Abstract
Steady background light and bright bleaching illumination produce changes in rod photoreceptor sensitivity and response waveform that are collectively known as adaptation. In this chapter, we review the phenomenology of both background and bleaching adaptation. We then describe the evidence for our present understanding of the molecular mechanisms of adaptation in vertebrate rods to both background light and bleaching, including the role of Ca2+ as a second messenger and modulation of guanylyl cyclase and phosphodiesterase. We also describe continuing areas of uncertainty awaiting resolution from future experimentation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
4.1 Introduction
Stimulation of a sensory receptor produces an electrical response that is communicated to higher levels of the nervous system to signal detection (Fain 2003). Receptor responses are graded, which means that their amplitudes increase in proportion to the intensity of the stimulus. The magnitude of the proportionality constant varies as the mean level of stimulation is altered. This process, known as sensory adaptation, is a time-dependent change in the sensitivity or responsiveness of the receptor in the presence of a constant stimulus. It produces an alteration in the amplitude of the response to constant stimulation as well as to any stimulus given in the presence of the maintained stimulus. Adaptation modulates the amount of neurotransmitter released onto postsynaptic neurons and regulates the message conveyed to the central nervous system (CNS).
Vertebrate photoreceptors respond to changes in light intensity over an enormous range of ambient light levels. Rod cells, which are responsible for detection of dim light, can signal the absorption of a single photon of visible light by their photopigment rhodopsin (Baylor et al. 1979). These same cells can adapt to brighter light and remain responsive at intensities that activate more than 1,000 rhodopsins per second (for example, see Mendez et al. 2001; Woodruff et al. 2008). The adaptation of photoreceptors in the presence of constant background illumination is called background adaptation. Photoreceptors also adapt after they are exposed to light bright enough to bleach a significant fraction of their visual pigment. The decrease in sensitivity after such bright light exposure is called “bleaching adaptation” and resembles in many respects the adaptation produced by background light. Intact rods slowly recover their sensitivity after bleaching as the photopigment is regenerated, a phenomenon known as dark adaptation. Background and bleaching adaptation are collectively referred to as light adaptation. The mechanisms of light adaptation have been extensively investigated, and many of these earlier studies have been previously reviewed (Fain et al. 2001; Lamb and Pugh 2004; Fain 2011; Reuter 2011; Arshavsky and Burns 2012; Kefalov 2012).
In this chapter, we summarize our present knowledge of the molecular mechanism of adaptation in rod photoreceptors, emphasizing more recent results on mammalian (mouse) rods. A summary of visual transduction, fundamental to our understanding of adaptation, is given first. We then describe the regulation of key components of the transduction cascade likely to be responsible for the modulation of sensitivity.
4.2 Molecular Mechanism Visual Transduction
One of the hallmarks of phototransduction in rods is its extreme sensitivity. This sensitivity is achieved through a highly regulated, multistep amplification process that results in a significant increase in signal gain (Fain 2003). Transduction begins with an opsin protein molecule that contains 11-cis retinal, a small organic molecule called the chromophore. The opsin and chromophore together make up the rhodopsin photopigment. This pigment has a broad absorption spectrum peaking, in a mammalian rod, at wavelengths near 500 nm. When a molecule of rhodopsin absorbs a photon of light, 11-cis retinal undergoes photoisomerization to all-trans retinal, which in turn causes a change in the conformation of the opsin molecule (Choe et al. 2011; Standfuss et al. 2011) to produce a photoproduct called meta II or Rh*, which triggers visual excitation (Fig. 4.1).
Rh* then binds to and activates a heterotrimeric G protein called transducin, whose α-subunit is a member of the αi/αo family. Binding of transducin to Rh* causes an exchange of GTP for GDP on the transducin-α guanosine-binding site, producing activated transducin-α or Tα⋅GTP. Each Rh* can produce many Tα⋅GTPs, which bind to and activate the effector enzyme phosphodiesterase 6 (PDE6). The PDE6 then hydrolyzes many cytoplasmic cGMP molecules in the next phase of amplification. PDE6 continues to hydrolyze and decrease the internal concentration of cGMP for as long as transducin remains in its active GTP-bound state. The reduction in concentration of cGMP via light-activated PDE6 results in the closure of cGMP-gated cation channels in the plasma membrane, which reduces the permeability of the rod membrane to Na+, hyperpolarizes the membrane potential, and decreases the release of the synaptic transmitter glutamate from the photoreceptor synapse.
The recovery of the light response is achieved when all the activated components of the cascade are turned off and the concentration of intracellular cGMP is returned to its pre-stimulus level. Activated rhodopsin is extinguished by phosphorylation and binding of arrestin, which prevents further binding of transducin. Phosphodiesterase remains active until Tα⋅GTP is hydrolyzed to Tα⋅GDP. Although Tα can hydrolyze bound GTP without assistance, the rate is quite slow and is greatly accelerated by a complex of proteins called the GTPase-activating proteins or GAPs, of which there are three in photoreceptors: RGS9-1 (He et al. 1998), Gβ5L (Makino et al. 1999; He et al. 2000) and the R9AP protein, which anchors the GAP complex to the disc membrane (Hu and Wensel 2002). Tα⋅GDP then recombines with the G-protein beta and gamma subunits to form the inactive heterotrimer.
The reopening of the cGMP-gated channels requires restoration of the cGMP concentration, which is achieved by the activity of an outer segment, membrane-bound guanylyl cyclase. The activity of guanylyl cyclase is regulated by the cytoplasmic free Ca2+ concentration (Koch and Stryer 1988) by means of small molecular weight, Ca2+-binding proteins called guanylyl cyclase-activating proteins (GCAPs) (Polans et al. 1996; Dizhoor 2000). This regulation occurs in the following way. The closing of the cGMP-gated channels in the light produces a decreases in Ca2+ concentration in the rod outer segment. In a mouse rod the concentration declines from about 250 nM in darkness to about 25 nM in light bright enough to close all the channels, a range of concentration of about tenfold (Woodruff et al. 2002). In the dark, when the free Ca2+ concentration is high, the Ca2+-bound form of GCAP inhibits the cyclase. The decrease in free Ca2+ concentration produced by the closing of the cyclic nucleotide-gated channels causes Ca2+ to be released from the GCAPs, greatly increasing the rate of synthesis of cGMP. The Ca2+ dependence of the cyclase produces a feedback loop: light decreases cGMP, closes the channels, and decreases the Ca2+ concentration, and the decrease in Ca2+ then accelerates the cyclase and increases cGMP, counteracting the decrease in cGMP concentration produced by light. When the activity of the cyclase restores the cGMP concentration to its pre-stimulus level, the channels rapidly reopen (Karpen et al. 1988).
4.3 Background Adaptation
A single Rh* has been estimated to produce approximately 100 activated Tα⋅GTPs in an amphibian rod (Leskov et al. 2000; Makino et al. 2003) and perhaps closer to 20 in a mouse rod (Krispel et al. 2006). Each Tα⋅GTP activates a single catalytic subunit of PDE6, and each activated PDE6 catalytic subunit hydrolyzes several hundred molecules of cGMP per second. A single photon response at its peak, which occurs in a mouse rod about 250 ms after light absorption, can close about 5 % of the channels. Assuming photoreceptors simply summed the effects of each photon absorbed by rhodopsin molecules, fewer than 100 activated rhodopsins per second would lead to the closure of all the light-sensitive channels and would render the rods unresponsive to further illumination.
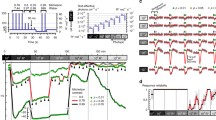
To prevent saturation, the rod response during exposure to continuous light immediately rises to a peak and then partially recovers. This relaxation is the result of reopening of some of the previously closed channels and is particularly evident at brighter intensities. Figure 4.2a demonstrates this phenomenon in suction-electrode recordings from the outer segments of mouse rods exposed to prolonged illumination. The rods adapt to the steady light; some of the channels begin to reopen, resulting in an increase in inward current. This process occurs in two phases: an initial rapid relaxation, followed by a more prolonged sag in current. Both the rapid and the slow decays can be fitted by exponential functions with time constants in mouse rods of a few hundred milliseconds and a few tens of seconds.
Adaptation of wild-type (WT) mouse rods. (a) Superimposed suction-electrode recordings of means of currents of seven WT rods, each exposed three times to steps of 500-nm light beginning at t = 0, of intensity 440 photons μm−2 s−1 and 10, 30, 60, and 120 s in duration. Inset: Same data at faster time resolution showing only first 4 s. (b) Flash responses recorded in background light. Means from five presentations each of ten WT rods to 20-ms flashes of 500-nm light beginning at t = 0; intensity was kept at 453 photons μm−2 for flashes in darkness and in backgrounds of the following intensities (in photons μm−2 s−1): 12, 38, 118, 438, and 1,354. Responses have been normalized cell by cell to the peak amplitude of the response and averaged. The most slowly decaying response is with no background; decay time course progressively accelerated as background intensity was increased. (c) Weber–Fechner plot. Sensitivity (S F) in pA photon−1 μm2 was calculated as the peak amplitude of the response in the linear range divided by the flash intensity. Graph gives mean sensitivity divided by sensitivity in the absence of a background (S DF ) as a function of background intensity (I B), averaged from ten WT rods. Solid line is given by Eq. (4.1) with a best-fitting value of I 0 = 77 photons μm−2 s−1. [Reprinted from Fain (2011) with permission of Springer US]
When rods are stimulated with a brief flash, first in darkness and then in the presence of continuous background light, the amplitude of the response to the flash becomes smaller in the presence of the background, reflecting a decrease in photoreceptor sensitivity (e.g., Baylor and Hodgkin 1974; Woodruff et al. 2008). Moreover, the time course of decay is accelerated, such that the brighter the background, the greater the acceleration. Figure 4.2b shows normalized responses to 20-ms flashes of constant intensity, which have been superimposed. The most slowly decaying response is the one recorded in the absence of a background light, and the other responses were recorded in progressively increasing background intensities. The decay of the flash response, at least for small-amplitude responses, follows a time course well fitted by a single exponential decay function, whose time constant is given as τREC. In a dark-adapted mouse rod, τREC has a value of about 200 ms, but this value decreases by a factor of 2 to about 100 ms in bright background light. A similar decrease occurs in the limiting time constant of response decay τD (Woodruff et al. 2008), indicating an acceleration in the rate of decay of light-activated PDE6 (Krispel et al. 2006; Tsang et al. 2006; Chen et al. 2010a). Response decay is slow in the dark to permit the cell to acquire and simultaneously sum as many photons as possible to maximize sensitivity. In brighter light, an abundance of photons allows rods to afford some reduction in the time over which the photoreceptors sum single photons, so that the photoreceptor can more readily detect change and motion. The acceleration of the time course of decay is partly responsible for the well-known increase in rod flicker-fusion frequency in background light.
For responses to brief flashes in the presence of a continuous background, the sensitivity decreases so that a brighter flash intensity is required to produce responses of same amplitude. In Fig. 4.2c, we show for mouse rods the sensitivity to a brief flash, S F, divided by the sensitivity in darkness, S DF . Sensitivities were calculated by dividing the response to a flash (in pA of current) by the flash intensity (in photons μm−2). The curve fitted to the data is known as the Weber–Fechner relationship:
where I B is the intensity of the background and I 0 is a constant called the dark light, which in Fig. 4.2c was 77 photons μm−2 s−1 or between 35 and 40 rhodopsin molecules bleached per second.
4.4 Bleaching Adaptation
In the experiments of Fig. 4.2, the changes in steady inward current, response decay time, and sensitivity all occurred in relatively dim light; the brightest light would have bleached much less than 1 % of the total amount of visual pigment. Once even the brightest background light used in the experiments of Fig. 4.2 was turned off, the rods rapidly recovered to their dark-adapted sensitivity. With brighter light exposures that bleach a significant fraction of the visual pigment, significant adaptation can occur even after the light has been extinguished. Some of the decrease in sensitivity after bleaching is simply the result of a decrease in the concentration of the photopigment, which reduces the “quantum catch” or probability of absorption of a photon. A significant sensitivity decrease is, however, also produced by a mechanism much like the one that decreases sensitivity in background light.
Stiles and Crawford (1932) first suggested that light bright enough to produce significant bleaching can lead to photoreceptor desensitization by producing an “equivalent” background light generated by the bleached pigment, which would then persist until the pigment is regenerated (Barlow 1964). One way of exploring the effect of bleaching and the equivalent background is to expose a rod, isolated from the rest of the retina and pigment epithelium, to light bleaching a significant fraction of the rhodopsin; and then to wait from 30 to 60 min for the circulating current and sensitivity of the rod to come to steady state. A rod isolated in this way can regenerate only a small fraction of its rhodopsin; as a consequence, large bleaches produce a significant amount of opsin without chromophore, which can act as an equivalent background and stimulate the visual cascade (Cornwall and Fain 1994).
The responses in Fig. 4.3 (from Nymark et al. 2012) were recorded from mouse rods in darkness and in steady state after bleaching from 5 % to 90 % of the photopigment. Bleaching produces a sustained decrease in circulating current caused by the closing of the cGMP-gated channels in the outer segment, very similar to that produced by continuous background light exposure. This decrease in circulating current is reflected in the progressive decrease in the maximum amplitude of the response to flashes in the records of Fig. 4.3. Bleaching also produces an acceleration in the decay of the response. In darkness, a half-maximal response requires approximately 1 s to return to baseline (Fig. 4.3a), whereas a half-maximal response after a 90 % bleach returns to baseline in about half the time (Fig. 4.3f).
Responses of mouse rods before and after exposure to bright bleaching light. Flashes were 20 ms in duration. (a) Averages of 18 dark-adapted rods. Flash intensities: 4, 17, 43, 159, 453, and 1,122 photons μm−2. (b) Averages of 12 rods at steady state after bleach of 5 % of photopigment. Flash intensities: 4, 17, 43, 159, 453, and 1,122 photons μm−2. (c) Averages of 8 rods at steady state after bleach of 10 % of photopigment. Flash intensities: 17, 43, 159, 453, and 1,122 photons μm−2. (d) Averages of 28 rods at steady state after bleach of 20 % of photopigment. Flash intensities: 17, 43, 159, 453, 1,120, and 1,870 photons μm−2. (e) Averages of 6 rods at steady state after bleach of 50 % of photopigment. Flash intensities: 43, 159, 453, 1,120, 3,250, and 10,500 photons μm−2. (f) Averages of 7 rods at steady state after bleach of 90 % of photopigment. Flash intensities: 159, 453, 1,120, 2,430, 4,230, and 10,500 photons μm−2. [Reprinted from Nymark et al. (2012) with permission of the Physiological Society]
Bleaches also produce a decrease in sensitivity. In Fig. 4.4 (also from Nymark et al. 2012), we have plotted on the ordinate the sensitivity as a fraction of sensitivity in darkness, as in Fig. 4.2c but now from rods at steady state after exposure to bleaches; and we have plotted on the abscissa the fraction of pigment bleached. The dashed line is the theoretical decrease in sensitivity expected only from the loss of quantum catch: when 90 % of the pigment is bleached, the sensitivity is decreased by a factor of ten because the rod has only one-tenth of its pigment remaining. The data lie uniformly above the dashed line, indicating that some additional process is reducing the sensitivity of the rods.
Sensitivity of mouse rods as a function of percent pigment bleached (calculated as in Fig. 4.2). Means with SE are plotted as a function of percent bleached for rods that were dark adapted (n = 38) or at steady state after bleaches of 5 % (n = 11), 10 % (n = 9), 20 % (n = 26), 50 % (n = 6), and 90 % (n = 7). Continuous curve is Eq. (4.4) with best-fitting value of k of 35. Dashed curve is change in sensitivity predicted by loss of quantum catch (S F/S FD = 1 − F). [Reprinted from Nymark et al. (2012) with permission of the Physiological Society]
For the solid line in Fig. 4.4, we assumed that the decrease in sensitivity consists of two components, one caused by the loss of quantum catch and an extra component from adaptation produced by activation of the cascade by the bleached pigment (Jones et al. 1996). Because the relative loss in sensitivity produced by the loss in quantum catch is equal simply to (1−F), the fraction of pigment remaining, we calculated the component from cascade activation, ΔS F/S DF , by removing the component caused by loss in quantum catch from the total loss in sensitivity by dividing by (1−F):
Following Jones et al. (1996), we then assumed that bleached pigment activates the cascade as does background light, that is, according to the Weber–Fechner relationship of Eq. (4.1). Moreover, we assumed that activation of transduction is linearly proportional to the fraction bleached, that is, to the opsin concentration (Cornwall and Fain 1994), with a proportionality constant given by k. From these assumptions and Eq. (4.1), the decrease in sensitivity ΔSF should be given by
Combining Eqs. (4.2) and (4.3), we have for the total decrease in sensitivity after a bleach
This relationship is the solid curve in Fig. 4.4 with a best-fitting value of k of 35.
The results in Fig. 4.4 show that desensitization after bleaching is a linear combination of the loss in quantum catch and adaptation produced by an equivalent background whose intensity is proportional to the fraction of pigment bleached. In an isolated rod at steady state, the equivalent background is produced by opsin (Cornwall and Fain 1994), probably phosphorylated and bound to arrestin. In an intact rod, other intermediates of bleaching can also contribute to the equivalent background, including (for small bleaches) reversion of the bleached pigment to Rh* and the binding of all-trans retinal to opsin (see Fain et al. 2001; Lamb and Pugh 2004). An additional component of desensitization can also be produced by translocation of transducin from the outer segment to the inner segment during bright light exposure, which reduces the gain of transduction (Sokolov et al. 2002; Majumder et al. 2013). The translocation of transducin is, however, unlikely to make any significant contribution to background adaptation, because translocation takes many minutes to complete and the light intensity required to produce significant migration of the protein is orders of magnitude brighter than the intensities used for the experiments of Fig. 4.2.
4.5 Calcium as the Second Messenger of Adaptation
Although it would be possible to imagine a mechanism of adaptation that was local and restricted, several observations indicate that the signal for the change in sensitivity can spread throughout the rod outer segment. Rods are desensitized by very dim illumination. The background intensity required to reduce sensitivity by half is given by I 0 in Eq. (4.1) and is only 35–40 Rh* per second in the mouse (see Fig. 4.2c) and 5–10 Rh* per second in amphibians (Fain 1986). The change in sensitivity occurs rather quickly after turning on the background light. Our experiments (in preparation) show that for background light even as dim as 12 photons μm−2 s−1 the decay of the flash response begins to be accelerated within 500 ms of the beginning of the background exposure. Because only a few tens of excited pigment molecules are sufficient to reduce sensitivity by a factor of two, and rods have of the order of 1,000–2,000 disks, some molecule must diffuse between the disks so that a pigment molecule bleached in one disk can affect the response of pigment molecules subsequently bleached in other disks. Outer segment current measurements with spatially localized adapting lights and test stimuli also indicate that the change in sensitivity can migrate up and down the outer segment (Lamb et al. 1981; Cornwall et al. 1990; Gray-Keller et al. 1999).
Deric Bownds (1980) first proposed that steady light causes a reduction in intracellular calcium concentration, which in some way regulates components of the transduction cascade to produce desensitization of the photoreceptor response. The role of Ca2+ in adaptation was first tested by Torre and collaborators (Torre et al. 1986), who incorporated the Ca2+ chelator BAPTA into rods with whole-cell patch recording and showed that an increase in Ca2+ buffering resulted in an increase in the maximum amplitude of the light response and a slowing of its decay—the inverse of the effect of background light. Stronger evidence of a role of Ca2+ was obtained by perfusing rods with an external solution in which Ca2+ was buffered to a low level (to prevent Ca2+ influx) and Na+ was substituted with Li+ or guanidinium (to block Na+/K+–Ca2+ exchange and prevent Ca2+ efflux). In such a low-Ca2+/zero-Na+ solution, the outer segment Ca2+ concentration is kept nearly constant for at least about 10 s (Matthews and Fain 2001), and, within this timeframe, responses to single photons sum linearly and adaptation is effectively eliminated (Matthews et al. 1988; Nakatani and Yau 1988).
Figure 4.5a compares responses of a salamander rod to a brief flash of light in Ringer solution and in low-Ca2+/zero-Na+ solution. Exposure to low-Ca2+/zero-Na+ produces an increase in the peak amplitude of the response and a marked prolongation of the time to peak and time course of decay, similar to incorporation of BAPTA in these cells. Other experiments show that the “sag” in current in response to steady background light no longer occurs, flash–response decay ceases to accelerate, and sensitivity declines with background intensity, not according to the Weber–Fechner relationship as in Fig. 4.2c but according to a theoretical curve predicted by the simple summation of responses to absorbed photons without any regulation of sensitivity (Matthews et al. 1988; Fain et al. 1989). Records similar to those in Fig. 4.5a have also been obtained from mammalian rods (unpublished data of M.L. Woodruff and G.L. Fain; see also Tamura et al. 1991), but more extensive investigations of the role of Ca2+ in mammalian photoreceptors have not yet been performed. The decrease in Ca2+ in a mouse rod occurs with two time constants of about 150 and 500–600 ms (Woodruff et al. 2002); in consequence, most of the reduction of Ca2+ will have taken place within 500 ms of turning on the background, a time course roughly consistent with the time course of background adaptation.
Ca2+ and guanylyl cyclase-activating protein (GCAP) regulation of photoreceptor response. (a) Small-amplitude responses of a salamander rod to 20-ms flashes of intensity of 2.8 photons μm−2 in Ringer (smaller response) and in low-Ca2+/zero-Na+ solution. (Modified from Fain et al. 1989.) (b) Averaged single-photon responses of wild-type mouse rod (+/+) and rod for which genes of GCAP proteins had been disrupted (−/−). (Modified from Mendez et al. 2001)
4.6 Regulation of Components of Signal Transduction by Ca2+
Several components of the photoreceptor signal cascade have been reported to be altered by changes in the free Ca2+ concentration. One of the most important is guanylyl cyclase, which, as we explained earlier, is regulated by Ca2+ binding GCAP molecules. When the genes for the GCAP proteins are knocked out (Mendez et al. 2001), the rod response is larger and decays more slowly (Fig. 4.5b), much as in low-Ca2+/zero-Na+ solution. When responses to flashes are recorded in the presence of background illumination in GCAPs −/− rods, sensitivity no longer declines according to the Weber–Fechner relationship (open circles in Fig. 4.6c), indicating that elimination of Ca2+ modulation of cyclase has a profound effect on adaptation. Adaptation is not however eliminated: the dotted and dashed lines in Fig. 4.6c are two alternative predictions of the relationship between sensitivity and background illumination in the absence of adaptation (see legend to figure). Both predictions give values of sensitivity that are orders of magnitude smaller than the sensitivity measured in GCAPs −/− rods (Mendez et al. 2001; Chen et al. 2010b), indicating that modulation of guanylyl cyclase cannot by itself explain the changes in rod response properties produced by background light.
Adaptation of GCAP −/− mouse rods. (a) Means of currents from three presentations each of 5 GCAP −/− rods to steps of light of intensity 38 photons μm−2 s−1 beginning at t = 0 for the following durations: 10, 30, 60, and 120 s. (b) Means from five presentations each of 7 GCAPs −/− rods to 20-ms flashes beginning at t = 0; intensity was kept at 17 photons μm−2 for flashes in darkness and backgrounds of the following intensities (in photons μm−2 s−1): 4, 13, 38, and 118. Responses have been normalized cell by cell to peak amplitude of the response and averaged. The most slowly decaying response is with no background; decay time course progressively accelerated as background intensity was increased. (c) Weber–Fechner plot as in Fig. 4.2c. Graph gives mean relative sensitivity as a function of background intensity, averaged from 10 WT rods (filled circles), 14 rods lacking the protein recoverin (filled squares), 13 rods from which the Ca2+-calmodulin-binding site of the cGMP-gated channel had been deleted (filled triangles), and 5 GCAPs−/− rods (open circles). Solid line is best-fitting Weber–Fechner function for WT rods given by Eq. (4.1) with I 0 = 77 photons μm−2 s−1. Black dashed and dotted curves give theoretical predictions of change of sensitivity produced by simple saturation (dashed, from Mendez et al. 2001) or in the absence of cyclase and PDE feedback (dotted, from Chen et al. 2010b). [Reprinted from Fain (2011) with permission of Springer US]
Further evidence for this claim is given in the remainder of Fig. 4.6. Figure 4.6a shows responses of GCAPs −/− rods to prolonged exposure to continuous illumination, much as in Fig. 4.2a for wild-type rods. The rapid component of response “sag” is eliminated when the GCAPs are deleted, suggesting that this component is produced by cyclase modulation (see also Calvert et al. 2002). The slower phase of the “sag” seems however to be entirely intact (Burns et al. 2002). The records in Fig. 4.6b show that response decay continues to accelerate in background light even after the GCAPs have been deleted. This aspect of light adaptation also seems to depend upon some other mechanism of transduction regulation.
What other components of transduction are affected by changes in Ca2+? Kawamura and Murakami (1991; Kawamura 1993) first described a small molecular weight protein that they called S-modulin but which is now more often referred to as recoverin (Dizhoor et al. 1991; Hurley et al. 1993; Kawamura et al. 1993). Recoverin has been shown to regulate the rate of rhodopsin kinase (Kawamura 1993; Chen et al. 1995) and modulate the lifetime of Rh* (Chen et al. 2010a). When the gene for recoverin is deleted, however, the relationship between sensitivity and background intensity is unaffected. The filled circles in Fig. 4.6c are from wild-type rods and the filled squares are from recoverin knockout animals (see Makino et al. 2004; Chen et al. 2010b). Regulation of rhodopsin lifetime seems to play little or no role in rod photoreceptor adaptation: recent experiments show that large increases and decreases in the expression level of rhodopsin kinase have minimal effects on rod transduction gain (Chen et al. 2012; Gross et al. 2012).
The channel can also be modulated: Ca2+ with calmodulin can alter the effective affinity of the rod cyclic nucleotide-gated channel for cGMP (Hsu and Molday 1993, 1994; Nakatani et al. 1995). The cyclic nucleotide-gated channel in a rod is a tetramer composed of CNGA1 and CNGB1 subunits (Weitz et al. 2002; Zheng et al. 2002; Zhong et al. 2002), and the CNGB1 subunit has a binding site for Ca2+-calmodulin (Grunwald et al. 1998; Weitz et al. 1998). When Ca2+ is relatively high in darkness, Ca2+-calmodulin is bound to the channel, and the affinity of the channel for cGMP is comparatively low. As Ca2+ falls in the light, Ca2+-calmodulin falls off of the CNGB1 subunit, increasing the affinity of the channel for cGMP and counteracting the light-dependent fall in cGMP concentration. The change in channel affinity is in a direction to produce channel reopening as the cGMP level decreases during illumination, so channel modulation could potentially contribute to the regulation of steady current and sensitivity in background light. When however the Ca2+-calmodulin binding site of the CNGB1 subunit was genetically deleted, there was a small change in the rate of decay of the photoreceptor response, especially to bright flashes, but little or no effect on the relationship between sensitivity and illumination in steady light (Fig. 4.6c, filled triangles; from Chen et al. 2010b).
4.7 Regulation of Phosphodiesterase
The results in Fig. 4.6 show that modulation of guanylyl cyclase by Ca2+ and the GCAPs makes a significant contribution to adaptation in background illumination, but that much of the adaptation nevertheless survives the genetic deletion of the GCAPs. Moreover, two further Ca2+-dependent mechanisms of transduction regulation that have been discovered, namely, modulation of the rate of rhodopsin phosphorylation by rhodopsin kinase and recoverin and alteration of the effective affinity of the channel for cGMP by Ca2+-calmodulin, have little role in the control of sensitivity during background illumination.
What is left? Four observations suggest that adaptation to background light in a mammalian rod can be produced in part by modulation of PDE6. These results are as follows. (1) Background light produces a decrease in the limiting time constant of response decay τD (Woodruff et al. 2008). As considerable evidence now indicates that the limiting time constant is a reflection of the rate of decay of light-activated PDE6 (Krispel et al. 2006; Tsang et al. 2006; Chen et al. 2010a), the rate of decay of PDE6 is likely to be accelerated in background light. (2) Mutations of the PDE6 γ-subunit can alter the relationship between sensitivity and background intensity (Woodruff et al. 2008). Although the interpretation of these experiments is not wholly free of difficulty, they do suggest that PDE6 modulation may play a role in sensitivity regulation. (3) The response of GCAPs −/− rods to prolonged illumination is followed by a pronounced current overshoot (see Fig. 4.6a). This overshoot cannot be produced by modulation of Rh*. Modulation of Rh* could not cause the current to become larger after the light is turned off than it was before the rod was illuminated; nor can the undershoots be produced by channel modulation, because they are even larger in GCAPs −/− rods that also lack the binding site for Ca2+-calmodulin (Chen et al. 2010b). There is practically nothing left but regulation of PDE6. (4) A model incorporating modulation of both spontaneous and light-activated PDE6 activity by Ca2+ can successfully account for the changes in sensitivity and time course of rod responses in background light (Chen et al. 2010b).
Although none of these observations is definitive, in aggregate they make a relatively strong case for light-dependent modulation of PDE6. Recent experiments suggest that at least part of this modulation may be produced by rhodopsin kinase (Chen et al. 2012). Both increases in kinase expression and the knocking out of recoverin can accelerate the limiting time constant of rod decay, which, as we have said, is apparently a measure of the rate of decay of PDE6. Moreover, the modulation of the time course of rod response decay in background light is nearly eliminated by deletion of the gene for recoverin, raising the interesting possibility that regulation of sensitivity and regulation of response decay are produced by somewhat different mechanisms.
4.8 Molecular Mechanism of Adaptation
The evidence that we presently have supports the following tentative conception of adaptation in mammalian rods. Background light and bleaches produce a steady decrease in circulating current, which reduces the outer segment free Ca2+ concentration. The decrease in Ca2+ increases the activity of guanylyl cyclase via the GCAP proteins, and activation of cyclase causes an increase in cGMP and a reopening of the channels. The modulation of cyclase is responsible for a rapid component of current recovery during maintained light exposure and some part of the modulation of sensitivity in background light, but it is not responsible for all the modulation of sensitivity, nor the slower component of current recovery, nor the acceleration of the time course of response decay in backgrounds or after bleaches. Neither modulation of rhodopsin lifetime nor regulation of the cGMP-gated channels by Ca2+-calmodulin seems to make any significant contribution to adaptation. In contrast, there is increasing evidence for an important component produced by direct modulation of PDE6, perhaps in concert with rhodopsin kinase and recoverin. Rhodopsin kinase has the well known role of phosphorylating and turning off Rh*, but it may also phosphorylate PDE6 or some other protein that regulates PDE6. Translocation of transducin may have a small effect on sensitivity during recovery from bleaching but makes no contribution to adaptation in steady background light. The principal functions of transducin translocation seem to be conservation of energy, protection from photoreceptor degeneration, and modulation of the gain of the photoreceptor synapse (Fain 2006; Majumder et al. 2013).
Where do we go from here? One of the biggest difficulties that confronts us is the paucity of information about the biochemistry of PDE6. The rod PDE6 enzyme is a tetramer, consisting of catalytic α- and β-subunits and two inhibitory γ-subunits. All these proteins are potential sites of phosphorylation. Other mechanisms of regulation are also conceivable. Until we have a more detailed understanding of this intriguing molecule, we will have difficulty designing experiments to elucidate its role in photoreceptor physiology. The challenge for the future is to discover further mechanisms of regulation, and then to combine this knowledge with genetic and physiological approaches to discover the role of PDE6 and other possible modulators in background and bleaching adaptation. Ten years ago, we thought that adaptation was mostly solved. We now understand that there is still much work to do.
References
Arshavsky VY, Burns ME (2012) Photoreceptor signaling: supporting vision across a wide range of light intensities. J Biol Chem 287:1620–1626
Barlow HB (1964) Dark-adaptation: a new hypothesis. Vision Res 4:47–58
Baylor DA, Hodgkin AL (1974) Changes in time scale and sensitivity in turtle photoreceptors. J Physiol (Lond) 242:729–758
Baylor DA, Lamb TD, Yau KW (1979) Responses of retinal rods to single photons. J Physiol (Lond) 288:613–634
Bownds MD (1980) Biochemical steps in visual transduction: roles for nucleotides and calcium ions. Photochem Photobiol 32:487–490
Burns ME, Mendez A, Chen J, Baylor DA (2002) Dynamics of cyclic GMP synthesis in retinal rods. Neuron 36:81–91
Calvert PD, Govardovskii VI, Arshavsky VY, Makino CL (2002) Two temporal phases of light adaptation in retinal rods. J Gen Physiol 119:129–145
Chen CK, Inglese J, Lefkowitz RJ, Hurley JB (1995) Ca(2+)-dependent interaction of recoverin with rhodopsin kinase. J Biol Chem 270:18060–18066
Chen CK, Woodruff ML, Chen FS, Chen D, Fain GL (2010a) Background light produces a recoverin-dependent modulation of activated-rhodopsin lifetime in mouse rods. J Neurosci 30:1213–1220
Chen J, Woodruff ML, Wang T, Concepcion F, Tranchina D, Fain GL (2010b) Channel modulation and the mechanism of light adaptation in mouse rods. J Neurosci 30:16232–16240
Chen CK, Woodruff ML, Chen FS, Chen Y, Cilluffo MC, Tranchina D, Fain GL (2012) Modulation of mouse rod response decay by rhodopsin kinase and recoverin. J Neurosci 32:15998–16006
Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP (2011) Crystal structure of metarhodopsin II. Nature (Lond) 471:651–655
Cornwall MC, Fain GL (1994) Bleached pigment activates transduction in isolated rods of the salamander retina. J Physiol (Lond) 480:261–279
Cornwall MC, Fein A, MacNichol EF Jr (1990) Cellular mechanisms that underlie bleaching and background adaptation. J Gen Physiol 96:345–372
Dizhoor AM (2000) Regulation of cGMP synthesis in photoreceptors: role in signal transduction and congenital diseases of the retina. Cell Signal 12:711–719
Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L (1991) Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science 251:915–918
Fain GL (1986) Evidence for a role of messenger substances in phototransduction. In: Stieve H (ed) The molecular mechanism of photoreception. Springer-Verlag, Berlin, pp 67–77
Fain GL (2003) Sensory transduction. Sinauer, Sunderland, MA
Fain GL (2006) Why photoreceptors die (and why they don’t). Bioessays 28:344–354
Fain GL (2011) Adaptation of mammalian photoreceptors to background light: putative role for direct modulation of phosphodiesterase. Mol Neurobiol 44:374–382
Fain GL, Lamb TD, Matthews HR, Murphy RL (1989) Cytoplasmic calcium as the messenger for light adaptation in salamander rods. J Physiol (Lond) 416:215–243
Fain GL, Matthews HR, Cornwall MC, Koutalos Y (2001) Adaptation in vertebrate photoreceptors. Physiol Rev 81:117–151
Gray-Keller M, Denk W, Shraiman B, Detwiler PB (1999) Longitudinal spread of second messenger signals in isolated rod outer segments of lizards. J Physiol (Lond) 519(pt 3):679–692
Gross OP, Pugh EN Jr, Burns ME (2012) Calcium feedback to cGMP synthesis strongly attenuates single-photon responses driven by long rhodopsin lifetimes. Neuron 76:370–382
Grunwald ME, Yu WP, Yu HH, Yau KW (1998) Identification of a domain on the beta-subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium-calmodulin. J Biol Chem 273:9148–9157
He W, Cowan CW, Wensel TG (1998) RGS9, a GTPase accelerator for phototransduction. Neuron 20:95–102
He W, Lu L, Zhang X, El-Hodiri HM, Chen CK, Slep KC, Simon MI, Jamrich M, Wensel TG (2000) Modules in the photoreceptor RGS9-1.Gbeta 5L GTPase-accelerating protein complex control effector coupling, GTPase acceleration, protein folding, and stability. J Biol Chem 275:37093–37100
Hsu YT, Molday RS (1993) Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin [published erratum appears in Nature 1993 Sep 16;365(6443):279] [see comments]. Nature (Lond) 361:76–79
Hsu YT, Molday RS (1994) Interaction of calmodulin with the cyclic GMP-gated channel of rod photoreceptor cells. Modulation of activity, affinity purification, and localization. J Biol Chem 269:29765–29770
Hu G, Wensel TG (2002) R9AP, a membrane anchor for the photoreceptor GTPase accelerating protein, RGS9-1. Proc Natl Acad Sci 99:9755–9760
Hurley JB, Dizhoor AM, Ray S, Stryer L (1993) Recoverin’s role: conclusion withdrawn [letter; comment]. Science 260:740
Jones GJ, Cornwall MC, Fain GL (1996) Equivalence of background and bleaching desensitization in isolated rod photoreceptors of the larval tiger salamander. J Gen Physiol 108:333–340
Karpen JW, Zimmerman AL, Stryer L, Baylor DA (1988) Gating kinetics of the cyclic-GMP-activated channel of retinal rods: flash photolysis and voltage-jump studies. Proc Natl Acad Sci USA 85:1287–1291
Kawamura S (1993) Rhodopsin phosphorylation as a mechanism of cyclic GMP phosphodiesterase regulation by S-modulin. Nature (Lond) 362:855–857
Kawamura S, Murakami M (1991) Calcium-dependent regulation of cyclic GMP phosphodiesterase by a protein from frog retinal rods. Nature (Lond) 349:420–423
Kawamura S, Hisatomi O, Kayada S, Tokunaga F, Kuo CH (1993) Recoverin has S-modulin activity in frog rods. J Biol Chem 268:14579–14582
Kefalov VJ (2012) Rod and cone visual pigments and phototransduction through pharmacological, genetic, and physiological approaches. J Biol Chem 287:1635–1641
Koch KW, Stryer L (1988) Highly cooperative feedback control of retinal rod guanylate cyclase by calcium ions. Nature (Lond) 334:64–66
Krispel CM, Chen D, Melling N, Chen YJ, Martemyanov KA, Quillinan N, Arshavsky VY, Wensel TG, Chen CK, Burns ME (2006) RGS expression rate-limits recovery of rod photoresponses. Neuron 51:409–416
Lamb TD, Pugh EN Jr (2004) Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res 23:307–380
Lamb TD, McNaughton PA, Yau KW (1981) Spatial spread of activation and background desensitization in toad rod outer segments. J Physiol (Lond) 319:463–496
Leskov IB, Klenchin VA, Handy JW, Whitlock GG, Govardovskii VI, Bownds MD, Lamb TD, Pugh EN Jr, Arshavsky VY (2000) The gain of rod phototransduction: reconciliation of biochemical and electrophysiological measurements. Neuron 27:525–537
Majumder A, Pahlberg J, Boyd KK, Kerov V, Kolandaivelu S, Ramamurthy V, Sampath AP, Artemyev NO (2013) Transducin translocation contributes to rod survival and enhances synaptic transmission from rods to rod bipolar cells. Proc Natl Acad Sci USA 25(1):341–348
Makino ER, Handy JW, Li T, Arshavsky VY (1999) The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5G protein beta subunit. Proc Natl Acad Sci USA 96:1947–1952
Makino CL, Wen XH, Lem J (2003) Piecing together the timetable for visual transduction with transgenic animals. Curr Opin Neurobiol 13:404–412
Makino CL, Dodd RL, Chen J, Burns ME, Roca A, Simon MI, Baylor DA (2004) Recoverin regulates light-dependent phosphodiesterase activity in retinal rods. J Gen Physiol 123:729–741
Matthews HR, Fain GL (2001) A light-dependent increase in free Ca2+ concentration in the salamander rod outer segment. J Physiol (Lond) 532:305–321
Matthews HR, Murphy RL, Fain GL, Lamb TD (1988) Photoreceptor light adaptation is mediated by cytoplasmic calcium concentration. Nature (Lond) 334:67–69
Mendez A, Burns ME, Sokal I, Dizhoor AM, Baehr W, Palczewski K, Baylor DA, Chen J (2001) Role of guanylate cyclase-activating proteins (GCAPs) in setting the flash sensitivity of rod photoreceptors. Proc Natl Acad Sci USA 98:9948–9953
Nakatani K, Yau KW (1988) Calcium and light adaptation in retinal rods and cones. Nature (Lond) 334:69–71
Nakatani K, Koutalos Y, Yau KW (1995) Ca2+ modulation of the cGMP-gated channel of bullfrog retinal rod photoreceptors. J Physiol (Lond) 484:69–76
Nymark S, Frederiksen R, Woodruff ML, Cornwall MC, Fain GL (2012) Bleaching of mouse rods: microspectrophotometry and suction-electrode recording. J Physiol (Lond) 590:2353–2364
Polans A, Baehr W, Palczewski K (1996) Turned on by Ca2+! The physiology and pathology of Ca2+-binding proteins in the retina. Trends Neurosci 19:547–554
Reuter T (2011) Fifty years of dark adaptation: 1961–2011. Vision Res 51:2243–2262
Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN Jr, Arshavsky VY (2002) Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron 34:95–106
Standfuss J, Edwards PC, D’Antona A, Fransen M, Xie G, Oprian DD, Schertler GF (2011) The structural basis of agonist-induced activation in constitutively active rhodopsin. Nature (Lond) 471:656–660
Stiles WS, Crawford BH (1932) Equivalent adaptation levels in localized retinal areas. In: Report of a joint discussion on vision, June 3, 1932, Imperial College of Science. Physical Society, London, pp 194–211
Tamura T, Nakatani K, Yau KW (1991) Calcium feedback and sensitivity regulation in primate rods. J Gen Physiol 98:95–130
Torre V, Matthews HR, Lamb TD (1986) Role of calcium in regulating the cyclic GMP cascade of phototransduction in retinal rods. Proc Natl Acad Sci USA 83:7109–7113
Tsang SH, Woodruff ML, Chen CK, Yamashita CY, Cilluffo MC, Rao AL, Farber DB, Fain GL (2006) GAP-independent termination of photoreceptor light response by excess gamma subunit of the c-GMP-phosphodiesterase. J Neurosci 26:4472–4480
Weitz D, Zoche M, Muller F, Beyermann M, Korschen HG, Kaupp UB, Koch KW (1998) Calmodulin controls the rod photoreceptor CNG channel through an unconventional binding site in the N-terminus of the beta-subunit. EMBO J 17:2273–2284
Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB (2002) Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron 36:881–889
Woodruff ML, Sampath AP, Matthews HR, Krasnoperova NV, Lem J, Fain GL (2002) Measurement of cytoplasmic calcium concentration in the rods of wild-type and transducin knock-out mice. J Physiol (Lond) 542:843–854
Woodruff ML, Janisch KM, Peshenko IV, Dizhoor AM, Tsang SH, Fain GL (2008) Modulation of phosphodiesterase 6 turnoff during background illumination in mouse rod photoreceptors. J Neurosci 28:2064–2074
Zheng J, Trudeau MC, Zagotta WN (2002) Rod cyclic nucleotide-gated channels have a stoichiometry of three CNGA1 subunits and one CNGB1 subunit. Neuron 36:891–896
Zhong H, Molday LL, Molday RS, Yau KW (2002) The heteromeric cyclic nucleotide-gated channel adopts a 3A:1B stoichiometry. Nature (Lond) 420:193–198
Acknowledgments
Work in our laboratory would not have been possible without the assistance of many able (and congenial) collaborators, including (in alphabetical order) Jason Chen, Jeannie Chen, Carter Cornwall, Alex Dizhoor, Hugh Matthews, Dan Tranchina, and Steve Tsang. We are also grateful to Margery J. Fain for drawing Fig. 4.1. Our research has been generously supported during the last 37 years by NIH R01 EY01844.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2014 Springer Japan
About this chapter
Cite this chapter
Morshedian, A., Fain, G.L. (2014). Molecular Mechanism of Adaptation in Vertebrate Rods. In: Furukawa, T., Hurley, J., Kawamura, S. (eds) Vertebrate Photoreceptors. Springer, Tokyo. https://doi.org/10.1007/978-4-431-54880-5_4
Download citation
DOI: https://doi.org/10.1007/978-4-431-54880-5_4
Published:
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-54879-9
Online ISBN: 978-4-431-54880-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)