the development of BER. The availability of a high-quality reference genome and whole genome sequencing allowed us to identify selected loci that can cause BER, facilitating genetic dissection and a deeper comprehension of the molecular mechanisms underlying this disorder. This review summarized the various factors and genes involved in BER development and management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, as the world’s population continuously grows, the demand for food is proportionally increasing (Lobell et al. 2009). Several agriculture-based companies and farmer's incomes depend on the production of edible crops, mainly from leguminous and solanaceous plants. One of the most common solanaceous edible fruits, the tomato (Solanum lyopersicum), is prevalent due to its high carotenoid content and antioxidant properties. Several physiological factors affect the growth and productivity of tomato crops, including biotic and abiotic stresses that can negatively impact yield and result in financial losses (Atkinson et al. 2011; Jolánkai et al. 2016).
BER is a physiological disorder that is influenced by multiple factors, affecting various vegetable crops such as eggplant (Solanum melongena L.) watermelon (Citrullus lanatus (Thunb.), pepper (Capsicum annuum L.), and tomato (Solanum lycopersicum L.) (Taylor and Locascio 2004; Zhang et al. 2019). BER can grow in any tomato-producing region of the world and has been shown to cause 50% yield reductions. BER symptoms first appear as a water-soaked area at the fruit's blossom end, typically, the area develops quickly, resulting in a blackened, dry, sunken leathery spot (Fig. 1a) (Ho and White 2005; Topcu et al. 2022). BER can also affect the placenta's blossom end, the surrounding locular contents, and the outside wall. There is a limited variation of the disorder that manifests as an internal browning or blackening of the internal tissue within the adjacent parenchyma surrounding the immature seeds and the distal portion of the placenta (Ho and White 2005). These disorders primarily affect the fruits, but they can also damage other organs like leaves and flower buds (Barta and Tibbitts 2000; Francois et al. 2019). A variety of leafy vegetables experience tip-burn due to defective Ca2+ distribution (Kuo et al. 1981; Su et al. 2019). Other vegetables, including celery and cauliflower, are also affected by illnesses that resemble BER (Aloni and Pressman 1987; Bianco et al. 2015). Bitter pit is a similar type of disorder that affects apple fruits (de Freitas et al. 2010).
a BER symptoms at the distal end of tomato fruits. The tomato fruit displays a sunken, leathery patch with a dark brown color as a sign of external blossom end rot (BER), marked with arrows b Symplasts are formed by connecting neighboring cells through plasmodesmata, whereas apoplasts are formed by extracellular gaps that exist between the cell walls of neighboring cells c A schematic model illustrating the basic role of Ca2+ in cells and how a defect/stress/change in normal cellular functioning leads to the development of Ca2+ deficiency-related physiological disorders
BER disorder was originally described as “Black rot” and was poorly understood and thought to be caused by a parasitic organism from the late 1800s until the early 1940s (Taylor and Locascio 2004). However, the initial evidence indicated that BER is a Ca2+ deficiency disorder based on the following data: (i) BER usually occurs when the fruit's Ca2+ content is less throughout its tissues and (ii) the incidence of BER was significantly decreased when Ca2+ fertilizer and sprays were applied to field and greenhouse tomatoes (Ho et al. 1993).
The total amount of Ca2+ concentration present on the earth's crust is approximately 3.6% while soils contain 1.37%, both play an important role in soil maintenance and stability (Hawkesford et al. 2011). Ca2+ is a key nutrient required for the cell wall structure, signalling and stress response, cell growth, plant tissue development, and membrane stability (Dodd et al. 2010). Plants primarily absorb Ca2+ from the soil and transfer it to the xylem of the root tissue and further transport in fruit or leaf tissue occurs via both apoplastic (extracellular space between cells) and symplastic (neighboring cells connected by plasmodesmata) pathways (Yang and Jie 2005) (Fig. 1b). In cells and subcellular organelles, Ca2+ uptake and transport are mainly facilitated by Ca2+ channels, Ca2+-ATPase, and Ca2+/H+ antiporter, so Ca2+ concentration at the cellular and extracellular levels will be highly regulated (Yang and Jie 2005; Tuteja and Mahajan 2007; Stael et al. 2012; Pathak et al. 2021). It has been demonstrated that greater free Ca2+ in the apoplastic pool (1-10 mM) is necessary to maintain proper membrane structure and function, but cytosolic Ca2+ levels are maintained at low concentrations (less than 1 mM) in comparison to the apoplastic and organelle pools (Hanson 1960; Plieth 2001). An increase in the basal Ca2+ concentration may result in anomalies in development and growth, increased rigidity of the fruit cell wall, and cellular toxicity while reduced basal Ca2+ concentrations have been linked to the breakdown of fruit cell walls and the emergence of many physiological diseases (Hocking et al. 2016). Ca2+ treatment could improve the fruit quality and yield in the postharvest Ca2+ treatment which reduces the physiological disorders (Irfan et al. 2013; Madani et al. 2016; Zhang et al. 2019).
Tomato fruit growth and development are predominantly associated with the cytosolic Ca2+ concentration. Several studies signify that the main cause of BER is a localized deficiency of Ca2+ in the distal fruit tissue, due to defective local calcium transport or imbalanced incorporation of Ca2+ with the pectin, a cell wall component (Adams and Ho 1993; Taylor and Locascio 2004; de Freitas et al. 2012a). During cellular growth, mainly two types of cellular processes regulate the effective concentration of cytoplasmic Ca2+ in cells. The first involves Ca2+ distribution and partition in the cell and apoplast using transporters, secondly, Ca2+ can create a link with cell wall components pectin, improving cell wall mechanical strength. However, in conditions of stress or Ca2+ deprivation, the functions of these two pathways are compromised, resulting in a failure in Ca2+ partitioning, decreased membrane selective permeability, and cellular content leakage, which influences the incidence of BER (Fig. 1c). The difference in Ca2+ concentrations between the proximal and distal regions of the fruit is linked to the development of BER during fruit growth; larger the difference, higher is the incidence of BER.
Research indicates that in healthy tomato fruit, Ca2+ binding to the cell membrane increases during rapid cell development, whereas in fruit damaged by BER, it decreases (Suzuki et al. 2003). Several studies suggest that even though the Ca2+ concentration in the fruit is adequate when rapidly growing fruit tissue requires Ca2+, an inadequate Ca2+ supply to rapidly growing tissue leads to the development of BER (de Freitas et al. 2018; Ho and White 2005; Ikeda and Kanayama 2015). Fruit exhibiting BER symptoms have much less Ca2+ than comparable unaffected fruits, indicating that fruit produced by plants cultivated in lower Ca2+ environments have a higher incidence of BER than fruit grown in higher Ca2+ environments.
Abiotic stress-induced BER has an influence on tomato output, and ineffective management measures increase the problem in terms of the frequency and severity of BER (Hagassou et al. 2019). The tomato susceptibility to BER also varies among cultivars, generally large-sized tomatoes are more susceptible to BER than small-sized tomatoes (Ho and White 2005). Variability appears to be caused by differences in fruit growth rate, Ca2+ content, and fruit xylem tissue development. Because BER only affects the top truss, which contains the fruits, it affects both green and ripe tomatoes, it is becoming a serious issue in tomato cultivars (Adams and Ho 1992). Abiotic stresses, phytohormones, high salinity, low humidity, high temperature, inadequate xylem tissue development, unfavorable moisture, and oxidative stress are additional factors that contribute to the BER disorder in the distal end of tomato fruits in addition to the Ca2+ deficiency (Taylor and Locascio 2004; Hagassou et al. 2019). This review summarizes the various factors that induce BER in tomato fruit, and different BER management techniques.
Symptoms and Occurrence of BER
The external symptoms of BER in fruit tissue begin with discolored, water-soaked, necrotic tissue at the distal end, which is associated with the collapse of epidermal and subepidermal parenchyma, following turgor loss which ultimately manifests as dark-brown and depressed abscesses on the fruit surface (Spurr 1959; Marcelis and Ho 1999; Ho and White 2005) (Fig. 1a). The first sign or external symptoms of BER in tomato fruits usually occur within two weeks of fruit set, when young fruit’s relative growth rate is at its maximum (Spurr 1959; Adams and El-Gizawy 1988), however, the symptoms of BER in tomato fruit can potentially emerge later in development, at five weeks after pollination (Marcelis and Ho 1999; Rached et al. 2018; de Freitas et al. 2018). Cells in the water-soaked area have interruption of the plasma membrane and tonoplast (vacuolar membrane), wavy-shaped cell walls, broken endoplasmic reticulum, and swollen plastids. BER signs often appear externally on the pericarp near the distal end of the placenta, while they can also persist internally in the tissue and remain undetected (Adams and Ho 1992; Ho and White 2005). It is believed that internal BER is a mild form or an earlier stage of the development of external BER. Both are assumed to be the outcome of cellular death and the spilling of its contents into the extracellular space. After induction, the fruit’s BER-affected patches often get bigger, turn into brown necrotic regions that cover a significant area of the fruit, and occasionally, they even infect the whole fruit. Soon after pollination, the symptoms could get worse and the fruit would never grow to its full size. Cells in the zone of necrotic BER tissue revealed normal internal structure, but the cell membrane was detached from the cell wall, indicating cell plasmolysis, which was not detected in other sections of the fruit or normal fruit cells (Taylor and Locascio 2004; Ho and White 2005).
Calcium Partitioning and Induction of BER
The plant cell wall contains 60–70% of the Ca2+ in plant tissue, making it the largest Ca2+ pool (Aghdam et al. 2012). Ca2+ is a well-known essential nutrient involved in many metabolic processes and signaling in plants. It also functions as a secondary messenger in diverse organisms and is critical for the stability and integrity of the cell wall and cell membrane (Gao et al. 2019). The uptake and transportation of Ca2+ from the soil to the shoot, fruit, and leaves of the plant depends upon the availability of Ca2+ content in the soil. The root takes up Ca2+ and is transported to the shoot and fruit utilizing apoplastic as well as symplastic pathways (Pathak et al. 2021). To cross the Casparian strip, it must enter the cytosol of endodermal cells via Ca2+-channel proteins and then be exported back into the apoplast via Ca2+-ATPases (Pittman et al. 2011; Pandey and Sanyal 2021). In tomatoes, the Ca2+ influx into fruit cells after vascular unloading has been postulated to occur via cyclic nucleotide-gated channel (CNGC) proteins (Thor 2019).
In plants, Ca2+ is utilized to perform mainly three primary functions throughout fruit growth and development (a) the partitioning and distribution of Ca2+ concentration in subcellular compartments, cytosol, and apoplast compartments, controls the activation of various proteins and signaling (b) Ca2+ interacts with the cell wall component pectin, forming a crosslinking that makes cell walls rigid. (c) Ca2+ also contributes significantly to cell membrane integrity by binding proteins and phospholipids in the plasma membrane (Hepler and Winship 2010). Furthermore, various types of biotic or abiotic stresses trigger signaling cascades in which calcium acts as a secondary messenger. Ca2+ enters into the cytosol from higher concentration compartments (apoplast, organelles) via channel proteins to induce an increase in the cytosolic Ca2+ concentration ([Ca2+]cyt) involved in various downstream signaling pathways. However, if Ca2+ is transported out of the cytosol by Ca2+-ATPases or Ca2+/H+-antiporters in the plasma or organelle membranes then it terminates the downstream signaling events (Fig. 2).
Coordination between cytosolic Ca2+ concentration and cellular expansion during tomato fruit development. The various subcellular Ca2+ transporter involved in transport leads to the accumulation or depletion of Ca2+ depending on stimuli in plant cells. Similarly, in the vacuole localized Ca2+-permeable channel, Ca2+-ATPase is involved in increasing cytosolic Ca2+, while CAX (Ca2+/H+ antiporter) is involved in the storage of Ca2+ from the apoplastic region to the vacuole. (1) Treatment with phytohormone causes the production of ROS, which activates hyperpolarization-activated Ca2+ channel (HACC) proteins (denoted by a circled positive symbol) (2) HACC proteins involved in Ca2+ transport from apoplast to cytoplasm region lead to an increase in cytosolic Ca2+ (3) Ca2+ increases the expression of phytohormone responsive, cell wall modifying, and expansin genes (4) Expression of various genes responsible for hydrolyzing the bond between cellulose microfibril and other polysaccharides, causing cell wall loosening (5) Increased cytosolic Ca2+ causes enhanced incorporation of vesicle-containing enzymes required for cell wall synthesis
Because of the poor phloem movement of Ca2+, the transportation of Ca2+ mainly depends upon the xylemic and transpirational water flow (Bukovac and Wittwer 1957; Montanaro et al. 2012). During the early stage of fruit development, Ca2+ plays an important role in cell division, responsible for the formation of cell plates and cellular metabolism. In the subsequent stage of fruit development, Ca2+ plays an important role in cell–cell interaction (Hocking et al. 2016). The accumulation of Ca2+ in tomato fruit is regulated by growth rates and transpiration. The tomato fruit develops at a high rate in its early stages, as compared to later stages, this suggests that Ca2+ is essential for the early stages of fruit development (Gao et al. 2019). Since, the xylem is the in-charge of supplying Ca2+ to aerial sink organs such as fruit, where it accumulates, the fruit may have localized Ca2+ shortages as a result of reduced xylem Ca2+ mobility or an abnormality in xylem development (White 2001; Kumar et al. 2015).
As the fruit develops, xylem vessels transport Ca2+ to the fruit tissues, these vessels are smaller and less in numbers near the fruit's blossom end, than they are near its proximal end. The xylem:phloem ratio also decreases toward the fruit's distal end. Additionally, during the crucial stage of rapid fruit cell expansion, the xylem network in the pericarp tissue grows while just two single working strands remain in the placental tissue. These characteristics are thought to reflect the anatomical origin of the distant placental tissues linked to the initial appearance of BER (Riboldi et al. 2022). Ca2+ therefore contributes to cell growth, and aberrant [Ca2+]cyt signals may arise from a local deficiency of accessible Ca2+. During the later phases of fruit ripening, the phloem plays an important role in the transport of water, sugar, and basic nutritional inputs to most species (Dražeta et al. 2004).
One of the first indications of BER observed in tomato fruit is cell membrane rupture. This could happen if the [Ca2+]apoplast around a cell was depleted too much, or it could be caused by increasing Ca2+ flow into the vacuole, a Ca2+ storage organelle that acts as a Ca2+ sink, demonstrating a strong inverse relationship between the incidence of BER and the apoplastic Ca2+ concentration in the distal half of tomato fruits tissues. Low calcium levels in the plant could lead to necrosis and curling in the leaf, bitter pit, BER, fruit cracking, and other physiological disorders during the development of fruit (Combrink 2013). A previous study suggests that the application of Ca2+ in the grapes enhances the flesh density and decreases the occurrence of Botrytis cinerea in grapes (Ciccarese et al. 2013; Gao et al. 2019). Another study suggested that the altered activity of Ca2+ channels, Ca2+/H+-exchanger, Ca2+-ATPase, and Ca2+ sensors or inappropriate cellular Ca2+ partitioning and distribution in the apoplast of cells in early fruit developmental stages can induce BER (Ho and White 2005; de Freitas and Mitcham 2012).
Ca2+ transport proteins are found in plant cells, and research on these proteins has developed in Arabidopsis thaliana (Hirschi et al. 1996). Ca2+-ATPases are integral membrane proteins found largely in the plasma membrane that transfer Ca2+ to the apoplast in plant cells utilizing ATP despite considerable concentration gradients. CAXs (Cation exchangers) are membrane proteins that transfer Ca2+ in exchange for proton (H+) or sodium (Na+) gradients generated by major transporters, which can disturb Ca2+ homeostasis (Hirschi 1999; Pittman and Hirschi 2001) (Fig. 2). In yeast (Saccharomyces cerevisiae) mutants, two Arabidopsis Ca2+/H+ antiporter genes, CAX1 and CAX2, can act as vacuolar Ca2+ transporters (Hirschi et al. 1996). Arabidopsis thaliana expresses the Ca2+/H+ antiporter CAX1 (Cation Exchanger 1), which is found in the vacuolar membrane. In the sCAX1 encoded version, the full-length gene is truncated at the N-terminus and lacks its regulatory region, resulting in a constitutively active Ca2+/H+-antiporter (de Freitas et al. 2011; Pittman and Hirschi 2001). When sCAX1 was expressed in tomatoes, most of the fruit displayed BER symptoms (Cheng et al. 2005). This is because the overexpression of the sCAX1 gene in the tomato plant causes Ca2+ to move from the apoplastic region to the vacuolar region of the cell, which lowers the Ca2+ level in the apoplastic region and causes membrane disintegration, a BER symptom, even in the presence of a "sufficient" Ca2+ concentration (de Freitas et al. 2011).
Even though there is enough Ca2+ in every part of the plant, transgenic tomato plants that have overexpressed Arabidopsis CAX1 and CAX2 exhibit severe Ca2+ deficiency-like symptoms like tip-burn and/or BER. Previous research has shown that Ca2+/H+-antiporter sCAX2A, a mutant variation of the sCAX2 gene, causes increased Ca2+ accumulation in tomatoes; additionally, the incidence of BER was the same in both the wild-type control line and the sCAX2A-expressing lines (Chung et al. 2010). This conclusion was supported by the fact that numerous transmembrane Ca2+-exchanger genes are involved in Ca2+ transport and the upregulation of one of these genes may cause the downregulation of other genes to preserve the Ca2+ flow into cellular storage organelles.
A similar study created transgenic plants expressing sCAX1 and CAX4 and discovered that the Ca2+ content of the transgenic plants was significantly higher than that of the vector controls, conversely, only the tomatoes expressing sCAX1 had a significantly higher incidence of BER (Park et al. 2005). The sCAX1-expressing tomato had lower apoplastic and cytosolic Ca2+ concentrations than the wild-type tomato due to excessive Ca2+ accumulation in the vacuole. These results clearly showed that elevated sCAX1 expression decreases Ca2+ mobility, which leads to the development of BER in fruits. Through simultaneous expression of sCAX1 and calreticulin (CRT), a Ca2+ binding protein located in endoplasmic reticulum, a study was able to correlate the frequency of Ca2+ accumulation with deficiency disorders in plants (Wu et al. 2012). The sCAX + CRT-expressing and sCAX-expressing lines' Ca2+ concentrations, however, did not differ noticeably. These results showed that sCAX1 + CRT-expressing tobacco and tomato lines exhibited lower levels of Ca2+ deficiency symptoms (leaf tip necrosis and BER) than the line expressing sCAX1 alone. Table 1 provides an overview of research that looked into the genes responsible for transporting Ca2+ and their role in the emergence of disorders related to Ca2+.
Another important component to which Ca2+ contributes is the fruit's cell membrane. The phosphate and carboxylate groups found in the phospholipids and proteins, in the cell membrane provide a bridge that Ca2+ crosses, reducing the permeability of the membrane and boosting its integrity. In strawberries and apricots, Ca2+ therapy delays fruit ripening and reduces cell wall disintegration (Figueroa et al. 2012; H. Liu et al. 2017). When applied to pear fruit, calcium chloride (CaCl2) decreases permeability in the cell membrane, controls metabolism in the cell membrane, and lessens lipid peroxidation in the cell membrane, which is brought on by reactive oxygen species (ROS). Moreover, the efficacy of the plant's various Ca2+ sinks might influence Ca2+ accumulation in the tomato and produce Ca2+-related physiological disorders such as blossom end rot (BER) (Ho and White 2005; Kumar et al. 2015). In conclusion, Ca2+ is a critical nutrient for cell wall structure, cell growth, plant tissue development, and membrane function, and it also acts as a counter-cation inside storage organelles, so Ca2+ concentrations at the cellular and extracellular levels will be highly regulated. Increased Ca2+ concentrations can cause cellular toxicity, increased rigidity of the fruit cell wall, and developmental and growth defects. Ca2+ deficiency can cause fruit cell walls to collapse and the development of a variety of physiological disorders including induction of BER in tomato fruits. In postharvest Ca2+ treatment, which lowers physiological abnormalities, fruit quality, and yield may be improved.
Calcium Interaction with de-Esterified Pectin and Induction of BER
The plant cell wall contains three layers the middle layer or middle lamella, the inner or secondary wall, and the outer layer, also known as the primary wall. The primary cell wall and middle lamella are primarily composed of cellulose microfibril which are crosslinked by branched polysaccharides such as hemicelluloses, and pectins (Cosgrove 2005; Lampugnani et al. 2018). The biosynthesis of pectins takes place in the Golgi apparatus, after that it is transferred to the fruit's cell wall (Lampugnani et al. 2018). The role of Ca2+ is very important for the cell wall during the development of the tomato fruit by binding with the de-esterified pectin which is present in the middle lamella of the plant cell (de Freitas et al. 2012a). During the growth period of the tomato fruit, the secreted pectin undergoes some modification, which is caused by the pectin methyl esterase, after which Ca2+ will interact with the carboxy-terminal of the demethylated pectin, where the interaction of Ca2+ and pectin leads to the thickening of the tomato fruit cell wall, when the interaction of pectin and Ca2+ changes, the stability of the cell wall collapses, resulting in BER.
Pectins are a highly diverse and complex family of heteropolysaccharides that are enriched in α-1,4 galacturonic acid (a sugar acid derived from galactose) subunits as principal components (Harholt et al. 2010). For example, homogalacturonans (HGs) are α-(1–4)-linked D-galacturonic acid linear chains polymer. The other structural type of pectin includes rhamnose/galacturonan disaccharide repeat rhamnogalacturonan-I (Mohnen 2008). The backbone pectin galacturonan residues can be decorated with other oligosaccharides such as α-(1,5)- arabinans and β-(1,4)-galactans with their methyl-esterified or acetyl derivatives. Highly esterified HGs are delivered to the wall where they undergo selective de-esterification, which facilitates polymer crosslinking via Ca2+ (Fig. 3).
Diagrammatic representation illustrating the function of the enzyme pectin methyl esterase in controlling the mechanical strength of cell walls. PMEs can act in two ways: either randomly, activating cell wall hydrolases like polygalacturonases (PG) and pectin lyase to cause the cell wall to loosen, or linearly, creating blocks of free carboxyl groups in homogalacturonan (pectin) that interact with bivalent ions (Ca2+) to cause the cell wall strengthening. The cross-linking and interaction between Ca2+ and the pectin polymer is depicted in an "egg box" model. An expanded version of the "egg box" can be seen in the inset. (Gray hexagon-methylated and, White hexagon-demethylated residue of galacturonic acid)
A wide variety of branching and linear side chain derivatives of pectins are also observed, and they together constitute the pectin structural classes xylogalacturonan, rhamnogalacturonan-I & II, and apiogalacturonan (Zdunek et al. 2021). Rhamnogalacturonan-II is the most complex pectin, with up to 12 different sugar residues and over 20 different connection types. The expression of several enzymes that contribute to pectin modifications such as polygalacturonases and pectin methylesterases during growth and development contributes to its structural complexity, which implies that pectin may have several potential interactions and functional roles in plants (Bonnin et al. 2014). Pectin methylesterases can demethylesterify HGs, which affects cell wall flexibility because this change is required for HG cross-linking to other pectic polysaccharides and cell wall proteins. Pectin methylesterase inhibitors enhance pectin methylesterification and therefore wall loosening by inhibiting pectin methylesterases (Caffall and Mohnen 2009).
Pectin methylesterase (PME) is an enzyme found in the cell wall that modifies the binding of Ca2+ to the pectic acid in the cell wall (Micheli 2001). Pectin methyl esterase causes fluctuations in the amount of pectin secreted by tomato fruits during their growth phase (Micheli 2001). The amount of PME activity and the availability of Ca2+ in the apoplast has a direct effect on the strength and expansion of the cell wall. PMEs de-esterify the pectin polymers found in fruit cell walls. This results in the formation of a negative carboxyl group on which positive Ca2+ will bind. This interaction between Ca2+ and the pectin polymer resembles an "egg-box" which provides stability and integrity to the cell wall (Fig. 3). This cross-linking between the pectin and Ca2+ forms the structural part of the fruit and it also inhibits the BER and pathogen susceptibility in tomato fruit (de Freitas et al. 2012a, b; Hocking et al. 2016; Zhang et al. 2019; Gao et al. 2019). On the other hand, BER results from the collapse of the cell wall due to a change in the interaction between pectin and Ca2+ (de Freitas et al. 2012a). Tomato fruit differs from leaves in that its xylem moves more slowly and experiences less transpiration. This causes the fruit to become deficient in Ca2+, which can result in many physiological illnesses.
A previous study also evaluated the effects of PME expression, the amount of Ca2+ bound to the cell wall, and the esterified pectin concentration on tomato fruit during BER development (de Freitas et al. 2012a). The expression of PME genes (PMEU1, LOC544090, LOC544289, Les.9028, Les.10790, and Les.10560), was analyzed in the tomato fruit (Lycopersicon esculentum) cultivar “Rutgers” and demonstrated that the key period for BER development in tomato fruit is exactly coincident with an increase in PME expression and activity. The research reveals a considerable decrease in the incidence of BER in PME-silenced tomato fruits that have less Ca2+ bound to the cell wall and exhibited higher total and apoplastic water-soluble Ca2+ concentrations in the pericarp. The total tissue, Ca2+ concentrations of wild-type and PME-silenced fruit were equivalent; however, the wild-type fruit had more membrane leakage, one of the early symptoms of BER, and reduced water-soluble apoplastic Ca2+ content. The study's findings suggest that the concentration of apoplastic water-soluble Ca2+ influences fruit sensitivity to Ca2+ deficient illnesses (de Freitas et al. 2012a).
In summary, the [Ca2+]cyt can regulate the rapid cell expansion that occurs during the early stages of tomato fruit growth and development. Because expansion growth is a Ca2+-dependent process that incorporates cell wall biosynthetic material into the cell wall and plasma membrane, a higher [Ca2+]cyt has been linked to growth in a variety of cell types. A simplified and schematic representation of the regulation of cell growth and expansion by apoplastic and cytoplasmic Ca2+ is presented in Fig. 4a. The early stages of tomato fruit growth, when cells expand quickly leads to an increase in the relative volume of their vacuoles. Appropriate maintenance of [Ca2+]apoplast and [Ca2+]cyt, along with regulated solute accumulation that is osmotically active, are necessary for cell expansion. As the osmotically active solutes accumulate, hydrostatic pressure is produced as a result of vacuolar enlargement. In typical circumstances, the [Ca2+]cyt is smaller than the [Ca2+]apoplast. However, the internal Ca2+ level rises during cell expansion due to the cytoplasm's elevated Ca2+ level brought on by the apoplasts' import of Ca2+ into the cytosol and the vacuoles' efflux of Ca2+. The apoplast acidification caused by this elevated Ca2+ causes xyloglucan endo-transglycosylases/hydrolase (XETs) and expansin protein to hydrolyze the link between cellulose microfibril with other polysaccharide components of the cell wall. This leads to a loosening of the cell wall and cell expansion. Pectin methyl esterase promotes pectin de-esterification and cross-linking with Ca2+, which strengthens the cell wall as the wall expands. Pectins also seem to prevent proteins like expansin proteins and xyloglucan endo-transglycosylases/hydrolase (XETs) from accessing their substrates, which can alter cell wall rigidity. Furthermore, as cytosol Ca2+ concentration rises, vesicles containing enzymes and materials for cell wall synthesis are transported from the endoplasmic reticulum to the plasma membrane at an enhanced rate, further strengthening the cell wall (Fig. 4a).
A simplified and schematic illustration of how apoplastic and cytoplasmic Ca2+ regulates tomato fruit cell growth and expansion (a). Cells expand rapidly during the early stages of tomato fruit growth and development, resulting in an increased cellular demand for calcium. Under normal conditions, which govern cell wall loosening and expansion, however, further expansion is controlled by pectin-methyl esterase by de-esterifying and interacting with Ca2+. The schematic model is based on a previous study (Ho and White 2005).. (b) Excessive cell enlargement is caused by defects in the Ca2+ movement between the [Ca2+]cyto and [Ca2+]vacuole, as well as an insufficient apoplastic Ca2+ concentration. As a result of a Ca2+ deficiency in the fruit cells and an ill-formed cell wall, BER develops. The schematic model is based on a previous study (Ho and White 2005)
Ca2+ is associated with abnormal regulation of its partitioning in different cellular compartments during BER development. The binding of Ca2+ to negatively charged lipids maintains the structural integrity of the plasma membrane. In the case of cytosolic depletion of Ca2+, membranes become semi-permeable, resulting in the "leaky" state of charged solutes and cellular death. In addition, rapid fruit growth associated with increased pectin biosynthesis and de-esterification may lead to altered Ca2+ distribution and partitioning within the cell, resulting in lower apoplastic Ca2+ concentrations that damage membrane integrity and structure and increase fruit vulnerability to BER development. Furthermore, the plasma membrane and walls surrounding cells in BER fruit that collapse during rapid cell expansion have lower Ca2+ concentrations (Fig. 4b).
Phytohormones Mediated BER Development
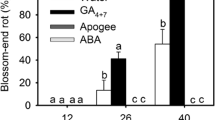
Phytohormones are signal molecules produced by plants in extremely low concentrations. Plant hormones regulate all aspects of plant growth and development, including pathogenic defense and tolerance to stress. Several studies have shown a strong correlation between the occurrence of Ca2+ deficiency disorders in plants, especially in the early stages of fruit development, and phytohormones (Gao et al. 2019). Many physiological processes have been reported to involve Gibberellins (GA) in cellular Ca2+ partitioning and distribution, moreover it has been shown to increase the expression of CAX and Ca2+-ATPase in the membranes of rice aleurone cells (Bush et al. 1989; Gilroy and Jones 1992; Chen et al. 1997). Consequently, in plants treated with GA, the concentration of water-soluble apoplastic Ca2+ dropped while the frequency of BER increased (de Freitas et al. 2012b).
A transcriptomics study was carried out to determine the possible mechanisms of inducing or preventing BER development in tomato fruit in response to phytohormones such as gibberellins (GA) and abscisic acid (ABA) or the GA biosynthesis inhibitor "Apogee". From 26 to 40 days after pollination, the incidence rate of BER was negligible in Apogee and ABA-treated plants, but high in GA-treated plants (de Freitas et al. 2018). They have the opposite effect when it comes to Ca2+ uptake: whereas GA can lower Ca2+ concentration, increasing stress sensitivity and the likelihood of BER, ABA increases Ca2+ translocation into the fruit, inhibiting the occurrence of BER (de Freitas et al. 2018). During the early growth and development of the tomato fruits, the GAs is high, whereas the level of Ca2+ is low. This high level of GAs is responsible for the rapid cell expansion which causes the blocking of xylem tissue in the distal end of the fruit, which is later developed as BER. The application of GAs in fruit decreases the Ca2+ content in the apoplastic region of the fruit and increases the function of the Ca2+/H+ exchanger and Ca2+-ATPase transporter which are present in the tonoplast plays an important role in the transport of Ca2+ into the storage organelle such as vacuole, which induces BER in tomato fruit. The use of GAs also raises ROS levels while decreasing the expression of many antioxidant genes like catalase, peroxidase, and superoxide dismutase (Fath et al. 2001).
Abscisic acid (ABA) has the opposite effect, reduced cell expansion, and strengthened cell structures. ABA promotes Ca2+ mobility into the fruit, leading to higher and water-soluble extracellular Ca2+ concentrations that stop the spread of BER in the fruit tissue. Furthermore, combined treatment of ABA and Apogee (an inhibitor of GA biosynthesis) can inhibit the development of BER by increasing the Ca2+ level in the fruit tissues (Heuvelink and Körner 2001). The foliar application of ABA increases the Ca2+ accumulation in the shoot and the xylem tissue at the distal end of the tomato fruit which reduces the incidence of BER. ABA treatment directly on fruit does not affect xylem sap flow rates or Ca2+ movement into the fruit, but it did raise water-soluble apoplastic Ca2+ concentrations and somewhat reduce fruit susceptibility to BER. During the fruit's early growth and development, ABA treatment increased the number of functional xylem vessels, which facilitated the translocation of Ca2+ toward the tissue close to the blossom end. The blossom-end tissue experienced a greater accumulation of Ca2+ following the whole-plant ABA treatment in contrast to the fruit-specific ABA treatment. This could be because the ABA treatment also increased the fruit's flow rate of xylem sap and its absorption of Ca2+-containing xylem sap. Fruit's susceptibility to BER development decreased, membrane leakage decreased, and concentrations of water-soluble apoplastic Ca2+ increased as a result of this accumulation. In this scenario, selecting new tomato cultivars with enhanced ABA biosynthesis for reduced fruit susceptibility to BER may be an option in addition to conserving water.
A disruption in the fruit's polar auxin transport could also influence the development of BER. It's interesting to think that a decrease in xylogenesis and a subsequent poorer transport of calcium to the distal tissue could be the cause of this phenomenon. A high Ca2+ concentration rate in apples and pears decreases ethylene production by inhibiting the 1-aminocyclopropane-1- carboxylic acid oxidases (ACO) which is a precursor for the biosynthesis of ethylene (Gao et al. 2019). Ca2+ regulates the cell wall degrading enzymes such as cellulase, pectin esterase, pectin lyase, β- and galactosidase. If pectin degradation occurs, the free Ca2+ increases, leading to the loss of cell–cell interaction and fruit softening (Sasanuma and Suzuki 2016). It has also been suggested that ethylene plays a role in BER induction. However, it's also possible that ethylene and other "stress" factors that raise ROS production could affect the occurrence of BER by activating hyperpolarization-activated Ca2+ channels (HACCs), an annexin protein, which would raise [Ca2+]cyt and cause rapid cell expansion (Fig. 2, follow steps 1, 2, 3). A summary of genes involved in the induction or inhibition of BER upon phytohormone treatment is given in Table 2.
Oxidative Stress Induces BER Development
Previous research has examined the potential link between oxidative stress and BER incidence in tomato fruits to identify a major cause of BER development other than Ca2+ deficiency (Schmitz-Eiberger et al. 2002). Oxidative stress induced by reactive oxygen species (ROS) can result from any circumstance that disrupts the redox balance within cells (De Gara et al. 2010; Schieber and Chandel 2014). ROS overproduction is one of the main reasons for decreased productivity, damage, and plant death in response to environmental stress. Superoxide radicals (O2°─), hydroxyl radicals (OH.), and hydrogen peroxide (H2O2) are the most dangerous ROS produced by plants because of their capacity to cross membranes and accumulate at varying degrees within cells (Sharma et al. 2012; Turhan et al. 2006). Membrane fluidity is often reduced as a result of lipid peroxidation caused by the formation of free oxygen radicals and H2O2, which can exacerbate membrane leakage and tissue degradation (Dhindsa et al. 1981; Chen and Yu 1994; Alché 2019) Both Ca2+ and ROS signaling are interconnected secondary messengers that respond to a variety of environmental stresses. Ca2+ controls ROS production, whereas ROS controls Ca2+ homeostasis (Kobayashi et al. 2007; Görlach et al. 2015; Marcec et al. 2019).
During abiotic and biotic stress ROS generated by the plasma membrane NADPH oxidase, by-products of mitochondrial, endoplasmic reticulum, and chloroplast respiratory chain (Turhan et al. 2006; Jiang et al. 2011). The balance between ROS generation and detoxification determines whether ROS is a threat to cells or plays a role in response signaling (Ayer et al. 2014). Plant cells can reduce the negative effects of ROS by utilizing enzymatic and non-enzymatic antioxidant pathways (Fig. 5) (Das and Roychoudhury 2014). Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPX), ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) are the example of enzymatic antioxidants. Non-enzymatic antioxidants are ascorbic acid (vitamin C), α- Tocopherols, glutathione, carotenoids, and phenolic compounds such as flavonoids, tannins, lignin, and hydroxycinnamate esters which are abundant in the plant tissue (Ahmad et al. 2010; Ayer et al. 2014; Topcu et al. 2022). The Ascorbate–Glutathione pathway, which consists of four major enzymes (APX, MDHAR, DHAR, and GR) and two antioxidants Ascorbate–Glutathione (AsA- GSH), is important in the detoxification of ROS in plants (Foyer and Noctor 2011). BER is associated with excessive ROS and unbalanced glutathione homeostasis which causes lipid and protein peroxidation, enzyme inhibition, and cellular membrane leakage (Mestre et al. 2012). Tomatoes cultivated in Ca2+-deficiency environments have increased BER occurrence which is related to the increased expression of NADPH oxidase and SOD (Mestre et al. 2012). Similarly, peppers cultivated under high saline environments have elevated ROS accumulation in the apoplast because of elevated NADPH oxidase activity (Aktas et al. 2005). Conversely, tomatoes cultivated in Ca2+-deficient environments exhibit down-regulation of numerous antioxidant genes, including CAT, APX, and GR (Gong and Li 1995; Schmitz-Eiberger et al. 2002; Yang and Poovaiah 2002). As a result, ROS is regarded as an important factor in the onset and progression of BER (Fig. 5) (Van Breusegem and Dat 2006; Sharma et al. 2012; Topcu et al. 2022). According to studies, GSH is essential for shielding plants from the majority of environmental stresses in multiple ways (Chakraborty et al. 2021). The likelihood of this physiological disorder is dependent on the degree of GSH homeostasis and its cellular concentration, both of which are influenced by GR activity (Mestre et al. 2012). Tomato cultivars with naturally high ascorbate and anti-oxidant levels during the most susceptible stage of BER are more resilient to the disorder than those with lower levels of antioxidants, regardless of fruit Ca2+ concentration. Additionally, BER does not always affect the whole fruit. This could be due to higher lignification, antioxidants, and oxidative stress-related proteins, which prevent BER from spreading to surrounding healthy tissues (Mestre et al. 2012; Rached et al. 2018; Reitz and Mitcham 2021; Topcu et al. 2022). Antioxidants and ROS-generating enzymes work together to greatly accelerate the development of BER, which is made worse by low Ca2+ concentration and abiotic stress (Aloni et al. 2008; Rached et al. 2018).
Increased membrane lipid peroxidation and BER formation in fruit cells are caused by an increase in oxidative stress molecules (ROS), which alter the cellular redox level because of Ca2+ deficiency or abiotic stress. Ca2+ deficiency inhibits several enzymes, including catalase, ascorbate peroxidase, and glutathione reductase, and induces NADPH oxidase, superoxide dismutase, and dehydro- and mono-dehydro ascorbate reductase. These conditions cause an increase in the production of reactive oxygen species (ROS) and a decrease in cellular redox, both of which have an impact on the development of BER via lipid peroxidation
A proteomic approach to BER in tomato (Lycopersicon esculentum M.) fruits revealed the induction of proteins from the pentose phosphate pathway as well as specific antioxidant (ascorbate–glutathione cycle) enzymes, implying that these two metabolic pathways limit the blackening of the entire fruit by acting as scavengers of ROS in BER-affected fruits (Casado-Vela et al. 2005). Furthermore, a comparative transcriptome study in tomato fruits from an introgression line found a correlation between higher fruit ascorbic acid concentration and high expression of pectinesterase family proteins and two polygalacturonases related to pectin degradation. This is primarily accomplished by increasing flux through the L-galactonic acid pathway, which is driven by pectin degradation and may be triggered by ethylene (Di Matteo et al. 2010). According to a prior study, pepper (Capsicum annuum L.) fruit that is susceptible to BER may exhibit alterations in apoplastic pH, a decrease in apoplastic AsA concentration, and an increase in apoplastic ascorbate oxidase activity as a contributing factor to the development of BER symptoms (Aloni et al. 2008). One of the most popular processing tomatoes in California, USA, HM 4885, had an 85% BER incidence, which contributed to catalase activity suppression, resulting in higher ROS accumulation (Reitz and Mitcham 2021). In conclusion, decreased activity of the ROS detoxification pathway can result in massive H2O2 deposition, lipid peroxidation, and membrane degradation, DNA breakdown, protein degradation resulting in increased BER incidence (Fig. 5).
Abiotic Stress-Mediated BER Induction
Even though Ca2+ deficiency is a main contributor, BER can be induced by a variety of abiotic stresses. Numerous studies demonstrate the connection between BER and abiotic stressors like excessive salinity, drought, relative humidity, shoot and root temperatures, high light levels, and mineral imbalance (Taylor and Locascio 2004; Ho and White 2005). The induction of BER by stress factors does not rule out the possibility of Ca2+ involvement, because changes in cytosolic Ca2+ ([Ca2+]cyt) will most likely play a role in coordinating the cellular reactions to all of these stressors. Many studies have been conducted to examine the impact of abiotic stresses on the Ca2+ transport to the fruit, particularly in the distal end of the fruit, and has been found to regulates plant Ca2+ uptake, Ca2+ motility to the fruit, Ca2+ role in the cell, and fruit susceptibility to Ca2+ deficiency disorders.
High light and high air temperature conditions speed up fruit expansion, which may be related to higher photosynthetic rates and photoassimilated fruit delivery. Plants exposed to high temperatures and light levels experience a reduction in cell differentiation, an alteration in membrane permeability, and an increase in ROS production, which ultimately results in lipid peroxidation within the plant cell (Ho and White 2005; Roeber et al. 2021; Sachdev et al. 2021). The ideal range for root temperature to transfer Ca2+ into a plant is between 15 °C and 27 °C. Root temperature also controls the amount of Ca2+ that enters the plant. The amount of Ca2+ transported to the plant is reduced if this temperature changes (de Freitas and Mitcham 2012).
Some nutrients are resistant to absorption while others are not. High soil monovalent cation concentrations, such as potassium (K+), magnesium (Mg+), sodium (Na+), and ammonium (NH4+), inhibit divalent cation Ca2+ uptake, increasing the frequency of BER. Increased NH4+ concentration in the nitrate/ammonium ratio, for example, reduced Ca2+ absorption and increased BER formation. Fruits obtained from a BER-resistant cultivar exhibited a strong link between boron (B+) and Ca2+ concentrations in the distal region of each fruit, whereas susceptible accessions exhibited no association between the two elements. In this scenario, the relationship between boron (B+) and Ca2+ could disclose a role in cell wall pectin structural stabilization (Watanabe et al. 2021). High nitrogen (N) levels promote shoot growth, which has been linked to increased Ca2+ transport towards the leaves and away from the fruit, presumably due to higher leaf transpiration rates at the whole plant level (Ho and White 2005). High quantities of nitrogen are also known to cause rapid fruit and cell development, which may result in further diluting of the fruit's limited Ca2+ content which can lead to BER (de Freitas and Mitcham 2012). K+ is found to be involved in cell-expansion-related processes, high levels of K+ may stimulate rapid plant and fruit growth, which could reduce fruit Ca2+ uptake, dilute fruit Ca2+ content, and increase fruit sensitivity to BER. Ca2+ might theoretically be replaced by large quantities of K+ and Mg2+ at the plasma membrane surface, but not for its function in membranes, this can cause a leaky plasma membrane and increases the incidence of BER in fruit tissue (de Freitas and Mitcham 2012).
To adapt to different abiotic stressors and survive in low light, they must be able to sense both the quality and quantity of light and respond appropriately (Roeber et al. 2021). Climate change also exposes plants to more unfavorable environmental conditions, which reduces fitness and crop production. Drought and salinity have been shown to promote Ca2+ deficiency disorders in fruits (Bashir et al. 2021; Manishankar et al. 2018). The effect of drought, salinity, and low relative humidity on enhancing fruit sensitivity to Ca2+ deficit-related disorders may be due to plant water stress, which has been proposed as a means of restricting xylemic Ca2+ mobility into the fruit. Furthermore, drought and salinity have been shown to decrease the number of functional xylem vessels in fruit, lowering fruit Ca2+ uptake. Both drought and salinity produce ROS in the plant which is the major cause of developing BER in the tomato fruit (Ho and White 2005). Fruit Ca2+ concentrations are typically lowered simultaneously by the effects of excessive light and air temperatures. Plants exposed to high temperatures and light cause their cells to become less differentiated, alter the permeability of their membranes, and produce more ROS, which in turn causes lipid peroxidation in the plant's cells. Alteration in temperature also causes inhibition of Ca2+ uptake by the plant and influences the development of BER. A summary of various abiotic factor-mediated cellular alterations that lead to the development of BER in tomatoes is provided in Fig. 6a.
a A simplified model for the impact of various abiotic stress factors involved in the occurrence of BER in distal fruit tissue. Blossom end-rot is promoted by environmental factors such as high light and temperature, low humidity, or high nitrogen, low Ca2+ supply or high ammonium, and high salinity. A common mechanism is a decrease in local Ca2+ content in the distal end of tissue, which affects the Ca2+ demand required for fruit cell expansion and induces the occurrence of BER. The schematic model is adapted and modified from a previous study (Ho and White 2005). b A schematic representation of how different environmental and genetic factors cause tomato fruit to develop blossom-end rot (BER), which results in cell death. In the distal section of young tomato fruits, environmental and genetic factors facilitate the rapid expansion of cells with enhanced delivery of Ca2+ to vacuoles from the cytosol. Reduced apoplastic Ca2+ concentration is the result of an imbalance between Ca2+ supply and Ca2+ demand brought on by increased cell expansion. This has an impact on cytosolic Ca2+ signals, ROS generation, compromised cell wall, and membrane integrity which can affect solute leakage which ultimately leads to cell death. The schematic model is adapted and modified from a previous study (Ho and White 2005)
In conclusion, the majority of environmental conditions that influence the incidence of BER disrupt the link between the rate of cell expansion and Ca2+ buildup in growing fruit. Similarly, Ca2+ sequestration in expanding vacuoles of a fruit cell during a period of low Ca2+ supply may deprive the cytoplasm or apoplast of Ca2+ required for intracellular signaling or cellular function. This may be exacerbated by specific mineral stressors that inhibit calcium absorption or promote the synthesis of organic acids that chelate Ca2+. Decreases in [Ca2+]apoplast can lead to altered responses to environmental or developmental stimuli that are triggered by Ca2+ influx; modifications in plasma membrane permeability that result in uncontrollable solute fluxes and solute leakage; and compromised cell wall properties that lead to premature cell expansion and structural weakness. This would ultimately lead to an irreversible cell death (Fig. 6b).
Genetic Factor's Contribution towards BER
The genomic techniques that, with the aid of more extensively accessible genomic resources for tomatoes, can be utilized to look into the genetic mechanisms supporting BER. A simple approach would be to use association analysis to correlate phenotypic data with allelic diversity by surveying allele variation in the entire population (Caballero and Rodríguez-Ramilo 2010). The chromosomal loci influencing the occurrence of BER can also be identified using genomic regions that contribute to variation in quantitative trait locus (QTL) or quantitative phenotype analyses (Corre and Kremer 2003; Powder 2020). This will provide insights into the physiological mechanisms causing BER and will direct future breeding efforts and research. Previous studies using tomato introgression lines (ILs) provided the first information about the genetic foundations of BER. The genomic segments of Solanum pennellii LA716 that comprise ILs were introduced into Solanum lycopersicum cv. M82 (Eshed and Zamir 1995). Of these lines, IL8–3 has a lower BER incidence as compared to the parent M82, and fine mapping this region revealed a segment of 610 kb regions containing 78 genes (Uozumi et al. 2012; Watanabe et al. 2021). These results suggested that IL8-3 may harbor gene(s) affecting Ca2+ concentration and growth rate in the early stages of fruit development because of the higher Ca2+ concentration in the distal portion of the fruit and the initially slower growth rate in the BER-resistant line.
Additional examination of these IL8–3 lines ten days after flowering also showed that M82 and IL8–3 differed from each other in the expression of several genes related to Ca2+ transport, such as Ca2+/H+-exchanger (CAX), Na+/Ca2+-exchanger (NCX) Ca2+-ATPase, and Ca2+-channel however, none of these genes were mapped to the region where IL8–3 is located on chr08 (Ikeda et al. 2017). Possibly the genes located in the 610 kb region of IL8-3 controls, Ca2+ transport-related genes in other chromosomes, could explain these findings. Similarly IL5-4, which is situated on chr05, BER differences were also noted; however, in this case, the BER differences are more pronounced in the IL than in the control M82 (Matsumoto et al. 2021). Through the enrichment of SNPs linked to the trait, molecular markers to map BER loci in the population are created by employing the QTL seq approach (Topcu et al. 2021). Populations resulting from crosses between Solanum lycopersicum var. cerasiforme (SLC) and S. lycopersicum var. lycopersicum (SLL) contained four loci: BER3.1 and BER3.2 on chr03, BER4.1 on chr04, and BER11.1 on chr11 (Topcu et al. 2021). BER3.2 and BER11.1 were fine-mapped to 1.58 and 1.13 Mb, respectively, even though BER11.1 had previously been mapped in a different population derived from SLL cv Ailsa Craig and SLL cv Kentucky Beefsteak (Prinzenberg et al. 2021). According to the findings, BER3.2 is most likely related to FW3.2/KLUH, the fruit weight gene that was found to be segregating in one of the populations.
A comparison of large vs mid-sized tomato cultivars revealed that the difference in BER susceptibility is most likely driven by changes in genetic factors, which may be closely related to potential fruit size and mostly determines water-soluble Ca2+ delivery in the tissue. The size of the tomato fruit and the occurrence of BER are co-related to each other (Heuvelink and Körner 2001; Ho et al. 2015), because the incidence of BER does not occur in the small tomato fruits that don’t have the phase of rapid cell expansion, but it affects the large fruits that have the phase of rapid cell expansion (Topcu et al. 2022). The susceptibility of BER is different in different sizes of fruit. Except for FW3.2/KLUH, five loci in tomatoes have been identified as being involved in the genetic basis of BER studies: Chr 03, Chr 04, Chr 05, Chr 08, and Chr 11. The function of these various loci could be examined using a biotechnological method, which can offer fresh perspectives on BER developments.
The tomato gene Cell Size Regulator (FW11.3/CSR) causes cells to enlarge, which increases fruit weight (Mu et al. 2017). FW11.3 near-isogenic lines (NILs) carrying the derived allele of CSR had a significantly higher BER incidence than FW11.3 NILs carrying the wild-type allele, indicating that FW11.3/CSR may be involved in the development of BER. The relationship between BER and these fruit weight genes is most likely indirect rather than causative, as many tomato types with the derived fruit weight alleles are resistant to BER. In addition to fruit size, elongated fruit forms are more prone to BER than round-fruited cultivars (Grandillo et al. 1996; Ku et al. 2000; Riboldi et al. 2018). A small number of genes—SUN, OVATE, OFP20, and FS8.1—are responsible for controlling the elongated fruit form of tomatoes. Among these genes, the minimal BER incidence is associated with the fs8.1 round fruit allele (Ku et al. 2000; Liu et al. 2002; Xiao et al. 2008; Wu et al. 2018). Furthermore, the variations in OVATE mutation-carrying San Marzano or banana legs with the SUN mutation have a very high potential for BER (Riboldi et al. 2018).
Prevention and Control Strategies for BER
Increasing crop yield under target conditions should always begin with the adoption of so-called good agricultural practices (Kılıç et al. 2020). Even though the exact mechanism responsible for BER onset remains unknown, many management strategies have been employed to prevent and control the frequency of BER, beginning with an assessment of the characteristics of the soil, proper irrigation, Calicum spray, utilizing BER resistant genetic variety, and possible use of genetically engineered plants. These methods could be paired with efficient irrigation strategies that are dependent on the water's quality, growth regulators, and maintaining balanced nutrition which are crucial components in preventing abiotic stressors. Figure 7 provides an overview of the several approaches that can be used to avoid and manage BER.
Improve the Soil Qualities
Both directly and indirectly, biochemical mineral and biological soil factors (symbiotic bacteria and fungi) influence the availability of nutrients, which impacts the prevalence of BER. Since tomato is a crop that is only moderately sensitive to soil salinity, the only practical way to avoid BER and significant output losses is to remove salt from the root zone using a process known as "reclamation" (Shaygan and Baumgartl 2022). In addition, a widely used farming practice “fertigation” the process of applying fertilizers with irrigation water, improves the timing and availability of nutrients while making it simple to regulate the concentration of the fertilizer (Machado and Serralheiro 2019). Drip irrigation, which reduces inputs and delivers nutrients to the root zone, is used in the most efficient fertigation systems. This helps to reduce the effects of salt stress and prevent soil salinization (Costa et al. 2018). According to earlier research, there may be relationships between the rhizosphere microbiome, enzyme activity (mainly phosphatase, catalase, and invertase), and BER in tomatoes (Shao et al. 2018). The incidence rate of tomato BER was significantly inversely related to the relative abundance of soil microorganisms but positively related to the abundance of bacteroides.
Proper Irrigation
An adequate water supply is required to maximize the quality and productivity of tomato crops (de Pascale et al. 2011; Halli et al. 2021). Conversely, BER occurrence may significantly increase in response to a high water supply (Zhai et al. 2016). A reduced irrigation technique known as partial root-zone drying (PRD) irrigates only half of the root system. PRD can lower the incidence of BER in tomato plants and increase water use efficiency without appreciably affecting yields (Jovanovic and Stikic 2018; Iqbal et al. 2020). Many tomato-growing regions around the world struggle with high salinity levels in irrigation water, which have an adverse effect on fruit quality and yield. Irrigation with saline water is highly associated with lower yields, primarily due to a corresponding drop in fruit weight and a notable rise in the percentage of fruit with BER symptoms (Jha et al. 2017; El-Mogy et al. 2018). To avoid waterlogging and root hypoxia, which both lower fruit yields, tomatoes grown in saline irrigation require less frequent watering (Ide et al. 2022; Daniel and Hartman 2023).
Application of Calcium-based Nutrition
The most popular and initial agronomic technique to prevent BER development has been controlling the soil's Ca2+ accessibility. Soils containing high concentrations of monovalent cations, such as K+, Na+, and NH4+, can have a detrimental effect on the intake of Ca2+ (Hagassou et al. 2019). Consequently, these results imply that calcium is an essential component for the plant that can lower the frequency of BER in tomato fruit. The foliar application of 2% CaCl2 decreased the incidence of BER and produced a large yield of tomatoes (Mazumder et al. 2021). Tomato fruit BER incidence is reduced by exogenous application of calcium nitrate or calcium liquid formulation (Syahren et al. 2012). Applying phosphorous solution to tomato plants increases their uptake of Ca2+, reduces the incidence of BER in tomato fruit, and promotes vegetative growth and fruit yield (Alam et al. 2017). Similarly, applying a humic acid and calcium mixture helps tomato plants grow less stressed in saline water and lessens the likelihood of BER in tomato fruit (Kazemi 2014; Kataoka et al. 2017). Furthermore, applying nickel solution to tomato plants raises fruit yield, lowers BER incidence, and increases Ca2+ accumulation in the shoots (Macedo et al. 2022). Moreover, the application of ascorbic acid decreases the incidence of BER in tomato fruit, because it acts as an antioxidant that protects the plant tissue from ROS (Rached et al. 2018). In actuality, BER can be avoided by decreasing canopy management and enhancing Ca2+ transport toward fruit transpiration or through canopy Ca2+ sprays (Wani et al. 2021; Karlsons et al. 2023).
Application of Phytohormones
During the early fruit development, the amount of GAs is high which promotes rapid cell expansion, due to rapid cell expansion the obstruction of xylem tissue occurs which can lead to BER in tomato fruits (Topcu et al. 2022). The exogenous application of GA inhibitor decreases the incidence of BER in tomato fruit (de Freitas et al. 2012b). In addition, the root and foliar application of ABA increases the Ca2+ accumulation in the tomato shoots and fruits, it also increases the xylem tissue development at the distal end of the tomato fruit which reduces the incidence of BER.
BER-Resistant Variety Selection
Certain tomato cultivars are more prone to BER than others such as cherry tomatoes have not been reported to have BER, but plum and pear-shaped tomatoes are more prone than round-fruited varieties. This has led to the hypothesis that the varied ways in which phloem-borne leaf assimilate and xylem-borne calcium are delivered to the distal end of the fruit in response to the growth environment are linked to the vulnerability of cultivars to BER. Fruit from BER-susceptible cultivars does, in fact, typically contain less calcium than fruit from non-susceptible cultivars, especially right after anthesis (Franco et al. 1994). In a previous study, to elucidate the relationship between BER incidence and oxidative stress, two BER-resistant cultivars, “Managua RZ” and “House Momotaro,” and one BER-susceptible cultivar, “Reiyoh,” were cultivated under salinity or standard nutrient solution (control) conditions. It was suggested that, in response to BER-inductive growth conditions, BER-resistant cultivars showed a larger increase in their ROS scavenging capacity, represented by ascorbate than BER-susceptible cultivars (Rached et al. 2018). Thus, choosing cultivars resistant to BER may be another strategy to reduce BER. Like this, Marmande-type tomato varieties had a higher prevalence of BER than cherry, cocktail, or round tomato varieties, which indicates that the genetic architecture of fruit is important (Hagassou et al. 2019). Grafting tomato plants could be a viable solution to mitigate the effects of salt and drought stress (He et al. 2009). To avoid BER, rootstock breeding with improved nutrient absorption, tolerance to salt stress, and fruit quality can be used (King et al. 2010).
Conclusions
Ca2+ is essential for fruit growth and development because it regulates various cellular activities and metabolism. BER is a Ca2+ deficiency disorder that primarily affects fruit tissues at the blossom end. Plants with altered [Ca2+]cyt homeostasis play a role in the onset of BER. BER is induced despite an adequate Ca2+ level in tomato fruits due to a defect in Ca2+ distribution and partitioning by the Ca2+/H+-exchangers, Ca2+-ATPase, and Ca2+-channels and a defective distribution of the xylemic vessel at the distal end of the fruit. Deregulated expression of an Arabidopsis Ca2+/H+ antiporter (sCAX1) raises total Ca2+ in crops but may cause yield losses because of symptoms resembling a Ca2+ deficiency. Given that sCAX1 expression can significantly increase Ca2+ accumulation in edible crop tissues (such as lettuce, tomato, potato, and carrot), the use of sCAX1 for Ca2+ biofortification has been thoroughly studied in a variety of horticultural crop species. Ca2+ deficiency can also influence the degree of methylation of homogalacuronan pectin in the cell wall, which modulates apoplastic Ca2+ level and governs membrane stability and BER appearance. Any abiotic factors, that increase cell expansion and decrease the apoplastic Ca2+ level in the cell wall can cause the development of BER in tomato fruits. The primary antioxidant defense mechanism is suppressed by Ca2+ deficiency or oxidative stress, resulting in an excess of ROS. The accumulation of ROS leads to increased lipid peroxidation, resulting in increased membrane leakage and the development of the BER in tomato fruits.
Because physiological and environmental factors both have a significant impact on the incidence of BER, many agricultural settings find it difficult to manage this disorder under field and greenhouse growth conditions. Soil quality-improving techniques can help to reduce the induction of BER. However, to reduce BER, more emphasis must be placed on utilizing crop germplasm's genetic diversity. Uniform soil moisture using proper mulching techniques, a suitable drainage system, appropriate irrigation, soil quality improvement, proper irrigation, and balanced application of fertilizer are all effective ways to manage BER. In addition, there are other tactics like resistant cultivar selection, enhancing Ca2+ uptake, and application of ABA and GA inhibitors that also help to reduce the incidence of BER in tomato fruits. It is anticipated that genetic research and the identification of genes linked to BER in tomato fruit will advance our knowledge and mechanism of BER in tomatoes. Meanwhile, increasing the apoplastic Ca2+ concentration in susceptible fruit tissue should provide a simple reliable, practical solution for the prevention of BER in tomatoes. Biofertilizers are also can be used to diminish the effect of climatic change on crops. Novel approaches for crop improvement in many vegetables are becoming possible as genetic studies are shedding more and more light on the underlying genes that cause BER. For example, downregulating or eliminating BER susceptibility tomato genes through CRISPR/Cas9 mediated gene or promoter editing should lead to commercially produced varieties that are more resistant to BER. Consequently, it is expected that the toolkit for suppressing BER will expand, offering breeders fresh methods to produce varieties more resistant to this often-fatal physiological disorder.
Abbreviations
- BER:
-
Blossom End Rot
- PME:
-
Pectin methylesterase
- ACO:
-
1-Aminocyclopropne-1-Carboxylic acid oxidase
- GAs:
-
Gibberellins
- ABA:
-
Abscisic acid
- GPX:
-
Glutathione peroxidase
- APX:
-
Ascorbate peroxidase
- GR:
-
Glutathione reductase
- MDHAR:
-
Monodehydro ascorbate reductase
- DHAR:
-
Dehydro ascorbate reductase
- AsA-GSH:
-
Ascorbate- Glutathione
References
Adams P, El-Gizawy AM (1988) Effect of calcium stress on the calcium status of tomatoes grown in NFT. Acta Horticult 222(1):15–22. https://doi.org/10.17660/actahortic.1988.222.1
Adams P, Ho LC (1992) The susceptibility of modern tomato cultivars to blossom- end rot in relation to salinity. J Horticult Sci 67(6):827–839. https://doi.org/10.1080/00221589.1992.11516315
Adams P, Ho LC (1993) Effects of environment on the uptake and distribution of calcium in tomato and on the incidence of blossom-end rot. Plant Soil 154(1):127–132. https://doi.org/10.1007/BF00011081
Aghdam MS, Hassanpouraghdam MB, Paliyath G, Farmani B (2012) The language of calcium in postharvest life of fruits, vegetables and flowers. Sci Hortic 144:102–115. https://doi.org/10.1016/j.scienta.2012.07.007
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175. https://doi.org/10.3109/07388550903524243
Aktas H, Karni L, Chang DC, Turhan E, Bar-Tal A, Aloni B (2005) The suppression of salinity-associated oxygen radicals production, in pepper (Capsicum annuum) fruit, by manganese, zinc and calcium in relation to its sensitivity to blossom-end rot. Physiol Plant 123(1):67–74. https://doi.org/10.1111/j.1399-3054.2004.00435.x
Alam M, Billah KMM, Prince MH, Hasan KMM (2017) Effect of Nitrogen and Phosphorous Fertilizer Application on the Growth and Yield of Tomato: A Mini-Review. Int J Adv Agric Sci 2(12):1–5
Alché J de D (2019) A concise appraisal of lipid oxidation and lipoxidation in higher plants. Redox Biol 23:101136. https://doi.org/10.1016/j.redox.2019.101136
Aloni B, Pressman E (1987) The effects of salinity and gibberellic acid on blackheart disorder in celery (Apium graveolens L.). J Horticult Sci 62(2):205–209. https://doi.org/10.1080/14620316.1987.11515770
Aloni B, Karni L, Deventurero G, Turhan E, Aktas H (2008) Changes in ascorbic acid concentration, ascorbate oxidase activity, and apoplastic pH in relation to fruit development in pepper (Capsicum annuum L.) and the occurrence of blossom-end rot. J Horticult Sci Biotechnol 83(1):100–105. https://doi.org/10.1080/14620316.2008.11512353
Aouini A, Hernould M, Ariizumi T, Matsukura C, Ezura H, Asamizu E (2012) Overexpression of the tomato glutamate receptor-like genes SlGLR1.1 and SlGLR3.5 hinders Ca2+ utilization and promotes hypersensitivity to Na+ and K+ stresses. Plant Biotechnol 29(3):229–235. https://doi.org/10.5511/plantbiotechnology.12.0213a
Atkinson NJ, Dew TP, Orfila C, Urwin PE (2011) Influence of combined biotic and abiotic stress on nutritional quality parameters in tomato (Solanum lycopersicum). J Agric Food Chem 59(17):9673–9682. https://doi.org/10.1021/jf202081t
Ayer A, Gourlay CW, Dawes IW (2014) Cellular redox homeostasis, reactive oxygen species and replicative ageing in Saccharomyces cerevisiae. FEMS Yeast Res 14(1):60–72. https://doi.org/10.1111/1567-1364.12114
Barta DJ, Tibbitts TW (2000) Calcium localization and tipburn development in lettuce leaves during early enlargement. J Am Soc Horticult Sci 125(3):294–298. https://doi.org/10.21273/jashs.125.3.294
Bashir SS, Hussain A, Hussain SJ, Wani OA, Zahid Nabi S, Dar NA, Baloch FS, Mansoor S (2021) Plant drought stress tolerance: understanding its physiological, biochemical and molecular mechanisms. Biotechnol Biotechnol Equip 35(1):1912–1925. https://doi.org/10.1080/13102818.2021.2020161
Bianco MS, Filho ABC, De Carvalho LB (2015) Nutritional status of the cauliflower cultivar “Verona” grown with omission of out added macronutrients. PLoS ONE 10(4):e0123500. https://doi.org/10.1371/journal.pone.0123500
Bonnin E, Garnier C, Ralet MC (2014) Pectin-modifying enzymes and pectin-derived materials: Applications and impacts. Appl Microbiol Biotechnol 98:519–532. https://doi.org/10.1007/s00253-013-5388-6
Bukovac MJ, Wittwer SH (1957) Absorption and Mobility of Foliar Applied Nutrients. Plant Physiol 32(5):428–435. https://doi.org/10.1104/pp.32.5.428
Bush DS, Biswas AK, Jones RL (1989) Gibberellic-acid-stimulated Ca2+ accumulation in endoplasmic reticulum of barley aleurone: Ca2+ transport and steady-state levels. Planta 178(3):411–420. https://doi.org/10.1007/BF00391870
Caballero A, Rodríguez-Ramilo ST (2010) A new method for the partition of allelic diversity within and between subpopulations. Conserv Genet 11(6):2219–2229. https://doi.org/10.1007/s10592-010-0107-7
Caffall KH, Mohnen D (2009) The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohyd Res 344(14):1879–1890. https://doi.org/10.1016/j.carres.2009.05.021
Casado-Vela J, Sellés S, Martínez RB (2005) Proteomic approach to blossom-end rot in tomato fruits (Lycopersicon esculentum M.): Antioxidant enzymes and the pentose phosphate pathway. Proteomics 5(10):2488–2496. https://doi.org/10.1002/pmic.200401146
Chakraborty N, Mukherjee S, Shaw P, Acharya K (2021) Role of glutathione transporter in plants under stress. Transporters and Plant Osmotic Stress, 345–364, Academic Press. https://doi.org/10.1016/B978-0-12-817958-1.00021-9
Chen JJ, Yu BP (1994) Alterations in mitochondrial membrane fluidity by lipid peroxidation products. Free Radical Biol Med 17(5):411–418. https://doi.org/10.1016/0891-5849(94)90167-8
Chen X, Chang M, Wang B, Wu R (1997) Cloning of a Ca2+-ATPase gene and the role of cytosolic Ca2+ in the gibberellin-dependent signaling pathway in aleurone cells. Plant J 11(3):363–371. https://doi.org/10.1046/j.1365-313X.1997.11030363.x
Cheng NH, Pittman JK, Shigaki T, Lachmansingh J, LeClere S, Lahner B, Salt DE, Hirschi KD (2005) Functional association of Arabidopsis CAX1 and CAX3 is required for normal growth and ion homeostasis. Plant Physiol 138(4):2048–2060. https://doi.org/10.1104/pp.105.061218
Chung MY, Han JS, Giovannoni J, Liu Y, Kim CK, Lim KB, Chung JD (2010) Modest calcium increase in tomatoes expressing a variant of Arabidopsis cation/H+ antiporter. Plant Biotechnol Rep 4(1):15–21. https://doi.org/10.1007/S11816-009-0112-9
Ciccarese A, Stellacci AM, Gentilesco G, Rubino P (2013) Effectiveness of pre- and post-veraison calcium applications to control decay and maintain table grape fruit quality during storage. Postharvest Biol Technol 75:135–141. https://doi.org/10.1016/j.postharvbio.2012.08.010
Combrink NJJ (2013) Calcium-Related plant physiological disorders. Acta Horticult 1014:7–11. https://doi.org/10.17660/actahortic.2013.1014.2
Corre L, Kremer A (2003) Genetic Variability at Neutral Markers, Quantitative Trait Loci and Trait. Genetics 164(3):1205–1219. https://doi.org/10.1093/genetics/164.3.1205
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6(11):850–861. https://doi.org/10.1038/nrm1746
Costa SF, Martins D, Agacka-Mołdoch M, Czubacka A, de Sousa Araújo S (2018) Strategies to Alleviate Salinity Stress in Plants. In: Kumar V, Wani S, Suprasanna P, Tran LS (eds) Salinity Responses and Tolerance in Plants. Springer, Cham. https://doi.org/10.1007/978-3-319-75671-4_12
Daniel K, Hartman S (2023) How plant roots respond to waterlogging. J Exp Bot. https://doi.org/10.1093/jxb/erad332
Das K, Roychoudhury A (2014) Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front Environ Sci 2:53. https://doi.org/10.3389/fenvs.2014.00053
de Freitas ST, Mitcham EJ (2012) Factors involved in fruit calcium deficiency disorders. Hortic Rev 40(1):107–146. https://doi.org/10.1002/9781118351871.ch3
de Freitas ST, do Amarante CVT, Labavitch JM, Mitcham EJ (2010) Cellular approach to understand bitter pit development in apple fruit. Postharvest Biol Technol 57(1):3–13. https://doi.org/10.1016/j.postharvbio.2010.02.006
de Freitas ST, Padda M, Wu Q, Park S, Mitcham EJ (2011) Dynamic Alternations in Cellular and Molecular Components during Blossom-End Rot Development in Tomatoes Expressing sCAX1, a Constitutively Active Ca2+/H+ Antiporter from Arabidopsis. Plant Physiol 156(2):844–855. https://doi.org/10.1104/PP.111.175208
de Freitas ST, Handa AK, Wu Q, Park S, Mitcham EJ (2012a) Role of pectin methylesterases in cellular calcium distribution and blossom-end rot development in tomato fruit. Plant J 71(5):824–835. https://doi.org/10.1111/j.1365-313X.2012.05034.x
de Freitas ST, Jiang CZ, Mitcham EJ (2012b) Mechanisms Involved in Calcium Deficiency Development in Tomato Fruit in Response to Gibberellins. J Plant Growth Regul 31(2):221–234. https://doi.org/10.1007/S00344-011-9233-9
de Freitas ST, Martinelli F, Feng B, Reitz NF, Mitcham EJ (2018) Transcriptome Approach to Understand the Potential Mechanisms Inhibiting or Triggering Blossom-End Rot Development in Tomato Fruit in Response to Plant Growth Regulators. J Plant Growth Regul 37(1):183–198. https://doi.org/10.1007/s00344-017-9718-2
de Pascale S, Costa LD, Vallone S, Barbieri G, Maggio A (2011) Increasing water use efficiency in vegetable crop production: From plant to irrigation systems efficiency. HortTechnology 21(3):308–321. https://doi.org/10.21273/horttech.21.3.301
De Gara L, Locato V, Dipierro S, de Pinto MC (2010) Redox homeostasis in plants. The challenge of living with endogenous oxygen production. Respir Physiol Neurobiol 173:S13–S19. https://doi.org/10.1016/j.resp.2010.02.007
Dhindsa RS, Plumb-dhindsa P, Thorpe TA (1981) Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot 32(1):93–101. https://doi.org/10.1093/jxb/32.1.93
Di Matteo A, Sacco A, Anacleria M, Pezzotti M, Delledonne M, Ferrarini A, Frusciante L, Barone A (2010) The ascorbic acid content of tomato fruits is associated with the expression of genes involved in pectin degradation. BMC Plant Biol 10:163. https://doi.org/10.1186/1471-2229-10-163
Dodd AN, Kudla J, Sanders D (2010) The language of calcium signaling. Annu Rev Plant Biol 61:593–620. https://doi.org/10.1146/annurev-arplant-070109-104628
Dražeta L, Lang A, Hall AJ, Volz RK, Jameson PE (2004) Causes and effects of changes in xylem functionality in apple fruit. Ann Bot 93(3):275–282. https://doi.org/10.1093/aob/mch040
El-Mogy MM, Garchery C, Stevens R (2018) Irrigation with salt water affects growth, yield, fruit quality, storability and marker-gene expression in cherry tomato. Acta Agric Scand Sect B Soil Plant Sci 68(8):727–737. https://doi.org/10.1080/09064710.2018.1473482
Eshed Y, Zamir D (1995) An introgression line population of Lycopersicon pennellii in the cultivated tomato enables the identification and fine mapping of yield- associated QTL. Genetics 141(3):1147–1162. https://doi.org/10.1093/genetics/141.3.1147
Fath A, Bethke PC, Jones RL (2001) Enzymes That Scavenge Reactive Oxygen Species Are Down-Regulated Prior to Gibberellic Acid-Induced Programmed Cell Death in Barley Aleurone. Plant Physiol 126(1):156–166. https://doi.org/10.1104/PP.126.1.156
Figueroa CR, Opazo MC, Vera P, Arriagada O, Díaz M, Moya-León MA (2012) Effect of postharvest treatment of calcium and auxin on cell wall composition and expression of cell wall-modifying genes in the Chilean strawberry (Fragaria chiloensis) fruit. Food Chem 132(4):2014–2020. https://doi.org/10.1016/j.foodchem.2011.12.041
Foyer CH, Noctor G (2011) Ascorbate and glutathione: the heart of the redox hub. Plant Physiol 155(1):2–18. https://doi.org/10.1104/PP.110.167569
Franco JA, Bañón S, Madrid R (1994) Effects of a protein hydrolysate applied by fertigation on the effectiveness of calcium as a corrector of blossom-end rot in tomato cultivated under saline conditions. Sci Hortic 57(4):283–292. https://doi.org/10.1016/0304-4238(94)90111-2
Francois LE, Donovan TJ, Maas EV (2019) Calcium Deficiency of Artichoke Buds in Relation to Salinity. HortScience 26(5):549–553. https://doi.org/10.21273/hortsci.26.5.549
Gao Q, Xiong T, Li X, Chen W, Zhu X (2019) Calcium and calcium sensors in fruit development and ripening. Sci Hortic 253:412–421. https://doi.org/10.1016/j.scienta.2019.04.069
Gilroy S, Jones RL (1992) Gibberellic acid and abscisic acid coordinately regulate cytoplasmic calcium and secretory activity in barley aleurone protoplasts. Proc Natl Acad Sci U S A 89(8):3591–3595. https://doi.org/10.1073/pnas.89.8.3591
Gong M, Li Z-G (1995) Calmodulin-binding proteins from Zea mays germs. Phytochemistry 40(5):1335–1339. https://doi.org/10.1016/0031-9422(95)00381-G
Gonzalo MJ, Li YC, Chen KY, Gil D, Montoro T, Nájera I, Baixauli C, Granell A, Monforte AJ (2020) Genetic Control of Reproductive Traits in Tomatoes Under High Temperature. Front Plant Sci 11:326. https://doi.org/10.3389/fpls.2020.00326
Görlach A, Bertram K, Hudecova S, Krizanova O (2015) Calcium and ROS: A mutual interplay. Redox Biol 6:260–271. https://doi.org/10.1016/j.redox.2015.08.010
Grandillo S, Ku HM, Tanksley SD (1996) Characterization of fs8.1, a major QTL influencing fruit shape in tomato. Molec Breed 2(3):251–260. https://doi.org/10.1007/BF00564202
Hagassou D, Francia E, Ronga D, Buti M (2019) Blossom end-rot in tomato (Solanum lycopersicum L.): A multi-disciplinary overview of inducing factors and control strategies. Sci Hortic 249:49–58. https://doi.org/10.1016/j.scienta.2019.01.042
Halli HM, Govindasamy P, Sannagoudar MS, Wasnik VK, Raghavendra M, Hatti V, Goud RB, Rajpoot SK (2021) Water management for efficient crop production. In: Jatav HS (ed) Sustainable Soil Fertility Management. Nova Science Publishers, Inc., pp 201–219
Hanson JB (1960) Impairment of Respiration, Ion Accumulation, and Ion Retention in Root Tissue Treated with Ribonuclease and Ethylenediamine Tetraacetic Acid. Plant Physiol 35(3):372–379. https://doi.org/10.1104/pp.35.3.372
Harholt J, Suttangkakul A, Scheller HV (2010) Biosynthesis of pectin. Plant Physiol 153(2):384–395. https://doi.org/10.1104/pp.110.156588
Hawkesford M, Horst W, Kichey T, Lambers H, Schjoerring J, Møller IS, White P (2011) Functions of Macronutrients. Marschner’s Min Nutr Higher Plants 3:135–189. https://doi.org/10.1016/B978-0-12-384905-2.00006-6
He Y, Zhu Z, Yang J, Ni X, Zhu B (2009) Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ Exp Bot 66(2):270–278. https://doi.org/10.1016/j.envexpbot.2009.02.007
Hepler PK, Winship LJ (2010) Calcium at the cell wall-cytoplast interface. J Integr Plant Biol 52(2):147–160. https://doi.org/10.1111/j.1744-7909.2010.00923.x
Heuvelink E, Körner O (2001) Parthenocarpic Fruit Growth Reduces Yield Fluctuation and Blossom-end Rot in Sweet Pepper. Ann Bot 88(1):69–74. https://doi.org/10.1006/anbo.2001.1427
Hirschi KD (1999) Expression of Arabidopsis CAX1 in Tobacco: Altered Calcium Homeostasis and Increased Stress Sensitivity. Plant Cell 11(11):2113–2122. https://doi.org/10.1105/tpc.11.11.2113
Hirschi KD, Zhen RG, Cunningham KW, Rea PA, Fink GR (1996) CAX1, an H+/Ca2+ antiporter from Arabidopsis. Proc Natl Acad Sci U S A 93(16):8782–8786. https://doi.org/10.1073/pnas.93.16.8782
Ho LC, White PJ (2005) A cellular hypothesis for the induction of blossom-end rot in tomato fruit. Ann Bot 95(4):571–581. https://doi.org/10.1093/aob/mci065
Ho LC, Belda R, Brown M, Andrews J, Adams P (1993) Uptake and transport of calcium and the possible causes of blossom-end rot in tomato. J Exp Bot 44(2):509–518. https://doi.org/10.1093/jxb/44.2.509
Ho LC, Adams P, Li XZ, Shen H, Andrews J, Xu ZH (2015) Responses of Ca-efficient and Ca-inefficient tomato cultivars to salinity in plant growth, calcium accumulation and blossom-end rot. 70(6):909–918. https://doi.org/10.1080/14620316.1995.11515366
Hocking B, Tyerman SD, Burton RA, Gilliham M (2016) Fruit calcium: Transport and physiology. Front Plant Sci 7:569. https://doi.org/10.3389/fpls.2016.00569
Ide R, Ichiki A, Suzuki T, Jitsuyama Y (2022) Analysis of yield reduction factors in processing tomatoes under waterlogging conditions. Sci Hortic 295:110840. https://doi.org/10.1016/j.scienta.2021.110840
Ikeda H, Shibuya T, Nishiyama M, Nakata Y, Kanayama Y (2017) Physiological Mechanisms Accounting for the Lower Incidence of Blossom-end Rot in Tomato Introgression Line IL8-3 Fruit. Horticult J 86(3):327–333. https://doi.org/10.2503/hortj.OKD-015
Ikeda H, Kanayama Y (2015) Blossom-End Rot in Fruit Vegetables. Kanayama Y, Kochetov A (eds) Abiotic Stress Biology in Horticultural Plants. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55251-2_9
Iqbal R, Raza MAS, Toleikiene M, Ayaz M, Hashemi F, Habib-ur-Rahman M, Zaheer MS, Ahmad S, Riaz U, Ali M, Aslam MU, Haider I (2020) Partial root-zone drying (PRD), its effects and agricultural significance: a review. Bull Nat Res Centre 44(1):159. https://doi.org/10.1186/s42269-020-00413-w
Irfan PK, Vanjakshi V, Prakash MNK, Ravi R, Kudachikar VB (2013) Calcium chloride extends the keeping quality of fig fruit (Ficus carica L.) during storage and shelf-life. Postharvest Biol Technol 82:70–75. https://doi.org/10.1016/j.postharvbio.2013.02.008
Jha G, Choudhary OP, Sharda R (2017) Comparative effects of saline water on yield and quality of potato under drip and furrow irrigation. Cogent Food Agric 3(1):1369345. https://doi.org/10.1080/23311932.2017.1369345
Jiang F, Zhang Y, Dusting GJ (2011) NADPH oxidase-mediated redox signaling: Roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev 63(1):218–242. https://doi.org/10.1124/pr.110.002980
Jolánkai M, Tarnawa Á, Horváth C, Nyárai FH, Kassai K (2016) Impact of climatic factors on yield quantity and quality of grain crops. Idojaras 120(1):73–84
Jovanovic Z, Stikic R (2018) Partial Root-Zone Drying Technique: from Water Saving to the Improvement of a Fruit Quality. Front Sustain Food Syst 1:3. https://doi.org/10.3389/fsufs.2017.00003
Karlsons A, Osvalde A, Cekstere G, Āboliņa L (2023) Effects of Ca Sprays on Fruit Ca Content and Yield of Tomato Variety Susceptible to Blossom-End Rot. Plants 12(8):1640. https://doi.org/10.3390/plants12081640
Kataoka K, Sugimoto K, Ohashi H, Yamada H (2017) Effect of organo-mineral fertilizer on tomato fruit production and incidence of blossom-end rot under salinity. Horticult J 86(3):357–364. https://doi.org/10.2503/hortj.OKD-041
Kazemi M (2014) Effect of foliar application of humic acid and calcium chloride on tomato growth. Bull Environ, Pharmacol Life Sci 3(3):41–46
Kılıç O, Boz İ, Eryılmaz GA (2020) Comparison of conventional and good agricultural practices farms: A socio-economic and technical perspective. J Clean Prod 258:120666. https://doi.org/10.1016/j.jclepro.2020.120666
King SR, Davis AR, Zhang X, Crosby K (2010) Genetics, breeding and selection of rootstocks for solanaceae and cucurbitaceae. Sci Hortic 127(2):106–111. https://doi.org/10.1016/j.scienta.2010.08.001
Kobayashi M, Ohura I, Kawakita K, Yokota N, Fujiwara M, Shimamoto K, Doke N, Yoshioka H (2007) Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. Plant Cell 19(3):1065–1080. https://doi.org/10.1105/tpc.106.048884
Ku HM, Grandillo S, Tanksley SD (2000) fs8.1, a major QTL, sets the pattern of tomato carpel shape well before anthesis. Theor Appl Genet 101:873–878. https://doi.org/10.1007/s001220051555
Kumar A, Singh UM, Manohar M, Gaur VS (2015) Calcium transport from source to sink: understanding the mechanism(s) of acquisition, translocation, and accumulation for crop biofortification. Acta Physiol Plant 37(1):1722. https://doi.org/10.1007/s11738-014-1722-6
Kuo CG, Tsay JS, Tsai CL, Chen RJ (1981) Tipburn of Chinese cabbage in relation to calcium nutrition and distribution. Sci Hortic 14(2):131–138. https://doi.org/10.1016/0304-4238(81)90004-2
Lampugnani ER, Khan GA, Somssich M, Persson S (2018) Building a plant cell wall at a glance. J Cell Sci 131(2):1–6. https://doi.org/10.1242/jcs.207373
Liu J, Van Eck J, Cong B, Tanksley SD (2002) A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc Nat Acad Sci U S A 99(20):13302–13306. https://doi.org/10.1073/pnas.162485999
Liu H, Chen F, Lai S, Tao J, Yang H, Jiao Z (2017) Effects of calcium treatment and low temperature storage on cell wall polysaccharide nanostructures and quality of postharvest apricot (Prunus armeniaca). Food Chem 225:87–97. https://doi.org/10.1016/j.foodchem.2017.01.008
Lobell DB, Cassman KG, Field CB (2009) Crop yield gaps: Their importance, magnitudes, and causes. Annu Rev Environ Resour 34:179–204. https://doi.org/10.1146/annurev.environ.041008.093740
Macedo FG, De Melo WJ, Delarica DDL, Cruz RB, Santos EF, De Melo GMP, Mattiuz BH, da Cruz MCP, Cecílio Filho AB (2022) Nickel increases productivity, Ca accumulation and reduces blossom-end rot in tomato. Arch Agron Soil Sci 68(11):1543–1553. https://doi.org/10.1080/03650340.2021.1912322
Machado R, Serralheiro R (2019) Salt stress alleviation through fertilization in fruit crops. Fruit Crops: Diagn Manage Nutrient Constraints. 465–480. https://doi.org/10.1016/B978-0-12-818732-6.00033-2
Madani B, Mirshekari A, Sofo A, Tengku Muda Mohamed M (2016) Preharvest calcium applications improve postharvest quality of papaya fruits (Carica papaya L. cv. Eksotika II). J Plant Nutr 39(10):1483–1492. https://doi.org/10.1080/01904167.2016.1143500
Manishankar P, Wang N, Köster P, Alatar AA, Kudla J (2018) Calcium signaling during salt stress and in the regulation of ion homeostasis. J Exp Bot 69(17):4215–4226. https://doi.org/10.1093/jxb/ery201
Marcec MJ, Gilroy S, Poovaiah BW, Tanaka K (2019) Mutual interplay of Ca2+ and ROS signaling in plant immune response. Plant Sci 283:343–354. https://doi.org/10.1016/j.plantsci.2019.03.004
Marcelis LFM, Ho LC (1999) Blossom-end rot in relation to growth rate and calcium content in fruits of sweet pepper (Capsicum annuum L.). J Experiment Botany 50(332):357–363. https://doi.org/10.1093/jxb/50.332.357
Matsumoto C, Yada H, Hayakawa C, Hoshino K, Hirai H, Kato K, Ikeda H (2021) Physiological characterization of tomato introgression line IL5-4 that increases brix and blossom-end rot in ripening fruit. Horticult J 90(2):215–222. https://doi.org/10.2503/hortj.UTD-264
Mazumder MNN, Misran A, Ding P, Wahab PEM, Mohamad A (2021) Preharvest foliar spray of calcium chloride on growth, yield, quality, and shelf life extension of different lowland tomato varieties in Malaysia. Horticulturae 7(11):466. https://doi.org/10.3390/horticulturae7110466
Mestre TC, Garcia-Sanchez F, Rubio F, Martinez V, Rivero RM (2012) Glutathione homeostasis as an important and novel factor controlling blossom-end rot development in calcium-deficient tomato fruits. J Plant Physiol 169(17):1719–1727. https://doi.org/10.1016/j.jplph.2012.07.013
Micheli F (2001) Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci 6(9):414–419. https://doi.org/10.1016/S1360-1385(01)02045-3
Mohnen D (2008) Pectin structure and biosynthesis. Curr Opin Plant Biol 11(3):266–277. https://doi.org/10.1016/j.pbi.2008.03.006
Montanaro G, Dichio B, Xiloyannis C (2012) Fruit Transpiration: Mechanisms and Significance for Fruit Nutrition and Growth. Adv Selected Plant Physiol Aspects. https://doi.org/10.5772/21411
Mu Q, Huang Z, Chakrabarti M, Illa-Berenguer E, Liu X, Wang Y, Ramos A, van der Knaap E (2017) Fruit weight is controlled by Cell Size Regulator encoding a novel protein that is expressed in maturing tomato fruits. PLoS Genet 13(8):e1006930. https://doi.org/10.1371/journal.pgen.1006930
Pandey GK, Sanyal SK (2021) Ca2+-ATPase and Ca2+/Cation Antiporters. In: Functional Dissection of Calcium Homeostasis and Transport Machinery in Plants. SpringerBriefs in Plant Science. Springer, Cham. https://doi.org/10.1007/978-3-030-58502-0_9
Park S, Cheng NH, Pittman JK, Yoo KS, Park J, Smith RH, Hirschi KD (2005) Increased calcium levels and prolonged shelf life in tomatoes expressing Arabidopsis H+/Ca2+ transporters. Plant Physiol 139(3):1194–1206. https://doi.org/10.1104/pp.105.066266
Pathak RK, Singh DB, Sharma H, Pandey D, Dwivedi S (2021) Calcium uptake and translocation in plants. Calcium Transport Elements in Plants. 373–386. https://doi.org/10.1016/B978-0-12-821792-4.00018-7
Pittman JK, Bonza MC, De Michelis MI (2011) Ca2+ Pumps and Ca2+ Antiporters in Plant Development. Geisler, M., Venema, K. (eds) Transporters and Pumps in Plant Signaling. Signaling and Communication in Plants, 7. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-14369-4_5
Pittman JK, Hirschi KD (2001) Regulation of CAX1, an arabidopsis Ca2+/H+ antiporter. Identification of an N-terminal autoinhibitory domain. Plant Physiol 127(3):1020–1029. https://doi.org/10.1104/pp.010409
Plieth C (2001) Plant calcium signaling and monitoring: Pros and cons and recent experimental approaches. Protoplasma 218:1–23. https://doi.org/10.1007/BF01288356
Powder KE (2020) Quantitative Trait Loci (QTL) Mapping. In: Shi, X. (eds) eQTL Analysis Methods in Molecular Biology, 2082. New York: Humana. https://doi.org/10.1007/978-1-0716-0026-9_15
Prinzenberg AE, van der Schoot H, Visser RGF, Marcelis LFM, Heuvelink E, Schouten HJ (2021) Genetic mapping of the tomato quality traits brix and blossom-end rot under supplemental LED and HPS lighting conditions. Euphytica 217(12):213. https://doi.org/10.1007/s10681-021-02946-1
Rached M, Pierre B, Yves G, Matsukura C, Ariizumi T, Ezura H, Fukuda N (2018) Differences in blossom-end rot resistance in tomato cultivars is associated with total ascorbate rather than calcium concentration in the distal end part of fruits per se. Horticult J 87(3):372–381. https://doi.org/10.2503/hortj.OKD-150
Reitz NF, Mitcham EJ (2021) Lignification of tomato (Solanum lycopersicum) pericarp tissue during blossom-end rot development. Sci Hortic 276:109759. https://doi.org/10.1016/j.scienta.2020.109759
Riboldi LB, Araújo SH da C, de Freitas ST, E Castro PR de C (2018) Incidence of blossom-end rot in elongated tomato fruit. Botany 96(10):663–676. https://doi.org/10.1139/cjb-2018-0021
Riboldi LB, de Freitas ST, Norris AM, Jiang CZ (2022) Xylem functionality controlling blossom-end rot incidence in transgenic ALC::NCED tomato plants. S Afr J Bot 150:120–128. https://doi.org/10.1016/j.sajb.2022.07.015
Roeber VM, Bajaj I, Rohde M, Schmülling T, Cortleven A (2021) Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant, Cell Environ 44(3):645–664. https://doi.org/10.1111/pce.13948
Sachdev S, Ansari SA, Ansari MI, Fujita M, Hasanuzzaman M (2021) Abiotic stress and reactive oxygen species: Generation, signaling, and defense mechanisms. Antioxidants 10(2):277. https://doi.org/10.3390/antiox10020277
Sasanuma I, Suzuki T (2016) Effect of calcium on cell-wall degrading enzymes of Botrytis cinerea. Biosci Biotechnol Biochem 80(9):1730–1736. https://doi.org/10.1080/09168451.2016.1146064
Schieber M, Chandel NS (2014) ROS function in redox signaling and oxidative stress. Curr Biol 24(10):R453–R462. https://doi.org/10.1016/j.cub.2014.03.034
Schmitz-Eiberger M, Haefs R, Noga G (2002) Calcium deficiency - Influence on the antioxidative defense system in tomato plants. J Plant Physiol 159(7):733–742. https://doi.org/10.1078/0176-1617-0621
Shao T, Zhao J, Zhu T, Chen M, Wu Y, Long X, Gao X (2018) Relationship between rhizosphere soil properties and blossom-end rot of tomatoes in coastal saline-alkali land. Appl Soil Ecol 127:96–101. https://doi.org/10.1016/j.apsoil.2018.03.012
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive Oxygen Species, Oxidative Damage, and Antioxidative Defense Mechanism in Plants under Stressful Conditions. J Botany, 217037. https://doi.org/10.1155/2012/217037
Shaygan M, Baumgartl T (2022) Reclamation of Salt-Affected Land: A Review. Soil Syst 6(3):61. https://doi.org/10.3390/soilsystems6030061
Spurr AR (1959) Anatomical aspects of blossom-end rot in the tomato with special reference to calcium nutrition. Hilgardia 28(12):269–295. https://doi.org/10.3733/hilg.v28n12p269
Stael S, Wurzinger B, Mair A, Mehlmer N, Vothknecht UC, Teige M (2012) Plant organellar calcium signalling: An emerging field. J Exp Bot 63(4):1525–1542. https://doi.org/10.1093/jxb/err394
Su T, Li P, Wang H, Wang W, Zhao X, Yu Y, Zhang D, Yu S, Zhang F (2019) Natural variation in a calreticulin gene causes reduced resistance to Ca2+ deficiency-induced tipburn in Chinese cabbage (Brassica rapa ssp pekinensis). Plant Cell Environ 42(11):3044–3060. https://doi.org/10.1111/pce.13612
Suzuki K, Shono M, Egawa Y (2003) Localization of calcium in the pericarp cells of tomato fruits during the development of blossom-end rot. Protoplasma 222:149–156. https://doi.org/10.1007/s00709-003-0018-2
Syahren AM, Wong NC, Mahamud S (2012) The efficacy of calcium formulation for treatment of tomato blossom-end rot. J Tropic Agric Food Sci 40(1):89–98
Taylor MD, Locascio SJ (2004) Blossom-End Rot: A Calcium Deficiency. J Plant Nutr 27(1):123–139. https://doi.org/10.1081/PLN-120027551
Thor K (2019) Calcium—nutrient and messenger. Front Plant Sci 10:440. https://doi.org/10.3389/fpls.2019.00440
Topcu Y, Sapkota M, Illa-Berenguer E, Nambeesan SU, van der Knaap E (2021) Identification of blossom-end rot loci using joint QTL-seq and linkage-based QTL mapping in tomato. Theor Appl Genet 134(9):2931–2945. https://doi.org/10.1007/s00122-021-03869-0
Topcu Y, Nambeesan SU, van der Knaap E (2022) Blossom-end rot: a century-old problem in tomato (Solanum lycopersicum L.) and other vegetables. Molec Horticult 2(1). https://doi.org/10.1186/s43897-021-00022-9
Turhan E, Karni L, Aktas H, Deventurero G, Chang DC, Bar-Tal A, Aloni B (2006) Apoplastic anti-oxidants in pepper (Capsicum annuum L.) fruit and their relationship to blossom-end rot. J Horticult Sci Biotechnol 81(4):661–667. https://doi.org/10.1080/14620316.2006.11512121
Tuteja N, Mahajan S (2007) Calcium signaling network in plants: An overview. Plant Signal Behav 2(2):79–85. https://doi.org/10.4161/psb.2.2.4176
Uozumi A, Ikeda H, Hiraga M, Kanno H, Nanzyo M, Nishiyama M, Kanahama K, Kanayama Y (2012) Tolerance to salt stress and blossom-end rot in an introgression line, IL8-3, of tomato. Sci Hortic 138:1–6. https://doi.org/10.1016/j.scienta.2012.01.036
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141(2):384–390. https://doi.org/10.1104/pp.106.078295
Wani RA, Din S, Khan M, Hakeem SA, Jahan N, Lone RA, Baba JA, Parray GN, Khan IJ (2021) Canopy management in fruit crops for maximizing productivity. Int J Chem Stud 9(3):160–165
Watanabe T, Tomizaki R, Watanabe R, Maruyama H, Shinano T, Urayama M, Kanayama Y (2021) Ionomic differences between tomato introgression line IL8–3 and its parent cultivar M82 with different trends to the incidence of blossom-end rot. Sci Hortic 287:110266. https://doi.org/10.1016/j.scienta.2021.110266
White PJ (2001) The pathways of calcium movement to the xylem. J Exp Bot 52(358):89–899. https://doi.org/10.1093/jexbot/52.358.891
Wu Q, Shigaki T, Han JS, Kim CK, Hirschi KD, Park S (2012) Ectopic expression of a maize calreticulin mitigates calcium deficiency-like disorders in sCAX1-expressing tobacco and tomato. Plant Mol Biol 80:609–619. https://doi.org/10.1007/s11103-012-9970-6
Wu S, Zhang B, Keyhaninejad N, Rodríguez GR, Kim HJ, Chakrabarti M, Illa-Berenguer E, Taitano NK, Gonzalo MJ, Díaz A, Pan Y, Leisner CP, Halterman D, Buell CR, Weng Y, Jansky SH, van Eck H, Willemsen J, Monforte AJ, … van der Knaap E (2018) A common genetic mechanism underlies morphological diversity in fruits and other plant organs. Nat Commun 9(1):4734. https://doi.org/10.1038/s41467-018-07216-8
Xiao H, Jiang N, Schaffner E, Stockinger EJ, Van Der Knaap E (2008) A retrotransposon-mediated gene duplication underlies morphological variation of tomato fruit. Science 319(5869):1527–1530. https://doi.org/10.1126/science.1153040
Yang HQ, Jie YL (2005) Uptake and transport of calcium in plants. J Plant Physiol Molec Biol 31(3):227
Yang T, Poovaiah BW (2002) Hydrogen peroxide homeostasis: Activation of plant catalase by calcium/calmodulin. Proc Natl Acad Sci U S A 99(6):4097–4102. https://doi.org/10.1073/pnas.052564899
Zdunek A, Pieczywek PM, Cybulska J (2021) The primary, secondary, and structures of higher levels of pectin polysaccharides. Comprehens Rev Food Sci Food Safety 20(1):1101–1117. https://doi.org/10.1111/1541-4337.12689
Zhai Y, Yang Q, Wu Y (2016) Soil salt distribution and tomato response to saline water irrigation under straw mulching. PLoS ONE 11(11):e0165985. https://doi.org/10.1371/journal.pone.0165985
Zhang L, Wang JW, Chen JY, Song T, Jiang YG, Zhang YF, Wang LJ, Li FL (2019) Preharvest spraying calcium ameliorated aroma weakening and kept higher aroma-related genes expression level in postharvest ‘Nanguo’ pears after long-term refrigerated storage. Sci Horticult 247:287–295. https://doi.org/10.1016/j.scienta.2018.12.038
Funding
The authors acknowledge the Department of Science and Technology, New Delhi (DST-FIST-SR/FST/LS-I/2018/125), for financial assistance to the Department of Biochemistry, Central University of Punjab, through the DST-FIST programs. The funding agencies had no role in the preparation of the manuscript or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
KS: Prepared the initial draft, SSD provided guidance on management techniques, and VKB finalized the content and wrote the manuscript
Corresponding author
Ethics declarations
Ethical Approval
This article does not contain any studies with human participants or animals by any of the authors.
Consent for Publication
Written informed consent for publication was obtained from all the authors.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sethi, K., Dhaka, S.S. & Bari, V.K. Insights into Blossom End-Rot Disorder in Tomato (Solanum lycopersicum). Plant Mol Biol Rep (2024). https://doi.org/10.1007/s11105-024-01442-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11105-024-01442-9