Abstract
The retinal pigment epithelium (RPE) is a single layer of cuboidal cells that develops from the neural tube as well as neural retina does, and is therefore considered part of the central nervous system. While the neural retina receives light and converts it to electrical signals to process information and transfer it to the brain, the RPE acts as an essential coordinator of the retinal microenvironment for appropriate retinal neural function. Reactive oxygen species (ROS) are generated during basal cellular metabolic processes. Moreover, daily exposure to light stimuli enhances the production of ROS in the RPE and the neural retina, the excessive accumulation of which contributes to retinal pathogenesis. In this section, oxidative stress in the RPE and its proposed contribution to age-related macular degeneration (AMD) are discussed, reviewing recent in vivo and in vitro studies that support the role of oxidative stress in the development and progression of AMD.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Inflammation
- Light exposure
- Reactive oxygen species (ROS)
- Retinal pigment epithelium (RPE)
- Tight junction
1 Roles of the RPE and Oxidative Stress
1.1 Physiological Roles of the RPE
The retinal pigment epithelium (RPE) is a multifunctional regulator of the retinal microenvironment. Its physiological and cellular functions include the recycling of retinal pigment during the visual cycle, the directional transport of oxygen and nutrients, the formation of the blood–retinal barrier (BRB) which regulates the infiltration of molecules and cells, and the polarized secretion of cytokines and chemokines [1, 2].
The RPE plays a central role in regulating the visual cycle of rhodopsin. During phototransduction, the rhodopsin chromophore 11-cis retinal is converted into all-trans retinol, which accumulates in the outer segments of photoreceptor cells. The RPE cells phagocytose the outer segments containing all-trans retinol, which is then sequentially metabolized to regenerate 11-cis retinal and the rhodopsin pigment. During this process, N-retinylidene-N-retinylethanolamine (A2E) is generated, which can accumulate and lead to lipofuscin formation in the aging RPE. A2E also induces production of reactive oxygen species (ROS) in response to blue light [3], which can result in RPE cell death [4]. An abnormality in the gene encoding a transmembrane transporter, ATP-binding cassette, subfamily A (ABC1), member 4 (ABCA4), causes A2E and lipofuscin accumulation, leading to the retinal degenerative condition known as Stargardt disease [5]. Light-induced photoreceptor cell death is suppressed in the absence of rhodopsin [6] or in the presence of a mutation in an enzyme of the visual cycle, RPE65 [7]. These findings suggest that cellular metabolism during the visual cycle in the RPE contributes to increases in ROS and the development of tissue damage in the retina.
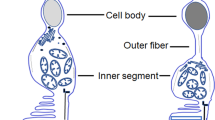
The RPE controls the flow of fluid which contains oxygen and nutrients from the highly vascularized choroid into the outer retina. Together the vascular and epithelial components of the BRB maintain the specialized environment of the neural retina [8]. The tight junctions and adherens junctions (Fig. 17.1) form connections between cells and regulate the localization of membrane proteins and cell polarity, and in the retinal environment, these structures are important for establishing the BRB. Thus, breakdown of the BRB can result in abnormal infiltrations of molecules and/or cells, including vascular endothelial cells and inflammatory cells, which may contribute to the development and/or progression of AMD lesions in the subretinal space.
The RPE secretes vascular endothelial growth factor (VEGF), which is indispensable for maintaining the choroidal vessels that provide oxygen and nutrients to the outer neural retina, mainly the photoreceptor cells [9, 10]. In mice that have been engineered to express only the membrane-bound, non-soluble isoform of VEGF (VEGF188), vessels in the choroid undergo degeneration, followed by photoreceptor cell apoptosis [9]. Targeted depletion of VEGF in the adult RPE also causes the similar changes [10]. The RPE also secretes a neuroprotective and vascular suppressive factor, pigment epithelium-derived factor (PEDF), which inhibits excessive VEGF signaling; insufficient PEDF may also be associated with AMD pathogenesis [11, 12]. In addition to these physiological cytokines, the RPE also secretes monocyte chemotactic factor-1 (MCP-1), which can recruit inflammatory cells and promote AMD lesions [13, 14]. Various studies have shown that ROS cross talk with inflammatory reactions can lead to a vicious cycle [15–18] that contributes to the development of AMD [19, 20].
1.2 ROS and Oxidative Stress
Oxidative stress occurs when excessive ROS accumulate and are not eliminated by the normal biological self-defense systems (see below), resulting in tissue/organ damage and various pathological conditions. ROS are highly reactive chemicals derived from oxygen following exposure to high-energy or electron-transferring chemical reactions [21, 22]. ROS include singlet oxygen (1O2), superoxide anion radical (O⋅‐), hydroxyl radical (HO˙), and hydrogen peroxide (H2O2) (Fig. 17.2). ROS are toxic to biological organisms due to their oxidation of lipids, proteins, DNA, and carbohydrates, which results in the loss of normal membrane, metabolic, and reproductive functions.
1.3 Generation, Elimination, and Accumulation of ROS
ROS are unavoidable by-products of the biochemical pathways involved in energy production and storage, such as glycolysis, the electron transport chain, and photosynthesis [22]. Mitochondrial oxidants are formed predominantly at complexes I and III of the cytochrome chain when electrons initially derived from NADH or FADH2 react with oxygen to produce superoxide anions [23]. Nicotinamide adenine dinucleotide phosphate (NADPH)-dependent oxidases also generate ROS. The NADPH-dependent oxidases comprise a seven-member family of membrane-bound enzymes (Nox1–5 and Duox1–2) that are widely expressed and evolutionarily conserved [23]. NADPH-dependent oxidase-produced ROS directly eliminate invading pathogens and play a central role in the pathobiology of sepsis by regulating the bactericidal activity of phagocytes [24]. Additional cellular sources of ROS production include a number of intracellular enzymes, such as xanthine oxidase, cyclooxygenases, cytochrome p450 enzymes, and lipoxygenases, all of which produce oxidants as part of their normal enzymatic function [23].
ROS levels are regulated by several enzymes that eliminate ROS in the cytosol or mitochondria (Fig. 17.3). These enzymes include the superoxide dismutase (SOD) family. SOD1 (Cu–Zn SOD) is cytosolic, while SOD2 (Mn SOD) localizes to the mitochondria, and SOD3 (a Cu–Zn SOD encoded by another gene and with a different protein structure compared to SOD1) localizes to the extracellular space. In the peroxisome, catalases are expressed that eliminate the H2O2 produced during the β-oxidation-mediated breakdown of long-chain fatty acids which are subsequently shuttled to mitochondria to generate ATP. One of the most important cellular antioxidants is glutathione, a cysteine-containing peptide found in most forms of aerobic life. Glutathione’s antioxidant properties are due to a thiol group in its cysteine moiety that functions as a reducing agent and can be reversibly oxidized and reduced.
Antioxidative reactions. SOD converts O⋅‐ to H2O2, which is further converted to H2O by catalase. H2O2 is also converted to H2O by glutathione peroxidase. In this system, GSH acts as an antioxidant reducing H2O2 and is oxidized to become GSSG. GSSG is then reduced by glutathione reductase to replenish GSH. SOD superoxide dismutase, GSH reduced glutathione, GSSG oxidized glutathione representing glutathione-S-S-glutathione
An additional means of regulating oxidation is by the uptake of exogenous antioxidative molecules. Examples of exogenous antioxidants include the carotenoid, lutein [17, 25–27], and vitamins. Lutein is not synthesized in humans and is considered to be a micronutrient found in certain foods, such as spinach and kale. Lutein is delivered to the retina and concentrated in the macula, the center of the retina; it also accumulates in the RPE [17, 28]. Two large clinical studies, the Age-Related Eye Disease Study (AREDS) [29] and AREDS 2 [30], demonstrated that lutein intake protects against the progression of AMD.
The cellular accumulation of ROS is determined by the net balance of ROS-generating and ROS-eliminating reactions. When ROS generation is excessive or when elimination fails, high levels of ROS will promote tissue/organ damage. Excessive ROS production can result from a metabolic imbalance induced by diabetes or in response to external environmental insults such as exposure to light or smoke, while the reduced elimination of ROS occurs when ROS-eliminating enzymes are disabled due to genetic abnormalities [31–33] or aging-related reductions in enzymatic activity [34]. For example, the SOD activity in mesenteric lymphatic vessels was shown to be reduced in 24- versus 9-month-old rats [34]. Interestingly, excessive ROS accumulation may lead to the reduced expression of antioxidative enzymes; Yuki et al. showed that oxidative stress caused by N-methyl-d-aspartate (NMDA) results in reduced SOD1 mRNA and protein levels in the retina [33]. Thus, ROS accumulation is mediated by multiple mechanisms.
2 Association of Oxidative Stress in the RPE with Aging and AMD
Although the ROS generated during normal metabolic processes are eliminated through the pathways described above, oxidative stress occurs constantly, and the effects of residual ROS that are not completely removed may contribute to the aging process [32, 35, 36]. The age-related increase in oxidative stress leads to an increased accumulation of autoxidative lipofuscin in the lysosomes of RPE cells, as well as drusen formation in the extracellular space between the RPE and Bruch’s membrane [2, 37]. The accumulation of oxidized low-density lipoproteins and lipid peroxidation end products reduces the degradation of phagocytosed photoreceptor outer segments and increases cellular stress in the RPE cells [38]. Excess ROS can lead to protein damage and unfolding, especially in age-related conditions [39]. Oxidative stress can also lead to mitochondrial DNA damage, which further increases ROS generation and reduces the metabolic capacity, thereby enhancing age-related degenerative tissue changes [2]. Moreover, these ROS-induced cellular changes may lead to the further production of ROS that are not properly eliminated, thus accelerating the ROS-induced pathogenic changes [32, 33].
These aging-related events are believed to lead to subsequent immunological responses, including the production of several types of inflammation-related molecules, the recruitment of leukocytes such as macrophages and dendritic cells, and the activation of complement pathways, and all of these processes are related to the pathogenesis of AMD [19]. AMD risk factors include smoking, metabolic syndromes (including hypertension and arteriosclerosis), single nucleotide polymorphisms (SNPs) [20, 40–42], and light exposure [43], all of which are proposed sources of ROS accumulation and can lead to consecutive and chronic inflammation. Recent studies indicate that the RPE is a primary target of oxidative stress and that changes in the RPE represent early changes in the pathogenesis of AMD. In the following sections, specific experimental studies are introduced.
3 In Vitro Analysis of Oxidative Stress Using the ARPE-19 Human RPE Cell Line
ARPE-19 is a spontaneously arising RPE cell line derived from the normal eyes of a 19-year-old male. To explore the underlying mechanisms of AMD, oxidative stress in the RPE is often studied using this cell line. ROS are induced by treating the cells with H2O2 or paraquat or by exposing them to ultraviolet or visible light [44, 45]. ARPE-19 cells exhibit barrier functions mediated by tight and adherens junctions and secrete cytokines, consistent with the RPE’s functions in vivo. However, the ARPE-19 cells exhibit reduced transepithelial resistance (TER), reduced levels of secreted cytokines, and limited formation of the hexagonal shape, a distinctive characteristic of the RPE, compared with the primary RPE culture known as fetal human RPE (fhRPE) [11, 35].
Despite these limitations, ARPE-19 cells are still commonly used for studying the influence of oxidative stress on the RPE. When ARPE-19 cells are treated intermittently and repeatedly with tert-butylhydroperoxide (tert-BHP), an organic peroxide, they exhibit four well-known senescence biomarkers: hypertrophy, senescence-associated β-galactosidase activity, growth arrest, and cell-cycle arrest in G1. This chronic oxidative stress leads to modifications of the transcriptome and cellular functions, which are involved in the cellular aging phenotype and pathophysiology of AMD [46]. The cells under this treatment also show increased amyloidogenesis, which can contribute to drusen formation, and an angiogenic molecular expression profile associated with AMD pathogenesis [47]. Alternatively, another group showed that treating ARPE-19 cells with A2E combined with blue light irradiation induces MCP-1, interleukin-8 (IL-8), and complement factor H (CFH), which are also proposed to be involved in AMD pathogenesis [48].
ARPE-19 cells have also been used to investigate the effects of oxidative stress on cell–cell junctions and their association with AMD pathogenesis [49, 50]. The cadherin proteins are essential components of the adherens junctions and have important roles in cell adhesion. Studies using ARPE-19 cells showed that ROS-mediated Src kinase activation increases the tyrosine phosphorylation of p120 catenin, a cellular protein that associates with and regulates cadherin turnover at the cell surface, thereby controlling the level of cadherin available for cell–cell adhesion. The induction of ROS rapidly triggers the translocation of p120 catenin and the internalization of N-cadherin from the cell–cell adhesion sites to an early endosomal compartment. The endosomal accumulation of p120 catenin results in stress fiber formation and cell–cell dissociation through activation of the Rho/Rho-associated protein kinase (ROCK) signaling pathway. Another group reported that white light exposure disrupts the expression of zona occludens (ZO-1), a component of tight junctions, in the ARPE19 cells and simultaneously activates the Wnt/β-catenin pathway [45]. These results are consistent with other studies showing that p120 and β-catenin bind to the intracellular domain of cadherin and are released when Wnt ligand binds to its receptor, Dvl, a human homolog of the Drosophila dishevelled. In fact, the oxidative stress-induced cytoskeletal remodeling and cell–cell dissociation were transient in their study, due to the induction of SOD and activation of a nuclear factor-κB (NF-κB)-induced negative feedback loop [49]; the ROS generation–elimination systems can be balanced under their study condition; therefore, the junction may be repaired.
Much of the research investigating the effects of oxidative stress on the RPE has been performed using ARPE-19 cells, in part because of the challenge in isolating sufficient primary RPE for mechanistic studies. However, since AMD pathogenesis involves whole ocular and systemic interactions, in vivo studies are indispensable for understanding this disease.
4 Proposed Mechanisms Linking Oxidative Stress in the RPE to the Pathogenesis of AMD
4.1 Analysis of AMD Pathogenesis in Animal Models
In vivo AMD pathogenesis studies are often performed with laser-induced choroidal neovascularization (CNV) models using wild-type or genetically modified mice [51–60]. These models are well accepted and have shed light on the inflammatory cytokines and signaling molecules contributing to neovascularization, including IL-6 [52], IL-17 [51], CCR3 [57], the renin–angiotensin system (including angiotensin II type 1 receptor [55], bradykinin [55], and the prorenin receptor [58]), JNK [59], and hyaluronan-CD44 [54]. In contrast, IL-18 has been shown to be a protective factor that inhibits CNV development [61].
The impact of MCP-1 in generating CNV has been reported in a model of CNV generated by injecting oxidized phospholipids into the subretinal space [14]. Notably, the CNV induction in this model is not observed in MCP-1 knockout mice. The study also showed that mild, chronic light exposure for 6 months induces the accumulation of oxidized phospholipids in the retina (including the RPE), which induces CNV in 50 % of the time (they found CNVs in four eyes in the eight analyzed eyes), while MCP-1 knockout mice never show CNV generation under the same condition. Since light exposure is associated with AMD [43], this model may be more physiologically relevant than the artificially laser-induced CNV model.
There are certain gene-targeted mice which develop AMD-related pathogenesis in the absence of CNV development. Neprilysin-deficient mice, in which amyloid β accumulates, exhibit RPE vacuolization and VEGF–PEDF imbalance [47], and Dicer1-deficient mice accumulate Alu RNA and show subsequent RPE degeneration and geographic atrophy, which is characteristic of an AMD subtype [62].
Inflammation and accumulated materials are highly intertwined with oxidative stress that could be involved in the AMD pathogenesis [35].
4.2 Influence of Light-Induced ROS in the RPE
In addition to the abovementioned studies, the significance of ROS and the underlying molecular mechanism in the development of AMD-related pathological changes in the RPE was investigated by Narimatsu et al., using another model in which wild-type Balb/c mice are acutely exposed to moderate light [63]; Balb/c mice have a polymorphism in the RPE65 gene, which is required for the visual cycle, and are thus more susceptible to light-induced retinal degeneration than C57B/6J mice [7]. In this study, the examination of flat-mounted RPE samples revealed that light exposure leads to the disruption of cell–cell junctions and the actin cytoskeleton. The analysis of specific membrane-bound molecules, including ZO-1 (a tight junction marker), N-cadherin and β-catenin (both adherens junction markers), and F-actin (an actin cytoskeleton marker detected by phalloidin), revealed the disruption of cell junctions (Fig. 17.4). These observations suggested that light exposure leads to breakdown of the BRB, which may promote CNV invasion and related exudative changes, as well as an infiltration of inflammatory cells, from the choroid into the subretinal space.
In this model, analysis using DCF-DH, a fluorescent probe that detects hydroxyl and peroxyl radicals, and other ROS, after deacetylation by endogenous esterases, revealed that the ROS levels in the RPE–choroid are elevated immediately after light exposure. (Separation of the RPE and choroid was not technically feasible; thus the two tissues were analyzed as a complex.) To determine if the altered cell junctions and cytoskeletal disruptions are mediated by the excessive ROS accumulation, light-exposed mice were treated with the antioxidant N-acetylcysteine (NAC). NAC is a by-product of glutathione and plays a role in glutathione maintenance and metabolism [64]; it is also an approved pharmaceutical drug and nutritional supplement used primarily as a mucolytic agent and in the detoxification of paracetamol (acetaminophen) overdose. NAC treatment was shown to prevent the light-induced cell–cell junction and actin cytoskeletal disruption, as indicated by the attenuated loss of membrane-bound ZO-1, N-cadherin, β-catenin, and F-actin. These findings indicated that excessive ROS levels are responsible for these changes and implicate oxidative stress-induced breakdown of the BRB in the pathogenesis of AMD.
In general, cell–cell junctions are regulated by Rho/ROCK signaling [65]. ROCK, activated by GTP-bound RhoA, phosphorylates LIM kinase, myosin light chain (MLC), and MLC phosphatase, all of which are key regulators of actin organization. Notably, ROCK activity increased after light exposure, and NAC treatment reduced the ROCK activation, in the above-mentioned study, indicating that ROCK is activated by excessive ROS accumulation [63]. A ROCK inhibitor, Y27632, suppressed the light-induced cell junction and actin cytoskeleton disruption in the RPE. Taken together, these findings suggest that light-induced ROS accumulation leads to ROCK activation, which disrupts cell–cell junctions and the actin cytoskeleton, resulting in breakdown of the BRB, in vivo. This mechanism may be involved in AMD progression.
Inflammatory cytokines and macrophages have also been implicated in AMD pathogenesis [13, 14, 57]. To investigate the involvement of light-induced ROS in promoting cytokine expression in the RPE–choroid, Narimatsu et al. also analyzed cytokine mRNA and protein levels after light exposure [63]; MCP-1 is strongly induced by light exposure at both the mRNA and protein levels, and macrophage infiltration is increased in the RPE–choroid, as shown by both F4/80 mRNA induction and the recruitment of F4/80-immunopositive cells to the choroid and subretinal space. The macrophage infiltration may result from the disrupted RPE and may contribute to CNV invasion into the subretinal space, since VEGF is secreted by macrophages. In addition, all of the inflammatory changes were found to be inhibited by NAC-mediated ROS suppression or Y27632-mediated ROCK inhibition. Another inflammatory cytokine, IL-6, which is also implicated in AMD pathogenesis [52], increases at the mRNA and protein levels in the RPE–choroid after light exposure, and these increases are suppressed by NAC or Y27632 treatment. Ccl-11, another AMD-related cytokine, was also found to increase after light exposure and to decrease by NAC administration, but was not regulated by ROCK activity. Taken together, these findings demonstrate that the light-induced inflammatory changes associated with AMD pathogenesis are mediated by ROS accumulation and in many cases ROCK activation.
Although light exposure is a widely accepted risk factor for AMD development, the underlying mechanisms contributing to AMD’s pathogenesis have not been fully defined. However, the study by Narimatsu et al. suggests that light exposure acts on the RPE–choroid and increases the risk of AMD through ROS accumulation and ROCK activation (Fig. 17.5). To develop treatments for age-related retinal pathologies, it is important to better understand the biological effects of light exposure on the RPE, which plays a number of important roles in supporting retinal function but may cause AMD when oxidative stress accumulates. Therapeutic approaches designed to block the molecular mechanisms involved in light-induced retinal changes would be of great value. The study discussed here raises the possibility that pharmaceuticals targeting ROS and ROCK signaling pathways may provide additional benefit when used in combination with light-blocking strategies [66, 67]. Further studies investigating the underlying molecular mechanisms and exploring potential therapeutic approaches are required.
Proposed model of the mechanism linking light exposure in the RPE–choroid to the pathogenesis of AMD. Intense light produces excessive ROS, thereby promoting ROCK activation, which leads to the disruption of cell–cell junctions and breakdown of the BRB, pathological cytokine production, and macrophage recruitment to the RPE and/or choroid. These changes may promote AMD development and progression
5 Summary
It is well accepted that ROS accumulation in the RPE contributes to AMD pathogenesis. The studies reviewed here suggest that light stimuli may be one of the important factors that cause ROS accumulation. Light-induced ROS accelerate the pathological changes in the RPE, at least in part, by altering the BRB structure, the molecular expression of inflammatory molecules, and the subsequent recruitment of inflammatory cells. These in vivo cellular changes observed in mice and the underlying molecular mechanisms contribute to our understanding of the pathogenesis of AMD and to the investigation of new treatment targets. Further biological studies are warranted.
References
Athanasiou D, Aguila M, Bevilacqua D, Novoselov SS, Parfitt DA, Cheetham ME (2013) The cell stress machinery and retinal degeneration. FEBS Lett 587(13):2008–2017. doi:10.1016/j.febslet.2013.05.020
Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Boulton ME, Petrovski G (2013) Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy 9(7):973–984. doi:10.4161/auto.24546
Sparrow JR, Zhou J, Ben-Shabat S, Vollmer H, Itagaki Y, Nakanishi K (2002) Involvement of oxidative mechanisms in blue-light-induced damage to A2E-laden RPE. Invest Ophthalmol Vis Sci 43(4):1222–1227
Maeda A, Maeda T, Golczak M, Palczewski K (2008) Retinopathy in mice induced by disrupted all-trans-retinal clearance. J Biol Chem 283(39):26684–26693. doi:10.1074/jbc.M804505200
Fujinami K, Zernant J, Chana RK, Wright GA, Tsunoda K, Ozawa Y, Tsubota K, Webster AR, Moore AT, Allikmets R, Michaelides M (2013) ABCA4 gene screening by next-generation sequencing in a British cohort. Invest Ophthalmol Vis Sci 54(10):6662–6674. doi:10.1167/iovs.13-12570
Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Reme CE (2000) Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet 25(1):63–66. doi:10.1038/75614
Wenzel A, Grimm C, Samardzija M, Reme CE (2003) The genetic modifier Rpe65Leu(450): effect on light damage susceptibility in c-Fos-deficient mice. Invest Ophthalmol Vis Sci 44(6):2798–2802
Runkle EA, Antonetti DA (2011) The blood-retinal barrier: structure and functional significance. Methods Mol Biol 686:133–148. doi:10.1007/978-1-60761-938-3_5
Saint-Geniez M, Kurihara T, Sekiyama E, Maldonado AE, D'Amore PA (2009) An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci USA 106(44):18751–18756. doi:10.1073/pnas.0905010106
Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M (2012) Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest 122(11):4213–4217. doi:10.1172/JCI65157
Ablonczy Z, Prakasam A, Fant J, Fauq A, Crosson C, Sambamurti K (2009) Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through gamma-secretase. J Biol Chem 284(44):30177–30186. doi:10.1074/jbc.M109.032391
Sonoda S, Sreekumar PG, Kase S, Spee C, Ryan SJ, Kannan R, Hinton DR (2010) Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging 2(1):28–42
Sakurai E, Anand A, Ambati BK, van Rooijen N, Ambati J (2003) Macrophage depletion inhibits experimental choroidal neovascularization. Invest Ophthalmol Vis Sci 44(8):3578–3585
Suzuki M, Tsujikawa M, Itabe H, Du ZJ, Xie P, Matsumura N, Fu X, Zhang R, Sonoda KH, Egashira K, Hazen SL, Kamei M (2012) Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J Cell Sci 125(Pt 10):2407–2415. doi:10.1242/jcs.097683
Miyake S, Takahashi N, Sasaki M, Kobayashi S, Tsubota K, Ozawa Y (2012) Vision preservation during retinal inflammation by anthocyanin-rich bilberry extract: cellular and molecular mechanism. Lab Invest J Tech Meth Pathol 92(1):102–109. doi:10.1038/labinvest.2011.132
Ozawa Y, Kurihara T, Sasaki M, Ban N, Yuki K, Kubota S, Tsubota K (2011) Neural degeneration in the retina of the streptozotocin-induced type 1 diabetes model. Exp Diabetes Res 2011:108328. doi:10.1155/2011/108328
Ozawa Y, Sasaki M, Takahashi N, Kamoshita M, Miyake S, Tsubota K (2012) Neuroprotective effects of lutein in the retina. Curr Pharm Des 18(1):51–56
Ozawa Y, Yuki K, Yamagishi R, Tsubota K, Aihara M (2013) Renin-angiotensin system involvement in the oxidative stress-induced neurodegeneration of cultured retinal ganglion cells. Jpn J Ophthalmol 57(1):126–132. doi:10.1007/s10384-012-0204-x
Grisanti S, Tatar O (2008) The role of vascular endothelial growth factor and other endogenous interplayers in age-related macular degeneration. Prog Retin Eye Res 27(4):372–390. doi:10.1016/j.preteyeres.2008.05.002
Ozawa Y, Ishida S, Tsubota K (2008) Age-related macular degeneration (AMD); from pathogenesis and approved therapies to proposed treatments for prevention. Anti-Aging Med 5:87–92, http://www.anti-aging.gr.jp/webjournal/thesis/vol0509/PDF/5-9r_2008.pdf
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. doi:10.1146/annurev.arplant.55.031903.141701
Rodriguez R, Redman R (2005) Balancing the generation and elimination of reactive oxygen species. Proc Natl Acad Sci USA 102(9):3175–3176. doi:10.1073/pnas.0500367102
Finkel T (2011) Signal transduction by reactive oxygen species. J Cell Biol 194(1):7–15. doi:10.1083/jcb.201102095
Kong X, Thimmulappa R, Kombairaju P, Biswal S (2010) NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice. J Immunol 185(1):569–577. doi:10.4049/jimmunol.0902315
Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K (2010) Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 53(5):971–979. doi:10.1007/s00125-009-1655-6
Sasaki M, Ozawa Y, Kurihara T, Noda K, Imamura Y, Kobayashi S, Ishida S, Tsubota K (2009) Neuroprotective effect of an antioxidant, lutein, during retinal inflammation. Invest Ophthalmol Vis Sci 50(3):1433–1439. doi:10.1167/iovs.08-2493
Sasaki M, Yuki K, Kurihara T, Miyake S, Noda K, Kobayashi S, Ishida S, Tsubota K, Ozawa Y (2012) Biological role of lutein in the light-induced retinal degeneration. J Nutr Biochem 23(5):423–429. doi:10.1016/j.jnutbio.2011.01.006
Ozawa Y, Sasaki M (2013) Lutein and oxidative stress-mediated retinal neurodegeneration in diabetes. In: Preedy V (ed) Diabetes: oxidative stress and dietary antioxidants. Academic, New York, pp 223–229
Age-Related Eye Disease Study Research G, SanGiovanni JP, Chew EY, Clemons TE, Ferris FL 3rd, Gensler G, Lindblad AS, Milton RC, Seddon JM, Sperduto RD (2007) The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report No. 22. Arch Ophthalmol 125(9):1225–1232. doi:10.1001/archopht.125.9.1225
Age-Related Eye Disease Study 2 Research G (2013) Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 309(19):2005–2015. doi:10.1001/jama.2013.4997
Hashizume K, Hirasawa M, Imamura Y, Noda S, Shimizu T, Shinoda K, Kurihara T, Noda K, Ozawa Y, Ishida S, Miyake Y, Shirasawa T, Tsubota K (2008) Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am J Pathol 172(5):1325–1331. doi:10.2353/ajpath.2008.070730
Yuki K, Ozawa Y, Yoshida T, Kurihara T, Hirasawa M, Ozeki N, Shiba D, Noda K, Ishida S, Tsubota K (2011) Retinal ganglion cell loss in superoxide dismutase 1 deficiency. Invest Ophthalmol Vis Sci 52(7):4143–4150. doi:10.1167/iovs.10-6294
Yuki K, Yoshida T, Miyake S, Tsubota K, Ozawa Y (2013) Neuroprotective role of superoxide dismutase 1 in retinal ganglion cells and inner nuclear layer cells against N-methyl-d-aspartate-induced cytotoxicity. Exp Eye Res 115:230–238. doi:10.1016/j.exer.2013.07.002
Thangaswamy S, Bridenbaugh EA, Gashev AA (2012) Evidence of increased oxidative stress in aged mesenteric lymphatic vessels. Lymphatic Res Biol 10(2):53–62. doi:10.1089/lrb.2011.0022
Ardeljan D, Chan CC (2013) Aging is not a disease: Distinguishing age-related macular degeneration from aging. Prog Retin Eye Res 37:68–89. doi:10.1016/j.preteyeres.2013.07.003
Gu X, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, Rayborn ME, Hollyfield JG, Bonilha VL (2012) Age-related changes in the retinal pigment epithelium (RPE). PLoS One 7(6):e38673. doi:10.1371/journal.pone.0038673
Plafker SM, O'Mealey GB, Szweda LI (2012) Mechanisms for countering oxidative stress and damage in retinal pigment epithelium. Int Rev Cell Mol Biol 298:135–177. doi:10.1016/B978-0-12-394309-5.00004-3
Finnemann SC, Leung LW, Rodriguez-Boulan E (2002) The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc Natl Acad Sci USA 99(6):3842–3847. doi:10.1073/pnas.052025899
Alexeyev MF (2009) Is there more to aging than mitochondrial DNA and reactive oxygen species? FEBS J 276(20):5768–5787. doi:10.1111/j.1742-4658.2009.07269.x
Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA (2005) Complement factor H polymorphism and age-related macular degeneration. Science 308(5720):421–424. doi:10.1126/science.1110189
Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA (2005) Complement factor H variant increases the risk of age-related macular degeneration. Science 308(5720):419–421. doi:10.1126/science.1110359
Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J (2005) Complement factor H polymorphism in age-related macular degeneration. Science 308(5720):385–389. doi:10.1126/science.1109557
Clemons TE, Milton RC, Klein R, Seddon JM, Ferris FL 3rd, Age-Related Eye Disease Study Research G (2005) Risk factors for the incidence of advanced age-related macular degeneration in the age-related eye disease study (AREDS) AREDS report no. 19. Ophthalmology 112(4):533–539. doi:10.1016/j.ophtha.2004.10.047
Relvas LJ, Bouffioux C, Marcet B, Communi D, Makhoul M, Horckmans M, Blero D, Bruyns C, Caspers L, Boeynaems JM, Willermain F (2009) Extracellular nucleotides and interleukin-8 production by ARPE cells: potential role of danger signals in blood-retinal barrier activation. Invest Ophthalmol Vis Sci 50(3):1241–1246. doi:10.1167/iovs.08-1902
Iriyama A, Iriyama T, Tamaki Y, Yanagi Y (2008) Effects of white light on beta-catenin signaling pathway in retinal pigment epithelium. Biochem Biophys Res Comm 375(1):173–177. doi:10.1016/j.bbrc.2008.07.158
Glotin AL, Debacq-Chainiaux F, Brossas JY, Faussat AM, Treton J, Zubielewicz A, Toussaint O, Mascarelli F (2008) Prematurely senescent ARPE-19 cells display features of age-related macular degeneration. Free Radic Biol Med 44(7):1348–1361. doi:10.1016/j.freeradbiomed.2007.12.023
Yoshida T, Ohno-Matsui K, Ichinose S, Sato T, Iwata N, Saido TC, Hisatomi T, Mochizuki M, Morita I (2005) The potential role of amyloid beta in the pathogenesis of age-related macular degeneration. J Clin Invest 115(10):2793–2800. doi:10.1172/JCI24635
Bian Q, Gao S, Zhou J, Qin J, Taylor A, Johnson EJ, Tang G, Sparrow JR, Gierhart D, Shang F (2012) Lutein and zeaxanthin supplementation reduces photooxidative damage and modulates the expression of inflammation-related genes in retinal pigment epithelial cells. Free Radic Biol Med 53(6):1298–1307. doi:10.1016/j.freeradbiomed.2012.06.024
Inumaru J, Nagano O, Takahashi E, Ishimoto T, Nakamura S, Suzuki Y, Niwa S, Umezawa K, Tanihara H, Saya H (2009) Molecular mechanisms regulating dissociation of cell–cell junction of epithelial cells by oxidative stress. Genes Cells Devoted Mol Cell Mech 14(6):703–716. doi:10.1111/j.1365-2443.2009.01303.x
Bailey TA (2004) Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 45(2):675–684. doi:10.1167/iovs.03-0351
Hasegawa E, Sonoda KH, Shichita T, Morita R, Sekiya T, Kimura A, Oshima Y, Takeda A, Yoshimura T, Yoshida S, Ishibashi T, Yoshimura A (2013) IL-23-independent induction of IL-17 from gammadeltaT cells and innate lymphoid cells promotes experimental intraocular neovascularization. J Immunol 190(4):1778–1787. doi:10.4049/jimmunol.1202495
Izumi-Nagai K, Nagai N, Ohgami K, Satofuka S, Ozawa Y, Tsubota K, Umezawa K, Ohno S, Oike Y, Ishida S (2007) Macular pigment lutein is antiinflammatory in preventing choroidal neovascularization. Arterioscler Thromb Vasc Biol 27(12):2555–2562. doi:10.1161/ATVBAHA.107.151431
Lambert V, Lecomte J, Hansen S, Blacher S, Gonzalez ML, Struman I, Sounni NE, Rozet E, de Tullio P, Foidart JM, Rakic JM, Noel A (2013) Laser-induced choroidal neovascularization model to study age-related macular degeneration in mice. Nat Protoc 8(11):2197–2211. doi:10.1038/nprot.2013.135
Mochimaru H, Takahashi E, Tsukamoto N, Miyazaki J, Yaguchi T, Koto T, Kurihara T, Noda K, Ozawa Y, Ishimoto T, Kawakami Y, Tanihara H, Saya H, Ishida S, Tsubota K (2009) Involvement of hyaluronan and its receptor CD44 with choroidal neovascularization. Invest Ophthalmol Vis Sci 50(9):4410–4415. doi:10.1167/iovs.08-3044
Nagai N, Oike Y, Izumi-Nagai K, Koto T, Satofuka S, Shinoda H, Noda K, Ozawa Y, Inoue M, Tsubota K, Ishida S (2007) Suppression of choroidal neovascularization by inhibiting angiotensin-converting enzyme: minimal role of bradykinin. Invest Ophthalmol Vis Sci 48(5):2321–2326. doi:10.1167/iovs.06-1296
Nagai N, Oike Y, Izumi-Nagai K, Urano T, Kubota Y, Noda K, Ozawa Y, Inoue M, Tsubota K, Suda T, Ishida S (2006) Angiotensin II type 1 receptor-mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Biol 26(10):2252–2259. doi:10.1161/01.ATV.0000240050.15321.fe
Takeda A, Baffi JZ, Kleinman ME, Cho WG, Nozaki M, Yamada K, Kaneko H, Albuquerque RJ, Dridi S, Saito K, Raisler BJ, Budd SJ, Geisen P, Munitz A, Ambati BK, Green MG, Ishibashi T, Wright JD, Humbles AA, Gerard CJ, Ogura Y, Pan Y, Smith JR, Grisanti S, Hartnett ME, Rothenberg ME, Ambati J (2009) CCR3 is a target for age-related macular degeneration diagnosis and therapy. Nature 460(7252):225–230. doi:10.1038/nature08151
Satofuka S, Ichihara A, Nagai N, Noda K, Ozawa Y, Fukamizu A, Tsubota K, Itoh H, Oike Y, Ishida S (2008) (Pro)renin receptor promotes choroidal neovascularization by activating its signal transduction and tissue renin-angiotensin system. Am J Pathol 173(6):1911–1918. doi:10.2353/ajpath.2008.080457
Du H, Sun X, Guma M, Luo J, Ouyang H, Zhang X, Zeng J, Quach J, Nguyen DH, Shaw PX, Karin M, Zhang K (2013) JNK inhibition reduces apoptosis and neovascularization in a murine model of age-related macular degeneration. Proc Natl Acad Sci USA 110(6):2377–2382. doi:10.1073/pnas.1221729110
Sheu SJ, Liu NC, Ou CC, Bee YS, Chen SC, Lin HC, Chan JY (2013) Resveratrol stimulates mitochondrial bioenergetics to protect retinal pigment epithelial cells from oxidative damage. Invest Ophthalmol Vis Sci 54(9):6426–6438. doi:10.1167/iovs.13-12024
Doyle SL, Campbell M, Ozaki E, Salomon RG, Mori A, Kenna PF, Farrar GJ, Kiang AS, Humphries MM, Lavelle EC, O'Neill LA, Hollyfield JG, Humphries P (2012) NLRP3 has a protective role in age-related macular degeneration through the induction of IL-18 by drusen components. Nat Med 18(5):791–798. doi:10.1038/nm.2717
Kaneko H, Dridi S, Tarallo V, Gelfand BD, Fowler BJ, Cho WG, Kleinman ME, Ponicsan SL, Hauswirth WW, Chiodo VA, Kariko K, Yoo JW, Lee DK, Hadziahmetovic M, Song Y, Misra S, Chaudhuri G, Buaas FW, Braun RE, Hinton DR, Zhang Q, Grossniklaus HE, Provis JM, Madigan MC, Milam AH, Justice NL, Albuquerque RJ, Blandford AD, Bogdanovich S, Hirano Y, Witta J, Fuchs E, Littman DR, Ambati BK, Rudin CM, Chong MM, Provost P, Kugel JF, Goodrich JA, Dunaief JL, Baffi JZ, Ambati J (2011) DICER1 deficit induces Alu RNA toxicity in age-related macular degeneration. Nature 471(7338):325–330. doi:10.1038/nature09830
Narimatsu T, Ozawa Y, Miyake S, Kubota S, Hirasawa M, Nagai N, Shimmura S, Tsubota K (2013) Disruption of cell–cell junctions and induction of pathological cytokines in the retinal pigment epithelium of light-exposed mice. Invest Ophthalmol Vis Sci 54(7):4555–4562. doi:10.1167/iovs.12-11572
Kerksick C, Willoughby D (2005) The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J Int Soc Sports Nutr 2:38–44. doi:10.1186/1550-2783-2-2-38
Riento K, Ridley AJ (2003) Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 4(6):446–456. doi:10.1038/nrm1128
Narimatsu T, Ozawa Y, Miyake S, Kubota S, Yuki K, Nagai N, Tsubota K (2013) Biological effects of blocking blue and other visible light on the mouse retina. Clin Exp Ophthalmol. doi:10.1111/ceo.12253
Tanito M, Kaidzu S, Anderson RE (2006) Protective effects of soft acrylic yellow filter against blue light-induced retinal damage in rats. Exp Eye Res 83(6):1493–1504. doi:10.1016/j.exer.2006.08.006
Acknowledgment
I wish to thank all the members of the Laboratory of Retinal Cell Biology (RCB lab) and Professor Kazuo Tsubota for supporting the retinal research program.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Ozawa, Y. (2014). Oxidative Stress in the RPE and Its Contribution to AMD Pathogenesis: Implication of Light Exposure. In: Nakazawa, T., Kitaoka, Y., Harada, T. (eds) Neuroprotection and Neuroregeneration for Retinal Diseases. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54965-9_17
Download citation
DOI: https://doi.org/10.1007/978-4-431-54965-9_17
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54964-2
Online ISBN: 978-4-431-54965-9
eBook Packages: MedicineMedicine (R0)